Abstract
We herein report a case of anti-myelin oligodendrocyte glycoprotein (MOG) antibody-related myelitis caused by coronavirus disease (COVID-19) infection in 2021. A 22-year-old man with no history of any related illness contracted COVID-19. Eight days later, he developed bladder problems, paraplegia and sensory disturbances. Cervical spinal cord magnetic resonance imaging revealed extensive hyperintensity at T2 and spinal cord lesions extending from C4 to Th1. The patient was diagnosed with transverse myelitis and started on intravenous methylprednisolone, plasma exchange and intravenous immunoglobulin therapy. The symptoms improved only after intravenous methylprednisolone therapy. Anti-MOG antibodies were found in his serum and cerebrospinal fluid during routine screening. As this observation is unusual and could cause serious health problems, we wonder if COVID-19 triggered this autoimmune response.
Keywords: oligodendrocyte myelin glycoprotein, COVID-19, transverse myelitis, prednisolone, autoimmune
Introduction
In 2019, coronavirus disease (COVID-19) became a pandemic, triggered by infection with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Cases of autoimmune diseases, such as Guillain-Barré syndrome, have been mentioned as complications of COVID-19 infection that emerged in China in December 2019 (1). We herein report a case of anti-myelin oligodendrocyte glycoprotein (MOG) antibody-related transverse myelitis caused by COVID-19 infection and propose that it might have been caused by an autoimmune mechanism triggered by the virus.
Case Report
A 22-year-old man with no underlying disease developed a fever in September 2021. Polymerase chain reaction (PCR) with a nasopharyngeal swab revealed that the patient had SARS-CoV-2. The fever subsided within a few days but reappeared nine days after the initial onset. Symptoms of bladder and bowel disturbance appeared, along with muscle weakness in both lower limbs, and the patient reported increasing difficulty moving. His blood pressure was 119/62 mmHg, pulse 71/min, temperature 39.4°C, respiratory rate 21/min and oxygen saturation 98% (room air). On admission, his neurological findings revealed that he was alert and fully conscious, with no speech disorder or cranial nerve abnormalities, and with a regular upper limb muscle strength.
Both his lower limbs had a muscle strength equivalent to MMT 2/5. However, the sensation in both hands' fingers was found to be abnormal. There was a loss of pain and temperature sensation below Th5. Deep tendon reflexes were absent in both lower extremities, but the pathological reflex was negative. Bladder and bowel disturbance was noted, and Lhermitte's sign was positive, with no neck stiffness. General blood tests revealed no obvious abnormalities except a leukocyte count of 10,700 /μL, and C-reactive protein level of 0.40 mg/dL (<0.14 mg/dL). HbA1c and thyroid hormone levels were normal, and anti-thyroid and anti-nuclear antibody tests were negative. The patient was negative for anti-ganglioside antibodies, myeloperoxidase anti-neutrophil cytoplasmic antibody (MPO-ANCA), proteinase3 anti-neutrophil cytoplasmic antibody (PR3-ANCA), IL-2R and syphilis infection.
A cerebrospinal fluid examination revealed a white blood cell count of 94 /μL (mononuclear cells 67 /μL, polynuclear cells 27 /μL), 49 mg/dL protein. Myelin basic protein (MBP) was 2,250 pg/mL (<102 pg/mL), and the IgG index was 0.62. A cerebrospinal fluid SARS-CoV-2 PCR test was negative. An enzyme immunoassay of the cerebrospinal fluid was negative for human herpes simplex virus, cytomegalovirus, mumps virus and Epstein-Barr virus. Oligoclonal bands and anti-AQP4 antibodies were negative. Anti-AQP4 antibody was evaluated by an enzyme-linked immunosorbent assay and found to be normal.
Chest computed tomography (CT) revealed a faint ground-glass shadow in part of the left lung field. Cervical and thoracic magnetic resonance imaging (MRI) revealed longitudinal extensive transverse myelitis (LETM) from C4 to Th1 (Fig. 1). Brain MRI-fluid-attenuated inversion recovery (FLAIR) and diffusion-weighted imaging (DWI) revealed a high-intensity area in the right thalamus (Fig. 2). Nasopharyngeal antigen quantitative PCR for SARS-CoV-2 conducted on admission was slightly positive (1.84 pg/mL). Nasopharynx loop-mediated isothermal amplification (LAMP) PCR for SARS-CoV-2 conducted on the fourth day of hospitalisation was negative.
