Abstract
Exosomes are extracellular vesicles with diameters of about 100 nm that are naturally secreted by cells into body fluids. They are derived from endosomes and are wrapped in lipid membranes. Exosomes are involved in intracellular metabolism and intercellular communication. They contain nucleic acids, proteins, lipids, and metabolites from the cell microenvironment and cytoplasm. The contents of exosomes can reflect their cells’ origin and allow the observation of tissue changes and cell states under disease conditions. Naturally derived exosomes have specific biomolecules that act as the “fingerprint” of the parent cells, and the contents changed under pathological conditions can be used as biomarkers for disease diagnosis. Exosomes have low immunogenicity, are small in size, and can cross the blood–brain barrier. These characteristics make exosomes unique as engineering carriers. They can incorporate therapeutic drugs and achieve targeted drug delivery. Exosomes as carriers for targeted disease therapy are still in their infancy, but exosome engineering provides a new perspective for cell‐free disease therapy. This review discussed exosomes and their relationship with the occurrence and treatment of some neuropsychiatric diseases. In addition, future applications of exosomes in the diagnosis and treatment of neuropsychiatric disorders were evaluated in this review.
Keywords: biomarker, diagnosis, exosomes, neuropsychiatric diseases, treatment
Exosomes from neural cells can cross the blood–brain barrier, which can be used for diagnosis. Meanwhile, exosomes can be modified and engineered by loading specific nucleic acids, proteins, and drugs, which can be peripherally injected and play a therapeutic role to restore the health of the brain.
1. INTRODUCTION
Exosomes are extracellular vesicles (EVs) secreted by almost all cell types. 1 , 2 , 3 They are formed by indenting the cell membrane, enveloping the extracellular microenvironment and cytoplasmic material, and exiting the cell before extracellular action. 4 , 5 , 6 , 7 , 8 , 9 Exosomes contain sugars, lipids, proteins, nucleic acids, and bioactive substances in the extracellular matrix, 10 which was initially thought to be a cellular mechanism to eliminate metabolic waste. 11 Recent studies have shown that released exosomes can be used by other cells to mediate intercellular communication and participate in the physiological and pathological processes of the body. 12 , 13 , 14 , 15 The function of exosomes depends on the type of cell origin. 16 They are involved in the immune response, 15 , 17 , 18 , 19 antigen presentation, cell migration, differentiation and angiogenesis, 20 , 21 inflammatory induction, 22 oxidative stress and apoptosis, 23 atherosclerosis, 24 , 25 tumor initiation, progression, invasion and metastasis, and drug resistance. 26 , 27 Given the ability of exosomes to carry and transfer bioactive substances, 28 the potential of exosomes to reveal the pathogenesis of diseases such as cancer, 29 , 30 , 31 , 32 , 33 brain diseases, 34 , 35 , 36 , 37 cardiovascular diseases, 38 , 39 , 40 , 41 , 42 , 43 , 44 and metabolic diseases, 45 , 46 , 47 as well as to diagnose and treat diseases as biomarkers are being studied and explored. 48 , 49 , 50 , 51 , 52
The prevalence of neuropsychiatric disorders, the leading cause of disability in humans, has increased in the last few decades 53 and continues to grow due to the coronavirus disease (COVID‐19) outbreak in 2019. 54 , 55 Despite numerous studies, the exact etiology of neuropsychiatric disorders is still unclear. Diagnosis is based primarily on subjective cognitive evaluation. There are no widely recognized objective indicators or biomarkers for early diagnosis, limiting the effective diagnosis and treatment of neuropsychiatric disorders. 53 Exosomes, as a new type of liquid biopsy, can potentially diagnose and predict the prognosis of diseases. 56 , 57 , 58 , 59 At the same time, exosomes are also high‐quality carriers of bioactive molecules or therapeutic drugs. 60 , 61 , 62 , 63 , 64 , 65 , 66 Their modification can target the injury site for precise treatment. 67 , 68 , 69 , 70 , 71 , 72 The contents of exosomes undergo specific changes when the internal and external environment changes, causing tissue dysfunction and disease. 73 , 74 , 75 , 76 , 77 , 78 The detection and modification of exosomes provide a new perspective for diagnosing and treating neuropsychiatric diseases, and their appearance offers a fresh approach for managing neuropsychiatric disorders.
Exosomes are involved in the pathophysiological processes of neuropsychiatric disorders, but their role in different neuropsychiatric disorders is not fully understood. 79 , 80 , 81 , 82 , 83 Therefore, this review explores the mechanism of the pathogenesis of exosomes and therapeutic targets in brain diseases. Finally, the problems and challenges facing exosomes in future diagnoses and treatments of neuropsychiatric disorders are discussed.
2. EXOSOMES
2.1. Discovery and the structure of exosomes
Exosomes are small membrane vesicles with diameters of 30–150 nm. They are naturally found in body fluids, such as blood, cerebrospinal fluid (CSF), saliva, breast milk, alveolar fluid, bile, joint fluid, amniotic fluid, semen, and urine. They were first discovered in 1983 from sheep's red blood cells before Johnstone coined “exosomes” in 1987. 84 , 85 In vitro experiments demonstrated that almost all cultured cells could secrete exosomes under normal and pathological conditions. 86 As EVs, exosomes are relatively similar to microvesicles and apoptotic bodies (Figure 1). 87 In contrast, the composition and structure of exosomes are more complex and contain additional components from the extracellular matrix. 88
FIGURE 1.
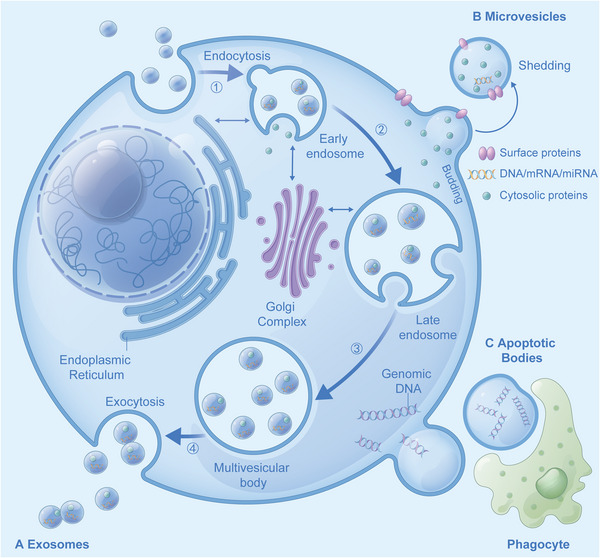
(A) Biogenesis of exosomes: (1) membrane invagination forms early endosomes, (2) early endosomes were loaded with particulate matter, (3) late endosomes and multivesicular body were formed successively, (4) multivesicular body membrane fuses with the cell membrane and releases intracavitary vesicles (exosomes) through exocytosis; (B) microvesicles (EVs): cell membrane sprouts or bubbles to form microvesicles; (C) apoptotic bodies: cells that initiate the apoptotic process form apoptotic bodies by germinating and shedding or forming autophagosomes, which are eventually engulfed by phagocytes.
Lipids and proteins are the main components of exosomal membranes and are involved in the formation and release of exosomes. 89 , 90 , 91 , 92 Exosomes contain essential biologically active molecules 93 , 94 (Figure 2, Table 1). Lipid bilayers can stabilize the activity of biological molecules such as nucleic acids and proteins contained in these exosomes.
FIGURE 2.
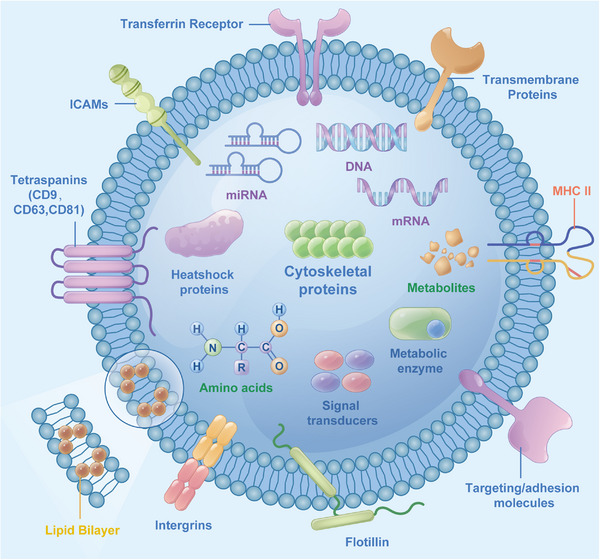
Exosome composition: (1) Lipids: lipid bilayers form the exosome membrane (yellow label); (2) nucleic acids: DNA and RNA (mRNA, miRNA) (purple label); (3) immunoregulatory molecules: major histocompatibility complex (MHC): MHC‐I, MHC‐II (orange label); (4) proteins: transmembrane proteins: tetraspanins (CD9, CD63, CD81), adhesion factors: intercellular adhesion molecules (ICAM), integrin, transferrin receptor, flotillin; heat shock protein, cytoskeletal protein, metabolic enzymes, signal transducers (blue label); (5) metabolites: derived from maternal cells and the surrounding microenvironment, such as amino acids (green label).
TABLE 1.
General composition of exosomes.
| Composition | Samples | Reference |
|---|---|---|
| Lipids | Ceramide, cholesterol, GM3, phosphatidylserine, phosphoglycerides | 2,5,10,93 |
| Phosphatidylcholine, phosphatidylethanolamine, phosphatidylinositol, sphingomyelin | 2,60,89 | |
| Nucleic acids | RNA: mRNA, ncRNA (microRNA, lncRNA, circRNA) | 2,5,50,94 |
| DNA: tumor susceptibility genes | 2,89,94 | |
| Immune regulatory molecules | MHC: MHC‐I, MHC‐II | 2,49 |
| Proteins | Inner membrane fusion/transport proteins: annexins, Rab proteins | 2,10,93 |
| Cytoskeletal proteins: tubulin protein, actin protein | 9,10,93 | |
| Heat shock proteins: Hsp70, Hsp90 | 9,10 | |
| A variety of metabolic enzymes: peroxidase, GAPDH, DPP4 | 9 | |
| Involved in the formation of multiple vesicle bodies: Alix, TSG101 | 2,9 | |
| Integrins and the four transmembrane protein family: CD9, C063, CD81, CD82 | 2,9 | |
| Signal transduction proteins: protein kinases, G proteins | 10,89 | |
| Metabolites | Derived from maternal cells and the surrounding microenvironment | 10 |
Abbreviations: circRNA, circular RNA; lncRNA, long noncoding RNA; MHC, major histocompatibility complex; mRNA, messenger RNA; ncRNA, noncoding RNA.
The various RNAs contained in exosomes are essential for the regulatory role of exosomes. 95 , 96 , 97 MicroRNAs (miRNAs or miR) are highly conserved noncoding single‐stranded RNAs (20–22 nucleotides in length) encoded by endogenous genes. 98 The binding of miRNAs onto the 3′ untranslated region of messenger RNA (mRNAs) causes deadenylation and forms RNA silencing complexes, which results in mRNA degradation and translation inhibition. 99 , 100 Long noncoding RNA (lncRNA) is a type of noncoding RNA (ncRNA) with a length of more than 200 nucleotides that induces DNA‐related effects that regulate transcription, epigenetics, protein/RNA stability, translational, and posttranslational modification. 101 Circular RNA (circRNA) is a covalently closed single‐stranded RNA that acts as a transcriptional regulator, miRNA sponge, and protein scaffold. 102 , 103 , 104 Its unique circular structure makes it less susceptible to degradation by RNA hydrolases. 105 , 106 , 107 Hence, circRNAs have a longer half‐life than linear RNAs, making them potential biomarkers and therapeutic targets.
2.2. Mechanism of action of exosomes in the brain
The structure and function of the brain are complex. Research on the molecular mechanism of encephalopathy is still in its infancy, restricting the development of diagnosis and treatment. 108 Exosome study in encephalopathy has attracted extensive attention in recent years. 109 , 110 Maintaining the normal physiological function of the brain depends on intercellular communication. 111 Exosomes of various cell types can penetrate the blood–brain barrier and act as important messengers in brain cell–cell communication, 112 , 113 , 114 regulating physiological and pathological processes in the brain. 115 Exosomes crosstalk brain cells through three main mechanisms: (1) cell internalization: endocytosis by recipient cells; (2) fusion with the cell membrane: direct fusion with the cell membrane through vesicles; and (3) protein–protein or receptor–ligand interaction: adhesion to the cell surface through ligands. 116 , 117 , 118 The contents of the exosomes are then released into the cytoplasm of receptor cells, 119 mediating intracellular signaling pathways, 120 , 121 and influencing biological effects (Figure 3).
FIGURE 3.
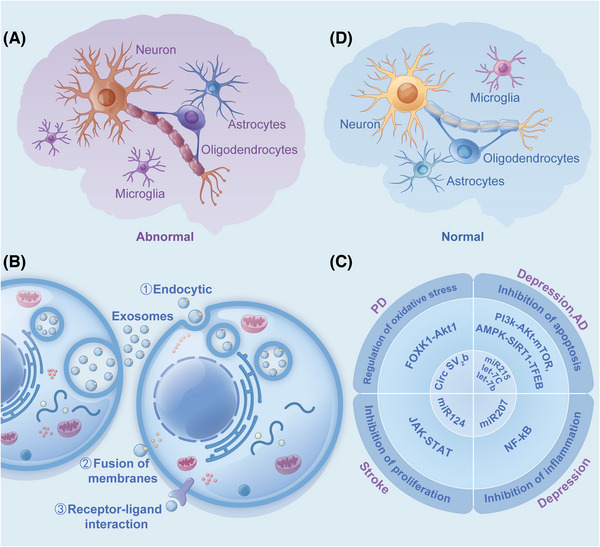
(A) Nerve cells that undergo inflammation and oxidative stress; (B) exosomes interact with target cells; (C) exosomes mediate intracellular signaling pathways; (D) nerve cells that have experienced biological effects.
Both let‐7c and let‐7b are involved in the expression regulation of insulin‐like growth factor 1 (IGF‐1) gene in the PI3K‐Akt‐mTOR (phosphatidylinositol trihydroxykinase–protein kinase B‐mammalian target of rapamycin) signaling pathway and are significantly downregulated in patients with major depressive disorder (MDD) as compared to healthy subjects. The overexpression of IGF‐1 relays an antidepressant‐like behavior that promotes brain‐derived neurotrophic factor (BDNF) signal transduction. 122 In vivo experiments demonstrated that exosomes with low levels of miR‐207 had reduced antidepressant activity. miR‐207 was overexpressed in normal mouse exosomes, directly targeted toll‐like receptor 4 (TLR4) to regulate TLR4 interactor with leucine‐rich repeats (Tril), inhibit NF‐κB (κ‐light chain was enhanced in B cells activated by nuclear factor) signaling pathway in astrocytes, and reduced the release of proinflammatory cytokines such as interleukin (IL) (IL‐1β, IL‐6) and tumor necrosis factor (TNF)‐α, alleviate depressive symptoms of mice. 123 In the M2 microglia, exosomal miR‐124 reduces the formation of glial scar after stroke by inhibiting the expression of signal transducer and activator of transcription 3 (STAT3), promotes poststroke recovery, and alleviates the migration and proliferation of astrocytes. 124 , 125 In Parkinson's disease (PD) model, circSV2b overexpression can reduce oxidative stress injury, protect dopaminergic neuron loss, maintain nigrostriatal function, and improve motor defects through miR‐5107‐5p‐FOXK1 (fork‐head box protein K1)‐AKT1 (serine/threonine protein kinase) signaling pathways. 126 miR‐215 targets the apoptosis‐related genes (BCL2L11 and SIRT1) and functions to regulate the AMPK‐SIRT1‐TFEB (adenosine 5′‐monophosphate [AMP] activated protein kinase [AMPK]‐silent message regulatory factor 1‐transcription factor EB) signaling pathway and prevent neuronal apoptosis. The exercise‐induced upregulation of exosomal miR‐215 may have preventive effects on Alzheimer's disease (AD). 127
However, exosomes can be hostile to or supportive of the brain, depending on the biological signals they carry. 127 , 128 Studies have found that exosomes are involved in the occurrence and development of various encephalopathies, 129 , 130 , 131 , 132 , 133 , 134 providing biomarkers and therapeutic targets for disease diagnosis and treatment. 135 , 136 , 137 Analysis of exosomes in body fluids can identify the subtle changes in the pathophysiological process of neuropsychiatric disorders, providing new means for diagnosing and predicting diseases and a new perspective for treatment.
3. EXOSOMES IN NEUROPSYCHIATRIC DISORDERS
Brain cells damaged by inflammation and oxidative stress exhibit morphological abnormalities and dysfunction. Exosomes derived from diseased nerve cells cross the blood–brain barrier into the peripheral circulation to release their contents, which can be used as biomarkers to diagnose and predict diseases. At the same time, exosomes with altered contents are modified, or therapeutic drugs are loaded into exosomes. As a natural source of body fluids, exosomes cross the blood–brain barrier and participate in the pathophysiological processes of neuropsychiatric diseases (Figure 4). In summary, altered exosomes in disease states can be used as potential biomarkers for disease diagnosis (Table 2). Engineered exosomes can also be used as carriers of bioactive molecules involved in signaling pathways and become potential targets for disease treatment (Table 3). This section summarizes the progress of exosomes' role in diagnosing and treating various neuropsychiatric disorders.
