Abstract
Background
Biological sex, gender, and race are important considerations in patients with interstitial lung diseases (ILDs).
Research Question
Does a patient’s sex assigned at birth, and race, influence ILD treatment initiation?
Study Design and Methods
Patients with ILD from three longitudinal prospective registries were compared in this observational study. ILD-related medications included antifibrotics and immunomodulating medications. Race was dichotomized as “White” vs “non-White.” Time to treatment initiation was determined from the date of the initial ILD registry visit to the date of first medication initiation. Proportions of treated patients were compared between groups by χ2 test. Cox proportional analysis was used to determine how sex and race were associated with time to treatment initiation stratified by ILD diagnosis.
Results
A total of 4,572 patients were included across all cohorts. The proportion of men who received treatment was higher than for women in the Canadian cohort (47% vs 40%; P < .001), and the proportion of White patients who received treatment was also higher compared with non-White patients (46% vs 36%; P < .001). In contrast, the proportion of treated men in the Chicago cohort was lower compared with women (56% vs 64%; P = .005), and that of White patients was lower compared with non-White patients (56% vs 69%; P < .001). No sex- or race-based differences in proportions of patients treated were found in the Australasian cohort. White race was significantly associated with earlier treatment initiation compared with non-White race across diagnoses in the Canadian cohort, whereas the opposite association was found in the Australasian cohort.
Interpretation
Sex- and race-based differences exist in the initiation of ILD treatment, with variability across different cohorts in different countries. Reasons for these differences need to be further explored in future studies.
Key Words: idiopathic pulmonary fibrosis, interstitial lung disease, race, sex and gender, treatment
Take-home Points.
Study Question: Does a patient’s sex assigned at birth, and race influence interstitial lung disease treatment initiation?
Results: Treatment initiation for interstitial lung disease varied widely across different prospective cohorts. In the Canadian cohort, the proportions of men and patients of White race were significantly higher than for women and non-White patients, but the opposite was found in the Chicago cohort. No sex or race differences were identified in the Australasian cohort.
Interpretation: Substantial heterogeneity exists in treatment initiation for interstitial lung disease across prospective cohorts. Although patient sex and race influence treatment in some cases, further research needs to explore the reasons behind geographical and sex- and race-based discrepancies.
Biological sex and gender are important considerations in patients with interstitial lung disease (ILD).1 Biological sex assigned at birth refers to a set of anatomic and physiological features typically encompassing chromosomes, gene expression, and hormonal function, and is categorized as male or female. Gender is a self-identified, self-determined construct that refers to a set of roles, behaviors, and expression of identity that exists within a society and culture.2,3 Certain types of ILD are more prevalent in men, including idiopathic pulmonary fibrosis (IPF) and certain pneumoconioses, whereas others, such as connective tissue disease-associated ILD, are more prevalent in women.4 These differences may be due to gender-related differential exposures, whereby men are more likely to have occupational exposures to dusts, silica, or asbestos,5,6 or to certain biological or genetic predispositions. Given the strong predominance of men in multiple epidemiological and registry studies and trials of IPF,7, 8, 9, 10 patient sex influences the diagnostic impression of clinicians when making a diagnosis of IPF, especially when the radiological pattern is anything but the usual interstitial pneumonia pattern.11 Other sex- and gender-based differences in care have not yet been adequately explored in ILD, but preliminary data and a few database studies suggest that this bias is present in IPF as well as other ILDs, with men treated earlier, and more frequently, than women.12,13 Whether this trend is true across cohorts around the world remains uncertain.
Race is a construct that refers to the concept of categorizing people into groups based on arbitrarily chosen physical differences and characteristics (such as skin tone, features, and so on), and to the process of attributing social meaning to those groups.14 There is no evidence or biological basis to support the identification of distinct racial groups. However, race has been shown to influence medical care and clinical outcomes across a broad group of diseases, especially as it pertains to structural racism within the medical establishment. These racially based disparities in care and outcomes are also seen in ILD.15 Prior work has shown that African American race is associated with earlier ILD diagnosis, and that it impacts prognosis.16,17 The incidence of ILD has also been shown to be higher in some Canadian Indigenous populations.18 Racial and ethnic distribution of IPF and ILD treatment have also not been well evaluated in the literature so far.
The objectives of this study were to determine if sex and race affect ILD treatment initiation among patients enrolled in three large prospective cohorts in North America and Australasia (Australia and New Zealand). We hypothesized that women and non-White patients would be less likely to receive treatment for ILD, and that treatment initiation would be delayed.
Study Design and Methods
Three prospective ILD registries were used for this study:
-
1.
The Canadian Registry for Pulmonary Fibrosis (CARE-PF) is a prospective, longitudinal multicenter registry of patients with ILD that was initiated in January 2015, with both prevalent and incident cases included.19 At the time of data extraction (November 2020), there were eight participating ILD centers from five Canadian provinces. All patients who are assessed at a participating ILD center are approached regarding participation in the registry.
