Abstract
Due to the pandemic of COVID -19 disease and the fact that the effective variables in the severity and control of the disease have not been established, numerous factors have been investigated, including the study of inflammatory factors. A cross-sectional study was carried out to investigate the proinflammatory cytokines in patients with COVID -19, conducted in Baghdad, Iraq. The age of the patients was above > (15) years old, with confirmed infection documented by polymerase chain reaction (PCR). The subjects were 132 patients, 69 (52.3%) males, and 63 (47.7%) females. Patients were divided into three pathological groups: mild patients (45), moderate patients (34), and severe patients (53), each group was divided into four weeks according to symptoms onset date. The most common clinical symptoms were cough, fever, and headache, while sore throat, gastrointestinal symptoms, chest pain, and loss of taste and smell were less common in COVID -19 patients. Sandwich-Enzyme-Linked Immunosorbent Assay kits were used to evaluate levels of proinflammatory cytokines, including IL-1β, IL-6, IL-8, and TNF-α. The results IL-6 and TNF-α were significantly elevated in mild during the four weeks with (P=0.0071) and (0.0266) respectively, levels of IL-1β were increased with highly significant differences (P=0.0001) while levels of IL-8 were decreased with highly significant differences (P=0.0001) during the four weeks. In moderate patients, levels of (IL-1β, IL-6, and IL-8) increased without significance (P=0.661, 0.074, 0.0651), respectively; in contrast, the levels of TNF-α increased with significant (P=0.0452) across four weeks. Severe COVID-19 patients showed significantly increased differences in levels of (IL-6, IL-8, and TNFα) (P=0.0438, 0.0348, 0.0447), respectively, while no significant differences in the level of IL-1β (P=0.0774). This study showed that investigating inflammatory factors in the COVID-19 pandemic is crucial in controlling and treating.
Keywords: Covid-19, Proinflammatory cytokines, IL-1β, IL-6, IL- 8, TNF-α
1. Introduction
Acute Respiratory Syndrome, Severe Coronavirus - 2 (SARS-CoV-2) causing Coronavirus Disease - 2019 (COVID-19) has rapidly mutated from an epidemic outbreak in Wuhan, China, to a global pandemic infecting over one million people ( 1 ). The fate of COVID-19 disease depends on various factors, including the immunological condition of the person. Most individuals infected with SARS-CoV-2 will develop mild to moderate respiratory infections and recover without specific treatment. The severe variants of COVID-19 are marked by a hyperinflammatory condition, sometimes known as a “cytokine storm" or "cytokine storm syndrome" (CSS). CSS, also known as cytokine storm, is not listed in the International Classification of Diseases (ICD). Cron and Behrens ( 2 ) demonstrated that a "cytokine storm" is an activation cascade of auto-amplifying cytokine production resulting from an unregulated host immunological response to diverse stimuli. Important causes of the cytokine storm include infections, malignancies, rheumatic illnesses, etc. It is also known that a "cytokine storm" is a systemic inflammatory response to infections and medications that results in the overactivation of immune cells and the release of proinflammatory cytokines ( 3 ). Cytokines are low-molecular-weight proteins with cell-specific and pleiotropic effects regulating cellular communication and coordinating cellular responses. In various immunological responses, such as inflammation, homeostatic conditions regulate the gene expression of cytokines, whereas different stimuli influence their transcription and translation. Immune responses, such as communication, proliferation, and differentiation, are the cellular functions regulated by cytokines ( 4 ). All immune cells can produce cytokines, including phagocytic cells, B and T lymphocytes, and NK cells. In systemic diseases, endotoxemia, septicemia, and the current pandemic respiratory infection coronavirus disease 19, where cytokines can cause severe clinical symptoms, the phrase 'cytokine storm' has been coined to describe the excessive production of cytokines ( 5 ). According to clinical trials, the levels of several cytokines and chemokines have risen significantly in COVID-19. Jones and Jenkins ( 6 ) examined the association between TNF-, IL-6, IL-17, IL-8, and IL-1 production with the etiology of COVID-19. Therefore, IL-6 has garnered considerable interest, and its high level appears to be significantly associated with the clinical manifestations of a severe patient type. This study investigates the proinflammatory cytokines IL-1, IL-6, IL-8, and TNF-in COVID-19 patients in Baghdad, Iraq.