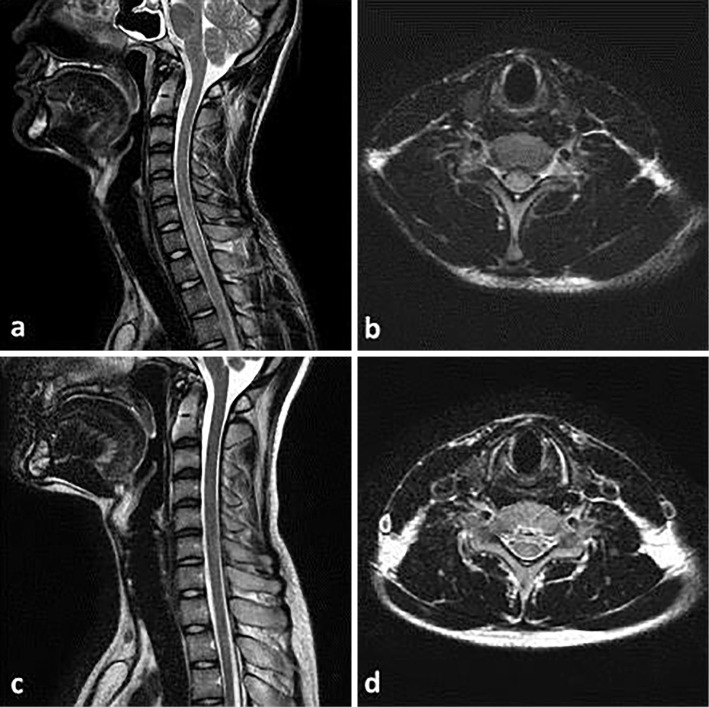
Figure 1.
Images of cervical spine MRI. (a) Pre-treatment T2-weighted sagittal images of the cervical spine reveal longitudinally extensive transverse myelitis. (b) Pre-treatment T2-weighted axial images of the cervical spine reveal transverse myelitis. (c) Post-treatment T2-weighted sagittal images of the cervical spine reveal the disappearance of myelitis. (d) Post-treatment T2-weighted axial images of the cervical spine reveal the disappearance of myelitis.
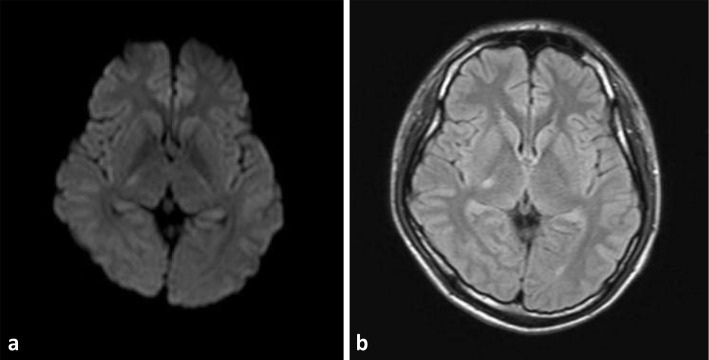
Figure 2.
Image of pre-treatment brain MRI. (a) DWI MRI of the brain showed hyperintensity in the right thalamus. (b) T2-weighted MRI of the brain showed hyperintensity in the right thalamus.
The patient was admitted to the intensive-care unit with an initial diagnosis of COVID-19 infection and transverse myelitis. Intravenous methylprednisolone (IVMP) was given from the first day of admission. A lower limb examination after the first IVMP administration revealed that the Medical Research Council (MRC) (2) muscle strength scale score had increased from 14 to 26 points, and Lhermitte's sign had disappeared. This improvement, however, was only temporary, and the MRC score of the lower extremities worsened to 6 after a few days. On the eighth day of illness, plasma exchange (PE) was combined with a second round of IVMP to enhance immunotherapy. Both lower extremity MRC scores improved slightly to 15 points. Subsequently, only PE was repeated.
The MRC score for both lower limbs dropped to 8 once more. After the third course of IVMP on the 14th day of hospitalisation, the MRC score for both lower extremities improved considerably to 25 points. Anti-MOG antibody levels were determined using a cell-based assay. The patient had anti-MOG antibodies in both the serum and cerebrospinal fluid. The anti-MOG antibody titre was 1:512 in serum and 1:8 in cerebrospinal fluid. The therapeutic effect of PE was deemed to be poor, so it was discontinued after six courses.