-
2.
The University of Chicago ILD registry (UChicago) is a prospective longitudinal single-center cohort study of incident and prevalent cases. All patients seen in an ILD tertiary care clinic are offered enrollment in this cohort. Data for this study were extracted in May 2021.
-
3.
The Australasian ILD registry (AILDR) is a prospective longitudinal cohort study from Australia and New Zealand that was initiated in 2015, predominantly of patients with IPF, but inclusive of all ILD subtypes.20 Data extraction was performed in October 2021.
For this study, all consenting patients with a diagnosis of IPF, connective tissue disease (CTD)-associated ILD (including interstitial pneumonia with autoimmune features), hypersensitivity pneumonitis (HP), and unclassifiable ILD across all three cohorts were included from inception of cohort until the date of data extraction. Patients with IPF were included if they had been enrolled from year 2015 onward, as no effective IPF treatment was available before that year. All patients provided informed consent for participation in the registry. Institutional review board approval was obtained at each participating site for this study (CARE-PF, McGill University Health Center REB, MP-02-2021-8881; AILDR, Sydney Local Health District Research Ethics and Governance Office, Protocol No. X16-0275 and 2019/ETH06440 “The Australasian Interstitial Lung Disease Registry”; UChicago, Natural History of ILD, IRB #14163A).
Variables
Data were collected at the baseline visit (visit of registry enrollment) and longitudinally. Patient sex assigned at birth was captured as male or female by the investigator. In CARE-PF, race was self-identified by patients among the following options: White, Black or African American, Indigenous including Pacific Islander and Alaskan Native, and Asian. In the UChicago cohort, patients self-identified into race/ethnic categories per the federally defined US Census Bureau standards on race (White, Black or African American, American Indian or Alaska Native, Asian, and Native Hawaiian or Other Pacific Islander) and ethnicity (Hispanic or not Hispanic). In the AILDR, race was self-identified as White, Black or African, Indigenous (from Australia or New Zealand), and Asian. Race was also dichotomized as White and non-White to account for the small number of participants from racial or ethnic minority groups. Other key variables included ILD diagnosis and date of diagnosis, demographics, smoking history, ILD-related investigations (eg, pulmonary function tests, surgical lung biopsy, autoimmune serologies), and comorbidities. All participating centers reviewed cases in a multidisciplinary discussion, with diagnoses established as per clinical practice guidelines and consensus recommendations, where available.21,22 For this study, patient demographics, pulmonary function tests including FVC and diffusion capacity of the lungs for carbon monoxide (Dlco), initial ILD clinic visit date, date of treatment initiation, type of treatment, and date of mortality or lung transplantation were extracted from the registry data sets.
Outcomes
ILD-related treatment medications included antifibrotics (pirfenidone, nintedanib) as well as long-term steroid-sparing immunomodulating medications (azathioprine, mycophenolate mofetil, cyclophosphamide, rituximab, cyclosporine, tacrolimus). Prednisone (any dosage or duration) when used alone for treatment of ILD was not included in the analysis given the high variation in dosing, duration, and indications for use, as well as it generally being inappropriate as a long-term single agent for treatment of the included ILD subtypes. The date of initiation for the first ILD-related medication was recorded for each patient to establish the time to treatment initiation. Subsequent ILD-related medications were not evaluated.
Statistical Analysis
Baseline characteristics at the initial visit were compared according to patient sex across cohorts, and included race. Proportions of treated patients during follow-up were compared between men and women, and across race categories, using the χ2 test. To account for differences in follow-up time, a sensitivity analysis was performed to compare proportions of treated patients within the first year of the initial visit date. Time to treatment initiation was determined from the date of the initial visit to the date of first initiation of an ILD-related medication. Median time between initial clinic visit and treatment initiation was compared between men and women, using the Wilcoxon Mann-Whitney test. Cox proportional analysis was used to determine the association of patient sex with time to treatment initiation, and then between patient race as a dichotomized variable and time to treatment initiation. This analysis was stratified by ILD diagnosis and adjusted for predetermined confounders in the relationship between sex, race, and treatment, including age and disease severity as measured by FVC and Dlco.
Results
A total of 4,572 patients were included in this study across all three cohorts: 3,060 patients from the CARE-PF, 1,046 from the UChicago, and 466 from the AILDR. Baseline characteristics for all patients are provided in Table 1. Women and men were nearly equally distributed among the cohorts, although women were younger and less likely to have smoked compared with men. Men more frequently had a diagnosis of IPF compared with women in all three cohorts, whereas women more frequently had diagnoses of CTD-ILD or HP compared with men. Patients in the AILDR cohort were older and had generally higher FVC % predicted compared with patients in the other two cohorts, whereas those in the UChicago cohort had the lowest FVC % predicted at baseline. Although all three cohorts had predominantly White patients, the UChicago cohort had the highest proportion of Black patients, whereas the CARE-PF and AILDR cohorts had more patients of Asian descent. There were slightly more women who were Black or Asian compared with men across cohorts. Surgical lung biopsies were rarely performed in the patients enrolled in the AILDR, and most often in patients from UChicago.
