Highlights
-
•
GLP1-RA have been shown to reduce ASCVD in cardiovascular outcome trials.
-
•
Effects of GLP1-RA on potential kidney outcomes are currently being explored.
-
•
Ongoing trials focus on GLP1-RA MOA and effect on kidney inflammation and perfusion.
-
•
GLP1-RA may offer protection via effects on natriuresis, diuresis, oxidative stress.
Keywords: Cardiovascular disease, Chronic kidney disease, Diabetic kidney disease, GLP1-RA, Type 2 diabetes
Abstract
Importance
Atherosclerotic cardiovascular disease (ASCVD) remains the leading cause of morbidity and mortality for patients with type 2 diabetes (T2D) and chronic kidney disease (CKD). However, testing for albuminuria among patients with T2D is substantially underutilized in clinical practice; many patients with CKD go unrecognized. For patients with T2D at high cardiovascular risk, or with established CVD, the glucagon-like peptide-1 receptor agonists (GLP1-RA) have been shown to reduce ASCVD in cardiovascular outcome trials, while potential kidney outcomes are being explored.
Observations
A recent meta-analysis found that GLP1-RA reduced 3-point major adverse cardiovascular events by 14% [HR, 0.86 (95% CI, 0.80–0.93)] in patients with T2D. The benefits of GLP1-RA to reduce ASCVD were at least as large among people with estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2. GLP1-RA also conferred a 21% reduction in the composite kidney outcome [HR, 0.79 (0.73–0.87)]; however, this result was achieved largely through reduction in albuminuria. It remains uncertain whether GLP1-RA would confer similar favorable results for eGFR decline and/or progression to end-stage kidney disease. Postulated mechanisms by which GLP1-RA confer protection against CVD and CKD include blood pressure lowering, weight loss, improved glucose control, and decreasing oxidative stress. Ongoing studies in T2D and CKD include a kidney outcome trial with semaglutide (FLOW, NCT03819153) and a mechanism of action study (REMODEL, NCT04865770) examining semaglutide's effect on kidney inflammation and fibrosis. Ongoing cardiovascular outcome studies are examining an oral GLP1-RA (NCT03914326), GLP1-RA in patients without T2D (NCT03574597), and dual GIP/GLP1-RA agonists (NCT04255433); the secondary kidney outcomes of these trials will be informative.
Conclusions and relevance
Despite their well-described ASCVD benefits and potential kidney protective mechanisms, GLP1-RA remain underutilized in clinical practice. This highlights the need for cardiovascular clinicians to influence and implement use of GLP1-RA in appropriate patients, including those with T2D and CKD at higher risk for ASCVD.
1. Introduction
People with diabetes are at heightened risk for developing adverse cardiovascular outcomes, compared with people without diabetes. Cardiovascular disease (CVD) remains the leading cause of death among people with diabetes, with their risk of death from coronary heart disease being almost 3-fold higher than that of people without diabetes [1]. The difference in life expectancy free of CVD for men and women aged ≥50 years with diabetes is 7.8 and 8.4 years shorter, respectively, compared with people without diabetes [2]. The risk of mortality is even greater when both diabetes and atherosclerotic CVD (ASCVD) are present, conferring 12 to 15 years of reduced life expectancy [3].
People with diabetes are also at increased risk of kidney failure, with approximately 40% developing chronic kidney disease (CKD) [4]. Furthermore, the presence of CKD among people with diabetes substantially increases the risk of developing CVD complications. Nearly two-thirds of adults aged ≥65 years with CKD also have CVD, a rate that is nearly double that of similarly aged adults without CKD [4]. Cardiovascular disease, rather than end-stage kidney disease (ESKD), remains the leading cause of death in the CKD population. The pro-inflammatory state of CKD can lead to deterioration of cardiovascular function through multiple mechanisms, such as propagation of atherosclerosis, vascular and valvular calcification, and myocardial fibrosis [5]. In patients with both diabetes and CKD, the adjusted risk difference for cardiovascular mortality is 16% (95% CI, 11%−21%), compared with the risks conferred by diabetes alone (3%) or CKD alone (6%) [6]. Thus, it is paramount to implement preventive strategies among patients with diabetes that confer both cardiovascular and kidney benefits.
1.1. Recognizing CKD
The first step in implementing such preventive interventions is to recognize when CKD is present at an early stage in the disease process. Notably, CKD can be defined by either, or both, a reduced estimated glomerular filtration rate (eGFR) of <60 mL/min/1.73 m2 for 3 months or more, regardless of underlying cause, and/or by presence of albuminuria defined as a urine albumin-to-creatinine ratio (UACR) ≥30 mg/g in at least 2 spot urine specimens [7]. Both a reduction in eGFR and the presence of albuminuria are independently associated with an increased risk of CVD and mortality in a graded fashion [8,9]. Therefore, the American Diabetes Association (ADA) has endorsed measuring both eGFR and UACR annually in patients with type 2 diabetes (T2D); however, this is sub-optimally done, particularly the UACR assessment. In a recent analysis of electronic health record data from 1164 clinical practices including over 500,000 adults with T2D in primary care clinics, the testing for eGFR was 89.5%, UACR 52.9%, and only 51.6% for both tests at a median of 1 year [10]. Thus, without intentional assessment for albuminuria, many patients with diabetic kidney disease (DKD) go unrecognized.
1.2. New treatment options for CVD and CKD
Progress has been made with therapeutic agents that can improve both cardiovascular and kidney outcomes among patients with DKD. For many years, the only agents that had demonstrated benefit in slowing kidney disease progression were the renin angiotensin aldosterone system blockers of angiotensin-converting enzyme inhibitors (ACEi) or angiotensin receptor blockers (ARBs) [11], [12], [13]. More recently, the sodium-glucose cotransporter-2 inhibitors (SGLT2i) have been shown to reduce both major adverse cardiovascular events (MACE), primarily, a reduction in heart failure hospitalizations and cardiovascular death (by ∼22%) and CKD progression (by ∼38%) among patients with T2D. Notably, these effects occurred in patients with or without ASCVD [14]. Furthermore, SGLT2i have been studied specifically among patients with CKD with or without T2D in 3 completed primary kidney outcome trials (CREDENCE[15], DAPA-CKD[16], and EMPA-KIDNEY[17]), which showed benefit for both a significant reduction in the primary kidney outcomes and cardiovascular outcomes [18,19]. Importantly, the DAPA-CKD and EMPA-KIDNEY trials both enrolled patients with CKD with or without diabetes, and the EMPA-KIDNEY trial enrolled patients with an eGFR nadir as low as 20 mL/min/1.73 m2 [16,17]. This led to the 2022 Kidney Disease: Improving Global Outcomes (KDIGO) guideline for management of patients with both T2D and CKD recommending SGLT2i as first-line therapy for kidney and cardiovascular protection in patients with eGFR ≥20 mL/min/1.73 m2 [20,21].