IVIg was initiated on the 20th day of hospitalisation without post-steroid therapy. The lower limb MRC scores improved to 27 points, and the bladder and bowel disturbances were finally alleviated. We conducted a cerebrospinal fluid test following IVMP and PE acute immunotherapy. The cerebrospinal fluid cell count and MBP were normalised. Anti-MOG antibody levels were also measured, but the serum and cerebrospinal fluid titres remained unchanged from prior treatment. Oral prednisolone (65 mg: 1.0 mg/kg) was initiated after the fifth IVMP administration. The oral prednisolone dose was decreased by 10 mg every 5 days, and the symptoms did not worsen. About two months later, the patient was transferred to a rehabilitation unit (Fig. 3). He had mild clonus in his lower limbs upon transfer but could walk and urinate independently.
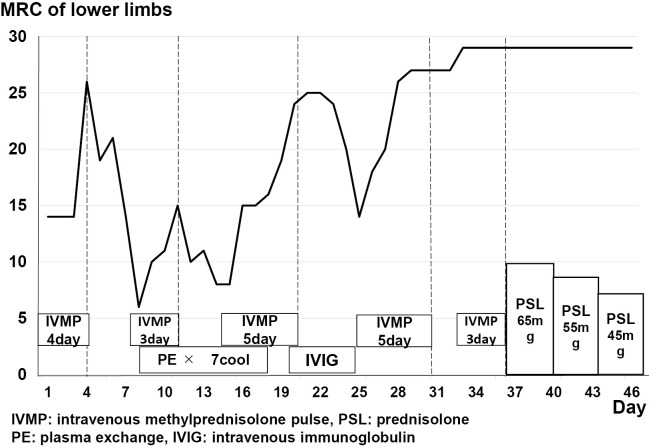
Figure 3.
Course of immunotherapy treatment after hospitalisation. This patient was repeatedly treated with IVMP and PE. IVIG was also administered but did not improve the symptoms. This case was ultimately successfully treated with IVMP.
Discussion
Anti-MOG antibody-associated myelitis developed in the present patient as a result of an autoimmune reaction triggered by COVID-19 infection. During the pandemic, many myelitis cases have been reported to be associated with COVID-19 (3). However, anti-MOG antibody-positive myelitis, as seen in this case, is uncommon. COVID-19 can enter the nervous system, making it a possible cause of neuroinflammatory conditions (4). Nevertheless, the mechanism by which the virus causes inflammation remains unclear.
MOG protein comprises 252 amino acids, has a molecular weight of approximately 28 kDa, and is a major component protein of the myelin sheath. Immunisation against MOGs and peptides induces autoimmune neuromyelitis in animal models (5). In addition to optic neuritis, MOG antibody-related positive inflammatory demyelination disease induces myelitis in the long axis. In some cases, optic neuritis and myelitis coexist and can be difficult to distinguish (6,7). Our patient had bladder and bowel disturbances and paraplegia with LETM. Initially, we also suspected that COVID-19 might have directly infiltrated the central nervous system, causing myelitis. However, SARS-CoV-2 PCR of the cerebrospinal fluid showed negative findings.
Eleven cases of MOG-related disease developing within a few weeks of COVID-19 infection have been reported (Table) (8-18). Nine of the reports involved men who presented several days after the COVID-19 onset, and MOG emerged within a few weeks of the infection. The lesions were prevalently optic neuritis, with only two cases of transverse myelitis. Zhou et al. (10) and Dias da Costa et al. (18) reported treating most of the cases positively with IVMP or oral prednisolone.
Table.
Reported Cases of COVID-19 Related Anti-MOG Antibody-related Diseases.
