Abstract
Background:
Existing models to predict fall-related injuries (FRI) in nursing homes (NH) focus on hip fracture, yet hip fractures comprise less than half of all FRIs. We developed and validated a series of models to predict absolute risk of FRIs in NH residents.
Methods:
Retrospective cohort study of long-stay US NH residents (≥100 days in same facility) between January 1, 2016 and December 31, 2017 (n=733,427) using Medicare claims and Minimum Data Set v3.0 clinical assessments. Predictors of FRIs were selected through LASSO logistic regression in a 2/3 random derivation sample and tested in a 1/3 validation sample. Sub-distribution hazard ratios (HR) and 95% confidence intervals (95% CI) were estimated for 6-month and 2-year follow-up. Discrimination was evaluated via C-statistic and calibration compared the predicted rate of FRI to the observed rate. To develop a parsimonious clinical tool, we calculated a score using the five strongest predictors in the Fine-Gray model. Model performance was repeated in the validation sample.
Results:
Mean (Q1, Q3) age was 85.0 (77.5, 90.6) years and 69.6% were women. Within 2 years follow-up, 43,976 (6.0%) residents experienced ≥1 FRI. 70 predictors were included in the model. Discrimination of the 2-year prediction model was good (C-index = 0.70), and calibration was excellent. Calibration and discrimination of the 6-month model were similar (C-index = 0.71). In the clinical tool to predict 2-year risk, the 5 characteristics included independence in ADLs (HR 2.27; 95% CI 2.14–2.41) and history of non-hip fracture (HR 2.02; 95% CI 1.94–2.12). Performance results were similar in the validation sample.
Conclusions:
We developed and validated a series of risk prediction models that can identify NH residents at greatest risk for FRI. In nursing homes, these models should help target preventive strategies.
Keywords: fall-related injuries, fracture, functional assessment, long-term care, risk prediction
Introduction
Falls are the leading cause of injuries and injury-related deaths in adults aged 65 years and older.1 Falls are particularly problematic in the nursing home (NH) setting, where 50% of residents fall each year.2–5 Approximately 10–25% result in a fall-related injury (FRI), such as fractures or concussions.6 The risk of one-year mortality following a hip fracture, the most common FRI resulting in hospitalization, is 40% among long-stay NH residents.7, 8 In addition to the significant morbidity and mortality, FRIs result in high costs, estimated to account for 6% of the total annual Medicare spending.3, 9
CMS requires reporting of FRIs as a quality measure for long-stay NH residents.10 While most NHs engage in local fall prevention efforts, considerable variation occurs in FRI rates across facilities that cannot be explained by patient or facility characteristics.11 Effective interventions to reduce recurrent falls exist12, but these programs are resource intensive, relying on multifactorial interventions that target an individual patient’s greatest risk factors for falls and injury.12–14 Deprescribing initiatives (i.e., reducing the dose or stopping medications associated with falls and injury) show promise in reducing recurrent falls, yet these initiatives are seldom sustained.15–17 Other resource intensive interventions, like exercise programs, review of all fall-related risk factors, and increased staffing could potentially derive greater benefit if targeted to individuals at higher risk of injury when falls occur.18, 19 While it may be impractical to prevent all falls in high-risk residents, the goal should be to reduce the number of falls, particularly those resulting in injury.20
Identification of high-risk individuals who would derive the greatest benefit from tailored FRI prevention is important to guide the allocation of scarce resources. Previously our group developed the Fracture Risk Assessment in Long Term Care (FRAiL) model, which estimates 2-year absolute risk of hip fracture in long-stay residents.21, 22 The model was derived from the Minimum Data Set (MDS) version 2.0 assessments and included a total of 15 characteristics associated with hip fracture, including independence in activities of daily living and wandering.21 While effective in predicting hip fractures, model performance diminished when including other fracture types, and it was not intended to predict other injuries such as intracranial bleeding, concussions, and joint dislocations.22 Further, this did not include a short prediction tool that providers could use quickly without a more lengthy assessment. Finally, since the development of FRAiL, the MDS has been updated to version 3.0, which includes new clinical characteristics.23 Therefore, it was timely to develop a new series of risk prediction models that could be used to identify NH residents at greatest risk of FRI.
Our objective was to develop and validate the Fall-Related Injury Risk in Nursing Homes (INJURE-NH) model to predict the short-term (6-month) and long-term (2-year) absolute risk of FRIs in long-stay NH residents. We also created a 5-item clinical tool (INJURE-NH-Short) for use in clinical practice.
Methods
Study Design and Data Sources
We conducted a national retrospective cohort study of long-stay NH residents using Medicare claims data linked to the MDS version 3.0 clinical assessment records from January 1, 2016 to December 31, 2017. The Residential History File, which summarizes information from Medicare claims and MDS assessments, was used to track residents across health care locations.24 Adjudicated claims for inpatient services (Medicare Provider Analysis and Review or MedPAR) and outpatient services (Outpatient and Carrier claims) were included. MedPAR claims included short-stay inpatient, long-stay inpatient, and skilled nursing facility claims. The Medicare Master Beneficiary Summary File (MBSF) was used to identify insurance enrollment and demographic characteristics.
