Abstract
Objective:
To investigate whether symptoms of gastroesophageal reflux (GERD) and radiographic measures of esophageal dilation are associated with radiographic progression of systemic sclerosis-related interstitial lung disease (SSc-ILD).
Methods:
Participants of Scleroderma Lung Study II, which compared mycophenolate versus cyclophosphamide for SSc-ILD, completed the reflux domain of the UCLA-SCTC GIT 2.0 at baseline. The diameter and area of the esophagus in the region of maximum dilation was measured by quantitative image analysis. Univariate and multivariable linear regression analyses were created to evaluate the relationship between these measures of esophageal involvement and progression of SSc-ILD over 2 years based on the quantitative radiographic extent of ILD (QILD) and fibrosis (QLF) in the lobe of maximum involvement (LM). All multivariable models controlled for treatment arm, baseline ILD severity and proton pump inhibitor use.
Results:
Baseline mean patient-reported reflux score was 0.57, indicating moderate reflux (N=141). Baseline mean maximal esophageal diameter and area were 22 mm and 242 mm2, respectively. Baseline reflux scores were significantly associated with the change in QLF-LM and QILD-LM in the univariate and multivariable models. Neither radiographic measure of esophageal dilation was associated with the change in radiographic measures of lung involvement.
Conclusion:
Severity of reflux symptoms as measured by an SSc-specific questionnaire was independently associated with the change in the radiographic extent of ILD and fibrosis over two years in patients with SSc-ILD. Two objective measures of esophageal dilation were not associated with radiographic progression of ILD, highlighting the need for improved objective measures of esophageal dysfunction in SSc.
The majority of patients with systemic sclerosis (SSc) (approximately 90%) have some form of esophageal involvement [1, 2]. Symptoms of esophageal involvement (e.g., heartburn from gastroesophageal reflux disease [GERD], dysphagia, odynonphagia, chronic cough, regurgitation and hoarseness) often arise early in the course of SSc and can progressively worsen over time [3]. The findings of studies in SSc [4–7] and other disease states, such as idiopathic pulmonary fibrosis (IPF) [8], have suggested an association between microaspiration due to esophageal reflux and interstitial lung disease (ILD).
ILD is the leading cause of death in SSc [9]. Cross-sectional studies have demonstrated an association between esophageal involvement and severity of SSc-ILD [10–13]. For example, a retrospective study of 79 patients with SSc demonstrated that patients with a complete loss of (absent) contractility on high-resolution manometry (N=40) had a significantly lower forced vital capacity (FVC) and diffusing capacity for carbon monoxide (DLCO) than patients with either measurable, but ineffective esophageal motility, or normal motility [10].
The cross-sectional nature of the aforementioned studies makes it challenging to conclude whether the findings reflect an actual causal relationship between esophageal involvement and the presence/severity of SSc-ILD. While the microaspiration theory seems plausible, it is also conceivable that the evolution of esophageal disease and ILD occurs in parallel and is an overall marker of a more severe SSc phenotype.
To address the limitations of prior cross-sectional reports, Scleroderma Lung Study (SLS) II prospectively collected patient reported outcomes related to gastrointestinal symptoms and high-resolution computerized tomography (HRCT) images of the chest, from which quantitative esophageal measurements could be abstracted [14]. All participants of SLS II received treatment for their underlying ILD with either mycophenolate (MMF, administered twice daily for 2 years) or oral cyclophosphamide (CYC, administered daily for 12 months followed by 12 months of placebo) and underwent serial assessments of spirometry and a repeat HRCT chest scan at 2 years [14]. Using three separate measures of esophageal involvement (patient-reported symptoms, quantitative radiographic assessment of maximum esophageal diameter and area), the present study hypothesized that increased severity of esophageal involvement is associated with progression of ILD independent of baseline severity of ILD and proton-pump inhibitor (PPI) use.
PATIENTS AND METHODS
Patient population
SLS II was an NIH-funded, multi-center, randomized controlled trial (RCT) that enrolled adults with SSc (≥18 years) with a disease duration of less than or equal to 7 years from the onset of the first non-Raynaud symptom attributable to SSc. Eligible patients had active ILD based on the presence of restrictive ventilatory impairment (FVC <80–85% but ≥45% predicted) and the presence of any ground glass opacity (GGO; hazy opacity through which normal lung markings can be discerned) on HRCT [14]. Key exclusion criteria included pulmonary hypertension requiring treatment, clinically significant abnormalities on HRCT not attributable to SSc, smoking within the past 6 months, and evidence of significant airflow obstruction. The study was approved by the Office of Human Research Protection Program at UCLA (IRB#11–002659-CR-00005) and by the IRBs of all 14 participating centers.
