To the Editor:
Early interstitial lung disease (ILD) has been detected in 14% to 22% of screened relatives of patients with familial pulmonary fibrosis.1,2 Relatives of patients with sporadic IPF have been found with a higher prevalence of interstitial lung abnormalities (ILA) and preclinical ILD than the general population.3 Despite advances made in screening, many previous cohorts have excluded individuals with nonidiopathic pulmonary fibrosis (IPF) ILD. Therefore, we sought to determine the prevalence of subclinical ILD in family members of patients with fibrotic ILD, regardless of subtype.
Methods
The Columbia University Medical Center institutional review board approved this study (AAAR1916). Written informed consent was obtained from all participants. We enrolled asymptomatic first-degree relatives, hereafter referred to as at-risk participants (ARPs), of patients with IPF, chronic hypersensitivity pneumonitis, connective tissue disease-associated ILD, or unclassifiable fibrotic ILD. Familial pulmonary fibrosis kindreds included those with at least two family members with a diagnosis of a fibrotic ILD.
Each ARP had a high-resolution CT scan of the chest at full inspiration. The presence of an ILA, defined as nondependent ground-glass abnormalities, reticulations, nonemphysematous cysts, traction bronchiectasis, or honeycombing that affected >5% of any lung zone was determined by consensus of two thoracic radiologists. Spirometry was performed in accordance with standard guidelines.
We purified genomic DNA from blood using the Gentra Puregene Blood kit (Qiagen,). The MUC5B single nucleotide polymorphism (rs35705950) was genotyped by Sanger sequencing.
In comparing clinical characteristics of ARPs with and without ILA, we used generalized linear models to account for family clustering. Within these models, we included the variable of interest in addition to a random intercept for household number to account for the hierarchic nature of the data.
Results
We enrolled 98 asymptomatic relatives of 57 patients with different fibrotic ILDs (Fig 1). Fifty one ARPs (52%) were related to sporadic cases, and 49 ARPs (48%) were related to those with familial pulmonary fibrosis. Altogether, 48 of the ARPs (49%) were related to patients with a diagnosis of IPF, and 50 ARPs (51%) were related to those with a non-IPF diagnosis. The average (SD) age was 51.3 (8.1) year; 63% were female, and 71% self-identified as non-Hispanic White (Table). We enrolled a mean ± SD number of 1.7 ± 1.2 ARPs from each kindred.
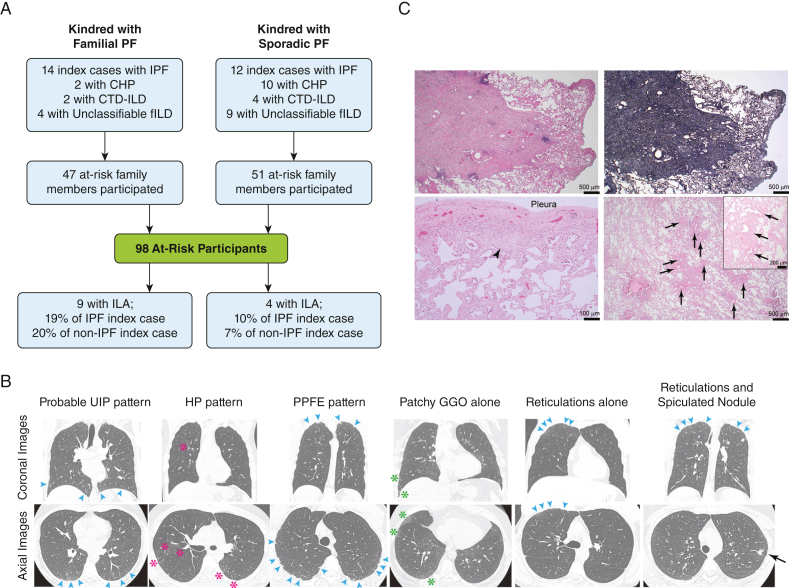
Figure 1.