The MDS, a clinical assessment with more than 400 items, is federally mandated for all NHs receiving Medicare or Medicaid funding at admission, quarterly thereafter, and upon any significant change in health status and discharge.23, 25, 26
Study Population
Supplemental Figure 1 describes the selection of the study population. The cohort included all long-stay NH residents in the year 2016 identified by the Residential History File algorithm.24 Long-stay was defined as residing within a NH facility for ≥ 100 days with < 10 consecutive days outside the facility.4, 24 Follow-up began the date a resident qualified as long-stay (cohort entry date); for residents who qualified as long-stay prior to 2016, follow-up began on January 1, 2016. Exclusion criteria included: age <65 years; < 6 months of continuous Medicare Part A and B enrollment before the start of follow up; enrollment in Medicare Advantage or hospice in the 6 months prior to cohort entry; persistent vegetative state; and no valid MDS assessment (i.e., admission, quarterly) within 1 year prior to the start of follow-up (Supplemental Figure 1).
Residents were not excluded if they experienced a FRI during the 100 days before the start of follow-up; however, if a resident had a FRI during this period, any subsequent FRI with the same injury type must have occurred at least 100 days from the first injury to avoid counting hospital readmissions for a complication related to the first injury.
Fall-related Injuries
FRIs included fractures (excluding fingers and toes), dislocations, concussions, intracranial hemorrhage, and other organ traumas (e.g., pneumothorax). We previously developed a series of case-qualifying (CQ) algorithms to identify FRIs using Medicare claims (Inpatient [CQ1], Outpatient and Provider with Procedure [CQ2], Outpatient and Provider with Fall [CQ3], or Inpatient or Outpatient and Provider with Fall [CQ4]).27 In this analysis, we utilized 10 separate definitions of FRI to help with variable selection and model training: presence of any FRIs identified by any of the four CQ algorithms (1 definition), any FRIs identified by the Inpatient and Outpatient and Provider claims with Fall algorithms together (i.e., union of sets CQ1 and CQ3) (1 definition), any FRIs identified by each of the four algorithms individually (4 definitions), and any hip fracture identified by each of the four algorithms individually (4 definitions). The 10 definitions were each used as the outcome in the initial models, allowing our team to select a set of predictors that are central to all FRIs, including hip fractures. We used this combination to be inclusive of major injuries treated in both inpatient and outpatient settings and to ensure the model included predictors for non-hip fracture injuries, as risk factors have been shown to differ between hip and non-hip fracture.22, 28 For all subsequent analyses, we used a combination of CQ1 and CQ3 as the definition of FRI, which encompassed inpatient and outpatient claims without the use of repair procedure codes.
Follow-up:
Each resident was followed from start of follow-up until the earliest event of FRI, death, Medicare disenrollment (from Parts A or B), enrollment in Medicare Advantage, or administrative censoring [6 months of follow-up without an FRI for the short-term FRI risk modeling, or 2 years of follow-up without an FRI for the long-term FRI risk modeling, or end of the study (December 31, 2017)]. Residents who were discharged from the NH (n=4,401; 0.6%) were followed until they reached one of the listed endpoints.
Essential Predictors
Using a modified Delphi approach, our team identified a core set of 9 essential predictors to be included in the model. Research team members, including four pharmacists and three geriatricians, were asked to vote on predictors they considered essential for inclusion based on clinical and research experience. Disagreements were reconciled through group discussion until a ≥80% consensus was reached. The final set of core predictors were gender, age, visual impairment, cognitive impairment as measured by the Cognitive Function Scale (CFS), activities of daily living (ADLs), orthostatic hypotension, diabetes, history of hip fracture, and recent falls.
Potential Predictors and Selection
In addition to the essential set of predictors detailed above, we also considered a wide array of 223 potential predictors. Characteristics from several domains were considered to allow for selection of predictors potentially associated with FRIs that have not previously been reported. These domains included sensory abnormalities (i.e., hearing, speech), mood, behaviors, continence, comorbidities, pain, nutrition, dental status, skin conditions, medication classes, and use of restraints. Most characteristics were ascertained using the MDS assessment closest to and preceding the cohort entry date. We considered only one predictor obtained from Medicare claims: hospitalization in the previous year. Supplemental Table S1 details all predictors considered for inclusion.
Statistical Analysis:
The cohort was randomly divided into a 2/3 development cohort and a 1/3 validation cohort. Within the development cohort, we utilized machine learning techniques to perform variable selection. Specifically, a LASSO logistic regression model was fit for the 10 FRI outcome definitions described above. Variable lists were compared across the 10 models to identify a unified list of variables selected in all 10 models. Only these variables (the core predictors and those selected in all 10 models) were included in the final model. We additionally used random forest performance to generate a model with the same variables. Model performance (C-index) was nearly identical to the LASSO logistic model, so no interaction terms were included in subsequent models. We used complete case method analysis, excluding individuals with missing data from the analysis (n=43,413, 6.0%; see Supplemental Figure S1).
To estimate the association of individual variables with 2-year risk of FRI we used Fine-Gray sub-distribution hazards regression.29 Discrimination was evaluated by the concordance index (C-index), whereas calibration was evaluated by comparing the predicted rate of FRI with the observed rate, according to deciles of estimated risk.30 Tests of discrimination and calibration were repeated in the validation cohort. To estimate the effect of individual variables on 6-month risk of FRI, we again used the same modelling and performance measures.
