Abstract
Considering the rarity and aggressive nature of retroperitoneal leiomyosarcoma (RLMS), several prognostic factors might contribute to the cancer-specific mortality of these patients. This study aimed to construct a competing risk-based nomogram to predict cancer-specific survival (CSS) for patients with RLMS. In total, 788 cases from the Surveillance, Epidemiology, and End Results (SEER) database (2000–2015) were included. Based on the Fine & Gray's method, independent predictors were screened to develop a nomogram for predicting 1-, 3-, and 5-year CSS. After multivariate analysis, CSS was found significantly associated with tumor characteristics (tumor grade, size, range), as well as surgery status. The nomogram showed solid prediction power and was well calibrated. Through decision curve analysis (DCA), a favorable clinical utility of the nomogram was demonstrated. Additionally, a risk stratification system was developed and distinctive survival between risk groups was observed. In summary, this nomogram showed a better performance than the AJCC 8th staging system and can assist in the clinical management of RLMS.
Keywords: Retroperitoneal leiomyosarcoma, Nomogram, Prognosis, Risk stratification system
1. Introduction
Retroperitoneal sarcomas (RPS) account for 10%–15% of all soft tissue sarcomas with high heterogeneity and different oncologic outcomes [1]. For decades, over 50 histological types of RPS have been acknowledged and each exhibits variable biological characteristics [2,3]. Patients with retroperitoneal leiomyosarcoma (RLMS) are exposed to increased cancer-specific death (CSD) and decreased cancer-specific survival (CSS) due to its aggressive and invasive behavior [4].
Surgical resection under the management of a multidisciplinary team remains the cornerstone of its treatment [[5], [6], [7]]. Although the local recurrence rate of RLMS ranges from 6% to 10% five years after surgery, its distant recurrence rate is as high as 50% [2,8]. For advanced or metastatic leiomyosarcomas (LMS), a first-line chemotherapy regimen based on anthracycline (Doxorubicin or Epirubicin) displayed certain efficacy in the clinical setting [9,10]. The combination of Gemcitabine and Docetaxel or Gemcitabine alone has also shown encouraging efficacy but higher toxicity in LMS [9,11]. However, lacking definitive evidence, the benefit of chemotherapy for LMS in the retroperitoneum site is still controversial. Several studies even noticed a worse survival outcome while using perioperative chemotherapy in RLMS [12,13]. Other non-surgical therapies, such as radiotherapy and immunotherapy, have failed to improve the prognosis of RLMS patients in clinical trials known as STRASS I, STREXIT, and SARC028 [[14], [15], [16]]. Therefore, the prognosis of RLMS patients remains poor, even though multiple treatments have been utilized.
Previous studies have identified risk factors, such as histology, tumor size, grade, surgery, and metastasis status, to predict the prognosis of patients with RPS [17,18]. However, nomograms to predict the probability of CSS for RLMS patients have not yet been constructed. Compared with traditional Cox analysis, the Fine & Gray method [19] is more accurate in survival analysis for considering the existence of competing events and has been applied in various tumors, including retroperitoneal liposarcoma [20,21].
The Surveillance, Epidemiology, and End Results (SEER) database is publicly available and includes 18 cancer registries in the United States, covering around 28% of the national population [22]. Considering the rarity of RLMS, the SEER database provides an ideal basis and long-term follow-up data for research on this disease. Based on this large-population database, our study aimed to establish a competing risk-based nomogram to obtain unbiased estimates of CSS for RLMS.