2. Materials and Methods
2.1. Sample Collection
From February to July 2020, a cross-sectional study was conducted to evaluate the proinflammatory cytokines patients with COVID-19 were hospitalized in Al-Yarmouk Hospital and Dar Al Salam field Hospital 1 in Baghdad city. 132 Iraqi patients with COVID-19 infection documented by PCR participated in this study, these groups were age and gender-matched, and all participants were adults > 15 years old. All patients were confirmed positive for SARS-CoV-2 using PCR from a nasopharyngeal swab. Those patients were classified into 3 groups according to the severity of the disease: mild, moderate, and severe, and each group was classified into 4 weeks according to the date of symptoms onset. Mild disease: includes patients without pneumonia or hypoxia, SpO2 95 on room air; Moderate disease: includes patients with clinical signs of pneumonia, SpO2 90 on room air; and Severe disease: includes patients with severe respiratory distress, SpO2 90 on room air, who is hospitalized in a respiratory care unit (ICU) and thus require supplementation of oxygen. The severity of COVID-19 disease was classified into the following groups in accordance with WHO recommendations.
2.2. Measurement of Inflammatory Cytokines
COVID-19 patients' blood samples for serum extraction were obtained as follows: venous blood samples of approximately 5 ml were collected with disposable syringes, placed in gel tubes, and allowed to clot (for at least 30 minutes) at room temperature (20-25°C); then sera were separated by centrifugation (The samples were centrifuged at 2000-3000 rpm for 20 minutes.) chilled to -20°C before being analyzed. Serum levels of IL-1β, IL-6, IL-8, and TNF-α in the samples were measured by sandwich. Enzyme-Linked Immunosorbent Assay (ELISA) at a wavelength of 450 nm. A commercial ELISA kit was used to quantitatively determine IL-1β, IL-6, IL-8, and TNF-α (CUSABIO, Chine). This test was performed following the procedure protocol of the Manufacturer Company. The detection ranges of the kits are given as follows:
•IL-1β: 125 pg/ml-8000 pg/ml ; Sensitivity= 31.25 pg/mL.
•IL-6: 7.8 pg/ml-500 pg/ml ; sensitivity= 2.453 pg/ml
•IL-8: 31.25 pg/ml-2000 pg/ml ; sensitivity= 7.110 pg/ml
•TNF-α: 7.8 pg/ml-500 pg/ml ; sensitivity= 1.953 pg/ml
2.3. Statistical Analysis
The Statistical Analysis System- SAS ( 7 ) program was used to detect the effect of different factors on study parameters. The least significant difference –LSD test (Analysis of Variation-ANOVA) was used to compare between means significantly. The Chi-square test significantly compared the percentage (0.05 and 0.01) probability. Estimate the correlation coefficient between variables in this study.
3. Results
The present study evaluated the role of proinflammatory cytokines in COVID-19 in adult Iraqi patients. The number of patients in the mild, moderate, and severe groups was 45, 34, and 53, respectively. The disease progression in mild, moderate, and severe patients with COVID-19 was divided into four weeks (1st week, 2nd week, 3rd week, and 4th week). Patients were divided by sex into 69 (52.3%) males and 63 (47.7 %) females (Figure 1).
Figure 1.
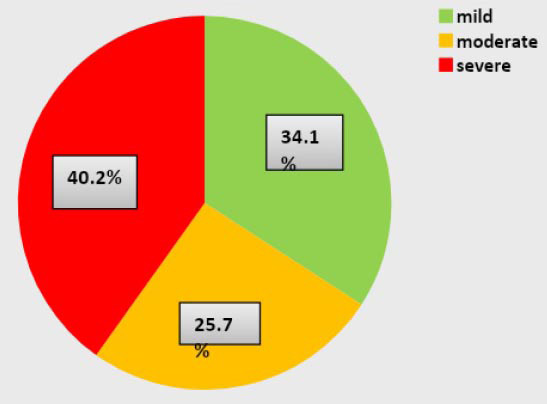
COVID-19 patients are distributed according to the severity of the disease
The signs and symptoms of COVID-19 are different at the onset of the disease. Our study indicated that COVID-19 patients showed that the most common clinical symptoms were cough, fever, and headache. Fever was observed in the first and second weeks during initial symptoms onset in different stages of the disease course. The shortness of breath was more commonly observed among people with severe and moderate COVID-19 than people with mild disease. Fatigue and muscle pain were the most frequent symptoms among patients diagnosed as mild and moderate to severe. Chest pain was a prominent symptom in severe patients. Sore throat and gastrointestinal symptoms such as nausea, vomiting, or diarrhea were less common in all patients. Loss of smell or taste has been expected, especially in non-severe patients (Table 1).
