Abstract
Wastewater-based epidemiology (WBE) is a promising approach for monitoring the spread of SARS-CoV-2 within communities. Although qPCR-based WBE is powerful in that it allows quick and highly sensitive detection of this virus, it can provide limited information about which variants are responsible for the overall increase or decrease of this virus in sewage, and this hinders accurate risk assessments. To resolve this problem, we developed a next generation sequencing (NGS)-based method to determine the identity and composition of individual SARS-CoV-2 variants in wastewater samples. Combination and optimization of targeted amplicon-sequencing and nested PCR allowed detection of each variant with sensitivity comparable to that of qPCR. In addition, by targeting the receptor binding domain (RBD) of the S protein, which has mutations informative for variant classification, we could discriminate most variants of concern (VOC) and even sublineages of Omicron (BA.1, BA.2, BA.4/5, BA.2.75, BQ.1.1 and XBB.1). Focusing on a limited domain has a benefit of decreasing the sequencing reads. We applied this method to wastewater samples collected from a wastewater treatment plant in Kyoto city throughout 13 months (from January 2021 to February 2022) and successfully identified lineages of wild-type, alpha, delta, omicron BA.1 and BA.2 as well as their compositions in the samples. The transition of these variants was in good agreement with the epidemic situation reported in Kyoto city during that period based on clinical testing. These data indicate that our NGS-based method is useful for detecting and tracking emerging variants of SARS-CoV-2 in sewage samples. Coupled with the advantages of WBE, this method has the potential to serve as an efficient and low cost means for the community risk assessment of SARS-CoV-2 infection.
Keywords: Wastewater-based epidemiology, Wastewater surveillance, Spike RBD, Next generation sequencing, Omicron BQ.1.1, Omicron XBB.1
Graphical abstract
1. Introduction
Wastewater-based epidemiology (WBE; also referred to as wastewater surveillance) of SARS-CoV-2, which measures molecular signatures of this virus in sewage, is an emerging approach for inferring COVID-19 status in local communities (Daughton, 2020; Haramoto et al., 2020). WBE has been expected to be a powerful public health tool that could enable early detection of outbreaks and unbiased monitoring of COVID-19 patients in particular geographical areas, both of which are difficult with clinical testing at the individual case level (people with inapparent infection basically do not undergo clinical testing and complete inspection is practically impossible for this testing method). Indeed, the feasibility and utility of WBE has been demonstrated in several studies: rise of SARS-CoV-2 RNA load in sewage can be a leading indicator of increased positive cases (Peccia et al., 2020) or hospitalization and ICU admissions (Galani et al., 2022). Furthermore, WBE has detected novel cryptic lineages of SARS-CoV-2 in urban wastewater that are not registered in GISAID's EpiCoV database (Smyth et al., 2022). Thus, WBE is becoming an indispensable public health tool for tracking the spread of COVID-19.
Quantitative PCR (qPCR) has thus far been a primary molecular technique for WBE purposes and is described in many reports (Ahmed et al., 2020; Lu et al., 2020). The ability of rapid and highly sensitive detection makes qPCR suitable to routinely track the rise and fall of SARS-CoV-2 RNA concentrations in wastewater over the course of a pandemic. In addition, digital PCR (dPCR), a more advanced PCR-based methodology, has also been applied to WBE and provided a detection rate even greater than that of standard qPCR (Ahmed et al., 2022). However, qPCR-based methods in principle can provide limited information about which variants/lineages are responsible for the overall increase or decrease of SARS-CoV-2 in sewage, thus hindering accurate risk assessments and effective countermeasures. Allele-specific qPCR protocols have been developed for some variants, but simultaneous detection of multiple variants in the same sewage sample would be complicated even if it is possible (Xu et al., 2022). Furthermore, it is becoming necessary to consider not a single but multiple mutations on a consecutive sequence because recently emerging variants share many core mutations, making variant-specific single mutations rare.
High-throughput sequencing of wastewater can overcome the limitations associated with PCR-based WBE, and its ability to detect mutations can help understanding the dynamics of variants (Tamáš et al., 2022). Most studies have utilized amplicon sequencing with the use of pooled primer panels covering nearly the entire genome of SARS-CoV-2. For example, the ARTIC V3 or V4 primer panel for Illumina platforms consists of about 100 primer pairs and is used as two pools in amplification reactions, with the average lengths of amplicons of 400 bp (Tamáš et al., 2022). Although pooling PCR primers has several significant benefits (e.g., cost, time, reagent amount, and labor), it can be prone to uneven amplification and insufficient coverage depth, resulting in fragmented assembled genomes and poor sequence quality (Silva et al., 2022). This is particularly true when analyzing wastewater with low-abundance and fragmented RNAs, and these hurdles reduce the sensitivity and reproducibility of detected mutations (Amman et al., 2022; Otero et al., 2022; Silva et al., 2022; Spurbeck et al., 2021).
To address these issues, we developed a targeted amplicon sequencing protocol to determine the identity and composition of individual variants of SARS-CoV-2 with sensitivity comparable to qPCR. We focused on the receptor binding domain (RBD) of the spike gene because the combination of mutations in this domain allows us to distinguish most variants of SARS-CoV-2. The domain also determines clinically important characteristics of variants, including infectivity and immune escape (Focosi et al., 2023; Uriu et al., 2023), and hence fulfills the practical requirements for WBE. Optimized nested PCR and highly conserved primers were employed to dramatically enhance the sensitivity and to cover as many variants as possible. We evaluated the performance of this protocol by conducting model experiments, where RNA mixtures of up to six Omicron sublineages with known compositions were analyzed, and by applying the protocol to 25 samples collected from a wastewater treatment plant in Kyoto city, which resulted in confirming its utility.
2. Materials and methods
2.1. Preparation of synthetic SARS-CoV-2 RNA
Synthetic SARS-CoV-2 RNA Control (wild-type), which was artificially synthesized based on GenBank Accession MN908947.3, was purchased from TwistBiosciences (CA, USA). To determine accurate copy number, the synthetic SARS-CoV-2 RNA Control was quantified by one-step qRT-PCR using One Step TB Green PrimeScript RT-PCR Kit II (TakaraBio, Japan) with SARS-CoV-2 nucleocapsid primers (Integrated DNA Technologies, IA, USA, S001 and S002) listed in Supplementary Table 1. The synthetic SARS-CoV-2 RNA Control was diluted to 500 copies/reaction and mixed for NGS library preparation.
2.2. Preparation of authentic SARS-CoV-2 RNA
Transmembrane serine protease 2 (TMPRSS-2)-expressing VeroE6 (VeroE6/TMPRSS2) cells were obtained from the Japanese Collection of Research Bioresources Cell Bank (Osaka, Japan) and maintained as described previously (Hashimoto et al., 2022). SARS-CoV-2 wild-type strain: 2019-nCoV/Japan/TY/WK-521/2020 (WK-521, accession no. EPI_ISL_408667), Omicron BA.1 strain: hCoV-19/Japan/TY38-873/2021 (TY38-873, accession no. EPI_ISL_7418017), Omicron BA.2 strain: hCoV-19/Japan/TY40-385/2022 (TY40-385, accession no. EPI_ISL_9595859), Omicron BA.5 strain: hCoV-19/Japan/TY41-702/2022 (TY41-702, accession no. EPI_ISL_13241867), Omicron BA.2.75 strain: hCoV-19/Japan/TY41-716/2022 (TY41-716, accession no. EPI_ISL_13969765), Omicron BQ1.1 strain: hCoV-19/Japan/TY41-796/2022 (TY41-796, accession no. EPI_ISL_15579783) and Omicron XBB.1 strain: hCoV-19/Japan/TY41-795/2022 (TY41-795, accession no. EPI_ISL_15669344) were provided by the National Institute of Infectious Diseases (NIID), Japan (Matsuyama et al., 2020). These authentic SARS-CoV-2 strains were propagated on VeroE6/TMPRSS2 cells and then subjected to RNA extraction using Direct-zol RNA Microprep Kits (Zymo Research, CA, USA) according to the manufacturer's instructions. The copy number in extracted RNA of each strain was quantitated by one-step qRT-PCR using One Step TB Green PrimeScript RT-PCR Kit II (TakaraBio, Japan) with S001 and S002 primers (Supplementary Table 1) for the nucleocapsid gene. The qPCR was conducted following manufacturer's instructions except that the reaction volume was 15 μL. The thermal cycling conditions were as follows: 42 °C for 5 min, 95 °C for 10 s, and 40 cycles of 95 °C for 3 s and 60 °C for 30 s, followed by a dissociation curve step. The 2019-nCoV Positive Control (Integrated DNA Technologies, IA, USA) was used as the calibration standard. The resultant live SARS-CoV-2 RNAs were diluted to 1000 copies/reaction and mixed for NGS library preparation.