FIGURE 4.
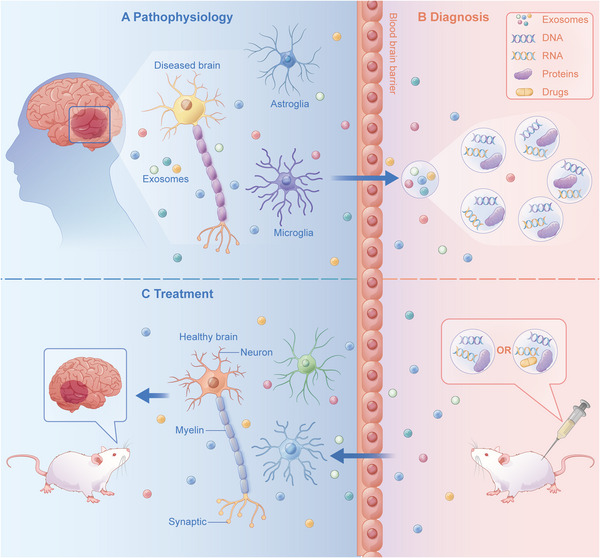
(A) Exosome crossing: exosomes from diseased nerve cells cross the blood–brain barrier and enter the peripheral circulation, releasing their contents such as RNA and protein; (B) extraction and modification of exosomes: exosomes are extracted from body fluids. Their contents are analyzed by high‐throughput sequencing qRT‐PCR and quantitative analysis tools to find clinical biomarkers, diagnose and predict the occurrence and development of diseases, provide a basis for clinical decision‐making, and identify and develop treatments based on exosome contents, such as increasing or decreasing a specific substance, or introduce therapeutic drugs, modify exosomes into engineered exosomes, and inject the engineered exosomes into model animals; (C) therapeutic effects of exosomes: engineered exosomes carrying therapeutic substances enter model animals, cross the blood–brain barrier, and reach the center to play a therapeutic role and restore nerve cell health.
TABLE 2.
Exosomal contents with pathogenicity in neuropsychiatric disorders.
| Disease | Exosome derivation | Contents | Pathogenesis | References |
|---|---|---|---|---|
| Depression | Serum from patients with depression | miR‐9‐5p | Promote M1 polarization of microglia cells | 144 |
| Schizophrenia | Plasma from EPP | miR‐137 | Induce autophagy and aggravating nerve damage | 152 |
| Plasma from patients with TRS | miR‐675‐3p | Involved in neuroapoptosis | 154 | |
| PTSD | Peripheral blood of soldiers with mTBI | NFL | Involve in neurodegenerative and inflammatory processes | 168,169 |
| AD | Plasma of Chinese AD patients | miR‐185‐5p | Involve in the production and accumulation of Aβ | 186,187 |
| PD | Serum from PD patients | TNF‐α, IL‐1β | Induce dopaminergic neuron death | 166 |
| ALS | Motor neurons of fALS | miR‐625‐3p | Mediate neuroinflammation, oxidative stress and apoptosis | 243 |
| Epilepsy | Plasma from patients with mTLE + HS | hsa‐miR‐184 | Modulate immunity, inflammation and apoptosis, and exacerbates neuronal death | 261 |
| Stroke | Serum from AIS patients | miR‐9 | Involved in the inflammatory process, regulating angiogenesis | 268 |
| Serum from AIS patients | circ_0006896 | Promote vascular migration and proliferation, resulting in plaque instability | 273 |
Abbreviations: AD, Alzheimer's disease; AIS, acute ischemic strokes; ALS, amyotrophic lateral sclerosis; Aβ, β‐amyloid; EPP, early psychosis patients; fALS, familial ALS; hsa: Homo sapiens; IL, interleukin; mTBI, mild traumatic brain injury; mTLE + HS, mesial temporal lobe epilepsy with hippocampal sclerosis; NFL, neurofilament; PD, Parkinson's disease; PTSD, posttraumatic stress disorder; TNF, tumor necrosis factor; TRS, treatment‐resistant schizophrenia.
TABLE 3.
Exosomal contents with therapeutic potential in neuropsychiatric disorders.
| Diseases | Exosome derivation | Contents | Therapeutic targets | References |
|---|---|---|---|---|
| Depression | Patients with refractory depression | let‐7c and let‐7b | Inhibit apoptosis | 122 |
| Non‐stressed mouse NK cells | miR‐207 | Inhibit inflammation | 123 | |
| BMSCs | miR‐26a | Improve hippocampal neuron damage | 147 | |
| AD | Blood | miR‐215 | Prevent neuronal apoptosis | 188 |
| BMSCs | miR‐21, miR‐29b, and miR‐146a | Improve synaptic dysfunction and suppress inflammation | 191 | |
| ADSCs | miR‐770‐3p | Promote the transformation of hippocampal microglia from M1 to M2 phenotype | 193 | |
| M2 microglia | miR‐124‐3p | Inhibit neuronal inflammation and promote nerve growth | 194 | |
| PD | ADSCs | miR‐188‐3p | Reverse the apoptosis | 205 |
| Serum of PD mice | CircSV2b | Resist oxidative stress injury | 206 | |
| HD | ADSCs | Neurotrophic factor | Reduce mHTT aggregation, mitochondrial dysfunction and apoptosis | 223,224 |
| Astrocytes | αB‐Crystallin | Reduce the aggregation of mHTT and the cytotoxicity of misfolded proteins | 225 | |
| ALS | Mouse ADSCs | SOD‐1 | Regulate SOD aggregation, participate in cell adhesion | 252 |
| Stroke | Astrocytes | miR‐190b | Regulate autophagy | 278 |
| Microglial | miR‐21 | Inhibit inflammation | 280 | |
| BMSCs | miR138‐5p | Promote astrocyte proliferation, inhibit inflammation and apoptosis | 281 | |
| BMSCs | miR‐133b | Promote functional recovery | 282 | |
| BMSCs | miR‐21‐5p | Promote neurological function | 282 | |
| BMSCs | miR‐150‐5p | Inhibit TLR5, protect cerebral I/R injury | 283 | |
| M2 microglial | miR124 | Inhibit proliferation | 125 | |
| BMSCs | miR‐124‐3p | Reduce neuronal damage, inhibit oxidative stress, and reduce neuronal apoptosis | 284,285 | |
| NSCs | miR‐150‐3p | Reduce infarct size and inhibit neuronal apoptosis | 286 |
Abbreviations: AD, Alzheimer's disease; ADSCs, adipose‐derived stem cells; ALS, amyotrophic lateral sclerosis; BMSCs, bone mesenchymal stem cells; circ, circular; HD, Huntington's disease; I/R, ischemia/reperfusion; mHTT, mutant huntingtin; NK cells, natural killer cells; NSCs, neural stem cells; SOD, superoxide dismutase; TLR, toll‐like receptor.
3.1. Depression
Mental disorders constitute a global health burden, with depression among the common one. 138 Depression is the second leading cause of disability in China and is normally characterized by low mood, loss of interest, fatigue, and in some cases, suicidal tendencies. 139 The currently available treatments for depression include medication, psychotherapy, and physical therapy. Nevertheless, the cause of depression remains unclear, with limited treatment efficacy and a high recurrence rate.
Blood exosomes from depression patients induced depression‐like behavior in normal mice. 140 Compared with healthy controls, plasma exosomes of patients with refractory depression were significantly upregulated by Homo sapiens (hsa)‐miR‐335‐5p and downregulated by hsa‐miR‐1292‐3p. These two miRNAs are associated with synaptic functions and treatment‐resistant depression. 141 The expression levels of miRNAs (hsa‐miR‐16‐5p, hsa‐miR‐129‐5p, hsa‐miR‐363‐3p, and hsa‐miR‐92a‐3p) in exosomes from drug‐dependent depressed patients were negatively correlated with both the Hamilton Rating Scale for Depression (HRSD) and Hamilton Anxiety Rating Scale (HARS). 142 Some studies isolated serum exosomes from patients with severe depression and healthy controls, and the expression level of miR‐146a was higher in patients with severe depression than in healthy controls. Compared with the non‐remission group, the levels of let‐7e, miR‐21‐5p, miR‐145, miR‐146a, and miR‐155 were significantly decreased before treatment in the remission group and increased after antidepressant treatment. 143 These exosomal miRNAs may play an important role in predicting the effects of antidepressants. There were significantly higher miR‐9‐5p expression levels in serum exosomes from depressed patients. miR‐9‐5p was transferred from neurons to microglia through exosomes, leading to microglia M1 polarization, further neuronal injury, and exacerbated depressive symptoms. 144 Genome‐wide miRNA expression profiling of blood‐derived exosomes from depressed patients and healthy subjects reported that the most differentially expressed exosomal miRNA, hsa‐miR‐139‐5p, was upregulated and could distinguish patients with MDD from controls. Exosomes from the blood of patients with depression can induce depression‐like behavior in normal mice by tail vein injection. However, the injection of blood exosomes isolated from healthy volunteers into chronic unpredictable mild stress (CUMS) mice alleviated depression‐like behavior. CUMS mice also exhibited significantly higher levels of exosomal miR‐139‐5p in the blood and brain. Treatment with exosomes from healthy blood and miR‐139‐5p antagonists both increased hippocampal neurogenesis in CUMS mice, whereas treatment with exosomes from depressed patients decreased hippocampal neurogenesis in normal mice. 145 , 146 Therefore, exosomal miRNAs may be potential targets for the diagnosis and treatment of depression.
Bone marrow stem cell (BMSC)‐derived exosomes (BMSC‐Exos) improved hippocampal neuronal injury in depression‐induced rats by upregulating miR‐26a. 147 miR‐207 is overexpressed in normal mouse exosomes, inhibiting the NF‐κB signaling pathway in astrocytes, reducing the release of pro‐inflammatory factors, and alleviating depressive symptoms in mice. 123 Plasma exosomes exerted antidepressant‐like effects on sigma‐1 receptors in lipopolysaccharide (LPS)‐induced depression. 148 , 149 Therefore, further research can facilitate the development of new exosome‐based treatments for MDD in the future.
3.2. Schizophrenia
Schizophrenia (SCZ) is a chronic mental illness of unknown etiology, affecting nearly 1% of the population. It mostly affects young adults and the elderly, with symptoms such as irregularities in physical behavior, logical thinking, and emotional experience. Antipsychotics have limited effects on their treatment and severe psychiatric and metabolic side effects. 150 , 151 Likewise, these drugs mainly improve positive symptoms such as hallucinations and thought disorders, but not negative (emotional flatness and social withdrawal) and cognitive (learning and attention disorders) symptoms.
The identification of biomarkers in the early stages of SCZ can help elucidate its pathogenesis and treatment. The expression of exosomal miR‐137 increased, whereas COX6A2 decreased in patients with early psychosis. This induces autophagy by regulating the expression of mitophagy markers (Fundc1, NIX, and LC3B), and aggravates the damage of parvalbumin interneurons in the prefrontal cortex of mice under oxidative stress. 152 Hence, the level of plasma exosomal miR‐137/COX6A2 is closely related to the pathology of SCZ and can be used as a biomarker for early diagnosis. BDNF plays a vital role in the development and maintenance of the nervous system. The miRNA expression profile of serum exosomes in patients with SCZ reported that hsa‐miR‐206 directly targets the BDNF mRNA, which negatively affects cognitive functions. 153 Microarray analysis indicated that 13 miRNAs were upregulated, and 18 miRNAs were downregulated in plasma exosomes of treatment‐resistant SCZ (TRS) patients. The target genes (PTRO, SRSF2, and CPEB4) were associated with neuronal apoptosis and brain development. The expression of miR‐675‐3p was upregulated in TRS patients and exosomes of clozapine‐treated SH‐SY5Y cells. The results suggested that clozapine can affect the expression of exosomal miRNAs. 154 miR‐223 is enriched in astrocytes and highly expressed in glial and neuronal exosomes. The levels of miR‐223 in the orbitofrontal cortex were significantly increased after SCZ patient deaths. miR‐223 is secreted by exosomes and enriched in astrocytes, which targets glutamate ionotropic receptor NMDA (N‐methyl‐d‐aspartic acid) type subunit 2B (GRIN2B) and glutamate ionotropic receptor AMPA (alpha‐amino‐3‐hydroxy‐5‐methyl‐4‐isooxazopropionic acid) type subunit 2 (GRIA2), its expression was significantly increased in SCZ patients. Studies have shown that miR‐223 inhibited the expression of neuronal glutamate receptor genes through specific binding to mRNA sites, thereby reducing the levels of GRIN2B and GRIA2 in mouse hippocampal neurons and decreasing synaptic activity. 155 This may be a mechanism of the pathophysiology of SCZ. Low NMDA receptor signaling is consistent with cognitive and behavioral disorders in SCZ. 156
Compared with healthy controls, plasma exosomes of SCZ patients showed significantly higher concentrations of glial fibrillary acid protein (GFAP) and lower concentrations of α‐II‐spectrin. Upregulation of GFAP indicates astrocyte injury. α‐II‐Spectrin is a cytoskeletal protein that makes up axons and synapses. It is abundant in brain neurons, and its downregulation suggests sustained nerve cell damage. 75 Therefore, an in‐depth study of protein and miRNA contents in exosomes can better elucidate the pathogenesis of SCZ and provide a basis for better treatment strategies.
3.3. Autism spectrum disorder
Autism spectrum disorder (ASD) is a central neurodevelopmental disorder caused by various genetic and environmental factors. ASD usually develops within 3 years of age, and its main symptoms include social communication disorder, communication disorder, and cognitive inflexibility. 157 ASD lacks specific treatments and requires lifelong care and maintenance.
Bioinformatics has revealed the genes and biological networks associated with ASD. 158 An exosomal proteomic analysis in an animal model of Rett syndrome–ASD suggested that the disruption of MECP2 function can affect brain development. 159 Active synaptic vesicle (SV)‐associated transcripts (SVATs) are present in the central nervous system (CNS) and the peripheral circulation, which play an important role in SV release and recycling. Abnormal SVAT expression has been found in patients with ASD. Alterations in the genetic expressions of lncRNAs and mRNAs related to SVATs were detected in the peripheral blood exosomes of children with ASD. 160 Thus, monitoring SVAT changes during pregnancy can predict the development and function of neural circuits in the fetal brain.
Elevated levels of proinflammatory cytokines and abnormal microglia/astrocyte responses were observed in the brains of animal models of ASD, suggesting that inflammation is involved in the pathogenesis of ASD. 161 The pathogenesis of ASD is associated with dysfunctional inflammatory pathways and macrophages. Studies have validated that the increased level of IL‐1 in exosomes of ASD patients regulates inflammation via miRNAs.
Stem cell‐derived exosomes polarize macrophages toward the M2 phenotype and stimulate anti‐inflammatory cytokines/proteins, thus reducing the pro‐inflammatory conditions associated with ASD. 162 Exosomal‐content recipient cells (RNA and protein) have the potential to repair damaged cells in ASD. BTBR T+ tf/J (BTBR) mice exhibit symptoms of ASD, which facilitates the assessment of ASD behaviors. In a BTBR mouse model, exosomes derived from mesenchymal stem cells (MSCs) that cross the blood–brain barrier and enter the brain via nasal administration ameliorated ASD‐related behaviors and symptoms. 163 , 164 Transplantation of human BMSCs (bone MSCs) into the lateral ventricles of BTBR mice resulted in the long‐term improvement of the behavioral phenotype of autism, and exosomes were the primary mediators of the therapeutic effect. 157 The beneficial effect of exosomes in BTBR mice may be a novel noninvasive treatment strategy for ASD symptoms.
3.4. Posttraumatic stress disorder
Posttraumatic stress disorder (PTSD) refers to a mental disorder related to trauma and stress that develops after experiencing a traumatic event. 165 , 166 The common symptoms are traumatic reexperience, avoidance, negative cognitive and emotional changes, and increased alertness.
PTSD is associated with mild traumatic brain injury (mTBI). A specific type of neurofilament (NFL) miRNA is associated with neurodegenerative and inflammatory processes and is a major component of the neuronal cytoskeleton. This miRNA is particularly expressed in myelinated nerve axons of large neurons, and its elevated expression level is a hallmark of axonal injury. 167 The levels of hsa‐miRNA‐139‐5p, tau, Aβ42, IL‐10, and NFL were elevated in peripheral blood EVs of soldiers with mTBI. As a significant component of the neuronal skeleton, NFL is a marker of axonal damage. It is involved in neurodegenerative and inflammatory processes and correlates with the severity of PTSD symptoms. 168 , 169 NFL in exosomes may be a new diagnostic tool and therapeutic target for PTSD.