Study Design
SLS II participants were randomized to treatment with either oral CYC for one year followed by one year of placebo or MMF for 2 years [14]. The primary endpoint for the study was the course of the FVC% predicted measured every 3 months over 2 years. A key secondary endpoint was the change in quantitative radiographic fibrosis at 2 years. A Computer Aided Design (CAD) scoring system was employed to quantitatively assess different patterns and extent of ILD as previously described [15]. The present study investigated the change (from baseline to two years) in quantitative lung fibrosis (QLF) in both the whole lung (WL) and lobe of maximum involvement (LM), as well as the change in the total quantitative extent of ILD (QILD) in the LM and WL. The QILD score includes the sum of all abnormally classified scores, including scores for QLF, GGO and honeycomb changes.
To evaluate patient reported respiratory symptoms, the following patient-reported outcomes (PRO) measures were used: Baseline and Transitional Dyspnea Index, (BDI) [16] and TDI [17], the Leicester Cough Questionnaire (LCQ) [18] and the St. George’s Respiratory Questionnaire (SGRQ) [19]. Each targets a unique aspect of patients’ perception of respiratory symptoms, such as breathlessness (BDI/TDI), chronic cough (LCQ), overall health and well-being related to respiratory disease (SGRQ). The complete details of the SLS II protocol and these PROs appear in the supplementary web appendix accompanying the main SLS II publication [14].
GERD Symptoms and Treatment
The UCLA Scleroderma Clinical Trials Consortium Gastrointestinal Tract (SCTC-GIT) 2.0 [20] was used to assess severity of SSc-related gastrointestinal tract symptoms at baseline. The UCLA SCTC-GIT 2.0 is a valid, patient-reported outcome measure that has been translated into several languages. Among the 7 scales, the reflux scale can discriminate between patients with and without objective evidence of upper gastrointestinal tract involvement attributable to SSc [21] and is sensitive to change in SSc patients with active GERD [22].
At baseline, the type, dosage and frequency of proton pump inhibitor (PPI) and pro-motility agents used were collected by individual site investigators. Patients were categorized as consumers of high dose PPIs based on previously published definitions [23] (Supplementary Table 1).
Quantitative Radiographic Assessment of Esophageal Dilation
Quantitative image analysis was used to calculate both the diameter and the area of the esophagus in the area of maximum esophageal dilation. The esophagus was contoured using a semi-automated technique on the image slice with the maximum dilatation. Following review and confirmation of the accuracy of the contour, the maximum bidimensional diameters and area were calculated.
Statistical Analysis
Summary statistics were generated for baseline characteristics. A two-sample t-test or Wilcoxon rank-sum test was used to compare continuous variables, and a chi-square test or Fisher’s exact test was used to compare categorical variables.
Pearson correlation coefficient was used to examine the relationship between baseline reflux score, esophageal diameter and esophageal area with the baseline extent of physiologic impairment (baseline FVC% predicted and DLCO% predicted), the baseline extent of radiographic lung disease (QLF and QILD), as well as baseline self-reported respiratory symptoms, modified Rodnan skin score (mRSS) and disease duration. Group differences in reflux scores (e.g., males versus females, limited versus diffuse cutaneous disease) were evaluated using a two-sample t-test.
Linear regression analysis was used to examine the relationship between baseline reflux score, esophageal diameter and esophageal area and the change (from baseline to two years) in radiographic fibrosis as measured by the QLF and QILD in the WL and LM. The multivariable linear regression analyses included the following additional pre-specified covariates: baseline QLF/QILD score, treatment arm, PPI use (Y/N). An exploratory multivariable linear regression analysis included the covariate of high dose PPI use (Y/N) in place of PPI use.
An inferential joint model was used to examine the relationship between baseline reflux score and the course of the FVC%-predicted over 2 years. The joint model consisted of a mixed effects model for longitudinal outcomes and a survival model. The survival model was included to handle non-ignorable missing FVC data due to premature treatment discontinuation, treatment failure or death (i.e., likely related to disease or treatment and therefore not random) [24]. Since missing data for FVC increased over time, all missing measurements for FVC were deemed missing not at random. Fixed effects for the longitudinal portion of the joint model included treatment assignment, baseline FVC%-predicted, baseline PPI use (Y/N), baseline reflux score and a time trend. The time trend was modeled by linear splines with knots at 12 and 21 months.