A, Study flow diagram shows familial and sporadic cases related to at-risk participants who were recruited to the study. A total of 13 at-risk participants were found to have evidence of interstitial lung abnormality (9 from the familial group; 4 from the sporadic group). B, Coronal and axial high-resolution CT imaging of the chest with examples of interstitial lung abnormality that were detected in at-risk participants. The patterns of interstitial lung abnormality included (from left to right): probable usual interstitial pneumonia pattern (bilateral, lower lobe- and basilar-predominant, peripheral reticulations [blue arrowheads]), hypersensitivity pneumonitis pattern (centrilobular nodules with airway-centric ground-glass opacities and traction bronchiolectasis [pink asterisks]), pleuroparenchymal fibroelastosis pattern (peripheral fibrosis [blue arrowheads]), patchy ground-glass opacities alone (green asterisks), focal reticulations alone (blue arrowheads), apical reticulations (blue arrowheads), and spiculated nodular opacity (black arrow). C, Histopathologic evidence of surgical lung biopsy from one at-risk participant from a familial kindred shows a well-delineated area of elastosis within the lung parenchyma that corresponds to the spiculated nodule seen in the radiographic study (B, far right). Additional finding included patchy, mild, pleural thickening and associated fibroblast focus-like changes (arrowhead) with adjacent reactive type 2 pneumocytes and mild smooth muscle hyperplasia within the bronchovascular bundles (arrows in lower- and higher-power images). Representative hemoxylin and eosin-stained and elastin-stained (upper right) sections are shown. Bars represent the indicated distances. CHP = chronic hypersensitivity pneumonitis; CTD-ILD = connective tissue disease associated interstitial lung disease; HP = hypersensitivity pneumonitis; ILA = interstitial lung abnormalities; ILD = interstitial lung disease; IPF = idiopathic pulmonary fibrosis; PPFE = pleuroparenchymal fibroelastosis; UIP = usual interstitial pneumonitis.
Table 1.
Baseline Characteristics of Cohort and the Effect of Each Characteristic on Interstitial Lung Abnormality Risk
| Characteristics | Total (N = 98) | Interstitial Lung Abnormality/Preclinical Interstitial Lung Disease (N = 13) | No Interstitial Lung Abnormality/Preclinical Interstitial Lung Disease (N = 85) | Effect of Characteristic on Interstitial Lung Abnormality Riska |
|
|---|---|---|---|---|---|
| OR (95% CI) | P Value | ||||
| Age, y, mean ± SD | 51.3 ± 8.1 | 55.7 ± 10.9 | 50.8 ± 7.4 | 1.09b (1.01-1.18) | .04 |
| Female, No. (%) | 61 (62) | 10 (77) | 51 (60) | 0.45 (0.12-1.77) | .25 |
| Race/ethnicity, No. (%) | … | … | … | 1.39c (0.35-5.42) | .64 |
| Non-Hispanic white | 70 (71) | 10 (77) | 60 (71) | … | … |
| Hispanic | 22 (23) | 3 (23) | 19 (22) | … | … |
| Asian | 2 (2) | 0 | 2 (2) | … | … |
| Black | 1 (1) | 0 | 1 (1) | … | … |
| Other | 3 (3) | 0 | 3 (4) | … | … |
| BMI, kg/m2, mean ± SD | 29.6 ± 6.4 | 28.0 ± 5.4 | 30.0 ± 6.4 | 0.94 (0.84-1.05) | .95 |
| Smoking status | … | … | … | 1.11 (0.31-3.90) | .88 |
| Ever smoker, No. (%) | 32 (33) | 4 (30) | 28 (33) | … | … |
| Subtype of fibrotic interstitial lung disease in index case,d No. (%) | … | … | … | 1.71 (0.52-5.67) | .38 |
| Idiopathic pulmonary fibrosis | 48 (48) | 7 (54) | 41 (48) | … | … |
| Non-idiopathic pulmonary fibrosisd | 50 (52) | 6 (46) | 44 (52) | … | … |
| Family history, No. (%) | … | … | … | 4.26 (0.86-5.67) | .12 |
| Familial | 48 (49) | 9 (69) | 38 (45) | … | … |
| Sporadic | 50 (51) | 4 (31) | 47 (55) | … | … |
| Physiologic measurements | |||||
| FVC, % predicted, mean ± SD | 100.3 ± 14.8 | 100.6 ± 15.9 | 100.2 ± 14.8 | 1.01 (0.96-1.04) | .92 |
| Diffusion capacity,e % predicted (SD) | 85.6 ± 11.5 | 87.9 ± 12.2 | 85.3 ± 11.4 | 1.02 (0.96-1.08) | .48 |
| Six-minute walk distance, m, mean ± SD | 485 ± 96 | 512 ± 61 | 479 ± 99 | 1.23f (0.86-1.74) | .25 |
| Genomic marker: MUC5B single nucleotide polymorphism (rs35705950) minor allele frequencyg | 0.18 (24 of 136) | 0.30 (6 of 20) | 0.16 (18 of 116) | 3.00 (1.08-8.39) | .03 |
Model included random intercept for household No. to adjust for family clustering/relatedness.
Per 10-y increase in age.