3.5. Alzheimer's disease
AD is a progressive neurodegenerative disease with insidious onset and is characterized by memory, cognitive and behavioral impairments. Dementia affected approximately 50 million people in 2018, which is projected to triple by 2050. 170 Its pathogenesis is associated with a variety of factors, including genetic alterations, deposition of β‐amyloid (Aβ) protein, accumulation of tau into neurofibrillary tangles in neurons, and abnormal phosphorylation of tau. The accumulation of Aβ 1–42 in the brain and the phosphorylation of tau protein are the two main pathological features of AD. 171
Exosomes are involved in neuroinflammation, 172 productions and clearance of Aβ, 173 , 174 transport of Aβ, tau, prions, and α‐synaptic nucleoproteins, 175 , 176 which trigger the hyperphosphorylation of Aβ and tau. 177 Intracranial injection of exosomes from AD mouse brains in wild‐type (WT) mice resulted in mitochondrial damage and neurotoxicity. The levels of tau protein and Aβ1–42 in exosomes of the AD group were significantly increased. 115 Exosomal tau protein can be used as biomarkers for the preclinical diagnosis of AD. 178 Aβ1–42, t‐tau, p‐T181‐tau, and P‐S96‐tau may be used as biomarkers for AD. 179
More and more evidences emphasize the regulatory role of miRNAs in the pathogenesis of AD. The expressions of miR‐135a and miR‐384 were upregulated, whereas miR‐193b was downregulated in the serum exosomes of AD patients. 180 Likewise, miR‐342‐3p was downregulated in plasma exosomes, whereas miR‐212 and miR‐132 were downregulated in neural exosomes. 181 miRNAs, such as miR‐9‐5p, miR‐598, miR‐125b, miR‐29, miR‐342‐3p, and miR‐193b, are highly stable and resistant to degradation in exosomes before the onset of AD, suggesting their promising roles as biomarkers for the early clinical diagnosis and monitoring of AD. 182 , 183 miR‐29c‐3p, miR‐384, and nerve cell adhesion molecule in double‐labeled exosomes have the potential for early diagnosis of AD. 184 , 185 Serum exosomal miR‐185‐5p was significantly downregulated in AD patients and mice as compared with the control group. Overexpression of neuronal APP (amyloid precursor protein) regulates the levels of miR‐185‐5p in exosomes, thereby preventing the miR‐185‐5p‐mediated translational repression of APP transcripts. The regulatory role of exosomes in APP expression suggests that exosomes and their miRNAs may be potential therapeutic targets and biomarkers for the treatment and diagnosis of AD. 186 , 187 The exercise‐induced upregulation of exosomal miR‐215 had preventive effects on AD. 188 Compared with normal controls, the expression of circular AXL (circAXL) in the exosomes of AD serum samples was significantly increased, whereas the expression of miR‐1306‐5p was significantly decreased, indicating the prospective diagnosis of AD. An AD cell model was constructed by treating SK‐N‐SH cells with Aβ1–42, and the results indicated that circAXL and PDE4A (phosphodiesterase 4A), a key regulator of cAMP (cyclic adenosine monophosphate) degradation, was upregulated, whereas the expression of miR‐1306‐5p was downregulated. CircAXL participates in Aβ1–42‐induced neuronal injury by targeting the miR‐1306‐5p/PDE4A axis. CircAXL knockdown can inhibit PDE4A by releasing miR‐1306‐5p, reduce apoptosis, inflammation, oxidative stress, and endoplasmic reticulum stress in AD cells, and reduce Aβ1–42‐induced neurotoxicity in AD pathology. 189 This approach provides a new treatment concept for AD.
The proteins contained in exosomes can be used as biomarkers of AD. The SNAP25 (synaptosome‐associated protein of 25 kDa) and synaptophysin‐I binding protein in neuron‐derived exosomes can predict the development of AD. The levels of complement proteins (C1q, C3b, and C3d) and cytokines (IL‐6, IL‐1β, and TNF‐α) in astrocyte‐derived exosomes (ADEs) were significantly different between AD patients and healthy controls. 181 AD patients had higher levels of complement proteins in ADEs when compared with healthy controls. However, the ADEs of complement regulatory proteins (CD59, CD46, decay‐accelerating factor, and complement receptor type 1 [CR1]) were lower in AD patients than in healthy controls and further decreased as the disease progressed. Thus, measuring complement protein levels in exosomes can predict disease progression. Growth‐associated protein‐43 (GAP‐43), neurogranin, synaptotagmin (Rab3A), and SNAP25 in neurogenic exosomes have been studied as blood biomarkers for AD and mild cognitive impairment. 134
Different exosomes injected into AD mouse models exert different therapeutic effects. 81 , 190 BMSC‐Exos contain miR‐21, miR‐29b, and miR‐146a, which can improve synaptic function and inhibit inflammation, thus improving cognitive function in AD animal models. 191 BMSC‐Exos isolated from the femur and tibia of adult C57BL/6 mice can improve the AD‐like behavior of the streptozotocin‐injected mouse model. The mechanism is related to the inhibition of microglial activation and astrocytes in the hippocampus of the mouse model. The expressions of neuroinflammatory factors (IL‐1β, IL‐6, TNF‐α, Aβ1–42, and p‐tau) decreased, while the protein expressions of synaptophysin and BDNF were upregulated. 192 Hypoxia‐treated adipose‐derived stem cell (ADSC)‐derived exosomes (ADSC‐Exos) promoted the transformation of hippocampal microglia from the pro‐inflammatory M1 phenotype to the anti‐inflammatory M2 phenotype in AD mice, targeted miR‐770‐3p and TREM2 (trigger receptor 2 expressed in bone marrow cells), reduced damage to hippocampal neurons, and improved cognitive function. 193 On the contrary, the administration of M2 microglia‐derived exosomes (M2‐Exos) in AD cell models (HT‐22 cells and MAP2‐positive neuronal cells of APP/PS1 AD mice models) reduced Aβ plaque deposition and oligomer expression, partially improved cell viability, and restored the mitochondrial membrane potential. In addition, it can reduce the accumulation of reactive oxygen species (ROS) in mitochondria and cells in a dose‐dependent manner and play a neuroprotective role. Exosomal miR‐124‐3p can inhibit neuronal inflammation and promote neuronal growth. Similarly, miR‐124 can change the level of autophagy in APP/PS1 mice and alleviate the pathological progression of AD. Therefore, we hypothesize that the protective effect of M2‐Exos in AD may be mediated by miR‐124. 194 Exosomes from human amniotic fluid stem cells (hAFSC‐Exos) targeting LPS‐activated BV‐2 microglia as a model of neuroinflammation. A study was conducted to investigate the effect of exosomes on the SH‐SY5Y interaction between AD neurons and microglia. The results demonstrated that hAFSC‐Exos could inhibit the inflammatory response, oxidative stress, and microglial apoptosis and could be a potential therapeutic drug for diseases related to inflammation such as AD in the future. 195
Neuroinflammation is associated with neuronal cell death in AD. Coenzyme Q10 (CoQ10), as a supplement to anti‐inflammatory and antioxidant stress, can improve AD‐related inflammation and oxidative stress. Furthermore, exosomal CoQ10 promotes neuronal differentiation by increasing hippocampal BDNF and SOX2 levels and also enhances AD cognitive and memory deficits. 196 Quercetin‐loaded exosomes potently ameliorate cognitive function in AD mice by inhibiting Tau phosphorylation and reducing neurofibrillary tangle formation. 197 With the deepening of the research on engineering exosomes, the optimal ratio of exosome contents may be a new idea for treating AD in the future.
3.6. Parkinson's disease
PD, also known as parkinsonism, is a neurodegenerative disease in which a lesion of dopaminergic neurons in the substantia nigra of the midbrain causes a decrease in dopamine levels and prevents the brain from stimulating the muscles. 198 The neuropathological features of PD commonly include the degeneration of substantia nigra dopaminergic neurons, extensive misfolding and accumulation of α‐synuclein (α‐syn), and the formation of eosinophilic inclusion bodies (Lewy bodies) in the cytoplasm of residual substantia nigra neurons. 199 Genetic mutation, α‐syn, oxidative stress, mitochondrial dysfunction, autophagy, and infection are hallmarks in the pathogenesis of PD.
Glucocerebrosidase (GBA) gene mutation is the most common genetic risk factor for PD. GBA is cleaved into glucose and ceramide, and ceramide is involved in exosome biogenesis. GBA activity decreases in PD patients, resulting in significant increases in the number of exosomes released from the brain. 200 POU3F3 in neurogenic exosomes, containing L1 cell adhesion molecule, is significantly positively correlated with α‐syn and negatively correlated with the activity of lysosomal enzyme β‐GBA encoded by the gene, which can lead to the degeneration of dopamine neurons and accelerate the progression of PD. 200 , 201
Exosomes are involved in the internalization and transport of α‐syn oligomer. α‐syn aggregation is a result of exosome internalization, which leads to the loss of dopaminergic neurons and motor dysfunction. α‐syn oligomers can be transferred in exosomes from damaged neurons to normal neurons. 181 This promotes the transmission of pathological synuclein and induces aggregation and neuronal apoptosis, thus promoting the pathological changes of PD. 202 α‐syn oligomers are present in microglia/macrophage‐derived exosomes in the CSF of PD patients, where they aggregate and multiply through dysregulation of autophagy. A study injected exosomes containing α‐syn from PD patients into the striatum of mice and reported dopaminergic neuronal degeneration and motor deficits. 203 The ratio of α‐syn oligomers to α‐syn in plasma exosomes was correlated with the severity of PD. 204
Abnormal miRNA expression levels in serum and CSF‐derived exosomes in PD patients can be used as biomarkers for PD diagnosis. In PD patients, the expression levels of exosomal let7f‐5p and miR‐125a‐5p were increased, whereas the expression levels of miR‐27a‐3p, miR‐423‐5p, miR‐151a‐3p, miR‐1, and miR‐19b‐3p were decreased. 205 The reduced expression of miR‐23b‐3p in PD exosomes led to the upregulation of α‐syn. 206 miR‐151a‐5p, miR‐24, miR‐485‐5p, miR‐331‐5p, miR‐214, miR‐99a‐5p, miR‐126‐5p, miR‐1, miR‐19b‐3p, miR‐153, miR‐409‐3p, miR‐10a‐5p, and miR‐501‐3p were upregulated in the serum and CSF‐derived exosomes of PD patients and could be used as biomarkers for the diagnosis of PD. 207 LncRNAs are enriched in dopaminergic neurons, which can also be used as diagnostic markers of PD. When compared with healthy controls, one mRNA (NDUFB7) and three lncRNAs (ENST00000564683, ENST00000570408, and ENST 00000628340) extracted from blood exosomes of PD patients were differentially expressed. NDUFB7, which encodes reduced nicotinamide adenine dinucleotide ubiquitin oxidoreductase (Complex I), is positively correlated with the three lncRNAs. These are markers related to mitochondrial respiration and may be a potential biomarker to evaluate the effect of mitochondrial function recovery in PD rehabilitation. 208 These findings provided valuable potential biomarkers for evaluating future treatment strategies for PD. Overexpression of TNF‐α and IL‐1β in serum exosomes of PD patients directly induced dopaminergic neuron death. 166 Glial exosomes containing inflammatory molecules can communicate with neurons and promote the development of PD.
Regarding treatment, exosomes can be used as drug carriers for PD, with a natural brain‐targeting ability. Dopamine‐loaded exosomes reported better therapeutic efficacy and lower systemic toxicity than direct intravenous dopamine administration in the PD mouse model. In PD models, exosomes are delivered with therapeutic catalase mRNA to attenuate neurotoxicity and neuroinflammation. 209 , 210 , 211 Blood‐derived exosomes from healthy volunteers can reduce dopaminergic neuron damage and improve the motor coordination ability of PD mice. Intracerebroventricular injection of exosomes loaded with an antisense oligonucleotide (ASO)‐4 significantly increased α‐syn aggregation and attenuated dopaminergic neuron degeneration in PD mice, thus improving motor functions. 212 , 213 ADSC‐Exos are rich in miR‐188‐3p and can reverse PD‐related apoptosis in animal models. 205 In the PD model, circSV2b overexpression can reduce oxidative stress injury, protect dopaminergic neuron loss, maintain nigrostriatal function, and improve motor defects through miR‐5107‐5p‐FOXK1‐AKT1 signaling pathways. CircSV2b may help to better understand the pathogenesis of PD and become the new target for the diagnosis and treatment of PD. 126 These findings provide valuable potential biomarkers for evaluating treatment strategies for PD.
3.7. Huntington's disease
Huntington's disease (HD) is a rare monogenic dominant neurodegenerative disease. The cytosine–adenine–guanine (CAG) repeat expansion in exon 1 of the huntingtin gene causes fragmentation and aggregation of mutant huntingtin (mHTT) proteins. This affects neuronal cell function and causes cell death, resulting in cognitive impairment and involuntary movement. HD usually develops in middle childhood with insidious onset, and the main clinical manifestations are involuntary dance movements, cognitive disorders, and neuropsychiatric symptoms. 214 , 215 , 216 In HD, the incomplete expansion of the CAG repeat in exon 1 of the HTT gene results in the production of polyglutamine (polyQ) protein. Both polyQ protein and amplified repeat RNAs can cause toxicity and neurodegeneration. 217
Huntington protein is a large 350 kDa protein, which makes it difficult to load into exosomes. Unconventional mHTTS contain many duplicates of glutamine, which can be transported, transported, and eventually cause nerve cell death through exosomes. 129 Exosomes can load, carry, and transfer mHTTS between cells, leading to nerve cell death and causing HD‐related behaviors and pathological manifestations. 218 Fibroblasts from HD patients have 72, 143, and 180 CAG repeats, whereas induced pluripotent stem cells have 143 CAG repeats. When their exosomes are injected into the ventricles of neonatal WT mice, mHTT can be delivered to genetically unrelated healthy tissues, causing loss of striatal spines. Inflammation in related brain regions increased gliosis, motor and cognitive impairments, HD‐related behavior, and phenotypic characteristics. 219 The results indicated that exosomes could load mHTT between cells and transmit prion‐like proteins.
Exosomes have recently been identified as carriers of siRNAs (small interfering RNAs). miR‐214, miR‐150, miR‐146a, and miR‐125b in exosomes regulate the HTT gene. 220 The differential expression of miRNAs in exosomes provides the potential for diagnosing and treating neurodegenerative diseases. Hydrophobic‐modified siRNAs can be efficiently loaded into exosomes during co‐incubation or internalized by mouse primary cortical neurons to promote dose‐dependent silencing of huntingtin mRNA and protein expressions. 221 This supports using exosomes in developing effective therapeutic strategies for HD.
Serum exosomes can be a valuable tool in treating HD. 222 Transplantation of serum exosomes from mice into HD cell models in vitro effectively improved mHTT mutation and mitochondrial biogenesis and inhibited cell apoptosis. ADSC‐Exos release neurotrophic factors, which reduce mHTT aggregation and alleviate mitochondrial dysfunction and apoptosis. 223 , 224 αB‐crystallin is a small heat shock protein enriched in ADEs. It is involved in the secretion of exosomes to reduce the cytotoxicity of misfolded proteins and contribute to neuron survival. In HD astrocytes, mHTT reduced levels of αb‐crystallin and exosome secretion, leading to noncellular autonomic neurotoxicity in HD. When exosomes containing αB‐crystallin were injected into the striatum of HD mice, the accumulation of mHTT in the striatum was reduced. 225 This approach may provide a breakthrough for HD treatment.
The active mutation of silent transcription factor leads to no more silence of HTT, resulting in transcriptional dysfunction. Many treatments attempt to restore normal silent transcription factor expression. However, when exosomes containing miR‐124 were injected into the striatum of R6/2 transgenic HD mice in an attempt to restore the normal expression of RE1‐silencing transcription factor and reduce Huntington protein activation, there was no significant improvement in HD‐like behavior, possibly due to the limited therapeutic effect of miR‐124 or the inadequate dosage in exosomes. 226 , 227 There is still a long way to go to effectively apply engineered exosomes to the treatment of HD.
