Abstract
Electroencephalography (EEG) can detect changes in cerebral activity during spaceflight. This study evaluates the effect of spaceflight on brain networks through analysis of the Default Mode Network (DMN)'s alpha frequency band power and functional connectivity (FC), and the persistence of these changes. Five astronauts' resting state EEGs under three conditions were analyzed (pre-flight, in-flight, and post-flight). DMN’s alpha band power and FC were computed using eLORETA and phase-locking value. Eyes-opened (EO) and eyes-closed (EC) conditions were differentiated. We found a DMN alpha band power reduction during in-flight (EC: p < 0.001; EO: p < 0.05) and post-flight (EC: p < 0.001; EO: p < 0.01) when compared to pre-flight condition. FC strength decreased during in-flight (EC: p < 0.01; EO: p < 0.01) and post-flight (EC: ns; EO: p < 0.01) compared to pre-flight condition. The DMN alpha band power and FC strength reduction persisted until 20 days after landing. Spaceflight caused electrocerebral alterations that persisted after return to earth. Periodic assessment by EEG-derived DMN analysis has the potential to become a neurophysiologic marker of cerebral functional integrity during exploration missions to space.
Subject terms: Neuroscience, Neurology
Introduction
Spaceflights expose crew members to factors that can negatively affect their health and performance1,2. Understanding this environmental impact on human physiology is essential to ensure personnel well-being and mission success. The effects of spaceflight conditions on many organ systems have been studied, and the central nervous system is of particular interest3,4 as it plays an integral role in cognitive function5–9 and behavioral performance10–12. The advance in the understanding of neurobehavioral biology during spaceflight is conceivably critical to the development of countermeasures mitigating detrimental effects during exploratory missions13,14. There has not been documented instances of overt or severe functional impairment in crew members of the US space program. However, transient disorientation, spatial illusions and visual disturbances, sleep alterations, and substandard performance have been reported15,16.
The main factors affecting human brain organization during long-duration space flights are isolation, radiation and microgravity. Isolation is a well-known factor that negatively influences the generation of synaptic contacts due to the reduced social interactions17. Microgravity has been associated with brain atrophy18,19. Animal models have shown that radiation can cause dendritic pruning20, affecting the ability of neurons to develop and maintain an enriched network. Magnetic resonance imaging (MRI) pre-flight and post-flight studies have reported morphological changes in different regions of the brain due to the effects of microgravity and radiation18. Therefore, it appears beneficial to monitor functional brain changes during all phases of space mission. This will help address the questions regarding the occurrence and reversibility of such changes, and their potential clinical significance in both short and long-term.
Due to the complexity of cognitive function, neurobehavioral assessments of astronauts, cosmonauts, and taikonauts have relied primarily on neuropsychological tests3,5,10,21,22. These neuropsychological tests are immensely useful in evaluating the subject's performance, but their capabilities in assessing preclinical and subclinical changes can be limited23. Structural changes have been detected in brain MRIs of astronauts a few days after having returned from a 6-month mission aboard the International Space Station (ISS). It has been estimated that greater than 50% of the personnel may be affected by such structural changes18,19,21. We view that the development of auxiliary assessment and monitoring methods and neuropsychological tests is critical. However, mission weight limit and microgravity environment are technical constraints. In this context, electroencephalography (EEG) has proven to be a technically feasible procedure in both transport and operational aspects24,25.
EEG, a reliable neurophysiological technique that measures neuronal electrical activity, has been used in spaceflight as a part of polysomnography26. An in-flight microgravity environment has been reported to affect EEG findings by Cheron et al. in the form of a transient alpha power increase during the arrest reaction27. Although the significance of this finding is not well understood, the alpha band is known to be a crucial frequency domain of oscillatory brain activity, and has been associated with attention, inhibition, and working memory28,29.
Two common measures used to describe oscillatory brain signals are power and functional connectivity30. Power represents the amount of activity or energy rate in a specific frequency band [delta (2–4 Hz), theta (4–8 Hz), alpha (8–12 Hz), beta (12–30 Hz), and gamma (30–45 Hz)]. Functional connectivity (FC) is the synchronization of two or more regions in phase or amplitude described by Lejko et al. as statistical dependencies between brain regions30. The alpha band is the most dominant and strongest brain rhythm in healthy adults during resting state31. It is identified by its frequency band, spatial topography (posterior distribution showing high amplitudes at occipital and parietal regions), behavioral correlates, and reactivity to stimuli31. Disruptions of the alpha band, such as decrease in power and functional connectivity, have been associated with cognitive alterations and neurodegenerative pathologies32–36, stressful situations and fatigue37–39, and increasing task demands40. Changes in alpha power and synchronization are crucial in cognitive neuroscience. Given the findings that EEG can detect brain activity changes, it seems important to advance our understanding of cognitive processes affected by the unique microgravity environment.
In this context, the EEG-derived Default Mode Network (DMN) is of particular interest41. As laid out by Cheron et al. the DMN is a global workspace that cannot be regarded as "a simple resting state" but as a complex process involving dynamic interplay between conscious and unconscious states42. Moreover, this network includes several cortical regions (e.g., posterior cingulate cortex, precuneus, medial prefrontal, and inferior parietal cortices)43 related to different neurocognitive disorders44–46. Furthermore, EEG measurements of these networks are readily feasible from an operational relevance point of view. The importance of functional assessment using EEG is further supported by the fact that alterations in brain function can precede structural brain abnormalities47. The validity of this method has been proven in clinical subjects32. We view that the brain's functional connectivity evaluation has excellent potential to be used as a practical assessment and monitoring tool during spaceflight.
The analyzed dataset used in this study is a part of the NEUROSPAT experiment6,25. We performed a retrospective analysis of the EEG data, obtained during long-duration ISS missions, to evaluate functional brain networks under three different conditions: pre-fight ground level, in-flight extraterrestrial, and post-flight ground level. Our objective is to assess the alterations of DMN's alpha frequency band power and FC, and the persistence of these changes. We hypothesize that EEG-derived DMN would be disrupted by the flight condition, and FC between brain nodes would be reduced.
