Abstract
We aimed to evaluate the effect of dietary calcium (Ca)-octanoate supplementation on concentrations of ghrelin, growth hormone (GH), insulin-like growth factor-1 (IGF-1), and insulin in plasma and milk of beef cattle during late gestation and early postpartum. Twelve Japanese Black cattle were offered concentrate without (CON, n = 6) or with Ca-octanoate supplementation at 1.5% of dietary dry matter (OCT, n = 6). Blood samples were collected at −60, −30, and −7 d relative to the expected parturition date and daily from d 0 to 3 after parturition. Milk samples were collected daily postpartum. Compared to the CON group, concentrations of acylated ghrelin increased in plasma as parturition approached in the OCT group (P = 0.02). However, concentrations of GH, IGF-1, and insulin in plasma and milk were not affected by treatment groups throughout the study. Additionally, we showed for the first time that bovine colostrum and transition milk contain acylated ghrelin at a significantly higher concentration than plasma (P = 0.01). Interestingly, concentrations of acylated ghrelin in milk were negatively correlated with those in plasma postpartum (r = −0.50, P < 0.01). Feeding Ca-octanoate increased concentrations of total cholesterol (T-cho) in plasma and milk (P < 0.05), tended to increase those of glucose in plasma at postpartum and milk (P < 0.1). We conclude that feeding Ca-octanoate in late gestation and early postpartum may contribute to increased concentrations of glucose and T-cho in plasma and milk without affecting concentrations of ghrelin, GH, IGF-1, and insulin in plasma and milk.
Keywords: Ghrelin, Calcium-octanoate, Colostrum, Transition milk, Japanese black cattle
1. Introduction
Bovine colostrum is rich in hormones, cytokines, and growth factors as well as immunoglobulins [1,2]. As proposed by the lactocrine hypothesis [3], these bioactive factors in maternal colostrum have long-lasting effects on the development and physiological function of offspring [4]. For example, growth hormone (GH) in colostrum is reported to be essential for the growth of neonatal calves [5]. Similarly, insulin-like growth factor-1 (IGF-1) and insulin in colostrum stimulate intestinal development in calves [5,6]. Therefore, it is vital to ensure that colostrum has adequate concentrations of these hormones to support post-natal growth of calves.
Phomvisith et al. reported that concentrations of GH in milk were lower for cows that had lower concentrations of GH in plasma [7], indicating that GH in milk is derived from maternal circulation. Similarly, it has been reported that the principle source of IGF-1 in milk is from the transfer from maternal circulation because synthesis in mammary glands is low [8,9]. In addition, it was reported that concentrations of insulin in colostrum were derived from maternal circulation and that there was a positive correlation between colostrum and plasma concentrations of insulin in prepartum cows [10]. These findings indicate that concentrations of hormones in milk are closely related to those in maternal blood. Therefore, it may be possible to increase concentrations of hormones in colostrum through an increase of those in maternal blood.
Ghrelin is a gastrointestinal peptide hormone synthesized in the abomasum and it stimulates secretion of GH and insulin in ruminants [11,12]. Growth hormone also stimulates secretion of IGF-1 [5], thus an increase in concentrations of ghrelin in blood has the potential to increase concentrations of GH, insulin, and IGF-1 in both blood and colostrum simultaneously. Ghrelin is present in two forms, acylated and desacyl-ghrelin. The third serine residue at the N-terminus of acylated ghrelin is modified with medium chain fatty acids (MCFAs), which are responsible for its physiological activity [13,14]. In mice, feeding MCFAs increased concentrations of acylated ghrelin modified with MCFAs, whose carbon chain length corresponded to that of the ingested one, in their stomachs [15]. Therefore, ingested MCFAs can be utilized directly for acylation of ghrelin. Ghrelin modified with octanoate is the most abundant form in mammalian species [14,16,17]. It was previously reported that feeding octanoate to cachectic patients increased concentrations of acylated ghrelin in plasma [18]. Octanoate is easily absorbed [19] and used as a supplementary energy source to improve performance in calves [20,21]. Furthermore, calcium salts of fatty acids bypass the rumen without fermentation [22]. Thus, we hypothesize that feeding calcium (Ca)-octanoate would increase circulating concentrations of acylated ghrelin, which would result in increased concentrations of GH, IGF-1, and insulin in blood and milk of cows. Currently, there are no reports on the effect of feeding octanoate on concentrations of ghrelin, GH, IGF-1, and insulin in blood and milk of cattle. Moreover, to the best of our knowledge, ghrelin has never been reported as being present in bovine colostrum and transition milk. Given that concentrations of ghrelin in colostrum are derived from maternal blood in humans [23], it is likely that concentrations could be increased by feeding octanoate to cows.
The objective of this study was to evaluate the effect of dietary Ca-octanoate supplementation on concentrations of ghrelin, GH, IGF-1, insulin, and metabolites in plasma, colostrum, and transition milk in beef cattle during periparturient period.
2. Material & methods
2.1. Animals and diets
All procedures conducted in this experiment were performed according to the Guidelines for the Animal Experiments by the Faculty of Agriculture in Kyushu University and with the approval of the Kyushu University Laboratory Animal Care and Use Committee (Approval ID: A-19-194-3 and A-21-042-0).
