Abstract
Background
The role of sex differences in clinical presentation, TB drug pharmacokinetic variables, and treatment outcomes is unclear.
Research Question
What is the effect of sex on TB disease severity, drug exposure, and treatment outcome?
Study Design and Methods
This study was a prospective cohort study conducted in India. It assessed TB disease severity; risk of unfavorable treatment outcomes (failure, recurrence, and death) according to sex; and risk factors for unfavorable outcomes stratified according to sex. Effects of sex on the pharmacokinetic variables (maximum concentration and area under the curve) of rifampicin, isoniazid, and pyrazinamide were estimated by using noncompartmental analyses.
Results
Of 1,541 people with microbiologically confirmed TB, 567 (37%) were women. Women had a lower risk of high mycobacterial burden (smear grade ≥ 2 and/or time to detection < 7 days) with an adjusted OR of 0.70 (95% CI, 0.56-0.87). Among the 744 participants who were followed up prospectively, 261 (35%) were women. Women had a lower risk of unfavorable treatment outcomes (adjusted incidence risk ratio, 0.60; 95% CI, 0.43-0.85), mostly because recurrence was lower (adjusted incidence risk ratio, 0.45; 95% CI, 0.23-0.86). Isoniazid (but not rifampicin and pyrazinamide) maximum concentration and area under the curve were significantly higher among women (P < .01) than men. Among women, unfavorable outcomes were more likely among those with cavitary disease, but among men, increased risk of unfavorable outcomes was associated with alcohol use, higher BMI, and lower glycated hemoglobin level.
Interpretation
Women present with lower mycobacterial burden, achieve higher TB drug exposure, and are less likely to have unfavorable treatment outcomes than men. Strategies to improve TB treatment success should take into account sex differences in risk factors for unfavorable outcomes.
Key Words: clinical presentation, drug exposure, mycobacterial burden, sex, TB treatment outcomes
Take-home Points.
StudyQuestion: What is the effect of sex on TB clinical presentation, drug exposure, and treatment outcome?
Results: The prospective cohort found that women had a 30% lower risk of high mycobacterial burden at presentation, achieved higher Cmax and AUC of isoniazid (a key TB drug), and reported a 40% lower risk of unfavorable TB treatment outcomes.
Interpretation: TB disease severity at presentation and treatment outcomes are lower among women compared with men, likely explained by the higher TB drugs’ target attainments among women.
TB is among the top 10 causes of death worldwide.1 In 2019, among 8.8 million adults who developed TB disease, 36% were women. India has the highest burden of new TB cases and TB deaths,2 with women constituting about one-third of cases.3 Notably, in India, TB has become the fifth leading cause of death among women, ahead of maternal death.2 Understanding sex differences in TB presentation and treatment outcomes is critical to successful diagnosis and treatment of TB and to achieving global TB control goals.
Historically, notification of TB cases has been significantly higher among men than women in most regions of the world.4,5 Biological mechanisms, including augmented susceptibility,6 as well as behavioral differences may play a significant role in higher detection of TB among men.7, 8, 9 In addition, women may be at risk of undernotification due to socioeconomic and cultural factors that lead to barriers in accessing health care.10 Regarding treatment outcomes, however, men tend to do worse.11,12 The reasons for this have not been fully elucidated but seem to vary by setting and are multifactorial.13,14
The majority of sex-specific TB research has focused on differences in women’s access to health care and delays in seeking health services.10,15 Limited evidence exists on sex-specific clinical presentation and treatment outcomes in resource-limited settings such as India.16, 17, 18, 19 Specifically, data on TB drug exposure, by sex, are scarce.20, 21, 22 Understanding sex differences in clinical presentation, drug exposure, and TB treatment outcomes will help identify specific subgroups that may benefit from close monitoring during and beyond TB treatment and sex-specific targeted public health interventions. Therefore, using an established prospective cohort of active TB in Pune, India, the aim of the current study was to assess the impact of sex on the clinical presentation of TB, exposure to TB drugs, and TB treatment outcomes.
Study Design and Methods
Study Design
This analysis was conducted by using data collected as part of two linked studies funded under the same National Institutes of Health (NIH) grant. The first study was a cross-sectional study evaluating patients with presumptive TB accessing care at the Revised National TB Control Programme centers in the Pune and Pimpri-Chinchwad Municipal Corporation areas. In the first study, hereafter referred to as the “Prevalence Study,” participants were screened to assess the prevalence of diabetes mellitus (DM).23 Building from the Prevalence Study, the second study, a prospective cohort, was established that enrolled adult patients with drug-sensitive pulmonary TB, with and without DM, and followed them up for 18 months (hereafter referred to as “TB-DM Cohort Study”)24 (e-Fig 1).
The TB-DM Cohort Study enrollment occurred at Byramjee Jeejeebhoy Clinical Research Site and Dr. D. Y. Patil Medical College in Pune, India. The government’s TB units provided routine TB treatment as per national program guidelines. The thrice-weekly regimen was directly observed therapy. It included 450 mg (600 mg for ≥ 60 kg body weight) of rifampin, 600 mg of isoniazid, 1,200 mg of ethambutol, and 1,500 mg of pyrazinamide during the intensive phase followed by rifampin and isoniazid at the same doses during the continuation phase.24 Baseline testing included sputum smear for acid-fast bacilli (AFB) staining, Xpert MTB/RIF (Cepheid) assay, culture (in Mycobacterial Growth Indicator Tube liquid culture and Lowenstein-Jensen media), chest radiography, and glycated hemoglobin (HbA1c) (Bio-Rad Laboratories). Follow-up visits occurred biweekly in the intensive phase (first 8 weeks) of anti-TB treatment, every 4 weeks during the continuation phase (up to 6 months), and at 12 and 18 months, and included AFB smear, sputum culture, and HbA1c testing. Semi-intensive plasma pharmacokinetic sampling was performed 4 to 6 weeks postinitiation of TB treatment, at predose, and at 0.5, 2, and 6 hours’ postdose for rifampicin, isoniazid, and pyrazinamide; sparse sampling was then performed 10 to 12 weeks postinitiation of TB treatment, 2 hours’ postdose for rifampicin and isoniazid, as previously described.25
Rifampicin, isoniazid, and pyrazinamide drug levels were measured by using high-performance liquid chromatography (Shimadzu Corporation). Acetonitrile was used for rifampin extraction, and a C18 column at 254 nm was used for analysis. Para-hydrobenzaldehyde and trifluoroacetic acid were used for isoniazid and pyrazinamide extraction, respectively; column C8 at 267 nm was used for the analysis. The lower limits of quantification were 0.25, 1.25, and 0.25 μg/mL, respectively. All of the drugs had < 10% within- and between-run variation.26, 27, 28
Study Outcomes
The primary outcomes for this analysis were: (1) severity of clinical presentation; and (2) composite unfavorable TB treatment (TB treatment failure, recurrence, or all-cause mortality). Clinical presentation comprised radiographic and microbiological findings collected at screening. Severe radiologic findings were defined as the presence of cavitary lung lesions and/or the involvement of two or more lung lobes. Microbiologically “severe” TB was defined as ≥ 2+ sputum AFB smear and/or time to TB detection (TTD) in liquid culture of less than the median value of 7 days.