Methods
The dataset originated from NEUROSPAT experiment (AO-2004, 118)6,8,16. Five male astronauts (54.2 ± 2.6 years old) with 6 months in low earth orbit (174.6 ± 19.9 days) participated in this experiment. The Ethics Committee of the Faculty of Medicine of the Université Libre de Bruxelles, the European Space Agency Medical Care Committee, and the NASA Johnson Space Center Institutional Review Board for Human Testing approved all experimental protocols and procedures, which were performed following the Helsinki Declaration of 1964. To ensure comparable levels of sleep quantity the night before the recordings, a sleep questionnaire was filled out by astronauts. Astronauts were allocated 8.5 h of sleep the night before the experiment. The experiments were not performed in the 48 h following air travel that involved a change of > 4 time zones, nor following work shifts inducing > 4 h of time shift, nor the day after imposed sleep deprivation, nor after a highly strenuous mental or physical task such as centrifuge training, vestibular counter-measures experiments, and extravehicular activities. Astronauts were asked to maintain their normal consumption of caffeine and were not allowed to take alcohol or medication 16 h before the experiment. Each astronaut was asked to execute the experiments on approximately the same day time preferably in the morning ± 2 h. Participants were assessed in three conditions: (1) pre-flight (on earth; three times: 66.8 ± 9.0, 42.6 ± 0.9, and 28.0 ± 0.4 days before departure), (2) in-flight (aboard ISS station in space; two times: 8.8 ± 1.8 and 54.6 ± 3.7 days during mission) and 3) post-flight (on earth; four times: 3.0 ± 0.4, 7.0 ± 1.2, 16.8 ± 0.64 and 20.2 ± 1.04 days after arrival).
Ethics statement
The European Space Agency Medical Care Committee and the NASA Johnson Space Centre Institutional Review Board for Human Testing approved all experimental procedures, which were performed in accordance with the Helsinki Declaration. All participants gave written, informed consent prior to starting the experiment.
EEG data acquisition
The brain activity of all participants was measured by electroencephalography (EEG) during 2-min resting-state eyes-closed (EC) and 2-min resting-state eyes-open (EO) conditions6. On earth, the participants were seated comfortably in a chair. During microgravity in space, they stayed in a free-floating condition where significant trajectory shifts were prevented using a belt around the subject's waist, which was attached to straps fixed to metal rings located at the racks on both sides of the Columbus module of the ISS. To avoid any external visual distractors, a cylindrical tube attached to the laptop screen including a face mask was fitted to the astronaut’s head. This recording setup was used both for the ground and ISS measurements.
EEG data were recorded at a sampling frequency of 1116 Hz using the 59-channel electroencephalogram mapping module (MEEMM) of the European physiology module, installed in the Columbus module of the ISS, at the European Astronaut Center (Köln, Germany) or in Star City (Moscow). The MEEMM uses a dedicated physical reference electrode (right earlobe). For some post-flight recordings at the Johnson Space Center (Houston), an asalab 64-channel amplifier (ANT Neuro BV, Hengelo, Netherlands) was used at ground level in a standard lab environment. The asalab amplifier is a stationary DC-EEG amplifier with a common average reference. Scalp electrode impedance was measured and kept below 5 kΩ for all recordings.
EEG data preprocessing
Fifty-five common EEG channels (based on the 10–10 system) were extracted from both systems (MEEMM and asalab) to provide a homogeneous layout and spatial coverage of the head. Data were re-referenced to a standard average reference. Bad channels were automatically identified by evaluating the mean power spectral density (PSD) of a given channel in the frequency band (70–100) Hz. A given channel was identified as a bad channel if its PSD is higher than the mean PSD + threefold standard deviation of all 55 channels of a given dataset25. Subsequently, bad channels were interpolated using spherical splines48, data were resampled to 512 Hz, and the DC offset of each individual channel was removed. Ocular artifacts were detected and removed using principal component analysis (PCA) (ASA software, ANT Neuro BV, Hengelo, Netherlands), where 95% of the calculated components explained the noise subspace. Muscle and jump artifacts were automatically detected and rejected using the FieldTrip package49. Any other residual artifacts were rejected by expert visual inspection. The remaining artifact-free data were segmented into four-second epochs. The final data set had on average across all subjects and conditions 23 ± 3 epochs for EC and 18 ± 4 epochs for EO. EEG data were filtered in the classical frequency bands: delta (2–4 Hz), theta (4–8 Hz), alpha (8–12 Hz), beta (12–30 Hz), and gamma (30–45 Hz).
EEG data analysis
Source activity was estimated using a template (Montreal Neurological Institute—MNI—space) with a regular volumetric grid of 10 mm spacing. The template was linearly transformed to fit the head shape of each participant (individual subjects’ T1 MRI were not available). Sources were reconstructed independently for each subject through the exact low-resolution brain electromagnetic tomography (eLORETA) method50 using a regularization factor of 10−8. Each source position was labeled using the Automated Anatomical Labeling (AAL) atlas51. Only those sources labeled as part of one of the 22 cortical areas involved in the default mode network (DMN) were included in subsequent analyses (215 sources).
The power spectrum of each source was computed for each epoch using the fast Fourier transform with Hanning tapers with 0.25 Hz smoothing. Relative power was calculated by normalizing each ROI’s spectrum by the total power over the 2–45 Hz range. The average power of the DMN per each classical frequency band was calculated by averaging all epochs, all sources, and all corresponding frequency steps, resulting in a source-reconstructed power matrix of 9 stages of 5 frequency bands in 5 participants.
FCs between the 22 DMN ROIs (representing the 22 cortical areas involved in the DMN) were assessed with the corrected imaginary phase-locking value (ciPLV)52, a phase synchronization measure that evaluates the distribution of phase differences extracted from two ROIs time series and is insensitive to zero-lag effects, as it removes the contribution of the zero phase differences52. Symmetrical, whole-brain matrices of 215 × 215 sources were thus obtained by averaging ciPLV values across epochs for each participant and frequency band. Then, DMN 22 × 22 ROIs FC matrices were constructed by averaging the FC weights of the corresponding sources for each pair of ROIs. Lastly, we computed the strength of each ROI (also known as weighted global connectivity), defined as the average FC across all links that belong to the DMN network. Then each ROI's strength was normalized by dividing the number of links connected to it, obtaining one brain map of normalized ROI strengths per participant and frequency band to account for the number of links.