Even more recently, the non-steroidal mineralocorticoid receptor antagonist (MRA) finerenone has been shown to confer both cardiovascular and kidney protection in patients with T2D and CKD, in both a dedicated kidney outcome trial and a dedicated cardiovascular outcome trial [22,23]. Notably, in the FIDELITY pre-specified pooled analysis of both these finerenone outcome trials, finerenone significantly reduced the composite cardiovascular outcome by 14%, which was driven by a 22% reduction in heart failure hospitalizations. Additionally, finerenone reduced the kidney outcome (a composite of kidney failure, a sustained decrease of at least 57% in the eGFR from baseline, or death from kidney causes) by 23% [24]. This benefit of finerenone was on the backdrop of ACEi/ARB therapy, although the use of SGLT2i in those trials was infrequent. The updated 2022 KDIGO statement further included a recommendation for the consideration of use of a non-steroidal MRA with proven kidney or cardiovascular benefits in patients with T2D, an eGFR ≥25 mL/min/1.73 m2, and UACR ≥30 mg/g [20].
Thus, ACEi/ARBs, SGLT2i, and finerenone all play important roles in kidney and cardiovascular protection among patients with DKD, but neither ACEi/ARBs nor finerenone treat blood glucose elevation, and the effect of SGLT2i on glucose lowering becomes diminished with eGFR <60 mL/min/1.73 m2. The DAPA-CKD and FIDELIO trials enrolled patients with eGFR ≥25 mL/min/1.73 m2 and the EMPA-KIDNEY trial enrolled patients with eGFR ≥20 mL/min/1.73 m2; results demonstrated that dapagliflozin, finerenone and empagliflozin are effective and safe in these patients, although safety data are lacking for initiating therapy below these thresholds [16,17,22]. However, if already initiated, these agents can be continued even if the eGFR dips below these thresholds up to the point of initiating kidney replacement therapy by dialysis or transplant.
Glucagon-like peptide-1 receptor agonists (GLP1-RA) are another class of treatment for T2D that have demonstrated cardiovascular benefits in large cardiovascular outcome trials (CVOTs), particularly for the reduction of 3-point MACE [25,26], and notably the reduction of stroke (the latter has not been shown for SGLT2i). The benefits of GLP1-RA to reduce 3-point MACE risk are at least as large even in the subset of patients with eGFR <60 mL/min/1.73 m2, similar to those with T2D with eGFR ≥60 mL/min/1.73 m2 [26]. Additionally, GLP1-RA confer more A1c lowering, lipid lowering, and weight loss than do SGLT2i irrespective of lower eGFR. The cardiovascular outcome and glycemic-lowering trials of GLP1-RA have included patients with T2D, with and without CKD, and with eGFR as low as 15 mL/min/1.73 m2.
As noted previously, SGLT2i are recommended as a first-line therapy for patients with T2D and CKD with an eGFR ≥20 mL/min/1.73 m2 [21], and they can be used alone or in combination with GLP1-RA. Due to the consistent reduction in heart failure hospitalizations with SGLT2i, patients with T2D and heart failure may benefit more from this therapy than from GLP1-RA. Alternatively, given the consistent favorable reductions in ASCVD with GLP1-RA, patients with T2D and ASCVD or high cardiovascular risk may benefit more from GLP1-RA than from SGLT2i. In addition, for patients with T2D and obesity, GLP1-RA are recommended to promote weight loss [21]. Importantly, GLP1-RA can be used in combination with SGLT2i, and many patients with DKD may benefit from both therapies although this has not yet been explored in detail in clinical trials [27,28]. The addition of GLP-1RA therapy to SGLT2i is recommended for patients who are looking to further reduce their HbA1c and/or albuminuria [21]. The combination therapy may also benefit unique patient populations, such as those with atherosclerosis, heart failure, and obesity. The diagnoses of heart failure, ASCVD, T2D, CKD, and/or obesity frequently overlap in clinical practice, and many high-risk patients would benefit from both SGL2i and GLP1-RA in combination.
While the ASCVD benefits of GLP1-RA have clearly been established in patients with T2D, there has been considerable interest in also identifying their potential role in DKD for kidney protection, given their favorable macrovascular and microvascular properties. Most of the CVOTs have also reported secondary kidney outcomes, although the potential kidney benefits are still being explored with a dedicated GLP1-RA kidney outcome trial ongoing (FLOW, NCT03819153) [29]. This review article will focus specifically on GLP1-RA therapy among patients with DKD, focusing on the potential for kidney as well as heart protection.
1.3. GLP1-RA mechanism of action
Glucagon-like peptide-1 (GLP1) is an intestinal hormone that plays an important role in glycemia regulation. It is an incretin hormone secreted from the intestine after ingestion of glucose or other food nutrients and stimulates release of insulin from the pancreatic islet cells in a glucose-dependent fashion [30]. GLP1 hormones can facilitate weight loss, by slowing gastric emptying and decreasing appetite stimulation in the brain. Patients with T2D have a reduced or absent natural incretin effect, and GLP1-RA mimic this pathway.
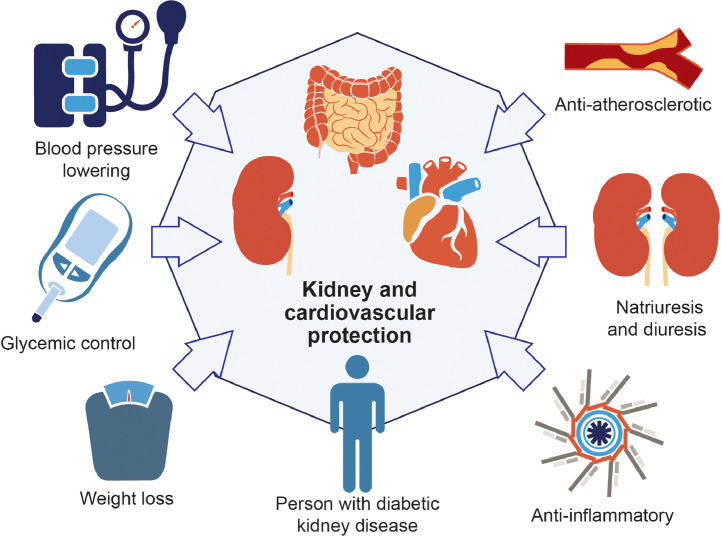
GLP1-RA may exert favorable effects on the kidneys through direct or indirect effects (Fig. 1/Central Illustration) [31], [32], [33], [34]. Direct effects include increasing natriuresis and diuresis, decreasing oxidative stress and inflammation, and possible glomerular hemodynamic effects. Specifically, GLP1-RA inhibit the sodium-hydrogen exchanger 3 in proximal tubular cells to ultimately increase natriuresis and diuresis [31]. In addition, GLP1-RA stimulate pathways responsible for reducing reactive oxygen species in the kidneys [31]. GLP1-RA also reduce inflammation by decreasing cytokine production and infiltration of immune cells [35]. Indirectly, GLP1-RA influence the kidneys via the above-mentioned weight loss and improved glucose control. GLP1-RA also lower blood pressure; the mechanisms underlying this effect are still under investigation[36] but may be partially attributed to the increase in natriuresis [31].
Fig. 1.
Potential mechanisms by which glucagon-like peptide-1 receptor agonists (GLP1-RA) confer kidney and cardiovascular protection.