This experiment was conducted at the Kuju Agricultural Research Center, Kyushu University, Oita Prefecture, Japan. Twelve, pregnant, Japanese Black cattle were used for the present study that was carried out from 60 d before the expected date of parturition (d −60) to 3 d after parturition. All cows were randomly assigned to either a CON (n = 6) or an OCT (n = 6) treatment group. Ages, parity, and initial body weight (BW) of the CON group were 6.5 ± 1.6 years old, 5.3 ± 1.5, and 507.8 ± 27.5 kg, respectively, and those of the OCT group were 3.8 ± 0.9 years old, 2.7 ± 0.9, 481.3 ± 29.4 kg, respectively. There was no difference in age, parity, or BW between the two treatment groups (P = 0.17, 0.16, and 0.53, respectively). Cows were fed hay (Italian rye-grass), concentrate (Yamato, JA Nishinihon Kumiai Shiryo Corp, Hyogo, Japan), and soybean meal (Daizu Kasu flake, JA Nishinihon Kumiai Shiryo Corp, Hyogo, Japan) separately once a day at 16:00 h using individually secured self-locking stanchions. Nutrient composition of hay, concentrate, and soybean meal is shown in Table 1, which was analyzed at Zen-Raku-Ren Analysis Center (Kamisu, Ibaraki, Japan) according to AOAC (1990) and AOAC International (2002) [24,25]. All cows were provided 85–90% of dry matter (DM), 140–155% of crude protein (CP), and 100% of total digestible nutrients (TDN) according to the Japanese Feeding Standard for Beef Cattle [26]. Hay and concentrate feed were offered at a ratio of 2.5:1. In addition, soybean meal was supplemented when the amount of CP provided was insufficient for the above targets. In the OCT group, Ca-octanoate (Kanematsu Agritech Co., Ltd., Saitama, Japan) was provided as a supplemented to the diet at 1.5% of dietary DM by mixing with concentrate feed. Refused feed was collected and weighed daily at 10:00 h. Dry matter intake (DMI) was calculated as the difference between feed offered and feed refused. All cows had free access to water and salt-mineral blocks (Nippon Zennyaku Kogyo Co., Ltd., Fukushima, Japan) throughout the study.
Table 1.
Nutrient composition of hay, concentrate, and soybean meal.
| Nutrient composition | Hay | Concentrate | Soybean meal |
|---|---|---|---|
| DM, % | 71.7 | 88.4 | 85.9 |
| Nutrient composition, % DM | |||
| CP | 17.2 | 16.9 | 52.5 |
| Ether extract | 3.10 | 4.00 | 1.40 |
| NDF | 63.1 | 25.8 | 13.1 |
| Crude ash | 7.60 | 7.70 | 8.20 |
| TDN | 60.0 | 77.0 | 75.0 |
| NEM, Mcal/kg | 1.21 | 1.84 | 1.80 |
| NEL, Mcal/kg | 1.15 | 1.76 | 1.72 |
DM = dry matter, CP = crude protein, NDF = neutral detergent fiber, TDN = total digestible nutrients, NEM = net energy for maintenance, NEL = net energy for lactation.
2.2. Sample collection and processing
Cows were weighed weekly from d 60 to d 11 prior to expected parturition, at d 7 prior to expected parturition, and at parturition (d 0). It is well known that circulating concentrations of acylated ghrelin in blood exhibit a diurnal rhythm, with peak concentrations corresponding to the onset of feeding [27]. It has been reported that feeding octanoate and MCFAs increased preprandial concentrations of ghrelin in blood of cachectic patients and dairy cows, respectively [18,28]. In addition, secretion of GH is synchronized around feeding, with a burst immediately before feeding, followed by no secretion for at least 1 h after feeding period [29]. Therefore, we collected blood samples before feeding to evaluate the effect of feeding octanoate on concentrations of peak ghrelin and to coincide with the pre-feeding secretory episode of GH. Blood samples were collected by jugular venipuncture (Venoject II VP-H100K with heparin sodium; Terumo Corporation, Tokyo, Japan) into 10 mL heparinized vacuum tubes 1 h before feeding (15:00 h). Twenty mL of blood was collected on d 60, 30, and 7 before the expected parturition date and daily from d 0 to 3 after parturition. Colostrum samples were obtained within 24 h after parturition (d 0) and transition milk samples were collected 24, 48, and 72 h after collecting samples of colostrum (d 1, 2, and 3, respectively). Milk samples were collected immediately after taking a sample of blood postpartum. Calves were separated from their dams 4 h before sampling to collect sufficient milk for analysis and to avoid a possible confounding effect of suckling. Before collecting the milk samples, teats were washed with water and then with 70% ethanol. Teats were milked several times prior to sampling to remove water and ethanol from the teat canal. Thereafter, 20 mL of milk was collected and transferred to collecting tubes. Both blood and milk samples were placed on ice immediately after sampling. Blood samples were transferred to a collection tube containing heparin (10 IU/mL of blood; AY Pharmaceuticals Co., Ltd., Tokyo, Japan) and aprotinin (100 Kallikrein Inhibitor Unit/mL of blood; Wako Pure Chemical, Osaka, Japan) and centrifuged at 4 °C, 2330×g for 20 min. Milk samples were transferred to a collection tube supplemented with aprotinin (100 Kallikrein Inhibitor Unit/mL of milk). The plasma and milk samples were stored at −80 °C until analysis.
2.3. Hormone and metabolite assay
Concentrations of hormones in plasma and milk were measured with time-resolved fluoroimmunoassays (TR-FIA).
Concentrations of acylated ghrelin in plasma were measured using europium (PerkinElmer Japan, Kanagawa, Japan)-labeled rat ghrelin (Peptide Institute Inc., Osaka, Japan), anti-ghrelin (Rabbit) serum (Yanaihara Institute Inc., Shizuoka, Japan) and polystyrene microtiter strips (Nalgene Nunc International, Tokyo, Japan) coated with anti-rabbit γ-globulin (Invitrogen, Thermo Fisher Scientific K. K., Tokyo, Japan), according to a previous study [27]. Intra- and Inter-assay coefficients of variability (CVs) of acylated ghrelin in plasma were 9.0 and 7.9%, respectively. Concentrations of GH, IGF-1, and insulin in plasma and milk were measured as previously described [7,11,30]. Intra-assay CVs for concentrations of GH, IGF-1, and insulin in plasma and milk were 6.1, 7.7, and 6.5%, respectively. Inter-assay CVs for concentrations of GH, IGF-1, and insulin in plasma and milk were 6.2, 4.4, and 7.3%, respectively.