Statistical analysis
Statistical analysis was performed using Prism 9 software (GraphPad version 9.0.0 https://www.graphpad.com/, San Diego, CA, USA). The final values of power spectrum and FC obtained under the three conditions pre-flight, in-flight, and post-flight were statistically compared. Eyes-closed and eyes-opened sub conditions were compared to determine differences in the DMN alpha band relative power and FC strength. Depending on the number of independent variables, normally distributed data (Shapiro–Wilk normality test), and equality of variances (sphericity test) of the groups compared, we used one-way ANOVA or two-way Repeated Measure ANOVAs by Tukey's multiple comparison test. Geisser and Greenhouse’s correction method was used for repeated measures with reduced sphericity. F values, t values, and degrees of freedom are reported in Table S1 of the supplementary material. Results are reported as mean ± standard error (SEM) and p values coded as follows: *p < 0.05, **p < 0.01, ***p < 0.001.
Results
Alpha band power changes in the DMN
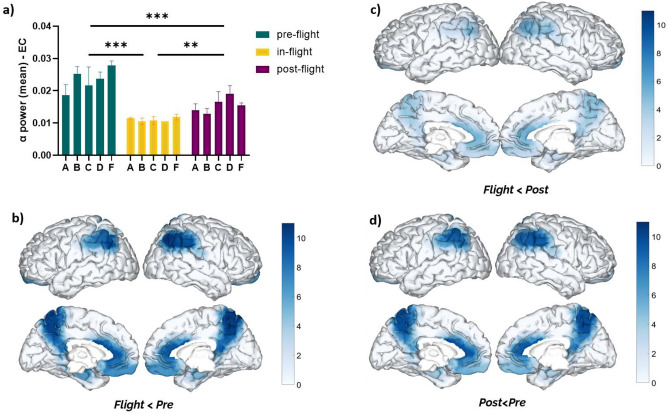
All individual subjects showed a reduction of DMN alpha band power during eyes-closed (EC) under in-flight conditions (aboard ISS station) (Fig. 1). As a cohort, the DMN alpha band power (EC) was found to be significantly decreased (F = 24.09, p < 0.001, eta2 = 0.53) during the in-flight condition when compared to the pre-flight (p < 0.001) and post-flight conditions (p < 0.01) (Fig. 1a). These changes were observed across different DMN regions (Fig. 1b–d). Significant changes were mainly observed during the in-flight condition compared to the pre-flight condition (Fig. 1b). Precuneus, the anterior cingulate cortex, and parietal regions showed the most considerable differences in alpha band power (higher q value). For a detailed description of the statistical analysis of the ANOVA, effect size of all the DMN regions refer to Table S1 of the supplementary material.
Figure 1.
Changes in DMN alpha band power (eyes closed) between flight conditions. (a) Statistical comparison between conditions. The bar graph depicts the mean ± SEM of the DMN alpha band power for each flight condition (*p < 0.05, **p < 0.01, ***p < 0.001). (b–d) Brain figures in the dashed boxes represent the DMN areas with higher statistical power changes in the alpha band comparing DMN ROIs between (b) in-flight versus pre-flight conditions, (c) in-flight versus post-flight conditions, (d) post-flight versus pre-flight conditions. The colorbar is displayed as a family-wise corrected significance level of q value > 3, corresponding with a minimum p value of 0.05. The q statistic value was obtained from the results of the post-hoc Tuckey test of the multiple comparison corrections. Thus, the darker blue color represents brain regions with higher statistical power. The five subjects are mentioned by the respective code letter under each bar.
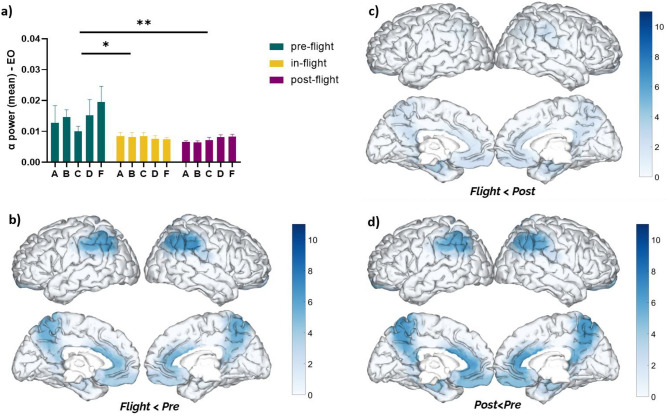
Additionally, we evaluated the DMN alpha band power during eyes-open (EO) and found a reduction of alpha power (F = 12.37, p < 0.001, eta2 = 0.39) across all subjects under in-flight conditions compared to pre-flight conditions (Fig. 2). As a cohort, the DMN alpha band power (EO) was found to be significantly decreased during the in-flight condition when compared to pre-flight (p < 0.05) (Fig. 2a).
Figure 2.
Changes in DMN alpha band power (eyes open) between flight conditions. (a) Statistical comparison between conditions. The bar graph depicts the mean ± SEM of the DMN alpha band power for each flight condition (*p < 0.05, **p < 0.01, ***p < 0.001). (b–d) Brain figures in the dashed boxes represent the DMN areas with higher statistical power changes in the alpha band comparing DMN ROIs between (b) in-flight versus pre-flight conditions, (c) in-flight versus post-flight conditions, (d) post-flight versus pre-flight conditions. The color bar is displayed as a family-wise corrected significance level of q value > 3, corresponding with a minimum p value of 0.05. The q statistic value was obtained from the results of the post-hoc Tuckey test of the multiple comparison corrections. Thus, the darker blue color represents brain regions with higher statistical power. The five subjects are mentioned by the respective code letter under each bar.