OR represents non-Hispanic White vs all others.
Non-idiopathic pulmonary fibrosis diagnoses included chronic hypersensitivity pneumonitis, connective tissue disease-associated interstitial lung disease, unclassifiable or other fibrotic interstitial lung disease.
Twelve participants with missing data.
OR per 50-m increase in distance.
Minor allele frequency was calculated only for those who self-identified as being White (N = 68).
We identified 13 ARPs with ILA (Fig 1). Five ARPs had bilateral ILA, which included one participant with a probable usual interstitial pneumonitis pattern, two participants with a hypersensitivity pneumonitis pattern, and two participants with a pleuroparenchymal fibroelastosis pattern. Four cases had ground-glass opacities alone; two cases had reticulations alone; one case had reticulations plus traction bronchiectasis, and one case had ground-glass opacities, reticulations, and traction bronchiectasis.
One ARP with an ILA was found to have upper lobe reticulations and a spiculated nodule, which was resected surgically because of a concern for cancer. The biopsy showed a well-delineated area of elastosis with pleural thickening, fibroblast focus-like changes, reactive type 2 pneumocytes, and mild smooth muscle hyperplasia (Fig 1).
Those ARPs with an ILA were older than those without an ILA (Table 1). There was no difference in sex, race/ethnicity, or smoking status between groups. Overall prevalence of ILA was 15% (7 of 48 participants) in those related to patients with IPF and 12% (6 of 50) in those related to patients with non-IPF ILD. ARPs with a family history of lung fibrosis had higher prevalence of ILA (19% [9 of 47 participants]) than those without a family history (8% [4 of 51 participants]). The prevalence of ILA in the familial group did not differ by ILD subtype (19% [5 of 27 participants] for IPF; 20% [4 of 201 participants] for non-IPF). Similarly, the prevalence of ILA in the sporadic group did not differ by ILD subtype (10% [2 of 21 participants] for IPF; 7% [2 of 30 participants] for non-IPF). There was no difference in lung function in ARPs with or without ILA. In adjusted analyses that accounted for familial clustering, increased age (OR 1.09 [95% CI, 1.01 to 1.14]; P = .04), and the presence of the MUC5B risk allele (OR 3.00 [95% CI, 1.08 to 8.39]; P = .03) were associated with ILA (Table 1). We were underpowered to find an association between ILA and family history after adjusting for familial clustering. We found no association between ILA and the subtype of fibrotic ILD (IPF vs non-IPF).
Discussion
This is the first study to our knowledge to examine the prevalence of radiographic abnormalities in first-degree relatives of patients to be diagnosed with a range of different fibrotic lung diseases. Overall, we find the prevalence of ILA to be 13%, with no difference if the index case had IPF or a non-IPF fibrotic ILD. Prior literature supports the broad phenotypic heterogeneity that is associated with familial pulmonary fibrosis4 and progressive pulmonary fibrosis.5,6 This study similarly highlights the importance of maintaining a broad definition of pulmonary fibrosis when screening for ILA.
Like prior studies, this study also finds an increased prevalence of ILA in those with a family history of pulmonary fibrosis.1, 2, 3,7 ILA are found in approximately one in five ARPs with a family history. In contrast, the prevalence of ILA in relatives of sporadic cases (8%) falls within the upper range that has been observed for the general population.8 In this study, we used the term “ILA” regardless of family history, although we recognize that radiographic abnormalities in familial cases are much more likely to represent preclinical disease.8 The prevalence of ILAs found in this cohort is lower than what has been observed in others,2,3,9 which may be related to the younger age and fewer familial cases. Although some studies have excluded pleuroparenchymal fibroelastosis as a manifestation of ILA,10 we have included cases in which the reticulations extend beyond the lung apex. Because the surgical biopsy of the highlighted case demonstrates active fibrosis, this ILA pattern may deserve future investigation.
This study has several limitations. The small numbers limit power to determine differences in ILA prevalence by specific non-IPF diagnoses. Approximately 20% of the index cases were referred by outside pulmonologists, so their diagnoses were ascertained by review of outside medical records. The nonrandom recruitment of multiple ARPs from families may have skewed results. Finally, missing diffusion capacity testing (12%) may have contributed to nonsignificant findings.
Because existing Food and Drug Administration-approved medications for pulmonary fibrosis reduce the rate of progression, screening protocols for detection of the earliest manifestations of subclinical disease and close follow up are of critical importance.
Funding/Support
This study was funded by National Institutes of Health: T32HL105323 (C. F. M.), Stony Wold-Herbert Fund (D. Z.), and R01HL103676 and R01HL093096 (C. K. G.).