Before measuring concentrations of acylated ghrelin in milk, milk samples were centrifuged at 4 °C, 20,630×g for 60 min to remove lipids according to Ganmaa et al. [31], and the intermediate layer was harvested and extracted according to a previous study [27]. Concentrations of acylated ghrelin in milk were assayed using the same method as that for those in plasma [27]. The calibration curve of the competitive TR-FIA assay for acylated ghrelin is shown in Fig. 1. The standard curve ranged from 0.01 to 100 ng/mL. Intra- and Inter-assay CVs of acylated ghrelin in milk were 9.8 and 13.8%, respectively. The least detectable level and 50% inhibitory concentration were 0.0389 and 1.27 ng/mL, respectively. The mean recovery rate of acylated ghrelin from bovine colostrum was 108.3%. The acylated ghrelin tracer was displaced by bovine colostrum in a dose-dependent manner (Fig. 1). The quality control criteria in our TR-FIA protocol were satisfactory; therefore, the assay was deemed suitable for determining concentrations of acylated ghrelin in bovine colostrum.
Fig. 1.
A competitive time-resolved fluoroimmunoassay calibration curve for rat ghrelin standard and bovine colostrum using the Logit-log method. Each point represents the average of triplicate measurements.
Concentrations of metabolites in bovine plasma and milk were measured using commercial assay kits by the mutarotase-glucose oxidase test (glucose C2 test) for glucose, the acyl coenzyme A (CoA) synthetase-acyl CoA oxidase test (NEFA C test) for nonesterified fatty acids (NEFA), and the cholesterol oxidase-N-ethyl-N-(2-hydroxy-3-sulfopropyl)-3,5-dimethoxyaniline test (Cholesterol E-test) for total cholesterol (T-cho) (Wako Pure Chemical, Osaka, Japan).
2.4. Statistical analysis
Data for concentrations of hormones and metabolites in plasma and milk from one cow in the CON group were removed from the statistical analyses for d 0 because insufficient amount of colostrum was collected. It was previously reported that only a limited volume of colostrum is able to be collected from Japanese Black cattle within 24 h after birth [7].
Data for concentrations of hormones and metabolites in plasma and milk were analyzed using the fit model procedure of JMP 14 (SAS Institute Inc., NC, USA) according to the following model (Eq. (1)):
| Yijk= μ + Treatmenti+ Timej+ (Treatment × Time)ij+ Cattlek+ eijk | (1) |
where Yijk is the dependent variable, μ is the overall mean, Treatmenti is the fixed treatment effect, Timej is the fixed time, (Treatment × Time)ij is the treatment interaction by time, Cattlek is the random effect of the cattle, and eijk is a random error. A simple main effect test was performed when treatment × time was P < 0.05 or 0.05 ≤ P < 0.10 in order to detect differences among treatment groups at the same time point.
Data for feed and nutrient intake and BW were further analyzed as per the following model (Eq. (2)):
| Yij= μ + Treatmenti+ Cattlej+ eij | (2) |
where Yij is the dependent variable, μ is the overall mean, Treatmenti is the fixed treatment effect, Cattlej is the random effect of the cattle, and eij is a random error.
Data for concentrations of ghrelin in milk and plasma were analyzed as per the following model (Eq. (3)):
| Yijk= μ + Samplei+ Timej+ (Sample × Time)ij+ Cattlek+ eijk | (3) |
where Yijk is the dependent variable, μ is the overall mean, Samplei is the fixed sample effect, Timej is the fixed time, (Sample × Time)ij is the sample interaction by time, Cattlek is the random effect of the cattle, and eijk is a random error.
Pearson's correlation coefficient (r) was determined between concentrations of ghrelin and IGF-1 in milk and plasma, and between concentrations of ghrelin in milk and elapsed time from parturition to sample collection. The effects of treatment, time, and treatment by time interaction were considered significant at P < 0.05 and tendencies were assumed at 0.05 ≤ P < 0.10. Data are presented as the mean ± SEM.
3. Results
3.1. Feed intake and body weight
Dry matter intake was not different between treatment groups throughout the study (Table 2). There was also no difference between treatment groups in CP, TDN, or in the net energy intake for maintenance and lactation (NEM and NEL, respectively) in both the pre- and postpartum periods. The intake of Ca-octanoate for the OCT group was 91.5 ± 4.62 g/d and 118 ± 5.14 g/d at pre- and postpartum period, respectively. Body weight did not differ between treatment groups (Table 2).
Table 2.
Feed and nutrient intake and body weight during pre- and postpartum in cows fed concentrate without (CON) or with Ca-octanoate supplementation (OCT).
| Treatments |
||||
|---|---|---|---|---|
| Item | CON | OCT | SEM | P-value |
| Prepartum | ||||
| Total DMI, kg/d | 6.05 | 5.93 | 0.20 | 0.78 |
| Hay DMI, kg/d | 4.16 | 4.23 | 0.19 | 0.87 |
| Concentrate DMI, kg/d | 1.84 | 1.69 | 0.06 | 0.20 |
| Soybean meal DMI, g/d | 48.7 | 11.0 | 15.2 | 0.23 |
| CP intake, kg/d | 1.05 | 1.02 | 0.03 | 0.64 |
| Ca-octanoate intake, g/d | – | 91.5 | 4.62 | – |
| TDN intake, kg/d | 3.95 | 3.85 | 0.13 | 0.70 |
| NEM intake, Mcal/d | 7.32 | 7.18 | 0.25 | 0.78 |
| NEL intake, Mcal/d | 6.96 | 6.82 | 0.23 | 0.78 |
| BW, kg | 511 | 486 | 20.3 | 0.56 |
| Postpartum | ||||
| Total DMI, kg/d | 8.02 | 7.59 | 0.22 | 0.36 |
| Hay DMI, kg/d | 5.45 | 5.23 | 0.26 | 0.69 |
| Concentrate DMI, kg/d | 2.08 | 1.86 | 0.12 | 0.36 |
| Soybean meal DMI, g/d | 478 | 496 | 107 | 0.94 |
| CP intake, kg/d | 1.54 | 1.47 | 0.06 | 0.58 |
| Ca-octanoate intake, g/d | – | 118 | 5.14 | – |
| TDN intake, kg/d | 5.24 | 4.94 | 0.14 | 0.30 |
| NEM intake, Mcal/d | 11.29 | 10.65 | 0.28 | 0.27 |
| NEL intake, Mcal/d | 10.8 | 10.1 | 0.27 | 0.27 |
| BW, kg | 465 | 442 | 20.1 | 0.59 |
DMI = dry matter intake, CP = crude protein, TDN = total digestible nutrients, NEM = net energy for maintenance, NEL = net energy for lactation, BW = body weight.