2.3. Wastewater sampling
Twenty-five influent wastewater samples were collected from a wastewater treatment plant in Kyoto city from 27 January 2021 to 25 February 2022 (Supplementary Table 2). All samples were collected in sterile plastic bottles via 24-h composite sewage sampling with flow-proportional mixing and immediately transported at 4 °C to the laboratory and stored at −80 °C.
2.4. RNA extraction from wastewater samples
RNA from each water sample was extracted by the EPISENS-S method (Efficient and Practical virus Identification System with an ENhanced Sensitivity for Solids method) as described previously (Ando et al., 2022). Briefly, approximately 40 mL of an influent sewage sample was centrifuged at 3000g for 10 min at 4 °C to collect solids suspended in the wastewater as pellets. The pellets were subjected to RNA extraction using RNeasy PowerMicrobiome Kit (QIAGEN, Hilden, Germany) to obtain a final RNA extract volume of 50 μL according to the manufacturer's instructions.
2.5. Primer design and conservation analysis for GISAID registered SARS-CoV-2 accessions
We downloaded GISAID EpiCoV dataset (https://www.epicov.org/), which includes all registered SARS-CoV-2 genomes (Elbe and Buckland-Merrett, 2017; Khare et al., 2021; Shu and McCauley, 2017). The EPI SET identifier of GISAID was EPI_SET_230124br. Those genome sequences were aligned by MAFFT version 7.487 (Katoh et al., 2002; Katoh and Standley, 2013). Next, partial sequences corresponding to the spike gene were extracted and sequences containing ambiguous bases were excluded using SeqKit (Shen et al., 2016). Also, we chose sequences that were derived from human hosts. For primer design, we calculated the conservation rates among strains registered with GISAID at each base coordinate of the Spike gene, and contiguous regions with relatively high conservation were selected. From those regions, we picked primer candidates using the Primer3 (Koressaar and Remm, 2007; Untergasser et al., 2012), and introduced some degenerate bases in order to increase conservation rates at each base. The designed primers S006, S007, S008, S009, S012, and S013 are listed in Supplementary Table 1. To evaluate conservation, we calculated the conservation rates among GISAID-registered strains (EPI SET identifier was EPI_SET_230124sz) at nucleotide coordinates corresponding to the primer design sites.
2.6. Preparation of spike gene amplicon
The RNA extracted from sewage was reverse transcribed to cDNA and amplified by either of the following two methods.
2.6.1. One-step method
Out of the 50 μL of RNA solution obtained from 40 mL of sewage, 13.5 μL were sequentially reverse transcribed and amplified using iScript Explore One-Step RT and PreAmp Kit (Bio-Rad, CA, USA) with SARS-CoV-2 spike gene primers S006 and S008 (Supplementary Table 1), according to the manufacturer's protocols with only the reaction volume being modified to 30 uL. These primers were designed to amplify a partial region of RBD (368–535 aa). The first PCR cycling conditions were as follows: 25 °C for 5 min, 45 °C for 60 min, 95 °C for 3 min, followed by 10 cycles of denaturation at 95 °C for 15 s and annealing/extension at 55 °C for 4 min. Using 2.5 μL of the reaction mixture as a template, an internal region (398–504 aa) was further amplified and Illumina overhang sequences were added with the use of KOD One PCR Master Mix (TOYOBO, Osaka, Japan) and primers S007 and S009 (Supplementary Table 1). The second PCR cycling conditions were as follows: 98 °C for 3 s followed by 45 cycles of denaturation at 98 °C for 10 s, annealing at 61 °C for 5 s, and extension at 68 °C for 2 s. The amplicons were purified using Agencourt AMPure XP beads (Beckman Coulter, Brea, CA, USA) and eluted with 10 mM Tris-HCl (pH 8.5).
2.6.2. Two-step method
Out of the 50 μL of RNA solution obtained from 40 mL of sewage, 13.5 μL were transferred to reverse transcription reaction of 20 μL using Reliance Select cDNA Synthesis Kit (Bio-Rad, CA, USA) and SARS-CoV-2 spike gene primer S008 (Supplementary Table 1), according to the manufacturer's protocols. The thermal conditions were as follows: 50 °C for 60 min, 95 °C for 1 min, and 4 °C until the next step. Then, 20 μL of the cDNA samples were subjected to nested PCR reaction using KOD One PCR Master Mix (TOYOBO, Osaka, Japan) or Platinum SuperFi II PCR Master Mix (Thermo Fisher Scientific, MA, USA). In the first PCR, the partial region of RBD (368–535 aa) or RBD (329–535 aa) of the SARS-CoV-2 spike gene were amplified using primers S006 and S008 for version 1 or primers S012 and S008 for version 2 (Supplementary Table 1). In both methods, reaction solutions were prepared according to the manufacturer's guidelines, and the reaction volume was 41.2 μL. The PCR thermal cycling conditions were as follows. For KOD One PCR Master Mix, the initial denaturation was performed at 98 °C for 3 s, followed by 10 cycles of denaturation at 98 °C for 10 s, annealing at 55 °C for 5 s, and extension at 68 °C for 2 s. For Platinum SuperFi II PCR Master Mix, the initial denaturation was conducted at 98 °C for 30 s, followed by 10 cycles of denaturation at 98 °C for 10 s, annealing at 60 °C for 10 s, and extension at 72 °C for 15 s.
In the second PCR step, using 3.4 μL of the reaction mixture of the first PCR as template, the gene sequences of internal region (398–504 aa) for version 1 and region (337–504 aa) for version 2 were amplified and Illumina overhang sequences were added using the same reaction composition as in the first PCR, and the primers S007 and S009 for version 1 and primers S013 and S009 for version 2 (Supplementary Table 1), respectively. The PCR thermal cycling conditions were as follows. For KOD One PCR Master Mix, the initial denaturation was performed at 98 °C for 3 s, followed by 45 cycles of denaturation at 98 °C for 10 s, annealing at 61 °C for 5 s, and extension at 68 °C for 2 s. For Platinum SuperFi II PCR Master Mix, the initial denaturation was conducted at 98 °C for 30 s, followed by 45 cycles of denaturation at 98 °C for 10 s, annealing at 60 °C for 10 s, and extension at 72 °C for 15 s. Amplicons were purified using Agencourt AMPure XP beads (Beckman Coulter, Brea, CA, USA) and eluted with 10 mM Tris-HCl (pH 8.5).
2.7. Library preparation and sequencing
The spike gene amplicon of each sewage was index-tagged using Nextera XT Index Kit v2 (Illumina, CA, USA), and purified using Agencourt AMPure XP beads (Beckman Coulter, Brea, CA, USA) according to the manufacturer's instructions. The size and DNA concentration of the purified pooled DNA libraries were measured using 4200 TapeStation (Agilent Technologies, CA, USA), and the libraries were sequenced using the Illumina MiSeq system with 250-base or 300-base paired-end reads. The target sequence depth was roughly 40,000 read pairs or more per sample.
2.8. RT-qPCR of sewage samples
SARS-CoV-2 RNA concentration in sewage samples was measured using the COPMAN method as described previously (Adachi Katayama et al., 2023). Briefly, the RNA extracted from sewage was reverse transcribed to cDNA using the Reliance Select cDNA Synthesis Kit (BIORAD, CA, USA) with the use of SARS-CoV-2 nucleocapsid gene primer S002 (Supplementary Table 1). Following cDNA synthesis, the nucleocapsid gene was pre-amplified using BIOTAQ HS DNA Polymerase (Nippon Genetics, Tokyo, Japan) with primer S001 and S002 (Supplementary Table 1). Copies of the SARS-CoV-2 virus were quantified using TaqMan™ Environmental Master Mix 2.0 (Thermo Fisher Scientific, MA, USA), primers S001 and S002 and probe S003 (Supplementary Table 1).