Notably, DMN alpha band power (EC/EO) continued to be reduced in all subjects during post-flight conditions compared to pre-flight (Figs. 1a, 2a). As a cohort, the reduction of DMN alpha band power during the post-flight condition was statistically significant when compared to in-flight condition (EC: p < 0.001; EO: p < 0.01). These changes were observed across different DMN regions (Fig. 2b–d).
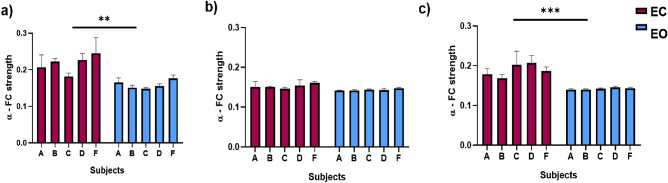
There were DMN alpha band power differences between eye-closed and eyes-opened conditions. Higher relative power was found during eye-closed in all flight conditions, which were statistically significant as a cohort (pre-flight: p = 0.0008; in-flight: p < 0.0001; post-flight: p = 0.0033) (Fig. 3a–c).
Figure 3.
Differences between eyes closed and open in DMN Alpha band relative power between flight conditions. (a) Comparison between eyes closed and eyes open in the pre-flight condition (p = 0.0008). (b) Comparison between closed and open eyes in the in-flight condition (p = 0.0033). (c) Comparison between closed and open eyes in the post-flight condition (p < 0.0001). Bar graphs depict the mean ± SEM of the DMN alpha band power for each flight condition per subject. The red color in a bar indicates the eyes closed condition, whereas the blue bar indicates eyes open condition. The five subjects are mentioned by the respective code letter under each bar. (*p < 0.05, **p < 0.01, ***p < 0.001).
Changes in alpha band FC strength in the DMN
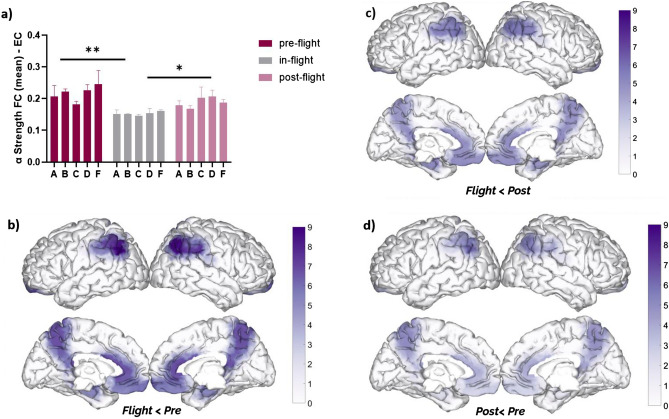
As a cohort, DMN alpha band FC strength significantly decreased (F = 8.10, p = 0.015, eta2 = 0.30) during the in-flight condition (aboard ISS station) when compared to the pre-flight (p < 0.01) and post-flight (p < 0.05) conditions (Fig. 4a). These changes were observed across different DMN regions (Fig. 4b–d). Similar to alpha band power alterations, significant changes in alpha band FC strength were mainly observed during in-flight conditions compared to the pre-flight condition (Fig. 4b), with the parietal regions showing the most significant differences (highest q value). For a detailed description of the statistical analysis refer to Table S1 of the supplementary material.
Figure 4.
Changes in DMN alpha Strength (FC—eyes closed) between flight conditions. (a) Statistical comparison between conditions. The bar graph depicts the mean ± SEM of the DMN alpha band FC strength for each flight condition (*p < 0.05, **p < 0.01, ***p < 0.001). (b–d) Brain figures in the dashed boxes represent the DMN areas with higher statistical power changes in the alpha band comparing DMN ROIs between (b) in-flight versus pre-flight conditions, (c) in-flight versus post-flight conditions, (d) post-flight versus pre-flight conditions. The color bar is displayed as a family-wise corrected significance level of q value > 3, corresponding with a minimum p value of 0.05. The q statistic value was obtained from the results of the post-hoc Tukey test of the multiple comparison corrections. Thus, the darker purple color represents brain regions with higher statistical power. The five subjects are mentioned by the respective code letter under each bar.
Additionally, we evaluated the DMN alpha band FC strength during eyes-opened (EO) and found a reduction of FC strength (F = 17.21, p < 0.001, eta2 = 0.39) in all subjects under in-flight conditions compared to pre-flight conditions (Fig. 5). As a cohort, the DMN alpha band FC strength (EO) was found to be significantly decreased during the in-flight condition when compared to pre-flight (p < 0.01) (Fig. 5a). Moreover, post-flight conditions (EO) also showed a significant decrease in FC strength compared to pre-flight (p < 0.01). These patterns were noticed across different DMN regions (Fig. 5b–d).
Figure 5.
Changes in DMN alpha strength (FC—eyes open) between flight conditions. (a) Statistical comparison between conditions. The bar graph depicts the mean ± SEM of the DMN alpha band FC strength for each flight condition (*p < 0.05, **p < 0.01, ***p < 0.001). (b–d) Brain figures in the dashed boxes represent the DMN areas with higher statistical power changes in the alpha band comparing DMN ROIs between (b) in-flight versus pre-flight conditions, (c) in-flight versus post-flight conditions, (d) post-flight versus pre-flight conditions. The color bar is displayed as a family-wise corrected significance level of q value > 3, corresponding with a minimum p value of 0.05. The q statistic value was obtained from the results of the post-hoc Tuckey test of the multiple comparison corrections. Thus, the darker purple color represents brain regions with higher statistical power. The five subjects are mentioned by the respective code letter under each bar.
There were DMN alpha band FC strength differences between eye-closed and eyes-opened conditions. Higher FC strength was found during eye-closed in all flight conditions (Fig. 6a–c). However, these differences were only statistically significant as a cohort at ground level (pre-flight: p = 0.0059; post-flight: p = 0.0002), but not during in-flight aboard ISS (p = 0.1245).
Figure 6.