Table 1.
Clinical characteristics in COVID-19 patients across the four weeks
| Clinical Characteristic | Mild | Moderate | Severe | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Week1 N=12 | Week2 N=8 | Week3 N=8 | Week4 N=17 | Week1 N=9 | Week2 N=8 | Week3 N=10 | Week4 N=7 | Week1 N=12 | Week2 N=17 | Week3 N=15 | Week4 N=9 | |
| Signs and symptoms (%) | ||||||||||||
| Fever | 10(83.3) | 4(50) | 7(77.7) | 3(37.5) | 11(91.6) | 10(58.8.) | ||||||
| Cough | 8(66.6) | 4(50) | 3(33.3) | 5(62.5) | 5(50) | 3(42.6) | 10(83.3) | 13(76.5) | 12(80) | 3(33.3) | ||
| Sore throat | 5(41.4) | 1(12.5) | 7(77.7) | 2(25) | 6(50) | 3(17.6) | ||||||
| Headache | 8(66.6) | 5(62.5) | 4(50) | 7(41.2) | 4(44.4) | 6(75) | 2(20) | 2(28.6) | 8(66.6) | 12(70.5) | 6(40) | 3(33.3) |
| Shortness of breath | 1(12.5) | 7(77.7) | 8(100) | 6(60) | 3(42.8) | 12(100) | 17(100) | 15(100) | 9(100) | |||
| Fatigue | 4(33.3) | 4(50) | 5(62.5) | 9(52.9) | 3(33.3) | 2(25) | 2(20) | 3(42.6) | 2(16.6) | 3(17.6) | 2(13.3) | |
| Muscle pain | 8(66.6) | 4(50) | 5(62.5) | 9(52.9) | 4(44.4) | 3(37.5) | 4(00) | 2(28.6) | 3(25) | 5(29.4) | ||
| Chest pain | 1(5.8) | 1(12.5) | 3(30) | 1(14.3) | 1(8.3) | 8(47.1) | 7(46.7) | 5(55.5) | ||||
| Nausea and vomiting | 5(41.4) | 1(12.5) | 1(11.1) | 3(37.5) | 3(25) | 3(17.6) | ||||||
| Diarrhea | 5(41.4) | 1(12.5) | 1(11.1) | 5(62.5) | 4(33.3) | 7(41.1) | ||||||
| Loss of taste and smell | 4(33.3) | 4(50) | 2(25) | 8(47.0) | 5(55.5) | 3(37.5) | 4(40) | 2(28.6) | 2(16.6) | |||
The study showed that proinflammatory cytokine levels varied across four weeks. The serum concentrations of common proinflammatory cytokines, including IL-1β, IL-6, IL-8, and TNF-α, were measured in COVID-19 groups across four weeks, and the mean±SD of IL-1β, IL-6, IL-8, and TNF-α levels for covid-19 groups as shown in table 2.
Table 2.
Levels of proinflammatory cytokines in COVID-19 groups across four weeks
| Variables | 1st Week | 2nd Week | 3rd Week | 4th Week | P-value |
|---|---|---|---|---|---|
| Mild | |||||
| IL-6 | 64.82±6.27ab | 55.14±7.18b | 68.83±5.52a | 53.13±5.0b | 0.0071 |
| IL-1β | 3714.88±547.45b | 6722.02±366.37a | 4963.62±856.44b | 6310.31±446.96a | 0.0001 |
| TNF-α | 151.08±24.33a | 99.35±13.49b | 117.96±19.20ab | 120.61±7.02ab | 0.0266 |