Composite unfavorable TB treatment outcome was a primary end point, whereas each component (failure, recurrence, and mortality) was assessed independently in secondary analyses. TB treatment failure was defined as positive smear or culture at months 5 or 6, and TB recurrence was indicated by a new diagnosis of TB following cure or completion of TB treatment. Mortality was defined as all-cause mortality measured through 18 months following initiation of TB treatment. We also evaluated the effect of sex on TB drug concentrations for rifampicin, isoniazid, and pyrazinamide. Additional outcomes assessed include 2- and 1-month culture conversion, time to culture conversion on TB treatment, and unfavorable outcomes stratified according to sex.
Statistical Analysis
To assess the impact of sex on TB clinical presentation, study data from the Prevalence Study were used. Baseline categorical and continuous variables were used to compare sociodemographic factors and clinical characteristics according to sex. These were summarized by using proportions and median values with interquartile range (IQR) and were compared by using χ2 test/Fisher exact test for calculating P values. Univariable and multivariable logistic regression was used to compare the radiographic and microbiological features of TB disease between both sexes. The prevalence study had > 80% power to detect the difference observed in the clinical presentation, smear grade ≥ 2, and/or TTD between both sexes.
To assess the effect of sex on TB treatment outcomes, the TB-DM Cohort Study data were used. Sex differences for unfavorable composite TB treatment outcomes (death, treatment failure, and recurrence) were calculated by using univariable and multivariable Poisson regression analysis. We adjusted the variables age (linear), education, BMI (linear), HBA1c (linear), and HIV to estimate effect of sex on TB clinical presentation, and age (linear), education, BMI (linear), HBA1c (linear), TTD, and smear grade were adjusted to estimate effect of sex on TB treatment outcomes. The hazard ratio (HR) was calculated to analyze the impact of sex on TB recurrence, all-cause mortality, 2- and 1-month culture conversion, and time to culture conversion. A Kaplan-Meier curve was plotted to compare time to death between men and women. Univariable Poisson regression was performed to determine the association between treatment outcomes and the various exposure variables stratified according to sex. In all tests, a P value < .05 was considered statistically significant and included in the multivariate model. With 483 men and 261 women in the TB-DM Cohort Study, the analysis had > 90% power to detect the observed differences in a composite unfavorable outcome between men and women.
The therapeutic target concentrations considered in this analysis were 8 μg/mL, 3 μg/mL, and 35 μg/mL for rifampicin, isoniazid, and pyrazinamide, respectively.29, 30, 31, 32 To estimate and compare the maximum concentration (Cmax) and area under the curve (AUC) of rifampicin, isoniazid, and pyrazinamide between sexes, noncompartmental analysis using the Phoenix WinNonlin program was conducted. Concentrations below the limit of quantitation were assigned a value of below the limit of quantitation divided by 2 in analyses. Cmax and AUC were displayed by the geometric mean, and the estimated parameter is displayed by geometric mean ratio (GMR). Data were entered on the tablet using the emocha mobile health platform (emocha Health) and were analyzed by using Stata version 14.2 (StataCorp).
Patient Consent and Ethics Approvals
Each participant provided written informed consent. Ethics approval was obtained from the Institutional Review Board of Johns Hopkins School of Medicine and the ethics committees at Byramjee Jeejeebhoy Medical College-Sassoon General Hospital and Dr. D. Y. Patil Vidyapeeth.