Statistical analyses were performed using SAS version 9.4 except for the joint modeling which was conducted in R. P-values of less than 0.05 were considered statistically significant. We did not correct for multiple hypothesis testing as these analyses were considered exploratory.
RESULTS
Baseline characteristics
The majority of participants in SLS II (N=142) were women (74%) with a mean age of 52 years and a mean disease duration of 2.6 years [14]. Participants had a moderate degree of ventilatory restriction on pulmonary function testing (mean FVC% predicted of 66.5%) and a mean extent of QLF-WL of 9% and QILD-WL of 30% (Table 1). The mean score for the reflux scale was 0.57 (N=141), indicating moderate reflux.
Table 1.
Baseline characteristics of study participants
| Variable | CYC (N=73) | MMF (N=69) |
|---|---|---|
| Age (years)- Mean (SD) | 52.0 (9.8) | 52.6 (9.7) |
| Female- % | 78.1% | 69.6% |
| SSc Duration (years)- Mean (SD) | 2.5 (1.8) | 2.6 (1.7) |
| Diffuse SSc- % | 54.8% | 62.3% |
| FVC % Predicted- Mean (SD) | 66.5 (9.9) | 66.5 (8.3) |
| DLCO % Predicted- Mean (SD) | 54.1 (14.1) | 54.0 (11.1) |
| MRSS- Mean (SD) | 14.0 (10.6) | 15.3 (10.4) |
| GIT 2.0 Reflux Score- Mean (SD) | 0.5 (0.1) | 0.6 (0.1) |
| PPI Use- % | 78.1% | 79.7% |
| QLF, % WL- Mean ± SD | 8.4 ± 7.3 | 8.9 ± 7.1 |
| QILD, % WL- Mean ± SD | 30.8 ± 14.4 | 29.3 ± 14.1 |
Abbreviations: CYC=cyclophosphamide; MMF=mycophenolate; SSc=systemic sclerosis; FVC=forced vital capacity; DLCO=diffusing capacity for carbon monoxide; mRSS=modified Rodnan skin score; PPI=proton pump inhibitor; QLF=quantitative lung fibrosis; QILD=quantitative interstitial lung disease; WL=whole lung.
Seventy-six percent (N=107) of all participants were taking a PPI at baseline. Among them, 36 participants reported taking high dose PPIs. PPI use varied significantly based on the severity of reflux (95% with severe to very severe reflux symptoms; 82% with moderate reflux symptoms; 65% with none to mild symptoms; P=0.007). Only four participants were taking a pro-motility agent, all of whom were taking metoclopramide.
Radiographic esophageal parameters
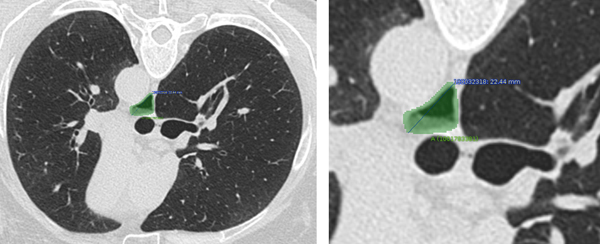
Baseline mean maximal esophageal diameter and area were 22 (SD 8.16; Range 6.30 to 51.60) mm and 242 (SD 161.07; Range 20.60 to 906.59) mm2, respectively (N=121). Figure 1 provides an example of how the esophagus was contoured and the maximum bidimensional diameter and area were calculated. Neither maximum esophageal diameter (r= 0.08, P=0.21), nor esophageal area (r=0.11; P=0.10) correlated with reflux scores.
Figure 1. Example of CAD approach to quantifying the widest esophageal diameter and maximal esophageal area.
In this SLS II subject, the widest esophageal diameter was 22.44 mm and the maximum esophageal area was 214.31 mm.
Esophageal involvement, ILD severity and SSc disease features
At baseline, no significant correlations were observed between the reflux score and measures of ILD severity (FVC%-predicted, DLCO%-predicted, QLF-LM/WL, QILD-LM/WL, GGO-WL), with the exception of a relatively weak positive correlation with GGO-LM (r=0.13; P=0.02). Baseline reflux score was significantly associated with self-reported dyspnea and cough, as measured by the BDI (r= −0.28, P=0.0009), SGRQ (r=0.38, P<0.0001), and LCQ (r= −0.31, P=0.0002). No significant associations were observed between reflux score and mRSS, disease duration, sex or SSc cutaneous subtype (i.e., limited versus diffuse cutaneous disease).
