Abstract
Objective
This study was performed to clarify the association of the degree of gastric mucosal atrophy (GMA) with the occurrence of gastric cancer in patients with Helicobacter pylori-eradicated status.
Methods
The subjects were 3,058 patients (2,035 men, 1,023 women; mean age 57.9 ± 9.5 years old) with H. pylori eradication who underwent esophago-gastroduodenal endoscopy examinations as part of medical checkups conducted between April 2013 and March 2022. The gender, age at eradication, time since eradication, usage of anti-secretory drugs, degree of endoscopic GMA, and the fundic gland polyp (FGP) prevalence were compared between subjects with and without gastric cancer occurrence.
Results
Gastric cancer was newly detected in 26 subjects (0.85%) during the study period, with an older age at H. pylori eradication and severe grade of endoscopic GMA being significant risk factors for its occurrence. The gender, smoking history, and usage of anti-secretory drugs were not significantly different between subjects with and without gastric cancer occurrence. A Cox regression analysis showed that an older age at eradication and the degree of GMA were risk factors significantly related to occurrence. Furthermore, the degree of GMA was inversely correlated with FGP development, and gastric cancer was not detected in 467 subjects with FGP prevalence.
Conclusion
An older age at the time of H. pylori eradication and the degree of GMA are significant risk factors for gastric cancer occurrence in H. pylori-eradicated patients. The FGP prevalence in subjects with H. pylori eradication was inversely associated with GMA, suggesting it was negatively related with gastric cancer occurrence.
Keywords: gastric cancer, gastric mucosal atrophy, fundic gland polyp, Helicobacter pylori, eradication
Introduction
Gastric cancer is a life-threatening malignancy seen worldwide. A close association between its development and Helicobacter pylori infection has been repeatedly demonstrated, with H. pylori eradication recognized to effectively decrease gastric cancer occurrence (1-6). The national health insurance system of Japan began coverage for H. pylori eradication therapy to treat H. pylori-associated chronic gastritis in February 2013 (7), which has resulted in a rapid increase in number of patients undergoing associated treatments since that time. Therefore, the occurrence of gastric cancer following H. pylori eradication is an important issue for clinicians who perform esophagogastroduodenoscopy (EGD) examinations.
The development of gastric cancer after successful eradication has been reported to be influenced by several factors, such as the age, time since eradication, smoking history, proton pump inhibitor (PPI) usage, and degree of gastric mucosal atrophy (GMA) (4-6,8-10).
The present study was conducted to clarify significant risk factors related to the occurrence of gastric cancer in H. pylori-eradicated patients who underwent EGD examinations as part of their annual checkups. In addition, the fundic gland polyp (FGP) prevalence in H. pylori-eradicated cases was also investigated to elucidate its association with the degree of GMA and gastric cancer occurrence, since the FGP prevalence was recently found to be increased in association with a longer time since eradication (11).
Materials and Methods
The subjects were selected from individuals who visited the Health Center of Shimane Environment and Health Public Corporation for a detailed medical checkup between April 2013 and March 2022, the majority of whom were socially active and productive and considered to be socioeconomically middle class. A total of 41,715 EGD examinations were conducted during the study period, and an accurate medical history concerning the status of H. pylori infection (negative, positive, post-eradication) was obtained in an interview with each individual conducted by a public health nurse. Subjects who had undergone therapy without successful eradication were not included in the post-eradication group. When eradication therapy could not be confirmed as successful, the individual was recommended to undergo an H. pylori stool antigen test at our institution. Successful eradication was confirmed in all subjects based on endoscopic findings, such as absence of sticky mucous, diffuse or spotty redness and enlarged folds, or the appearance of map-like redness, black spots, or multiple white flat elevated lesions (12-16).
A total of 11,359 EGD examinations were performed for patients confirmed to have successful eradication of H. pylori. Regular usage of a PPI or H2 receptor antagonist (H2RA) at the time of EGD was also determined by an interview, although details related to the duration or dosage of anti-secretory drugs were not obtained.