| IL-8 | 619.91±175.62a | 301.47±237.76a | 75.36±20.86b | 41.65±2.83b | 0.0001 |
| Moderate | |||||
| IL-6 | 74.30±12.01 | 71.19±13.80 | 62.14±9.09 | 86.91±18.91 | 0.074 |
| IL-1β | 2110.05±308.94 | 2421.00±844.38 | 2448.83±596.39 | 2734.45±881.46 | 0.661 |
| TNF-α | 147.82±22.26ab | 175.21±38.40a | 148.32±27.92ab | 122.78±16.52b | 0.0452 |
| IL-8 | 864.39±283.97 | 750.70±251.03 | 953.71±229.40 | 590.49±235.87 | 0.0651 |
| Severe | |||||
| IL-6 | 70.89±6.56b | 91.41±10.24a | 74.23±9.87b | 84.77±14.36ab | 0.0438 |
| IL-1β | 1653.43±213.52 | 1798.17±159.10 | 2245.75±468.77 | 1727.19±116.87 | 0.0774 |
| TNF-α | 131.89±22.67ab | 118.38±13.22b | 169.98±15.17a | 106.94±20.47b | 0.0447 |
| IL-8 | 691.03±200.80b | 798.83±192.07b | 1355.72±195.06a | 797.36±247.37b | 0.0348 |
The means that having the different letters in the same row differed significantly
The means that having similar letters in the same row did not differ significantly
The means that did not have letters in the same row did not differ significantly
IL-6 was significantly elevated in mild during the four weeks with (P-value=0.0071), levels of IL-1β high increased during the four weeks and was higher significantly after the 1stweek, (P-value=0.0001). Serum concentrations of TNF-α across the four weeks of COVID-19 mild patients were evaluated with significant differences (P-value=0.0266). Mild patients showed a gradual decrease in IL-8 with high significance (P-value =0.0001). The results showed an increase in (IL-1β, IL-6, and IL-8) levels without significance in moderate patients across four weeks (P-value=0.661, 0.074, 0.0651), respectively. In contrast, the data observed significant changes in the levels of TNF-α over time (P-value=0.0452). Patients of severe COVID 19 showed increased with significant differences in (IL-6, IL-8 and TNFα) (P-value=0.0438, 0.0348, 0.0447) respectively, while the results showed no significant differences in IL-1β (P-value=0.0774) (Figures 2-5).
Figure 2.
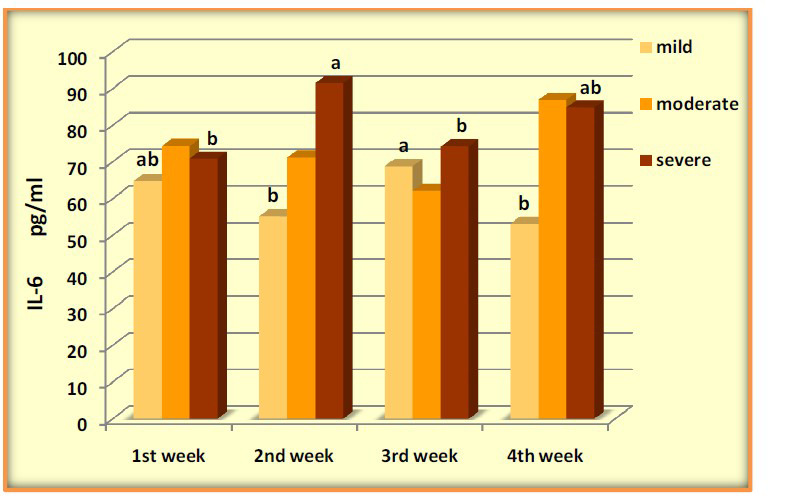
Levels of IL-6 pg/ml in COVID-19 groups across four weeks
Figure 3.
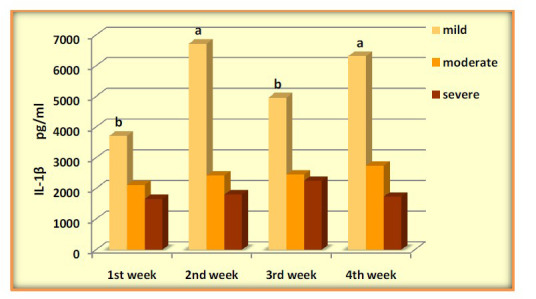
Levels of IL-1β pg/ml in COVID-19 groups across four weeks
Figure 4.
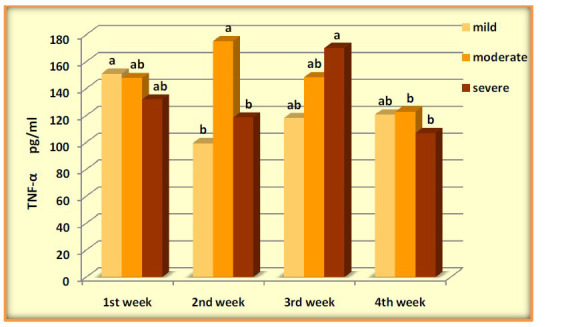
Levels of TNF-α pg/ml in COVID-19 groups across four weeks
Figure 5.