| References | Age | Gender | Symptom | Time relation between SARS-COV-2 infection and NP |
Leision | Treatment | Response to treatment |
|---|---|---|---|---|---|---|---|
| (8) | 4.17 | M | Seizures, facial palsy, | NA | ADEM | High dose steroids | improvement |
| four limb dysfunctions | |||||||
| (9) | 15 | M | Bilateral optic neuritis | Few weeks | ON | IVMP | Almost complete |
| recovery | |||||||
| (10) | 26 | M | Eye pain, visual loss, | Few days | ON+myelitis | IVMP, oral | Visual acuity |
| lower limb numbness | prednisolone | improved | |||||
| (11) | 44 | M | Eye pain, loss of vision | 2 weeks | ON | IVMP, orale | Improvement |
| prednisolone | |||||||
| (12) | 44 | F | Dysphasia, weakness | 7 days | CNS | IVMP, PE, oral | Improvement |
| in right arm and leg | prednisolone | ||||||
| (13) | 39 | F | Eye pain, loss of vision | 6 days | ON | IVMP, PE, oral | Partial |
| prednisolone, MMF | |||||||
| (14) | 11 | M | Eye pain, loss of vision | 4 days-2 weeks | ON | IVMP, oral | Improvement |
| prednisolone | |||||||
| (15) | 47 | M | Eye pain, upper visual | 2 days | ON | IVMP, oral | Partial |
| field defect | prednisolone | ||||||
| (16) | 63 | M | Loss of vision, | 4 weeks | ON | IVMP, oral | Improvement |
| headache | prednisolone | ||||||
| (17) | 7 | F | Epilepsy, aphasia, | NA | CNS | IVIG | Improvement |
| encephalopathy | |||||||
| paralysis | |||||||
| (18) | 31 | M | Bladder and rectal | 21 days | Myelitis | IVMP, oral | Improvement |
| disorder, lower limbs | prednisolone | ||||||
| pain | |||||||
| This case | 22 | M | Bladder and rectal | 8 days | Myelitis | IVMP, PE, IVIG, | Improvement |
| disorder, lower limbs | oral prednisolone | ||||||
| dysfunctions |
NA: not applicable, NP: neurological presentation, ADEM: acute disseminated encephalomyelitis, ON: optic neuritis, CNS: central nervous system, IVMP: intravenous methylprednisolone, IVIG: intravenous immunoglobulin, PE: plasma exchange, MMF: mycophenolate mofetil
While IVMP treatment showed a positive response in the present patient, PE and IVIg treatment elicited a poor response, and symptoms worsened after about five days when IVMP was stopped. In anti-MOG antibody-positive optic neuritis and myelitis, relapse following steroid treatment was observed at a rate of 44%. PE has also been reported to be ineffective at times (19). According to the same study, recurrence of MOG antibody-positive disease is common following acute treatment with IVMP and oral prednisolone (19). One limitation associated with the interpretation of the findings of this case is the fact that the time when anti-MOG antibody production began is unknown. Although we assumed that anti-MOG antibodies appeared in association with COVID-19 infection, they could have existed before the COVID-19 onset. However, there was no indication of any clinical symptoms, such as optic nerve symptoms, movement or sensory disorders, before the onset of COVID-19 in our patient. Because there were no inflammation-related symptoms expected to be associated with an antibody disease, this case was diagnosed as one of antibody-related myelitis with anti-MOG that had recently developed by an immune-mediated mechanism triggered by COVID-19. Furthermore, we suspect that the pathology may have been due to the influence of the immune response prior to COVID-19. COVID-19 patients have a dramatically increased autoantibody response compared to non-infected individuals. Indeed, in COVID-19 patients, autoantibodies to immunomodulatory proteins, such as cytokines, chemokines, complement and cell surface proteins, are frequently present (20).
Conclusion
We encountered a case of anti-MOG antibody-related transverse myelitis following COVID-19 infection. This uncommon case was thought to be one of antibody-related anti-MOG myelitis triggered by COVID-19. Only two cases of anti-MOG antibody-related myelitis have been previously reported following COVID-19 infection. While the response to PE and IVIg was poor in our patient, the response to steroid therapy was excellent. The global COVID-19 pandemic is expected to increase the number of cases of immune-mediated central nervous system inflammation. In rare cases, if paraplegia develops following COVID-19 infection, anti-MOG antibody-related transverse myelitis should be considered in addition to GBS.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
The authors wish to thank Toshiyuki Takahashi of the Department of Multiple Sclerosis Therapeutics, Tohoku University Graduate School of Medicine, for testing the anti-MOG antibody.