Understanding that models containing large numbers of predictors are less likely to be utilized at the point of care, we also created a clinical prediction tool. For this tool, no essential predictors were forced in the model. Instead, the lambda penalty in the LASSO model was calibrated to identify the 5 most influential predictors for FRI. Next, we constructed a Fine-Gray sub-distribution hazards regression model and standardized the coefficients of each covariate by dividing by the smallest coefficient in the model. These standardized coefficients were then summed to create a risk prediction score (range: 0–9). Discrimination of the score was evaluated by the C-index. Calibration for the clinical prediction tool was evaluated by dividing the score into eight groups, combining the 3 highest risk scores together. The predicted risk was then plotted against the actual risk of FRI. All analyses were conducted in Stata (17.0, StataCorp LLC, College Station, TX) or SAS version 9.4.
Results
Cohort characteristics
Among the 733,427 long-stay residents, the median (Q1, Q3) age was 85.0 (77.5, 90.6) years and 69.6% were female (Table 1). The cohort was predominantly non-Hispanic white (81.4%). Comorbidities were common, with 78.8% having hypertension, 32.7% having diabetes, and 30.5% having arthritis. 58.3% had a diagnosis of dementia, and 45.7% were classified as having moderate to severe cognitive impairment by the CFS. Characteristics were well-balanced between the derivation and validation cohorts, as indicated by the low standardized mean differences (Table 1).
Table 1.
Baseline characteristics of long-stay nursing home residents in the study sample, 2016–2017
| Full cohort | Development | Validation | SMDa | |
|---|---|---|---|---|
| Age, years, median (Q1, Q3) | 85.0 (77.5, 90.6) | 85.0 (77.5, 90.6) | 85.0 (77.5, 90.6) | 0.002 |
| Female sex, n (%) | 510,363 (69.6) | 336,472 (69.5) | 173,891 (69.7) | 0.005 |
| Other/Unknown | 4,546 (0.6) | 3,011 (0.6) | 1,535 (0.6) | |
| Number of active conditions, median (Q1, Q3)b | 6 (5, 9) | 6 (5, 9) | 6 (5, 9) | 0.002 |
| History of falls, n (%) | 133,422 (18.2) | 88,417 (18.3) | 45,005 (18.0) | −0.006 |
| Activities of Daily Living Scale, median (Q1, Q3)c | 9 (7, 11) | 9 (7, 11) | 9 (7, 11) | 0.001 |
| At least 1 hospitalization in the past year, n (%) | 359,224 (49.0) | 236,903 (48.9) | 122,321 (49.1) | 0.002 |
| Moderate to severe cognitive impairment (CFS ≥ 3), n (%) | 334,765 (45.7) | 220,821 (45.6) | 113,944 (45.7) | 0.001 |
| Alzheimer’s disease and related dementias, n (%) | 427,753 (58.3) | 282,382 (58.3) | 145,371 (58.3) | −0.001 |
| Diabetes mellitus, n (%) | 239,589 (32.7) | 157,865 (32.6) | 81,724 (32.8) | 0.003 |
| Visual impairment, n (%)d | 232,649 (31.7) | 153,444 (31.7) | 79,205 (31.8) | <0.001 |
Abbreviations: ADL = Activities of Daily Living, CFS = Cognitive Function Scale, IQR = interquartile range, SMD = standardized mean difference
Standardized mean differences are calculated for the difference between the Development and Validation samples
Number of active conditions represents the sum of comorbidities noted in the active diagnosis checkbox on the Minimum Data Set within the previous year.
Physical function was measured using Activities of Daily Living (ADL) via the Minimum Data Set Morris 16-point ADL Short Form score. The ADL scores range from 0 to 16, with 0 indicating total independence and 16 indicating total dependence in all ADLs.
Obtained from question B1000. Vision on the Minimum Data Set version 3.0. Visual impairment recorded as any value other than “Adequate”.
During the 6 months and 2 years of follow-up, 25,805 (3.5%) and 43,976 (6.0%) residents, respectively experienced at least 1 FRI. The most common type of FRI was hip fracture (n=15,922, 2.2%) followed by non-hip femur fracture (n=5,890, 0.8%) and intracranial bleeding (n=5,762, 0.8%). FRI incidence was similar within the development and validation samples [e.g., at 2 years 14,860 (6.0%) FRIs in the validation sample]. Death was common: overall, 333,814 (45.5%) residents died without FRI during follow-up.
Model performance
Supplemental Table S2 presents the sub-distribution hazard ratio (HR) and 95% confidence interval (CI) of all 70 characteristics included in the INJURE-NH model for the development sample (n=484,062). Fifty-one of the 70 variables remained significant predictors of FRI in the 2-year fully adjusted model. Notably, female sex (HR 1.38; 95%CI 1.34–1.42), previous hip fracture (HR 1.41; 95%CI 1.32–1.49), recent injurious fall (HR 1.66; 95%CI 1.61–1.71), and history of fracture other than hip (HR 1.69; 95%CI 1.61–1.77) were predictive of FRI within 2 years. Severity of cognitive impairment was not associated with FRI risk, whereas several functional characteristics were associated with FRIs. Specifically, greater independence in walking in one’s room (HR 1.37; 95%CI 1.27–1.47) and urinary continence (HR 1.34; 95%CI 1.26–1.42) were significant predictors of FRI. Within the development sample, the C-index was 0.70 (95% CI 0.70–0.71). Calibration of the model using nonparametric estimates of FRI was excellent, as shown in Figure 1a.
Figure 1. Calibration plots of the observed risk of fall-related injury and the predicted risk from the INJURE-NH tool among long-stay nursing home residents.