3.8. Amyotrophic lateral sclerosis
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease that causes progressive muscle weakness, atrophy, and paralysis. Progressive ALS culminates in respiratory failure and death, resulting from damaged upper motor neurons from the cortex to the brainstem and lower motor neurons that project from the spinal cord to the muscles. ALS is characterized by progressive paralysis and motor neuron death, and the median survival is only 2–5 years. Most cases are sporadic and 5%–10% are familial ALS (fALS). 131 The most common pathogenic genes for ALS include superoxide dismutase (SOD1), TAR DNA binding protein 43 (TARDBP/TDP43), chromosome 9 open reading frame 72 (C9orf72), and fusion protein (FUS). 228 , 229 ALS is also considered to be a protein disease, and its pathological proteins (SOD1, TDP‐43, and FUS) may accumulate and interfere with neuronal function, ultimately leading to cell death. 230
SOD1 mutants are present in exosomes isolated from the ALS model, 231 which is taken up by neighboring cells via endocytosis or released into the extracellular environment via exosomes. Their transmission is involved in ALS progression. 232 It was also reported that astroglia‐derived exosomes efficiently transported SOD1 to spinal cord neurons and selectively induced motor neuron death. 233 TDP‐43 is an RNA/DNA‐binding protein that has an aggregation tendency and cytotoxicity after hyperphosphorylation and ubiquitination. 234 TDP‐43 levels are elevated in ALS brain exosomes and can also be secreted by exosomes from nerve cells and primary neurons. TDP‐43 protein spreads like a “prion” through the secretion and endocytosis of exosomes to stimulate the cytoplasmic accumulation of TDP‐43 in recipient cells. 235 Exosomes play an essential role in TDP‐43 transport and clearance of TDP‐43. In addition, TDP‐43 level was positively correlated with ALS progression and can be used as a biomarker for diagnosis and progression of ALS. 236 The sarcoma FUS is an RNA/DNA‐binding protein that is dysregulated in ALS and is associated with the most aggressive type in young adults. 229 The CUEDC2 gene has a ubiquitin‐binding motif and regulates the ubiquitin‐proteasome pathway in CSF exosomes. It is considered a candidate disease biomarker for ALS. 230 The C9orf72 gene has a GGGGCC repeat expansion in intron 1, which is the most common cause of fALS. 237 RNA transcripts with repetitive sequences can accumulate repetitive RNAs into toxic dipeptide repeat proteins (DRPs), which disrupt normal cellular functions and lead to ALS. Exosomes can bind DRPs and deliver them to cortical neurons. 238 Although the mechanism of DRPs release and cellular uptake remains to be investigated, 239 the involvement of exosomes in the propagation of DRPs has been demonstrated. 236 , 240 Furthermore, miRNA dysfunction may be involved in the pathogenesis of ALS through TDP‐43.
miR‐27a‐3p is downregulated in serum exosomes of ALS patients. It stimulated myoblasts, promoted osteoblast mineralization through the Wnt signaling pathway, and participated in the progression of ALS. 230 miR‐124‐3p is highly enriched in neuronal exosomes, and its expression in exosomes derived from spinal motor neurons in ALS patients is increased and is significantly correlated with ALS severity. 236 , 241 , 242 This provides preliminary evidence that miR‐124‐3p can be used as an indicator of ALS progression. miR‐34a is involved in neuronal differentiation and neurogenesis. Its dysregulation leads to early neurodegeneration. miR‐34a and miR‐335 increased ROS production and impaired mitochondrial antioxidant function. Similarly, miR‐625‐3p targets lncRNA‐p21 and mediates neuroinflammation, oxidative stress, and apoptosis. 243 Nucleolar complex protein 2 homologs (NOC2L) are overexpressed in CSF exosomes of patients with sporadic ALS. Novel INHAT (inhibitor of histone acetyltransferase) repressor (NIR) inhibits p53‐mediated gene transfer activation by blocking histone acetylation. Therefore, the inhibition of NIR increases p53‐dependent apoptosis. Nucleolar stress may play a role in the pathogenesis of sporadic ALS. 244 Therefore, NIR can be used as a biomarker of homeostasis in ALS patients. These findings can provide valuable insights into the pathogenesis of ALS via specific pathways and facilitate the search for new therapeutic targets.
Protein dysfunction is present in ALS, and the best treatment is to restore protein homeostasis for proper protein folding. 245 , 246 S2RM stem cells were injected into the nervous system and spread through exosomes to restore protein homeostasis. 167 This suggests that exosome contents can be protected in vivo before reaching the neural targets. Exosomes can stably transfer genes into primary neurons without immunogenicity, which can realize effective management of ALS. 247 Glutamate is the major excitatory neurotransmitter in the CNS, and excessive or long‐term activation of glutamate receptors leads to neuron degeneration and death. 248 Exosomes isolated from neurons were internalized into astrocytes to increase the levels of miR‐124a and glutamate transporter 1 (GLT1). The subsequent in vivo and exogenous delivery of miR‐124a by stereotaxic injection into SOD1‐G93A mice significantly prevented the pathological loss of GLT1. 249 , 250 This highlights the association of other exosome‐related biomarkers with inflammatory pathways and ALS.
The NSC (neural stem cell)‐34 cell line is a common in vitro model of ALS, which overexpresses the human SOD1‐G93A mutant protein and has reduced mitochondrial oxidative capacity. Stromal cell exosomes protected ALS‐mutated NSC‐34 cells from oxidative damage and increased cell viability. 251 Mouse adipose stem cell (ASC) derived exosomes (ASC‐Exos) can repair the mitochondrial damage in NSC‐34 cells by regulating SOD1 aggregation, reducing oxidative damage, and restoring mitochondrial function. 252 Exosomal proteins isolated from ALS mice negatively regulated cell adhesion and apoptosis and played a neuroprotective role. 253 Repeated administration of ASC‐Exos in SOD1‐G93A transgenic ALS mice can improve motor performance, protect lumbar motor neurons, neuromuscular junctions, and muscles, and reduce glial cell activation. 254 , 255 This provides a new perspective for the application of ASC‐Exos in the treatment of ALS without the risks of immune rejection, genetic instability, and malignant transformation associated with the use of native or engineered stem cells.
Astrocytes are most abundant in the CNS and function to regulate neuron development. The miRNA expression profiles of astrocytes and exosomes in ALS models are different and not affected by the expression of mutant SOD1‐G93A. 256 Thus, miRNA compatibility should be established for better ALS treatment strategies in the future.
3.9. Epilepsy
Epilepsy occurs when an abnormal discharge of neurons in the brain leads to transient dysfunction of the CNS. 257 The presence of epilepsy in neurodegenerative diseases is increasingly recognized, 258 and its incidence is still underestimated.
In recent years, several studies have established the relationship among exosomes, miRNAs, and epilepsy. 259 , 260 hsa‐miR‐184 in exosomes of patients with mesial temporal lobe epilepsy with hippocampal sclerosis (mTLE + HS) modulate immunity, inflammation, apoptosis, and exacerbate neuronal death after epileptic state. 261 Its downregulation has predictive and diagnostic value for mTLE + HS. Expressions of miR‐194‐2 and miR‐15a in serum exosomes of epileptic patients were significantly downregulated. 262 In a rat model of epilepsy, miR‐346 and miR‐331‐3p were significantly downregulated in exosomes from anterior brain cells, 263 which may provide new targets for the pathogenesis and treatment of epilepsy. The expression of coagulation factor IX (F9) in serum exosomes of epileptic patients was higher than that of healthy controls, whereas thrombospondin 1 (TSP‐1) was lower than that of healthy controls. F9 is a vitamin K‐dependent plasma protein that has beneficial effects on synaptic plasticity. TSP‐1 is an adhesion glycoprotein secreted by astrocytes to promote synaptic development, neuronal migration, and axon growth. 264 This is the first study of exosomal proteins in epilepsy, and the results have proven that proteins in exosomes may be a new tool for epilepsy diagnosis and treatment.
Status epilepticus (SE) can cause neuronal damage and glioma. Intranasal administration of BMSC‐Exos in epileptic mice improved neuronal loss, memory, and cognitive impairment. Treatment with BMSC‐Exos can also alleviate astrogliosis, inflammation, and mitochondrial dysfunction in LPS‐induced inflammation. Intraventricular injection of BMSC‐Exos in pilocarpine‐induced SE mice improved learning and memory impairment. Nrf2 (nuclear factor erythroid 2‐related factor 2) is an intermediary factor for neuroinflammation and oxidative stress. BMSC‐Exos can inhibit this factor, regulate the Nrf2‐NF‐κB signaling pathway, restore hippocampal astrocyte activation, and play an anti‐inflammatory role in treating epilepsy. 265
3.10. Stroke
Stroke is an acute cerebrovascular disease, which is caused by the sudden rupture of blood vessels in the brain or blockage of blood circulation in the brain, resulting in focal neurological deficits. It is the second leading cause of death and disability in the world. 266 The incidence of stroke is increasing due to an aging population. Cerebrovascular diseases are divided into hemorrhagic and ischemic, depending on their pathogenesis. 267 Ischemic strokes (IS) are more common, but hemorrhagic strokes (HS) lead to higher rates of mortality and disability.
Circulating exosomal miR‐223 and miR‐134 were positively correlated with the occurrence, severity, and short‐term prognosis of IS. 124 As biomarkers of IS, the value of exosomal miRNAs is higher than that of plasma miRNAs. The serum exosomal miR‐152‐3p level decreased in patients with IS, related to the degree of neurological deficit. 267 As an indicator of nerve damage and neurotoxicity, serum exosome miR‐9 in patients with IS was associated with infarct volume, inflammatory status, and poor prognosis. Serum exosomal miR‐152‐3p is decreased in IS patients, which is related to the degree of neurological deficit. 268 Large artery atherosclerosis (LAA) stroke is the most common type of IS. Analysis of exosomal miRNAs and LAA stroke revealed that miR‐369‐3p, miR‐493‐3p, miR‐379‐5p, and miR‐1296‐5p were differentially expressed. miR‐369‐3p is associated with low‐density lipoprotein and mononuclear macrophage differentiation, and its targeted genes are involved in the inflammatory process. miR‐493‐3p can regulate angiogenesis in IS rat model, and both miR‐493‐3p and miR‐379‐5p are involved in fatty acid metabolism and the pathogenesis of LAA. The expression levels of miR‐493‐3p and miR‐1296‐5p were negatively correlated with the National Institutes of Health Stroke Scale (NIHSS). miR‐1296‐5p is associated with cell adhesion molecules and plays a key role in atherosclerosis. miR‐369‐3p, miR‐493‐3p, miR‐379‐5p, and miR‐1296‐5p are promising biomarkers for the diagnosis of LAA stroke. 269 Another study reported that lnc_000048, lnc_001350, and lnc_016442 in exosomes could effectively diagnose LAA stroke. lnc_001350 and lnc_016442 significantly improved the prediction of NIHSS in the prognosis of LAA stroke. 270 Exosomal lncRNA can be a valuable biomarker for the prognosis of LAA stroke and change the outcome of LAA stroke.
Carotid plaque instability is an independent risk factor for IS. Circ_0043837 in plasma exosomes is related to mitochondrial function. Hence, mitochondrial damage affects the function of macrophages and participates in atherosclerosis. Circ_0001801 is involved in gene regulation and cellular protein modification. The expressions of both circ_0043837 and circ_0001801 are negatively correlated with NIHSS and stroke severity, prompting a protective effect on IS. 271 , 272 These biomarkers have better diagnostic effects than other plasma circRNAs in predicting atherosclerotic plaque rupture. Upregulation of circ_0006896 in serum exosomes promoted the migration and proliferation of human umbilical vein endothelial cells, resulting in carotid plaque instability. The downregulated expression of miR‐1264 leads to the increase of DNMT1 (DNA methyltransferase 1) and STAT3 phosphorylation levels, and the downregulation of SOCS3 (suppressors of cytokine signaling 3). The effects are reflected in the reduced inhibition of the JNK (c‐Jun N‐terminal kinase)/STAT3 pathway and the formation of vulnerable carotid plaques. 273 The existence of the circ_0006896‐miR‐1264‐DNMT1 pathway in exosomes provides a new perspective for the study of carotid‐related IS. hsa_circ_0112036 and hsa_circ_0066867 in serum exosomes of IS patients regulated AMPK and PI3K‐AKT signaling pathways associated with IS pathogenesis. Upregulation of hsa‐circ_0093708 in plasma exosomes of IS patients increases the chance of brain injury by mediating hsa_circ_0093708‐miR‐4533‐AQP4 (aquaporin protein‐4) axis, and downregulating AQP4 can improve brain astrocyte injury. Upregulation of hsa_circ_0066867 and hsa_circ_0041685 regulated chemokine signaling through miR‐6737‐5p‐CCL2 and miR‐3192‐5p‐CXCL12, respectively, affecting leukocyte migration during inflammation and atherosclerosis development. Upregulation of hsa_circ_0041685 led to endocytosis and neuronal death and aggravated brain injury. 274 The roles of exosomal ncRNAs, lncRNAs, and circRNAs in IS are not fully understood. Thus, it is feasible to further study them and improve the exosome‐based treatments for IS.
Injection of exosomes into aged ischemic rats reduced synaptic damage and improved the functional outcome of IS. The levels of proinflammatory mediators complement components 1Q (C1q), C3a, and C3b in serum exosomes increase with age. C3a receptors (C3aRs) regulate the degree of phagocytosis in microglia, which affects the prognosis of stroke. Selective microglial C3aR inhibitors can alleviate synaptic dysfunction and promote functional recovery after IS in elderly rats. 275
Middle cerebral artery occlusion (MCAO) damages the structure and function of synapses, resulting in neurological dysfunction. Serum exosomes from the treatment group effectively inhibited microglia activation, increased the number of synapses and the expression of plasticity‐related proteins, reduced infarct volume, and improved neural function. 276 , 277 Thus, exosomes can provide a new therapeutic strategy for neuroprotection and repair after stroke. miR‐190b in ADEs inhibited apoptosis and increased neuronal survival under ischemic conditions. 278 Exosome miR‐21 reduced TNF expression by inhibiting the NF‐κB pathway and increased the anti‐inflammatory factor IL‐10. 279 It promoted the polarization of microglia cells toward the M2 phenotype. 280 Macrophages are involved in the immune regulation of strokes, and exosomes secreted by macrophages also participate in the treatment of stroke. Administration of M2 macrophage exosomes after MCAO significantly inhibited glial scar formation and the activation and proliferation of astrocytes.
miR‐138‐5p carried by BMSC‐Exos targets lipocalin‐2 (LCN2) in astrocytes, which promotes cell proliferation and inhibits inflammation and apoptosis, thus reducing nerve injury after stroke. 281 In addition, miR‐133b promotes functional recovery, whereas miR‐21‐5p promotes angiogenesis, 282 both of which can be used in treating IS. In a rat model of intracerebral hemorrhage, BMSCs promoted neural function by transferring exosomal miR‐21 to neurons. miR‐193b attenuated brain edema and blood‐brain barrier injury. 191 miR‐150‐3p is the most expressed exosomal miRNA, which has neuroprotective effects in vivo and in vitro. The expression of IS miR‐150‐5p was downregulated in BMSC‐Exos in MCAO rats and IS patients, leading to a poor prognosis and high mortality from IS. BMSC‐Exos improved nerve function and pathological changes in MCAO rats and reduced neuronal apoptosis and inflammatory factors. 283 miR‐150‐5p protects cerebral ischemia–reperfusion (I/R) injury and provides a new therapeutic target for treatment. miR‐124 secreted from the M2 microglia can reduce astrocyte migration, proliferation, and scar formation by inhibiting STAT3 expression. 125 Upregulation of exosomal miR‐124‐3p improved hypoxic–ischemic brain damage in neonatal rats by targeting TNF receptor–associated factor 6 (TRAF6). 284 , 285 Similarly, the upregulation of miR‐124‐3p in BMSC‐EVs was suggested as a therapeutic method for the targeted repair of damaged neurons after stroke. Caspases (CASP) 2, the most conserved cysteine‐rich proteases, are important in initiating apoptosis. Neural stem cell–derived exosomes (NSC‐Exo) inhibited neuronal apoptosis after brain injury by targeting CASP2 with miR‐150‐3p, reducing the infarct size in MCAO models, and playing a neuroprotective role. This could inform the development of new treatments for brain injury. 286 BDNF was loaded into NSC‐Exos to construct the engineered BDNF‐HNSC‐Exos. In the MCAO rat model, BDNF‐HNSC‐Exos can inhibit microglial activation, promote endogenous NSC differentiation in neurons, inhibit the inflammatory response, create a suitable microenvironment for nerve regeneration, and alleviate NSC stress injury. 287 Neuronal exosomes loaded with quercetin target ischemic brain tissue, improve neuronal survival, and reduce I/R damage by scavenging ROS production. 288 Macrophage‐derived exosomes loaded with curcumin have an antioxidant capacity, which can target cerebral ischemic areas, reduce ROS accumulation, inhibit mitochondria‐mediated neuronal apoptosis, and positively affect I/R therapy. 289
The content of exosomes and their contents also changes when IS occurs, 290 and involved in the pathophysiological progression of IS, contribute to the diagnosis of the disease. 291 Stem cell–derived exosomes play an important role in the treatment of stroke models. Therefore, engineered exosomes may be a potentially effective repair strategy for IS injury. However, there is a long way to go to further study on how to target the delivered exosomes to the responsible lesions. 292 This is a research direction to improve the clinical value of exosomes.
4. CONCLUSIONS AND PROSPECTS
The occurrence of neuropsychiatric disorders is increasing day by day, 293 but there are limitations in diagnosis, 294 , 295 limited means of treatment, and unsafe drugs. 296 , 297 , 298 Naturally derived biological humoral exosomes possess specific biomolecules that can be used as a “signature” of parent cells to reflect changes in their contents under pathological conditions. The occurrence and pathophysiological mechanism of the disease can be understood by detecting its changing contents. Blood‐derived exosomes as liquid biopsy specimens have the potential to be a new biomarker for the diagnosis of neuropsychiatric disorders. 299 Exosomes have the unique properties of being safe, low immunogenicity, biodegradable, and crossing the blood–brain barrier. The lipid bilayer escorts the loading and transport of bioactive molecules in exosomes, enables them to mediate the communication between brain cells stably, and crosstalk the physiological and pathological processes of the brain. In addition to that, exosomes can also be therapeutic carriers of drugs, RNAs, or proteins through direct loading, passive incubation, and active loading, 300 which have great clinical application value in the treatment of neuropsychiatric disorders.
