Abstract
Objective
To investigate the frequency and clinical characteristics of ischemic stroke patients with early seizures, especially with cortical involvement contralateral to their focal seizures.
Methods
We retrospectively studied patients with ischemic stroke admitted to our hospital. We compared the clinical characteristics of patients with and without early seizures (occurring within seven days of the stroke onset). In addition, we divided the patients with early focal seizures into two groups (patients with and without cortical involvement of a recent infarct contralateral to their focal seizure) and compared the clinical characteristics of the groups.
Results
Of the 5,806 patients with ischemic stroke, 65 (1.2%) were diagnosed with early seizures. A history of ischemic stroke [odds ratio (OR) 1.71], a history of seizures (OR 27.58), and a National Institutes of Health Stroke Scale score on admission (OR 1.07) were significant and independent factors associated with the presence of early seizures. Of these 65 patients, 56 had focal seizures, while the others had generalized or undetermined seizures. Cortical involvement of a recent infarct contralateral to their focal seizures was observed in 24 of these 56 patients (43%). Glucose and hemoglobin A1c levels were significantly higher in patients with cortical involvement of a recent infarct contralateral to their focal seizures than in those with infarcts in other regions.
Conclusion
These findings suggest that recent infarcts play a role as systemic causes of acute symptomatic seizures as well as an epileptogenic lesion in ischemic stroke patients with early focal seizures.
Keywords: early seizure, ischemic stroke, epileptogenic lesion, acute symptomatic seizure
Introduction
Early seizures are observed in 1-14% of patients with ischemic stroke and 3-24% of patients with hemorrhagic stroke (Table 1) (1-24). The presence of early seizures in stroke patients is a predictor of a poor outcome (1,2,15).
Table 1.
Early Seizures Reported in the Literature after 2000.
| Inclusion criteria | NIHSS on admission seizures present/absent |
Incidence of early seizures (%) |
Risk factor | Reference | |||
|---|---|---|---|---|---|---|---|
| Past seizures | After stroke day | Infra-tentorial stroke | Other conditions | ||||
| Including patients with past stroke | |||||||
| - | 14 | + | n.d. | n.d. | BI: 78/632 (5) | BI: cortical location, severe disability | 1 |
| ICH: 21/265 (8) | ICH: cortical location | ||||||
| n.d. | 2 | + | Atherothr- | n.d. | BI: 10/442 (2) | Occipital lobe involvement, decreased | 2 |
| ombotic | Oconsciousness | ||||||
| n.d. | 7 | n.d. | 18-55 y.o. | n.d. | BI: 14/582 (3) | Rankin Scale, cortical involvement | 3 |
| cryptogenic | |||||||
| + | 7 | + | TIA (-) ≥40 | n.d. | BI: 8/177 (5) | n.d. | 4 |
| y.o. | ICH: 3/25 (12) | ||||||
| - | 14 | n.d. | n.d. | n.d. | BI and ICH: | All: ICH, lesion size, stroke severity, age, | 5 |
| 4/1,195 (0.3) | early seizures, cortical involvement | ||||||
| n.d. | 7 | n.d. | n.d. | Mean 11/12 | BI: 12/1,235 (1) | None | 6 |
| - | 7 | + | n.d. | Onset seizure | ICH: 71/522 (14) | Early seizures: cortical involvement | 7 |
| median 22/16 | Onset seizure | onset seizures: previous ICH, cortical | |||||
| ICH: 38/522(7) | involvement, age, NIHSS | ||||||
| - | 7 | + | n.d. | n.d. | BI: 58/1,742 (3) | All: NIHSS, anterior circulation infarct, | 8 |
| ICH: 8/311 (3) | hemorrhagic transformation, hyperglycemia | ||||||
| + | 7 | n.d. | n.d. | Median 15/9 | BI: 28/2,327 (1) | Cortical involvement, thrombolysis | 9 |
| n.d. | 7 | n.d. | n.d. | Median 19/15 | ICH: 83/1,920 (4) | Early and late seizures: cortical involvement, | 10 |
| NIHSS, no hypertension | |||||||
| n.d. | 7 | - | n.d. | 11/8 | BI: 115/2,805 (1) | Early and late seizures: cardioembolism, | 11 |
| multiple territory infarct, recanalization, | |||||||
| hemorrhagic transformation | |||||||
| n.d. | 7 | + | n.d. | Median 34/16 | ICH: 9/297 (3) | NIHSS, coronary artery disease | 12 |
| n.d. | 7 | n.d. | Large vessel | Median 15/12 | BI: 38/979 (4) | NIHSS | 13 |
| occlusion | |||||||
| + | 7 | + | n.d. | Median 19/4 | BI: 65/5,806 (1) | Previous BI, previous seizures, NIHSS | Present |
| study | |||||||
| Excluding patients with past stroke | |||||||
| - | 14 | n.d. | n.d. | n.d. | BI: 26/2,742 (1) | n.d. | 14 |
| ICH: 27/463 (6) | |||||||
| n.d. | 7 | n.d. | ≥40 y.o. | Total mean 15/9 | BI: 22/704 (3) | All: cortical involvement, ICH | 15 |
| ICH: 11/150 (7) | |||||||
| SAH: 4/50 (8) | |||||||
| - | 7 | n.d. | TIA (-) | Mean 13/10 | BI: 26/543 (5) | All: large lesion size, hemorrhagic | 16 |
| ICH: 5/95 (5) | transformation | ||||||
| - | 7 | + | n.d. | n.d. | BI: 28/709 (5) | All: ICH, cortical involvement | 17 |
| ICH: 17/105 (16) | |||||||