Financial/Nonfinancial Disclosures
The authors have reported to CHEST the following: D. Z. reports relationship with Boehringer Ingelheim outside of this work. M. C. reports employment with Hoffman-La Roche. D. L. reports employment and equity in Regeneron Pharmaceuticals. A. S. reports relationships with Genentech, Roche, Bristol Myers Squibb, and Veracyte outside this work. A. J. P. reports relationships with Regeneron Pharmaceuticals, Boehringer Ingelheim, and Roche outside this work. M. M. S. reports relationships with Boehringer Ingelheim and Genentech outside of this work. C. K. G. reports relationships with Boehringer Ingelheim and AstraZeneca outside this work. None declared (E. A. F., C. F. M., K. M. C., B. D. S.).
Acknowledgments
Authorcontributions: C. K. G. had full access to all the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis. D. L. and C. K. G. contributed to study design; M. C., A. P., D. L., and C. K. G. contributed to patient recruitment; C. F. M., D. Z., E. A. H., A. S., K. M. C., B. D. S., M. M. S., and C. K. G. contributed to data analysis and interpretation; and C. F. M. and C. K. G. wrote the first draft of the manuscript. All authors contributed to manuscript review and approved the submitted draft.
Role ofsponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Othercontributions: The authors thank the subjects for their participation in this study; Chad Newton, MD, for helpful comments; Qi Li for bioinformatic expertise; Lesley Vickers and Mason Amelotte for excellent technical assistance; and Safety Monitoring Committee members Joao A. de Andrade, MD (chair), Anil Vachari, MD, and Anna Rozenshtein, MD, for their oversight of this study.
Footnotes
A. G. P. is currently affiliated with Weill Cornell Medical Center, New York, NY. D. L. is currently affiliated with Regeneron Pharmaceuticals, Tarrytown, NY.
References
- 1.Kropski J.A., Pritchett J.M., Zoz D.F., et al. Extensive phenotyping of individuals at risk for familial interstitial pneumonia reveals clues to the pathogenesis of interstitial lung disease. Am J Respir Crit Care Med. 2015;191(4):417–426. doi: 10.1164/rccm.201406-1162OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosas I.O., Ren P., Avila N.A., et al. Early interstitial lung disease in familial pulmonary fibrosis. Am J Respir Crit Care Med. 2007;176(7):698–705. doi: 10.1164/rccm.200702-254OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hunninghake G.M., Quesada-Arias L.D., Carmichael N.E., et al. Interstitial lung disease in relatives of patients with pulmonary fibrosis. Am J Respir Crit Care Med. 2020;201(10):1240–1248. doi: 10.1164/rccm.201908-1571OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newton C.A., Batra K., Torrealba J., et al. Telomere-related lung fibrosis is diagnostically heterogeneous but uniformly progressive. Eur Respir J. 2016;48(6):1710–1720. doi: 10.1183/13993003.00308-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flaherty K.R., Wells A.U., Cottin V., et al. Nintedanib in progressive fibrosing interstitial lung diseases. N Engl J Med. 2019;381(18):1718–1727. doi: 10.1056/NEJMoa1908681. [DOI] [PubMed] [Google Scholar]
- 6.Raghu G., Remy-Jardin M., Richeldi L., et al. Idiopathic pulmonary fibrosis (an update) and progressive pulmonary fibrosis in adults: an official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med. 2022;205(9):e18–e47. doi: 10.1164/rccm.202202-0399ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salisbury M.L., Hewlett J.C., Ding G., et al. Development and progression of radiologic abnormalities in individuals at risk for familial interstitial lung disease. Am J Respir Crit Care Med. 2020;201(10):1230–1239. doi: 10.1164/rccm.201909-1834OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hatabu H., Hunninghake G.M., Richeldi L., et al. Interstitial lung abnormalities detected incidentally on CT: a position paper from the Fleischner Society. Lancet Respir Med. 2020;8(7):726–737. doi: 10.1016/S2213-2600(20)30168-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diaz de Leon A., Cronkhite J.T., Yilmaz C., et al. Subclinical lung disease, macrocytosis, and premature graying in kindreds with telomerase (TERT) mutations. Chest. 2011;140(3):753–763. doi: 10.1378/chest.10-2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hata A., Hino T., Yanagawa M., et al. Interstitial lung abnormalities at CT: subtypes, clinical significance, and associations with lung cancer. Radiographics. 2022;42(7):1925–1939. doi: 10.1148/rg.220073. [DOI] [PMC free article] [PubMed] [Google Scholar]