Despite the unique advantages of exosomes as engineering vectors, there are still challenges to their clinical application. Mass production, isolation, and drug loading techniques are key factors that limit the therapeutic potential of exosomes once they are introduced into the clinic. 301 , 302 , 303 The loading efficiency of natural compounds was positively correlated with the molecular weight of exosomes. However, when molecular weight exceeds 1109 Da, exosomes coated with natural compounds cannot cross the blood–brain barrier, limiting therapeutic dose. 304 How to ensure the high quality and effectiveness of isolated and modified exosomes needs relevant scientific research and technical support. Exosomes from different sources have different characteristics and specificities for different cells. In the future, selecting exosomes suitable for loading bioactive molecules or drugs will be essential according to cell types and how to accurately direct exosomes to target cells. 305 Macrophages in mononuclear phagocytic system are responsible for the metabolic clearance of exosomes. Data from pharmacokinetic studies indicate that the residence time of exosomes in circulation affects the clinical transformation of therapeutic effect. Increasing the concentration of exosomes around target cells and allowing them to efficiently release bioactive molecules or drugs to achieve the expected therapeutic effect is an urgent problem that should be solved. 306 However, exosomes have not yet been approved for clinical use. 307 , 308 Whether this involves medical ethics and which departments are required to supervise them is subject to the formulation of guidelines or expert consensus and the introduction of relevant policies.
Some bioactive molecules that have protective effects on the CNS have unstable chemical structures under physiological conditions, easy to be hydrolyzed, and low bioavailability, which limits their clinical application. 309 , 310 , 311 The presence of the blood–brain barrier in turn blocks the delivery of bioactive molecules to the brain. Exosomes, as innovative drug carriers, can cross the blood–brain barrier, opening up the possibility for the treatment of neuropsychiatric disorders. 312 Exosomes as innovative nanostructures, 313 can “escort” bioactive molecules to be absorbed by neuron cells. 314 Currently, nanoscavenger carrying therapeutic drugs with controllable release characteristics can target diseased brain tissue through intranasal drug delivery system, trigger autophagy and calcium‐dependent exosome secretion, and clear pathological proteins, providing strong neuroprotective potential. 315 Or coating native immune cells with exosomes, 316 makes it become a scavenger to clear immune activation, as a new treatment platform for neuropsychiatric disorders. Experimental research focusing on increasing the number of exosomes extracted, such as promoting exosome biogenesis by gene knockout or adding reagents to the medium, 317 , 318 is trying to break through the low yield bottleneck and promote the clinical application of exosome transformation. Protein is effectively packaged into exosomes for photo‐controlled release to target cells, 319 and exosomes are used for bioimaging and disease therapy. 320 All these have created more opportunities for the diagnosis and treatment of neuropsychiatric disorders.
Research on engineering exosomes of bioactive molecules is rising, and how to efficiently use exosomes for the diagnosis and treatment of neuropsychiatric diseases is ongoing. Given that exosomes are important carriers for the transmission of genetic information and bioactive molecules from cell to cell, they are involved in intercellular communication, disease progression, and therapy. It is believed that exosomes can be a powerful tool in diagnosing and treating neuropsychiatric diseases in the future.
AUTHOR CONTRIBUTIONS
Qingying Si wrote and edited the manuscript. Linlin Wu conceived the manuscript. Deshui Pang and Pei Jiang provided significant assistance. All authors have read and approved the final manuscript.
CONFLICT OF INTEREST STATEMENT
Authors declare no conflicts of interest in this work.
ETHICS STATEMENT
Ethics approval was not needed for this study.
ACKNOWLEDGMENTS
All figures are created with BioRender (BioRender.com). The study was supported by the National Natural Science Foundation of China (P. Jiang, 81602846; P. Jiang, 82272253), the Natural Science Foundation of Shandong Province (ZR2021MH145), Taishan Scholar Project of Shandong Province (tsqn201812159), and China International Medical Foundation (No. Z‐2018‐35‐2002).
Si Q, Wu L, Pang D, Jiang P. Exosomes in brain diseases: Pathogenesis and therapeutic targets. MedComm. 2023;4:e287. 10.1002/mco2.287
DATA AVAILABILITY STATEMENT
REFERENCES
- 1. Gurunathan S, Kang MH, Kim JH. A comprehensive review on factors influences biogenesis, functions, therapeutic and clinical implications of exosomes. Int J Nanomedicine. 2021;16:1281‐1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alzhrani GN, Alanazi ST, Alsharif SY, et al. Exosomes: isolation, characterization, and biomedical applications. Cell Biol Int. 2021;45(9):1807‐1831. [DOI] [PubMed] [Google Scholar]
- 3. Chen J, Li P, Zhang T, et al. Review on strategies and technologies for exosome isolation and purification. Front Bioeng Biotechnol. 2021;9:811971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dyball LE, Smales CM. Exosomes: biogenesis, targeting, characterization and their potential as “Plug & Play” vaccine platforms. Biotechnol J. 2022;17(11):e2100646. [DOI] [PubMed] [Google Scholar]
- 5. Krylova SV, Feng D. The machinery of exosomes: biogenesis, release, and uptake. Int J Mol Sci. 2023;24(2):1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kang T, Atukorala I, Mathivanan S. Biogenesis of extracellular vesicles. Subcell Biochem. 2021;97:19‐43. [DOI] [PubMed] [Google Scholar]
- 7. Jadli AS, Ballasy N, Edalat P, et al. Inside(sight) of tiny communicator: exosome biogenesis, secretion, and uptake. Mol Cell Biochem. 2020;467(1‐2):77‐94. [DOI] [PubMed] [Google Scholar]
- 8. Rudraprasad D, Rawat A, Joseph J. Exosomes, extracellular vesicles and the eye. Exp Eye Res. 2022;214:108892. [DOI] [PubMed] [Google Scholar]
- 9. Fordjour FK, Guo C, Ai Y, et al. A shared, stochastic pathway mediates exosome protein budding along plasma and endosome membranes. J Biol Chem. 2022;298(10):102394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478):eaau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gurung S, Perocheau D, Touramanidou L, et al. The exosome journey: from biogenesis to uptake and intracellular signalling. Cell Commun Signal. 2021;19(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu Y, Wang Y, Lv Q, et al. Exosomes: from garbage bins to translational medicine. Int J Pharm. 2020;583:119333. [DOI] [PubMed] [Google Scholar]
- 13. Engin A. Dark‐side of exosomes. Adv Exp Med Biol. 2021;1275:101‐131. [DOI] [PubMed] [Google Scholar]
- 14. Hussain S, Fatima A, Fan XX, et al. REVIEW—The biological importance of cells secreted exosomes. Pak J Pharm Sci. 2021;34(6):2273‐2279. [PubMed] [Google Scholar]
- 15. Kavya ANVL, Subramanian S, Ramakrishna S. Therapeutic applications of exosomes in various diseases: a review. Biomater Adv. 2022;134:112579. [DOI] [PubMed] [Google Scholar]
- 16. Rasihashemi SZ, Sahrai H, Rezazadeh‐Gavgani E, et al. Exosomes carrying immune checkpoints, a promising therapeutic approach in cancer treatment. Med Oncol. 2022;39(12):183. [DOI] [PubMed] [Google Scholar]
- 17. Jia Y, Wei Y. Modulators of MicroRNA function in the immune system. Int J Mol Sci. 2020;21(7):2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hou Y, Liu Y, Liang S, et al. The novel target: exosoms derived from M2 macrophage. Int Rev Immunol. 2021;40(3):183‐196. [DOI] [PubMed] [Google Scholar]
- 19. Suh JH, Joo HS, Hong EB, et al. Therapeutic application of exosomes in inflammatory diseases. Int J Mol Sci. 2021;22(3):1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Qin J, Xu Q. Functions and application of exosomes. Acta Pol Pharm. 2014;71(4):537‐543. [PubMed] [Google Scholar]
- 21. Zhu D, Johnson TK, Wang Y, et al. Macrophage M2 polarization induced by exosomes from adipose‐derived stem cells contributes to the exosomal proangiogenic effect on mouse ischemic hindlimb. Stem Cell Res Ther. 2020;11(1):162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Marostica G, Gelibter S, Gironi M, et al. Extracellular vesicles in neuroinflammation. Front Cell Dev Biol. 2020;8:623039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nikdoust F, Pazoki M, Mohammadtaghizadeh M, et al. Exosomes: potential player in endothelial dysfunction in cardiovascular disease. Cardiovasc Toxicol. 2022;22(3):225‐235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang C, Li Z, Liu Y, et al. Exosomes in atherosclerosis: performers, bystanders, biomarkers, and therapeutic targets. Theranostics. 2021;11(8):3996‐4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Patel N, Chin DD, Chung EJ. Exosomes in atherosclerosis, a double‐edged sword: their role in disease pathogenesis and their potential as novel therapeutics. AAPS J. 2021;23(5):95. [DOI] [PubMed] [Google Scholar]
- 26. Li S, Yi M, Dong B, et al. The role of exosomes in liquid biopsy for cancer diagnosis and prognosis prediction. Int J Cancer. 2021;148(11):2640‐2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mashouri L, Yousefi H, Aref AR, et al. Exosomes: composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol Cancer. 2019;18(1):75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xing X, Han S, Li Z, et al. Emerging role of exosomes in craniofacial and dental applications. Theranostics. 2020;10(19):8648‐8664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kok VC, Yu CC. Cancer‐derived exosomes: their role in cancer biology and biomarker development. Int J Nanomedicine. 2020;15:8019‐8036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang H, Wang S, Sun M, et al. Exosomes as smart drug delivery vehicles for cancer immunotherapy. Front Immunol. 2022;13:1093607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Feng Q, Zhang Y, Fang Y, et al. Research progress of exosomes as drug carriers in cancer and inflammation. J Drug Target. 2023;31(4):335‐353. [DOI] [PubMed] [Google Scholar]
- 32. Tenchov R, Sasso JM, Wang X, et al. Exosomes horizontal line Nature's Lipid nanoparticles, a rising star in drug delivery and diagnostics. ACS Nano. 2022;16(11):17802‐17846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Panigrahi AR, Srinivas L, Panda J. Exosomes: insights and therapeutic applications in cancer. Transl Oncol. 2022;21:101439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Soares Martins T, Trindade D, Vaz M, et al. Diagnostic and therapeutic potential of exosomes in Alzheimer's disease. J Neurochem. 2021;156(2):162‐181. [DOI] [PubMed] [Google Scholar]
- 35. Li Y, Tang Y, Yang GY. Therapeutic application of exosomes in ischaemic stroke. Stroke Vasc Neurol. 2021;6(3):483‐495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bhimani AD, Kalagara R, Chennareddy S, et al. Exosomes in subarachnoid hemorrhage: a scoping review. J Clin Neurosci. 2022;105:58‐65. [DOI] [PubMed] [Google Scholar]
- 37. Rehman FU, Liu Y, Zheng M, et al. Exosomes based strategies for brain drug delivery. Biomaterials. 2023;293:121949. [DOI] [PubMed] [Google Scholar]
- 38. Chen H, Xue R, Huang P, et al. Modified exosomes: a good transporter for miRNAs within stem cells to treat ischemic heart disease. J Cardiovasc Transl Res. 2022;15(3):514‐523. [DOI] [PubMed] [Google Scholar]
- 39. Nakamura Y, Kita S, Tanaka Y, et al. Adiponectin stimulates exosome release to enhance mesenchymal stem‐cell‐driven therapy of heart failure in mice. Mol Ther. 2020;28(10):2203‐2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jia G, Sowers JR. Targeting endothelial exosomes for the prevention of cardiovascular disease. Biochim Biophys Acta Mol Basis Dis. 2020;1866(8):165833. [DOI] [PubMed] [Google Scholar]
- 41. Liu Q, Piao H, Wang Y, et al. Circulating exosomes in cardiovascular disease: novel carriers of biological information. Biomed Pharmacother. 2021;135:111148. [DOI] [PubMed] [Google Scholar]
- 42. Zhang J, Cui X, Guo J, et al. Small but significant: insights and new perspectives of exosomes in cardiovascular disease. J Cell Mol Med. 2020;24(15):8291‐8303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Guo D, Xu Y, Ding J, et al. Roles and clinical applications of exosomes in cardiovascular disease. Biomed Res Int. 2020;2020:5424281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Burtenshaw D, Regan B, Owen K, et al. Exosomal composition, biogenesis and profiling using point‐of‐care diagnostics‐implications for cardiovascular disease. Front Cell Dev Biol. 2022;10:853451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mei R, Qin W, Zheng Y, et al. Role of adipose tissue derived exosomes in metabolic disease. Front Endocrinol (Lausanne). 2022;13:873865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vlachakis D, Mitsis T, Nicolaides N, et al. Functions, pathophysiology and current insights of exosomal endocrinology (Review). Mol Med Rep. 2021;23(1):26. [DOI] [PubMed] [Google Scholar]
- 47. Chang W, Wang M, Zhang Y, et al. Roles of long noncoding RNAs and small extracellular vesicle‐long noncoding RNAs in type 2 diabetes. Traffic. 2022;23(11):526‐537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mosquera‐Heredia MI, Morales LC, Vidal OM, et al. Exosomes: potential disease biomarkers and new therapeutic targets. Biomedicines. 2021;9(8):1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Modani S, Tomar D, Tangirala S, et al. An updated review on exosomes: biosynthesis to clinical applications. J Drug Target. 2021;29(9):925‐940. [DOI] [PubMed] [Google Scholar]
- 50. Wang J, Yue BL, Huang YZ, et al. Exosomal RNAs: novel potential biomarkers for diseases‐a review. Int J Mol Sci. 2022;23(5):2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kapogiannis D. Exosome biomarkers revolutionize preclinical diagnosis of neurodegenerative diseases and assessment of treatment responses in clinical trials. Adv Exp Med Biol. 2020;1195:149. [DOI] [PubMed] [Google Scholar]
- 52. Moreira‐Costa L, Barros AS, Lourenco AP, et al. Exosome‐derived mediators as potential biomarkers for cardiovascular diseases: a network approach. Proteomes. 2021;9(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhuo CJ, Hou WH, Jiang DG, et al. Circular RNAs in early brain development and their influence and clinical significance in neuropsychiatric disorders. Neural Regen Res. 2020;15(5):817‐823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tondo G, Aprile D, Tesser F, et al. Increased prevalence of neuropsychiatric disorders during COVID‐19 pandemic in people needing a non‐deferrable neurological evaluation. J Clin Med. 2021;10(21):5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Premraj L, Kannapadi NV, Briggs J, et al. Mid and long‐term neurological and neuropsychiatric manifestations of post‐COVID‐19 syndrome: a meta‐analysis. J Neurol Sci. 2022;434:120162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wang Z, Wang H, Becker R, et al. Acoustofluidic separation enables early diagnosis of traumatic brain injury based on circulating exosomes. Microsyst Nanoeng. 2021;7:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhang Y, Xu C. Effects of exosomes on adult hippocampal neurogenesis and neuropsychiatric disorders. Mol Biol Rep. 2022;49(7):6763‐6777. [DOI] [PubMed] [Google Scholar]
- 58. Rastogi S, Sharma V, Bharti PS, et al. The evolving landscape of exosomes in neurodegenerative diseases: exosomes characteristics and a promising role in early diagnosis. Int J Mol Sci. 2021;22(1):440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gupta S, Mazumder PB. Exosomes as diagnostic tools. Adv Clin Chem. 2022;110:117‐144. [DOI] [PubMed] [Google Scholar]
- 60. Agarwal S, Agarwal V, Agarwal M, et al. Exosomes: structure, biogenesis, types and application in diagnosis and gene and drug delivery. Curr Gene Ther. 2020;20(3):195‐206. [DOI] [PubMed] [Google Scholar]
- 61. Gorshkov A, Purvinsh L, Brodskaia A, et al. Exosomes as natural nanocarriers for RNA‐based therapy and prophylaxis. Nanomaterials (Basel). 2022;12(3):524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Patil SM, Sawant SS, Kunda NK. Exosomes as drug delivery systems: a brief overview and progress update. Eur J Pharm Biopharm. 2020;154:259‐269. [DOI] [PubMed] [Google Scholar]
- 63. Rajput A, Varshney A, Bajaj R, et al. Exosomes as new generation vehicles for drug delivery: biomedical applications and future perspectives. Molecules. 2022;27(21):7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ke W, Afonin KA. Exosomes as natural delivery carriers for programmable therapeutic nucleic acid nanoparticles (NANPs). Adv Drug Deliv Rev. 2021;176:113835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ferreira D, Moreira JN, Rodrigues LR. New advances in exosome‐based targeted drug delivery systems. Crit Rev Oncol Hematol. 2022;172:103628. [DOI] [PubMed] [Google Scholar]
- 66. Guo ZY, Tang Y, Cheng YC. Exosomes as targeted delivery drug system: advances in exosome loading, surface functionalization and potential for clinical application. Curr Drug Deliv. 2022;19:PMID35702803. [DOI] [PubMed] [Google Scholar]
- 67. Li JY, Li QQ, Sheng R. The role and therapeutic potential of exosomes in ischemic stroke. Neurochem Int. 2021;151:105194. [DOI] [PubMed] [Google Scholar]
- 68. Lin Y, Lu Y, Li X. Biological characteristics of exosomes and genetically engineered exosomes for the targeted delivery of therapeutic agents. J Drug Target. 2020;28(2):129‐141. [DOI] [PubMed] [Google Scholar]