| - | 7 | n.d. | n.d. | Mean 18/14 | BI: 28/224 (13) | All: hemorrhagic stroke, NIHSS, alcoholism, | 18 |
| ICH: 51/217 (24) | cortical location | ||||||
| n.d. | 7 | - | n.d. | Mean 8/4 | BI: 39/375 (10) | Early and late seizures | 19 |
| ICH: 7/32 (22) | all: NIHSS, IVT | ||||||
| IVT: 8/14 (57) | BI: large artery disease | ||||||
| n.d. | 7 | n.d. | n.d. | Mean 13/8 | BI: 12/436 (3) | All: complication | 20 |
| ICH: 8/80 (10) | |||||||
| - | 7 | n.d. | ≤50 y.o. | Median 9/3 | BI: 21/631 (3) | Early and late seizures: age, NIHSS, | 21 |
| ICH: 4/66 (6) | cardioembolic stroke, normotension | ||||||
| - | 7 | n.d. | n.d. | n.d. | BI: 63/1,832 (3) | No statin administration | 22 |
| - | 7 | n.d. | TIA (-) | n.d. | BI: 12/140 (9) | Cortical involvement, female | 23 |
| Meta-analysis | |||||||
| n.d. | 1-28 | n.d. | n.d. | n.d. | BI: 1,031/34,018 (3) | n.d. | 24 |
BI: brain infarction, ICH: intracerebral hemorrhagic stroke, IVT: intracranial venous thrombosis, n.d.: not described, NIHSS: National Institutes of Health Stroke Scale, SAH: subarachnoid hemorrhage, TIA: transient ischemic attack
Beghi et al. defined an acute symptomatic seizure as a clinical seizure occurring at the time of a systemic insult or in close temporal association with a documented brain insult (17,25). They also defined an acute symptomatic seizure as an event that occurs within one week of stroke, traumatic brain injury, anoxic encephalopathy, or intracranial surgery.
Conversely, Lüders et al. defined and classified the concepts of brain regions associated with focal seizures into six zones: epileptogenic zone, seizure onset zone, irritable zone, ictal symptomatogenic zone, functional deficit zone, and epileptogenic lesion (26). Among these zones, an epileptogenic lesion was defined as a macroscopic lesion that causes epileptic seizures, as the lesion itself may be epileptogenic or may cause secondary hyperexcitability of the adjacent cortex. When a stroke patient develops early focal seizures, such seizure-associated brain regions are assumed to be located contralaterally to the seizures. In addition, the epileptogenic lesion directly responsible for early focal seizures in acute stroke may not be a new infarct but rather an old brain injury due to a past stroke or trauma, and such patients may not show neuro-radiologically apparent brain damage, such as dementia or metabolic disease.
We hypothesized that the three following brain regions might be responsible for early focal seizures: i) a recent infarct; ii) neuro-radiologically evident past brain injury, including stroke, tumor, and trauma; and iii) occult past brain damage, including dementia, autoimmune encephalitis, and metabolic disease. If a patient develops early focal seizures via mechanisms ii) or iii), then a recent infarct might be only one systemic cause of acute symptomatic seizures (25) and not the region directly responsible for early focal seizures as an epileptogenic lesion.
Whether or not prophylactic anti-epileptic agents should be administered to patients with early seizures is controversial, as they do not always develop late seizures (24,27). We believe that determining whether a recent infarct is a systemic cause of acute symptomatic seizures or an epileptogenic lesion directly responsible for early focal seizures would be useful for making decisions concerning prophylactic administration of anti-epileptic agents after early seizures. However, to our knowledge, no studies have established the frequency and clinical characteristics of patients with cortical involvement of a recent infarct contralateral to early focal seizures.
We conducted a study in which ischemic stroke patients with early seizures were evaluated with further neurological examinations and neuroimaging. Our aim was to investigate the clinical characteristics of ischemic stroke patients with early seizures, focusing on the cortical involvement of a recent infarct contralateral to their focal seizures.
Materials and Methods
This was a retrospective study of consecutive acute ischemic stroke patients who were admitted to our hospital within seven days of the onset of ischemic stroke or transient ischemic attack, from April 1, 2013, to March 31, 2021. Patients with a history of stroke or seizures were also included in the present study.
All of the patients were initially examined by neurologists on admission and evaluated with brain magnetic resonance imaging (MRI)/magnetic resonance angiography; if these modalities were not available, computed tomography was conducted.
The study protocol followed the principles outlined in the Declaration of Helsinki and was approved by the ethics committee of our hospital (Judgement number 524). All patients provided their written consent for the use of their medical data.