Table 1.
Baseline Characteristics According to Patient Sex, Across Cohorts
| Characteristic | Canadian Registry for Pulmonary Fibrosis |
Australasian Interstitial Lung Disease Registry |
University of Chicago Registrya |
||||||
|---|---|---|---|---|---|---|---|---|---|
| All Patients (N = 3,060) | Women (n = 1,608) | Men (n = 1,452) | All Patients (N = 466) | Women (n = 184) | Men (n = 282) | All Patients (N = 1,046) | Women (n = 560) | Men (n = 486) | |
| Age, mean (SD), y | 63 (13) | 60 (13) | 66 (11) | 68 (11) | 65 (12) | 70 (11) | 63 (13) | 61 (13) | 65 (12) |
| Smoker (ever) | 1,858 (61) | 833 (52) | 1,025 (71) | 275 (59) | 76 (41) | 199 (71) | 541 (52) | 242 (43) | 299 (62) |
| FVC % pred, mean (SD) | 77 (20) | 77 (20) | 76 (19) | 82 (20) | 83 (23) | 81 (19) | 67 (19) | 69 (20) | 65 (19) |
| Dlco % pred, mean (SD) | 60 (19) | 60 (19) | 58 (19) | 61 (17) | 60 (15) | 61 (19) | 59 (25) | 62 (25) | 55 (24) |
| Race | |||||||||
| White | 2,383 (78) | 1,174 (73) | 1,209 (83) | 384 (82) | 147 (80) | 237 (84) | 669 (64) | 313 (56) | 356 (73) |
| Black | 53 (2) | 30 (2) | 23 (2) | … | … | … | 221 (21) | 169 (30) | 52 (11) |
| Asian | 363 (12) | 235 (15) | 128 (9) | 49 (11) | 21 (11) | 28 (10) | 37 (4) | 16 (3) | 21 (4) |
| Indigenous | 81 (3) | 52 (3) | 29 (2) | 1 | 1 | 0 | … | … | … |
| Other | 26 (1) | 15 (1) | 11 (1) | 22 (5) | 9 (5) | 13 (5) | 92 (9) | 48 (9) | 44 (9) |
| Latino | 57 (2) | 32 (2) | 25 (2) | … | … | … | 27 (3) | 14 (3) | 13 (3) |
| Diagnosis | |||||||||
| IPF | 763 (25) | 195 (12) | 568 (40) | 192 (41) | 40 (22) | 152 (54) | 136 (13) | 33 (6) | 103 (21) |
| CTD-ILD | 1,368 (45) | 954 (59) | 414 (28) | 137 (31) | 86 (47) | 51 (18) | 196 (19) | 138 (25) | 58 (12) |
| HP | 264 (8) | 149 (9) | 115 (8) | 44 (9) | 28 (15) | 16 (6) | 135 (13) | 64 (13) | 71 (13) |
| Unclassifiableb | 665 (22) | 310 (19) | 355 (24) | 93 (20) | 30 (16) | 63 (22) | 344 (33) | 171 (31) | 173 (36) |
| Familial disease | 203 (7) | 98 (6) | 105 (7) | 26 (6) | 8 (4) | 18 (6) | 84 (8) | 39 (7) | 45 (9) |
| Surgical lung biopsy | 517 (17) | 243 (15) | 274 (19) | 26 (6) | 12 (7) | 14 (5) | 430 (41) | 217 (39) | 213 (44) |
| Follow-up, median (IQR), y | 2.9 (1.5-4.8) | 3.2 (1.6-5.5) | 2.5 (1.3-4.1) | 3.3 (2.2-4.8) | 3.7 (2.4-5.1) | 3.1 (2.1-4.6) | 4.8 (1.8-10) | 5.7 (2.1-11.3) | 3.9 (1.5-8.7) |
Data are presented as No. (%) unless otherwise indicated. CTD = connective tissue disease; Dlco = diffusion capacity of the lung for carbon monoxide; HP = hypersensitivity pneumonitis; ILD = interstitial lung disease; IPF = idiopathic pulmonary fibrosis; IQR = interquartile range.
Includes other, less frequent ILDs (n = 234, 22%): sarcoidosis, pneumoconiosis, lymphocytic interstitial pneumonia, Langerhans cell histiocytosis, cryptogenic organizing pneumonia, and less common ILDs with small sample sizes.
Includes patients classified as having interstitial pneumonia with autoimmune features.