Results
TB Clinical Presentation According to Sex
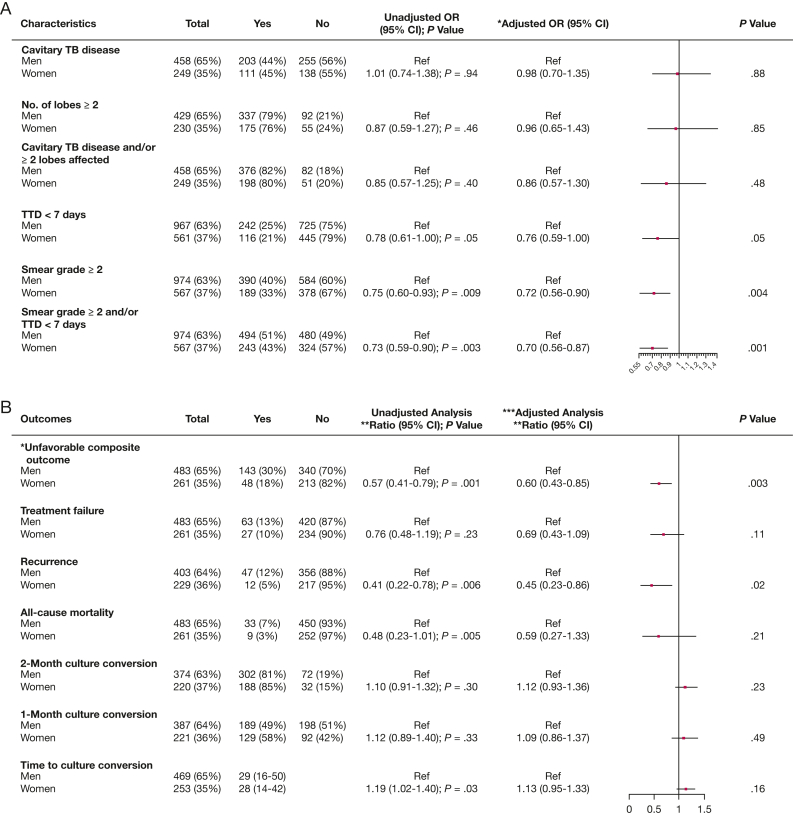
Between 2013 and 2018, a total of 1,541 patients with culture-confirmed pulmonary TB were enrolled in the Prevalence Study. Overall, the median age was 31 (IQR, 24-45) years, 567 (37%) were women, 95 (6%) were HIV-positive, and 311 (20%) had DM (e-Fig 1). As shown in Table 1, the sociodemographic and clinical characteristics of screened and microbiologically confirmed TB participants at baseline were comparable overall between men and women. However, compared with women, men were more likely to be older, be smokers, drink alcohol, and have DM. Compared with men, women were less likely to have microbiologically or radiologically severe TB (Fig 1A). Specifically, women had lower odds of high mycobacterial burden in both univariable analyses (OR, 0.73; 95% CI, 0.59-0.90; P = .003) and analyses adjusted for age, education, BMI, HbA1c, and HIV (adjusted OR, 0.70; 95% CI, 0.56-0.87).
Table 1.
Characteristics of TB Participants Enrolled in a Cross-Sectional Study and Subsequently Into a Prospective Cohort in Pune, India
| Characteristic | Total in Cross-Sectional Study (n = 1,541) |
Total Enrolled in Prospective Cohort Study (n = 744) |
||||
|---|---|---|---|---|---|---|
| Men | Women | P Value | Men | Women | P Value | |
| Total No. of patients | 974 (63%) | 567 (37%) | 483 (65%) | 261 (35%) | ||
| Age, y | ||||||
| 18-40 | 625 (64%) | 456 (80%) | 296 (61%) | 204 (78%) | ||
| > 40 | 349 (36%) | 111 (20%) | < .001 | 187 (39%) | 57 (22%) | < .001 |
| Education | ||||||
| Illiterate | 154 (16%) | 121 (21%) | 76 (16%) | 56 (21%) | ||
| Primary/more than primary | 820 (84%) | 446 (79%) | .006 | 407 (84%) | 205 (79%) | .05 |
| Smoking | ||||||
| No | 666 (68%) | 566 (99%) | 342 (71%) | 260 (99%) | ||
| Yes | 308 (32%) | 1 (1%) | .006 | 141 (29%) | 1 (1%) | < .001 |
| Alcohol | ||||||
| No | 533 (55%) | 265 (99%) | 264 (55%) | 259 (99%) | ||
| Yes | 441 (45%) | 2 (1%) | < .001 | 219 (45%) | 2 (1%) | < .001 |
| Clinical characteristics | ||||||
| BMI, kg/m2 | ||||||
| Normal (18.5-25) | 266 (28%) | 172 (30%) | 145 (30%) | 77 (30%) | ||
| Underweight (< 18.5) | 672 (70%) | 355 (63%) | 319 (67%) | 166 (64%) | ||
| Overweight (≥ 25) | 29 (3%) | 39 (7%) | < .001 | 16 (3%) | 17 (6%) | < .001 |
| Diabetic status | ||||||
| No DM | 560 (58%) | 385 (68%) | 233 (48%) | 146 (56%) | ||
| Pre-DM | 187 (19%) | 98 (17%) | 97 (20%) | 61 (23%) | ||
| DM | 227 (23%) | 84 (15%) | < .001 | 153 (32%) | 54 (21%) | < .001 |
| HbA1c, % | ||||||
| < 5.6 | 232 (33%) | 229 (40%) | 144 (30%) | 84 (32%) | ||
| 5.6-6.5 | 437 (45%) | 256 (45%) | 195 (40%) | 124 (48%) | ||
| ≥ 6.5 | 214 (22%) | 82 (15%) | < .001 | 144 (30%) | 53 (20%) | .02 |
| HbA1c, median (IQR), % | 5.8 (5.4-6.3) | 5.6 (5.4-6.0) | .0004 | 5.9 (5.4-6.9) | 5.7 (5.5-6.2) | .06 |
| HIV | ||||||
| Negative | 898 (94%) | 521 (93%) | ||||
| Positive | 58 (6%) | 37 (7%) | < .001 | |||
DM = diabetes mellitus; HbA1c = glycated hemoglobin; IQR = interquartile range.
Figure 1.
The estimated effect of sex on TB clinical presentation (A) and on TB treatment outcomes (B) in Pune, India. A, *Adjusted for age (linear), education, BMI (linear), HBA1c (linear), HIV. B, *Unfavorable composite outcome = composite outcome includes treatment failure, recurrence and all-cause mortality; **Measures of association (ratio) unfavorable composite outcomes and TB treatment failure: incidence risk ratio; Recurrence and all-cause mortality, 2-month culture conversion, 1-month culture conversion, time to culture conversion: hazard ratio; ***Adjusted for age (linear), education, BMI (linear), HBA1c (linear). TTD = time to TB detection.
Culture Conversion on TB Treatment According to Sex
Of 1,541 patients with culture-positive pulmonary TB, a total of 744 (48%) were enrolled in the prospective cohort study. The median age was 32 (IQR, 24-46) years, 261 (35%) were women, and 207 (28%) had DM. The sociodemographic and clinical characteristics were similar between men and women. However, compared with women, men were more likely to be smokers, drink alcohol, and have DM (Table 1).