Inclusion criteria and classification of early seizures
Seizure recording was based on the clinical diagnosis, regardless of the findings of electroencephalography (EEG). Seizures were defined according to the International League Against Epilepsy as paroxysmal disorders of the central nervous systems with or without loss of consciousness or awareness and with or without motor involvement (28). However, in the present study, seizures without motor involvement, such as non-convulsive status epilepticus, were excluded because they might have been missed by witnesses, even if they were medical staff. Indeed, non-convulsive status epilepticus can only be identified by EEG monitoring in patients with severe brain diseases (29). We also diagnosed early seizures regardless of the findings of diffusion-weighted imaging, in which hyperintense lesions are often observed in patients with status epilepticus (30).
Seizures were classified as focal (including focal seizures with secondary generalization) or generalized according to careful interviews with witnesses including caregivers and emergency medical service or hospital staff. Early seizures were defined according to the International League Against Epilepsy guidelines as those occurring within 7 days of stroke (31).
Attending physicians evaluated seizure classification. In patients with focal seizures, they also assessed the clinical laterality of seizures according to interviews with witnesses, the limbs where the seizures started and/or ended, eye position, Todd's palsy, and motor involvement. According to the above-mentioned medical information, the attending physicians diagnosed the subtype and laterality of early seizures. The physicians did not refer to the EEG findings of the patients in their diagnosis regarding the subtype and laterality of early seizures.
Neuro-radiological studies
MRI was performed using a Siemens MAGNETOM Vision 1.5-Tesla MR unit (Siemens, Munich, Germany) with echo-planar capability and was carried out on admission and in the subacute phase if the responsible lesions for seizures or neurological deficits were not detected. Recent and old brain lesions were classified into the following subgroups in relation to the side of the focal seizures: contralateral cortical group (involving the cerebral cortex), contralateral subcortical group, ipsilateral group, and infratentorial group (involving the brainstem or cerebellum). Lesions that involved both the contralateral cortex and subcortical area were included in the contralateral cortical group.
Statistical analyses
First, we divided the patients into two subgroups according to the presence of early seizures and compared the clinical characteristics between these groups with respect to the following: age, sex, medical history, premorbid modified Rankin Scale (mRS) score, vital signs and National Institutes of Health Stroke Scale (NIHSS) score on admission, stroke subtype according to the definitions given in the Trial of ORG 10172 in Acute Stroke Treatment study classification, lesion sites of recent infarcts and past brain injuries, arterial territory of recent infarcts, laboratory examinations, and NIHSS and mRS scores at discharge.
In addition, we divided the patients with early focal seizures into two groups (with and without cortical involvement of a recent infarct contralateral to their focal seizures) and compared the clinical characteristics between these groups.
The Mann-Whitney U test or Student's t-test was used to compare the age, vital signs, mRS and NIHSS scores, and laboratory test results between two groups. Other clinical characteristics evaluated by a nominal scale were compared between two groups using the chi-square test. Subsequently, the factors considered to be potentially important were entered into a multivariate logistic regression analysis to determine the adjusted odds ratio (OR).
All statistical analyses were performed using a commercially available software package (JMP, version 12; SAS Institute, Cary, USA). p values <0.05 were considered statistically significant.
Results
In total, 5,806 patients with ischemic stroke were admitted to our hospital during the 8-year study period. Sixty-five (1.2%) patients were diagnosed with early seizures (Supplementary Material 1). Two patients had simple focal seizures, 54 had focal seizures (11 with secondary generalization), and 9 had generalized seizures (3 with myoclonic seizures) or unknown laterality. No patients had bilateral focal seizures within their acute phase.
Clinical characteristics of patients with or without early seizures
The clinical characteristics of patients with or without early seizures are shown in Table 2. On a univariate analysis, current smoking, atrial fibrillation, a history of ischemic stroke, history of seizures, premorbid mRS score, NIHSS score on admission, stroke subtype, site of lesion, and hematocrit, brain natriuretic peptide, and D-dimer levels were significantly different between the groups.
Table 2.
Clinical Characteristics of Patients with/ without Early Seizures.