1.4. GLP1-RA and cardiovascular outcomes
GLP1-RA have been shown to reduce MACE in patients with T2D with A1c elevation >6.5%, who were at high cardiovascular risk or had established CVD (Table 1) [37], [38], [39], [40], [41]. A 2019 meta-analysis confirmed the cardiovascular benefits of GLP1-RA with a 12% reduction in the risk of MACE in patients with T2D [hazard ratio (HR), 0.88 (95% CI, 0.82–0.94)] [25]. This meta-analysis included the 7 CVOTs of ELIXA (lixisenatide) [42], LEADER (liraglutide) [37], SUSTAIN-6 (subcutaneous semaglutide) [38], PIONEER 6 (oral semaglutide) [43], EXSCEL (exenatide) [44], HARMONY Outcomes (albiglutide) [39], and REWIND (dulaglutide) [40].
Table 1.
CV and kidney outcomes of GLP1-RA CVOT trials.
| Trial | Drug | Population | CV outcomes | Kidney outcomes | Reference |
|---|---|---|---|---|---|
| ELIXA | lixisenatide | Patients with T2D and recent MI or unstable angina | Noninferior to placebo for primary end point including CV death, non-fatal MI, non-fatal stroke, hospitalization for unstable angina | Reduced risk of developing macroalbuminuria | Pfeffer et al. [42] |
| LEADER | liraglutide | Patients with T2D and high CV risk |
|
Reduced risk of composite kidney outcomeb | Marso et al. [37] |
| SUSTAIN-6 | subcutaneous semaglutide | Patients with T2D and CVD, chronic heart failure, or CKD with a CV risk factor |
|
Reduced risk of composite kidney outcomec | Marso et al. [38] |
| PIONEER 6 | oral semaglutide | Patients ≥50 years old with CVD or CKD, patients ≥60 years old with CV risk factors |
|
Not available | Husain et al. [43] |
| EXSCEL | exenatide | Patients with T2D with or without CVD | Noninferior to placebo for primary end point including CV death, non-fatal MI, non-fatal stroke | Reduced risk of composite kidney outcomed | Holman et al. [44] |
| Harmony Outcomes | albiglutide | Patients ≥40 years old with CVD |
|
Not available | Hernandez et al. [39] |
| REWIND | dulaglutide | Patients ≥50 years old with T2D and CVD or CV risk factors |
|
Reduced risk of composite kidney outcomee Reduced risk of worsening kidney function |
Gerstein et al. [40] |
| AMPLITUDE-O | efpeglenatide | Patients with T2D and CVD or CKD |
|
Reduced risk of composite kidney outcomef | Gerstein et al. [41] |
| FLOW | subcutaneous semaglutide | Patients with T2D and CKD | Ongoing trial | Ongoing trial | NCT03819153[29] |
| NCT03819153 | |||||
| SOUL | oral semaglutide | Patients with T2D | Ongoing trial | Ongoing trial | NCT03914326[49] |
| NCT03914326 |
CV, cardiovascular; CKD, chronic kidney disease; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; MACE, major adverse cardiac events; MI, myocardial infarction; T2D, type 2 diabetes.
Three-point MACE was comprised of CV death, MI, and stroke.
Composite kidney outcome was comprised of development of macroalbuminuria, doubling of serum creatinine or an eGFR of ≤40 mL/min/1.73 m2, kidney replacement therapy, or death due to kidney disease.
Composite kidney outcome was comprised of persistent macroalbuminuria, doubling of serum creatinine or an eGFR of ≤45 mL/min/1.73 m2, or kidney replacement therapy.
Composite kidney outcomes included kidney replacement therapy or death due to kidney disease.
Composite kidney outcome included development of macroalbuminuria, a sustained ≥30% decline in eGFR, or kidney replacement therapy.
Composite kidney outcome included development of macroalbuminuria, increase in albumin-to-creatinine ratio of >30%, a sustained ≥40% decline in eGFR, end-stage kidney disease, or death due to any cause.
In an updated 2021 meta-analysis that included the 7 aforementioned trials plus new data from the AMPLITUDE-O trial (efpeglenatide) [41], Sattar et al. confirmed that GLP1-RA provided favorable benefits for cardiovascular protection with a reduction in 3-point MACE [cardiovascular death, myocardial infarction (MI), or stroke] by 14% [HR, 0.86 (0.80–0.93)] [26]. The pooled GLP1-RA analysis demonstrated benefits in the secondary endpoints as well, with a 13% reduction in cardiovascular death [HR, 0.87 (0.80–0.94)], 10% reduction in MI [HR, 0.90 (0.83–0.98)], 17% reduction in stroke [HR, 0.83 (0.76–0.92)], 11% reduction in heart failure hospitalizations [HR, 0.89 (0.82–0.98)], and 12% reduction in all-cause mortality [HR, 0.88 (0.82–0.94)] [26]. These 8 trials enrolled patients with T2D with and without CKD, but there was no significant interaction by eGFR status for the primary outcome of 3-point MACE. Similar cardiovascular benefits were seen for those with reduced eGFR (<60 mL/min/1.73 m2) and preserved eGFR (≥60), with HR of 0.88 (0.77–1.01) and 0.83 (0.74–0.93), respectively [p-interaction 0.52] [26].
Notably, the benefits of GLP1-RA on cardiovascular protection extend well beyond A1c lowering. This has led to guidelines from the ADA [45], the American College of Cardiology (ACC) [46], and the European Society of Cardiology (ESC)[47] endorsing the use of GLP1-RA in treatment algorithms for the purposes of ASCVD prevention for patients with T2D at elevated cardiovascular risk. Additionally, the KDIGO guideline for managing diabetes in patients with CKD recommends GLP1-RA as the next-in-line therapy after initiation of SGLT2i for further A1c lowering or high-risk of ASCVD; this class of medication (GLP1-RA) was given a stronger preference over other glucose-lowering agents given its cardiovascular benefits [21,48].
A few caveats regarding the aforementioned CVOTs: efpeglenatide is not available in the US, and albiglutide has been withdrawn from all markets. In the PIONEER 6 trial (which was designed to rule out an excess in cardiovascular risk with oral semaglutide among patients with T2D and not powered to demonstrate superiority), oral semaglutide demonstrated cardiovascular safety compared with placebo, and although there was a trend toward significance for benefit (eg, a 21% risk reduction), statistical significance was not met [43]. Another cardiovascular outcome trial of oral semaglutide is ongoing (SOUL, NCT03914326) [49].
1.5. GLP1-RA and kidney outcomes
The cardiovascular benefits are well established for GLP1-RA among patients with T2D at elevated cardiovascular risk, including for patients with DKD. Additionally, the aforementioned updated 2021 meta-analysis that now included the AMPLITUDE-O trial also demonstrated favorable kidney outcomes [26]. GLP1-RA (compared with placebo) were shown to confer benefit on the kidney in the 6 available trials that reported kidney outcomes (Table 1), with a 21% reduction in the composite kidney outcome [HR, 0.79 (0.73–0.87)], which translated to a favorable number needed to treat of 47 (37–77) over an average of 3 years. Again, the kidney composite outcome was also defined broadly as development of new-onset macroalbuminuria, at least 40% decline in eGFR, kidney replacement therapy, or death from kidney disease. The authors also examined a “harder” kidney outcome of worsening kidney function, which was defined as either doubling of serum creatinine or at least 40% decline in eGFR (and for EXSCEL, worsening kidney outcomes included kidney replacement therapy or death due to kidney disease). GLP1-RA showed a trend for benefit with a 14% reduction, which did not meet statistical significance [HR, 0.86 (0.72–1.02)] [26].