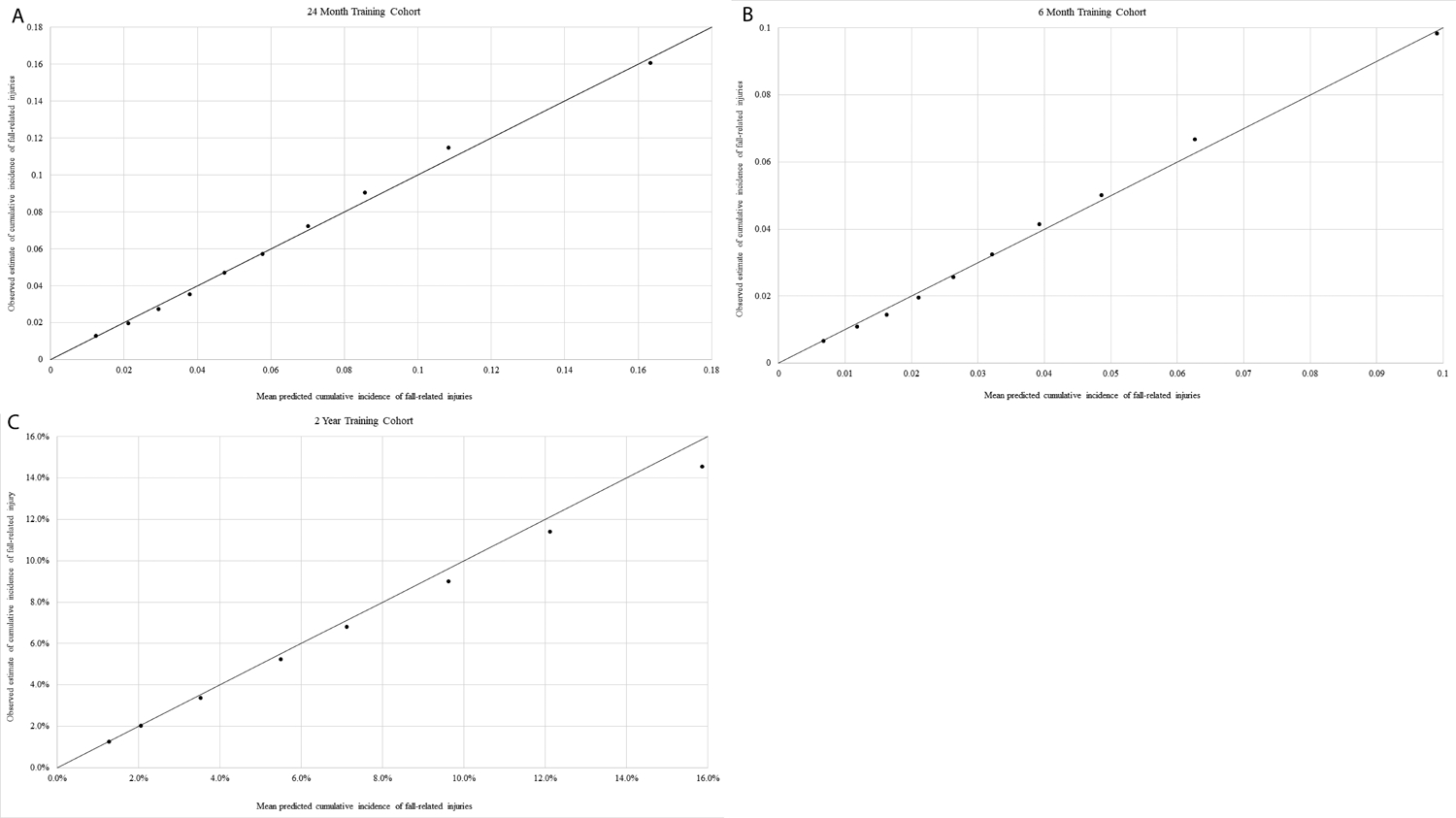
- 2-year risk, full model (INJURE-NH), development sample (n=484,062)
- 6-month risk, full model (INJURE-NH), development sample (n=484,062)
- 2-year risk, clinical model (INJURE-NH-Short), development sample (n=484,062)
In the validation sample (n=249,365), the associations between predictors and FRI remained similar (Supplemental Table S3). The C-index was 0.70 (95% CI 0.70–0.71) and calibration remained excellent (Supplemental Figure S2a).
When follow-up was shortened to 6 months, the model performed similarly (Supplemental Table S4). Of the 51 predictors in the 24-month model, 41 remained significant in the 6-monthmodel. Six additional predictors became significant with shorter follow-up: visual impairment (HR 1.06; 95%CI 1.01–1.12), disorganized thinking (HR 1.35; 95%CI 1.10–1.65), behavior interfering with care (HR 0.90; 95%CI 0.82–0.99), worsened behavior (HR 1.16; 95%CI 1.06–1.27), independence in on-unit locomotion (HR 1.23; 95%CI 1.06–1.43), and requiring support with setup for off-unit locomotion (HR 0.90; 95%CI 0.81–0.99). Discrimination was similar in both the development (C-index 0.71; 95% CI 0.70–0.71) and validation samples (C-index 0.71; 95% CI 0.70–0.72). Calibration, displayed visually in Figure 1b and Supplemental Figure S2b, remained excellent.