| With early seizures (n=65) | Without early seizures (n=5,741) | p value | Multivariate study* | |
|---|---|---|---|---|
| Age, years, median (IQR) | 78 (70-86) | 76 (68-85) | 0.267 | |
| Sex, male (%) | 35 (54) | 3,383 (59) | 0.408 | |
| Medical history (%) | ||||
| Hypertension | 48 (74) | 4,553 (79) | 0.272 | |
| Diabetes mellitus | 19 (29) | 1,783 (31) | 0.752 | |
| Dyslipidemia | 25 (39) | 2,600 (45) | 0.272 | |
| Current smoking | 3 (5) | 992 (17) | 0.004† | |
| Atrial fibrillation | 30 (46) | 1,615 (28) | 0.001 † | |
| Ischemic heart disease | 10 (15) | 703 (12) | 0.443 | |
| Ischemic cerebrovascular disease | 32 (48) | 1,688 (29) | <0.001 † | 1.71 (1.00-2.91) † |
| Seizures | 9 (14) | 13 (0.2) | <0.001 † | 27.58 (9.24-78.77) † |
| Premorbid mRS score, median (IQR) | 3 (0-4) | 0 (0-2) | <0.001 † | 1.10 (0.93-1.28) |
| Vital signs, mean±SD | ||||
| Systolic blood pressure, mmHg | 156±28 | 158±29 | 0.604 | |
| Diastolic blood pressure, mmHg | 85±18 | 86±17 | 0.744 | |
| Heart rate, /min | 87±24 | 78±17 | <0.001 † | |
| Body temperature, °C | 36.5±0.7 | 36.6±1.8 | 0.901 | |
| NIHSS score on admission, median (IQR) | 19 (12-29) | 4 (2-11) | <0.001† | 1.07 (1.05-1.10)† |
| Stroke subtype (%) | <0.001† | |||
| Cardioembolic stroke | 28 (43) | 1,538 (27) | 1.24 (0.71-2.23) | |
| Large artery disease | 16 (25) | 1,018 (18) | ||
| Small vessel occlusion | 4 (6) | 1,512 (26) | ||
| Other | 0 (0) | 225 (4) | ||
| Undetermined | 17 (26) | 1,168 (20) | ||
| Transient ischemic attack | 0 (0) | 280 (5) | ||
| Site of lesion (%) | 0.004† | |||
| Anterior circulation | 55 (85) | 3,871 (67) | 1.85 (0.97-2.92) | |
| Posterior circulation | 7 (11) | 1,337 (23) | ||
| Both circulations | 3 (5) | 134 (2) | ||
| Unknown | 0 (0) | 399 (7) | ||
| Laboratory examinations | ||||
| White blood cell count, 103/mm3, mean±SD | 7.4±3.3 | 7.2±2.7 | 0.546 | |
| Hematocrit, %, mean±SD | 37.9±5.5 | 39.3±5.4 | 0.028† | |
| Platelets, 103/mm3, mean±SD | 203±94 | 201±69 | 0.893 | |
| Glucose, mg/dL, mean±SD | 149±75 | 139±62 | 0.190 | |
| Blood urea nitrogen, mg/dL, mean±SD | 20±11 | 19±10 | 0.320 | |
| Creatinine, mg/dL, mean±SD | 1.2±1.2 | 1.1±1.2 | 0.707 | |
| C-reactive protein, mg/dL, mean±SD | 0.6±0.9 | 0.8±2.4 | 0.516 | |
| Hemoglobin A1c (%), mean±SD | 6.3±1.5 | 6.2±1.2 | 0.538 | |
| LDL-cholesterol, mg/dL, mean±SD | 107±39 | 114±36 | 0.143 | |
| Brain natriuretic peptide, pg/mL, median (IQR) | 87 (35-246) | 52 (19-147) | 0.001† | |
| D-dimer, μg/mL, median (IQR) | 1.5 (0.9-3.8) | 1.1 (0.7-2.3) | 0.021 † | |
| Hyperacute reperfusion therapy (%) | ||||
| Intravenous thrombolysis | 3 (5) | 447 (8) | 0.463 | |
| Endovascular thrombectomy | 8 (12) | 280 (5) | 0.006† | |
| Any reperfusion therapy | 10 (15) | 623 (11) | 0.244 | |
| Day of early seizures | ||||
| Onset | 37 (57) | |||
| Day 0-1 | 13 (20) | |||
| ≥Day 2 | 15 (23) | |||
| NIHSS score at discharge, median (IQR) | 15 (5-25) | 2 (0-7) | <0.001† | |
| mRS score at discharge, median (IQR) | 5 (4-5) | 3 (1-4) | <0.001† |
IQR: interquartile range, LDL: low-density lipoprotein, mRS: modified Rankin Scale, NIHSS: National Institutes of Health Stroke Scale, SD: standard deviation
*We performed multivariate analysis, including history of ischemic stroke, history of seizures, premorbid mRS score, NIHSS score on admission, cardioembolic stroke, and anterior circulation. Significant findings are highlighted in bold.
†p value <0.05
A multiple logistic regression model that included the following six explanatory factors was studied: a history of ischemic stroke, a history of seizures, premorbid mRS score, NIHSS score on admission, stroke subtype of cardioembolic stroke, and site of lesion in the anterior circulation. As a result, a history of ischemic stroke, history of seizures, and NIHSS score on admission were found to be significant and independent factors associated with the presence of early seizures.
The clinical outcome, indicated by the NIHSS and mRS scores at discharge, was worse in patients with early seizures than in patients without early seizures.