There was a trend toward higher 2-month (85% vs 81%; adjusted HR; 1.14; 95% CI, 0.95-1.38; P = .16) as well as 1-month (58% vs 49%; adjusted HR, 1.13; 95% CI, 0.89-1.42; P = .31) culture conversion in women compared with men, but these differences did not reach statistical significance (Fig 1B). Median time to culture conversion was 28 (IQR, 14-42) days for women and 29 (IQR, 16-50) days for men (P = .08) (Fig 2A).
Figure 2.
Kaplan-Meier curve showing time to culture conversion (A) and time to all-cause mortality (B) according to sex in Pune, India. ATT = anti-TB treatment.
Drug Concentration According to Sex
Pharmacokinetic values are shown according to sex in Table 2. The isoniazid Cmax (GMR, 1.3 [95% CI, 1.0-1.68, P = .046]; adjusted GMR, 1.29 [95% CI, 1.0-1.68; P = .047]) and AUC0-6 (GMR, 1.36 [95% CI, 1.07-1.7, P = .012]; adjusted GMR, 1.36 [95% CI, 1.07-1.73, P = .012]) were significantly higher in women compared with men during the intensive phase even after adjustment for age, BMI, and HbA1c. Similarly, during the continuation phase, the Cmax and AUC0-6 levels of isoniazid were higher among women (GMR of 1.3; 95% CI, 0.99-1.72; P = .063 [adjusted GMR, 1.29; 95% CI, 0.98-1.70; P = .072] and GMR of 1.47; 95% CI, 1.12-1.92; P = .005 [adjusted GMR, 1.46; 95% CI, 1.12-1.90; P = .005), respectively). No statistically significant differences by sex were observed for the Cmax and AUC0-6 levels of rifampicin and pyrazinamide during the intensive and continuation phases.
Table 2.
Sex-Wise Geometric Means With Univariate and Adjusted GMRs With 95% CIs Comparing the Cmax vs the AUC0-6 of Anti-TB Drugs in the Prospective Cohort, Pune, India
| Drug, Phase, and Parameter | Sex-Wise Geometric Mean Value |
GMR |
||||
|---|---|---|---|---|---|---|
| Men (n = 151) | Women (n = 72) | GMR (95% CI) | P Value | Adjusted GMR (95% CI)a | P Value | |
| Rifampin | ||||||
| Intensive phase | ||||||
| Cmax, μg/mL | 4.09 (3.45-4.85) | 4.26 (3.34-5.45) | 1.02 (0.75-1.38) | .92 | 1.04 (0.76-1.42) | .24 |
| AUC0-6, μg•h/mL | 13.80 (11.55-16.50) | 14.99 (11.87-18.91) | 1.09 (0.81-1.45) | .58 | 1.09 (0.81-1.46) | .57 |
| Continuation phase | ||||||
| Cmax, μg/mL | 3.72 (3.08-4.49) | 3.34 (2.46-4.53) | 0.96 (0.66-1.39) | .81 | 1.01 (0.71-1.44) | .97 |
| AUC0-6, μg•h/mL | 11.92 (9.64-14.73) | 12.53 (8.82-17.80) | 1.05 (0.70-1.57) | .81 | 1.09 (0.75-1.60) | .65 |
| Isoniazid | ||||||
| Intensive phase | ||||||
| Cmax, μg/mL | 6.44 (5.53-7.52) | 8.38 (6.77-10.38) | 1.3 (1.0-1.68) | .046 | 1.29 (1.0-1.68) | .047 |
| AUC0-6, μg•h/mL | 23.24 (19.88-27.17) | 31.67 (26.23-38.24) | 1.36 (1.07-1.7) | .012 | 1.36 (1.07-1.73) | .012 |
| Continuation phase | ||||||
| Cmax, μg/mL | 6.85 (5.61-8.37) | 8.93 (7.30-10.93) | 1.30 (0.99-1.72) | .063 | 1.29 (0.98-1.70) | .072 |
| AUC0-6, μg•h/mL | 24.67 (20.48-29.72) | 36.23 (29.71-44.17) | 1.47 (1.12-1.92) | .005 | 1.46 (1.12-1.90) | .005 |
| Pyrazinamide | ||||||
| Intensive phase | ||||||
| Cmax | 26.55 (22.93-30.74) | 29.44 (23.27-37.25) | 1.11 (0.84-1.46) | .46 | 1.04 (0.80-1.36) | .78 |
| AUC0-6, μg•h/mL | 97.01 (82.31-114.35) | 112.97 (86.81-147.03) | 1.16 (0.86-1.58) | .33 | 1.09 (0.81-1.47) | .57 |
The models are adjusted for age, sex, and glycated hemoglobin value. Visit 1 was during the intensive phase of TB treatment, while visit 2 took place during the continuation phase of TB treatment.
AUC = area under the curve; Cmax = maximum concentration; GMR = geometric mean ratio.
As shown in Figure 3, the overall proportion of women achieving therapeutic target concentrations was higher than in men, reaching statistical significance only for pyrazinamide (47% of men and 65% of women achieving the target; P = .012). Sex-wise, the proportion of patients who achieved a therapeutic target for isoniazid was 82.7% of men vs 88.9% of women (P = .23) in the intensive phase and 84.9% of men vs 91.7% of women (P = .17) in the continuation phase. For rifampicin, 19% of men and 21% of women (P = .77) during the intensive phase and 20% of men and 24% of women (P = .52) during the continuation phase achieved therapeutic target values.
Figure 3.
Sex-wise proportion of participants above the therapeutic target concentration in the enrolled cohort, Pune, India. INH = isoniazid; PZA = pyrazinamide; RMP = rifampin.
TB Treatment Outcomes According to Sex
Overall, 191 patients (26%) had unfavorable composite TB treatment outcomes; 143 (76%) were men, and 48 (25%) were women. Of the 191, a total of 90 (47%) had treatment failure, 59 (31%) had recurrence, and 42 (22%) died. Compared with men, women had a lower probability of an unfavorable composite outcome in both univariable (incidence risk ratio [IRR], 0.57; 95% CI, 0.41-0.79; P = .001) and multivariable (adjusted IRR, 0.60; 95% CI, 0.43-0.85; P = .003) analyses adjusted for age, education, BMI, TTD, and smear grade at baseline and HbA1c analyses. Furthermore, women had a lower likelihood of experiencing a recurrent TB event (adjusted HR, 0.45; 95% CI, 0.23-0.86; P = .02) (Fig 1B). The median time to death was 175 days among men compared with 149 days in women (P = .05) (Fig 2B).