References
- 1.Toscano G, Palmerini F, Ravaglia S, et al. Guillain-Barré syndrome associated with SARS-CoV-2. N Engl J Med 382: 2574-2576, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kleyweg RP, van der Meché FG, Schmitz PI. Interobserver agreement in the assessment of muscle strength and functional abilities in Guillain-Barré syndrome. Muscle Nerve 14: 1103-1109, 1991. [DOI] [PubMed] [Google Scholar]
- 3.Rodríguez de Antonio LA, González-Suárez I, Fernández-Barriuso I, Rabasa Pérez M, et al. Para-infectious anti-GD2/GD3 IgM myelitis during the COVID-19 pandemic: case report and literature review. Mult Scler Relat Disord 49: 102783, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pezzini A, Padovani A. Lifting the mask on neurological manifestations of COVID-19. Nat Rev Neurol 16: 636-644, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakashima I. MOG-IgG related disease. Neurol Ther 36: 220-224, 2019. [Google Scholar]
- 6.Imai T, Shibata S, Shinohara K, Sakurai K, Horiuchi M, Hasegawa Y. Longitudinally extensive transverse myelitis involving fifteen vertebral bodies positive for anti-myelin oligodendrocyte glycoprotein (MOG) antibody: a case report. Rinsho Shinkeigaku 59: 375-378, 2019. [DOI] [PubMed] [Google Scholar]
- 7.Ikeda K, Kiyota N, Kuroda H, et al. Severe demyelination but no astrocytopathy in clinically definite neuromyelitis optica with anti-myelin-oligodendrocyte glycoprotein antibody. Mult Scler 21: 656-659, 2015. [DOI] [PubMed] [Google Scholar]
- 8.Lindan CE, Mankad K, Ram D, et al. Neuroimaging manifestations in children with SARS-CoV-2 infection: a multinational, multicentre collaborative study. Lancet Child Adolesc Health 5: 167-177, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Ruijter NS, Kramer G, Gons RAR, Hengstman GJD. Neuromyelitis optica spectrum disorder after presumed coronavirus (COVID-19) infection: a case report. Mult Scler Relat Disord 46: 102474, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou S, Jones-Lopez EC, Soneji DJ, Azevedo CJ, Patel VR. Myelin oligodendrocyte glycoprotein antibody-associated optic neuritis and myelitis in COVID-19. J Neuroophthalmol 40: 398-402, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sawalha K, Adeodokun S, Kamoga GR. COVID-19-induced acute bilateral optic neuritis. J Investig Med High Impact Case Rep 8: 2324709620976018, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pinto AA, Carroll LS, Nar V, Varatharaj A, Galea I. CNS inflammatory vasculopathy with anti-myelin oligodendrocyte glycoprotein antibodies in COVID-19. Neurol Neuroimmunol Neuroinflamm 10: e813, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woodhall M, Mitchell JW, Gibbons E, Healy S, Waters P, Huda S. Case report: myelin oligodendrocyte glycoprotein antibody-associated relapse with COVID-19. Front Neurol 11: 598531, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khan A, Panwala H, Ramadoss D, Khubchandani R. Myelin oligodendrocyte glycoprotein (MOG) antibody disease in an 11-year-old with COVID-19 infection. Indian J Pediatr 88: 488-489, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kogure C, Kikushima W, Fukuda Y, et al. Myelin oligodendrocyte glycoprotein antibody-associated optic neuritis in a COVID-19 patient: a case report. Medicine 14: e25865, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Žorić L, Rajović-Mrkić I, Čolak E, Mirić D, Kisić B. Optic neuritis in a patient with seropositive myelin oligodendrocyte glycoprotein antibody during the post-COVID-19 period. Int Med Case Rep J 14: 349-355, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahsan N, Jafarpour S, Santoro JD. Myelin oligodendrocyte glycoprotein antibody encephalitis following severe acute respiratory syndrome coronavirus 2 in a pediatric patient. Clin Exp Pediatr 64: 310-312, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dias da Costa M, Leal Rato ML, Cruz D, Valadas A, Antunes AP, Albuquerque L. Longitudinally extensive transverse myelitis with anti-myelin oligodendrocyte glycoprotein antibodies following SARS-CoV-2 infection. J Neuroimmunol 361: 577739, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jarius S, Ruprecht K, Kleiter I, et al. MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 2: epidemiology, clinical presentation, radiological and laboratory features, treatment responses, and long-term outcome. J Neuroinflammation 13: 280, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang EY, Mao T, Klein J, et al. Diverse functional autoantibodies in patients with COVID-19. Nature 595: 283-288, 2021. [DOI] [PubMed] [Google Scholar]