The five variables included in the short clinical tool (INJURE-NH-Short) were ADL Score, recent fall, hospitalization in the previous year, ability to walk in room, and history of non-hip fractures (Table 2). Of these, history of non-hip fractures (HR 2.02; 95%CI 1.94–2.12), independence in ADLs (HR 2.27; 95%CI 2.14–2.41), and independence in walking in the room (HR 1.95, 95% CI 1.89–2.01) were most predictive of a future FRI. The INJURE-NH-Short score ranged from 0–9, with a median (Q1, Q3) score of 3 (2, 5). In the derivation cohort, 85,765 residents (17.7%) had an INJURE-NH-Short score ≤ 1, corresponding with a predicted 2-year risk of FRI of ≤ 2.1%, while 53,694 residents (11.1%) received a score ≥ 6, corresponding with an average predicted 2-year FRI risk of ≥ 12.1%. Residents with a score ≥ 7 had an average predicted 2-year FRI risk of 15.9%. Discrimination for 2-year follow-up was moderate (C-index = 0.67; 95% CI 0.66–0.67). Calibration remained excellent, with the clinical model slightly overestimating observed FRI rates with scores above 4 (Figure 1c).
Table 2.
Predictors of fall-related injuries included in the final clinical 2-year risk prediction tool (INJURE-NH-Short)a
| Characteristic | Multivariable SHR (95% CI) | MDS v3.0 Item(s)b | Score Assigned |
|---|---|---|---|
| ADL Short Form Scorec | G0110E1, H1, I1, J1 | ||
| 13–16 | REF | 0 | |
| 9–12 | 1.82 (1.72–1.92) | 1 | |
| 0–8 | 2.27 (2.14–2.41) | 2 | |
| Recent fall | J1800, 1900A-C | ||
| No fall | REF | 0 | |
| Fall without injury | 1.41 (1.37–1.45) | 1 | |
| Fall resulting in injury | 1.76 (1.71–1.82) | 2 | |
| Hospitalized in 1 year baselined | n/a | ||
| No | REF | 0 | |
| Yes | 1.48 (1.45–1.52) | 1 | |
| Ability to walk in room | G0110C1 | ||
| Total dependence (4) | REF | 0 | |
| Extensive assistance (3) | 1.59 (1.53–1.65) | 1 | |
| Independent to limited assistance (0–2) | 1.95 (1.89–2.01) | 2 | |
| History of fractures other than hip | I4000 | ||
| No | REF | 0 | |
| Yes | 2.02 (1.94–2.12) | 2 |
Abbreviations: ADL, Activities of Daily Living; CI, Confidence Interval; MDS, Minimum Data Set; OR, Odds Ratio; REF, Reference category
Predictors included in this table are from the 5-variable clinical prediction tool (INJURE-NH-Clinical) designed for rapid calculation at the bedside without the need for a computer.
Item(s) in this column denote the corresponding items from the Minimum Data Set version 3.0 used in the calculation of the given feature. Numbers denote the levels of response that the given level is derived from.
Physical function was measured using Activities of Daily Living (ADL) via the Minimum Data Set Morris 16-point ADL Short Form score. The ADL scores range from 0 to 16, with 0 indicating total independence and 16 indicating total dependence in all ADLs.
Obtained from Medicare claims.
Discussion
This study, utilizing routinely collected clinical assessment data from the MDS and Medicare claims, developed and validated a series of prediction models to estimate FRI risk among NH residents. The prediction models achieved good discrimination and excellent calibration for both 2-year and 6-month outcomes. The short tool with just five predictors performed similarly. These models, particularly if automated, could provide researchers, clinicians, and policymakers with validated options to identify NH residents at greatest risk for FRI.
Falls and injuries are multifactorial in NH residents,31, 32 making them challenging to predict. Previously our group developed the FRAiL model to estimate hip fracture risk among NH residents.21 Discrimination of FRAiL was good in women in the original sample (C-index=0.71), but was more modest when the model was used to predict non-vertebral fractures (C-index=0.65).21, 22 Other hip fracture prediction models exist, including a Canadian model developed in MDS v2.0 including eight risk factors for fracture (C-index=0.64–0.67).33 The discrimination of these models is consistent with other commonly used clinical prediction tools, like Fracture Risk Assessment Tool (FRAX®), whose performance varies between C-indices of 0.60 to 0.69, depending on whether bone mineral density is available.34 Approximately 2/5 of the FRIs in our study were non-hip fracture, yet thirteen of the characteristics included in the INJURE-NH model are also included in FRAiL and eight in the Canadian model. Risk factors identified in INJURE-NH that were not included in FRAiL include recent hospitalization and history of non-hip fracture. Nonetheless, the strong association with higher functional status and falls is consistent when predicting hip fracture or FRI, and so targeting these residents for fall prevention efforts may be an effective way to reduce all FRIs.
Previous studies have found that risk factors for hip versus other fracture types can differ in nursing home residents.28, 31, 32 For example, obesity has been associated with increases in ankle, leg, humerus, and vertebral fracture while also being associated with reductions in wrist, hip, and pelvis fractures.35–37 It is notable then that INJURE-NH performed as well as the hip fracture models despite including additional FRI types. Our model included several domains of risk factors known to affect non-hip fracture risk, including independence in locomotion. This is notable as recent research in home care recipients has demonstrated locomotion outdoors as a risk factor unique to non-hip fractures.38While facilities that discourage locomotion may have fewer fall related injuries, such practices are not recommended as they lead to declines in other functional and cognitive domains.