Factors Associated With Unfavorable TB Treatment Outcomes Stratified According to Sex
We stratified the analysis by sex to further assess differences according to this factor. Among men, alcohol consumption was independently associated with unfavorable TB treatment outcomes (IRR, 1.93; 95% CI, 1.38-2.69; P < .001) (Table 3). In addition, among men, a unit increase in BMI (IRR, 0.93; 95% CI, 0.88-0.98; P = .005), and a unit decrease in HbA1c (IRR, 0.90; 95% CI, 0.82-0.98; P = .02) were each associated with a lower likelihood of unfavorable outcomes. Among women, only the presence of cavity at TB treatment initiation was significantly associated with unfavorable outcomes (IRR, 1.87; 95% CI, 1.01-3.46; P = .05).
Table 3.
Exposure Variables and Risk of Unfavorable Outcomes Stratified According to Sex in the Prospective Cohort, Pune, India (N = 744)
| Characteristic | Men (n = 483) |
Women (n = 261) |
||||
|---|---|---|---|---|---|---|
| Total (N = 483) | Unfavorable Outcome: Yes, No. (%) N = 143 (30%) |
Univariable IRR (95% CI); P Value |
Total (N = 261) | Unfavorable Outcome: Yes, No. (%) N = 48 (18%) |
Univariable IRR (95% CI); P Value |
|
| Age (linear) | 483 (100%) | 143 (30%) | 1 .0 (0.99-1.02); P = .40 | 261 (100%) | 48 (18%) | 0.99 (0.97-1.02); P = .62 |
| Smoking | ||||||
| No | 342 (71%) | 97 (28%) | 1 | 260 (99%) | 47 (18%) | -- |
| Yes | 141 (29%) | 46 (33%) | 1.18 (0.83-1.67); P = 0.36 | 1 (1%) | 1 (100%) | |
| Alcohol | ||||||
| No | 246 (55%) | 59 (22%) | 1 | 259 (99%) | 47 (18%) | -- |
| Yes | 219 (45%) | 84 (38%) | 1.93 (1.38-2.69); P < .001 | 2 (1%) | 1 (50%) | |
| BMI (linear) | 480 (99%) | 142 (30%) | 0.93 (0.88-0.98); P = .005 | 260 (99%) | 47 (18%) | 0.96 (0.89-1.04); P = .34 |
| Diabetic status | ||||||
| No DM | 233 (48%) | 66 (28%) | 1 | 146 (56%) | 24 (16%) | 1 |
| Pre-DM | 97 (20%) | 38 (39%) | 1.42 (0.95-2.11); P = .09 | 61 (23%) | 15 (25%) | 1.51 (0.79-2.87); P = .21 |
| DM | 153 (32%) | 39 (25%) | 0.94 (0.63-1.39); P = .74 | 54 (21%) | 9 (17%) | 1.07 (0.50-2.29); P = .87 |
| HbA1c (linear) | 483 (100%) | 143 (30%) | 0.90 (0.82-0.98); P = .02 | 261 (100%) | 48 (18%) | 0.96 (0.83-1.10); P = .53 |
| Time to culture positivity | ||||||
| ≥ 7 d | 351 (73%) | 101 (30%) | 1 | 214 (82%) | 36 (17%) | 1 |
| < 7 d | 130 (27%) | 41 (32%) | 1.08 (0.75-1.55); P = .68 | 47 (18%) | 12 (26%) | 1.45 (0.75-2.79); P = .27 |
| Smear grade | ||||||
| 1+, scanty | 290 (60%) | 80 (28%) | 1 | 177 (68%) | 30 (17%) | 1 |
| ≥ 2 | 193 (40%) | 63 (33%) | 1.20 (0.87-1.67); P = .27 | 84 (32%) | 18 (21%) | 1.34 (0.75-2.41); P = .32 |
| Cavity | ||||||
| No | 232 (56%) | 60 (26%) | 1 | 128 (57%) | 18 (14%) | 1 |
| Yes | 179 (44%) | 58 (32%) | 1.28 (0.89-1.83); P = .19 | 95 (43%) | 23 (24%) | 1.87 (1.01-3.46); P = .05 |
DM = diabetes mellitus; -- = cannot estimate as all had an event; HbA1c = glycated hemoglobin; IRR = incidence risk ratio.
Discussion
Our assessment of sex-specific differences in TB clinical presentation, drug exposure, and TB treatment outcomes in a prospective cohort in India revealed several important findings. At clinical presentation, we found that women were less likely to have a high mycobacterial burden. Furthermore, unfavorable TB treatment outcomes were less common among women than men, with recurrent TB events also lower among women. Notably, women generally had higher anti-TB drug levels and percent target attainment than men. Lastly, risk factors for unsuccessful TB treatment outcome were different for men than for women. Taken together, these findings indicate marked differences between sexes for TB, the possible explanations for which deserve further attention.
Male predisposition for higher incidence and worse outcomes has been reported for other infectious diseases such as hepatitis A, leishmaniasis, schistosomiasis, leptospirosis, and meningococcal meningitis.8 Consistent with prior studies, even with improved access to TB diagnostics and medical care worldwide, our report shows that disease severity at presentation and unfavorable TB treatment outcomes were greater among men than women.11,12 However, the biological basis for these differences is unclear. The higher mycobacterial burden at presentation in men may explain the higher risk of worse treatment outcomes in our cohort, but even after adjusting for this variable and many other demographic, clinical, and behavioral exposures, the risk of worse TB treatment outcomes persisted among men.