ILD Treatment and Sex
The proportion of patients who received ILD-related medications at any time during follow-up varied across cohorts, with patients from the AILDR having the highest proportion of treated patients (71%) compared with 61% in the Chicago cohort and 43% in the Canadian cohort. The proportion of patients who received any ILD-related treatment during follow-up was significantly higher for men than for women when pooling all diagnoses in the Canadian cohort (47% vs 40%; P < .001) (Table 2). Sex-based differences were statistically significant in patients with IPF and CTD-ILD, but not for patients with a diagnosis of HP or unclassifiable ILD. The proportions of male and female CARE-PF patients treated within the first year were slightly smaller (37% vs 32%; e-Table 1), but the sex-based differences remained. In contrast, men enrolled in the Chicago cohort were less likely to be treated compared with women overall (56% vs 64%; P = .005). This difference was more pronounced among patients with IPF or unclassifiable ILD. This remained true when restricting analysis to patients treated within the first year of follow-up. Treatment was balanced in the overall AILDR cohort, with equal proportions of men and women receiving ILD-related medications, except for patients with HP and unclassifiable disease, among whom women were somewhat more likely to receive treatment, although this did not reach statistical significance.
Table 2.
Proportion of Treated Patients at Any Time During Follow-up Period, Stratified by Sex
| Canadian Registry for Pulmonary Fibrosis |
Australasian Interstitial Lung Disease Registry |
University of Chicago Cohort |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Men (%) | Women (%) | P Value | Men (%) | Women (%) | P Value | Men (%) | Women (%) | P Value | |
| All diagnoses | 47 | 40 | < .001 | 71 | 70 | .79 | 56 | 64 | .005 |
| IPF | 55 | 46 | .02 | 82 | 83 | .89 | 74 | 88 | .09 |
| CTD-ILD | 47 | 41 | .04 | 80 | 69 | .13 | 59 | 62 | .63 |
| HP | 47 | 38 | .13 | 44 | 68 | .12 | 66 | 66 | .94 |
| Unclassifiable | 29 | 29 | .81 | 46 | 60 | .21 | 38 | 51 | .013 |
Boldface indicates statistical significance. CTD = connective tissue disease; HP = hypersensitivity pneumonitis; ILD = interstitial lung disease; IPF = idiopathic pulmonary fibrosis.
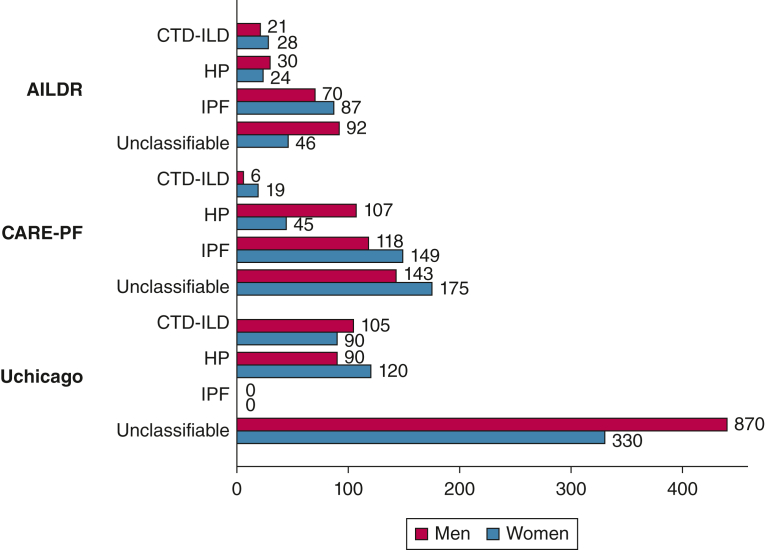
There were no significant differences in median time to treatment initiation between men and women across diagnoses in any of the three cohorts (Fig 1, e-Table 2). Across cohorts, there was substantial variability in median number of days before treatment initiation. However, in all cohorts, time to treatment initiation was generally longest in patients with unclassifiable ILD.
Figure 1.
Median time (days) to treatment initiation across cohorts and across diagnoses did not significantly differ between men and women. A median time to treatment initiation of zero occurred when more than one-half of the patients were receiving treatment at the time of their initial evaluation. Median time to treatment initiation in men with unclassifiable disease in UChicago is not to scale. AILDR = Australasian Interstitial Lung Disease Registry; CARE-PF = Canadian Registry for Pulmonary Fibrosis; CTD = connective tissue disease; HP = hypersensitivity pneumonitis; ILD = interstitial lung disease; IPF = idiopathic pulmonary fibrosis; UChicago = University of Chicago Registry.
ILD Treatment and Race
The proportion of White patients who received treatment during follow-up was significantly higher compared with non-White patients across all diagnoses in the CARE-PF cohort (46% vs 364%; P < .001) but the opposite was true in the UChicago cohort (56% vs 69%; P = 005) (Table 3). These race-based differences were especially marked in IPF and CTD-ILD for CARE-PF, and in CTD-ILD for UChicago. These differences remained when looking at treatment initiated within the first year of follow-up (e-Table 3). No significant racial differences in treatment proportions were found in the AILDR cohort. Median time to treatment initiation was significantly longer for non-White patients in CARE-PF (153 vs 28 days; P = .04) but was shorter in AILDR (5 vs 57 days; P < .001) and in UChicago (0 vs 210 days; P < .001) compared with White patients.