Risk factors for imminent FRI may be driven largely by fall risk, whereas longer-term risk of injury may the consequence of other factors related to injury in the setting of a fall. Community-based studies suggest that a recent fall and low self-rated health are strongly associated with imminent risk of osteoporotic fracture.39, 40 Our 6-month model identified six risk factors for imminent FRI that were not associated with 2-year risk. These factors, including disorganized thinking and visual impairment, are associated with falls and are potentially important to consider in new NH residents, as their presence signals imminent FRI risk.
The INJURE-NH models could be used in several ways to enhance clinical care in NHs. First, all predictors in the models are routinely collected in the EMR, allowing automated model calculation if EMR vendors and/or individual facilities adopted these models. Given the limited information technology support in most facilities, it would be preferable for CMS to modify the Resident Assessment Instruments (RAI) to allow for automated calculation within the MDS. Once automated these models could be used by facility administrators, regulatory entities, and researchers to compare predicted and observed FRI rates.11 Aggregated facility data has the additional benefit of being able to identify high-performing facilities, which can be studied to gain insights into best practices.
In cases where the score cannot be automated, the 5-item clinical tool could be used by providers to estimate risk. The utility of clinical tools to predict falls in this setting has been debated by some, as a meta-analysis of fall prediction tools suggests that nursing assessment is as valid in predicting falls as the clinical tools.41 While regular nursing staff may be able to quickly identify fallers, NH staff turnover is at an extreme high, and so standardized tools to assess risk are likely still useful. Further, communication between the direct care staff who know the patient best and the providers who order interventions is generally limited, and this is a barrier to implementing quality improvement initiatives in the NH.42 Rigorous studies that implement targeted fall prevention strategies using a prediction tool have not been conducted in the NH20; however, this approach is currently being evaluated to prevent pressure ulcers in this setting.43 Future research should be conducted to determine whether using these models to target resource intensive interventions, such as deprescribing medications associated with FRIs, treatment for osteoporosis, exercise programs, multifactorial interventions, or environmental interventions to reduce injury in the setting of a fall, are effective strategies to reduce injurious falls.
While the shortened prediction tool had somewhat reduced discrimination compared to the full model, it performed well despite containing just five predictors (C-index=0.67). Calibration remained excellent with the shortened clinical tool, with only slight overestimation of FRI risk among persons at greatest risk. Importantly our clinical tool resulted in a wide range of scores (0–9), with a median score of 3 and only 11.1% of residents with a score of 6–9. This is in marked contrast to application of community-based fracture screening tools in the nursing home setting: 98% of nursing home residents are identified as high risk for fracture when using the FRAX® with body mass index.44 Future investigation should identify the optimal cut-point for designating high risk using the INJURE-NH-Short tool taking into account the effectiveness of the proposed intervention, cost, and level of risk deemed too excessive by the given organization.
Limitations
Our work is not without limitations. The current model was developed and validated using data obtained from long-stay NH residents. While this model might also be useful in other at-risk populations, including assisted living and short-stay NH residents, future investigation should validate the model in these populations. Second, our model does not include all risk factors for FRIs, including environmental hazards or a comprehensive list of medication classes that have been associated with fall risk in older adults. Because our prediction models are intended to impact clinical decision making by targeting FRI-reduction initiatives, we purposefully did not include all classes of medications or environmental hazards associated with falls and injury in the models. Third, our models were designed to measure risk of FRIs, not risk of falls, and they do not include minor injuries (i.e., sprains, lacerations).27 We elected to focus on major injuries because prior studies found that the validity of ascertaining minor injuries with claims data is low. This is likely to be even more problematic in the NH setting.45 Future research that include electronic health records or a valid measure of minor injuries should evaluate the performance of the models when adding minor injuries.
Conclusions
As a result of the COVID-19 pandemic, the U.S. government has pledged a series of reforms to NH oversight including expanding the Value-Based Purchasing Program, a financial reimbursement system that incentivizes high quality care, to include measures capturing the resident experience.46 While details of what the resident experience includes are lacking, it is likely FRIs will be included. Our models can be used to accurately estimate 6-month and 2-year risk of FRI among long-stay NH residents. The full models can be automatically calculated with existing clinical data, while the short tool could be used by clinicians with similar performance. We believe these models will provide researchers, clinicians, and policymakers with a useful tool to improve the quality of care in NHs.
Supplementary Material
Key Points:
Routinely collected clinical assessment data can be used to identify nursing home residents at greatest risk for fall-related injuries (FRI).
Using data elements from the Minimum Data Set, we developed a series of models to estimate 6-month and 2-year absolute risk of FRI.
Discrimination of the 2-year model was good (C-index=0.70) with modest reduction for the 5-item clinical tool (C-index=0.67).
Why does this matter?
These models can be used by researchers and clinicians to accurately determine patient risk for FRI using routinely collected clinical assessment data. In nursing homes, these models should be used to target preventive strategies.
Acknowledgements
Conflict of Interest:
Dr. Zullo receives grant funding paid by Sanofi Pasteur directly to Brown University for research on the epidemiology of infections and vaccine use among nursing home residents. Dr. Kiel receives grant funding from Amgen to Hebrew SeniorLife for research on fracture prediction, serves on Scientific Advisory Boards for Reneo, Pfizer, and Solarea Bio, serves on a data and safety monitoring board for Agnovos, and receives royalties from Wolters Kluwer for publication on falls in UpToDate. Dr. Kim received personal fees from Alosa Health and VillageMD. The other authors report no commercial conflicts of interest.