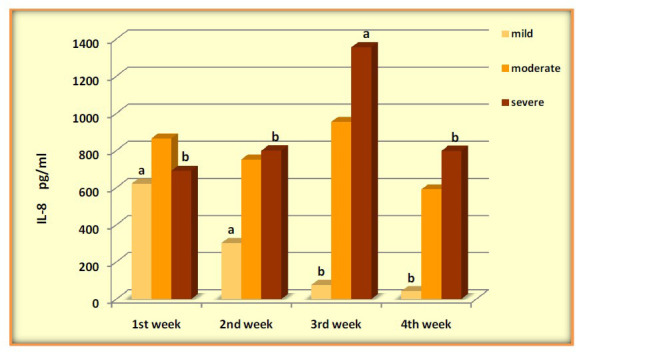
Levels of IL-8 pg/ml in COVID-19 groups across four weeks
4. Discussion
Severe symptoms of acute respiratory distress syndrome (ARDS) with high mortality have been observed in some patients with COVID-19, resulting from a cytokine storm ( 8 ). The current study showed that individuals of any age could acquire COVID-19 infection, although adults of middle age and older are most commonly affected. Previous studies documented that the effect of middle-aged and elderly patients infected with COVID-19 has been observed with more severe symptoms than younger-aged patients, and the median age ranged from 49 to 56 years ( 9 ). In an analysis from the United Kingdom, the risk of death among individuals 80 years and older was 20-fold that among individuals 50 to 59 years old ( 10 ). The COVID-19 disease was more prevalent in 69 males (52.3%) than in 63 (47.7 %) females. This study agrees with a study by Goyal, Choi ( 11 ) that also revealed that male patients with COVID-19 were higher than female patients. Badawi and Ryoo ( 12 ) found that the MERS-CoV and SARS-CoV have also infected more males than females. The reduced susceptibility of females to viral infections could be attributed to the protection from the X chromosome and sex hormones, which play an essential role in innate and adaptive immunity ( 13 ). Examining sex hormones on the mutual effect of immunity and infection has shown that estrogens strengthen innate and adaptive immunity. Testosterone as a suppressor affects the immune system's functioning ( 14 ).
A study conducted by Raimondi, Novelli ( 15 ) showed that biochemical factors such as coagulation factors and liver enzymes in women show a lower inflammatory state and minor tissue damage, which is caused by the role of the endothelium, as well as the importance of the estrogen hormone (for example, 17β-estradiol or E2) on the function of the vascular system, has also been suggested ( 16 ).
This study's results agree with Killerby, Link-Gelles ( 17 ), which explained that shortness of breath is more common in severe patients than in non-severe patients. Other studies in China found that the dominant clinical features of COVID-19 patients were cough and fever, similar to the current study on initial symptoms of COVID-19 patients ( 9 , 18 ). The data showed that headaches occurred more frequently in all stages of the disease. Guan, Ni ( 19 ) supported that some initial symptoms, including fatigue and muscle pain, are among the most common symptoms of mild disease. Sore throat and gastrointestinal symptoms did not differ between patients with non-severe and severe COVID-19. According to current results of clinical characteristics, severe patients tend to have Chest pain after 1st week in some moderate and severe cases, which was consistent with the findings of Zhang, Dong ( 20 ).
This study observed loss of smell or taste in non-severe patients. In agreement with Lechien, Chiesa-Estomba ( 21 ), they noticed that younger or middle-aged patients' changes in smell were strongly associated with COVID-19, mainly in women and patients with fever; more have been reported. Although the reasons for the loss of smell caused by SARS-CoV-2 are still unknown, the high tendency of the virus to multiply in the nasal cavity and the olfactory bulb, which have ACE-2 receptors, could be one of the reasons. Due to the high prevalence of this symptom in patients with taste disorders, it should be recognized by the international scientific community as an essential symptom of COVID-19 infection ( 21 ).
A study by Zeng, Yu ( 22 ) noticed that the levels of serum cytokines IL6, IL-8, and TNF-α, in survivors after 20 days of symptoms onsets were significantly lower than those of patients in the 1st week agrees with current results. The results showed an increase in the concentration of IL-1β in the 4th week as compared with the 1st week, in agreement with the results of Ling, Chen ( 8 ), who found levels of IL-1β in mild patients in the late phase had high than patients in early phase from symptoms onsets. Jøntvedt Jørgensen, Holter ( 23 ) showed an increase in IL-1β after 5 days and 10 days during hospitalization. It has been suggested that proinflammatory cytokines, especially IL-1α and IL-1β, play a prominent role in the development of central fatigue; therefore, we observed that fatigue was the most frequent symptom among mild to severe patients ( 23 ).