Similarly, no correlations were observed between esophageal area/diameter and any of the aforementioned measures of ILD severity (e.g., FVC, DLCO, QLF, QILD, GGO), self-reported respiratory symptoms, or SSc disease features (e.g, mRSS, cutaneous subtype, sex).
Esophageal involvement and change in radiographic measures of ILD over 24 months
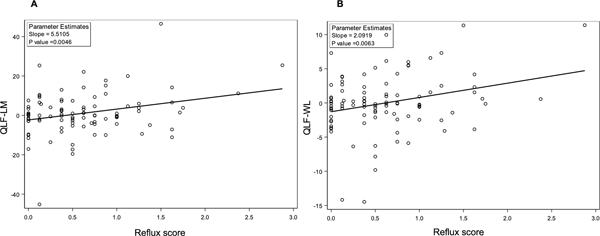
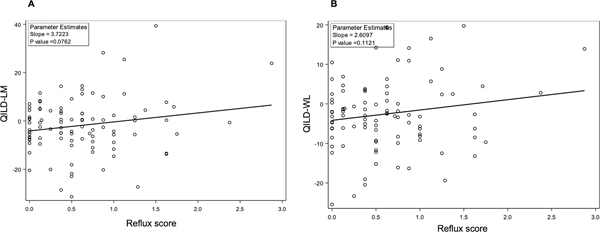
On univariate analysis, reflux score at baseline was positively correlated with the change in QLF-LM (Regression coefficient 5.51; P=0.0046) and QLF-WL (Regression coefficient 2.09; P=0.0063) at 2 years (Figure 2). In other words, worse reflux scores at baseline were associated with either less improvement or relative progression of the fibrotic measure of ILD on HRCT. The reflux score at baseline was also associated with the change in QILD-LM (Regression coefficient 3.72; P=0.076) and QILD-WL (Regression coefficient 2. 61; P=0.11) at 2 years (Figure 3), although these associations did not reach the pre-defined threshold for statistical significance (P<0.05). On univariate analysis, neither the esophageal diameter or area were significantly associated with change in QLF-WL, QLF-LM, QILD-WL, QILD-LM at 2 years (all P-values ≥0.2).
Figure 2. Increased reflux score at baseline is associated with increased progression of lung fibrosis in the lobe of maximum involvement (QLF-LM; Panel A) and in the whole lung (QLF-WL; Panel B) over 24 months.
Figure 3. Increased reflux score at baseline is associated with increased progression of ILD in the lobe of maximum involvement (QILD-LM; Panel A) and in the whole lung (QILD-WL; Panel B) over 24 months.
In the multivariable analysis, which adjusted for baseline QLF score, treatment arm and PPI use, the reflux score remained positively associated with the change in QLF-LM (Estimate 5.63; P=0.007) and QLF-WL (Estimate 2.19; P=0.005) at 2 years (Table 2). Reflux score at baseline was also associated the change in QILD-WL (Estimate 3.45; P=0.039) at 2 years, after adjusting for baseline QILD score, treatment arm and PPI use (Table 3). Substituting “high dose PPI” use as a covariate for “any PPI” use in the multivariable models did not affect the relationship between reflux score and the change in radiographic fibrosis or ILD scores (Supplementary Tables 2 and 3). No significant associations were observed between PPI use or high dose PPI use and changes in QLF or QILD in any of the multivariable models. No significant associations were observed between esophageal diameter or area and changes in QLF or QILD in any of the multivariable models. Furthermore, no significant interaction terms were observed between reflux score and PPI use in any of the multivariable models.
Table 2.
Baseline reflux score is associated with worsening QLF-LM and QLF-WL at 2 years in multivariable analysis
| Outcome: Change in QLF-LM | |||
|---|---|---|---|
|
| |||
| Estimate | 95% CI | P-value | |
| Baseline QLF-LM | −0.05 | −0.16, 0.04 | 0.271 |
| Treatment arm (CYC) | −0.77 | −5.04, 3.50 | 0.721 |
| Baseline reflux score | 5.63 | 1.63, 9.62 | 0.006 |
| PPI use (Yes) | −1.87 | −7.07, 3.33 | 0.477 |
| Outcome: Change in QLF-WL | |||
|
| |||
| Estimate | 95% CI | P-value | |
| Baseline QLF-WL | −0.18 | −0.30, −0.07 | 0.002 |
| Treatment arm (CYC) | −0.05 | −1.67, 1.57 | 0.951 |
| Baseline reflux score | 2.19 | 0.69, 3.69 | 0.005 |
| PPI use (Yes) | −0.26 | −2.33, 1.81 | 0.807 |
Abbreviations: CYC=cyclophosphamide; PPI=proton pump inhibitor; QLF=quantitative lung fibrosis; WL=whole lung.