Distribution of past and recent lesions and relationship with the laterality of early focal seizures
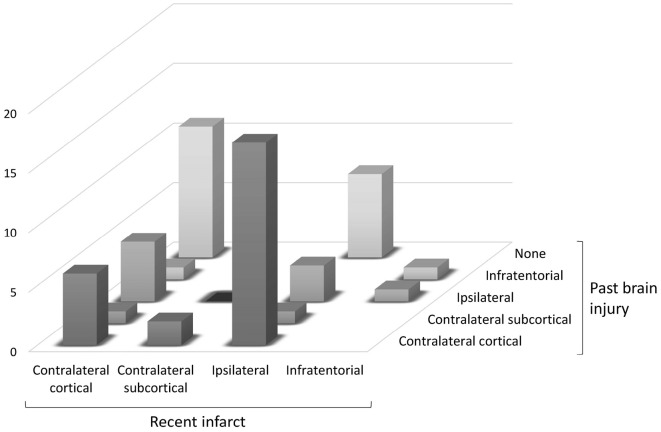
Figure and Supplementary Material 2 shows the distribution of lesions, including recent infarcts, and past brain injuries, in the patients with early focal seizures. Of the 56 patients with early focal seizures, contralateral cortical involvement of a recent infarct was observed in 24 (43%). Of these 24 patients, 7 also had past brain injuries in the cortex contralateral to early focal seizures. Of the 32 patients without cortical involvement of a recent infarct contralateral to their focal seizures, 18 had past brain injuries in the cortex contralateral to their early focal seizure. Furthermore, in 12 patients with early focal seizures, neither recent infarcts nor past brain injuries were observed in the cortex contralateral to their early focal seizures.
Figure.
Relationship between the side of a recent infarct and past injury in relation to early focal seizures. “Contralateral (or ipsilateral)” means that the (recent or past) lesion is situated in the cerebral hemisphere contralateral (or ipsilateral) to early focal seizure.
Clinical characteristics of early focal seizure patients with or without contralateral cortical involvement of a recent infarct
Table 3 shows the clinical characteristics of patients with early focal seizures and with or without contralateral cortical involvement of a recent infarct. There were no marked differences between the groups in other background factors, vital sign parameters, stroke subtype, lesion site, severity, or outcomes at discharge. Laboratory examinations revealed significantly higher glucose and hemoglobin A1c levels in the recent infarct group than in the no recent infarct group. However, there were no significant differences between groups with regard to other laboratory findings.
Table 3.
Clinical Characteristics of Patients with or without Recent Infarcts in the Contralateral Cortex of Focal Early Seizures.
| Contralateral cerebral cortex to early focal seizures | p value | ||
|---|---|---|---|
| With a recent infarct (n=24) | Without a recent infarct (n=32) | ||
| Age, years, median (IQR) | 79 (71-83) | 80 (74-86) | |
| Sex, male (%) | 15 (63) | 14 (44) | 0.163 |
| Medical history (%) | |||
| Hypertension | 16 (67) | 23 (72) | 0.675 |
| Diabetes mellitus | 8 (33) | 8 (25) | 0.495 |
| Dyslipidemia | 9 (38) | 11 (34) | 0.809 |
| Current smoking | 2 (8) | 1 (3) | 0.571 |
| Atrial fibrillation | 9 (38) | 17 (53) | 0.246 |
| Ischemic heart disease | 5 (21) | 4 (13) | 0.476 |
| Ischemic cerebrovascular disease | 10 (42) | 18 (56) | 0.280 |
| Seizures | 2 (8) | 5 (16) | 0.686 |
| Premorbid mRS score, median (IQR) | 2 (0-4) | 3 (2-4) | 0.225 |
| Vital signs, mean±SD | |||
| Systolic blood pressure, mmHg | 156±29 | 159±27 | 0.707 |
| Diastolic blood pressure, mmHg | 85±19 | 86±17 | 0.898 |
| Heart rate, /min | 97±30 | 81±16 | 0.015† |
| Body temperature, °C | 36.4±0.6 | 36.6±0.5 | 0.199 |
| NIHSS score on admission, median (IQR) | 19 (14-28) | 17 (11-30) | 0.875 |
| Stroke subtype (%) | 0.533 | ||
| Cardioembolic stroke | 11 (46) | 15 (47) | |
| Large artery disease | 7 (29) | 6 (19) | |
| Small vessel occlusion | 0 (0) | 2 (6) | |
| Other | 0 (0) | 0 (0) | |
| Undetermined | 6 (25) | 9 (28) | |
| Transient ischemic attack | 0 (0) | 0 (0) | |
| Site of lesion (%) | 0.440 | ||
| Anterior circulation | 21 (88) | 27 (84) | |
| Posterior circulation | 3 (13) | 3 (9) | |
| Both circulations | 0 (0) | 2 (6) | |
| Unknown | 0 (0) | 0 (0) | |
| Laboratory examinations | |||
| White blood cell count, 103/mm3, mean±SD | 7.4±2.3 | 7.5±4.0 | 0.864 |
| Hematocrit, %, mean±SD | 37.6±5.3 | 37.5±6.0 | 0.966 |
| Platelets, 103/mm3, mean±SD | 193±63 | 214±117 | 0.439 |
| Glucose, mg/dL, mean±SD | 184±107 | 126±30 | 0.005† |
| Blood urea nitrogen, mg/dL, mean±SD | 21.7±14.1 | 18.8±7.5 | 0.327 |
| Creatinine, mg/dL, mean±SD | 1.53±1.9 | 0.9±0.3 | 0.067 |
| C-reactive protein, mg/dL, mean±SD | 0.5±0.9 | 0.7±1.0 | 0.578 |
| Hemoglobin A1c (%), mean±SD | 6.8±2.2 | 6.0±7.5 | 0.045† |
| LDL-cholesterol, mg/dL, mean±SD | 108±36 | 104±37 | 0.687 |
| Brain natriuretic peptide, pg/mL, median (IQR) | 115 (53-459) | 86 (38-225) | 0.194 |
| D-dimer, μg/mL, median (IQR) | 1.6 (0.9-3.3) | 1.8 (1.0-4.8) | 0.349 |
| Day of early seizures (%) | 0.964 | ||
| Onset | 13 (54) | 17 (53) | |
| Day 0-1 | 5 (21) | 7 (22) | |
| ≥Day 2 | 6 (25) | 8 (25) | |
| NIHSS score at discharge, median (IQR) | 14 (3-21) | 18 (10-31) | 0.144 |
| mRS score at discharge, median (IQR) | 5 (4-5) | 5 (4-5) | 0.186 |
IQR: interquartile range, LDL: low-density lipoprotein, mRS: modified Rankin Scale, NIHSS: National Institutes of Health Stroke Scale, SD: standard deviation
Significant findings are highlighted in bold. †p value <0.05
Discussion
The present study had four major findings. First, 1.2% of patients with ischemic stroke were diagnosed with early seizures. Second, a history of ischemic stroke and seizures, and high NIHSS score on admission were significant and independent factors associated with the presence of early seizures. Third, the cortical involvement of a recent infarct contralateral to focal seizures was observed in 43% of patients with early focal seizures. Finally, glucose and hemoglobin A1c levels were significantly higher in patients with cortical involvement of a recent infarct contralateral to focal seizures than in those without such involvement.