In agreement with the previous study by Ling, Chen ( 8 ), which showed no significant differences in moderate patients in levels of (IL-1β, IL-6, and IL-8) in early and late phases during the disease progression of moderate COVID-19. No significant differences in levels of TNF-α were observed in moderately ill patients in a study conducted by Jing, Xu ( 24 ). Our results showed a fluctuation in levels of (IL-6 and TNF-α) during disease progression in patients of the severe group, IL-6 in the 2nd week showed significantly higher levels of serum IL-6 than those in other weeks, An increase in the level of TNF-α with high significance is noticed during the 3rd week. IL-8 was increased gradually after symptoms onset and decreased in the 4th week. At the same time, IL-1β gave similar levels over time. In agreement with Ling, Chen ( 8 ), IL-6 and IL-8 were higher in the early and late phases among patients with more severe diseases.
In contrast, two cytokines (IL-1β and IL-9) were lower only during the early phase among patients with more severe diseases. Another study showed that serum levels of IL-6 and IL-8 were higher in severe patients than in moderate patients, with significant differences in IL-6 (1st week to 5th week) and IL-8 (2nd week to 5th week), serum levels of TNF-α were slightly higher in severe patients ( 24 ). Whereas a study conducted by Tan, Zhang ( 25 ) observed IL-6 level was relatively normal at the onset of symptoms and then elevated and peaked at about 10 days, the IL-8 level gradually elevated between 1 and 30 days in severe and critical cases while remaining low in midland moderate cases. Immune cells in the host show different responses against infectious agents, including viruses ( 26 ). An effective immune response is essential in controlling and eliminating viral infection, but research has shown that a strong, exaggerated, or prolonged immune response can lead to organ dysfunction ( 27 ). Among the most important initiators of inflammatory responses are IL-6, IL-8, and TNF-α. The investigation of the pathophysiology of ARDS revealed that lung inflammation increases as inflammatory mediators such as IL-6, IL-8, and TNF- increase. In patients with SARS and MERS, proinflammatory cytokines increased in acute and severe cases compared to mild and moderate cases ( 28 ).
Studies have demonstrated a correlation between the blood levels of cytokines and chemokines and the disease severity and clinical symptoms of COVID-19. In many COVID-19 patients, serum levels of cytokines and chemokine (IL-6, IL-1α, IL-1β, IL-8, IL-10, and IL-37), tumor necrosis factor-α and TNF-α) Furthermore, the transforming growth factor-β has been increased ( 29 , 30 ). Important alterations in IL-6 in COVID-19 have been identified as an early indicator of inflammatory and immunological responses in these patients. The increase of IL-6 in serum can be seen in the first 10 days after the onset of symptoms ( 31 ). In patients with severe symptoms of Covid-19, proinflammatory factors (IL-18, IP-10, and IL-10) and ARDS-related cytokines (IL-6, IL-1RA, and IL-8) gradually increase. And then, seven days after the onset of symptoms, IL-8 and IP-10 levels increase ( 8 ); as other researchers have shown, serum levels of IL-6 and IL-8 increase with the severity of Covid-19 and mortality ( 32 ). As two IL-6 receptor antagonists, Tocilizumab and Sarilumab have been used to control COVID-19 ( 33 ). Ling, Chen ( 8 ) discovered that the serum level of IL-6 increased during the onset of the disease in patients identified with severe symptoms. This shows that the early measurements of IL-6 can be used to regulate the disease's symptoms and progression. Also, an increase in TNF-a has been observed in patients with severe COVID-19, which was not widespread ( 32 ). Measuring Th1 cytokines (IP-10 and IL-10) and ARDS (IL-6 and IL-8) in the early stages of the disease is beneficial in predicting the patient's condition. It can be used as an early marker in diagnosing and classifying severity. Also, recent research has shown that Tofacitinib, suppressing the Th1 response and IL-6 production, reduces mortality in patients ( 34 ).