Table 3.
Proportion of Treated Patients at Any Time During Follow-up Period, Stratified by Race (White vs Non-White)
| Canadian Registry for Pulmonary Fibrosis |
Australasian Interstitial Lung Disease Registry |
University of Chicago Cohort |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| White (%) | Non-White (%) | P Value | White (%) | Non-White (%) | P Value | White (%) | Non-White (%) | P Value | |
| All diagnoses | 46 | 36 | .001 | 72 | 66 | .28 | 56 | 69 | < .001 |
| IPF | 53 | 35 | .001 | 81 | 86 | .62 | 76 | 82 | .49 |
| CTD-ILD | 47 | 37 | .003 | 76 | 67 | .27 | 52 | 70 | .009 |
| HP | 44 | 27 | .1 | 63 | 25 | .15 | 64 | 71 | .44 |
| Unclassifiable | 30 | 29 | .81 | 51 | 47 | .74 | 43 | 48 | .42 |
Boldface indicates statistical significance. CTD = connective tissue disease; HP = hypersensitivity pneumonitis; ILD = interstitial lung disease; IPF = idiopathic pulmonary fibrosis.
Factors Associated With Treatment Initiation
Patient sex was not significantly associated with earlier time to treatment initiation in any of the three cohorts (Table 4). The cumulative proportion of patients who received treatment according to race is illustrated in Figure 2. White race was associated with earlier time to treatment initiation in the CARE-PF cohort for patients with IPF (hazard ratio [HR], 1.52; 95% CI, 1.04-2.23) and in those with non-IPF ILD (HR, 1.27; 95% CI, 1.0-1.60) on unadjusted analysis. This association was even stronger after adjusting for sex, age, FVC % predicted, and Dlco % predicted, whereby White patients with IPF were more than two times more likely to receive earlier treatment (HR, 2.07; 95% CI, 1.23-3.48), and those with non-IPF ILD were 60% more likely to receive earlier treatment (HR, 1.60; 95% CI, 1.13-2.25), compared with non-White patients. This association seemed to be driven by patients with CTD-ILD in CARE-PF (e-Table 4). The opposite association was found in the AILDR cohort. White patients were less likely to receive treatment compared with non-White patients on unadjusted analysis, with an HR for earlier treatment of 0.56 (95% CI, 0.33-0.95) in those with IPF, and 0.61 (95% CI, 0.41-0.91) in those with non-IPF ILD. After adjusting for confounders, the point estimate for this association remained similar, but statistical significance was lost due to the low number of non-White patients. There was no significant association between race and time to treatment initiation in the UChicago cohort. Across cohorts and for nearly all diagnostic subtypes, lower FVC and Dlco % predicted were associated with increased hazards of earlier treatment initiation.
Table 4.
Cox Proportional Hazards Model of Time to Treatment Initiation Stratified by IPF vs Non-IPF, Across Cohorts
| IPF |
Non-IPF ILD |
|||||
|---|---|---|---|---|---|---|
| CARE-PF | AILDR | UChicago | CARE-PF | AILDR | UChicago | |
| Male sex (unadjusted) | 1.17 (0.94-1.46) | 0.93 (0.64-1.39) | 0.59 (0.23-1.54) | 1.06 (0.89-1.26) | 0.96 (0.70-1.32) | 0.77 (0.57-1.04) |
| White race (unadjusted) | 1.52 (1.04-2.23) | 0.56 (0.33-0.95) | 3.03 (0.40-22.6) | 1.27 (1.0-1.60) | 0.61 (0.41-0.91) | 0.76 (0.56-1.02) |
| Adjusted model | ||||||
| Male sex | 0.96 (0.72-1.28) | 0.91 (0.61-1.36) | 0.38 (0.07-2.04) | 0.91 (0.74-1.13) | 0.96 (0.70-1.33) | 0.75 (0.54-1.04) |
| White race | 2.07 (1.23-3.48) | 0.54 (0.30-0.97) | 4.24 (0.49-36.99) | 1.60 (1.13-2.25) | 0.70 (0.46-1.07) | 0.95 (0.67-1.35) |
| FVC % predicted (per 10% decrease) | 1.11 (1.02-1.20) | 1.12 (1.02-1.25) | 1.20 (0.62-2.32) | 1.02 (0.96-1.09) | 1.16 (1.05-1.26) | 1.14 (1.00-1.30) |
| Dlco % predicted (per 10% decrease) | 1.02 (0.84-1.11) | 1.05 (0.95-1.16) | 1.14 (0.83-1.57) | 1.14 (1.06-1.22) | 1.03 (0.95-1.12) | 1.04 (0.94-1.16) |
| Age (per 10-y increase) | 0.96 (0.84-1.11) | 1.23 (1.0-1.52) | 0.37 (0.17-0.81) | 0.83 (0.76-1.92) | 0.96 (0.83-1.12) | 0.90 (0.79-1.02) |
Boldface indicates statistical significance. AILDR = Australasian Interstitial Lung Disease Registry; CARE-PF = Canadian Registry for Pulmonary Fibrosis; CTD = connective tissue disease; Dlco = diffusion capacity of the lungs for carbon monoxide; HP = hypersensitivity pneumonitis; ILD = interstitial lung disease; IPF = idiopathic pulmonary fibrosis; UChicago = University of Chicago Registry.