The incidence of early seizures was lower in the present study than in previous studies. One explanation for the low frequency of early seizures may be that our patients had milder neurological deficits as evaluated by the NIHSS on admission than those in previous studies. Indeed, stroke severity was a main risk factor for early seizures in the present and previous studies (1,7,8,10,18,19,21).
In addition, transient complete-occlusion recanalization (11), thrombolysis (9), and hemorrhagic transformation (8,11,16) are known to be risk factors of early seizures. Similarly, in the present study, hyperacute recanalization therapy was performed more frequently in the patients with early seizures than in those without them. We did not perform computed tomography/MRI in all patients to detect hemorrhagic transformation. However, we consider the frequency of hyperacute reperfusion therapy and hemorrhagic transformation to be lower in the present study (11%) than in previous studies. In our hospital, most patients with ischemic stroke are evaluated with MRI on admission to precisely assess the occlusion site, collateral flow, and presumed area of the ischemic core and penumbra. Therefore, the indication for hyperacute reperfusion therapy might be more strictly limited in our hospital than in other institutes that have reported their own findings.
In the present study, the cortical involvement of a recent infarct contralateral to focal seizures was observed in approximately 50% of patients with early focal seizures. If a recent infarct is located in the cerebral cortex contralateral to early focal seizures, it is likely to be an epileptogenic lesion directly responsible for the seizures. Conversely, if a recent infarct is located in the subcortical, ipsilateral, or infratentorial region, it is likely to be a systemic cause of acute symptomatic seizures rather than an epileptogenic lesion in ischemic stroke patients with early focal seizures. As the frequency of the contralateral cortical involvement of a recent infarct was approximately 50%, this indicates the clinical significance of a recent infarct as a systemic cause of acute symptomatic seizures may be equal to that of epileptogenic lesions in ischemic stroke patients with early focal seizures.
The glucose and hemoglobin A1c levels were significantly higher in patients with cortical involvement of a recent infarct contralateral to focal seizures than in those without such involvement in the present study. Hyperglycemia is reportedly a predictor of early seizures (8). The present findings suggest that early focal seizures are more likely to occur in a hyperglycemic condition when a recent infarct is an epileptogenic lesion in ischemic stroke patients with early focal seizures than in a normoglycemic condition.
Conversely, in patients whose recent infarcts are not located in the cortex contralateral to early focal seizures, an alternative epileptogenic lesion should be assumed. In the present study, of the 32 patients without the cortical involvement of a recent infarct contralateral to their focal seizures, 17 had a past brain injury in the cortex contralateral to early focal seizures. In these 17 patients, it is highly possible that these past lesions were epileptogenic. In addition, of the 24 patients with the cortical involvement of a recent infarct contralateral to focal seizures, 6 also had a past brain injury in the cortex contralateral to early focal seizures. The past brain injuries in these six patients may have been true epileptogenic lesions, with the recent infarcts merely a systemic cause of acute symptomatic seizures.
Furthermore, in 13 patients with early focal seizures, neither recent infarcts nor past brain injuries were observed in the cortex contralateral to the seizures. In these 13 patients, it is necessary to identify neuro-radiologically inapparent epileptogenic lesions. Some types of brain damage, such as cortical degeneration associated with dementia, are considered possible epileptogenic lesions of early focal seizures.
Focal seizures occur in 10% of patients during the entire course of dementia (32,33). Most of the subjects in the present study were elderly; therefore, our study population likely contained a certain number of patients with dementia, although we did not evaluate cognitive function before the onset of stroke. We suspect that epileptogenic lesions related to dementia may have coexisted in some of the subjects of the present study, especially in those without a recent infarct or past brain injury in the cortex contralateral to early focal seizures. These results suggest that it is necessary to consider not only recent infarcts but also past brain injuries and subclinical lesions, such as dementia-related degeneration, as epileptogenic lesions of early focal seizures.