Sponsor’s Role:
The sponsor had no role in the design, methods, data collection, analysis, or preparation of the manuscript.
Funding:
This work was funded by National Institute on Aging award RF1AG061221. Dr. Zullo is also funded in part by awards R21AG061632 and R01AG065722 from the National Institute on Aging and U54GM115677 from the National Institute of General Medical Sciences.
Footnotes
Supplemental Material Title:
Online only data for INJURE-NH Prediction Tool. Data includes patient flow diagram, additional calibration plots, and predictor parameters.
References
- [1].Moreland B, Kakara R, Henry A. Trends in Nonfatal Falls and Fall-Related Injuries Among Adults Aged >/=65 Years - United States, 2012–2018. MMWR Morb Mortal Wkly Rep. 2020;69: 875–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Rubenstein LZ, Josephson KR, Robbins AS. Falls in the nursing home. Ann Intern Med. 1994;121: 442–451. [DOI] [PubMed] [Google Scholar]
- [3].Florence CS, Bergen G, Atherly A, Burns E, Stevens J, Drake C. Medical costs of fatal and nonfatal falls in older adults. J Am Geriatr Soc. 2018;66: 693–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Berry SD, Lee Y, Zullo AR, Kiel DP, Dosa D, Mor V. Incidence of hip fracture in U.S. nursing homes. J Gerontol A Biol Sci Med Sci. 2016;71: 1230–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Rapp K, Becker C, Lamb SE, Icks A, Klenk J. Hip fractures in institutionalized elderly people: incidence rates and excess mortality. J Bone Miner Res. 2008;23: 1825–1831. [DOI] [PubMed] [Google Scholar]
- [6].Quigley PA, Campbell RR, Bulat T, Olney RL, Buerhaus P, Needleman J. Incidence and cost of serious fall-related injuries in nursing homes. Clin Nurs Res. 2012;21: 10–23. [DOI] [PubMed] [Google Scholar]
- [7].Neuman MD, Silber JH, Magaziner JS, Passarella MA, Mehta S, Werner RM. Survival and functional outcomes after hip fracture among nursing home residents. JAMA Intern Med. 2014;174: 1273–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Berry SD, Samelson EJ, Bordes M, Broe K, Kiel DP. Survival of aged nursing home residents with hip fracture. J Gerontol A Biol Sci Med Sci. 2009;64: 771–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ouslander JG, Lamb G, Perloe M, et al. Potentially avoidable hospitalizations of nursing home residents: frequency, causes, and costs: [see editorial comments by Drs. Jean F. Wyman and William R. Hazzard, pp 760–761]. J Am Geriatr Soc. 2010;58: 627–635. [DOI] [PubMed] [Google Scholar]
- [10].(CMS) CfMMS. Centers for Medicare & Medicaid Services (CMS). [PubMed]
- [11].Zullo AR, Zhang T, Banerjee G, et al. Facility and state variation in hip fracture in U.S. nursing home residents. J Am Geriatr Soc. 2018;66: 539–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cameron ID, Dyer SM, Panagoda CE, et al. Interventions for preventing falls in older people in care facilities and hospitals. Cochrane Database Syst Rev. 2018;9: CD005465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lee J, Negm A, Peters R, Wong EKC, Holbrook A. Deprescribing fall-risk increasing drugs (FRIDs) for the prevention of falls and fall-related complications: a systematic review and meta-analysis. BMJ Open. 2021;11: e035978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Vlaeyen E, Coussement J, Leysens G, et al. Characteristics and effectiveness of fall prevention programs in nursing homes: a systematic review and meta-analysis of randomized controlled trials. J Am Geriatr Soc. 2015;63: 211–221. [DOI] [PubMed] [Google Scholar]
- [15].Kua CH, Mak VSL, Huey Lee SW. Health Outcomes of Deprescribing Interventions Among Older Residents in Nursing Homes: A Systematic Review and Meta-analysis. J Am Med Dir Assoc. 2019;20: 362–372 e311. [DOI] [PubMed] [Google Scholar]
- [16].Thompson W, Jacobsen IT, Jarbol DE, Haastrup P, Nielsen JB, Lundby C. Nursing Home Residents’ Thoughts on Discussing Deprescribing of Preventive Medications. Drugs Aging. 2020;37: 187–192. [DOI] [PubMed] [Google Scholar]
- [17].Stone R, Oganesyan A, Marco N, Smith R, Hoffman J. The Impact of a Pharmacist-Led Hypertension Medication Management Program on Older People in a Skilled Nursing Facility. Sr Care Pharm. 2022;37: 62–72. [DOI] [PubMed] [Google Scholar]
- [18].Cameron ID, Dyer SM, Panagoda CE, et al. Interventions for preventing falls in older people in care facilities and hospitals. Cochrane Database Syst Rev. 2018;9: CD005465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gulka HJ, Patel V, Arora T, McArthur C, Iaboni A. Efficacy and Generalizability of Falls Prevention Interventions in Nursing Homes: A Systematic Review and Meta-analysis. J Am Med Dir Assoc. 2020;21: 1024–1035 e1024. [DOI] [PubMed] [Google Scholar]
- [20].Schoberer D, Breimaier HE, Zuschnegg J, Findling T, Schaffer S, Archan T. Fall prevention in hospitals and nursing homes: Clinical practice guideline. Worldviews Evid Based Nurs. 2022;19: 86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Berry SD, Zullo AR, Lee Y, et al. Fracture Risk Assessment in Long-term care (FRAiL): development and validation of a prediction model. J Gerontol A Biol Sci Med Sci. 2018;73: 763–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Berry SD, Zullo AR, Zhang T, Lee Y, McConeghy KW, Kiel DP. Validation of the FRAiL model to predict non-vertebral and hip fractures in nursing home residents. Bone. 2019;128: 115050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Saliba D, Jones M, Streim J, Ouslander J, Berlowitz D, Buchanan J. Overview of significant changes in the Minimum Data Set for nursing homes version 3.0. J Am Med Dir Assoc. 2012;13: 595–601. [DOI] [PubMed] [Google Scholar]