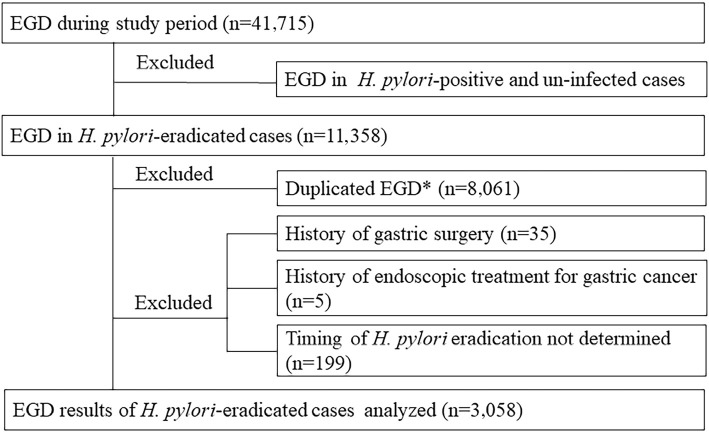
In patients with gastric cancer occurrence, an EGD examination was performed at the time of the diagnosis to determine the duration between gastric cancer occurrence and H. pylori eradication. For those not diagnosed during the study period, the most recent EGD findings were used. Patients with a history of gastric surgery or endoscopic treatment for gastric cancer were excluded from the present analysis, as were those whose timing of H. pylori eradication could not be determined. Therefore, EGD results for a total of 3,058 patients with H. pylori-eradicated status (2,035 men, 1,023 women; mean age 57.9±9.5 years old) were analyzed (Fig. 1).
Figure 1.
Selection of study subjects. *The present study analyzed EGD findings at the time of the diagnosis of gastric cancer (GC) in subjects with GC or the most recent EGD findings in those without GC.
All EGD examinations were performed by experienced licensed endoscopists using an EG-L580NW endoscope (Fujifilm, Tokyo, Japan). GMA was evaluated based on endoscopic findings using the classification of Kimura and Takemoto, with GMA classified into six groups (C1, C2, C3, O1, O2, O3), which has been shown to correlate well with histological features of atrophy (17). For the present study, C1-C2 was defined as mild, C3-O1 as moderate, and O2-O3 as severe GMA. Three expert endoscopists simultaneously reviewed the endoscopic images, with the presence of FGPs and degree of GMA determined by consensus. The presence of an FGP was determined based on endoscopic findings showing a sessile or pedunculated polyp with the same color as the fundic gland mucosa. Histological examinations for the diagnosis of FGP development were not performed for these patients, as the use of endoscopic findings allowed us to easily distinguish FGPs from other polyps, such as foveolar hyperplastic and adenomatous polyps and protruded-type carcinoma. For the present study, FGP occurrence was confirmed when one or more polyps were detected in the fundic area of the stomach.
Statistical analyses were performed using a chi-squared test, Fisher's exact probability test, and Mann-Whitney U test. The Kaplan-Meier method and a log-rank test were employed to analyze the time-course occurrence of gastric cancer after H. pylori eradication. A Cox regression analysis was also used to determine significant risk factors for the occurrence of gastric cancer after adjusting for co-founding factors. All calculations were performed using the StatView 5.0 software program for Macintosh (Abacus Concepts, Berkeley, USA), with a p level <0.05 considered to indicate statistical significance.
This study was performed in accordance with the Declaration of Helsinki and the protocol was approved by the ethics committee of the Shimane Environment and Health Public Corporation. Written informed consent indicating that the obtained clinical data would be used for a study without the release of individual information was received from all subjects before the medical checkups were performed. In addition, each study subject was allowed to opt out of this retrospective investigation at any time by informing the manager of the study.
Results
Gastric cancer was diagnosed based on endoscopy findings in 26 (0.85%) of the 3,058 enrolled subjects and then confirmed by histological examinations of endoscopically biopsied and endoscopically or surgically resected specimens. Two gastric cancer lesions were simultaneously discovered in one case. The 26 cases diagnosed with gastric cancer were divided into 17 with intestinal-type lesions and 9 with diffuse-type lesions, including signet-ring cell carcinoma, based on Lauren's criteria (18).