Therefore, there remains uncertainty whether GLP1-RA will confer similar benefits on “harder” kidney outcomes such as eGFR decline, progression to ESKD and death from kidney causes. However, it is important to note that there has not yet been a published dedicated GLP1-RA outcome trial in patients with CKD, although the FLOW trial (NCT03819153)[29] is on-going. Thus the data for the potential kidney benefits of GLP1-RA have been extrapolated from studies of the general T2D population which included patients with and without CKD, and none of these aforementioned trials were powered for “harder” kidney outcomes. This is in contrast to SGLT2i where there have been 3 dedicated kidney outcome trials that specifically enrolled a CKD population. The FLOW trial, when published, will be informative whether or not GLP1-RA reduce the risk of worsening kidney function and death from kidney causes.
In the meantime, additional exploratory analyses have been encouraging regarding kidney protection with GLP1-RA. A post hoc exploration of LEADER (liraglutide) and SUSTAIN 6 (semaglutide) trials found that the annual reduction in eGFR was slower for the GLP1-RA agents, compared with their respective placebos, for the overall populations; however, the effect was more marked in patients with CKD with baseline eGFR <60 mL/min/1.73 m2 [50]. Additionally, another pooled analysis from these 2 trials showed that patients assigned to liraglutide and semaglutide treatment were more likely to achieve a 30% reduction in UACR, compared with placebo, irrespective of baseline UACR [51]. In a recent post hoc analysis of the SUSTAIN 6 and PIONEER 6 trials encompassing 6480 participants at high cardiovascular risk, pooled data by treatment group were analyzed for differences in eGFR slope. In the overall population, the estimated treatment difference between semaglutide and placebo for annual eGFR slope was significant at 0.59 mL/min/1.73 m2 (95% confidence interval 0.29; 0.89), but numerically largest in the 30 to <60 mL/min/1.73 m2 eGFR group at 1.06 mL/min/1.73 m2 (0.45; 1.67) [52]. Thus, pooled analyses of clinical trial data in patients with T2D suggest that semaglutide may reduce the rate of eGFR decline and that kidney protection by GLP1-RA could be especially beneficial among those with established CKD characterized by low eGFR.
This is notable because reduction in albuminuria may translate to an associated reduction in clinical events. Another analysis of the LEADER trial found that a reduction in albuminuria during the first year of treatment was associated with fewer cardiovascular and kidney outcomes; patients who had a >30% reduction in UACR experienced an 18% lower risk of MACE [HR, 0.82 (0.71–0.74)] and 33% reduced risk for the composite kidney outcome [HR, 0.67 (0.49–0.93)] regardless of baseline UACR or treatment arm [53]. These data emphasize the importance of monitoring UACR on treatment for prognosis.
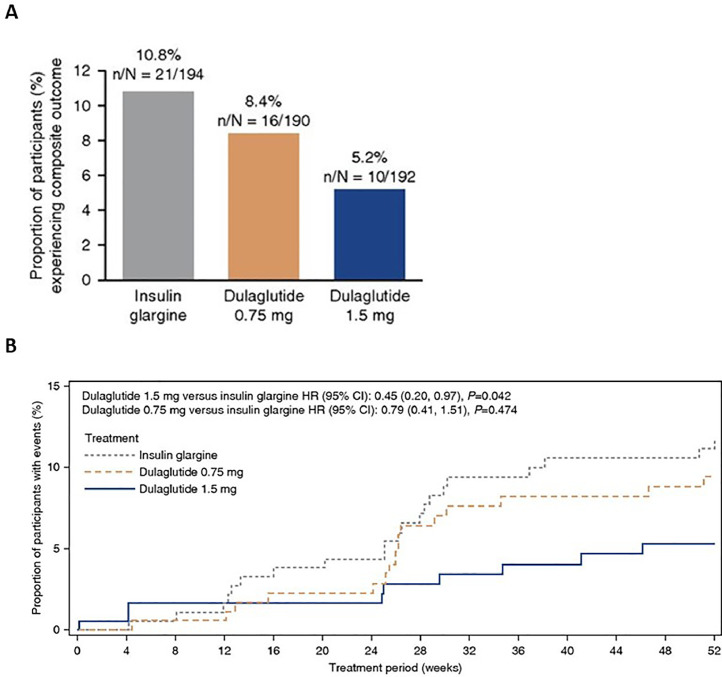
Another important study that established the safety and benefits of GLP1-RA among patients with moderate to severe CKD was the AWARD-7 trial, an open-label, randomized clinical trial of dulaglutide compared with insulin glargine as basal therapy for hyperglycemia [54]. AWARD-7 enrolled adult patients with T2D (A1c, 7.5%−10.5%) and CKD stages 3–4 who were being treated with ACEi or ARBs and found that dulaglutide (0.75 or 1.5 mg subcutaneous once weekly) achieved similar glycemic control to insulin glargine but had significantly less eGFR decline (mean −0.7 vs −3.3 mL/min/1.73 m2, respectively; P<0.001) over 52 weeks of follow-up. In a pre-specified exploratory analysis, the composite rate of ≥40% eGFR decline or kidney failure treated by dialysis or transplant was reduced by more than half, and in the macroalbuminuric group, the HR was 0.25 (95% CI, 0.10–0.68; P = 0.006) for these events over just 1 year of follow-up (Fig. 2) [55]. Although dulaglutide was associated with higher rates of gastrointestinal (GI) side effects, the rate of symptomatic hypoglycemia was reduced in half with dulaglutide vs insulin glargine. This adds to the safety data of GLP1-RA, specifically for patients with more advanced CKD down to an eGFR nadir of 15 mL/min/1.73 m2.
Fig. 2.
AWARD 7 trial: Composite kidney outcome of ≥40% decline in estimated glomerular filtration rate (eGFR) or end-stage kidney disease (ESKD) with dulaglutide 0.75 or 1.5 mg/wk vs insulin glargine. Panel A: Proportion experiencing primary outcome by treatment group. Panel B: Time to first event for primary outcome by treatment group. Used with permission of American Society of Nephrology, from Clinical Outcomes by Albuminuria Status with Dulaglutide versus Insulin Glargine in Participants with Diabetes and CKD: AWARD-7 Exploratory Analysis, Kidney360, Tuttle KR, et al., 2, 2 © 2021; permission conveyed through Copyright Clearance Center, Inc.
Notably, limited data exist for the use of GLP1-RA among patients with stage 5 CKD or on dialysis, and therefore, these agents should be used with caution in this group. There are also insufficient data for the use of GLP1-RA among kidney transplant recipients and patients with type 1 diabetes.
1.6. Definition of CKD progression in clinical trials
How CKD is defined in trials will be very important in establishing kidney efficacy for GLP1-RA and other agents. The Food and Drug Administration (FDA) and European Medicines Agency (EMA) have considered an eGFR decline of ≥40% as an acceptable end point highly predictive of kidney failure in clinical trials of CKD progression. Recently, a CKD working group determined that both early change in albuminuria (UACR reduction of ≥30%) and difference in eGFR slope (≥0.75 mL/min/1.73 m2 per year over ≥2 years) were associated with clinical outcomes and may be considered for use as end points in clinical trials of CKD progression [56].