- 69. Park JH. Regulation of in vivo fate of exosomes for therapeutic applications: new frontier in nanomedicines. J Control Release. 2022;348:483‐488. [DOI] [PubMed] [Google Scholar]
- 70. Zeng EZ, Chen I, Chen X, et al. Exosomal MicroRNAs as novel cell‐free therapeutics in tissue engineering and regenerative medicine. Biomedicines. 2022;10(10):2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hao Y, Song H, Zhou Z, et al. Promotion or inhibition of extracellular vesicle release: emerging therapeutic opportunities. J Control Release. 2021;340:136‐148. [DOI] [PubMed] [Google Scholar]
- 72. Zhao AG, Shah K, Cromer B, et al. Mesenchymal stem cell‐derived extracellular vesicles and their therapeutic potential. Stem Cells Int. 2020;2020:8825771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zhou B, Xu K, Zheng X, et al. Application of exosomes as liquid biopsy in clinical diagnosis. Signal Transduct Target Ther. 2020;5(1):144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Mathew B, Mansuri MS, Williams KR, et al. Exosomes as emerging biomarker tools in neurodegenerative and neuropsychiatric disorders‐a proteomics perspective. Brain Sci. 2021;11(2):258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ranganathan M, Rahman M, Ganesh S, et al. Analysis of circulating exosomes reveals a peripheral signature of astrocytic pathology in schizophrenia. World J Biol Psychiatry. 2022;23(1):33‐45. [DOI] [PubMed] [Google Scholar]
- 76. Deb A, Gupta S, Mazumder PB. Exosomes: a new horizon in modern medicine. Life Sci. 2021;264:118623. [DOI] [PubMed] [Google Scholar]
- 77. Xia X, Wang Y, Qin Y, et al. Exosome: a novel neurotransmission modulator or non‐canonical neurotransmitter? Ageing Res Rev. 2022;74:101558. [DOI] [PubMed] [Google Scholar]
- 78. Waqas MY, Javid MA, Nazir MM, et al. Extracellular vesicles and exosome: insight from physiological regulatory perspectives. J Physiol Biochem. 2022;78(3):573‐580. [DOI] [PubMed] [Google Scholar]
- 79. Du Y, Tan WL, Chen L, et al. Exosome transplantation from patients with schizophrenia causes schizophrenia‐relevant behaviors in mice: an integrative multi‐omics data analysis. Schizophr Bull. 2021;47(5):1288‐1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Andjus P, Kosanovic M, Milicevic K, et al. Extracellular vesicles as innovative tool for diagnosis, regeneration and protection against neurological damage. Int J Mol Sci. 2020;21(18):6859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Gao P, Li X, Du X, et al. Diagnostic and therapeutic potential of exosomes in neurodegenerative diseases. Front Aging Neurosci. 2021;13:790863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Yang Z, Li Y, Wang Z. Recent advances in the application of mesenchymal stem cell‐derived exosomes for cardiovascular and neurodegenerative disease therapies. Pharmaceutics. 2022;14(3):618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Xia X, Wang Y, Zheng JC. Extracellular vesicles, from the pathogenesis to the therapy of neurodegenerative diseases. Transl Neurodegener. 2022;11(1):53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33(3):967‐978. [DOI] [PubMed] [Google Scholar]
- 85. Johnstone RM, Adam M, Hammond JR, et al. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem. 1987;262(19):9412‐9420. [PubMed] [Google Scholar]
- 86. van Niel G, D'Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19(4):213‐228. [DOI] [PubMed] [Google Scholar]
- 87. Abels ER, Breakefield XO. Introduction to extracellular vesicles: biogenesis, RNA cargo selection, content, release, and uptake. Cell Mol Neurobiol. 2016;36(3):301‐312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373‐383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Skryabin GO, Komelkov AV, Savelyeva EE, et al. Lipid rafts in exosome biogenesis. Biochemistry (Mosc). 2020;85(2):177‐191. [DOI] [PubMed] [Google Scholar]
- 90. Chew BC, Liew FF, Tan HW, et al. Chemical advances in therapeutic application of exosomes and liposomes. Curr Med Chem. 2022;29(25):4445‐4473. [DOI] [PubMed] [Google Scholar]
- 91. Donoso‐Quezada J, Ayala‐Mar S, Gonzalez‐Valdez J. The role of lipids in exosome biology and intercellular communication: function, analytics and applications. Traffic. 2021;22(7):204‐220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Egea‐Jimenez AL, Zimmermann P. Lipids in exosome biology. Handb Exp Pharmacol. 2020;259:309‐336. [DOI] [PubMed] [Google Scholar]
- 93. Mathieu M, Martin‐Jaular L, Lavieu G, et al. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell‐to‐cell communication. Nat Cell Biol. 2019;21(1):9‐17. [DOI] [PubMed] [Google Scholar]
- 94. Gebeyehu A, Kommineni N. Role of exosomes for delivery of chemotherapeutic drugs. Crit Rev Ther Drug Carrier Syst. 2021;38(5):53‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Pegtel DM, Gould SJ. Exosomes. Annu Rev Biochem. 2019;88:487‐514. [DOI] [PubMed] [Google Scholar]
- 96. Qin T, Li J, Zhang KQ. Structure, regulation, and function of linear and circular long non‐coding RNAs. Front Genet. 2020;11:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Qiu Y, Li P, Zhang Z, et al. Insights into exosomal non‐coding RNAs sorting mechanism and clinical application. Front Oncol. 2021;11:664904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Zhang J, Li S, Li L, et al. Exosome and exosomal microRNA: trafficking, sorting, and function. Genomics Proteomics Bioinformatics. 2015;13(1):17‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Hamdan Y, Mazini L, Malka G. Exosomes and micro‐RNAs in aging process. Biomedicines. 2021;9(8):968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ali Syeda Z, Langden SSS, Munkhzul C, et al. Regulatory mechanism of MicroRNA expression in cancer. Int J Mol Sci. 2020;21(5):1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Bridges MC, Daulagala AC, Kourtidis A. LNCcation: lncRNA localization and function. J Cell Biol. 2021;220(2):e202009045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Zhou WY, Cai ZR, Liu J, et al. Circular RNA: metabolism, functions and interactions with proteins. Mol Cancer. 2020;19(1):172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Hu X, Qin H, Yan Y, et al. Exosomal circular RNAs: biogenesis, effect, and application in cardiovascular diseases. Front Cell Dev Biol. 2022;10:948256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Guo X, Tan W, Wang C. The emerging roles of exosomal circRNAs in diseases. Clin Transl Oncol. 2021;23(6):1020‐1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Guria A, Sharma P, Natesan S, et al. Circular RNAs‐the road less traveled. Front Mol Biosci. 2019;6:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Saaoud F, Drummer IVC, Shao Y, et al. Circular RNAs are a novel type of non‐coding RNAs in ROS regulation, cardiovascular metabolic inflammations and cancers. Pharmacol Ther. 2021;220:107715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Shao Y, Jiang Y. Circular RNAs in toxicology. Toxicol Sci. 2021;179(2):149‐161. [DOI] [PubMed] [Google Scholar]
- 108. Benameur T, Giacomucci G, Panaro MA, et al. New promising therapeutic avenues of curcumin in brain diseases. Molecules. 2021;27(1):236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Wang MM, Feng YS, Tan ZX, et al. The role of exosomes in stroke. Mol Biol Rep. 2020;47(8):6217‐6228. [DOI] [PubMed] [Google Scholar]
- 110. Polanco JC, Hand GR, Briner A, et al. Exosomes induce endolysosomal permeabilization as a gateway by which exosomal tau seeds escape into the cytosol. Acta Neuropathol. 2021;141(2):235‐256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Li XH, Zhang J, Li DF, et al. Physiological and pathological insights into exosomes in the brain. Zool Res. 2020;41(4):365‐372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Li C, Qin S, Wen Y, et al. Overcoming the blood‐brain barrier: exosomes as theranostic nanocarriers for precision neuroimaging. J Control Release. 2022;349:902‐916. [DOI] [PubMed] [Google Scholar]
- 113. Oliveira FD, Castanho M, Neves V. Exosomes and brain metastases: a review on their role and potential applications. Int J Mol Sci. 2021;22(19):10899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Mulvihill JJ, Cunnane EM, Ross AM, et al. Drug delivery across the blood‐brain barrier: recent advances in the use of nanocarriers. Nanomedicine (Lond). 2020;15(2):205‐214. [DOI] [PubMed] [Google Scholar]
- 115. Fan Y, Chen Z, Zhang M. Role of exosomes in the pathogenesis, diagnosis, and treatment of central nervous system diseases. J Transl Med. 2022;20(1):291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Tastan B, Tarakcioglu E, Birinci Y, et al. Role of exosomal MicroRNAs in cell‐to‐cell communication. Methods Mol Biol. 2022;2257:269‐292. [DOI] [PubMed] [Google Scholar]
- 117. Pascual M, Ibanez F, Guerri C. Exosomes as mediators of neuron‐glia communication in neuroinflammation. Neural Regen Res. 2020;15(5):796‐801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Huo L, Du X, Li X, et al. The emerging role of neural cell‐derived exosomes in intercellular communication in health and neurodegenerative diseases. Front Neurosci. 2021;15:738442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Hariharan H, Kesavan Y, Raja NS. Impact of native and external factors on exosome release: understanding reactive exosome secretion and its biogenesis. Mol Biol Rep. 2021;48(11):7559‐7573. [DOI] [PubMed] [Google Scholar]
- 120. Henning RJ. Cardiovascular exosomes and MicroRNAs in cardiovascular physiology and pathophysiology. J Cardiovasc Transl Res. 2021;14(2):195‐212. [DOI] [PubMed] [Google Scholar]
- 121. Isaac R, Reis FCG, Ying W, et al. Exosomes as mediators of intercellular crosstalk in metabolism. Cell Metab. 2021;33(9):1744‐1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Gururajan A, Naughton ME, Scott KA, et al. MicroRNAs as biomarkers for major depression: a role for let‐7b and let‐7c. Transl Psychiatry. 2016;6(8):e862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Li D, Wang Y, Jin X, et al. NK cell‐derived exosomes carry miR‐207 and alleviate depression‐like symptoms in mice. J Neuroinflammation. 2020;17(1):126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Jiang L, Chen W, Ye J, et al. Potential role of exosomes in ischemic stroke treatment. Biomolecules. 2022;12(1):115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Xiong Y, Song J, Huang X, et al. Exosomes derived from mesenchymal stem cells: novel effects in the treatment of ischemic stroke. Front Neurosci. 2022;16:899887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Cheng Q, Wang J, Li M, et al. CircSV2b participates in oxidative stress regulation through miR‐5107‐5p‐Foxk1‐Akt1 axis in Parkinson's disease. Redox Biol. 2022;56:102430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Kaur S, Verma H, Dhiman M, et al. Brain exosomes: friend or foe in Alzheimer's disease? Mol Neurobiol. 2021;58(12):6610‐6624. [DOI] [PubMed] [Google Scholar]
- 128. Yu Y, Hou K, Ji T, et al. The role of exosomal microRNAs in central nervous system diseases. Mol Cell Biochem. 2021;476(5):2111‐2124. [DOI] [PubMed] [Google Scholar]
- 129. Beatriz M, Vilaca R, Lopes C. Exosomes: innocent bystanders or critical culprits in neurodegenerative diseases. Front Cell Dev Biol. 2021;9:635104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Yuyama K, Igarashi Y. Physiological and pathological roles of exosomes in the nervous system. Biomol Concepts. 2016;7(1):53‐68. [DOI] [PubMed] [Google Scholar]
- 131. Kumari M, Anji A. Small but mighty‐exosomes, novel intercellular messengers in neurodegeneration. Biology (Basel). 2022;11(3):413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Peng C, Trojanowski JQ, Lee VM. Protein transmission in neurodegenerative disease. Nat Rev Neurol. 2020;16(4):199‐212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Yerrapragada SM, Bihl JC. Role of exosomes in mediating the cross‐talk between adipose tissue and the brain. Neuromolecular Med. 2022;24(2):57‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Zhang N, He F, Li T, et al. Role of exosomes in brain diseases. Front Cell Neurosci. 2021;15:743353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Shaimardanova AA, Solovyeva VV, Chulpanova DS, et al. Extracellular vesicles in the diagnosis and treatment of central nervous system diseases. Neural Regen Res. 2020;15(4):586‐596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Natale F, Fusco S, Grassi C. Dual role of brain‐derived extracellular vesicles in dementia‐related neurodegenerative disorders: cargo of disease spreading signals and diagnostic‐therapeutic molecules. Transl Neurodegener. 2022;11(1):50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Aheget H, Tristan‐Manzano M, Mazini L, et al. Exosome: a new player in translational nanomedicine. J Clin Med. 2020;9(8):2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Collaborators C‐MD . Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID‐19 pandemic. Lancet. 2021;398(10312):1700‐1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Lu J, Xu X, Huang Y, et al. Prevalence of depressive disorders and treatment in China: a cross‐sectional epidemiological study. Lancet Psychiatry. 2021;8(11):981‐990. [DOI] [PubMed] [Google Scholar]
- 140. Tavakolizadeh J, Roshanaei K, Salmaninejad A, et al. MicroRNAs and exosomes in depression: potential diagnostic biomarkers. J Cell Biochem. 2018;119(5):3783‐3797. [DOI] [PubMed] [Google Scholar]
- 141. Li LD, Naveed M, Du ZW, et al. Abnormal expression profile of plasma‐derived exosomal microRNAs in patients with treatment‐resistant depression. Hum Genomics. 2021;15(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Chen F, Zou L, Dai Y, et al. Prognostic plasma exosomal microRNA biomarkers in patients with substance use disorders presenting comorbid with anxiety and depression. Sci Rep. 2021;11(1):6271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Hung YY, Chou CK, Yang YC, et al. Exosomal let‐7e, miR‐21‐5p, miR‐145, miR‐146a and miR‐155 in predicting antidepressants response in patients with major depressive disorder. Biomedicines. 2021;9(10):1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Xian X, Cai LL, Li Y, et al. Neuron secrete exosomes containing miR‐9‐5p to promote polarization of M1 microglia in depression. J Nanobiotechnology. 2022;20(1):122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Wei ZX, Xie GJ, Mao X, et al. Exosomes from patients with major depression cause depressive‐like behaviors in mice with involvement of miR‐139‐5p‐regulated neurogenesis. Neuropsychopharmacology. 2020;45(6):1050‐1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Kouter K, Paska AV. Biomarkers for suicidal behavior: miRNAs and their potential for diagnostics through liquid biopsy – a systematic review. Epigenomics. 2020;12(24):2219‐2235. [DOI] [PubMed] [Google Scholar]
- 147. Guo H, Huang B, Wang Y, et al. Bone marrow mesenchymal stem cells‐derived exosomes improve injury of hippocampal neurons in rats with depression by upregulating microRNA‐26a expression. Int Immunopharmacol. 2020;82:106285. [DOI] [PubMed] [Google Scholar]
- 148. Wang Y, Gao C, Gao T, et al. Plasma exosomes from depression ameliorate inflammation‐induced depressive‐like behaviors via sigma‐1 receptor delivery. Brain Behav Immun. 2021;94:225‐234. [DOI] [PubMed] [Google Scholar]
- 149. Bhatt S, Kanoujia J, Dhar AK, et al. Exosomes: a novel therapeutic paradigm for the treatment of depression. Curr Drug Targets. 2021;22(2):183‐191. [DOI] [PubMed] [Google Scholar]
- 150. Stepnicki P, Kondej M, Kaczor AA. Current concepts and treatments of schizophrenia. Molecules. 2018;23(8):2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Wang Y, Amdanee N, Zhang X. Exosomes in schizophrenia: pathophysiological mechanisms, biomarkers, and therapeutic targets. Eur Psychiatry. 2022;65(1):e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Khadimallah I, Jenni R, Cabungcal JH, et al. Mitochondrial, exosomal miR137‐COX6A2 and gamma synchrony as biomarkers of parvalbumin interneurons, psychopathology, and neurocognition in schizophrenia. Mol Psychiatry. 2022;27(2):1192‐1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Du Y, Yu Y, Hu Y, et al. Genome‐wide, integrative analysis implicates exosome‐derived MicroRNA dysregulation in schizophrenia. Schizophr Bull. 2019;45(6):1257‐1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Funahashi Y, Yoshino Y, Iga JI, et al. Impact of clozapine on the expression of miR‐675‐3p in plasma exosomes derived from patients with schizophrenia. World J Biol Psychiatry. 2022;24(4):303‐313. [DOI] [PubMed] [Google Scholar]
- 155. Amoah SK, Rodriguez BA, Logothetis CN, et al. Exosomal secretion of a psychosis‐altered miRNA that regulates glutamate receptor expression is affected by antipsychotics. Neuropsychopharmacology. 2020;45(4):656‐665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Narahari A, Hussain M, Sreeram V. MicroRNAs as biomarkers for psychiatric conditions: a review of current research. Innov Clin Neurosci. 2017;14(1‐2):53‐55. [PMC free article] [PubMed] [Google Scholar]
- 157. Perets N, Hertz S, London M, et al. Intranasal administration of exosomes derived from mesenchymal stem cells ameliorates autistic‐like behaviors of BTBR mice. Mol Autism. 2018;9:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Banks WA. A spectrum of topics for 2019: advances in neuroinflammation, oxidative stress, obesity, diabetes mellitus, cardiovascular disease, autism, exosomes, and central nervous system diseases. Curr Pharm Des. 2020;26(1):1‐5. [DOI] [PubMed] [Google Scholar]