3.2. Hormone concentrations
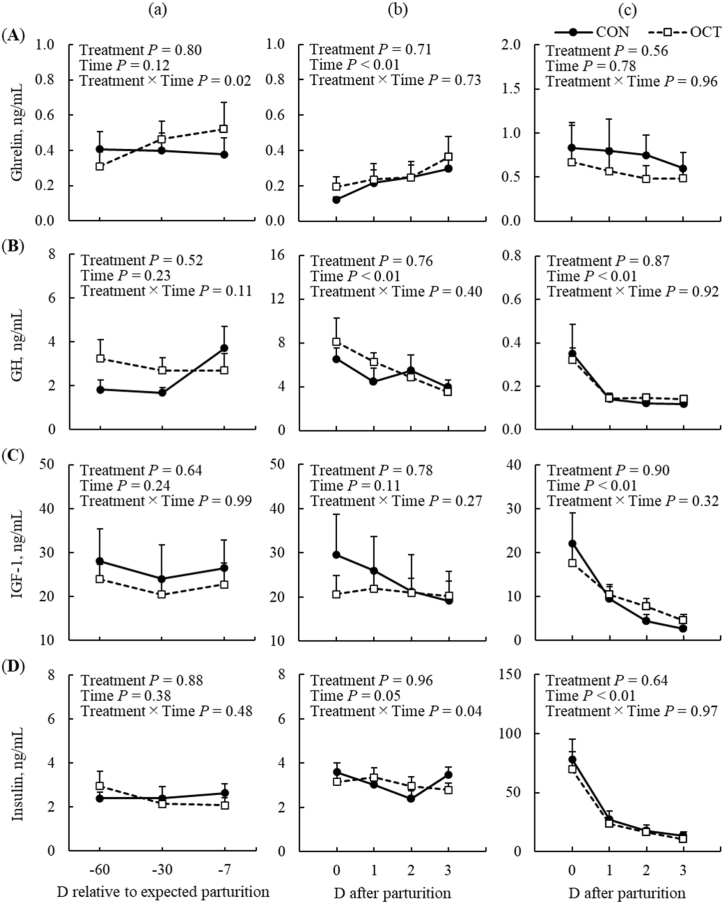
The concentrations of hormones in plasma and milk are shown for the two treatment groups in Fig. 2. There was a treatment by time interaction observed for concentrations of acylated ghrelin in plasma during the prepartum period (P = 0.02; Fig. 2Aa); there was a temporal increase in concentrations of acylated ghrelin in plasma of the OCT group (P < 0.01), but no change observed in the CON group during the pre-partum period. Concentrations of acylated ghrelin in plasma increased in both treatment groups with time postpartum (Fig. 2Ab), but concentrations in plasma and milk were unaffected by treatment during the postpartum period (Fig. 2Ab and Ac, respectively). No treatment effects were observed for concentrations of GH, IGF-1, and insulin in plasma during both the pre- and postpartum periods (Fig. 2Ba, Bb, Ca, Cb, Da, and Db, respectively). However, concentrations of GH and insulin in plasma decreased with time from 0 to 3 d after parturition (Time P < 0.01 and P = 0.05; Fig. 2Bb and Db, respectively). Similarly, concentrations of GH, IGF-1, and insulin in milk decreased time with from 0 to 3 d after parturition (Time P < 0.01) with no difference observed between treatment groups (Fig. 2Bc, Cc, and 2Dc, respectively).
Fig. 2.
Concentrations of hormones in plasma and milk: acylated ghrelin (A), growth hormone (GH) (B), insulin-like growth factor-1 (IGF-1) (C), and insulin (D) in cows fed concentrate without (CON) or with Ca-octanoate supplementation (OCT). Panels (a), (b), and (c) correspond to concentrations in plasma at prepartum, those at postpartum, and concentrations in milk, respectively. Data are presented as the mean ± SEM.
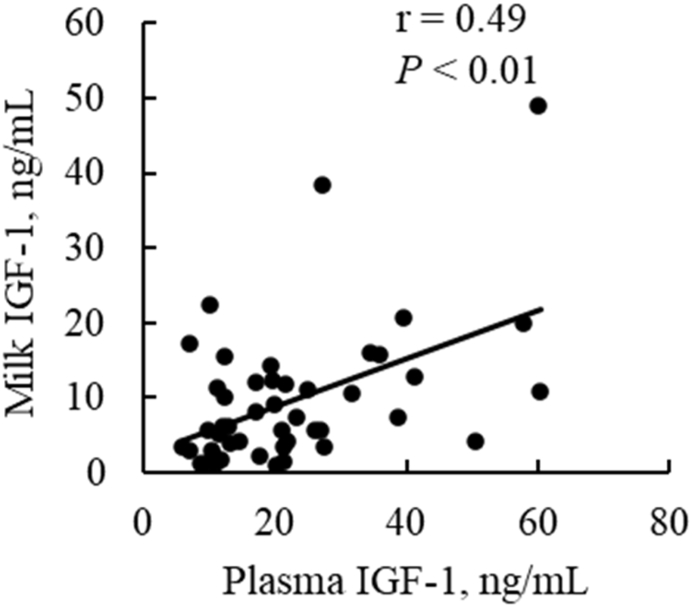
Fig. 3A shows concentrations of acylated ghrelin in milk and plasma during the postpartum period for all cows (n = 12) irrespective of treatment groups. Concentrations of acylated ghrelin were significantly higher (Sample P = 0.01) in milk (0.64 ± 0.09 ng/mL) than in plasma (0.25 ± 0.03 ng/mL) and did not change from d 0 to 3 postpartum. As shown in Fig. 3B, concentrations of acylated ghrelin in milk were negatively correlated with those in plasma (r = −0.50, P < 0.01). No significant correlation was detected between elapsed time from parturition to sample collection and concentrations of acylated ghrelin in milk (Fig. 3C). Concentrations of IGF-1 in milk were positively correlated with those in plasma (r = 0.49, P < 0.01; Fig. 4).