The levels of proinflammatory cytokines would change with the progression of the disease, and we found that IL-6 may be the cytokine of early elevation in COVID-19 patients. The serum concentration of IL-6, IL-8, and TNF-α was high in moderate and severe patients, while mild patients had higher IL-1β levels than moderate and severe patients. Due to the high prognostic values for proinflammatory cytokines, we could recommend its evaluation to differentiate the severity of covid-19. The proinflammatory cytokines (IL-1β, IL-6, IL-8, and TNF-α) and their receptors must be investigated at the molecular level to determine their gene expression in covid-19 patients. Other family members of anti-proinflammatory cytokines (IL-37 and IL-38) require intensive investigations at the phenotype (serum level) and genotype (SNPs) levels in covid-19 patients.
Authors' Contribution
Study concept and design: M. A. M.
Acquisition of data: M. A. M. and S. B. A.
Analysis and interpretation of data: W. M. R.
Drafting of the manuscript: S. B. A.
Critical revision of the manuscript for important intellectual content: W. M. R.
Statistical analysis: M. A. M.
Administrative, technical, and material support: S. B. A.
Ethics
The Ethics Committee (No.10223 on February 16/2021) at the Baghdad Al Karekh Health Directorate (Iraqi Ministry of Health and Environment) approved the study protocol.
Conflict of Interest
The authors declare that they have no conflict of interest.
Acknowledgment
We thank the medical staff of Al-Yarmouk Hospital and Dar Al Salam field Hospital 1 (Alkarech, Baghdad, Iraq) for their kind cooperation.
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020 doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cron RQ, Behrens EM. Cytokine storm syndrome: Springer; 2019. [Google Scholar]
- 3.Tang Y, Liu J, Zhang D, Xu Z, Ji J, Wen C. Cytokine storm in COVID-19: the current evidence and treatment strategies. Front Immunol. 2020;11:1708. doi: 10.3389/fimmu.2020.01708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takeuchi O. Posttranscriptional regulation of cytokine mRNA controls the initiation and resolution of inflammation. Chronic Inflammation: Springer; 2016. pp. 319–32. [DOI] [PubMed] [Google Scholar]
- 5.Chauhan P, Nair A, Patidar A, Dandapat J, Sarkar A, Saha B. A primer on cytokines. Cytokine. 2021;145:155458. doi: 10.1016/j.cyto.2021.155458. [DOI] [PubMed] [Google Scholar]
- 6.Jones SA, Jenkins BJ. Recent insights into targeting the IL-6 cytokine family in inflammatory diseases and cancer. Nat Rev Immunol. 2018;18(12):773–89. doi: 10.1038/s41577-018-0066-7. [DOI] [PubMed] [Google Scholar]
- 7.SAS J. Statistical Analysis System, v. 10.0. 2. Cary, North Carolina USA. 2012 [Google Scholar]
- 8.Ling L, Chen Z, Lui G, Wong CK, Wong WT, Ng RW, et al. Longitudinal cytokine profile in patients with mild to critical COVID-19. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.763292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–6. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goyal P, Choi JJ, Pinheiro LC, Schenck EJ, Chen R, Jabri A, et al. Clinical characteristics of Covid-19 in New York city. N Engl J Med. 2020;382(24):2372–4. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Badawi A, Ryoo SG. Prevalence of comorbidities in the Middle East respiratory syndrome coronavirus (MERS-CoV): a systematic review and meta-analysis. Int J Infect Dis. 2016;49:129–33. doi: 10.1016/j.ijid.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jaillon S, Berthenet K, Garlanda C. Sexual dimorphism in innate immunity. Clin Rev Allergy Immunol. 2019;56(3):308–21. doi: 10.1007/s12016-017-8648-x. [DOI] [PubMed] [Google Scholar]
- 14.Bartz D, Chitnis T, Kaiser UB, Rich-Edwards JW, Rexrode KM, Pennell PB, et al. Clinical advances in sex-and gender-informed medicine to improve the health of all: a review. JAMA Intern Med. 2020;180(4):574–83. doi: 10.1001/jamainternmed.2019.7194. [DOI] [PubMed] [Google Scholar]