Figure 2.
A-C, Cumulative proportion of treated patients according to race for (A) the Canadian Registry for Pulmonary Fibrosis (P = .005), (B) the Australasian Interstitial Lung Disease Registry (P = .90), and (C) the University of Chicago Registry (P = .14).
Discussion
Using three large prospective longitudinal cohorts, we show substantial geographic heterogeneity in the initiation of ILD-related treatment, when comparing men with women, and White with non-White patients. Interestingly, the direction of the differences identified varied across cohorts, with more frequent treatment for men and Whites in Canada, and greater likelihood of earlier treatment in White patients. In contrast, treatment was more common in women in Chicago, and non-Whites were more likely to receive earlier treatment. Although the differences in overall proportion of treated patients between men and women were smaller and perhaps less clinically meaningful, the differences in treatment were larger between White and non-White patients in both CARE-PF and UChicago.
These differences in treatment rates and disparities may be explained in part by regional variability in physician’s attitudes, preconceptions, and geographical distributions of ILD subtypes. A recent study has also shown important heterogeneity in the approach to ILD treatment globally.23 Other studies have demonstrated heterogeneity in rates of treatment for ILD, particularly in IPF. An earlier study of physician-reported treatment patterns across Europe has shown that less than one-half of the patients with IPF eligible for treatment received antifibrotic medications,24 similar to treatment rates seen in the CARE-PF registry. A large American study using administrative databases has shown even lower rates of treatment in IPF (26%).13 In contrast, a different American longitudinal cohort, the IPF-PRO registry, reported a rate of treatment for patients with IPF of 70%.25
This heterogeneity in treatment initiation across the cohorts included in this study is not easily explained. Patients in the Australasian cohort were older, and age is a risk factor for disease progression and death, which may partially explain their high rates of treatment.26, 27, 28 In addition, ILD centers included in this registry use nursing case managers, likely leading to higher treatment uptake. FVC % predicted was also overall higher in that cohort, and more patients may have fit the regional reimbursement criteria for antifibrotics or other restricted medications, whereas patients with lung function below a certain threshold may not have access to treatment. Universal health care and regional reimbursement criteria for ILD medications such as private insurance coverage in the United States may have impacted access to treatment.
Sex- or gender-based differences in the treatment of patients with ILD have been reported in prior studies. In a large US administrative study, women were significantly less likely to receive an antifibrotic prescription compared with men (22% vs 30%).13 In a French study of gender-based differences in patients with IPF, women were much less likely to undergo lung transplantation, with only one woman out of 51 (2%) receiving a lung transplant compared with 20 of 185 men (11%).29
In addition, racial differences also seem to impact treatment initiation, at least in some geographic areas and some cohorts; this was particularly striking in the CARE-PF cohort, where the association between earlier treatment and White patients was robust. Differences in ethnic populations captured at each center may explain some of this across-cohort variability. Whereas White patients in Canada, the United States, and Australia are likely consistent across cohorts and easily compared, non-White patients across these countries are different. The CARE-PF and AILDR cohorts have a greater number of Asians, whereas the UChicago cohort has the highest proportion of Black patients included in this study. In addition, the AILDR had one Indigenous Australasian patient (whereas this group comprises about 2% of the regional population), and the CARE-PF registry did include 3% of Indigenous patients.
Racial disparities in the treatment of ILD have not previously been explored. However, prior studies in lung cancer have shown that non-Hispanic Black patients are less likely to receive treatment.30 Black patients with COPD are also less likely to be referred for smoking cessation programs, vaccinations, or home oxygen initiation.31
Our study’s strength was to assess three distinct prospective longitudinal observational cohorts, which, analyzed in parallel, allowed us to uncover differences in the management of ILD across the disease spectrum, and across geographical areas. Some limitations of our study included the exclusion of prednisone from the ILD-related medications, due to the heterogeneity of prednisone use in terms of dosing, length of treatment, and indication for treatment. By focusing on steroid-sparing agents, we were able to assess long-term definitive management of ILD, especially pertaining to non-IPF ILD. We also had access to a limited number of covariates to understand the reasons behind heterogeneous findings on treatment initiation and sex- and race-based disparities. Gender, as self-identified by patients, was not captured in any of the three cohorts, and we therefore had to rely on sex assigned at birth. This may have led to identifying disparities that are in fact attributable to gender and societal gender roles, which we could not have identified. In addition, the heterogeneity of ethnic minority groups across cohorts and the limited number of patients led us to group them as “non-White” patients, understanding that this would limit our ability to detect treatment differences across specific groups. In addition, race is tightly linked to socioeconomic markers, which were missing in our cohort data. Finally, health care providers’ sex and race may have influenced their decision to initiate treatment, consciously or not. However, we were unable to ascertain providers’ sex/gender or race and therefore could not assess whether this had an impact on patient’s treatment initiation in our cohorts.