Fig. 3.
(A) Concentrations of acylated ghrelin in milk and plasma of cows. Data are presented as the mean ± SEM. (B) The correlation coefficient (r) between concentrations of acylated ghrelin in plasma and milk. (C) The correlation coefficient (r) between elapsed time from parturition to sample collection and concentrations of acylated ghrelin in milk.
Fig. 4.
The correlation coefficient (r) between concentrations of IGF-1 in plasma and milk.
3.3. Metabolite concentrations
Concentrations of metabolites in plasma and milk are shown in Fig. 5. Concentrations of T-cho were significantly higher in plasma (Treatment P = 0.02 and P = 0.01, respectively) during both pre- and postpartum periods in the OCT group than in the CON group; mean concentrations of T-cho in plasma during the prepartum period were 164.1 ± 3.9 mg/dL and 137.4 ± 5.0 mg/dL for the OCT and the CON group, respectively, and those for the postpartum period were 142.7 ± 2.2 mg/dL and 124.7 ± 2.6 mg/dL for the OCT and the CON group, respectively (Fig. 5Aa and Bb, respectively). There was a treatment by time interaction observed for concentrations of T-cho in milk (P = 0.05), wherein concentrations were higher in milk of the OCT group (461.5 ± 87.0 mg/dL) than that of the CON group (289.3 ± 87.4 mg/dL) at d 0 (Fig. 5Ac). There was no difference in concentrations of glucose between treatment groups during the prepartum period (Fig. 5Ba). However, concentrations of glucose in plasma during the postpartum period declined with time (P < 0.01) and tended to be higher (P = 0.09) in the OCT group (60.7 ± 1.0 mg/dL) than in the CON group (56.6 ± 1.2 mg/dL) (Fig. 5Bb). There was a tendency for a treatment by time interaction for concentrations of glucose in milk to be higher (P = 0.07) in the OCT group (253.1 ± 61.0 mg/dL) than in the CON group (153.8 ± 43.3 mg/dL) at d 0 (Fig. 5Bc). Concentrations of NEFA in plasma and milk did not differ between treatment groups (Fig. 5Ca–c).
Fig. 5.
Concentrations of metabolites in plasma and milk: total cholesterol (T-cho) (A), glucose (B), and nonesterified fatty acid (NEFA) (C) in cows fed concentrate without (CON) or with Ca-octanoate supplementation (OCT). Panels (a), (b), and (c) correspond to concentrations in plasma at prepartum, those at postpartum, and concentrations in milk, respectively. Data are presented as the mean ± SEM. Asterisks (*) indicate significant differences at P < 0.05.
4. Discussion
As reported in previous studies, feeding MCFAs to cows typically decreases DMI [28,32], which can cause impaired fetal growth, decreased milk yield, and postpartum metabolic disorders in dairy cows [[33], [34], [35]]. However, no adverse effects of Ca-octanoate supplementation on DMI and BW were observed in the current study. Inconsistent results between the present and previous studies likely results from the difference in animal species, feeding method, life stage, and the amount and composition of MCFAs offered.
No significant differences were observed for preprandial concentrations of acylated ghrelin in plasma between treatment groups during late gestation. However, it should be noted that an increase in concentrations of acylated ghrelin in plasma during prepartum period was observed for the OCT group but not for the CON group. Thus, it is suggested that feeding Ca-octanoate increases concentrations of acylated ghrelin in plasma of prepartum beef cattle, which is consistent with clinical observations [18].
In contrast to the pre-partum period, there were no treatment, or treatment × time effects observed in concentrations of acylated ghrelin in plasma during the postpartum period. Irrespective of treatment groups, intake of NEM and NEL was significantly higher in the postpartum than in the pre-partum period. In addition, concentrations of ghrelin and NEFA in plasma, which increase in negative energy balance [36], were significantly lower in the postpartum than in the pre-partum period, regardless of dietary treatment groups. Thus, energy balance may have been improved in the postpartum as compared to the prepartum period. It was previously reported that the expression of ghrelin O-acyltransferase (GOAT), an enzyme for ghrelin acylation, was increased when the nutritional status was reduced [37]. Therefore, we assume that the lack of effect of feeding Ca-octanoate on concentrations of acylated ghrelin in plasma during the postpartum period is associated with altered acylation of ghrelin, although the precise mechanism is unclear.
In previous studies, feeding MCFA and octanoate affected concentrations of GH and insulin in blood of pigs and IGF-1 in cachectic patients through increased concentrations of ghrelin [18,38]. However, as noted earlier concentrations of acylated ghrelin in plasma did not differ between treatment groups, which likely results in the lack of differences in concentrations of GH, IGF-1, and insulin in plasma throughout the study. Similarly, the same argument holds for unaltered concentrations of GH, IGF-1, and insulin in colostrum because these are derived from maternal circulation [7,9,10].