- 159. Sharma P, Mesci P, Carromeu C, et al. Exosomes regulate neurogenesis and circuit assembly. Proc Natl Acad Sci USA. 2019;116(32):16086‐16094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Fang Y, Wan C, Wen Y, et al. Autism‐associated synaptic vesicle transcripts are differentially expressed in maternal plasma exosomes of physiopathologic pregnancies. J Transl Med. 2021;19(1):154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Alessio N, Brigida AL, Peluso G, et al. Stem cell‐derived exosomes in autism spectrum disorder. Int J Environ Res Public Health. 2020;17(3):944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Mittal R, Bencie N, Langlie J, et al. Exosomes as drug delivery vehicles and biomarkers for neurological and auditory systems. J Cell Physiol. 2021;236(12):8035‐8049. [DOI] [PubMed] [Google Scholar]
- 163. Perets N, Betzer O, Shapira R, et al. Golden exosomes selectively target brain pathologies in neurodegenerative and neurodevelopmental disorders. Nano Lett. 2019;19(6):3422‐3431. [DOI] [PubMed] [Google Scholar]
- 164. Harrell CR, Volarevic A, Djonov V, et al. Mesenchymal stem cell‐derived exosomes as new remedy for the treatment of neurocognitive disorders. Int J Mol Sci. 2021;22(3):1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Guedes VA, Kenney K, Shahim P, et al. Exosomal neurofilament light: a prognostic biomarker for remote symptoms after mild traumatic brain injury? Neurology. 2020;94(23):e2412‐e2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Gupta S, Guleria RS, Szabo YZ. MicroRNAs as biomarker and novel therapeutic target for posttraumatic stress disorder in Veterans. Psychiatry Res. 2021;305:114252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Guedes VA, Lai C, Devoto C, et al. Extracellular Vesicle proteins and MicroRNAs are linked to chronic post‐traumatic stress disorder symptoms in service members and veterans with mild traumatic brain injury. Front Pharmacol. 2021;12:745348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Devoto C, Guedes VA, Lai C, et al. Remote blast‐related mild traumatic brain injury is associated with differential expression of exosomal microRNAs identified in neurodegenerative and immunological processes. Brain Inj. 2022;36(5):652‐661. [DOI] [PubMed] [Google Scholar]
- 169. Gill J, Mustapic M, Diaz‐Arrastia R, et al. Higher exosomal tau, amyloid‐beta 42 and IL‐10 are associated with mild TBIs and chronic symptoms in military personnel. Brain Inj. 2018;32(10):1277‐1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Scheltens P, De Strooper B, Kivipelto M, et al. Alzheimer's disease. Lancet. 2021;397(10284):1577‐1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Gong X, Zhang H, Liu X, et al. Is liquid biopsy mature enough for the diagnosis of Alzheimer's disease? Front Aging Neurosci. 2022;14:977999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Miyoshi E, Bilousova T, Melnik M, et al. Exosomal tau with seeding activity is released from Alzheimer's disease synapses, and seeding potential is associated with amyloid beta. Lab Invest. 2021;101(12):1605‐1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Izco M, Carlos E, Alvarez‐Erviti L. Impact of endolysosomal dysfunction upon exosomes in neurodegenerative diseases. Neurobiol Dis. 2022;166:105651. [DOI] [PubMed] [Google Scholar]
- 174. Soliman HM, Ghonaim GA, Gharib SM, et al. Exosomes in Alzheimer's disease: from being pathological players to potential diagnostics and therapeutics. Int J Mol Sci. 2021;22(19):10794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Manna I, De Benedittis S, Iaccino E, et al. Diagnostic and therapeutic potential of exosomal miRNAs in Alzheimer's disease. Neural Regen Res. 2021;16(11):2217‐2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Valappil DK, Mini NJ, Dilna A, et al. Membrane interaction to intercellular spread of pathology in Alzheimer's disease. Front Neurosci. 2022;16:936897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Weng S, Lai QL, Wang J, et al. The role of exosomes as mediators of neuroinflammation in the pathogenesis and treatment of Alzheimer's disease. Front Aging Neurosci. 2022;14:899944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Younas N, Fernandez Flores LC, Hopfner F, et al. A new paradigm for diagnosis of neurodegenerative diseases: peripheral exosomes of brain origin. Transl Neurodegener. 2022;11(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Liu WL, Lin HW, Lin MR, et al. Emerging blood exosome‐based biomarkers for preclinical and clinical Alzheimer's disease: a meta‐analysis and systematic review. Neural Regen Res. 2022;17(11):2381‐2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Tan YJ, Wong BYX, Vaidyanathan R, et al. Altered cerebrospinal fluid exosomal microRNA levels in young‐onset Alzheimer's disease and frontotemporal dementia. J Alzheimers Dis Rep. 2021;5(1):805‐813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Mattingly J, Li Y, Bihl JC, et al. The promise of exosome applications in treating central nervous system diseases. CNS Neurosci Ther. 2021;27(12):1437‐1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Zou Y, Mu D, Ma X, et al. Review on the roles of specific cell‐derived exosomes in Alzheimer's disease. Front Neurosci. 2022;16:936760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Li CC, Hsu WF, Wo AM. Exosomes‐potential for blood‐based marker in Alzheimer's disease. Acta Neurol Taiwan. 2022;31(1):1‐6. [PubMed] [Google Scholar]
- 184. Li Y, Xia M, Meng S, et al. MicroRNA‐29c‐3p in dual‐labeled exosome is a potential diagnostic marker of subjective cognitive decline. Neurobiol Dis. 2022;171:105800. [DOI] [PubMed] [Google Scholar]
- 185. Li Y, Meng S, Di W, et al. Amyloid‐beta protein and MicroRNA‐384 in NCAM‐Labeled exosomes from peripheral blood are potential diagnostic markers for Alzheimer's disease. CNS Neurosci Ther. 2022;28(7):1093‐1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Ding L, Yang X, Xia X, et al. Exosomes mediate APP dysregulation via APP‐miR‐185‐5p axis. Front Cell Dev Biol. 2022;10:793388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Wang L, Zhen H, Sun Y, et al. Plasma Exo‐miRNAs correlated with AD‐related factors of Chinese individuals involved in abeta accumulation and cognition decline. Mol Neurobiol. 2022;59(11):6790‐6804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Chen Y, Sun Y, Luo Z, et al. Potential mechanism underlying exercise upregulated circulating blood exosome miR‐215‐5p to prevent necroptosis of neuronal cells and a model for early diagnosis of Alzheimer's disease. Front Aging Neurosci. 2022;14:860364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Meng S, Wang B, Li W. CircAXL knockdown alleviates abeta(1‐42)‐induced neurotoxicity in Alzheimer's disease via repressing PDE4A by releasing miR‐1306‐5p. Neurochem Res. 2022;47(6):1707‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Kandimalla R, Saeed M, Tyagi N, et al. Exosome‐based approaches in the management of Alzheimer's disease. Neurosci Biobehav Rev. 2023;144:104974. [DOI] [PubMed] [Google Scholar]
- 191. Nakano M, Fujimiya M. Potential effects of mesenchymal stem cell derived extracellular vesicles and exosomal miRNAs in neurological disorders. Neural Regen Res. 2021;16(12):2359‐2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Liu S, Fan M, Xu JX, et al. Exosomes derived from bone‐marrow mesenchymal stem cells alleviate cognitive decline in AD‐like mice by improving BDNF‐related neuropathology. J Neuroinflammation. 2022;19(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Liu H, Jin M, Ji M, et al. Hypoxic pretreatment of adipose‐derived stem cell exosomes improved cognition by delivery of circ‐Epc1 and shifting microglial M1/M2 polarization in an Alzheimer's disease mice model. Aging (Albany NY). 2022;14(7):3070‐3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Li N, Shu J, Yang X, et al. Exosomes derived from M2 microglia cells attenuates neuronal impairment and mitochondrial dysfunction in Alzheimer's disease through the PINK1/Parkin pathway. Front Cell Neurosci. 2022;16:874102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Zavatti M, Gatti M, Beretti F, et al. Exosomes derived from human amniotic fluid mesenchymal stem cells preserve microglia and neuron cells from abeta. Int J Mol Sci. 2022;23(9):4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Sheykhhasan M, Amini R, Soleimani Asl S, et al. Neuroprotective effects of coenzyme Q10‐loaded exosomes obtained from adipose‐derived stem cells in a rat model of Alzheimer's disease. Biomed Pharmacother. 2022;152:113224. [DOI] [PubMed] [Google Scholar]
- 197. Qi Y, Guo L, Jiang Y, et al. Brain delivery of quercetin‐loaded exosomes improved cognitive function in AD mice by inhibiting phosphorylated tau‐mediated neurofibrillary tangles. Drug Deliv. 2020;27(1):745‐755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Cacabelos R. Parkinson's Disease: from pathogenesis to pharmacogenomics. Int J Mol Sci. 2017;18(3):551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Li KL, Huang HY, Ren H, et al. Role of exosomes in the pathogenesis of inflammation in Parkinson's disease. Neural Regen Res. 2022;17(9):1898‐1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Gan‐Or Z, Liong C, Alcalay RN. GBA‐associated Parkinson's disease and other synucleinopathies. Curr Neurol Neurosci Rep. 2018;18(8):44. [DOI] [PubMed] [Google Scholar]
- 201. Zhang P, Rasheed M, Liang J, et al. Emerging potential of exosomal non‐coding RNA in Parkinson's disease: a review. Front Aging Neurosci. 2022;14:819836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Ouerdane Y, Hassaballah MY, Nagah A, et al. Exosomes in Parkinson: revisiting their pathologic role and potential applications. Pharmaceuticals (Basel). 2022;15(1):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Sepulveda D, Cisternas‐Olmedo M, Arcos J, et al. Contribution of autophagy‐lysosomal pathway in the exosomal secretion of alpha‐synuclein and its impact in the progression of Parkinson's disease. Front Mol Neurosci. 2022;15:805087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Long J, Zhang Y, Liu X, et al. Exosomes in the field of neuroscience: a scientometric study and visualization analysis. Front Neurol. 2022;13:871491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Kuo MC, Liu SC, Hsu YF, et al. The role of noncoding RNAs in Parkinson's disease: biomarkers and associations with pathogenic pathways. J Biomed Sci. 2021;28(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Doroszkiewicz J, Groblewska M, Mroczko B. Molecular biomarkers and their implications for the early diagnosis of selected neurodegenerative diseases. Int J Mol Sci. 2022;23(9):4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Tong G, Zhang P, Hu W, et al. Diagnostic test to identify Parkinson's disease from the blood sera of Chinese population: a cross‐sectional study. Parkinsons Dis. 2022;2022:8683877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Wang Y, Liu Y, Jin Z, et al. Association between mitochondrial function and rehabilitation of Parkinson's disease: revealed by exosomal mRNA and lncRNA expression profiles. Front Aging Neurosci. 2022;14:909622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Leggio L, Paterno G, Vivarelli S, et al. Extracellular vesicles as novel diagnostic and prognostic biomarkers for Parkinson's disease. Aging Dis. 2021;12(6):1494‐1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Yang X, Ma Y, Xie H, et al. Extracellular vesicles in the treatment of Parkinson's disease: a review. Curr Med Chem. 2021;28(31):6375‐6394. [DOI] [PubMed] [Google Scholar]
- 211. Liu SF, Li LY, Zhuang JL, et al. Update on the application of mesenchymal stem cell‐derived exosomes in the treatment of Parkinson's disease: a systematic review. Front Neurol. 2022;13:950715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Luo S, Du L, Cui Y. Potential therapeutic applications and developments of exosomes in Parkinson's disease. Mol Pharm. 2020;17(5):1447‐1457. [DOI] [PubMed] [Google Scholar]
- 213. Leggio L, Paterno G, Vivarelli S, et al. Extracellular vesicles as nanotherapeutics for Parkinson's disease. Biomolecules. 2020;10(9):1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Zhang L, Wu T, Shan Y, et al. Therapeutic reversal of Huntington's disease by in vivo self‐assembled siRNAs. Brain. 2021;144(11):3421‐3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Ananbeh H, Novak J, Juhas S, et al. Huntingtin Co‐isolates with small extracellular vesicles from blood plasma of TgHD and KI‐HD pig models of Huntington's disease and human blood plasma. Int J Mol Sci. 2022;23(10):5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. He M, Zhang HN, Tang ZC, et al. Diagnostic and therapeutic potential of exosomal MicroRNAs for neurodegenerative diseases. Neural Plast. 2021;2021:8884642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Zhang X, Abels ER, Redzic JS, et al. Potential transfer of polyglutamine and CAG‐repeat RNA in extracellular vesicles in Huntington's disease: background and evaluation in cell culture. Cell Mol Neurobiol. 2016;36(3):459‐470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Ananbeh H, Vodicka P, Kupcova Skalnikova H. Emerging roles of exosomes in Huntington's disease. Int J Mol Sci. 2021;22(8):4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Jeon I, Cicchetti F, Cisbani G, et al. Human‐to‐mouse prion‐like propagation of mutant huntingtin protein. Acta Neuropathol. 2016;132(4):577‐592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Wang JKT, Langfelder P, Horvath S, et al. Exosomes and homeostatic synaptic plasticity are linked to each other and to Huntington's, Parkinson's, and other neurodegenerative diseases by database‐enabled analyses of comprehensively curated datasets. Front Neurosci. 2017;11:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Didiot MC, Hall LM, Coles AH, et al. Exosome‐mediated delivery of hydrophobically modified siRNA for huntingtin mRNA silencing. Mol Ther. 2016;24(10):1836‐1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Lee M, Im W, Kim M. Exosomes as a potential messenger unit during heterochronic parabiosis for amelioration of Huntington's disease. Neurobiol Dis. 2021;155:105374. [DOI] [PubMed] [Google Scholar]
- 223. Li D, Li YP, Li YX, et al. Effect of regulatory network of exosomes and microRNAs on neurodegenerative diseases. Chin Med J (Engl). 2018;131(18):2216‐2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Wang J, Hu WW, Jiang Z, et al. Advances in treatment of neurodegenerative diseases: perspectives for combination of stem cells with neurotrophic factors. World J Stem Cells. 2020;12(5):323‐338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Hong Y, Zhao T, Li XJ, et al. Mutant huntingtin inhibits alphaB‐crystallin expression and impairs exosome secretion from astrocytes. J Neurosci. 2017;37(39):9550‐9563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Lee ST, Im W, Ban JJ, et al. Exosome‐based delivery of miR‐124 in a Huntington's disease model. J Mov Disord. 2017;10(1):45‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Wang L, Zhang L. Circulating exosomal miRNA as diagnostic biomarkers of neurodegenerative diseases. Front Mol Neurosci. 2020;13:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Hosaka T, Yamashita T, Tamaoka A, et al. Extracellular RNAs as biomarkers of sporadic amyotrophic lateral sclerosis and other neurodegenerative diseases. Int J Mol Sci. 2019;20(13):3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Tsai YL, Mu YC, Manley JL. Nuclear RNA transcript levels modulate nucleocytoplasmic distribution of ALS/FTD‐associated protein FUS. Sci Rep. 2022;12(1):8180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Otake K, Kamiguchi H, Hirozane Y. Identification of biomarkers for amyotrophic lateral sclerosis by comprehensive analysis of exosomal mRNAs in human cerebrospinal fluid. BMC Med Genomics. 2019;12(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Ciregia F, Urbani A, Palmisano G. Extracellular vesicles in brain tumors and neurodegenerative diseases. Front Mol Neurosci. 2017;10:276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Grad LI, Pokrishevsky E, Silverman JM, et al. Exosome‐dependent and independent mechanisms are involved in prion‐like transmission of propagated Cu/Zn superoxide dismutase misfolding. Prion. 2014;8(5):331‐335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Basso M, Pozzi S, Tortarolo M, et al. Mutant copper‐zinc superoxide dismutase (SOD1) induces protein secretion pathway alterations and exosome release in astrocytes: implications for disease spreading and motor neuron pathology in amyotrophic lateral sclerosis. J Biol Chem. 2013;288(22):15699‐15711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Feiler MS, Strobel B, Freischmidt A, et al. TDP‐43 is intercellularly transmitted across axon terminals. J Cell Biol. 2015;211(4):897‐911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Iguchi Y, Eid L, Parent M, et al. Exosome secretion is a key pathway for clearance of pathological TDP‐43. Brain. 2016;139(Pt 12):3187‐3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Chen QY, Wen T, Wu P, et al. Exosomal proteins and miRNAs as mediators of amyotrophic lateral sclerosis. Front Cell Dev Biol. 2021;9:718803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Hanspal MA, Dobson CM, Yerbury JJ, et al. The relevance of contact‐independent cell‐to‐cell transfer of TDP‐43 and SOD1 in amyotrophic lateral sclerosis. Biochim Biophys Acta Mol Basis Dis. 2017;1863(11):2762‐2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Upadhya R, Zingg W, Shetty S, et al. Astrocyte‐derived extracellular vesicles: neuroreparative properties and role in the pathogenesis of neurodegenerative disorders. J Control Release. 2020;323:225‐239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Westergard T, Jensen BK, Wen X, et al. Cell‐to‐cell transmission of dipeptide repeat proteins linked to C9orf72‐ALS/FTD. Cell Rep. 2016;17(3):645‐652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Kawabe Y, Mori K, Yamashita T, et al. The RNA exosome complex degrades expanded hexanucleotide repeat RNA in C9orf72 FTLD/ALS. EMBO J. 2020;39(19):e102700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Yelick J, Men Y, Jin S, et al. Elevated exosomal secretion of miR‐124‐3p from spinal neurons positively associates with disease severity in ALS. Exp Neurol. 2020;333:113414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Pinto S, Cunha C, Barbosa M, et al. Exosomes from NSC‐34 cells transfected with hSOD1‐G93A are enriched in miR‐124 and drive alterations in microglia phenotype. Front Neurosci. 2017;11:273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Rizzuti M, Melzi V, Gagliardi D, et al. Insights into the identification of a molecular signature for amyotrophic lateral sclerosis exploiting integrated microRNA profiling of iPSC‐derived motor neurons and exosomes. Cell Mol Life Sci. 2022;79(3):189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Wang K, Li Y, Ren C, et al. Extracellular vesicles as innovative treatment strategy for amyotrophic lateral sclerosis. Front Cell Dev Biol. 2021;9:754630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. McCluskey G, Morrison KE, Donaghy C, et al. Extracellular vesicles in amyotrophic lateral sclerosis. Life (Basel). 2022;13(1):121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Gagliardi D, Bresolin N, Comi GP, et al. Extracellular vesicles and amyotrophic lateral sclerosis: from misfolded protein vehicles to promising clinical biomarkers. Cell Mol Life Sci. 2021;78(2):561‐572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Ediriweera GR, Chen L, Yerbury JJ, et al. Non‐viral vector‐mediated gene therapy for ALS: challenges and future perspectives. Mol Pharm. 2021;18(6):2142‐2160. [DOI] [PubMed] [Google Scholar]