2. Side effects
While meta-analyses of the pooled trials importantly demonstrate no significant serious adverse events of GLP1-RA, with no increase in the risk of retinopathy, severe hypoglycemia, or adverse pancreatic effects [26], there are some established side effects to be mindful of in clinical practice. Commonly, there can be initial GI side effects (mostly nausea and decreased appetite, but possibly also vomiting and diarrhea). GI side effects generally improve after 2 weeks on therapy [57]. A good rule is to start with a lower dose of GLP1-RA, titrate up as tolerated, and avoid large meals [58]. Some GLP1-RA may cause injection site reactions, with an incidence rate varying between 0% and 22% [57]. GLP1-RA may also cause an increase in heart rate of 1 to 10 beats per minute; this effect is usually transient [59]. While GLP1-RA do not cause hypoglycemia when used alone, there is a risk of hypoglycemia when combined with sulfonylureas or insulin; if a patient's A1c is already controlled, adjustment/withdrawal of other hypoglycemic agents may be needed. GLP1-RA should not be used in combination with dipeptidyl peptidase 4 (DPP-4) inhibitors known as “gliptins".[60] Because post-marketing surveillance has suggested a possible increased risk of pancreatitis with GLP1-RA, these agents should be used with caution in patients with prior history of pancreatitis [61]. An association between GLP1-RA and medullary thyroid cancer had been suggested from animal studies, and thus GLP1-RA are contraindicated in patients with this rare cancer [62].
2.1. Underutilization of GLP1-RA
Despite multiple diabetes, cardiovascular, and kidney organizations endorsing the use of GLP1-RA in their guideline recommendations [45], [46], [47], this class of medications remains underutilized in clinical practice. An analysis of the records of over 21,000 patients with T2D and CVD from a large academic medical center found that only 1.6% of patients received a GLP1-RA during 2013–2019 [63]. Of these GLP1-RA prescriptions, 45% were written by primary care and 45% by endocrinology, but only 1.4% were written by cardiologists. Another registry (the GOULD registry) of high-risk patients with both T2D and ASCVD found that GLP1-RA were used in only 7.9% of patients [64]. Cost and insurance pre-authorization approvals remain barriers to use in clinical practice, but unfamiliarity with the CVD benefits, discomfort by non-endocrinologists with potentially adjusting other glycemic agents (i.e., sulfonylureas, insulin) in order to initiate use of GLP1-RA, and treatment inertia also contribute to poor uptake of this class of medications into routine clinical practice.
Given the established cardiovascular benefits of GLP1-RA, cardiologists are well positioned to take greater ownership in ensuring that their patients with T2D are treated with medications with proven cardioprotective benefits. It is in the “wheelhouse” of cardiologists to ensure implementation of cardiovascular preventive medications. Analyses of 2 major US healthcare systems found that patients with T2D were 2 times more likely to see a cardiologist than an endocrinologist in clinic, and patients were 4 times more likely to see a cardiologist than an endocrinologist if they had T2D plus ASCVD [65]. The much greater frequency of visits to cardiologists highlights the need for cardiovascular clinicians to influence or implement the use of these evidence-based GLP1-RA in appropriate patients, as part of a team-based approach to management. Given that CKD is a “risk-enhancing” condition for CVD, cardiologists should also be better equipped to recognize CKD by assessing and monitoring eGFR and albuminuria in order to deliver goal-directed preventive strategies.
2.2. Future directions in DKD
Although many potential favorable mechanisms on the kidney are speculated, it is not exactly known how GLP1-RA may confer beneficial properties on the kidney. The mechanisms of action of GLP1-RA on the kidney are being explored in the REMODEL study (NCT04865770), which will include patients with T2D on ACEi/ARB treatment but with CKD defined as eGFR ≥30 to <75 mL/min/1.73 m2 and UACR ≥30 to <5000 mg/g. In this study, magnetic resonance imaging will be used to determine the effect of semaglutide on changes in kidney inflammation and fibrosis, perfusion, and oxygenation. A subset of patients in REMODEL will also undergo kidney biopsy for deep molecular phenotyping with a focus on single-cell transcriptomics to reveal molecular signatures, pathway activation, cellular targets, and responses to treatment with semaglutide [66].
FLOW is a complementary, traditional kidney outcomes trial (NCT03819153) designed to determine whether semaglutide is safe and effective in DKD. This randomized, interventional, multi-country study will establish whether semaglutide (once-weekly subcutaneous injection) vs placebo added to standard of care impacts the primary composite outcome, defined as persistent eGFR decline of ≥50% from trial start, reaching ESKD, death from kidney disease, or death from CVD. The FLOW trial enrolled patients with T2D, A1c ≤10%, and CKD defined as serum eGFR ≥50 to ≤75 mL/min/1.73 m2 and UACR >300 to <5000 mg/g or eGFR ≥25 to <50 mL/min/1.73 m2 with UACR >100 to <5000 mg/g. Participants must be treated with ACEi or ARBs, unless contraindicated or not tolerated [29].
The SOUL trial (NCT03914326) is examining the efficacy of oral semaglutide for a combined cardiovascular and kidney primary outcome defined as cardiovascular death, kidney death, onset of persistent reduction in eGFR ≥50%, or onset of ESKD (eGFR <15 mL/min/1.73 m2 or initiation of kidney replacement therapy) among more than 9600 people with T2D and established CVD or CKD and A1c 6.5% to 10% [49].
The SELECT trial (NCT03574597) is a CVOT establishing the efficacy of semaglutide (up to 2.4 mg subcutaneously/wk) vs placebo for preventing MACE among more than 17,000 adults with established ASCVD and overweight or obesity (body mass index >27 kg/m2) but without diabetes (A1c <6.5%) [67]. Secondary kidney outcomes in SELECT include the onset of persistent macroalbuminuria (UACR >300 mg/g), persistent ≥50% reduction in eGFR vs baseline, onset of persistent eGFR <15 mL/min/1.73 m2 or kidney replacement therapy, or death from kidney causes [68]. These new trials (FLOW [29], SOUL [49], SELECT[68]) will be informative about additional patient groups that may benefit from GLP1-RA for kidney and heart protection.
Tirzepatide is a dual glucose-dependent insulinotropic polypeptide (GIP) and GLP1-RA, which in a phase 3 trial (SURPASS) demonstrated superiority in reducing hemoglobin A1c, fasting blood glucose, and body weight vs placebo. The dose-dependent weight-loss effects were notable (7–9.5 kg greater than with placebo in the SURPASS 1 trial, which evaluated tirzepatide vs placebo in patients with T2D) [69]. The ongoing CVOT of tirzepatide (SURPASS-CVOT, NCT04255433) will compare tirzepatide with dulaglutide 1.5 mg/wk to determine efficacy for the primary outcome of 3-point MACE [70]. Another ongoing trial will also investigate the effects of tirzepatide on CKD in patients with or without T2D, with a primary outcome of change in kidney oxygenation (TREASURE-CKD, NCT05536804) [71].