The present study revealed for the first time that acylated ghrelin is present in bovine colostrum. Furthermore, concentrations of acylated ghrelin in colostrum were significantly higher than those in maternal plasma, which has been noted for other colostrum hormones, such as IGF-1 and insulin [8,39]. Higher concentrations of hormones in colostrum are not unusual because we have previously reported that concentrations of glucagon-like peptide 2, a gastrointestinal hormone, was also higher in colostrum than in maternal plasma of Japanese Black cattle [40]. In this study, concentrations of GH, IGF-1, and insulin in milk decreased with time after parturition. In addition, concentrations of IGF-1 in milk were positively correlated with those in plasma postpartum. These results are consistent with previous studies [10,39]. In contrast, concentrations of acylated ghrelin in milk were negatively correlated with those in plasma. In addition, concentrations of acylated ghrelin in milk did not fluctuate through 0–3 d postpartum and there was no significant relationship observed between concentrations of acylated ghrelin in milk and elapsed time after parturition. These characteristics are unique and unlike hormones such as IGF-1 and insulin [7,39].
There was no difference in concentrations of acylated ghrelin in milk between treatment groups. As described above, ghrelin in milk is derived from the maternal blood [23]. Therefore, the lack of a difference in concentrations of acylated ghrelin in milk is due to the unaltered concentrations of ghrelin in plasma.
In the present study, concentrations of T-cho in plasma were higher in the OCT group than in the CON group, which is consistent with the previous study, where feeding of MCFAs increased concentrations in dairy cows [28]. Ingested MCFAs are oxidized in the liver and degraded to acetyl-CoA, one of the substrates for cholesterol [19,41]. Therefore, supplemented Ca-octanoate may be absorbed and oxidized in the liver, resulting in increased concentrations of T-cho in plasma of cows in the OCT group.
Concentrations of glucose in plasma tended to be higher for the OCT group than for the CON group in the postpartum period. In a previous study, feeding medium chain triglycerides increases preprandial concentrations of glucose in plasma by enhancing glycolytic flux [42,43]. It is also reported that MCFAs offered to ruminants increased that of propionate, an indispensable precursor of gluconeogenesis [44], in in vitro and in vivo studies [45,46]. Thus, a possible enhancement of glycolysis and/or an increase in concentrations of propionate in the rumen likely account for increased concentrations of glucose in plasma of cows in the OCT group. In addition, the intake of Ca-octanoate in the OCT group was higher in the postpartum than in the prepartum period because of a greater intake of feed, which would also account for the trend for higher concentrations of glucose in blood of the OCT group during the postpartum period.
The tendency for increased concentrations of glucose in colostrum is likely due to a tendency for increased concentrations in plasma postpartum because glucose in milk is transported from maternal circulation by facilitated diffusion [47]. Similarly, higher concentrations of T-cho in colostrum of the OCT group may also be associated with higher concentrations of T-cho in plasma in both the pre- and postpartum periods as T-cho in milk are derived from maternal circulation [48]. Neonatal calves often experience marked hypoglycemia, which has a negative impact on subsequent growth [49], due to low ability of gluconeogenesis [49,50]. Thus, it is essential to provide calves with glucose via colostrum. Cholesterol is also a component of biological membranes, a precursor for steroid hormones, bile acid and vitamin D and has a role in cell signaling via hormones or growth factors [51,52]. Therefore, it is plausible that increased concentrations of glucose and T-cho in colostrum of cows fed Ca-octanoate have significant implications for the growth and health of neonatal beef calves.
In conclusion, dietary supplementation of Ca-octanoate during the late gestation and early postpartum periods did not affect preprandial concentrations of acylated ghrelin in plasma, which is consistent with unaltered concentrations of GH, IGF-1, and insulin in plasma. In addition, no difference was observed in concentrations of GH, IGF-1, and insulin in milk between treatment groups, which is likely due to a limited effect of Ca-octanoate on concentrations of GH, IGF-1, and insulin in plasma. Feeding Ca-octanoate increased concentrations of glucose and T-cho in plasma, which resulted in increased concentrations of glucose and T-cho in colostrum. These results extend our understanding of the effect of feeding octanoate on concentrations of ghrelin and other components in plasma and milk. The results may have implications for improved growth and health of beef cattle and calves.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was supported by the Japan Society for the Promotion of Science [Grant-in-Aid for Scientific Research no., 19K06355 and 20K15649].
References
- 1.Hammon H.M., Blum J.W. Metabolic and endocrine traits of neonatal calves are influenced by feeding colostrum for different durations or only milk replacer. J. Nutr. 1998;128(3):624–632. doi: 10.1093/jn/128.3.624. [DOI] [PubMed] [Google Scholar]
- 2.Godden S. Colostrum management for dairy calves. Vet. Clin. Food Anim. Pract. 2008;24(1):19–39. doi: 10.1016/j.cvfa.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartol F.F., Wiley A.A., Bagnell C.A. Epigenetic programming of porcine endometrial function and the lactocrine hypothesis. Reprod. Domest. Anim. 2008;43(s2):273–279. doi: 10.1111/j.1439-0531.2008.01174.x. [DOI] [PubMed] [Google Scholar]