2.9. Data analysis
After forward and reverse sequence reads were obtained, primer sequences were cut by cutadapt (Martin, 2011) (ver. 2.3), followed by trimming of low-quality sequences by Trimmomatic (Bolger et al., 2014) (ver. 0.39) with setting SLIDINGWINDOW parameter as 200:30. Then, DADA2 (Callahan et al., 2016) (ver. 1.10.0) was applied to filter and merge all paired reads as well as to correct amplicon sequence variant error. All merged reads were aligned to the reference sequence, that is, the subsequence of wild-type (MN908947.3) spike protein targeted by designed primers. Aligned sequences were translated into protein sequences and compared to the reference protein sequence to detect amino acid variations for each position. Individual sequences were assigned to specific variants/lineages by comparing the combination of amino acid variations and the definitions prepared in advance (Table 1, Table 2 ). The sequences which did not match any definitions were classified as “Others”. Finally, we calculated the frequency of each variant/lineage. Python3.6 was used for downstream procedures after DADA2.
Table 1.
Relationships between mutations in spike protein and lineages/variants assigned in workflow version 1.
| Version 1 | Wild-type | Omicron BA.1 | Omicron BA.2/3 | Omicron BA.2.75 | Omicron BA.4/5 | Omicron BA.2.12.1 | Alpha | Beta | Gamma | Delta |
|---|---|---|---|---|---|---|---|---|---|---|
| D405 | a | N | N | N | N | |||||
| R408 | X | X | X | X | ||||||
| K417 | Xb | N, T | X | N | N | N | T | |||
| G446 | S | S | ||||||||
| L452 | R | Q | R | |||||||
| N460 | K | |||||||||
| S477 | N | N | N | N | N | |||||
| T478 | K | K | K | K | K | K | ||||
| E484 | A | A | A | A | A | K | K | |||
| F486 | F, S | V | ||||||||
| Q493 | R | R | R | |||||||
| G496 | S | |||||||||
| Q498 | R | R | R | R | R | |||||
| N501 | Y | Y | Y | Y | Y | Y | Y | Y |
Table 2.
Relationships between mutations in spike protein and lineages/variants assigned in workflow version 2.
| Version 2 | Wild-type | Omicron BA.1 | Omicron BA.2/3 | Omicron BA.2/3 with R346X | Omicron BA.2/3 with K444X | Omicron BA.2/3 with V445X | Omicron BA.2/3 with R346X + K444X or V445X | Omicron BA.2.3.20 | Omicron BQ.1 | Omicron BQ.1.1 | Omicron XBB | Omicron XBB.1.5 | Omicron BA.2.12.1 | Omicron BA.2.75 | Omicron BA.4/5 | Omicron BA.4/5 with V445X | Omicron BA.4/5 with K444X | Omicron BA.4/5 with R346S | Omicron BA.4/5 with R346X + K444X or V445X | Omicron BA.4.6/BF7 | Omicron BA.5.9 | Delta | Alpha | Beta | Gamma |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G339 | a | D | N,D,H | N,D,H | N,D,H | N,D,H | N,D,H | D | D | D | H | H | D | H | D | D | D | D | D | D | D | ||||
| R346 | R,K | T,S,I | T,S,I | T | T | T | R,T,I | S | S,T,I | T | I | ||||||||||||||
| K356 | T,K | ||||||||||||||||||||||||
| L368 | L,I | L,I | L,I | L,I | L,I | I | I | ||||||||||||||||||
| D405 | N | N | N | N | N | N | N | N | N | N | N,D | N | N,D | N,D | N,D | N,D | N,D | N,D | N,D | ||||||
| R408 | Xb | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||||
| K417 | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | N | T | |||
| N440 | K | N,K | N,K | N,K | N,K | N,K | N,K | K | K | K | K | N,K | K | N,K | N,K | N,K | N,K | N,K | N,K | N,K | |||||
| K444 | R,T,M,N,R,Q,A | R,T,M,N,R,Q,A | R | T | T | K,M,T | R,T,M,N,R,Q,A | R,T,M,N,R,Q,A | |||||||||||||||||
| V445 | A,P,F | V,A,P,F | P | P | A,P,F | V,A,P,F | |||||||||||||||||||
| G446 | S | G,S | G,S | G,S | G,S | G,S | G,S | S | S | S | G,S | G,S | G,S | G,S | G,S | G,S | G,S | ||||||||
| N450 | D | N,D | N,D | N,D | N,D | N,D | N,D | N,D | N,D | ||||||||||||||||
| L452 | L,M,R | L,M,R | L,M,R | L,M,R | L,M,R | M | R | R | Q | L,R | X | X | X | X | X | X | X | R | |||||||
| N460 | N,K | N,K | N,K | N,K | N,K | K | K | K | K | K | K | N,K | |||||||||||||
| S477 | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | |||||
| T478 | K | K | K | K | K | K | K,E | K | K | K | K | K | K | K | K | K | K | K | K | K | K | ||||
| E484 | A | A | A | A | A | A | R | A | A | A | A | A | A | A | A | A | A | A | A | A | K | K | |||
| F486 | V | V | S | P | F,S | V,A | V,A | V,A | V,A | V,A | V,A | V,A | |||||||||||||
| F490 | S | S | F,S | ||||||||||||||||||||||
| Q493 | R | R | R | R | R | R | R | ||||||||||||||||||
| G496 | S | ||||||||||||||||||||||||
| Q498 | R | R | R | R | R | R | R,Q | R | R | R | R | R | R | R | R | R | R | R | R | R | |||||
| N501 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
This variant definition was used to obtain the data shown Fig. 5.
The same as Wild-type (null).
Any residues.
2.10. Analysis of prevalent variants in Kyoto
We downloaded the GISAID EpiCoV metadata (https://www.epicov.org/) for all registered SARS-CoV-2 genomes, and metadata with year, month and day were used for analysis of the variant transition. For variant transition in Japan, metadata with “Asia/Japan” were used and the EPI SET identifier of the GISAID dataset was EPI_SET_230201ye. For the variant transition in Kyoto, “Asia/Japan/Kyoto” metadata were used and the EPI SET identifier of the GISAID dataset was EPI_SET_230123hg. We aggregated the count of sequences by WHO label for each week and visualized with stacked bar chart using Python version 3.7.4 and TIBCO Spotfire Analyst version 11.4.3.
3. Results
3.1. Development of a workflow to analyze the composition ratios of SARS-CoV-2 variants from sewage
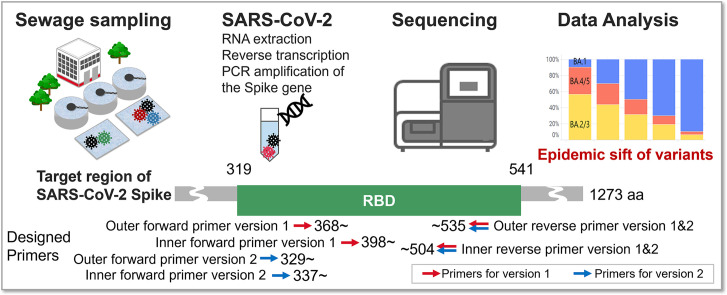
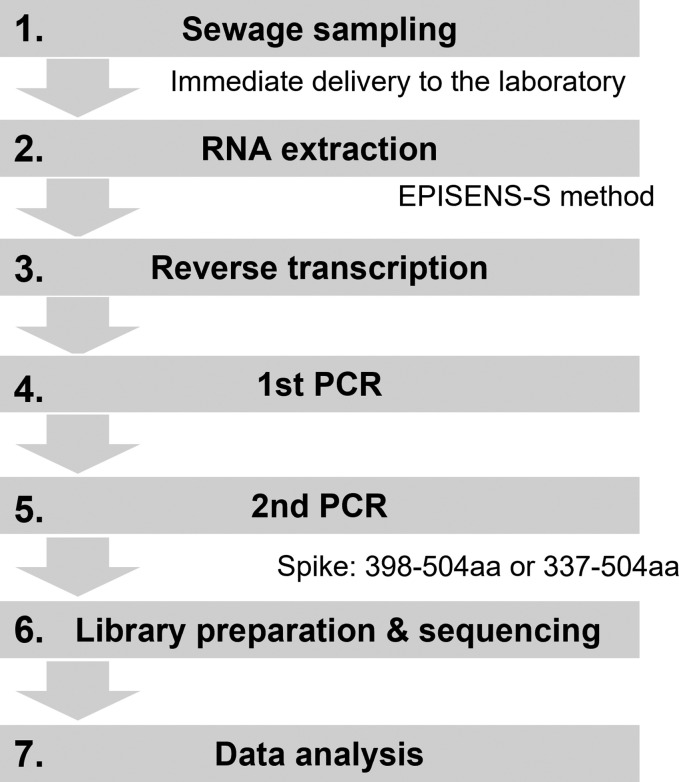
We developed a sensitive workflow for measuring the composition ratios of SARS-CoV-2 variants in wastewater. This workflow consists of the seven steps illustrated in Fig. 1 .