Conclusions
Patients with T2D and CKD are at high risk for adverse cardiovascular and kidney outcomes and require a multipronged approach to risk reduction. For patients with DKD, ACEi/ARBs, SGLT2i, and a non-steroidal MRA (finerenone) have demonstrated cardiovascular and kidney benefits in dedicated kidney disease outcome trials. Long-acting GLP1-RA have many favorable properties and have clear efficacy data for reducing cardiovascular risks, as well as safety data, for their use in patients with T2D at high cardiovascular risk, including the subset of patients with DKD. The kidney benefits of GLP1-RA include reduction in macroalbuminuria, which may be driven in part by reductions in blood pressure and A1c with these agents. Notably, secondary and exploratory analyses show a promising signal for slowing eGFR decline and reducing risk of progression to kidney failure. Further study is on-going in the kidney mechanism of action trial (REMODEL)[72] and a dedicated kidney disease outcome trial (FLOW) [29]. Greater use of GLP1-RA in current clinical practice is already needed, given their integration into multiple clinical practice guideline recommendations for patients with T2D and high CVD risk for the purposes of ASCVD prevention. Given the recent evidence for kidney benefits, GLP1-RA should also be considered for patients with T2D and CKD, especially those at higher risk for ASCVD.
Authorship
EDM wrote the initial draft of the manuscript. GLB, HWR, and KRT reviewed the document and provided critical scientific feedback.
Funding institutions
The authors received no specific funding for this work. Outside of this work, Dr. Michos is supported by an American Heart Association grant 946222. Development of this manuscript was supported financially by Novo Nordisk Inc., Plainsboro, NJ.
Declaration of Competing Interest
EDM reports participation in advisory boards for Amgen, Astra Zeneca, Amarin, Bayer, Boehringer Ingelheim, Esperion, Novartis, Novo Nordisk, and Pfizer. GLB reports receiving research support and steering committee membership from Alnylam, Bayer, Novo Nordisk, Quantum Genomics, and Vascular Dynamics. He also reports being a consultant with Alnylam, AstraZeneca, Bayer, Horizon, Ionis, KBP Biosciences, Merck, Novo Nordisk, Quantum Genomics, and Vascular Dynamics. HWR reports receiving research support for clinical trials, service as principal investigator, on advisory boards, speaking and consulting from Eli Lilly and Co, Esperion, Bayer, Boehringer Ingelheim, Novo Nordisk, Sanofi, and Zealand. KRT reports steering committee membership, principal investigator role, or consultant for research related to diabetes and kidney disease from Eli Lilly and Co, Boehringer Ingelheim, AstraZeneca, Gilead, Goldfinch Bio, Novo Nordisk, and Bayer.
Acknowledgment
The authors would like to thank medical writer Amy Ross, PhD, of PRECISION scientia for her assistance with reference gathering, outline development, and review of the draft and Sherif Mehanna, MD for providing a medical accuracy review. This assistance was supported by Novo Nordisk Inc.
Footnotes
Supplementary material associated with this article can be found in the online version, at doi:10.1016/j.ajpc.2023.100502.
Appendix. Supplementary materials
References
- 1.Baena-Diez J.M., Penafiel J., Subirana I., et al. Risk of cause-specific death in individuals with diabetes: a competing risks analysis. Diabetes Care. 2016;39(11):1987–1995. doi: 10.2337/dc16-0614. [DOI] [PubMed] [Google Scholar]
- 2.Franco O.H., Steyerberg E.W., Hu F.B., Mackenbach J., Nusselder W. Associations of diabetes mellitus with total life expectancy and life expectancy with and without cardiovascular disease. Arch Intern Med. 2007;167(11):1145–1151. doi: 10.1001/archinte.167.11.1145. [DOI] [PubMed] [Google Scholar]
- 3.Emerging Risk Factors Collaboration. Di Angelantonio E., Kaptoge S., et al. Association of cardiometabolic multimorbidity with mortality. JAMA. 2015;314(1):52–60. doi: 10.1001/jama.2015.7008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.United States Renal Data System . National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2018. 2018 USRDS annual data report: epidemiology of kidney disease in the United States. [Google Scholar]
- 5.Jankowski J., Floege J., Fliser D., Bohm M., Marx N. Cardiovascular disease in chronic kidney disease: pathophysiological insights and therapeutic options. Circulation. 2021;143(11):1157–1172. doi: 10.1161/CIRCULATIONAHA.120.050686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Afkarian M., Sachs M.C., Kestenbaum B., et al. Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol. 2013;24(2):302–308. doi: 10.1681/ASN.2012070718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levey A.S., Eckardt K.U., Tsukamoto Y., et al. Definition and classification of chronic kidney disease: a position statement from kidney disease: improving global outcomes (KDIGO) Kidney Int. 2005;67(6):2089–2100. doi: 10.1111/j.1523-1755.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- 8.Ninomiya T., Perkovic V., de Galan B.E., et al. Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J Am Soc Nephrol. 2009;20(8):1813–1821. doi: 10.1681/ASN.2008121270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fox C.S., Matsushita K., Woodward M., et al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. Lancet. 2012;380(9854):1662–1673. doi: 10.1016/S0140-6736(12)61350-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stempniewicz N., Vassalotti J.A., Cuddeback J.K., et al. Chronic kidney disease testing among primary care patients with type 2 diabetes across 24 U.S. Health Care Organizations. Diabetes Care. 2021;44(9):2000–2009. doi: 10.2337/dc20-2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brenner B.M., Cooper M.E., de Zeeuw D., et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345(12):861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 12.Lewis E.J., Hunsicker L.G., Clarke W.R., et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345(12):851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 13.Sica D.A., Bakris G.L. Type 2 diabetes: RENAAL and IDNT–the emergence of new treatment options. J Clin Hypertens (Greenwich) 2002;4(1):52–57. doi: 10.1111/j.1524-6175.2002.00749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGuire D.K., Shih W.J., Cosentino F., et al. Association of SGLT2 inhibitors with cardiovascular and kidney outcomes in patients with type 2 diabetes: a meta-analysis. JAMA Cardiol. 2021;6(2):148–158. doi: 10.1001/jamacardio.2020.4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perkovic V., Jardine M.J., Neal B., et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295–2306. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 16.Heerspink H.J.L., Stefansson B.V., Correa-Rotter R., et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436–1446. doi: 10.1056/NEJMoa2024816. [DOI] [PubMed] [Google Scholar]