- 4.Soberon F., et al. Preweaning milk replacer intake and effects on long-term productivity of dairy calves. J. Dairy Sci. 2012;95(2):783–793. doi: 10.3168/jds.2011-4391. [DOI] [PubMed] [Google Scholar]
- 5.Georgiev I.P. Effect of colostrum insulin-like growth factors on growth and development of neonatal calves. Bulg. J. Vet. Med. 2008;11(2):75–88. [Google Scholar]
- 6.Kirovski D., et al. Effects of peroral insulin and glucose on circulating insulin-like growth factor-1, its binding proteins and thyroid hormones in neonatal calves. Can. J. Vet. Res. 2008;72(3):253–258. [PMC free article] [PubMed] [Google Scholar]
- 7.Phomvisith O., et al. Effects of nutritional status on hormone concentrations of the somatotropin axis and metabolites in plasma and colostrum of Japanese Black cows. Anim. Sci. J. 2017;88(4):643–652. doi: 10.1111/asj.12686. [DOI] [PubMed] [Google Scholar]
- 8.Oda S., et al. Insulin-like growth factor-I, GH, insulin and glucagon concentrations in bovine colostrum and in plasma of dairy cows and neonatal calves around parturition. Comp. Biochem. Physiol., A: Comp. Physiol. 1989;94(4):805–808. doi: 10.1016/0300-9629(89)90638-5. [DOI] [PubMed] [Google Scholar]
- 9.Prosser C.G. Insulin-like growth factors in milk and mammary gland. J. Mammary Gland Biol. Neoplasia. 1996;1(3):297–306. doi: 10.1007/BF02018082. [DOI] [PubMed] [Google Scholar]
- 10.Mann S., et al. Effect of dry period dietary energy level in dairy cattle on volume, concentrations of immunoglobulin G, insulin, and fatty acid composition of colostrum. J. Dairy Sci. 2016;99(2):1515–1526. doi: 10.3168/jds.2015-9926. [DOI] [PubMed] [Google Scholar]
- 11.Takahashi H., et al. Ghrelin enhances glucose-induced insulin secretion in scheduled meal-fed sheep. J. Endocrinol. 2006;189(1):67–75. doi: 10.1677/joe.1.06310. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi H., et al. Leptin and ghrelin expressions in the gastrointestinal tracts of calves and cows. J. Vet. Med. Sci. 2020;82(4):475–478. doi: 10.1292/jvms.19-0680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kojima M., et al. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402(6762):656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 14.Nishi Y., et al. Structures and molecular forms of the ghrelin-family peptides. Peptides. 2011;32(11):2175–2182. doi: 10.1016/j.peptides.2011.07.024. [DOI] [PubMed] [Google Scholar]
- 15.Nishi Y., et al. Ingested medium-chain fatty acids are directly utilized for the acyl modification of ghrelin. Endocrinology. 2005;146(5):2255–2264. doi: 10.1210/en.2004-0695. [DOI] [PubMed] [Google Scholar]
- 16.Hosoda H., et al. Structural divergence of human ghrelin: identification of multiple ghrelin-derived molecules produced by post-translational processing. J. Biol. Chem. 2003;278(1):64–70. doi: 10.1074/jbc.M205366200. [DOI] [PubMed] [Google Scholar]
- 17.Ida T., et al. Purification and characterization of feline ghrelin and its possible role. Domest. Anim. Endocrinol. 2007;32(2):93–105. doi: 10.1016/j.domaniend.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Ashitani J.-I., Matsumoto N., Nakazato M. Effect of octanoic acid-rich formula on plasma ghrelin levels in cachectic patients with chronic respiratory disease. Nutr. J. 2009;8(1):25. doi: 10.1186/1475-2891-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lemarié F., et al. Dietary caprylic acid and ghrelin O-acyltransferase activity to modulate octanoylated ghrelin functions: what is new in this nutritional field? Prostagl. Leukot. Essent. Fat. Acids. 2018;135:121–127. doi: 10.1016/j.plefa.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 20.Matsui H., et al. Effects of the supplementation of a calcium soap containing medium-chain fatty acids on the fecal microbiota of pigs, lactating cows, and calves. Anim. Sci. J. 2021;92(1) doi: 10.1111/asj.13636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klopp R.N., et al. Effect of medium-chain fatty acids on growth, health, and immune response of dairy calves. J. Dairy Sci. 2022;105(9):7738–7749. doi: 10.3168/jds.2021-21567. [DOI] [PubMed] [Google Scholar]
- 22.Bayourthe C., Vernay M., Moncoulon R. Effect of calcium salts of fatty acids on rumen function and the digestibility of rations by sheep. J. Sci. Food Agric. 1994;64(3):341–347. [Google Scholar]
- 23.Dündar N.O., et al. Ghrelin and adiponectin levels in colostrum, cord blood and maternal serum. Pediatr. Int. 2010;52(4):622–625. doi: 10.1111/j.1442-200X.2010.03100.x. [DOI] [PubMed] [Google Scholar]
- 24.Aoac A. 1990. Official Methods of Analysis of the AOAC. International. vol. I. [Google Scholar]
- 25.International A. Moisture in sugars; 2000. Official Methods of Analysis. [Google Scholar]
- 26.Agriculture, Forestry and Fishery Research Council Secretariat . Japan Livestock Industry Association; Tokyo, Japan: 2008. MAFF, Japanese Feeding Standard for Beef Cattle. [Google Scholar]
- 27.Sugino T., et al. A transient ghrelin surge occurs just before feeding in a scheduled meal-fed sheep. Biochem. Biophys. Res. Commun. 2002;295(2):255–260. doi: 10.1016/s0006-291x(02)00654-x. [DOI] [PubMed] [Google Scholar]
- 28.Fukumori R., et al. Ingestion of medium chain fatty acids by lactating dairy cows increases concentrations of plasma ghrelin. Domest. Anim. Endocrinol. 2013;45(4):216–223. doi: 10.1016/j.domaniend.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 29.McMahon C.D., et al. Neuroregulation of growth hormone secretion in domestic animals. Domest. Anim. Endocrinol. 2001;20(2):65–87. doi: 10.1016/s0739-7240(01)00084-4. [DOI] [PubMed] [Google Scholar]