Differences between eyes closed and open in DMN alpha band FC strength between flight conditions. (a) Comparison between closed and open eyes in the pre-flight condition (p = 0.0059). (b) Comparison between closed and open eyes in the in-flight condition (p = 0.1245). (c) Comparison between closed and open eyes in the post-flight condition (p = 0.0002). Bar graphs depict the mean ± SEM of the DMN alpha band FC strength for each flight condition per subject. The red color in a bar indicates the eyes closed condition, whereas the blue bar indicates eyes open condition. The five subjects are mentioned by the respective code letter under each bar. (*p < 0.05, **p < 0.01, ***p < 0.001).
Discussion
The impact of spaceflight and microgravity on cognitive function has been addressed by researchers and medical experts in space agencies, as optimal performance can be critical to mission success9. Thus, there are several studies14 addressing neurophysiological alterations due to spaceflight, they include structural changes of grey matter and even in cerebrospinal fluid (CSF)4. Additionally, changes in visuo-attentional activity, visuospatial performance, brain activity, and effects of space travel on sleep quantity have been observed4. Our study findings indicate that spaceflight can produce neurophysiological alterations, as evidenced through EEG-derived DMN analysis. These changes result in a reduction of both DMN alpha band power and FC strength, notably over the frontoparietal regions, which occurred in space and persisted after return to earth across all five subjects.
We consider that these data can be of critical importance and warrant further investigation. The frontoparietal network has been implicated in task initiation and error control53. Changes to this network that we have observed for the in-flight condition may be of critical importance due to the direct impact on task performance aboard the spacecraft. Moreover, alpha band reduction in power or connectivity has been reported in various neurocognitive disorders such as dementia, stroke, and traumatic brain injury32,54. The persistent changes after return to earth in our cohort are notable, indicating that effects of spaceflight are present at least 20 days after return to earth.
The changes in our cohort cannot be readily explained by their expected high task demand and stressful environment. In contrast to the alpha power reduction observed in our astronaut cohort, commercial pilots whose jobs also involve multitasking and situations of high cognitive stress level were reported to have increased alpha band power55. Common occupational cognitive-related conditions such as high working memory load56, drowsiness during driving57, long periods of psychoacoustic stimulation58, and mental exhaustion59 are also associated with an increase in alpha power.
The unique spaceflight conditions are a probable cause of DMN alpha band power and FC strength reduction. Simulated microgravity environments such as head-down or bed rest60 have been associated with changes in alpha power (i.e., reduce alpha power)61. However, these alterations are transient and returned to baseline after a return to normal positioning, and there were no functional connectivity differences61. EEG has also been used in space to measure changes in neurocognitive performance and brain activity that result from exposure to microgravity and isolation62. Alterations in brain activity are commonly identified in the faster EEG frequencies, demonstrating inhibition of brain activity in the cortical regions under these conditions4. Additionally, long-term isolation and dry immersion studies have found alterations in sleep spectral content63 and changes in alpha power as well64. Nonetheless, these effects are expected to disappear after weeks of returning to normal social activity. In our study, we demonstrate both persistent reductions of alpha band power on DMN upon return to earth and frontoparietal functional connectivity changes, indicating that microgravity alone would not explain these findings. Our results showed reduced brain activity that persisted weeks after landing and resumption of social activity, making it unlikely that isolation is the sole contributing factor to the alpha band reduction.
Galactic cosmic radiation (GCR)65 is an important risk factor for human spaceflight, especially for expeditionary missions beyond lower earth orbit (LEO). Radiation-induced brain injury is a known condition and has been linked to cognitive impairment66. Gamma radiation exposure has been reported to trigger oxidative stress, increase inflammation, and cause neurotransmitter fluctuations in the central nervous system in rats, resulting in the presence of β-amyloid deposits in the cerebral cortex67. β-amyloid deposits have been notably associated with Alzheimer's disease68, and EEG findings indicate reduced alpha band power and abnormal frontoparietal coupling69,70. Although our data were recorded in lower earth orbit, the observed reduction of the alpha power and connectivity in our study could be in part caused by cascading patho-mechanisms induced by this proteinopathy, as it happens in Alzheimer's disease71.
These EEG results are similar to our cohort's observations, raising questions regarding the influence of radiation on our subjects and findings. Even under the protection of the earth's magnetic field, astronauts aboard ISS receive a 40–60 times higher effective yearly dose-rate than on earth due to galactic cosmic radiation, solar flares, and radiation from the Van Allen Belt72.
There are many remaining questions that the available data of this study alone cannot be fully addressed. The onset and progression of DMN changes during spaceflight cannot be reliably deduced from only two recordings, averaging 9 and 55 days, which were available for our analysis. The duration and return-to-baseline of these changes are also unclear, as follow-up recording continued only until 20-days after return to ground level. The number of participants and gender balance in this study is also a limiting factor. Due to the nature of our dataset, it was not possible to include both sexes. In 62 years of human spaceflight activities, only 6 female astronauts and cosmonauts were involved in inflight EEG studies compared to more than 70 males73,74. Our dataset includes only EEGs from male astronauts. Moreover, it is known in the literature that there are gender differences in neurophysiological data related to the alpha band in 1G conditions75,76 and even in hypo- and hyper-gravity conditions77. We expect to find differences between gender in future studies. These issues should be resolved as exploration missions beyond LEO and increased commercial spaceflights activities are at the near horizon. We notice that future studies are needed to determine the onset and persistence of these changes in a larger cohort. Traditionally used neuropsychological testing alone has limits, as neuropathology suggests cognitive impairment can develop prior to changes in performance23. Future studies of EEG-derived DMN analyses, in conjunction with neuropsychological testing and imaging, can promote understanding of the spaceflight effect on neurobiology, ensuring mission success and crew safety. Furthermore, EEG-derived DMN analysis carries excellent potential as a neurophysiologic marker with practical applications, as it can be readily performed and analyzed during spaceflight missions78.
Supplementary Information
Author contributions
S.P.: Methodology, Data processing, Statistical Analysis, Manuscript—original draft preparation and figures preparation. J.A.Z.V.: Methodology, Statistical Analysis, Manuscript—original draft preparation P.C.: Methodology, Data processing, Manuscript—original draft preparation. C.L.: Manuscript—original draft preparation, review and editing. P.F.: Data pre-processing, Manuscript—review and editing. A.C., J.H., M.F. and F.M.: Manuscript—review and editing. G.C.: Funding acquisition, Design and Implementation of the research, Manuscript—review and editing. All present authors contributed to the article and approved the submitted version.