Fig. 1.
Flowchart of targeted amplicon sequencing protocol developed in this study. The original protocol (version 1) and the updated protocol (version 2) target 398–504 aa and 337–504 aa of SARS-CoV-2 spike protein, respectively.
In the sewage sampling step (Step 1), it is ideal to use an abundant amount of sewage to calculate the stochastically plausible variant composition, but the amount of sewage required should be as small as possible in terms of tasks and costs for continuous water sampling, transportation and storage. Considering a balance between data accuracy and sampling burden, we employed a protocol that uses 40 mL of sewage.
In the RNA extraction step (Step 2), a method for extracting high-purity SARS-CoV-2 RNA from sewage is essential. Hence, we compared a few RNA extraction methods that have been published and found the RNA extraction protocol of the EPISENS-S method (Ando et al., 2022) to be suitable for this workflow (data not shown). Briefly, sewage samples are centrifuged to pellet the sediment, and the RNA of sewage organisms contained in the pellet are purified.
We then optimized the reverse transcription and pre-amplification (1st PCR) process (Steps 3 and 4). Following the EPISES-S method mentioned above, we first tried using the iScript Explore One-Step RT and PreAmp Kit, which allows reverse transcription and pre-amplification to be performed in one step. As a result, although we could obtain amplicons and composition data from mixtures of SARS-CoV-2 RNAs (Supplementary Fig. 1), no amplicons were detected in some sewage samples with low-copy number SARS-CoV-2 (data not shown). Therefore, to increase the amount of RNA brought into amplification reactions and to utilize different enzymes/kits, we tried a two-step method, where reverse transcription and amplification were conducted separately. The Reliance Select cDNA Synthesis Kit and KOD One PCR Master Mix were used for reverse transcription and amplification, respectively. As a result, we succeeded in detecting amplicons of variants from sewage that included only several tens to hundreds copies/L of SARS-CoV-2 (data not shown).
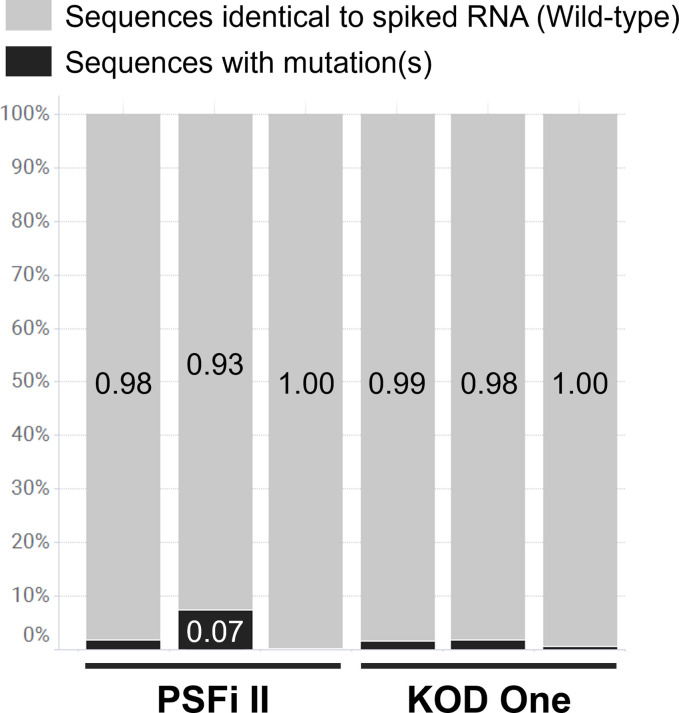
We also focused on the accuracy of mutation detection. PCR enzymes used in the amplification steps (Steps 4 and 5) should be of high fidelity (less error) and resistant to inhibition by contaminants in sewage. We selected two candidate enzymes, KOD One PCR Master Mix and Platinum SuperFi II PCR Master Mix and evaluated their fidelity using wild-type synthetic RNA. As a result, KOD One PCR Master Mix resulted in fewer PCR errors (Fig. 2 ) and hence was employed in this workflow. These two enzymes did not differ in success rates of amplicon detection from actual sewage samples (data not shown).
Fig. 2.
Results of optimization of PCR enzyme. Two PCR enzymes, Platinum SuperFi II PCR Master Mix (PSFi II, Thermo Fisher Scientific, MA, USA) and KOD One PCR Master Mix (KOD One, TOYOBO, Osaka, Japan) were tested as candidates. To evaluate the PCR fidelity of these enzymes, we tested whether the correct lineage can be detected using 500 copies per reaction of wild-type synthetic RNA (TwistBiosciences, CA, USA). Stacked bar chart indicates lineage composition identified using PSFi II (left half bars, n = 3) and KOD One (right half bars, n = 3). Grey and black show wild-type and undefined lineages (wild-type with mutation(s) caused by PCR errors), respectively. KOD One detected fewer undefined lineages, indicating fewer PCR errors.
To comprehensively detect variants/sublineages, we designed a primer set for nested PCR on the spike RBD region with emphasis on conservation among the sequences registered in GISAID. We succeeded in identifying a high-coverage primer set (S006, S007, S008, and S009 in Supplementary Table 1), which was conserved in most GISAID accessions as of 21 January 2023 (Supplementary Table 3 and Supplementary Fig. 4A). The inner primer set amplifies residues 398–504 of the spike protein. We also confirmed that the coverage of these series of primers was maintained during the sampling period of Kyoto city sewage (from January 2021 to February 2022), which was used for validation of our workflow using actual sewage, with a coverage of 99.18 % (Supplementary Table 3).
Finally, we prepared a definition table for assigning the NGS unique sequences to specific variants, with reference to covSPECTRUM (https://cov-spectrum.org/) (Chen et al., 2022) (Table 1). The definition consists of amino acid mutations characteristic of each variant to be detected and was designed to classify the sequences as accurately as possible at that time. It is supposed to be updated when novel variants important for public health emerge.
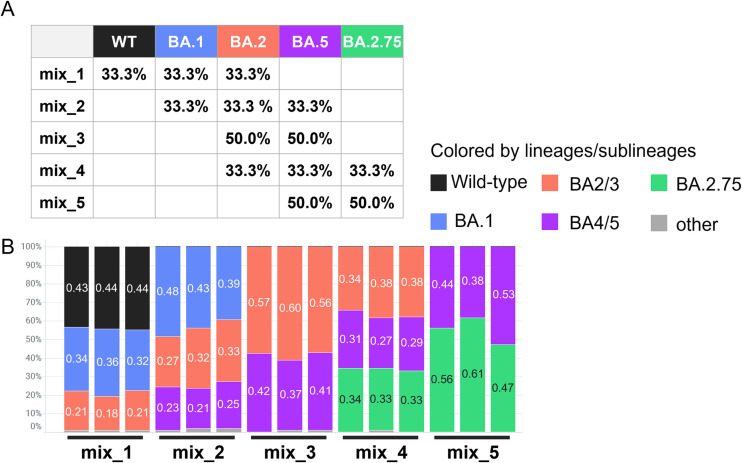
We validated this workflow using SARS-CoV-2 RNA mixtures which contained two or three variants (Fig. 3A). For each mixture sample, the measured compounding ratios were close to the theoretical value (Fig. 3B). Information on the NGS datasets corresponding to Fig. 2, Fig. 3 and Supplementary Fig. 1 have been deposited at DDBJ/GenBank under accession number DRA016444, BioProject number PRJDB15905, and BioSample number SAMD00612299 to SAMD00612328 and SAMD00617904 to SAMD00617909.
Fig. 3.
Evaluation of original workflow (version 1) using mixture of RNAs of SARS-CoV-2 variants. To validate our workflow, we first measured viral copy numbers of each live SAR-CoV-2 sublineage by qRT-PCR and mixed into a total of 1000 copies/reaction. Three replicates of each mixture were then analyzed according to the flowchart shown in Fig. 1 (from reverse transcription on step 3 to data analysis on step 7). (A) Theoretical composition ratios of RNA mixture of sublineages (mix_1 to 5). (B) Proportion of sublineages detected using the samples showed in (A). The colors represent individual lineages, which are shown in the legend.