Based on the results of the present study, the clinical significance of recent infarcts in early seizures may be diverse. Therefore, stratified analyses according to the laterality of recent infarcts and early focal seizures in patients with ischemic stroke may be useful when studying the prophylactic administration of anti-epileptic agents.
The present study had several limitations. We divided the subjects into two groups according to whether or not the cortical involvement of a recent infarct contralateral to focal seizures was observed, as we assumed that the epileptogenic lesions of early focal seizures would be located in the cerebral cortex contralateral to the side of the seizures. However, the significance of subcortical lesions as epileptogenic lesions was not established. Therefore, it will be necessary to establish that subcortical lesions cannot be epileptogenic lesions of focal early seizures in future studies.
In addition, we diagnosed early seizures without referring to EEG findings. In our hospital, EEG is only performed during daytime working hours, and the period from seizure occurrence to the examination was often several days. Therefore, we considered that an EEG study could not be used for the reliable detection of epilepsy findings. Indeed, the frequency of the detection of epilepsy findings on EEG is reportedly low in patients with early seizures (6). In the present study, we did not adopt continuous EEG monitoring for the detection of early seizures. In stroke patients, non-convulsive status epilepticus is detected at a high frequency using EEG monitoring (29,34). If EEG monitoring had been performed in all stroke patients, the detection rate, type classification, and laterality of early focal seizures might have been improved.
Conclusion
In the present study, the cortical involvement of a recent infarct contralateral to focal seizures was observed in approximately 50% of patients with early focal seizures. We suggested that a recent infarct was significant for early seizures as a systemic cause of acute symptomatic seizures as well as an epileptogenic lesion directly responsible for the development of early focal seizures in ischemic stroke patients. Therefore, we propose the performance of stratified analyses according to the laterality of a recent infarct and early focal seizures in patients with ischemic stroke when studying the prophylactic administration of anti-epileptic agents.
The authors state that they have no Conflict of Interest (COI).
Supplementary Material
Supplementary Material 1 shows clinical characteristics of the present patients with early seizures.
Supplementary Material 2 shows the distribution of lesions, including recent infarcts, and past brain injuries, in the patients with early focal seizures
References
- 1.Bladin CF, Alexandrov AV, Bellavance A, et al. Seizures after stroke: a prospective multicenter study. Arch Neurol 57: 1617-1622, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Arboix A, Comes E, García-Eroles L, et al. Prognostic value of very early seizures for in-hospital mortality in atherothrombotic infarction. Eur Neurol 50: 78-84, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Lamy C, Domigo V, Semah F, et al. Early and late seizures after cryptogenic ischemic stroke in young adults. Neurology 60: 400-404, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Cordonnier C, Hénon H, Derambure P, Pasquier F, Leys D. Influence of pre-existing dementia on the risk of post-stroke epileptic seizures. J Neurol Neurosurg Psychiatry 76: 1649-1653, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kammersgaard LP, Olsen TS. Poststroke epilepsy in the Copenhagen stroke study: incidence and predictors. J Stroke Cerebrovasc Dis 14: 210-214, 2005. [DOI] [PubMed] [Google Scholar]
- 6.De Reuck J, Goethals M, Claeys I, Van Maele G, De Clerck M. EEG findings after a cerebral territorial infarct in patients who develop early- and late-onset seizures. Eur Neurol 55: 209-213, 2006. [DOI] [PubMed] [Google Scholar]
- 7.De Herdt V, Dumont F, Hénon H, et al. Early seizures in intracerebral hemorrhage: incidence, associated factors, and outcome. Neurology 77: 1794-1800, 2011. [DOI] [PubMed] [Google Scholar]
- 8.Procaccianti G, Zaniboni A, Rondelli F, Crisci M, Sacquegna T. Seizures in acute stroke: incidence, risk factors and prognosis. Neuroepidemiology 39: 45-50, 2012. [DOI] [PubMed] [Google Scholar]
- 9.Alvarez V, Rossetti AO, Papavasileiou V, Michel P. Acute seizures in acute ischemic stroke: does thrombolysis have a role to play? J Neurol 260: 55-61, 2013. [DOI] [PubMed] [Google Scholar]
- 10.Neshige S, Kuriyama M, Yoshimoto T, Takeshima S, Himeno T, Takamatsu K. Seizures after intracerebral hemorrhage; risk factor, recurrence, efficacy of antiepileptic drug. J Neurol Sci 359: 318-322, 2015. [DOI] [PubMed] [Google Scholar]