Data availability
The data that support the findings of this study are available upon reasonable request to the corresponding author.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Sandra Pusil and Jonathan Zegarra-Valdivia.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-34744-1.
References
- 1.Clément GR, et al. Challenges to the central nervous system during human spaceflight missions to Mars. J. Neurophysiol. 2020;123:2037–2063. doi: 10.1152/jn.00476.2019. [DOI] [PubMed] [Google Scholar]
- 2.Afshinnekoo E, et al. Fundamental biological features of spaceflight: Advancing the field to enable deep-space exploration. Cell. 2020;183:1162–1184. doi: 10.1016/j.cell.2020.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basner M, et al. Development and validation of the cognition test battery for spaceflight. Aerosp. Med. Hum. Perform. 2015;86:942–952. doi: 10.3357/AMHP.4343.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dinatolo MF, Cohen LY. Monitoring the impact of spaceflight on the human brain. Life (Basel) 2022;12:1060. doi: 10.3390/life12071060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheron G, et al. Gravity influences top-down signals in visual processing. PLoS ONE. 2014;9:e82371. doi: 10.1371/journal.pone.0082371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cebolla AM, et al. Cerebellar contribution to visuo-attentional alpha rhythm: insights from weightlessness. Sci. Rep. 2016;6:1–10. doi: 10.1038/srep37824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cebolla AM, Petieau M, Palmero-Soler E, Cheron G. Brain potential responses involved in decision-making in weightlessness. Sci. Rep. 2022;12:1–11. doi: 10.1038/s41598-022-17234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takács E, et al. Persistent deterioration of visuospatial performance in spaceflight. Sci. Rep. 2021;11:1–11. doi: 10.1038/s41598-021-88938-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De la Torre GG. Cognitive neuroscience in space. Life (Basel) 2014;4:281–294. doi: 10.3390/life4030281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Saedeleer C, et al. Weightlessness alters up/down asymmetries in the perception of self-motion. Exp. Brain Res. 2013;226:95–106. doi: 10.1007/s00221-013-3414-7. [DOI] [PubMed] [Google Scholar]
- 11.Bourrelly A, McIntyre J, Morio C, Despretz P, Luyat M. Perception of affordance during short-term exposure to weightlessness in parabolic flight. PLoS ONE. 2016;11:e0153598. doi: 10.1371/journal.pone.0153598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desai RI, Limoli CL, Stark CEL, Stark SM. Impact of spaceflight stressors on behavior and cognition: A molecular, neurochemical, and neurobiological perspective. Neurosci. Biobehav. Rev. 2022;138:104676. doi: 10.1016/j.neubiorev.2022.104676. [DOI] [PubMed] [Google Scholar]
- 13.Tays GD, et al. The effects of long duration spaceflight on sensorimotor control and cognition. Front. Neural Circuits. 2021;15:110. doi: 10.3389/fncir.2021.723504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Ombergen A, et al. The effect of spaceflight and microgravity on the human brain. J. Neurol. 2017;264:18. doi: 10.1007/s00415-017-8427-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Christensen JM, Talbot J. A review of the psychological aspects of space flight. Aviat. Space Environ. Med. 1986;57:203–212. [PubMed] [Google Scholar]
- 16.Petit G, et al. Local sleep-like events during wakefulness and their relationship to decreased alertness in astronauts on ISS. NPJ Microgravity. 2019;5:10. doi: 10.1038/s41526-019-0069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamamuro K, et al. Social isolation during the critical period reduces synaptic and intrinsic excitability of a subtype of pyramidal cell in mouse prefrontal cortex. Cereb. Cortex. 2018;28:998–1010. doi: 10.1093/cercor/bhx010. [DOI] [PubMed] [Google Scholar]
- 18.Marshall-Goebel K, et al. Assessment of jugular venous blood flow stasis and thrombosis during spaceflight. JAMA Netw. Open. 2019;2:e1915011. doi: 10.1001/jamanetworkopen.2019.15011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koppelmans V, Bloomberg JJ, Mulavara AP, Seidler RD. Brain structural plasticity with spaceflight. NPJ Microgravity. 2016;2:1–8. doi: 10.1038/s41526-016-0001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parihar VK, et al. Persistent changes in neuronal structure and synaptic plasticity caused by proton irradiation. Brain Struct. Funct. 2015;220:1161. doi: 10.1007/s00429-014-0709-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roberts DR, et al. Effects of spaceflight on astronaut brain structure as indicated on MRI. N. Engl. J. Med. 2017;377:1746–1753. doi: 10.1056/NEJMoa1705129. [DOI] [PubMed] [Google Scholar]
- 22.Casario K, et al. Acceptability of the cognition test battery in astronaut and astronaut-surrogate populations. Acta Astronaut. 2022;190:14–23. doi: 10.1016/j.actaastro.2021.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakamura A, et al. Electromagnetic signatures of the preclinical and prodromal stages of Alzheimer’s disease. Brain. 2018;141:1470–1485. doi: 10.1093/brain/awy044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.La Torre GGD, et al. Future perspectives on space psychology: Recommendations on psychosocial and neurobehavioural aspects of human spaceflight. Acta Astronaut. 2012;81:587–599. doi: 10.1016/j.actaastro.2012.08.013. [DOI] [Google Scholar]
- 25.Fiedler P, et al. Noise characteristics in spaceflight multichannel EEG. PLoS ONE. 2023;18:e0280822. doi: 10.1371/journal.pone.0280822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monk TH, Buysse DJ, Billy BD, Kennedy KS, Willrich LM. Sleep and circadian rhythms in four orbiting astronauts. J. Biol. Rhythms. 1998;13:188–201. doi: 10.1177/074873098129000039. [DOI] [PubMed] [Google Scholar]