The characteristics of subjects with and without gastric cancer occurrence are shown in Table 1. The age at the time of the diagnosis of gastric cancer and H. pylori eradication was significantly older in those with gastric cancer occurrence than in those without it, while there was no significant difference in the time since H. pylori eradication between the groups. The gender, smoking history, and usage of anti-secretory drugs were not markedly different between those with and without gastric cancer occurrence. Furthermore, gastric cancer was not observed in subjects with H2RA administration, so a subsequent analysis was performed using PPI administration as a factor. The percentage value for endoscopically determined degree of GMA was higher in cases with gastric cancer occurrence than in those without it. FGPs were observed in 467 (15.3%) of the present subjects, none of whom had gastric cancer occurrence.
Table 1.
Characteristics of H. Pylor-eradicated Subjects with and without Gastric Cancer.
| Gastric cancer occurrence | p value | ||
|---|---|---|---|
| Positive | Negative | ||
| Number of subjects | 26 | 3,032 | |
| Gender, male/female | 13/13 | 2,022/1,010 | 0.073 |
| Age, years | 63.8±8.9 | 57.9±9.5 | 0.002 |
| Age at eradication, years | 56.0±10.0 | 50.6±10.1 | 0.004 |
| Duration after eradication, years | 7.3±5.9 | 7.3±5.4 | 0.808 |
| Smoking history | 17 (65.3) | 1,569 (51.7) | 0.082 |
| Anti-secretory drug usage | 3 (11.5) | 239 (7.9) | 0.419 |
| PPI usage | 3 (11.5) | 203 (6.7) | 0.327 |
| Gastric mucosal atrophy* | 0.001 | ||
| Mild | 7 (26.9) | 1,635 (53.9) | |
| Moderate | 11 (42.3) | 1,079 (35.6) | |
| Severe | 8 (30.8) | 318 (10.5) | |
| Fundic gland polyp, positive/negative | 0/26 | 467/2,565 | 0.030 |
Data are expressed as the mean±SD or number of subjects. Values in parentheses indicate percentage. Age at eradication: age at confirmation of successful eradication of H. pylori infection. Duration after eradication: duration after successful eradication of H. pylori. Anti-secretory drug usage: regular usage of proton pump inhibitor (PPI) or H2 receptor antagonist (H2RA) noted at esophago-gastrointestinal endoscopic examination.
*The degree of gastric mucosal atrophy (GMA) was endoscopically evaluated using the classification of Kimura and Takemoto, with C1-C2 indicating mild, C3-O1 moderate, and O2-O3 severe GMA.
A Cox regression analysis revealed the age at eradication and degree of GMA to be significant risk factors for occurrence of gastric cancer (Table 2). The risk of FGP prevalence could not be calculated using a Cox regression analysis, since gastric cancer was not observed in subjects with FGP. Results obtained with the Kaplan-Meier method regarding the time-course occurrence of gastric cancer in those with different degrees of GMA status are presented in Fig. 2. Log-rank test results showed significant differences among subjects with different degrees of GMA. The time-course occurrence of gastric cancer determined by the Kaplan-Meier method in subjects with and without FGP prevalence is presented in Fig. 3. Statistical analyses such as a log-rank test to compare subjects with and without FGPs could not be performed, as gastric cancer occurrence was not observed in any of the subjects with FGPs.
Table 2.
Cox’s Proportional Hazards Regression Analysis for Occurrence of Gastric Cancer
| Risk ratio | 95%CI | p value | |
|---|---|---|---|
| Gender (male) | 0.430 | 0.155-1.193 | 0.105 |
| Age at eradication | 1.089 | 1.039-1.141 | <0.001 |
| Smoking history | 0.595 | 0.206-1.717 | 0.337 |
| PPI usage | 1.329 | 0.393-4.496 | 0.647 |
| Gastric mucosal atrophy* | |||
| Moderate | 2.123 | 0.812-5.554 | 0.125 |
| Severe | 4.266 | 1.436-12.669 | 0.009 |
*The degree of gastric mucosal atrophy (GMA) was endoscopically evaluated using the classification of Kimura and Takemoto, with C1-C2 indicating mild, C3-O1 moderate, and O2-O3 severe GMA. Risk ratio was calculated by comparison with mild GMA.