- 248. Bonafede R, Mariotti R. ALS pathogenesis and therapeutic approaches: the role of mesenchymal stem cells and extracellular vesicles. Front Cell Neurosci. 2017;11:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Properzi F, Ferroni E, Poleggi A, et al. The regulation of exosome function in the CNS: implications for neurodegeneration. Swiss Med Wkly. 2015;145:w14204. [DOI] [PubMed] [Google Scholar]
- 250. Gharbi T, Zhang Z, Yang GY. The function of astrocyte mediated extracellular vesicles in central nervous system diseases. Front Cell Dev Biol. 2020;8:568889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Giunti D, Marini C, Parodi B, et al. Role of miRNAs shuttled by mesenchymal stem cell‐derived small extracellular vesicles in modulating neuroinflammation. Sci Rep. 2021;11(1):1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Calabria E, Scambi I, Bonafede R, et al. ASCs‐exosomes recover coupling efficiency and mitochondrial membrane potential in an in vitro model of ALS. Front Neurosci. 2019;13:1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Bonafede R, Brandi J, Manfredi M, et al. The anti‐apoptotic effect of ASC‐exosomes in an in vitro ALS model and their proteomic analysis. Cells. 2019;8(9):1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Bonafede R, Turano E, Scambi I, et al. ASC‐exosomes ameliorate the disease progression in SOD1(G93A) murine model underlining their potential therapeutic use in human ALS. Int J Mol Sci. 2020;21(10):3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Bonafede R, Scambi I, Peroni D, et al. Exosome derived from murine adipose‐derived stromal cells: neuroprotective effect on in vitro model of amyotrophic lateral sclerosis. Exp Cell Res. 2016;340(1):150‐158. [DOI] [PubMed] [Google Scholar]
- 256. Jovicic A, Gitler AD. Distinct repertoires of microRNAs present in mouse astrocytes compared to astrocyte‐secreted exosomes. PLoS One. 2017;12(2):e0171418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Wei N, Zhang H, Wang J, et al. The progress in diagnosis and treatment of exosomes and MicroRNAs on epileptic comorbidity depression. Front Psychiatry. 2020;11:405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Neri S, Mastroianni G, Gardella E, et al. Epilepsy in neurodegenerative diseases. Epileptic Disord. 2022;24(2):249‐273. [DOI] [PubMed] [Google Scholar]
- 259. Khalyfa A, Sanz‐Rubio D. Genetics and extracellular vesicles of pediatrics sleep disordered breathing and epilepsy. Int J Mol Sci. 2019;20(21):5483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Batool A, Hill TDM, Nguyen NT, et al. Altered biogenesis and MicroRNA content of hippocampal exosomes following experimental status epilepticus. Front Neurosci. 2019;13:1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Huang LG, Luo YH, Xu JW, et al. Plasma exosomal MiRNAs expression profile in mesial temporal lobe epilepsy with hippocampal sclerosis: case‐control study and analysis of potential functions. Front Mol Neurosci. 2020;13:584828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Chen SD, Pan HY, Huang JB, et al. Circulating MicroRNAs from serum exosomes may serve as a putative biomarker in the diagnosis and treatment of patients with focal cortical dysplasia. Cells. 2020;9(8):1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Xiao L, Hareendran S, Loh YP. Function of exosomes in neurological disorders and brain tumors. Extracell Vesicles Circ Nucl Acids. 2021;2:55‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Lin Z, Gu Y, Zhou R, et al. Serum exosomal proteins F9 and TSP‐1 as potential diagnostic biomarkers for newly diagnosed epilepsy. Front Neurosci. 2020;14:737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Xian P, Hei Y, Wang R, et al. Mesenchymal stem cell‐derived exosomes as a nanotherapeutic agent for amelioration of inflammation‐induced astrocyte alterations in mice. Theranostics. 2019;9(20):5956‐5975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Collaborators GBDS . Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021;20(10):795‐820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Xu Y, Hu Y, Xu S, et al. Exosomal microRNAs as potential biomarkers and therapeutic agents for acute ischemic stroke: new expectations. Front Neurol. 2021;12:747380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Venkat P, Chopp M. Exosome treatment for stroke with diabetic comorbidity. Neural Regen Res. 2022;17(2):315‐317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Niu M, Li H, Li X, et al. Circulating exosomal miRNAs as novel biomarkers perform superior diagnostic efficiency compared with plasma miRNAs for large‐artery atherosclerosis stroke. Front Pharmacol. 2021;12:791644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Zhang S, Wang X, Yin R, et al. Circulating exosomal lncRNAs as predictors of risk and unfavorable prognosis for large artery atherosclerotic stroke. Clin Transl Med. 2021;11(12):e555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Wang Q, Chen Y, Meng L, et al. A novel perspective on ischemic stroke: a review of exosome and noncoding RNA studies. Brain Sci. 2022;12(8):1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Xiao Q, Hou R, Li H, et al. Circulating exosomal circRNAs contribute to potential diagnostic value of large artery atherosclerotic stroke. Front Immunol. 2021;12:830018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Ye YC, Chang ZH, Wang P, et al. Infarct‐preconditioning exosomes of umbilical cord mesenchymal stem cells promoted vascular remodeling and neurological recovery after stroke in rats. Stem Cell Res Ther. 2022;13(1):378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Yang J, Hao J, Lin Y, et al. Profile and functional prediction of plasma exosome‐derived CircRNAs from acute ischemic stroke patients. Front Genet. 2022;13:810974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Zhang H, Lin S, McElroy CL, et al. Circulating pro‐inflammatory exosomes worsen stroke outcomes in aging. Circ Res. 2021;129(7):e121‐e140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Li C, Hu J, Liu W, et al. Exercise intervention modulates synaptic plasticity by inhibiting excessive microglial activation via exosomes. Front Cell Neurosci. 2022;16:953640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Li C, Ke C, Su Y, et al. Exercise intervention promotes the growth of synapses and regulates neuroplasticity in rats with ischemic stroke through exosomes. Front Neurol. 2021;12:752595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. He T, Yang GY, Zhang Z. Crosstalk of astrocytes and other cells during ischemic stroke. Life (Basel). 2022;12(6):910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Yang L, Qian J, Yang B, et al. Challenges and improvements of novel therapies for ischemic stroke. Front Pharmacol. 2021;12:721156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Wan T, Huang Y, Gao X, et al. Microglia polarization: a novel target of exosome for stroke treatment. Front Cell Dev Biol. 2022;10:842320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Cun Y, Jin Y, Wu D, et al. Exosome in Crosstalk between inflammation and angiogenesis: a potential therapeutic strategy for stroke. Mediators Inflamm. 2022;2022:7006281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Hu H, Hu X, Li L, et al. Exosomes derived from bone marrow mesenchymal stem cells promote angiogenesis in ischemic stroke mice via upregulation of MiR‐21‐5p. Biomolecules. 2022;12(7):883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Li X, Bi T, Yang S. Exosomal microRNA‐150‐5p from bone marrow mesenchymal stromal cells mitigates cerebral ischemia/reperfusion injury via targeting toll‐like receptor 5. Bioengineered. 2022;13(2):3030‐3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Min W, Wu Y, Fang Y, et al. Bone marrow mesenchymal stem cells‐derived exosomal microRNA‐124‐3p attenuates hypoxic‐ischemic brain damage through depressing tumor necrosis factor receptor associated factor 6 in newborn rats. Bioengineered. 2022;13(2):3194‐3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Norouzi‐Barough L, Asgari Khosroshahi A, Gorji A, et al. COVID‐19‐induced stroke and the potential of using mesenchymal stem cells‐derived extracellular vesicles in the regulation of neuroinflammation. Cell Mol Neurobiol. 2023;43(1):37‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Luo H, Ye G, Liu Y, et al. miR‐150‐3p enhances neuroprotective effects of neural stem cell exosomes after hypoxic‐ischemic brain injury by targeting CASP2. Neurosci Lett. 2022;779:136635. [DOI] [PubMed] [Google Scholar]
- 287. Zhu ZH, Jia F, Ahmed W, et al. Neural stem cell‐derived exosome as a nano‐sized carrier for BDNF delivery to a rat model of ischemic stroke. Neural Regen Res. 2023;18(2):404‐409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. Guo L, Huang Z, Huang L, et al. Surface‐modified engineered exosomes attenuated cerebral ischemia/reperfusion injury by targeting the delivery of quercetin towards impaired neurons. J Nanobiotechnology. 2021;19(1):141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. He R, Jiang Y, Shi Y, et al. Curcumin‐laden exosomes target ischemic brain tissue and alleviate cerebral ischemia‐reperfusion injury by inhibiting ROS‐mediated mitochondrial apoptosis. Mater Sci Eng C Mater Biol Appl. 2020;117:111314. [DOI] [PubMed] [Google Scholar]
- 290. Jin M, Zhang S, Wang M, et al. Exosomes in pathogenesis, diagnosis, and therapy of ischemic stroke. Front Bioeng Biotechnol. 2022;10:980548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Lee EC, Ha TW, Lee DH, et al. Utility of exosomes in ischemic and hemorrhagic stroke diagnosis and treatment. Int J Mol Sci. 2022;23(15):8367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Alptekin A, Khan MB, Ara R, et al. Pulsed focal ultrasound as a non‐invasive method to deliver exosomes in the brain/stroke. J Biomed Nanotechnol. 2021;17(6):1170‐1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Calabria M, Garcia‐Sanchez C, Grunden N, et al. Post‐COVID‐19 fatigue: the contribution of cognitive and neuropsychiatric symptoms. J Neurol. 2022;269(8):3990‐3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Weintraub D, Irwin D. Diagnosis and treatment of cognitive and neuropsychiatric symptoms in Parkinson disease and dementia with Lewy bodies. Continuum (Minneap Minn). 2022;28(5):1314‐1332. [DOI] [PubMed] [Google Scholar]
- 295. Magrath Guimet N, Zapata‐Restrepo LM, Miller BL. Advances in treatment of frontotemporal dementia. J Neuropsychiatry Clin Neurosci. 2022;34(4):316‐327. [DOI] [PubMed] [Google Scholar]
- 296. Mula M, Coleman H, Wilson SJ. Neuropsychiatric and cognitive comorbidities in epilepsy. Continuum (Minneap Minn). 2022;28(2):457‐482. [DOI] [PubMed] [Google Scholar]
- 297. Cacabelos R, Naidoo V, Martinez‐Iglesias O, et al. Personalized management and treatment of Alzheimer's disease. Life (Basel). 2022;12(3):460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. Mathur N, Bhatt H, Lidstone SC. Neuropsychiatric treatments for Parkinson's disease: nonpharmacological approaches. Semin Neurol. 2022;42(2):158‐167. [DOI] [PubMed] [Google Scholar]
- 299. Oraki Kohshour M, Papiol S, Delalle I, et al. Extracellular vesicle approach to major psychiatric disorders. Eur Arch Psychiatry Clin Neurosci. 2022:PMID36302978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. He C, Zheng S, Luo Y, et al. Exosome theranostics: biology and translational medicine. Theranostics. 2018;8(1):237‐255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Kimiz‐Gebologlu I, Oncel SS. Exosomes: large‐scale production, isolation, drug loading efficiency, and biodistribution and uptake. J Control Release. 2022;347:533‐543. [DOI] [PubMed] [Google Scholar]
- 302. Tschuschke M, Kocherova I, Bryja A, et al. Inclusion biogenesis, methods of isolation and clinical application of human cellular exosomes. J Clin Med. 2020;9(2):436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. Saludas L, Garbayo E, Ruiz‐Villalba A, et al. Isolation methods of large and small extracellular vesicles derived from cardiovascular progenitors: a comparative study. Eur J Pharm Biopharm. 2022;170:187‐196. [DOI] [PubMed] [Google Scholar]
- 304. Tang B, Zeng W, Song LL, et al. Extracellular vesicle delivery of Neferine for the attenuation of neurodegenerative disease proteins and motor deficit in an Alzheimer's disease mouse model. Pharmaceuticals (Basel). 2022;15(1):83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Zhao X, Wu D, Ma X, et al. Exosomes as drug carriers for cancer therapy and challenges regarding exosome uptake. Biomed Pharmacother. 2020;128:110237. [DOI] [PubMed] [Google Scholar]
- 306. Parada N, Romero‐Trujillo A, Georges N, et al. Camouflage strategies for therapeutic exosomes evasion from phagocytosis. J Adv Res. 2021;31:61‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307. Lakshmi S, Hughes TA, Priya S. Exosomes and exosomal RNAs in breast cancer: a status update. Eur J Cancer. 2021;144:252‐268. [DOI] [PubMed] [Google Scholar]
- 308. Ahn SH, Ryu SW, Choi H, et al. Manufacturing therapeutic exosomes: from bench to industry. Mol Cells. 2022;45(5):284‐290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Mobahat M, Sadroddiny E, Nooshabadi VT, et al. Curcumin‐loaded human endometrial stem cells derived exosomes as an effective carrier to suppress alpha‐synuclein aggregates in 6OHDA‐induced Parkinson's disease mouse model. Cell Tissue Bank. 2023;24(1):75‐91. [DOI] [PubMed] [Google Scholar]
- 310. Mohi‐Ud‐Din R, Mir RH, Wani TU, et al. Novel drug delivery system for curcumin: implementation to improve therapeutic efficacy against neurological disorders. Comb Chem High Throughput Screen. 2022;25(4):607‐615. [DOI] [PubMed] [Google Scholar]
- 311. Panzarini E, Mariano S, Tacconi S, et al. Novel therapeutic delivery of nanocurcumin in central nervous system related disorders. Nanomaterials (Basel). 2020;11(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312. Upadhya R, Shetty AK. Extracellular vesicles for the diagnosis and treatment of Parkinson's disease. Aging Dis. 2021;12(6):1438‐1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313. Ordonez‐Gutierrez L, Wandosell F. Nanoliposomes as a therapeutic tool for Alzheimer's disease. Front Synaptic Neurosci. 2020;12:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314. Fernandes M, Lopes I, Magalhaes L, et al. Novel concept of exosome‐like liposomes for the treatment of Alzheimer's disease. J Control Release. 2021;336:130‐143. [DOI] [PubMed] [Google Scholar]
- 315. Liu J, Liu C, Zhang J, et al. A self‐assembled alpha‐synuclein nanoscavenger for Parkinson's disease. ACS Nano. 2020;14(2):1533‐1549. [DOI] [PubMed] [Google Scholar]
- 316. Liu L, Li Y, Peng H, et al. Targeted exosome coating gene‐chem nanocomplex as “nanoscavenger” for clearing alpha‐synuclein and immune activation of Parkinson's disease. Sci Adv. 2020;6(50):eaba3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317. Jafari D, Malih S, Eini M, et al. Improvement, scaling‐up, and downstream analysis of exosome production. Crit Rev Biotechnol. 2020;40(8):1098‐1112. [DOI] [PubMed] [Google Scholar]
- 318. Zhang R, Bu T, Cao R, et al. An optimized exosome production strategy for enhanced yield while without sacrificing cargo loading efficiency. J Nanobiotechnology. 2022;20(1):463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319. Cheng Q, Dai Z, Shi X, et al. Expanding the toolbox of exosome‐based modulators of cell functions. Biomaterials. 2021;277:121129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320. Barjesteh T, Mansur S, Bao Y. Inorganic nanoparticle‐loaded exosomes for biomedical applications. Molecules. 2021;26(4):1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