Table 3.
Baseline reflux score is associated with worsening QILD-LM and QILD-WL at 2 years in multivariable analysis
| Outcome: Change in QILD-LM | |||
|---|---|---|---|
|
| |||
| Estimate | 95% CI | P-value | |
| Baseline QILD-LM | −0.05 | −0.16, 0.06 | 0.341 |
| Treatment arm (CYC) | −0.29 | −4.99, 4.40 | 0.902 |
| Baseline reflux score | 3.46 | −0.91, 7.83 | 0.119 |
| PPI use (Yes) | 1.63 | −4.08, 7.34 | 0.571 |
| Outcome: Change in QILD-WL | |||
|
| |||
| Estimate | 95% CI | P-value | |
| Baseline QILD-WL | −0.23 | −0.36, −0.09 | 0.001 |
| Treatment arm (CYC) | 1.09 | −2.45, 4.63 | 0.541 |
| Baseline reflux score | 3.45 | 0.18, 6.72 | 0.039 |
| PPI use (Yes) | 0.53 | −5.00, 3.95 | 0.815 |
Abbreviations: CYC=cyclophosphamide; QILD=quantitative interstitial lung disease; WL=whole lung.
In the joint model analysis, baseline reflux score was not independently associated with the course of the FVC%-predicted over 24 months (P=0.72), nor was PPI use (P=0.63) (Supplementary Table 4).
DISCUSSION
Identifying factors that predict ILD progression is central to risk stratification and management of patients with SSc. The current post-hoc analysis was conceived to determine whether baseline measures of esophageal disease might aid in the prediction of physiological and radiological changes over time in patients with SSc-ILD.
This study found that the severity of reflux symptoms at baseline was significantly associated with self-reported dyspnea and cough at baseline, but not with baseline measures of ILD as assessed by either quantitative image analysis or FVC. The observed relationship between reflux symptoms and respiratory symptoms is an interesting observation and may reflect the overlap of symptoms attributable to both ILD and reflux (e.g., cough). Indeed, in our prior study, we discovered that cough improved over the course of SLS II in parallel to improvements in both ILD and in reflux [25].
While studies have previously demonstrated a cross-sectional relationship between esophageal dysfunction and ILD severity in SSc [11, 13], this study provided a unique opportunity to examine how the severity of esophageal reflux at baseline is related to radiographic ILD progression over time in a clinical trial cohort receiving standardized therapy with MMF or CYC. Patients reporting increased symptoms of reflux at baseline had worse radiographic ILD outcomes, despite treatment with MMF or CYC. Another important observation was that the relationship between reflux symptoms and radiographic changes over time was independent of baseline ILD severity and PPI use. Finally, even though esophageal diameter and area have been proposed as independent objective measures of SSc-associated involvement, this study did not observe a significant relationship between radiographic measures of esophageal dilation and reflux symptoms, nor ILD progression, suggesting that symptoms may serve as a better measure of esophageal involvement in SSc than these specific objective tests. Evaluating symptoms using a valid questionnaire also represents a more patient-centric approach to approximating the burden of esophageal disease in SSc.
Interestingly, while the present study did not observe a significant relationship between baseline reflux symptoms and baseline ILD severity using radiographic and physiologic measures of ILD severity, a weak correlation was found between baseline reflux score and the extent of GGO in the lobe of maximal involvement, which may be related to aspiration events. These findings could suggest that aspiration due to esophageal reflux may perpetuate pulmonary inflammation and fibrosis in SSc; however, additional, prospective studies are needed to test this hypothesis.
The key finding that the severity baseline reflux symptoms was associated with either less improvement or relative progression of the fibrotic measure of ILD on HRCT warrants further evaluation. As mentioned above, it is unclear whether these two dimensions of SSc (e.g., esophageal dysfunction and ILD) progress in parallel to one another, or whether the esophageal dysfunction itself is a driver of ILD pathogenesis. Both treatment arms in this study experienced net improvements in lung function and radiographic measures of ILD over time. The fact that self-reported reflux scores did not change significantly over the course of SLS II [26] could point to a causal relationship; however, future longitudinal studies are needed which include patients with very early SSc, prior to the onset of ILD. Such a study could evaluate the relationship between baseline reflux symptoms and the future development of ILD.