Among several factors, sex-specific differential pharmacokinetic and pharmacodynamic variables may in part explain the observed differences in TB treatment outcomes. Women generally achieved higher concentrations of anti-TB drugs. Although fixed-dose combinations may result in higher drug dosing per body weight or BMI for women, drug levels among women remained higher than men even after adjusting for BMI in our cohort.12,21,22 Specifically, Cmax and AUC in the key bactericidal TB drug isoniazid were significantly lower in men throughout TB treatment. Furthermore, proportions achieving therapeutic levels of anti-TB drugs (particularly pyrazinamide, a drug that accelerates the sterilizing effect33 of isoniazid and rifampicin during the intensive phase) were significantly lower among men. It should be noted, however, that overall target drug level achievements have been lower in this population as previously published, likely due to suboptimal dosing, a finding that is more prominent for men.25,28 From a pharmacodynamic perspective, preclinical and clinical studies suggest that genetic, epigenetic,34,35 immunologic,36 hormonal,37,38 and metabolic factors may affect the presentation of infectious diseases and treatment outcomes, and these factors may, in theory, explain some sex differences in exposure-response relationships.
Complex interactions of behavioral risk factors such as smoking39 and alcohol consumption40 play a critical role in unsuccessful TB treatment. Indeed, alcohol use was an important risk factor for worse TB treatment outcomes among men,41 underscoring the need for additional interventions such as alcohol treatment programs40,42 to achieve optimal TB treatment success among men. However, the role of behavioral factors in outcomes among women could not be assessed as few women reported substance use, likely due to cultural pressure in India to limit use, but this can also be attributed to underreporting due to sociocultural stigma.43 Furthermore, as seen in prior studies,44, 45, 46 we found that decreasing HbA1c levels (although the median HbA1c was lower) was associated with favorable TB treatment outcomes but only among men, indicative of both biological and behavioral (lifestyle) risk for poor outcomes among men.24,46 Moreover, the association found in the overall cohort between high BMI and adverse TB treatment outcomes seems to be driven by men, not both sexes. This finding could be likely explained by the overall lower weight among men; the topic merits further investigation.
A significant finding of the current study is that among women, a cavitary lesion at baseline was associated with worse TB treatment outcomes. Interestingly, another study from our group found that women had a threefold higher risk47 for abnormal lung health at the end of TB treatment, which may increase the risk for recurrence. Taken together, these findings suggest that attention to cavitary lung disease and to lung damage is especially important in women, as these scenarios represent high-risk situations for recurrence in women. This is in addition to other known risk factors for poor outcomes in women, including diagnostic delay48 and lower access to treatment.49
The current study has limitations. We did not collect information about health-seeking behavior or stigmatization as potential factors influencing sex-specific differences in clinical presentation and treatment outcomes. More than one-quarter of participants had unfavorable treatment outcomes, significantly higher than the Indian programmatic data,17,19 which is likely explained by the inclusion of only culture-confirmed participants in our study. Furthermore, because the drugs were provided under the national program, we did not collect data on drug toxicity or adverse events that can influence the differential adherence and treatment outcomes among sexes. Although the analysis was powered to assess the effect of sex on TB clinical presentation and outcomes, this study is a post hoc analysis and should be considered exploratory.
Interpretation
There are significant differences in sex in TB disease presentation and TB treatment outcomes, and factors that influence overall outcomes vary according to sex. Ensuring that dosing achieves target exposures in men, and using strategies to address their factors for poor outcomes (eg, alcohol use, low body weight, poorly controlled diabetes), should be tenets of plans to improve TB treatment success in men. For women, careful follow-up of those with cavitary lung disease is most important, as that is their highest risk factor for unsuccessful outcomes. Future studies should further investigate the biological and behavioral basis for these differences and evaluate whether these sex differences are seen across geographic settings.
Funding/Support
This work was supported by the NIH [NIH 1R01A1I097494 to J. E. G.] and by the National Institute of Allergy and Infectious Diseases of the NIH [UM1AI069465]. Data in this article were also collected as part of the Regional Prospective Observational Research for Tuberculosis (RePORT) India Consortium under CRDF Global Agreement #OISE-15-614381-1 and #DAA3-18-64774-1. This project has been funded in whole or in part with federal funds from the Government of India’s Department of Biotechnology, the Indian Council of Medical Research, the NIH, the National Institute of Allergy and Infectious Diseases, and the Office of AIDS Research, and was distributed in part by CRDF Global. The authors also acknowledge support from Persistent Systems in kind.
Financial/Nonfinancial Disclosures
None declared.
Acknowledgments
Authorcontributions: V. M., N. G., A. Gupte, A. K., K. E. D., and J. E. G. conceived the study; J. E. G. obtained funding; V. M., S. G., R. L., S. Deshmukh, A. K., N. P., S. R., S. Dharmshale, T. S., and M. B. ran the study and collected data; N. G., M. S., and O. A. performed data analyses; V. M., N. G., S. Deshmukh., A. Gupte, A. Gupta, K. E. D., and J. E. G. conducted data interpretation; and S. Deshmukh., V. M., and J. E. G. drafted the initial manuscript. All authors assisted in manuscript preparation and approved the manuscript.
Role of the sponsors: The sponsors had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: The authors thank all the staff members at Byramjee Jeejeebhoy Medical College and Sassoon General Hospital, Pune, and Dr. D. Y. Patil Medical College, Pune, along with Dr Sachin Atre, for their contribution to this study. They also thank all the study participants, without whose support the study would not have been possible, and the local Revised National TB Control Programme staff for their cooperation.
Additional information: The e-Figure is available online under “Supplementary Data.”
Footnotes
DISCLAIMER: The contents of this publication are solely the responsibility of the authors and do not represent the official views of the Department of Biotechnology, the Indian Council of Medical Research, the National Institutes of Health, or CRDF Global. Any mention of trade names, commercial projects, or organizations does not imply endorsement by any of the sponsoring organizations.
Supplementary Data
e-Figure 1.