- 27.Cheron G, et al. Effect of gravity on human spontaneous 10-Hz electroencephalographic oscillations during the arrest reaction. Brain Res. 2006;1121:104–116. doi: 10.1016/j.brainres.2006.08.098. [DOI] [PubMed] [Google Scholar]
- 28.Foster JJ, Sutterer DW, Serences JT, Vogel EK, Awh E. The topography of alpha-band activity tracks the content of spatial working memory. J. Neurophysiol. 2016;115:168–177. doi: 10.1152/jn.00860.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klimesch W. α-band oscillations, attention, and controlled access to stored information. Trends Cogn. Sci. 2012;16:606–617. doi: 10.1016/j.tics.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lejko N, Larabi DI, Herrmann CS, Aleman A, Ćurčić-Blake B. Alpha power and functional connectivity in cognitive decline: A systematic review and meta-analysis. J. Alzheimers Dis. 2020;78:1047–1088. doi: 10.3233/JAD-200962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Britton, J. W. et al. Electroencephalography (EEG): An introductory text and atlas of normal and abnormal findings in adults, children, and infants. Electroencephalography (EEG): An Introductory Text and Atlas of Normal and Abnormal Findings in Adults, Children, and Infants (2016). [PubMed]
- 32.López-Sanz D, et al. Network disruption in the preclinical stages of Alzheimer’s disease: From subjective cognitive decline to mild cognitive impairment. Int. J. Neural Syst. 2017;27:1750041. doi: 10.1142/S0129065717500411. [DOI] [PubMed] [Google Scholar]
- 33.Brueggen K, et al. Early changes in alpha band power and DMN BOLD activity in Alzheimer’s disease: A simultaneous resting state EEG-fMRI study. Front. Aging Neurosci. 2017;9:319. doi: 10.3389/fnagi.2017.00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Locatelli T, Cursi M, Liberati D, Franceschi M, Comi G. EEG coherence in Alzheimer’s disease. Electroencephalogr. Clin. Neurophysiol. 1998;106:229–237. doi: 10.1016/S0013-4694(97)00129-6. [DOI] [PubMed] [Google Scholar]
- 35.Jeong J. EEG dynamics in patients with Alzheimer’s disease. Clin. Neurophysiol. 2004;115:1490–1505. doi: 10.1016/j.clinph.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 36.López-Sanz D, et al. Alpha band disruption in the AD-continuum starts in the subjective cognitive decline stage: A MEG study. Sci. Rep. 2016;6:37685. doi: 10.1038/srep37685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alonso JF, Romero S, Ballester MR, Antonijoan RM, Mañanas MA. Stress assessment based on EEG univariate features and functional connectivity measures. Physiol. Meas. 2015;36:1351. doi: 10.1088/0967-3334/36/7/1351. [DOI] [PubMed] [Google Scholar]
- 38.Leroy A, Cheron G. EEG dynamics and neural generators of psychological flow during one tightrope performance. Sci. Rep. 2020;10:1–13. doi: 10.1038/s41598-020-69448-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.di Fronso S, et al. Dry EEG in sports sciences: A fast and reliable tool to assess individual alpha peak frequency changes induced by physical effort. Front. Neurosci. 2019;13:982. doi: 10.3389/fnins.2019.00982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fink A, Grabner RH, Neuper C, Neubauer AC. EEG alpha band dissociation with increasing task demands. Brain Res. Cogn. Brain Res. 2005;24:252–259. doi: 10.1016/j.cogbrainres.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 41.Smallwood J, et al. The default mode network in cognition: A topographical perspective. Nat. Rev. Neurosci. 2021;22:503–513. doi: 10.1038/s41583-021-00474-4. [DOI] [PubMed] [Google Scholar]
- 42.Cheron G, et al. Adaptive changes of rhythmic EEG oscillations in space implications for brain-machine interface applications. Int. Rev. Neurobiol. 2009;86:171–187. doi: 10.1016/S0074-7742(09)86013-3. [DOI] [PubMed] [Google Scholar]
- 43.Raichle ME, et al. A default mode of brain function. Proc. Natl. Acad. Sci. USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Broyd SJ, et al. Default-mode brain dysfunction in mental disorders: A systematic review. Neurosci. Biobehav. Rev. 2009;33:279–296. doi: 10.1016/j.neubiorev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 45.Hohenfeld C, Werner CJ, Reetz K. Resting-state connectivity in neurodegenerative disorders: Is there potential for an imaging biomarker? Neuroimage Clin. 2018;18:849–870. doi: 10.1016/j.nicl.2018.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koelewijn L, et al. Alzheimer’s disease disrupts alpha and beta-band resting-state oscillatory network connectivity. Clin. Neurophysiol. 2017;128:2347–2357. doi: 10.1016/j.clinph.2017.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dunlop K, Talishinsky A, Liston C. Intrinsic brain network biomarkers of antidepressant response: A review. Curr. Psychiatry Rep. 2019;21:1–11. doi: 10.1007/s11920-019-1072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perrin F, Pernier J, Bertrand O, Echallier JF. Spherical splines for scalp potential and current density mapping. Electroencephalogr. Clin. Neurophysiol. 1989;72:184–187. doi: 10.1016/0013-4694(89)90180-6. [DOI] [PubMed] [Google Scholar]
- 49.Oostenveld R, Fries P, Maris E, Schoffelen JM. FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput. Intell. Neurosci. 2011;2011:1–9. doi: 10.1155/2011/156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pascual-Marqui RD, et al. Assessing interactions in the brain with exact low-resolution electromagnetic tomography. Philos. Trans. A Math. Phys. Eng. Sci. 2011;369:3768–3784. doi: 10.1098/rsta.2011.0081. [DOI] [PubMed] [Google Scholar]
- 51.Tzourio-Mazoyer N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 52.Bruña R, Maestú F, Pereda E. Phase locking value revisited: Teaching new tricks to an old dog. J. Neural Eng. 2018;15:056011. doi: 10.1088/1741-2552/aacfe4. [DOI] [PubMed] [Google Scholar]