It is interesting that the severity of baseline reflux symptoms was not associated with physiological progression of SSc-ILD, as measured by the FVC%-predicted. This finding is in line with a recent study, which also observed no relationship between esophageal dilation on HRCT and future FVC decline in patients with SSc [27]. These observations could be due to the fact that FVC measurement may reflect multiple behavioral and physiological processes independent from parenchymal lung disease (e.g., patient effort, respiratory muscle weakness and chest wall fibrosis).
While prior studies have demonstrated a relationship between the widest esophageal diameter in SSc and ILD severity [13, 28], this study did not observe a relationship between widest esophageal diameter and any measure of ILD severity at baseline. These findings are consistent with an earlier study, which also found no relationship between radiographic extent of ILD and esophageal dilation [29]. In addition, the present study observed no significant relationship between widest esophageal diameter or maximal esophageal area and severity of reflux symptoms or the progression of SSc-ILD. While the reasons for these findings are unknown, it is conceivable that these simple metrics of esophageal dilation are dynamic measures that can vary based on various factors, such as what a patient ate prior to the HRCT or whether their stomach is full at the time of the HRCT. It is also possible that functional esophageal studies (e.g., manometry) more accurately capture the breadth of esophageal involvement in SSc than a measure of esophageal dilation at a single point in time. These findings have important clinical implications as they suggest that relying solely on HRCT visualization to examine the extent of esophageal involvement at a single time point in SSc may be misleading.
Another important finding of this study was that neither PPI use, nor the interaction between PPI use and baseline reflux score, was associated with ILD progression. To our knowledge, no studies have previously examined whether PPI use affects the course of ILD in patients with SSc. In IPF, post-hoc analyses of large RCTs reported that baseline exposure to antacid drugs was not significantly associated with progression-free survival over 52 weeks [30, 31]. The present study suggests that PPI use does not play a disease-modifying role in SSc-ILD, although it is important to note that the vast majority of patients in the study were taking PPIs; thus, there may not have been adequate power to detect significant differences in ILD progression based on PPI use. Furthermore, patients were not randomized to PPI use in this study.
Additional limitations of the present study include the possibility that patients were not consistently adherent to their reported PPI dosing schedule. While the dose and frequency of PPI use remained relatively the same within subjects throughout the study, patients often self-increase or decrease their PPI dosage based on symptoms and lifestyle choices on a given day. Another short-coming was that this study did not include a functional measure of esophageal involvement, such as manometry. Since the purpose of SLS II was to compare the safety and efficacy of CYC and MMF for SSc-ILD, including such assessments were beyond the scope of the study. However, future studies are needed to evaluate whether functional measures of esophageal involvement, with or without radiographic assessment, could predict ILD outcomes in SSc. Furthermore, since these analyses were post-hoc, there are likely confounding factors that could affect the primary outcome. Finally, given the exploratory nature of these analyses, we did not correct for multiple hypothesis testing.
Strengths of the present study include the prospective design, the use of different metrics for defining ILD progression (radiographical, physiological metrics), the uniform collection of study data, and the ability to adjust for ILD treatment, PPI use and ILD severity in the multivariable models. Another strength was the use of a fully quantitative assessment method for objectively calculating the widest esophageal diameter and maximal esophageal area.
In conclusion, this study demonstrated that among patients with SSc-ILD, the severity of esophageal symptoms is associated with future radiographic ILD progression, even in the presence of PPI therapy. Future studies are needed to determine whether other treatments aimed at reducing aspiration events due to GERD (e.g., pro-motility agents), could improve outcomes for patients with SSc-ILD. Optimizing the treatment of esophageal involvement in SSc may not only provide symptomatic relief for patients, but it may also represent a life-extending measure for patients with this often-fatal disease.
Supplementary Material
Significance and Innovations.
The relationship between gastroesophageal reflux disease (GERD) and interstitial lung disease progression (ILD) in systemic sclerosis (SSc) is unclear. The present study is the first to demonstrate that increased symptoms of GERD are associated with worsening SSc-ILD, even after controlling for baseline ILD disease severity and proton pump inhibitor use.
Esophageal dilation is observed on high resolution computed tomography (HRCT) of the chest in the majority of patients with SSc. It is unknown whether the severity of esophageal dilation is associated with SSc-ILD progression. The present study found no relationship between baseline esophageal diameter or area and radiographic progression of ILD, suggesting that additional objective metrics of esophageal dilation and dysfunction in SSc are needed.