Interpretation
Overall, we have shown that ILD care, specifically treatment initiation, varies substantially across countries and registries. Patient sex and race may have a significant impact on the decision to initiate ILD treatment. Although we could not determine if patients are not started on medications because of physician factors or because of patient reluctance, we have shown that in Canada, men tend to be treated more than women, whereas the opposite was true in Chicago, and that White patients were more likely to be treated in Canada. Further research is needed to explore the reasons behind geographical and sex- and race-based discrepancies, but also across gender and gender roles, and across specific ethnic minority groups, throughout the trajectory of care for patients with ILD.
Funding/Support
The CARE-PF registry received funding from Boehringer-Ingelheim Canada. Author from the University of Chicago received funding for this work by the National Institutes of Health (NIH K23HL146942). The AILDR is supported by the NHMRC Centre of Research Excellence in Pulmonary Fibrosis (GNT1116371), which is supported by the Lung Foundation Australia, anonymous philanthropy, the Three Lakes Foundation, and Foundation Partner Boehringer Ingelheim.
Financial/Nonfinancial Disclosures
None declared.
Acknowledgments
Author contributions: D. A., A. A., R. S., and C. J. R. made substantial contributions to the design, analysis, and interpretation of the data, and to drafting the work. All authors have contributed to the acquisition of data, have critically revised the work for intellectual content, have given approval of the final version, and are accountable for the work.
Role of sponsors: The sponsors had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript. The sponsors had no role in this study beyond financial support.
Supplementary Data
References
- 1.Kawano-Dourado L., Glassberg M.K., Assayag D., Borie R., Johannson K.A. Sex and gender in interstitial lung diseases. Eur Respir Rev. 2021;30(162) doi: 10.1183/16000617.0105-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Institutes of Health Office of Research on Women’s Health. Sex & Gender. https://orwh.od.nih.gov/sex-gender
- 3.Canadian Institutes of Health Research What is Gender? What is Sex? 2020. https://cihr-irsc.gc.ca/e/48642.html
- 4.Raghu G., Collard H.R., Egan J.J., et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183(6):788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poinen-Rughooputh S., Rughooputh M.S., Guo Y., Lai H., Sun W., Chen W. Sex-related differences in the risk of silicosis among Chinese pottery workers: a cohort study. J Occup Environ Med. 2021;63(1):74–79. doi: 10.1097/JOM.0000000000002068. [DOI] [PubMed] [Google Scholar]
- 6.Blanc P.D., Eisner M.D., Balmes J.R., Trupin L., Yelin E.H., Katz P.P. Exposure to vapors, gas, dust, or fumes: assessment by a single survey item compared to a detailed exposure battery and a job exposure matrix. Am J Ind Med. 2005;48(2):110–117. doi: 10.1002/ajim.20187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrara G., Arnheim-Dahlström L., Bartley K., et al. Epidemiology of pulmonary fibrosis: a cohort study using healthcare data in Sweden. Pulm Ther. 2019;5(1):55–68. doi: 10.1007/s41030-019-0087-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hopkins R.B., Burke N., Fell C., Dion G., Kolb M. Epidemiology and survival of idiopathic pulmonary fibrosis from national data in Canada. Eur Respir J. 2016;48(1):187–195. doi: 10.1183/13993003.01504-2015. [DOI] [PubMed] [Google Scholar]
- 9.Raghu G., Chen S.Y., Yeh W.S., et al. Idiopathic pulmonary fibrosis in US Medicare beneficiaries aged 65 years and older: incidence, prevalence, and survival, 2001-11. Lancet Respir Med. 2014;2(7):566–572. doi: 10.1016/S2213-2600(14)70101-8. [DOI] [PubMed] [Google Scholar]
- 10.Jalbert A.C., Siafa L., Ramanakumar A.V., Assayag D. Gender and racial equity in clinical research for idiopathic pulmonary fibrosis: a systematic review and meta-analysis. Eur Respir J. 2022;59(3) doi: 10.1183/13993003.02969-2021. [DOI] [PubMed] [Google Scholar]
- 11.Assayag D., Morisset J., Johannson K.A., Wells A.U., Walsh S.L.F. Patient gender bias on the diagnosis of idiopathic pulmonary fibrosis. Thorax. 2020;75(5):407–412. doi: 10.1136/thoraxjnl-2019-213968. [DOI] [PubMed] [Google Scholar]
- 12.Assayag D., Garlick K., Johannson K.A., et al. Treatment initiation in patients with interstitial lung disease in Canada. Ann Am Thorac Soc. 2021;18(10):1661–1668. doi: 10.1513/AnnalsATS.202009-1122OC. [DOI] [PubMed] [Google Scholar]