References
- 1.World Health Organization Global tuberculosis report 2020. http://www.who.int/tb/publications/global_report/en/
- 2.TB Gender Assessment India. http://www.stoptb.org/assets/documents/communities/CRG/TB%20Gender%20Assessment%20India.pdf
- 3.World Health Organization Global tuberculosis report 2019. http://www.who.int/tb/publications/global_report/en/
- 4.Borgdorff M.W., Nagelkerke N.J.D., Dye C., Nunn P. Gender and tuberculosis: a comparison of prevalence surveys with notification data to explore sex differences in case detection. Int J Tuberc Lung Dis. 2000;4(2):123–132. [PubMed] [Google Scholar]
- 5.Horton K.C., MacPherson P., Houben R.M.G.J., White R.G., Corbett E.L. Sex differences in tuberculosis burden and notifications in low- and middle-income countries: a systematic review and meta-analysis. PLoS Med. 2016;13(9) doi: 10.1371/journal.pmed.1002119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nhamoyebonde S., Leslie A. Biological differences between the sexes and susceptibility to tuberculosis. J Infect Dis. 2014;209(suppl 3):S100–S106. doi: 10.1093/infdis/jiu147. [DOI] [PubMed] [Google Scholar]
- 7.Neyrolles O., Quintana-Murci L. Sexual inequality in tuberculosis. PLoS Med. 2009;6(12) doi: 10.1371/journal.pmed.1000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guerra-Silveira F., Abad-Franch F. Sex bias in infectious disease epidemiology: patterns and processes. PLoS One. 2013;8(4) doi: 10.1371/journal.pone.0062390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reardon S. Infections reveal inequality between the sexes. Nature. 2016;534(7608):447. doi: 10.1038/534447a. [DOI] [PubMed] [Google Scholar]
- 10.Allotey P., Gyapong M. Gender in tuberculosis research. Int J Tuberc Lung Dis. 2008;12(7):831–836. [PubMed] [Google Scholar]
- 11.Chidambaram V., Tun N.L., Majella M.G., et al. Male sex is associated with worse microbiological and clinical outcomes following tuberculosis treatment: a retrospective cohort study, a systematic review of the literature, and meta-analysis. Clin Infect Dis. 2021;73(9):1580–1588. doi: 10.1093/cid/ciab527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murphy M.E., Wills G.H., Murthy S., et al. Gender differences in tuberculosis treatment outcomes: a post hoc analysis of the REMoxTB study. BMC Mede. 2018;16(1):189. doi: 10.1186/s12916-018-1169-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Torres N.M.C., JJQ Rodríguez, Andrade P.S.P., Arriaga M.B., Netto E.M. Factors predictive of the success of tuberculosis treatment: a systematic review with meta-analysis. PLoS One. 2019;14(12) doi: 10.1371/journal.pone.0226507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tok P.S.K., Liew S.M., Wong L.P., et al. Determinants of unsuccessful treatment outcomes and mortality among tuberculosis patients in Malaysia: a registry-based cohort study. PLoS One. 2020;15(4) doi: 10.1371/journal.pone.0231986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weiss M.G., Auer C., Somma D., et al. Gender and tuberculosis: cross-site analysis and implications of a multi-country study in Bangladesh, India, Malawi, and Colombia. https://apps.who.int/iris/handle/10665/69355
- 16.Mundra A., Deshmukh P.R., Dawale A. Magnitude and determinants of adverse treatment outcomes among tuberculosis patients registered under Revised National Tuberculosis Control Program in a Tuberculosis Unit, Wardha, Central India: a record-based cohort study. J Epidemiol Glob Health. 2017;7(2):111–118. doi: 10.1016/j.jegh.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mukherjee A., Saha I., Sarkar A., Chowdhury R. Gender differences in notification rates, clinical forms and treatment outcome of tuberculosis patients under the RNTCP. Lung India. 2012;29(2):120–122. doi: 10.4103/0970-2113.95302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balasubramanian R., Garg R., Santha T., et al. Gender disparities in tuberculosis: report from a rural DOTS programme in south India. Int J Tuberc Lung Dis. 2004;8(3):323–332. [PubMed] [Google Scholar]
- 19.Ramachandran G., Kupparam H.K.A., Vedhachalam C., et al. Factors influencing tuberculosis treatment outcome in adult patients treated with thrice-weekly regimens in India. Antimicrob Agents Chemother. 2017;61(5):e02464–e02516. doi: 10.1128/AAC.02464-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Denti P., Jeremiah K., Chigutsa E., et al. Pharmacokinetics of isoniazid, pyrazinamide, and ethambutol in newly diagnosed pulmonary TB patients in Tanzania. PLoS One. 2015;10(10) doi: 10.1371/journal.pone.0141002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sileshi T., Tadesse E., Makonnen E., Aklillu E. The impact of first-line anti-tubercular drugs’ pharmacokinetics on treatment outcome: a systematic review. Clin Pharmacol. 2021;13:1–12. doi: 10.2147/CPAA.S289714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pasipanodya J.G., McIlleron H., Burger A., Wash P.A., Smith P., Gumbo T. Serum drug concentrations predictive of pulmonary tuberculosis outcomes. J Infect Dis. 2013;208(9):1464–1473. doi: 10.1093/infdis/jit352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mave V., Meshram S., Lokhande R., et al. Prevalence of dysglycemia and clinical presentation of pulmonary tuberculosis in Western India. Int J Tuberc Lung Dis. 2017;21(12):1280–1287. doi: 10.5588/ijtld.17.0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mave V., Gaikwad S., Barthwal M., et al. Diabetes mellitus and tuberculosis treatment outcomes in Pune, India. Open Forum Infect Dis. 2021;8(4):ofab097. doi: 10.1093/ofid/ofab097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alfarisi O., Mave V., Gaikwad S., et al. Effect of diabetes mellitus on the pharmacokinetics and pharmacodynamics of tuberculosis treatment. Antimicrob Agents Chemother. 2018;62(11):e01383–e01418. doi: 10.1128/AAC.01383-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hemanth Kumar A.K., Chandra I., Geetha R., Silambu Chelvi K., Lalitha V., Prema G. A validated high-performance liquid chromatography method for the determination of rifampicin and desacetyl rifampicin in plasma and urine. Indian J Pharmacol. 2004;36(4):231–233. [Google Scholar]