The findings of the present study suggest that patient-reported symptoms of GERD are associated with future SSc-ILD progression. Optimizing the treatment of GERD may lead to improved outcomes in patients with SSc-ILD.
Acknowledgements
The authors thank the patients and investigators for their participation in this study and acknowledge the following funding sources: NHLBI (K23 HL150237-01 [ERV]; U01 HL 60587 [DPT] and U01 HL 60606 [RME]. Hoffmann-LaRoche supplied mycophenolate mofetil for use in SLS II. For a list of all persons and institutions who participated in SLS I and II please see the Appendix.
Funding:
NIH/NHLBI: K23 HL150237 (ERV), R01 HL089758 (DPT), R01 HL089901 (RME). Hoffmann-LaRoche supplied mycophenolate mofetil for use in Scleroderma Lung Study II.
Financial support and interests:
None of the authors reports any financial support or interests that represent a conflict of interest with regard to the work. Outside of the scope of this work, ERV reports consulting fees from Boehringer Ingelheim and institutional grant support from Kadmon, Horizon, Corbus, Forbius and Boehringer Ingelheim; MDR reports institutional grant support from Genentech/Roche; JG reports founder of MedQIA LLC.
References
- 1.Kröner PT, Tolaymat OA, Bowman AW, et al. Gastrointestinal Manifestations of Rheumatological Diseases. Am J Gastroenterol 2019;114:1441–54. [DOI] [PubMed] [Google Scholar]
- 2.Li B, Yan J, Pu J, et al. Esophageal Dysfunction in Systemic Sclerosis: An Update. Rheumatol Ther 2021;8:1535–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McFarlane IM, Bhamra MS, Kreps A, et al. Gastrointestinal Manifestations of Systemic Sclerosis. Rheumatology (Sunnyvale) 2018;8:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bütikofer S, Jordan S, Sauter M, et al. Abnormal esophageal motility during a solid test meal in systemic sclerosis-detection even in very early disease and association with disease progression. Neurogastroenterol Motil 2019;31:e13480. [DOI] [PubMed] [Google Scholar]
- 5.Savarino E, Bazzica M, Zentilin P, et al. , Gastroesophageal reflux and pulmonary fibrosis in scleroderma: a study using pH-impedance monitoring. Am J Respir Crit Care Med 2009;179:408–13. [DOI] [PubMed] [Google Scholar]
- 6.Marie I, Dominique S, Levesque H, et al. Esophageal involvement and pulmonary manifestations in systemic sclerosis. Arthritis Rheum 2001;45:346–54. [DOI] [PubMed] [Google Scholar]
- 7.Christmann RB, Wells AU, Capelozzi VL, Silver RM. Gastroesophageal reflux incites interstitial lung disease in systemic sclerosis: clinical, radiologic, histopathologic, and treatment evidence. Semin Arthritis Rheum 2010;40:241–9. [DOI] [PubMed] [Google Scholar]
- 8.Ghisa M, Marinelli C, Savarino V, Savarino E. Idiopathic pulmonary fibrosis and GERD: links and risks. Ther Clin Risk Manag 2019;15:1081–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Volkmann ER, Fischer A. Update on Morbidity and Mortality in Systemic Sclerosis-Related Interstitial Lung Disease. J Scleroderma Relat Disord 2021;6:11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kimmel JN, Carlson DA, Hinchcliff M, et al. The association between systemic sclerosis disease manifestations and esophageal high-resolution manometry parameters. Neurogastroenterol Motil 2016;28:1157–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuribayashi S, Motegi SI, Hara K, et al. Relationship between esophageal motility abnormalities and skin or lung involvements in patients with systemic sclerosis. J Gastroenterol 2019;54:950–62. [DOI] [PubMed] [Google Scholar]
- 12.Takekoshi D, Arami S, Sheppard TJ, et al. Computed tomography of the esophagus in scleroderma and lung disease. Tohoku J Exp Med 2015;237:345–52. [DOI] [PubMed] [Google Scholar]
- 13.Salaffi F, Di Carlo M, Carotti M, et al. Relationship between interstitial lung disease and oesophageal dilatation on chest high-resolution computed tomography in patients with systemic sclerosis: a cross-sectional study. Radiol Med 201;123:655–63. [DOI] [PubMed] [Google Scholar]