Figure 2.
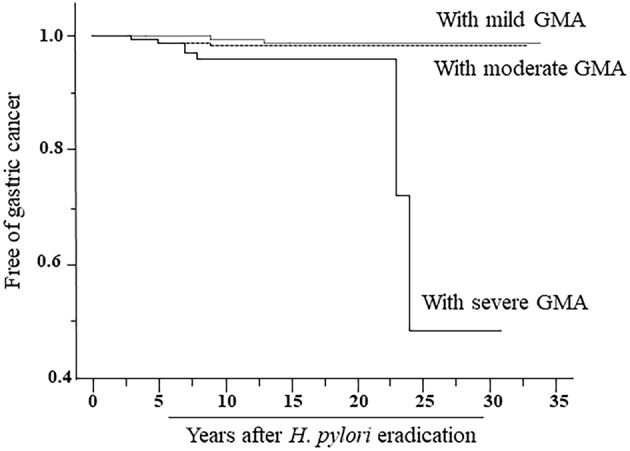
Results of the Kaplan-Meier analysis of the time-course occurrence of gastric cancer in subjects with different degrees of gastric mucosal atrophy (GMA). The degree of GMA was endoscopically evaluated using the classification of Kimura and Takemoto, with C1-C2 indicating mild, C3-O1 moderate, and O2-O3 severe GMA. p values for log-rank tests of mild vs. moderate, mild vs. moderate, and mild vs. moderate/severe GMA were 0.028, <0.001, and 0.027, respectively.
Figure 3.
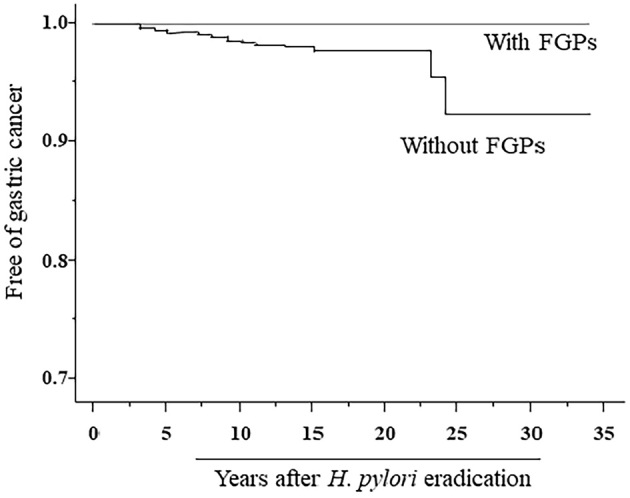
Results of the Kaplan-Meier analysis of the time-course occurrence of gastric cancer in subjects with and without FGP occurrence.
An analysis performed after dividing post-H. pylori-eradicated subjects by the degree of GMA showed that gastric cancer occurrence was more frequently observed in those with a higher degree of GMA than in those with a lower degree (p=0.001). The rates of prevalence of FGP in subjects with mild, moderate, and severe GMA were 20.8%, 11.0%, and 1.5%, respectively (p<0.001) (Table 3). In addition, FGP was more frequently observed in subjects with PPI administration than in those without it (35.4% vs. 13.8%). Among the mild, moderate, and severe GMA cases, FGP prevalence in those with and without PPI administration was 44.3% and 18.9% (p<0.001), 29.0% and 9.9% (p<0.001), and 4.5% and 1.3% (p=0.296), respectively.
Table 3.
Association of Degree of Gastric Mucosal Atrophy with Presence of Gastric Cancer and Fundic Gland Polyp.
| Gastric mucosal atrophy* | p value | |||
|---|---|---|---|---|
| Mild (n=1,642) | Moderate (n=1,090) | Severe (n=326) | ||
| Gastric cancer | 0.001 | |||
| Positive | 7 (0.4) | 11 (1.0) | 8 (2.5) | |
| Negative | 1,635 (99.6) | 1,079 (99.0) | 342 (97.5) | |
| Fundic gland polyp | <0.001 | |||
| Positive | 342 (20.8) | 120 (11.0) | 5 (1.5) | |
| Negative | 1,300 (79.1) | 970 (89.0) | 321 (98.5) | |
Data are expressed as number of subjects. Values in parentheses indicate percentage.