- 27.Hemanth A.K., Sudha V., Ramachandran G. Simple and rapid liquid chromatography method for simultaneous determination of isoniazid and pyrazinamide in plasma. SAARC J Tuberc Lung Dis HIV AIDS. 2012;9(1):13–18. [Google Scholar]
- 28.Ramachandran G., Chandrasekaran P., Gaikwad S., et al. Subtherapeutic rifampicin concentration is associated with unfavorable tuberculosis treatment outcomes. Clin Infect Dis. 2020;70(7):1463–1470. doi: 10.1093/cid/ciz380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Donald P.R., Maritz J.S., Diacon A.H. The pharmacokinetics and pharmacodynamics of rifampicin in adults and children in relation to the dosage recommended for children. Tuberculosis (Edinb) 2011;91(3):196–207. doi: 10.1016/j.tube.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 30.Chideya S., Winston C.A., Peloquin C.A., et al. Isoniazid, rifampin, ethambutol, and pyrazinamide pharmacokinetics and treatment outcomes among a predominantly HIV-infected cohort of adults with tuberculosis from Botswana. Clin Infect Dis. 2009;48(12):1685–1694. doi: 10.1086/599040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peloquin C.A. Therapeutic drug monitoring in the treatment of tuberculosis. Drugs. 2002;62(15):2169–2183. doi: 10.2165/00003495-200262150-00001. [DOI] [PubMed] [Google Scholar]
- 32.Alsultan A., Peloquin C.A. Therapeutic drug monitoring in the treatment of tuberculosis: an update. Drugs. 2014;74(8):839–854. doi: 10.1007/s40265-014-0222-8. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y., Shi W., Zhang W., Mitchison D. Mechanisms of pyrazinamide action and resistance. Microbiol Spectr. 2013;2(4):1–12. doi: 10.1128/microbiolspec.MGM2-0023-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Casanova J.L., Abel L. Human genetics of infectious diseases: a unified theory. EMBO J. 2007;26(4):915–922. doi: 10.1038/sj.emboj.7601558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leung K.H., Yip S.P., Wong W.S., et al. Sex- and age-dependent association of SLC11A1polymorphisms with tuberculosis in Chinese: a case control study. BMC Infect Dis. 2007;7(1):19. doi: 10.1186/1471-2334-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fish E.N. The X-files in immunity: sex-based differences predispose immune responses. Nat Rev Immunol. 2008;8(9):737–744. doi: 10.1038/nri2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vodo S., Bechi N., Petroni A., Muscoli C., Aloisi A.M. Testosterone-induced effects on lipids and inflammation. Mediators Inflamm. 2013;2013 doi: 10.1155/2013/183041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kluft C., Leuven J.A.G., Helmerhorst F.M., Krans H.M.J. Pro-inflammatory effects of oestrogens during use of oral contraceptives and hormone replacement treatment. Vascul Pharmacol. 2002;39(3):149–154. doi: 10.1016/s1537-1891(02)00304-x. [DOI] [PubMed] [Google Scholar]
- 39.Lin H.H., Ezzati M., Murray M. Tobacco smoke, indoor air pollution and tuberculosis: a systematic review and meta-analysis. PLoS Med. 2007;4(1) doi: 10.1371/journal.pmed.0040020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Silva M.R., Pereira J.C., Costa R.R., Dias J.A., Guimarães M.D.C., Leite I.C.G. Drug addiction and alcoholism as predictors for tuberculosis treatment default in Brazil: a prospective cohort study. Epidemiol Infect. 2017;145(16):3516–3524. doi: 10.1017/S0950268817002631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cox S.R., Gupte A.N., Thomas B., et al. Unhealthy alcohol use independently associated with unfavorable TB treatment outcomes among Indian men. Int J Tuberc Lung Dis. 2021;25(3):182–190. doi: 10.5588/ijtld.20.0778. [DOI] [PubMed] [Google Scholar]
- 42.Duraisamy K., Mrithyunjayan S., Ghosh S., et al. Does alcohol consumption during multidrug-resistant tuberculosis treatment affect outcome? A population-based study in Kerala, India. Ann Am Thorac Soc. 2014;11(5):712–718. doi: 10.1513/AnnalsATS.201312-447OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Malik K., Benegal V., Murthy P., Chand P., Arun K., Suman L.N. Clinical audit of women with substance use disorders: findings and implications. Indian J Psychol Med. 2015;37(2):195–200. doi: 10.4103/0253-7176.155620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Degner N.R., Wang J.Y., Golub J.E., Karakousis P.C. Metformin use reverses the increased mortality associated with diabetes mellitus during tuberculosis treatment. Clin Infect Dis. 2018;66(2):198–205. doi: 10.1093/cid/cix819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baker M.A., Harries A.D., Jeon C.Y., et al. The impact of diabetes on tuberculosis treatment outcomes: a systematic review. BMC Med. 2011;9(1):81. doi: 10.1186/1741-7015-9-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huangfu P., Ugarte-Gil C., Golub J., Pearson F., Critchley J. The effects of diabetes on tuberculosis treatment outcomes: an updated systematic review and meta-analysis. Int J Tuberc Lung Dis. 2019;23(7):783–796. doi: 10.5588/ijtld.18.0433. [DOI] [PubMed] [Google Scholar]
- 47.Gupte A.N., Paradkar M., Selvaraju S., et al. Assessment of lung function in successfully treated tuberculosis reveals high burden of ventilatory defects and COPD. PLoS One. 2019;14(5) doi: 10.1371/journal.pone.0217289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang W.T., Gounder C.R., Akande T., et al. Barriers and delays in tuberculosis diagnosis and treatment services: does gender matter? Tuberc Res Treat. 2014;2014 doi: 10.1155/2014/461935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cai J., Wang X., Ma A., Wang Q., Han X., Li Y. Factors associated with patient and provider delays for tuberculosis diagnosis and treatment in Asia: a systematic review and meta-analysis. PLoS One. 2015;10(3) doi: 10.1371/journal.pone.0120088. [DOI] [PMC free article] [PubMed] [Google Scholar]