- 53.Dosenbach NUF, et al. Distinct brain networks for adaptive and stable task control in humans. Proc. Natl. Acad. Sci. USA. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Castellanos NP, et al. Reorganization of functional connectivity as a correlate of cognitive recovery in acquired brain injury. Brain. 2010;133:2365–2381. doi: 10.1093/brain/awq174. [DOI] [PubMed] [Google Scholar]
- 55.Puma S, Matton N, Paubel PV, Raufaste É, El-Yagoubi R. Using theta and alpha band power to assess cognitive workload in multitasking environments. Int. J. Psychophysiol. 2018;123:111–120. doi: 10.1016/j.ijpsycho.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 56.Dehais F, et al. Monitoring pilot’s mental workload using ERPs and spectral power with a six-dry-electrode eeg system in real flight conditions. Sensors (Basel) 2019;19:1324. doi: 10.3390/s19061324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Di Flumeri G. EEG-based index for timely detecting user’s drowsiness occurrence in automotive applications. Front. Hum. Neurosci. 2022;16:866118. doi: 10.3389/fnhum.2022.866118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sadeghian M, Mohammadi Z, Mousavi SM. Investigation of electroencephalography variations of mental workload in the exposure of the psychoacoustic in both male and female groups. Cogn. Neurodyn. 2022;16:561–574. doi: 10.1007/s11571-021-09737-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zheng R, Wang Z, He Y, Zhang J. EEG-based brain functional connectivity representation using amplitude locking value for fatigue-driving recognition. Cogn. Neurodyn. 2022;16:325–336. doi: 10.1007/s11571-021-09714-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marušič U, Meeusen R, Pišot R, Kavcic V. The brain in micro- and hypergravity: The effects of changing gravity on the brain electrocortical activity. Eur. J. Sport Sci. 2014;14:813–822. doi: 10.1080/17461391.2014.908959. [DOI] [PubMed] [Google Scholar]
- 61.Brauns K, Friedl-Werner A, Maggioni MA, Gunga HC, Stahn AC. Head-down tilt position, but not the duration of bed rest affects resting state electrocortical activity. Front. Physiol. 2021;12:638669. doi: 10.3389/fphys.2021.638669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rosenberg SD, et al. EEG in adults in the laboratory or at the patient’s bedside. Neurophysiol. Clin. 2015;45:19–37. doi: 10.1016/j.neucli.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 63.Gemignani A, et al. How stressful are 105 days of isolation? Sleep EEG patterns and tonic cortisol in healthy volunteers simulating manned flight to Mars. Int. J. Psychophysiol. 2014;93:211–219. doi: 10.1016/j.ijpsycho.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 64.Lazarev IE, Tomilovskaya ES, Kozlovskaya IB. Resting state brain activity during long-term dry immersion. Aerosp. Med. Hum. Perform. 2018;89:642–647. doi: 10.3357/AMHP.4972.2018. [DOI] [PubMed] [Google Scholar]
- 65.Onorato G, Di Schiavi E, Di Cunto F. Understanding the effects of deep space radiation on nervous system: The role of genetically tractable experimental models. Front. Phys. 2020;8:362. doi: 10.3389/fphy.2020.00362. [DOI] [Google Scholar]
- 66.Turnquist C, Harris BT, Harris CC. Radiation-induced brain injury: Current concepts and therapeutic strategies targeting neuroinflammation. Neurooncol. Adv. 2020;2:vdaa057. doi: 10.1093/noajnl/vdaa057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Algeda FR, Eltahawy NA, Shedid SM, Saada HN. The impact of gamma-radiation on the cerebral- and cerebellar—Cortex of male rats’ brain. Brain Res. Bull. 2022;186:136–142. doi: 10.1016/j.brainresbull.2022.05.011. [DOI] [PubMed] [Google Scholar]
- 68.Murphy MP, Levine H. Alzheimer’s disease and the amyloid-beta peptide. J. Alzheimers Dis. 2010;19:311–323. doi: 10.3233/JAD-2010-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Caravaglios G, et al. EEG resting-state functional networks in amnestic mild cognitive impairment. Clin. EEG Neurosci. 2022 doi: 10.1177/15500594221110036. [DOI] [PubMed] [Google Scholar]
- 70.Babiloni C, et al. Abnormal fronto-parietal coupling of brain rhythms in mild Alzheimer’s disease: A multicentric EEG study. Eur. J. Neurosci. 2004;19:2583–2590. doi: 10.1111/j.0953-816X.2004.03333.x. [DOI] [PubMed] [Google Scholar]
- 71.Raz L, Knoefel J, Bhaskar K. The neuropathology and cerebrovascular mechanisms of dementia. J. Cereb. Blood Flow Metab. 2016;36:172. doi: 10.1038/jcbfm.2015.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Restier-Verlet J, et al. Radiation on earth or in space: What does it change? Int. J. Mol. Sci. 2021;22:3739. doi: 10.3390/ijms22073739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Parin VV, Volynkin YM, Vassilyev PV. Manned space flight–some scientific results. Life Sci. Space Res. 1965;3:3–22. [PubMed] [Google Scholar]
- 74.Dijk DJ, et al. Sleep, performance, circadian rhythms, and light-dark cycles during two space shuttle flights. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;281(5):R1647–64. doi: 10.1152/ajpregu.2001.281.5.R1647. [DOI] [PubMed] [Google Scholar]
- 75.Cantillo-Negrete J, et al. Gender differences in quantitative electroencephalogram during a simple hand movement task in young adults. Rev. Investig. Clin. 2017;68:245–255. [PubMed] [Google Scholar]
- 76.Jaušovec N, Jaušovec K. Resting brain activity: Differences between genders. Neuropsychologia. 2010;48:3918–3925. doi: 10.1016/j.neuropsychologia.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 77.Schneider S, Robinson R, Smith C, Von Der Wiesche M, Goswami N. Gender specific changes in cortical activation patterns during exposure to artificial gravity. Acta Astronaut. 2014;104:438–443. doi: 10.1016/j.actaastro.2014.03.003. [DOI] [Google Scholar]
- 78.Samogin J, et al. Frequency-dependent functional connectivity in resting state networks. Hum. Brain Mapp. 2020;41:5187–5198. doi: 10.1002/hbm.25184. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available upon reasonable request to the corresponding author.