*The degree of gastric mucosal atrophy (GMA) was endoscopically evaluated using the classification of Kimura and Takemoto, with C1-C2 indicating mild, C3-O1 moderate, and O2-O3 severe GMA.
Discussion
H. pylori eradication therapy is widely performed around the world to prevent several different types of gastroduodenal diseases, such as gastric cancer. However, successful eradication does not completely prevent gastric cancer occurrence, and the clarification of related risk factors in eradicated cases is clinically important for examiners using EGD. In previous investigations, several factors, such as an older age, smoking history, long-term usage of a PPI, and more severe GMA, have been demonstrated to be related to occurrence of gastric cancer (8-10). The results of the present study of H. pylori-eradicated patients who underwent EGD as part of an annual checkup showed that a smoking history and anti-secretory usage were not significant risk factors, whereas an older age at the time of H. pylori eradication and higher degree of GMA were demonstrated to be significant risk factors for gastric cancer occurrence.
The accumulation of DNA methylation in gastric mucosa due to continuous H. pylori infection has been demonstrated to be associated with gastric cancer development, and eradication of such an infection can result in a decreased gastric mucosal methylation level (8,19-22). H. pylori infection generally occurs during childhood, and long-term persistent infection can cause a high degree of GMA (7), while an older age at the time of H. pylori eradication and high degree of GMA are associated with the duration of infection. Therefore, these factors are considered to be related to the degree DNA methylation of gastric mucosa in H. pylori-eradicated patients who later develop gastric cancer.
In the present study, FGP prevalence was noted in 15.3% of the subjects with H. pylori-eradicated status. The degree of GMA was inversely correlated with the prevalence of FGP, and the prevalence of FGP in subjects with PPI administration was significantly higher than in those without it. Findings showing FGP prevalence in H. pylori-eradicated subjects were also presented in a previous study (11). In the present study, gastric cancer occurrence was not observed in H. pylori-eradicated cases with FGPs. This inverse relationship between FGP prevalence and the degree of GMA is thought to be closely associated with the absence of gastric cancer in H. pylori-eradicated patients with FGPs.
Several limitations associated with the present study warrant mention. This study was not performed in a prospective manner, as the investigation was conducted in a cross-sectional manner. The determination of H. pylori eradicated status was based on the medical history obtained by an interview as well as several endoscopic examinations showing H. pylori-positive and H. pylori-negative findings in the gastric mucosa. Therefore, there may have been H. pylori-positive patients among the study subjects, although an H. pylori stool antigen test was performed for those without confirmation of successful eradication. In addition, details related to the duration or dosage of PPI, which may influence the development of not only FGPs and but also gastric cancer, were not examined. Furthermore, this study was performed in a single medical center. Long-term large-scale multicenter studies are needed to confirm the factors related to gastric cancer occurrence, especially the negative association between FGP prevalence and gastric cancer occurrence noted in the present subjects who had undergone H. pylori eradication.
In conclusion, the results of this study revealed that an older age at eradication and the degree of GMA were significant risk factors for gastric cancer occurrence in patients with H. pylori-eradicated status. FGP prevalence in H. pylori-eradicated cases was inversely associated with GMA and suggested to be negatively related to the occurrence of gastric cancer.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We wish to thank Marie Ishida, Yukari Inoue, Riko Yamamoto, Noriko Yamauchi, Mayumi Iwatani, and Masatomo Aoki of the Shimane Environment and Health Public Corporation, as well as Keiko Masuzaki of the Second Department of Internal Medicine, Shimane University Faculty of Medicine, for their helpful technical support.