- 11.Leung T, Leung H, Soo YO, Mok VC, Wong KS. The prognosis of acute symptomatic seizures after ischaemic stroke. J Neurol Neurosurg Psychiatry 88: 86-94, 2017. [DOI] [PubMed] [Google Scholar]
- 12.Liao HC, Chen SH, Yang CD, Chen YW. Clinical profile and outcomes of early seizures in Asian patients with acute intracerebral hemorrhage. J Acute Med 9: 172-177, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tako LM, Strzelczyk A, Rosenow F, et al. Predictive factors of acute symptomatic seizures in patients with ischemic stroke due to large vessel occlusion. Front Neurol 13: 894173, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berges S, Moulin T, Berger E, et al. Seizures and epilepsy following strokes: recurrence factors. Eur Neurol 43: 3-8, 2000. [DOI] [PubMed] [Google Scholar]
- 15.Labovitz DL, Hauser WA, Sacco RL. Prevalence and predictors of early seizure and status epilepticus after first stroke. Neurology 57: 200-206, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Alberti A, Paciaroni M, Caso V, Venti M, Palmerini F, Agnelli G. Early seizures in patients with acute stroke: frequency, predictive factors, and effect on clinical outcome. Vasc Health Risk Manag 4: 715-720, 2008. [PMC free article] [PubMed] [Google Scholar]
- 17.Beghi E, D'Alessandro R, Beretta S, et al. Incidence and predictors of acute symptomatic seizures after stroke. Neurology 77: 1785-1793, 2011. [DOI] [PubMed] [Google Scholar]
- 18.Goswami RP, Karmakar PS, Ghosh A. Early seizures in first-ever acute stroke patients in India: incidence, predictive factors and impact on early outcome. Eur J Neurol 19: 1361-1366, 2012. [DOI] [PubMed] [Google Scholar]
- 19.Conrad J, Pawlowski M, Dogan M, Kovac S, Ritter MA, Evers S. Seizures after cerebrovascular events: risk factors and clinical features. Seizure 22: 275-282, 2013. [DOI] [PubMed] [Google Scholar]
- 20.Pezzini A, Grassi M, Del Zotto E, et al. Complications of acute stroke and the occurrence of early seizures. Cerebrovasc Dis 35: 444-450, 2013. [DOI] [PubMed] [Google Scholar]
- 21.Arntz R, Rutten-Jacobs L, Maaijwee N, et al. Post-stroke epilepsy in young adults: a long-term follow-up study. PLoS One 8: e55498, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo J, Guo J, Li J, et al. Statin treatment reduces the risk of poststroke seizures. Neurology 85: 701-707, 2015. [DOI] [PubMed] [Google Scholar]
- 23.Mansour S, Youness M, Cherri S, et al. Assessment of the incidence and risk factors of early poststroke seizures in Lebanese patients. Brain Behav 11: e02204, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang JZ, Vyas MV, Saposnik G, Burneo JG. Incidence and management of seizures after ischemic stroke: systematic review and meta-analysis. Neurology 89: 1220-1228, 2017. [DOI] [PubMed] [Google Scholar]
- 25.Beghi E, Carpio A, Forsgren L, et al. Recommendation for a definition of acute symptomatic seizure. Epilepsia 51: 671-675, 2010. [DOI] [PubMed] [Google Scholar]
- 26.Lüders HO, Najm I, Nair D, Widdess-Walsh P, Bingman W. The epileptogenic zone: general principles. Epileptic Disord 8(Suppl 2): S1-S9, 2006. [PubMed] [Google Scholar]
- 27.Sykes L, Wood E, Kwan J. Antiepileptic drugs for the primary and secondary prevention of seizures after stroke. Cochrane Database Syst Rev 1: CD005398, 2014. [DOI] [PubMed] [Google Scholar]
- 28.Berg AT, Berkovic SF, Brodie MJ, et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005-2009. Epilepsia 51: 676-685, 2010. [DOI] [PubMed] [Google Scholar]
- 29.Khan OI, Azevedo CJ, Hartshorn AL, et al. A comparison of continuous video-EEG monitoring and 30-minute EEG in an ICU. Epileptic Disord 16: 439-448, 2014. [DOI] [PubMed] [Google Scholar]
- 30.Nakae Y, Kudo Y, Yamamoto R, et al. Relationship between cortex and pulvinar abnormalities on diffusion-weighted imaging in status epilepticus. J Neurol 263: 127-132, 2016. [DOI] [PubMed] [Google Scholar]
- 31.Commission on Epidemiology and Prognosis, International League Against Epilepsy. Guidelines for epidemiologic studies on epilepsy. Epilepsia 34: 592-596, 1993. [DOI] [PubMed] [Google Scholar]
- 32.Imfeld P, Bodmer M, Schuerch M, Jick SS, Meier CR. Seizures in patients with Alzheimer's disease or vascular dementia: a population-based nested case-control analysis. Epilepsia 54: 700-707, 2013. [DOI] [PubMed] [Google Scholar]
- 33.Cook M, Baker N, Lanes S, Bullock R, Wentworth C, Arrighi HM. Incidence of stroke and seizure in Alzheimer's disease dementia. Age Ageing 44: 695-699, 2015. [DOI] [PubMed] [Google Scholar]
- 34.Belcastro V, Vidale S, Gorgone G, et al. Non-convulsive status epilepticus after ischemic stroke: a hospital-based stroke cohort study. J Neurol 261: 2136-2142, 2014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1 shows clinical characteristics of the present patients with early seizures.
Supplementary Material 2 shows the distribution of lesions, including recent infarcts, and past brain injuries, in the patients with early focal seizures