3.2. Transition of variants in Kyoto sewage from January 2021 to February 2022
We retrospectively analyzed the transition of SARS-CoV-2 variants in sewage samples from a wastewater treatment plant in Kyoto city from January 2021 to February 2022 (Supplementary Table 2). This period corresponded to the third to sixth waves of the COVID-19 pandemic in Japan. The most prevalent variants were B.1.1.214 (D614G mutant prevalent mainly in Japan) in the third wave (around January 2021), Alpha in the fourth wave (around May 2021), Delta in the fifth wave (around August 2021), and Omicron BA.1/BA.2 in the sixth wave (around February 2022). In particular, the number of infected people in the sixth wave was relatively high in Japan, and the peak number of newly confirmed cases per day on average for 7 days exceeded 87,000 in Japan and 2600 in Kyoto prefecture.
We used qRT-PCR to measure the concentration of SARS-CoV-2 in Kyoto sewage for the 25 samples collected over the time period. The copy numbers of SARS-CoV-2 per liter were calculated for each batch of sewage, and the resultant range was from below the lower limit of quantitation to over 140,000 copies/L and some samples seemed to be difficult to analyze because of low copy numbers (Supplementary Table 2).
Using our workflow, spike amplicons were detected from all 25 samples (Supplementary Table 2) with the expected length (Supplementary Fig. 2), and hence all the samples were subsequently NGS-sequenced and analyzed. As a result, we succeeded in detecting SARS-CoV-2 variant transition in sewage of Kyoto, which agreed well with that of clinically detected variants during the same period in Japan and in Kyoto (Fig. 4 and Supplementary Fig. 3A, B). These data demonstrated the high sensitivity and utility of our workflow for detection and relative quantification of SARS-CoV-2 variants in sewage. Information on the NGS datasets corresponding to Fig. 4 have been deposited at DDBJ/GenBank under accession number DRA016444, BioProject number PRJDB15905 and BioSample number SAMD00612274 to SAMD00612298.
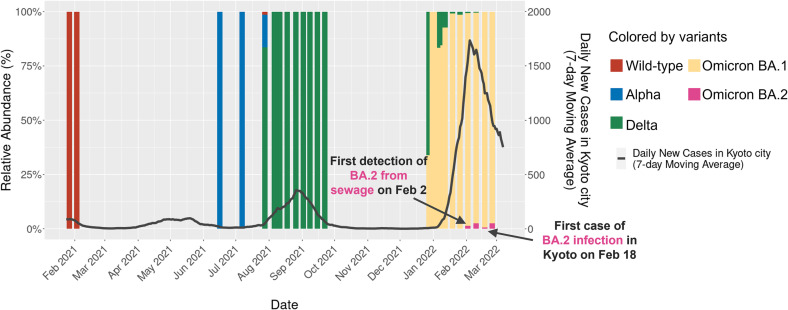
Fig. 4.
Transition of variants in the sewage of Kyoto city, Japan. Results of analyzing sewage samples collected in Kyoto from 27 January 2021 to 25 February 2022, using workflow version 1. The X-axis indicates the time series, the Y-axis (left) and the stacked bar graph indicate the relative abundance of variants detected in the sewage at each time point, and the Y-axis (right) and the line graph indicate the number of new COVID-19 infections per day (7-day average) in Kyoto City. The colors represent each detected variant, as shown in the legend. The lineage present in the two sewage samples at the beginning of 2021, was detected as the wild-type. Considering the infection situation in Japan at that time, it was likely to be of Japan origin lineage (B.1.1.214), which does not have mutations in the targeted region.
3.3. Update of workflow for SARS-CoV-2 variants with spike R346X, K444X, V445X and F486P
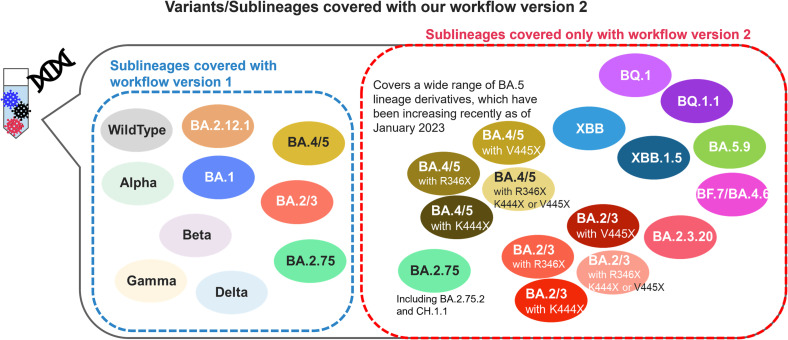
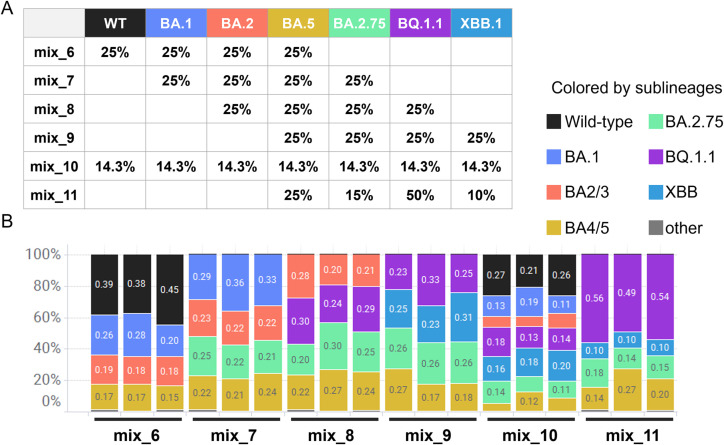
As of the end of December 2022 to the middle of January 2023, while this paper was being prepared, the Omicron BQ.1.1, BA.2.75, XBB.1, and XBB.1.5 were mainly prevalent globally, and variants with spike R346X, K444X, V445X and F486P were increasing (Focosi et al., 2023). In addition, XBB.1 and XBB.1.5 acquired a mutation that resulted in a single-base mismatch with an existing nested forward primer. Therefore, to cover these emerging variants, we extended the target region of amplicon sequencing to the 5′ side and changed the nested forward primers. New forward primers (S012 and S013 in Supplementary Table 1) were designed by searching for regions around spike 320–340 residues that are comprehensively conserved in the variant sequences registered in GISAID. We named the new primer set version 2. It amplifies the sequence of residues 337–504 of the spike gene, which corresponds to most of the RBD. We also updated the mutation definition table (Table 2), which enabled us to classify 25 variants, including the recently increasing BQ.1, BQ.1.1, XBB, XBB.1.5 and BA4/5 with R346X, K444X, and/or V445X as of January 2023 (Fig. 6). Using this updated workflow, we analyzed samples containing viral RNA of XBB.1 and BQ.1.1 (Fig. 5A) and succeeded in detecting each variant at a close composition ratio to the theoretical value (Fig. 5B). Notably, we could classify seven lineages with nearly accurate compositions for the authentic virus mixture which contained equal amounts of RNA from seven lineages, namely, wild-type, Omicron BA.1, BA.2, BA.5, BA.2.75, BQ.1.1, and XBB.1 (Fig. 5B, mix_10). Information on the NGS datasets corresponding to Fig. 4 have been deposited at DDBJ/GenBank under accession number DRA016444, BioProject number PRJDB15905 and BioSample number SAMD00612329 to SAMD00612346.
Fig. 6.
Conceptual diagram of detectable variants (comparison between version 1 and version 2).
Fig. 5.
Evaluation of updated workflow (version 2) using mixture of RNAs of SARS-CoV-2 variants. To validate our workflow, we first measured viral copy numbers of each live SAR-CoV-2 sublineage by qRT-PCR and mixed into a total of 1000 copies/reaction. Three replicates of each mixture were then analyzed according to the flowchart shown in Fig. 1 (from reverse transcription on step 3 to data analysis on step 7). (A) Theoretical composition ratios of RNA mixture of sublineages (mix_6 to 11). (B) Proportion of sublineages detected using the samples showed in (A). The colors represent each detected variant, as shown in the legend.
4. Discussion
In this study, we developed a highly sensitive, cost-effective sewage analysis workflow to measure SARS-CoV-2 variant compositions, which requires only 40 mL of sewage. In addition, we showed the validity of the workflow using past samples of sewage from Kyoto (Fig. 4 and Supplementary Fig. 3). Notably, we demonstrated that target variants could be updated in response to the epidemic shift through validation experiments (Fig. 5B) using RNA of SARS-CoV-2 variants including BQ.1.1 and XBB.1 (Fig. 5A).