- 15.Raimondi F, Novelli L, Ghirardi A, Russo FM, Pellegrini D, Biza R, et al. Covid-19 and gender: lower rate but same mortality of severe disease in women—an observational study. BMC Pulm Med. 2021;21(1):1–11. doi: 10.1186/s12890-021-01455-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knowlton AA, Lee A. Estrogen and the cardiovascular system. Pharmacol Ther. 2012;135(1):54–70. doi: 10.1016/j.pharmthera.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Killerby ME, Link-Gelles R, Haight SC, Schrodt CA, England L, Gomes DJ, et al. Characteristics associated with hospitalization among patients with COVID-19—Metropolitan Atlanta, Georgia, March–April 2020. Morb Mortal Wkly Rep. 2020;69(25):790. doi: 10.15585/mmwr.mm6925e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med. 2020 doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guan W-j, Ni Z-y, Hu Y, Liang W-h, Ou C-q, He J-x, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–20. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang J-j, Dong X, Cao Y-y, Yuan Y-d, Yang Y-b, Yan Y-q, et al. Clinical characteristics of 140 patients infected with SARS‐CoV‐2 in Wuhan, China. Allergy. 2020;75(7):1730–41. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 21.Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M, Le Bon SD, Rodriguez A, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Oto-Rhino L. 2020;277(8):2251–61. doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zeng Z, Yu H, Chen H, Qi W, Chen L, Chen G, et al. Longitudinal changes of inflammatory parameters and their correlation with disease severity and outcomes in patients with COVID-19 from Wuhan, China. Critic Care. 2020;24(1):1–12. doi: 10.1186/s13054-020-03255-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jøntvedt Jørgensen M, Holter JC, Christensen EE, Schjalm C, Tonby K, Pischke SE, et al. Increased interleukin-6 and macrophage chemoattractant protein-1 are associated with respiratory failure in COVID-19. Sci Rep. 2020;10(1):1–11. doi: 10.1038/s41598-020-78710-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jing X, Xu M, Song D, Yue T, Wang Y, Zhang P, et al. Association between inflammatory cytokines and anti-SARS-CoV-2 antibodies in hospitalized patients with COVID-19. Immun Ageing. 2022;19(1):1–17. doi: 10.1186/s12979-022-00271-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tan Y, Zhang W, Zhu Z, Qiao N, Ling Y, Guo M, et al. Integrating longitudinal clinical laboratory tests with targeted proteomic and transcriptomic analyses reveal the landscape of host responses in COVID-19. Cell Discov. 2021;7(1):1–19. doi: 10.1038/s41421-021-00274-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iwasaki A, Pillai PS. Innate immunity to influenza virus infection. Nat Rev Immunol. 2014;14(5):315–28. doi: 10.1038/nri3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ye Q, Wang B, Mao J. The pathogenesis and treatment of theCytokine Storm'in COVID-19. J infect. 2020;80(6):607–13. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Min C-K, Cheon S, Ha N-Y, Sohn KM, Kim Y, Aigerim A, et al. Comparative and kinetic analysis of viral shedding and immunological responses in MERS patients representing a broad spectrum of disease severity. Sci Rep. 2016;6(1):1–12. doi: 10.1038/srep25359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodrigues TS, de Sá KS, Ishimoto AY, Becerra A, Oliveira S, Almeida L, et al. Inflammasomes are activated in response to SARS-CoV-2 infection and are associated with COVID-19 severity in patients. J Exp Med. 2021;218(3) doi: 10.1084/jem.20201707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jurado A, Martín MC, Abad-Molina C, Orduña A, Martínez A, Ocaña E, et al. COVID-19: age, Interleukin-6, C-reactive protein, and lymphocytes as key clues from a multicentre retrospective study. Immun Ageing. 2020;17(1):1–15. doi: 10.1186/s12979-020-00194-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schultze JL, Aschenbrenner AC. COVID-19 and the human innate immune system. Cell. 2021;184(7):1671–92. doi: 10.1016/j.cell.2021.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hadjadj J, Yatim N, Barnabei L, Corneau A, Boussier J, Smith N, et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369(6504):718–24. doi: 10.1126/science.abc6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stone JH, Frigault MJ, Serling-Boyd NJ, Fernandes AD, Harvey L, Foulkes AS, et al. Efficacy of tocilizumab in patients hospitalized with Covid-19. N Engl J Med. 2020;383(24):2333–44. doi: 10.1056/NEJMoa2028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guimarães PO, Quirk D, Furtado RH, Maia LN, Saraiva JF, Antunes MO, et al. Tofacitinib in patients hospitalized with Covid-19 pneumonia. N Engl J Med. 2021;385(5):406–15. doi: 10.1056/NEJMoa2101643. [DOI] [PMC free article] [PubMed] [Google Scholar]