- 13.Dempsey T.M., Payne S., Sangaralingham L., Yao X., Shah N.D., Limper A.H. Adoption of the antifibrotic medications pirfenidone and nintedanib for patients with idiopathic pulmonary fibrosis. Ann Am Thorac Soc. 2021;18(7):1121–1128. doi: 10.1513/AnnalsATS.202007-901OC. [DOI] [PubMed] [Google Scholar]
- 14.Race. Merriam-Webster Dictionary. 2021. https://www.merriam-webster.com/dictionary/race?utm_campaign=sd&utm_medium=serp&utm_source=jsonld
- 15.Adegunsoye A., Vela M., Saunders M. Racial disparities in pulmonary fibrosis and the impact on the Black population. Arch Bronconeumol. 2022;58(8):590–592. doi: 10.1016/j.arbres.2021.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adegunsoye A., Oldham J.M., Bellam S.K., et al. African-American race and mortality in interstitial lung disease: a multicentre propensity-matched analysis. Eur Respir J. 2018;51(6) doi: 10.1183/13993003.00255-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swigris J.J., Olson A.L., Huie T.J., et al. Ethnic and racial differences in the presence of idiopathic pulmonary fibrosis at death. Respir Med. 2012;106(4):588–593. doi: 10.1016/j.rmed.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Storme M., Semionov A., Assayag D., et al. Estimating the incidence of interstitial lung diseases in the Cree of Eeyou Istchee, northern Québec. PLoS One. 2017;12(9) doi: 10.1371/journal.pone.0184548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ryerson C.J., Tan B., Fell C.D., et al. The Canadian Registry for Pulmonary Fibrosis: design and rationale of a national pulmonary fibrosis registry. Can Respir J. 2016;2016 doi: 10.1155/2016/3562923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jo H.E., Glaspole I., Grainge C., et al. Baseline characteristics of idiopathic pulmonary fibrosis: analysis from the Australian Idiopathic Pulmonary Fibrosis Registry. Eur Respir J. 2017;49(2) doi: 10.1183/13993003.01592-2016. [DOI] [PubMed] [Google Scholar]
- 21.Raghu G., Remy-Jardin M., Myers J.L., et al. Diagnosis of idiopathic pulmonary fibrosis: an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2018;198(5):e44–e68. doi: 10.1164/rccm.201807-1255ST. [DOI] [PubMed] [Google Scholar]
- 22.Travis W.D., Costabel U., Hansell D.M., et al. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188(6):733–748. doi: 10.1164/rccm.201308-1483ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boente R.D., White E.N., Baxter C.M., et al. PFF Best Practices Committee Differences in patient outcomes across the Pulmonary Fibrosis Foundation Care Center Network. Am J Respir Crit Care Med. 2023;207(2):214–218. doi: 10.1164/rccm.202206-1173LE. [DOI] [PubMed] [Google Scholar]
- 24.Maher T.M., Molina-Molina M., Russell A.M., et al. Unmet needs in the treatment of idiopathic pulmonary fibrosis—insights from patient chart review in five European countries. BMC Pulm Med. 2017;17(1):124. doi: 10.1186/s12890-017-0468-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salisbury M.L., Conoscenti C.S., Culver D.A., et al. IPF-PRO Registry Principal Investigators. Antifibrotic drug use in patients with IPF: data from the IPF-PRO Registry. Ann Am Thorac Soc. 2020;17(11):1413–1423. doi: 10.1513/AnnalsATS.201912-880OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ley B., Ryerson C.J., Vittinghoff E., et al. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann Internal Med. 2012;156(10):684–691. doi: 10.7326/0003-4819-156-10-201205150-00004. [DOI] [PubMed] [Google Scholar]
- 27.Morisset J., Vittinghoff E., Elicker B.M., et al. Mortality risk prediction in scleroderma-related interstitial lung disease: the SADL model. Chest. 2017;152(5):999–1007. doi: 10.1016/j.chest.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ryerson C.J., Vittinghoff E., Ley B., et al. Predicting survival across chronic interstitial lung disease: The ILD-GAP model. Chest. 2014;145(4):723–728. doi: 10.1378/chest.13-1474. [DOI] [PubMed] [Google Scholar]
- 29.Sesé L., Nunes H., Cottin V., et al. Gender differences in idiopathic pulmonary fibrosis: are men and women equal? Front Med (Lausanne) 2021;8 doi: 10.3389/fmed.2021.713698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blom E.F., Ten Haaf K., Arenberg D.A., de Koning H.J. Disparities in receiving guideline-concordant treatment for lung cancer in the United States. Ann Am Thorac Soc. 2020;17(2):186–194. doi: 10.1513/AnnalsATS.201901-094OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kirkpatrick d.P., Dransfield M.T. Racial and sex differences in chronic obstructive pulmonary disease susceptibility, diagnosis, and treatment. Curr Opin Pulm Med. 2009;15(2):100–104. doi: 10.1097/MCP.0b013e3283232825. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.