Early warning is one of the advantages of WBE in the monitoring of COVID-19 patients in communities (Daughton, 2020). In the case of measurement by qPCR, it has been reported that an increase in SARS-CoV-2 RNA concentrations in wastewater preceded a rise in clinical positive cases by 2–10 days (Nemudryi et al., 2020; Wu et al., 2022). In the feasibility experiment using past samples of Kyoto sewage, Omicron BA.2 was first detected in sewage collected on 2 February 2022 in Kyoto city, and the first collection date of BA.2 from an infected individual in Kyoto whose genome sequence is registered in GISAID was 18 February 2022. This shows that our workflow was able to detect this variant in sewage 16 days earlier than clinical sample sequencing. BA.2 was confirmed as early as 30 December 2021 at the quarantine area of a Japanese airport (Narita) and genome sequencing of clinical specimens from infected individuals is generally less common in Japan compared with other countries such as United States and United Kingdom. Preceding detection of newly arising variants in sewage one month prior to clinical testing is also reported in several studies (Joshi et al., 2022; Vo et al., 2022). Therefore, our findings and previous reports collectively indicate that the benefit of early detection by WBE may be greater in variant identification by sequencing than in quantification of total SARS-CoV-2 RNAs by qPCR. In addition, the value of early detection of emerging variants by wastewater sequencing may increase if the number of clinical tests for SARS-CoV-2 decreases, which will likely occur after May 2023 in Japan because the infectious disease law has been revised to lower the classification of COVID-19 to that of seasonal influenza.
We analyzed 25 sewage samples collected from the municipal wastewater treatment plant in Kyoto city. The copy numbers per liter of SARS-CoV-2 RNAs in these samples ranged from below the lower limit of quantitation to over 140,000 and some samples seemed to be difficult to analyze because of low copy numbers (Supplementary Table 2). Nevertheless, we could obtain amplicons from all sewage samples. The highly sensitive detection is likely to be due both to the RNA extraction protocol of the EPISENS-S method and to nested PCR with version 1 primers. EPISENS-S has been developed for routine COVID-19 wastewater surveillance and is characterized by highly sensitive and cost-effective detection. It has been reported that the method could detect a few hundred copies of SARS-CoV-2 per liter of sewage with qPCR targeted nucleocapsid (N1) gene using the CDC-N1 primer set (Ando et al., 2022) and our workflow showed a similar lower limit. It should be noted that, in principle, the detection of the long amplicon (e.g., the RBD region targeted in this study, 322 bp) is more difficult than that of the short fragment (e.g., N1 gene amplified by the CDC-N1 primer set, 72 bp) because RNA in sewage is considerably degraded. In fact, in some sewage with low concentrations of SARS-CoV-2, we observed that RNA extraction by EPISENS-S without nested PCR failed to detect Spike gene amplicons (data not shown). On the other hand, nested PCR is a well-known method for enabling the detection of low amounts of nucleic acids (Haff, 1994), and has been used in several studies for WBE (Gregory et al., 2022; Smyth et al., 2022). Primer design is one of the keys to nested PCR and hence we designed several primer candidates, evaluated their performance, and selected the best ones in terms of sensitivity and stable detection (data not shown).
Resolution of variant identification is an important factor for SARS-CoV-2 surveillance because recently emerging Omicron variants (e.g., BQ.1.1 and XBB.1.5) have originated from a common ancestral lineage BA.2 and share many key mutations, including R346T and N460K (Focosi et al., 2023). Focusing on a specific region generally leads to lower resolution and involves a risk of sacrificing the value of protocols. However, the RBD region targeted in this workflow (398–504 or 337–504 of the spike protein) encompasses most of the mutation sites important for infectivity and immune escape of the virus (Focosi et al., 2023; Starr et al., 2022), and hence can provide sufficient resolution for discriminating clinically important variants, such as BQ.1.1 and XBB.1 (Fig. 5B). Therefore, although there exist some significant mutations outside the region such as E156G/Δ157–158 (Mishra et al., 2022), our workflow can likely be used for most future variants.
There are some reports that employed targeted amplicon sequencing for analyzing SARS-CoV-2 RNA in sewages with or without nested PCR (Cancela et al., 2023; Iwamoto et al., 2023; Smyth et al., 2022). These researches successfully captured the transitions of major variants/lineages in wastewater of each community, but their methodologies did not cover the recent Omicron sublineages (e.g., BQ.1.1 and XBB.1) due to the limited regions of RBD analyzed. Therefore, we believe that our protocol has some advantages against these prior studies.
As a public health tool, it is critical to be able to measure samples simply and cost-effectively. In this sense, the workflow we have developed is suitable because it uses only four primers (both for reverse transcription and nested PCR) and can be conducted with widely distributed NGS (i.e. MiSeq, Nextseq). Additionally, focusing on a partial region of the spike protein considerably saves on the number of sequence reads required (ca. 40,000 reads/sample). These features offer advantages over competing methods, such as whole genome amplicon sequencing (Tamáš et al., 2022), whole SARS-CoV-2 capture sequencing (Doddapaneni et al., 2021) and long spike amplicon sequencing (Barbé et al., 2022), all of which require specialized reagents and/or instruments. Therefore, we conclude that our workflow can be widely adopted as a wastewater sequencing method of SARS-CoV-2.
Our workflow does have some limitations. In evaluating the accuracy of our workflow using SARS-CoV-2 RNA mixtures, we could detect composition ratios of variants close to the theoretical value. However, we observed a slight deviation from the theoretical value for some lineages (e.g., BA.4/5 in Fig. 3). This may be due to the difference of the genes amplified by qPCR and NGS; qPCR targeted the nucleocapsid (N1) gene using the CDC-N1 primer set, whereas NGS targeted the spike gene. In addition, it has been reported that the RNA expression level of each gene differs slightly among the variants (Niemeyer et al., 2022). Therefore, to more accurately evaluate our amplicon-based sequencing protocol, it would be better to use the spike gene (as done with NGS) for copy number measurement by qPCR.
Another limitation of our workflow is the time required to obtain sequence data, which is a general issue of NGS-based protocols. When using a short-read sequencer, it takes several days to conduct a series of experiment for data acquisition (e.g., library preparation and sequencing run), which results in a partial loss of the early detection feature of WBE. Therefore, in the future, we need to develop workflows that would enable the detection of emerging variants from sewage in several hours with sufficient resolution. Such a methodology would realize the full potential value of WBE.
5. Conclusions
-
•
We have developed a workflow with high sensitivity and sufficient resolution to measure SARS-CoV-2 variant compositions in sewage by using targeted amplicon sequencing.
-
•
The workflow succeeded in capturing the transition of VOCs in the sewage of Kyoto city, Japan, which was in good agreement with data from clinical testing.
-
•
Our workflow supports the latest closely related variants, such as BQ.1.1 and XBB.1.
CRediT authorship contribution statement
Miho Kuroiwa: Research design, Methodology, Investigation, Formal analysis, Writing – original draft, Writing – review & editing. Yoshinari Gahara: Methodology, Investigation, Validation, Writing – review & editing. Hirohito Kato: Primer design, Investigation, Validation, Writing – review & editing. Yuji Morikawa: Analysis system design and implementation, Bioinformatical analysis, Investigation, Writing – review & editing. Yuki Matsui: Analysis system design and implementation, Bioinformatical analysis, Investigation, Writing – review & editing. Takumi Adachi: Analysis system design and implementation, Bioinformatical analysis, Investigation, Writing – review & editing. Shin Kurosawa: Analysis system design and implementation, Writing – review & editing. Tomohiro Kuroita: Sampling, Investigation, Writing – review & editing. Yoshinori Ando: Sampling, Investigation, Writing – review & editing. Masatomo Rokushima: Supervision, Research design, Methodology, Investigation, Writing – original draft, Writing – review & editing.
Declaration of competing interest
All authors (Miho Kuroiwa, Yoshinari Gahara, Hirohito Kato, Yuji Morikawa, Yuki Matsui, Takumi Adachi, Shin Kurosawa, Tomohiro Kuroita, Yoshinori Ando, and Masatomo Rokushima) report a relationship with Shionogi & Co., Ltd. that includes: employment.