References
- 1.Infection with Helicobacter pylori. IARC Monogr Eval Carcinog Risks Hum 61: 177-240, 1994. [PMC free article] [PubMed] [Google Scholar]
- 2.Uemura N, Okamoto S, Yamamoto S, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med 345: 784-789, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Ohata H, Kitauchi S, Yoshimura N, et al. Progression of chronic atrophic gastritis associated with Helicobacter pylori infection increases risk of gastric cancer. Int J Cancer 109: 138-143, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Fukase K, Kato M, Kikuchi S, et al. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet 372: 392-397, 2008. [DOI] [PubMed] [Google Scholar]
- 5.Choi IJ, Kook MC, Kim YI, et al. Helicobacter pylori therapy for the prevention of metachronous gastric cancer. N Engl J Med 378: 1085-1095, 2018. [DOI] [PubMed] [Google Scholar]
- 6.Lee YC, Chiang TH, Chou CK, et al. Association between Helicobacter pylori eradication and gastric cancer incidence: a systematic review and meta-analysis. Gastroenterology 150: 1113-1124.e5, 2016. [DOI] [PubMed] [Google Scholar]
- 7.Asaka M, Kato M, Sakamoto N. Roadmap to eliminate gastric cancer with Helicobacter pylori eradication and consecutive surveillance in Japan. J Gastroenterol 49: 1-8, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asada K, Nakajima T, Shimazu T, et al. Demonstration of the usefulness of epigenetic cancer risk prediction by a multicentre prospective cohort study. Gut 64: 388-396, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shichijo S, Hirata Y. Characteristics and predictors of gastric cancer after Helicobacter pylori eradication. World J Gastroenterol 24: 2163-2172, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shichijo S, Uedo N, Michida T. Detection of early gastric cancer after Helicobacter pylori eradication. Digestion 103: 54-61, 2022. [DOI] [PubMed] [Google Scholar]
- 11.Notsu T, Adachi K, Mishiro T, Ishimura N, Ishihara S. Fundic gland polyp prevalence according to Helicobacter pylori infection status. J Gastroenterol Hepatol 35: 1158-1162, 2020. [DOI] [PubMed] [Google Scholar]
- 12.Kyoto Classification of Gastritis. Haruma K, Ed. Nihon Medical Center, Tokyo, 2017. [Google Scholar]
- 13.Watanabe K, Nagata N, Nakashima R, et al. Predictive findings for Helicobacter pylori-uninfected, -infected and -eradicated gastric mucosa: validation study. World J Gastroenterol 19: 4374-4379, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kato M, Terao S, Adachi K, et al.; Study Group for Establishing Endoscopic Diagnosisi of Chronic Gastritis. Changes in endoscopic findings of gastritis after cure of H. pylori infection: multicenter prospective trial. Dig Endosc 25: 264-273, 2013. [DOI] [PubMed] [Google Scholar]
- 15.Adachi K, Notsu T, Mishiro T, Kinoshita Y. Relationship of Helicobacter pylori infection with gastric black spots shown by endoscopy. Intern Med 58: 767-772, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adachi K, Mishiro T, Okada M, Kinoshita Y. Prevalence of multiple white and flat elevated lesions in individuals undergoing a medical checkup. Intern Med 57: 1213-1218, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kimura K, Takemoto T. An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy 1: 87-97, 1969. [Google Scholar]
- 18.Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand 64: 31-49, 1965. [DOI] [PubMed] [Google Scholar]
- 19.Maekita T, Nakazawa K, Mihara M, et al. High levels of aberrant DNA methylation in Helicobacter pylori-infected gastric mucosae and its possible association with gastric cancer risk. Clin Cancer Res 12: 989-995, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Chan AO, Peng JZ, Lam SK, et al. Eradication of Helicobacter pylori infection reverses E-cadherin promoter hypermethylation. Gut 55: 463-468, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perri F, Cotugno R, Piepoli A, et al. Aberrant DNA methylation in non-neoplastic gastric mucosa of H. pylori infected patients and effect of eradication. Am J Gastroenterol 102: 1361-1371, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Nakajima T, Enomoto S, Yamashita S, et al. Persistence of a component of DNA methylation in gastric mucosae after Helicobacter pylori eradication. J Gastroenterol 45: 37-44, 2010. [DOI] [PubMed] [Google Scholar]