- 14.Tashkin DP, Roth MD, Clements PJ, et al. ; Sclerodema Lung Study II Investigators. Mycophenolate mofetil versus oral cyclophosphamide in scleroderma-related interstitial lung disease (SLS II): a randomised controlled, double-blind, parallel group trial. Lancet Respir Med 2016;4:708–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim HG, Tashkin DP, Clements PJ, et al. A computer-aided diagnosis system for quantitative scoring of extent of lung fibrosis in scleroderma patients. Clin Exp Rheumatol 2010;28:S26–35. [PMC free article] [PubMed] [Google Scholar]
- 16.Mahler DA, Weinberg DH, Wells CK, Feinstein AR. The measurement of dyspnea. Contents, interobserver agreement and physiologic correlates of two new clinical indexes. Chest 1984;85:751–758. [DOI] [PubMed] [Google Scholar]
- 17.Mahler DA, Ward J, Fierro-Carrion G, et al. Development of self-administered versions of modified baseline and transition dyspnea indexes in COPD. J of COPD 2004;1:1–8. [DOI] [PubMed] [Google Scholar]
- 18.Birring SS, Prudon B, Carr AJ, et al. Development of a symptom specific health status measure for patients with chronic cough: Leicester Cough Questionnaire (LCQ). Thorax 2003;58:339–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beretta L, Santaniello A, Lemos A, et al. Validity of the Saint George’s Respiratory Questionnaire in the evaluation of the health-related QoL in patients with interstitial lung disease secondary to systemic sclerosis. Rheumatology (Oxford) 2007;46:296–301. [DOI] [PubMed] [Google Scholar]
- 20.Khanna D, Hays RD, Maranian P, et al. Reliability and validity of the University of California, Los Angeles Scleroderma Clinical Trial Consortium Gastrointestinal Tract Instrument. Arthritis Rheum 2009;61:1257–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bae S, Allanore Y, Furst DE, et al. Associations between a scleroderma-specific gastrointestinal instrument and objective tests of upper gastrointestinal involvements in systemic sclerosis. Clin Exp Rheumatol 2013;31:57–63. [PubMed] [Google Scholar]
- 22.McMahan ZH, Frech T, Berrocal V, et al. Longitudinal Assessment of Patient-reported Outcome Measures in Systemic Sclerosis Patients with Gastroesophageal Reflux Disease - Scleroderma Clinical Trials Consortium. J Rheumatol 2019;46:78–84. [DOI] [PubMed] [Google Scholar]
- 23.Hendrix I, Page AT, Korhonen MJ, et al. Patterns of High-Dose and Long-Term Proton Pump Inhibitor Use: A Cross-Sectional Study in Six South Australian Residential Aged Care Services. Drugs Real World Outcomes 2019;6:105–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li N, Elashoff RM, Li G, Tseng CH. Joint analysis of bivariate longitudinal ordinal outcomes and competing risks survival times with nonparametric distributions for random effects. Stat Med 2012;31:1707–21. [DOI] [PubMed] [Google Scholar]
- 25.Tashkin DP, Volkmann ER, Tseng CH, et al. Improved Cough and Cough-Specific Quality of Life in Patients Treated for Scleroderma-Related Interstitial Lung Disease: Results of Scleroderma Lung Study II. Chest 2017;151:813–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Volkmann ER, Tashkin DP, LeClair H, et al. Treatment With Mycophenolate and Cyclophosphamide Leads to Clinically Meaningful Improvements in Patient-Reported Outcomes in Scleroderma Lung Disease: Results of Scleroderma Lung Study II. ACR Open Rheumatol 2020;2:362–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Showalter K, Hoffmann A, Richardson C, et al. Esophageal Dilation and Other Clinical Factors Associated with Pulmonary Function Decline in Patients With Systemic Sclerosis. J Rheumatol 2021;48:1830–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richardson C, Agrawal R, Lee J, et al. Esophageal dilatation and interstitial lung disease in systemic sclerosis: A cross-sectional study. Semin Arthritis Rheum 2016;46:109–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pandey AK, Wilcox P, Mayo JR, et al. Oesophageal dilatation on high-resolution CT chest in systemic sclerosis: what does it signify? J Med Imaging Radiat Oncol 2011;55:551–5. [DOI] [PubMed] [Google Scholar]
- 31.Kreuter M, Spagnolo P, Wuyts W, et al. Antacid Therapy and Disease Progression in Patients with Idiopathic Pulmonary Fibrosis Who Received Pirfenidone. Respiration 2017;93:415–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kreuter M, Wuyts W, Renzoni E, et al. Antacid therapy and disease outcomes in idiopathic pulmonary fibrosis: a pooled analysis. Lancet Respir Med 2016;4:381–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.