Acknowledgements
We sincerely acknowledge the National Institute of Infectious Diseases (Japan) for providing the SARS-CoV-2 variants and Kyoto city for kindly providing the sewage samples. The authors also thank Ryo Iwamoto, Aya Uchida, and other members in AdvanSentinel Inc. for sewage sample collection and shipping. We thank Keiichi Taniguchi and Takao Shishido for preparing the SARS-CoV-2 variants. We thank Takashi Hashimoto and other members in Shionogi Techno Advance Research, Co., Ltd. for the culture and RNA extraction of live SARS-CoV-2 variants. We gratefully acknowledge all data contributors, i.e., the authors and their originating laboratories responsible for obtaining the specimens, and their Submitting laboratories for generating the genetic sequence and metadata and sharing via the GISAID Initiative, on which this research is based. This research was conducted as part of “Covid-19 AI & Simulation Project” run by Mitsubishi Research Institute commissioned by Cabinet Secretariat. This study was funded by Shionogi & Co., Ltd.
Editor: Warish Ahmed
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.scitotenv.2023.164766.
Appendix A. Supplementary data
Supplementary figures and tables
Data availability
The NJ/GenBank undeGS Raw datasets were deposited to DDBr accession number DRA016444, BioProject number PRJDB15905, and BioSample number SAMD00612274 to SAMD00612346 and SAMD00617904 to SAMD00617909.
References
- Adachi Katayama Y., Hayase S., Ando Y., Kuroita T., Okada K., Iwamoto R., Yanagimoto T., Kitajima M., Masago Y. COPMAN: a novel high-throughput and highly sensitive method to detect viral nucleic acids including SARS-CoV-2 RNA in wastewater. Sci. Total Environ. 2023;856(Pt 1) doi: 10.1016/j.scitotenv.2022.158966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Angel N., Edson J., Bibby K., Bivins A., O’Brien J.W., Choi P.M., Kitajima M., Simpson S.L., Li J., Tscharke B., Verhagen R., Smith W.J.M., Zaugg J., Dierens L., Hugenholtz P., Thomas K.V., Mueller J.F. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020;728 doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Smith W.J.M., Metcalfe S., Jackson G., Choi P.M., Morrison M., Field D., Gyawali P., Bivins A., Bibby K., Simpson S.L. Comparison of RT-qPCR and RT-dPCR platforms for the trace detection of SARS-CoV-2 RNA in wastewater. ACS ES&T Water. 2022 doi: 10.1021/acsestwater.1c00387. acsestwater.1c00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amman F., Markt R., Endler L., Hupfauf S., Agerer B., Schedl A., Richter L., Zechmeister M., Bicher M., Heiler G., Triska P., Thornton M., Penz T., Senekowitsch M., Laine J., Keszei Z., Klimek P., Nägele F., Mayr M.…Bergthaler A. Viral variant-resolved wastewater surveillance of SARS-CoV-2 at national scale. Nat. Biotechnol. 2022 doi: 10.1038/s41587-022-01387-y. [DOI] [PubMed] [Google Scholar]
- Ando H., Iwamoto R., Kobayashi H., Okabe S., Kitajima M. The efficient and practical virus identification system with ENhanced Sensitivity for Solids (EPISENS-S): a rapid and cost-effective SARS-CoV-2 RNA detection method for routine wastewater surveillance. Sci. Total Environ. 2022;843 doi: 10.1016/j.scitotenv.2022.157101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbé L., Schaeffer J., Besnard A., Jousse S., Wurtzer S., Moulin L., Le Guyader F.S., Desdouits M. SARS-CoV-2 whole-genome sequencing using Oxford nanopore technology for variant monitoring in wastewaters. Front. Microbiol. 2022;13 doi: 10.3389/fmicb.2022.889811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger A.M., Lohse M., Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics (Oxford, England) 2014;30(15):2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan B.J., McMurdie P.J., Rosen M.J., Han A.W., Johnson A.J.A., Holmes S.P. DADA2: high-resolution sample inference from Illumina amplicon data. Nat. Methods. 2016;13(7):581–583. doi: 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancela F., Ramos N., Smyth D.S., Etchebehere C., Berois M., Rodríguez J., Rufo C., Alemán A., Borzacconi L., López J., González E., Botto G., Thornhill S.G., Mirazo S., Trujillo M. Wastewater surveillance of SARS-CoV-2 genomic populations on a country-wide scale through targeted sequencing. PLoS One. 2023;18(4) doi: 10.1371/journal.pone.0284483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Nadeau S., Yared M., Voinov P., Xie N., Roemer C., Stadler T. CoV-Spectrum: analysis of globally shared SARS-CoV-2 data to identify and characterize new variants. Bioinformatics (Oxford, England) 2022;38(6):1735–1737. doi: 10.1093/bioinformatics/btab856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daughton C.G. Wastewater surveillance for population-wide Covid-19: the present and future. Sci. Total Environ. 2020;736 doi: 10.1016/j.scitotenv.2020.139631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doddapaneni H., Cregeen S.J., Sucgang R., Meng Q., Qin X., Avadhanula V., Chao H., Menon V., Nicholson E., Henke D., Piedra F.-A., Rajan A., Momin Z., Kottapalli K., Hoffman K.L., Sedlazeck F.J., Metcalf G., Piedra P.A., Muzny D.M.…Gibbs R.A. Oligonucleotide capture sequencing of the SARS-CoV-2 genome and subgenomic fragments from COVID-19 individuals. PLoS One. 2021;16(8) doi: 10.1371/journal.pone.0244468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbe S., Buckland-Merrett G. Data, disease and diplomacy: GISAID’s innovative contribution to global health. Glob. Chall. (Hoboken, NJ) 2017;1(1):33–46. doi: 10.1002/gch2.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Focosi D., Quiroga R., McConnell S., Johnson M.C., Casadevall A. Convergent evolution in SARS-CoV-2 spike creates a variant soup from which new COVID-19 waves emerge. Int. J. Mol. Sci. 2023;24(3) doi: 10.3390/ijms24032264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galani A., Aalizadeh R., Kostakis M., Markou A., Alygizakis N., Lytras T., Adamopoulos P.G., Peccia J., Thompson D.C., Kontou A., Karagiannidis A., Lianidou E.S., Avgeris M., Paraskevis D., Tsiodras S., Scorilas A., Vasiliou V., Dimopoulos M.-A., Thomaidis N.S. SARS-CoV-2 wastewater surveillance data can predict hospitalizations and ICU admissions. Sci. Total Environ. 2022;804 doi: 10.1016/j.scitotenv.2021.150151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory D.A., Trujillo M., Rushford C., Flury A., Kannoly S., San K.M., Lyfoung D., Wiseman R.W., Bromert K., Zhou M.-Y., Kesler E., Bivens N., Hoskins J., Lin C.-H., Oâ Connor D.H., Wieberg C., Wenzel J., Kantor R.S., Dennehy J.J., Johnson M.C. medRxiv: The reprint Server for Health Sciences. 2022. Genetic diversity and evolutionary convergence of cryptic SARS-CoV-2 lineages detected via wastewater sequencing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haff L.A. Improved quantitative PCR using nested primers. PCR Methods Appl. 1994;3(6):332–337. doi: 10.1101/gr.3.6.332. [DOI] [PubMed] [Google Scholar]