- [24].Intrator O, Hiris J, Berg K, Miller SC, Mor V. The residential history file: studying nursing home residents’ long-term care histories(*). Health Serv Res. 2011;46: 120–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Morris JN, Hawes C, Fries BE, et al. Designing the national resident assessment instrument for nursing homes. Gerontologist. 1990;30: 293–307. [DOI] [PubMed] [Google Scholar]
- [26].Thomas KS, Dosa D, Wysocki A, Mor V. The Minimum Data Set 3.0 Cognitive Function Scale. Med Care. 2017;55: e68–e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Mintz J, Duprey MS, Zullo AR, et al. Identification of Fall-related Injuries in Nursing Home Residents using Administrative Claims Data. J Gerontol A Biol Sci Med Sci. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sine K, Lee Y, Zullo AR, Daiello LA, Zhang T, Berry SD. Incidence of lower-extremity fractures in US nursing homes. J Am Geriatr Soc. 2019;67: 1253–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association. 1999;94: 496–509. [Google Scholar]
- [30].Lee M, Cronin KA, Gail MH, Feuer EJ. Predicting the absolute risk of dying from colorectal cancer and from other causes using population-based cancer registry data. Stat Med. 2012;31: 489–500. [DOI] [PubMed] [Google Scholar]
- [31].Ambrose AF, Paul G, Hausdorff JM. Risk factors for falls among older adults: a review of the literature. Maturitas. 2013;75: 51–61. [DOI] [PubMed] [Google Scholar]
- [32].Mangoni AA, Jackson SH. Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol. 2004;57: 6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Negm AM, Ioannidis G, Jantzi M, et al. Validation of a one year fracture prediction tool for absolute hip fracture risk in long term care residents. BMC Geriatr. 2018;18: 320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Briot K, Paternotte S, Kolta S, et al. FRAX(R): prediction of major osteoporotic fractures in women from the general population: the OPUS study. PLoS One. 2013;8: e83436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Premaor MO, Comim FV, Compston JE. Obesity and fractures. Arq Bras Endocrinol Metabol. 2014;58: 470–477. [DOI] [PubMed] [Google Scholar]
- [36].Pirro M, Fabbriciani G, Leli C, et al. High weight or body mass index increase the risk of vertebral fractures in postmenopausal osteoporotic women. J Bone Miner Metab. 2010;28: 88–93. [DOI] [PubMed] [Google Scholar]
- [37].Zhang T, Lary CW, Zullo AR, et al. Post-hip fracture mortality in nursing home residents by obesity status. J Am Geriatr Soc. 2019;67: 1983–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].McArthur C, Ioannidis G, Jantzi M, et al. Factors That Predict 1-Year Incident Hip and Non-Hip Fractures for Home Care Recipients: A Linked-Data Retrospective Cohort Study. J Am Med Dir Assoc. 2021;22: 1035–1042. [DOI] [PubMed] [Google Scholar]
- [39].Barron RL, Oster G, Grauer A, Crittenden DB, Weycker D. Determinants of imminent fracture risk in postmenopausal women with osteoporosis. Osteoporos Int. 2020;31: 2103–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Hannan MT, Weycker D, McLean RR, et al. Predictors of Imminent Risk of Nonvertebral Fracture in Older, High-Risk Women: The Framingham Osteoporosis Study. JBMR Plus. 2019;3: e10129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Haines TP, Hill K, Walsh W, Osborne R. Design-related bias in hospital fall risk screening tool predictive accuracy evaluations: systematic review and meta-analysis. J Gerontol A Biol Sci Med Sci. 2007;62: 664–672. [DOI] [PubMed] [Google Scholar]
- [42].Colon-Emeric CS, Casebeer L, Saag K, et al. Barriers to providing osteoporosis care in skilled nursing facilities: perceptions of medical directors and directors of nursing. J Am Med Dir Assoc. 2005;6: S61–66. [DOI] [PubMed] [Google Scholar]
- [43].Yap TL, Kennerly SM, Horn SD, Bergstrom N, Datta S, Colon-Emeric C. TEAM-UP for quality: a cluster randomized controlled trial protocol focused on preventing pressure ulcers through repositioning frequency and precipitating factors. BMC Geriatr. 2018;18: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Greenspan SL, Perera S, Nace D, et al. FRAX or fiction: determining optimal screening strategies for treatment of osteoporosis in residents in long-term care facilities. J Am Geriatr Soc. 2012;60: 684–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Min L, Tinetti M, Langa KM, Ha J, Alexander N, Hoffman GJ. Measurement of Fall Injury With Health Care System Data and Assessment of Inclusiveness and Validity of Measurement Models. JAMA Netw Open. 2019;2: e199679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].FACT SHEET: Protecting Seniors by Improving Safety and Quality of Care in the Nation’s Nursing Homes. The White House, 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