- 30.Fukumori R., et al. Effects of fat-enriched diet and methionine on insulin sensitivity in lactating cows1. J. Anim. Sci. 2015;93(6):2778–2784. doi: 10.2527/jas.2015-8868. [DOI] [PubMed] [Google Scholar]
- 31.Ganmaa D., et al. A two-generation reproduction study to assess the effects of cows' milk on reproductive development in male and female rats. Fertil. Steril. 2004;82:1106–1114. doi: 10.1016/j.fertnstert.2004.05.073. [DOI] [PubMed] [Google Scholar]
- 32.Reveneau C., et al. Interaction of unsaturated fat or coconut oil with monensin in lactating dairy cows fed 12 times daily. I. Protozoal abundance, nutrient digestibility, and microbial protein flow to the omasum1. J. Dairy Sci. 2012;95(4):2046–2060. doi: 10.3168/jds.2011-4887. [DOI] [PubMed] [Google Scholar]
- 33.Duske K., et al. Metabolism and lactation performance in dairy cows fed a diet containing rumen-protected fat during the last twelve weeks of gestation. J. Dairy Sci. 2009;92(4):1670–1684. doi: 10.3168/jds.2008-1543. [DOI] [PubMed] [Google Scholar]
- 34.Pérez-Báez J., et al. Association of dry matter intake and energy balance prepartum and postpartum with health disorders postpartum: Part I. Calving disorders and metritis. J. Dairy Sci. 2019;102(10):9138–9150. doi: 10.3168/jds.2018-15878. [DOI] [PubMed] [Google Scholar]
- 35.Seyed Almoosavi S.M.M., et al. Effects of late-gestation heat stress independent of reduced feed intake on colostrum, metabolism at calving, and milk yield in early lactation of dairy cows. J. Dairy Sci. 2021;104(2):1744–1758. doi: 10.3168/jds.2020-19115. [DOI] [PubMed] [Google Scholar]
- 36.Wertz-Lutz A.E., et al. Circulating ghrelin concentrations fluctuate relative to nutritional status and influence feeding behavior in cattle1,2. J. Anim. Sci. 2006;84(12):3285–3300. doi: 10.2527/jas.2006-053. [DOI] [PubMed] [Google Scholar]
- 37.González C.R., et al. Influence of chronic undernutrition and leptin on GOAT mRNA levels in rat stomach mucosa. J. Mol. Endocrinol. 2008;41(6):415–421. doi: 10.1677/JME-08-0102. [DOI] [PubMed] [Google Scholar]
- 38.Miller D.W., et al. Dietary stimulation of the endogenous somatotropic axis in weaner and grower-finisher pigs using medium chain triglycerides and cysteamine hydrochloride. J. Anim. Sci. Biotechnol. 2016;7(1) doi: 10.1186/s40104-016-0121-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hammon H.M., Zanker I.A., Blum J.W. Delayed colostrum feeding affects IGF-I and insulin plasma concentrations in neonatal calves. J. Dairy Sci. 2000;83(1):85–92. doi: 10.3168/jds.S0022-0302(00)74859-4. [DOI] [PubMed] [Google Scholar]
- 40.Inabu Y., et al. Glucagon-like peptide 2 (GLP-2) in bovine colostrum and transition milk. Heliyon. 2021;7(5) doi: 10.1016/j.heliyon.2021.e07046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nguyen P., et al. Liver lipid metabolism. J. Anim. Physiol. Anim. Nutr. 2008;92(3):272–283. doi: 10.1111/j.1439-0396.2007.00752.x. [DOI] [PubMed] [Google Scholar]
- 42.Tholstrup T., et al. Effects of medium-chain fatty acids and oleic acid on blood lipids, lipoproteins, glucose, insulin, and lipid transfer protein activities. Am. J. Clin. Nutr. 2004;79(4):564–569. doi: 10.1093/ajcn/79.4.564. [DOI] [PubMed] [Google Scholar]
- 43.Kojima K., Kasai M. Effects of dietary medium-chain triacylglycerol on mRNA level of gluconeogenic enzymes in malnourished rats. J. Nutr. Sci. Vitaminol. 2008;54(6):507–510. doi: 10.3177/jnsv.54.507. [DOI] [PubMed] [Google Scholar]
- 44.Zhang X., et al. Ruminal pH pattern, fermentation characteristics and related bacteria in response to dietary live yeast (Saccharomyces cerevisiae) supplementation in beef cattle. Anim. Biosci. 2022;35(2):184–195. doi: 10.5713/ab.21.0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Machmüller A., Soliva C.R., Kreuzer M. Methane-suppressing effect of myristic acid in sheep as affected by dietary calcium and forage proportion. Br. J. Nutr. 2003;90(3):529–540. doi: 10.1079/bjn2003932. [DOI] [PubMed] [Google Scholar]
- 46.Panyakaew P., et al. Medium-chain fatty acids from coconut or krabok oil inhibit in vitro rumen methanogenesis and conversion of non-conjugated dienoic biohydrogenation intermediates. Anim. Feed Sci. Technol. 2013;180(1):18–25. [Google Scholar]
- 47.McManaman J.L., Neville M.C. Mammary physiology and milk secretion. Adv. Drug Deliv. Rev. 2003;55(5):629–641. doi: 10.1016/s0169-409x(03)00033-4. [DOI] [PubMed] [Google Scholar]
- 48.Mani O., et al. Expression, localization, and functional model of cholesterol transporters in lactating and nonlactating mammary tissues of murine, bovine, and human origin. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010;299(2):R642–R654. doi: 10.1152/ajpregu.00723.2009. [DOI] [PubMed] [Google Scholar]
- 49.Klein K.A., Clark C., Allen A.L. Hypoglycemia in sick and moribund farmed elk calves. Can. Vet. J. 2002;43(10):778–781. [PMC free article] [PubMed] [Google Scholar]
- 50.Hammon H.M., et al. Lactation biology symposium: role of colostrum and colostrum components on glucose metabolism in neonatal calves1,2. J. Anim. Sci. 2013;91(2):685–695. doi: 10.2527/jas.2012-5758. [DOI] [PubMed] [Google Scholar]
- 51.Ontsouka E.C., Albrecht C., Bruckmaier R.M. Invited review: growth-promoting effects of colostrum in calves based on interaction with intestinal cell surface receptors and receptor-like transporters. J. Dairy Sci. 2016;99(6):4111–4123. doi: 10.3168/jds.2015-9741. [DOI] [PubMed] [Google Scholar]
- 52.Baardman M.E., et al. The role of maternal-fetal cholesterol transport in early fetal life: current Insights1. Biol. Reprod. 2013;88(1):24. doi: 10.1095/biolreprod.112.102442. 1–9. [DOI] [PubMed] [Google Scholar]