- Haramoto E., Malla B., Thakali O., Kitajima M. First environmental surveillance for the presence of SARS-CoV-2 RNA in wastewater and river water in Japan. Sci. Total Environ. 2020;737 doi: 10.1016/j.scitotenv.2020.140405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto M., Nagata N., Homma T., Maeda H., Dohi K., Seki N.M., Yoshihara K., Iwata-Yoshikawa N., Shiwa-Sudo N., Sakai Y., Shirakura M., Kishida N., Arita T., Suzuki Y., Watanabe S., Asanuma H., Sonoyama T., Suzuki T., Omoto S., Hasegawa H. Immunogenicity and protective efficacy of SARS-CoV-2 recombinant S-protein vaccine S-268019-b in cynomolgus monkeys. Vaccine. 2022;40(31):4231–4241. doi: 10.1016/j.vaccine.2022.05.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto R., Yamaguchi K., Katayama K., Ando H., Setsukinai K.-I., Kobayashi H., Okabe S., Imoto S., Kitajima M. Identification of SARS-CoV-2 variants in wastewater using targeted amplicon sequencing during a low COVID-19 prevalence period in Japan. Sci. Total Environ. 2023;887 doi: 10.1016/j.scitotenv.2023.163706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi M., Kumar M., Srivastava V., Kumar D., Rathore D.S., Pandit R., Graham D.W., Joshi C.G. Genetic sequencing detected the SARS-CoV-2 delta variant in wastewater a month prior to the first COVID-19 case in Ahmedabad (India) Environ. Pollut. (Barking, Essex: 1987) 2022;310:119757. doi: 10.1016/j.envpol.2022.119757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K., Standley D.M. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 2013;30(4):772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K., Misawa K., Kuma K., Miyata T. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002;30(14):3059–3066. doi: 10.1093/nar/gkf436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khare S., Gurry C., Freitas L., Schultz M.B., Bach G., Diallo A., Akite N., Ho J., Lee R.T., Yeo W., Curation Team G.C., Maurer-Stroh S. GISAID’s role in pandemic response. China CDC Wkly. 2021;3(49):1049–1051. doi: 10.46234/ccdcw2021.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koressaar T., Remm M. Enhancements and modifications of primer design program Primer3. Bioinformatics (Oxford, England) 2007;23(10):1289–1291. doi: 10.1093/bioinformatics/btm091. [DOI] [PubMed] [Google Scholar]
- Lu X., Wang L., Sakthivel S.K., Whitaker B., Murray J., Kamili S., Lynch B., Malapati L., Burke S.A., Harcourt J., Tamin A., Thornburg N.J., Villanueva J.M., Lindstrom S. US CDC real-time reverse transcription PCR panel for detection of severe acute respiratory syndrome coronavirus 2. Emerg. Infect. Dis. 2020;26(8):1654–1665. doi: 10.3201/eid2608.201246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet.J. 2011;17(1):10–12. doi: 10.14806/ej.17.1.200. [DOI] [Google Scholar]
- Matsuyama S., Nao N., Shirato K., Kawase M., Saito S., Takayama I., Nagata N., Sekizuka T., Katoh H., Kato F., Sakata M., Tahara M., Kutsuna S., Ohmagari N., Kuroda M., Suzuki T., Kageyama T., Takeda M. Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells. Proc. Natl. Acad. Sci. 2020;117(13):7001–7003. doi: 10.1073/pnas.2002589117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra T., Dalavi R., Joshi G., Kumar A., Pandey P., Shukla S., Mishra R.K., Chande A. SARS-CoV-2 spike E156G/Δ157-158 mutations contribute to increased infectivity and immune escape. Life Sci. Alliance. 2022;5(7) doi: 10.26508/lsa.202201415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemudryi A., Nemudraia A., Wiegand T., Surya K., Buyukyoruk M., Cicha C., Vanderwood K.K., Wilkinson R., Wiedenheft B. Temporal detection and phylogenetic assessment of SARS-CoV-2 in municipal wastewater. Cell Rep. Med. 2020;1(6) doi: 10.1016/j.xcrm.2020.100098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemeyer D., Stenzel S., Veith T., Schroeder S., Friedmann K., Weege F., Trimpert J., Heinze J., Richter A., Jansen J., Emanuel J., Kazmierski J., Pott F., Jeworowski L.M., Olmer R., Jaboreck M.-C., Tenner B., Papies J., Walper F., Drosten C. SARS-CoV-2 variant alpha has a spike-dependent replication advantage over the ancestral B.1 strain in human cells with low ACE2 expression. PLoS Biol. 2022;20(11) doi: 10.1371/journal.pbio.3001871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero M.C.B., Murao L.A.E., Limen M.A.G., Caalim D.R.A., Gaite P.L.A., Bacus M.G., Acaso J.T., Miguel R.M., Corazo K., Knot I.E., Sajonia H., 2nd, de Los Reyes F.L., 3rd, Jaraula, C. M. B., Baja, E. S., & Del Mundo, D. M. N. Multifaceted assessment of wastewater-based epidemiology for SARS-CoV-2 in selected urban communities in Davao City, Philippines: a pilot study. Int. J. Environ. Res. Public Health. 2022;19(14) doi: 10.3390/ijerph19148789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peccia J., Zulli A., Brackney D.E., Grubaugh N.D., Kaplan E.H., Casanovas-Massana A., Ko A.I., Malik A.A., Wang D., Wang M., Warren J.L., Weinberger D.M., Arnold W., Omer S.B. Measurement of SARS-CoV-2 RNA in wastewater tracks community infection dynamics. Nat. Biotechnol. 2020;38(10):1164–1167. doi: 10.1038/s41587-020-0684-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W., Le S., Li Y., Hu F. SeqKit: a cross-platform and ultrafast toolkit for FASTA/Q file manipulation. PLoS One. 2016;11(10) doi: 10.1371/journal.pone.0163962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu Y., McCauley J. GISAID: global initiative on sharing all influenza data - from vision to reality. Euro surveillance. 2017;22(13) doi: 10.2807/1560-7917.ES.2017.22.13.30494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva C.S., Tryndyak V.P., Camacho L., Orloff M.S., Porter A., Garner K., Mullis L., Azevedo M. Temporal dynamics of SARS-CoV-2 genome and detection of variants of concern in wastewater influent from two metropolitan areas in Arkansas. Sci. Total Environ. 2022;849 doi: 10.1016/j.scitotenv.2022.157546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth D.S., Trujillo M., Gregory D.A., Cheung K., Gao A., Graham M., Guan Y., Guldenpfennig C., Hoxie I., Kannoly S., Kubota N., Lyddon T.D., Markman M., Rushford C., San K.M., Sompanya G., Spagnolo F., Suarez R., Teixeiro E., Dennehy J.J. Tracking cryptic SARS-CoV-2 lineages detected in NYC wastewater. Nat. Commun. 2022;13(1):635. doi: 10.1038/s41467-022-28246-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurbeck R.R., Minard-Smith A., Catlin L. Feasibility of neighborhood and building scale wastewater-based genomic epidemiology for pathogen surveillance. Sci. Total Environ. 2021;789 doi: 10.1016/j.scitotenv.2021.147829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr T.N., Greaney A.J., Stewart C.M., Walls A.C., Hannon W.W., Veesler D., Bloom J.D. Deep mutational scans for ACE2 binding, RBD expression, and antibody escape in the SARS-CoV-2 omicron BA.1 and BA.2 receptor-binding domains. PLoS Pathog. 2022;18(11) doi: 10.1371/journal.ppat.1010951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamáš M., Potocarova A., Konecna B., Klucar Ľ., Mackulak T. Wastewater sequencing-an innovative method for variant monitoring of SARS-CoV-2 in populations. Int. J. Environ. Res. Public Health. 2022;19(15):9749. doi: 10.3390/ijerph19159749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Untergasser A., Cutcutache I., Koressaar T., Ye J., Faircloth B.C., Remm M., Rozen S.G. Primer3--new capabilities and interfaces. Nucleic Acids Res. 2012;40(15) doi: 10.1093/nar/gks596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uriu K., Ito J., Zahradnik J., Fujita S., Kosugi Y., Schreiber G., Sato K. Enhanced transmissibility, infectivity, and immune resistance of the SARS-CoV-2 omicron XBB.1.5 variant. Lancet Infect. Dis. 2023;23(3):280–281. doi: 10.1016/S1473-3099(23)00051-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vo V., Tillett R.L., Papp K., Shen S., Gu R., Gorzalski A., Siao D., Markland R., Chang C.-L., Baker H., Chen J., Schiller M., Betancourt W.Q., Buttery E., Pandori M., Picker M.A., Gerrity D., Oh E.C. Use of wastewater surveillance for early detection of alpha and epsilon SARS-CoV-2 variants of concern and estimation of overall COVID-19 infection burden. Sci. Total Environ. 2022;835 doi: 10.1016/j.scitotenv.2022.155410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., Xiao A., Zhang J., Moniz K., Endo N., Armas F., Bonneau R., Brown M.A., Bushman M., Chai P.R., Duvallet C., Erickson T.B., Foppe K., Ghaeli N., Gu X., Hanage W.P., Huang K.H., Lee W.L., Matus M.…Alm E.J. SARS-CoV-2 RNA concentrations in wastewater foreshadow dynamics and clinical presentation of new COVID-19 cases. Sci. Total Environ. 2022;805 doi: 10.1016/j.scitotenv.2021.150121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Deng Y., Ding J., Zheng X., Li S., Liu L., Chui H.-K., Poon L.L.M., Zhang T. Real-time allelic assays of SARS-CoV-2 variants to enhance sewage surveillance. Water Res. 2022;220 doi: 10.1016/j.watres.2022.118686. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures and tables
Data Availability Statement
The NJ/GenBank undeGS Raw datasets were deposited to DDBr accession number DRA016444, BioProject number PRJDB15905, and BioSample number SAMD00612274 to SAMD00612346 and SAMD00617904 to SAMD00617909.