- 17.The Empa-Kidney Collaborative Group. Herrington W.G., Staplin N., et al. Empagliflozin in patients with chronic kidney disease. N Engl J Med. 2023;388(2):117–127. doi: 10.1056/NEJMoa2204233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li N., Lv D., Zhu X., et al. Effects of SGLT2 inhibitors on renal outcomes in patients with chronic kidney disease: a meta-analysis. Front Med (Lausanne) 2021;8 doi: 10.3389/fmed.2021.728089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhatia K., Jain V., Gupta K., et al. Prevention of heart failure events with sodium-glucose co-transporter 2 inhibitors across a spectrum of cardio-renal-metabolic risk. Eur J Heart Fail. 2021;23(6):1002–1008. doi: 10.1002/ejhf.2135. [DOI] [PubMed] [Google Scholar]
- 20.Rossing P., Caramori M.L., Chan J.C.N., et al. Executive summary of the KDIGO 2022 clinical practice guideline for diabetes management in chronic kidney disease: an update based on rapidly emerging new evidence. Kidney Int. 2022;102(5):990–999. doi: 10.1016/j.kint.2022.06.013. [DOI] [PubMed] [Google Scholar]
- 21.Kidney disease: improving global outcomes diabetes work G. KDIGO 2022 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int. 2022;102(5S):S1–S127. doi: 10.1016/j.kint.2022.06.008. [DOI] [PubMed] [Google Scholar]
- 22.Bakris G.L., Agarwal R., Anker S.D., et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med. 2020;383(23):2219–2229. doi: 10.1056/NEJMoa2025845. [DOI] [PubMed] [Google Scholar]
- 23.Pitt B., Filippatos G., Agarwal R., et al. Cardiovascular events with finerenone in kidney disease and type 2 diabetes. N Engl J Med. 2021;385(24):2252–2263. doi: 10.1056/NEJMoa2110956. [DOI] [PubMed] [Google Scholar]
- 24.Agarwal R., Filippatos G., Pitt B., et al. Cardiovascular and kidney outcomes with finerenone in patients with type 2 diabetes and chronic kidney disease: the FIDELITY pooled analysis. Eur Heart J. 2022;43(6):474–484. doi: 10.1093/eurheartj/ehab777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kristensen S.L., Rorth R., Jhund P.S., et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;7(10):776–785. doi: 10.1016/S2213-8587(19)30249-9. [DOI] [PubMed] [Google Scholar]
- 26.Sattar N., Lee M.M.Y., Kristensen S.L., et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol. 2021;9(10):653–662. doi: 10.1016/S2213-8587(21)00203-5. [DOI] [PubMed] [Google Scholar]
- 27.Clegg L.E., Penland R.C., Bachina S., et al. Effects of exenatide and open-label SGLT2 inhibitor treatment, given in parallel or sequentially, on mortality and cardiovascular and renal outcomes in type 2 diabetes: insights from the EXSCEL trial. Cardiovasc Diabetol. 2019;18(1):138. doi: 10.1186/s12933-019-0942-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blonde L., Belousova L., Fainberg U., et al. Liraglutide as add-on to sodium-glucose co-transporter-2 inhibitors in patients with inadequately controlled type 2 diabetes: lIRA-ADD2SGLT2i, a 26-week, randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2020;22(6):929–937. doi: 10.1111/dom.13978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.A research study to see how semaglutide works compared to placebo in people with type 2 diabetes and chronic kidney disease (FLOW). ClinicalTrials.gov identifier: NCT03819153. ClinicalTrials.gov 2021 December 7, 2021 September 15, 2021]; Available from: https://clinicaltrials.gov/ct2/show/study/NCT03819153.
- 30.Alicic R.Z., Cox E.J., Neumiller J.J., Tuttle K.R. Incretin drugs in diabetic kidney disease: biological mechanisms and clinical evidence. Nat Rev Nephrol. 2021;17(4):227–244. doi: 10.1038/s41581-020-00367-2. [DOI] [PubMed] [Google Scholar]
- 31.Greco E.V., Russo G., Giandalia A., et al. GLP-1 receptor agonists and kidney protection. Medicina (Kaunas) 2019;55(6):233. doi: 10.3390/medicina55060233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DeFronzo R.A. Combination therapy with GLP-1 receptor agonist and SGLT2 inhibitor. Diabetes Obes Metab. 2017;19(10):1353–1362. doi: 10.1111/dom.12982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muskiet M.H.A., Tonneijck L., Smits M.M., et al. GLP-1 and the kidney: from physiology to pharmacology and outcomes in diabetes. Nat Rev Nephrol. 2017;13(10):605–628. doi: 10.1038/nrneph.2017.123. [DOI] [PubMed] [Google Scholar]
- 34.Rosenstock J., Capehorn M., De Remigis A., et al. Semaglutide resduces high-sensitivity CRP levels across different treatment formulations: exploratory analysis of SUSTAIN 3 and PIONEER 1, 2, and 5 trials. J. Am. Coll. Cardiol. 2021;77(1):1607. 18_Supplement_-1607. [Google Scholar]
- 35.Lee Y.S., Jun H.S. Anti-inflammatory effects of GLP-1-based therapies beyond glucose control. Mediators Inflamm. 2016;2016 doi: 10.1155/2016/3094642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yaribeygi H., Farrokhi F.R., Abdalla M.A., et al. The effects of glucagon-like peptide-1 receptor agonists and dipeptydilpeptidase-4 inhibitors on blood pressure and cardiovascular complications in diabetes. J Diabetes Res. 2021;2021 doi: 10.1155/2021/6518221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marso S.P., Daniels G.H., Brown-Frandsen K., et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marso S.P., Bain S.C., Consoli A., et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834–1844. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 39.Hernandez A.F., Green J.B., Janmohamed S., et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392(10157):1519–1529. doi: 10.1016/S0140-6736(18)32261-X. [DOI] [PubMed] [Google Scholar]
- 40.Gerstein H.C., Colhoun H.M., Dagenais G.R., et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394(10193):121–130. doi: 10.1016/S0140-6736(19)31149-3. [DOI] [PubMed] [Google Scholar]
- 41.Gerstein H.C., Sattar N., Rosenstock J., et al. Cardiovascular and renal outcomes with efpeglenatide in type 2 diabetes. N Engl J Med. 2021;385(10):896–907. doi: 10.1056/NEJMoa2108269. [DOI] [PubMed] [Google Scholar]
- 42.Pfeffer M.A., Claggett B., Diaz R., et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373(23):2247–2257. doi: 10.1056/NEJMoa1509225. [DOI] [PubMed] [Google Scholar]
- 43.Husain M., Birkenfeld A.L., Donsmark M., et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019;381(9):841–851. doi: 10.1056/NEJMoa1901118. [DOI] [PubMed] [Google Scholar]
- 44.Holman R.R., Bethel M.A., Mentz R.J., et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377(13):1228–1239. doi: 10.1056/NEJMoa1612917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.American Diabetes Association Professional Practice Committee. Draznin B., Aroda V.R., et al. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2022. Diabetes Care. 2022;45(1):S125–S143. doi: 10.2337/dc22-S009. Supplement_. [DOI] [PubMed] [Google Scholar]
- 46.Das S.R., Everett B.M., Birtcher K.K., et al. 2020 expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes: a report of the American College of Cardiology solution set oversight committee. J Am Coll Cardiol. 2020;76(9):1117–1145. doi: 10.1016/j.jacc.2020.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cosentino F., Grant P.J., Aboyans V., et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41(2):255–323. doi: 10.1093/eurheartj/ehz486. [DOI] [PubMed] [Google Scholar]
- 48.Michos E.D., Tuttle K.R. GLP-1 receptor agonists in diabetic kidney disease. Clin J Am Soc Nephrol. 2021;16(10):1578–1580. doi: 10.2215/CJN.18771220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.A heart disease study of semaglutide in patients with type 2 diabetes (SOUL). ClinicalTrials.gov identifier: NCT03914326. 2021 December 10, 2021 January 18, 2022]; Available from: https://clinicaltrials.gov/ct2/show/NCT03914326.
- 50.Perkovic V., Bain S., Bakris G., et al. eGFR loss with glucagon-like peptide-1 (GLP-1) analogue treatment: data from SUSTAIN 6 and LEADER. Nephrology Dialysis Transplantation. 2019;34(1):i205–i220. Supplement_. [Google Scholar]
- 51.Perkovic V., Bain S., Bakris G., et al. Effects of semaglutide and liraglutide on urinary albumin-to-creatinine ratio (UACR) - a pooled analysis of SUSTAIN 6 and LEADER. Nephrology Dialysis Transplantation. 2019;34(1):i205–i220. Supplement_. [Google Scholar]
- 52.Tuttle K.R., Bosch-Traberg H., Cherney D.Z.I., et al. Post hoc analysis of SUSTAIN 6 and PIONEER 6 trials suggests that people with type 2 diabetes at high cardiovascular risk treated with semaglutide experience more stable kidney function compared with placebo. Kidney Int. 2023;103(4):772–781. doi: 10.1016/j.kint.2022.12.028. [DOI] [PubMed] [Google Scholar]
- 53.Persson F., Bain S.C., Mosenzon O., et al. Changes in albuminuria predict cardiovascular and renal outcomes in type 2 diabetes: a post Hoc analysis of the LEADER trial. Diabetes Care. 2021;44(4):1020–1026. doi: 10.2337/dc20-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tuttle K.R., Lakshmanan M.C., Rayner B., et al. Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7): a multicentre, open-label, randomised trial. Lancet Diabetes Endocrinol. 2018;6(8):605–617. doi: 10.1016/S2213-8587(18)30104-9. [DOI] [PubMed] [Google Scholar]
- 55.Tuttle K.R., Rayner B., Lakshmanan M.C., et al. Clinical outcomes by albuminuria status with dulaglutide versus insulin glargine in participants with diabetes and CKD: AWARD-7 exploratory analysis. Kidney. 2021;360(2):254–262. doi: 10.34067/KID.0005852020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Levey A.S., Gansevoort R.T., Coresh J., et al. Change in albuminuria and GFR as end points for clinical trials in early stages of CKD: a scientific workshop sponsored by the National Kidney Foundation in collaboration with the US Food and Drug Administration and European Medicines Agency. Am J Kidney Dis. 2020;75(1):84–104. doi: 10.1053/j.ajkd.2019.06.009. [DOI] [PubMed] [Google Scholar]
- 57.Trujillo J.M., Nuffer W., Smith B.A. GLP-1 receptor agonists: an updated review of head-to-head clinical studies. Ther Adv Endocrinol Metab. 2021;12 doi: 10.1177/2042018821997320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Romera I., Cebrian-Cuenca A., Alvarez-Guisasola F., Gomez-Peralta F., Reviriego J. A review of practical issues on the use of glucagon-like peptide-1 receptor agonists for the management of type 2 diabetes. Diabetes Ther. 2019;10(1):5–19. doi: 10.1007/s13300-018-0535-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lorenz M., Lawson F., Owens D., et al. Differential effects of glucagon-like peptide-1 receptor agonists on heart rate. Cardiovasc Diabetol. 2017;16(1):6. doi: 10.1186/s12933-016-0490-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lajthia E., Bucheit J.D., Nadpara P.A., et al. Combination therapy with once-weekly glucagon like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes: a case series. Pharm Pract (Granada) 2019;17(4):1588. doi: 10.18549/PharmPract.2019.4.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Singh S., Chang H.Y., Richards T.M., et al. Glucagonlike peptide 1-based therapies and risk of hospitalization for acute pancreatitis in type 2 diabetes mellitus: a population-based matched case-control study. JAMA Intern Med. 2013;173(7):534–539. doi: 10.1001/jamainternmed.2013.2720. [DOI] [PubMed] [Google Scholar]
- 62.Bjerre Knudsen L., Madsen L.W., Andersen S., et al. Glucagon-like Peptide-1 receptor agonists activate rodent thyroid C-cells causing calcitonin release and C-cell proliferation. Endocrinology. 2010;151(4):1473–1486. doi: 10.1210/en.2009-1272. [DOI] [PubMed] [Google Scholar]
- 63.Hamid A., Vaduganathan M., Oshunbade A.A., et al. Antihyperglycemic therapies with expansions of US Food and Drug Administration indications to reduce cardiovascular events: prescribing patterns within an academic medical center. J Cardiovasc Pharmacol. 2020;76(3):313–320. doi: 10.1097/FJC.0000000000000864. [DOI] [PubMed] [Google Scholar]
- 64.Arnold S.V., de Lemos J.A., Rosenson R.S., et al. Use of guideline-recommended risk reduction strategies among patients with diabetes and atherosclerotic cardiovascular disease. Circulation. 2019;140(7):618–620. doi: 10.1161/CIRCULATIONAHA.119.041730. [DOI] [PubMed] [Google Scholar]
- 65.Gunawan F., Nassif M.E., Partridge C., et al. Relative frequency of cardiology vs. endocrinology visits by type 2 diabetes patients with cardiovascular disease in the USA: implications for implementing evidence-based use of glucose-lowering medications. Cardiovasc Endocrinol Metab. 2020;9(2):56–59. doi: 10.1097/XCE.0000000000000195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Anker S.D., Butler J., Filippatos G., et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385(16):1451–1461. doi: 10.1056/NEJMoa2107038. [DOI] [PubMed] [Google Scholar]
- 67.Ryan D.H., Lingvay I., Colhoun H.M., et al. Semaglutide effects on cardiovascular outcomes in people with overweight or obesity (SELECT) rationale and design. Am Heart J. 2020;229:61–69. doi: 10.1016/j.ahj.2020.07.008. [DOI] [PubMed] [Google Scholar]
- 68.Semaglutide effects on heart disease and stroke in patients with overweight or obesity (SELECT). ClinicalTrials.gov identifier: NCT03574597. 2021 November 30, 2021 January 18, 2022]; Available from: https://www.clinicaltrials.gov/ct2/show/NCT03574597.
- 69.Rosenstock J., Wysham C., Frias J.P., et al. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): a double-blind, randomised, phase 3 trial. Lancet. 2021;398(10295):143–155. doi: 10.1016/S0140-6736(21)01324-6. [DOI] [PubMed] [Google Scholar]
- 70.A study of tirzepatide (LY3298176) compared with dulaglutide on major cardiovascular events in participants with type 2 diabetes (SURPASS-CVOT). ClinicalTrials.gov identifier: NCT04255433. 2022 January 6, 2022 January 18, 2022]; Available from: https://www.clinicaltrials.gov/ct2/show/NCT04255433.
- 71.A study of tirzepatide (LY3298176) in participants with overweight or obesity and chronic kidney disease with or without type 2 diabetes (TREASURE-CKD). ClinicalTrial.gov identifier: NCT05536804. 2023 March 27, 2023 [cited 2023 March 28, 2023]; Available from: https://clinicaltrials.gov/ct2/show/NCT05536804.
- 72.A research study to find out how semaglutide works in the kidneys compared to placebo, in people with type 2 diabetes and chronic kidney disease (REMODEL). ClinicalTrials.gov identifier: NCT04865770. ClinicalTrials.gov 2021 December 15, 2021 August 25, 2021]; Available from: https://clinicaltrials.gov/ct2/show/NCT04865770.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.