2. Materials and methods
2.1. Study design and data collection
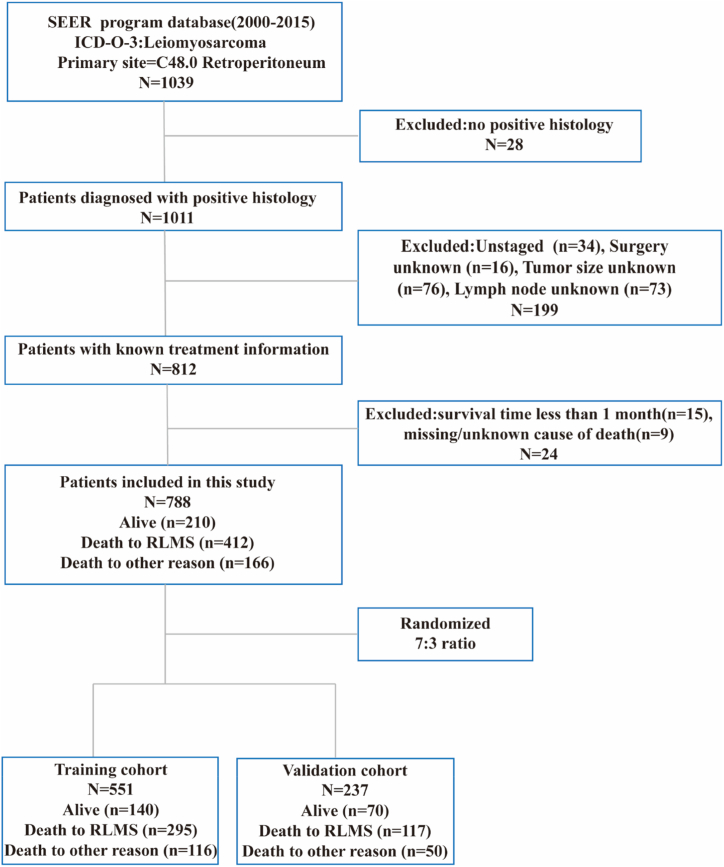
Data for patients diagnosed with RLMS were obtained from the SEER Research Plus Data, 18 Registries, Nov 2020 Sub (2000–2018). Inclusion criteria: (1) the primary site was C48.0 (retroperitoneum); (2) the ICD-O-3 was coded as leiomyosarcoma (8890/3, 8891/3, 8893/3, 8896/3); (3) “Year of diagnosis” was set from 2000 to 2015. Exclusion criteria: (1) patients without positive histology; (2) survival time less than 1 month; (2) missing/unknown information on tumor range, tumor size, lymph node, surgery status, and cause of death. A total of 788 cases diagnosed with RLMS were enrolled in the final cohort (Fig. 1), with all patients over 18 years old. Since the database is publicly available and patient data are de-identified, this study was exempt from approval by our hospital review board.
Fig. 1.
Flowchart of the inclusion and exclusion process.
2.2. Variable definitions
We extracted demographic information (age, sex, race, marital status), as well as tumor characteristics (grade, tumor size, tumor range), treatments (surgery, radiotherapy, chemotherapy), survival information, and cancer-specific death for each included patient. Stratification for continuous variables was conducted according to median age (≤60 vs. >60 years old) and median tumor size (≤10 cm vs. > 10 cm). Other categorical variables included marital status (married, no/unknown), race (White, Black, others), grade (I/II, III/IV, Unknown), tumor range (localized, regional, distant), surgery status (yes, no), radiation (yes, no/unknown), and chemotherapy (yes, no/unknown). Deaths from RLMS and competing risks were defined as cancer-specific death (CSD) and non-cancer-specific death (NCSD), respectively. The primary outcome of this study was cancer-specific survival (CSS), which was recorded as “Dead (attribute to this cancer dx)” in the SEER cause-specific death classification.
To compare our nomogram with the AJCC 8th staging system, the AJCC tumor stage for each patient was derived from tumor size (T1/2/3/4), lymph node metastasis (N0/1), distant metastasis (M0/1) and histologic grade (GX/1/2/3). The detailed staging system can be found in the NCCN guideline for retroperitoneal soft tissue sarcomas (2017 version).
2.3. Development and validation of the prognostic nomogram
The total cohort (n = 788) was randomly divided into the training set (n = 551) and the validation set (n = 237) at a ratio of 7:3. The training set was used to screen prognostic factors to generate the nomogram, whereas the validation set was used to verify the performance of the nomogram. The cumulative incidence function (CIF) was used to evaluate the overall incidence of CSD and NCSD, as well as the incidence for each single variable in the training cohort. After univariate and multivariate analysis, four predictors with significance (p < 0.05) were incorporated to develop a nomogram for predicting 1-, 3- and 5-year CSS. To further validate its predictive power, the following procedures were performed both in the training and validation cohorts. First, the receiver-operating characteristic (ROC) curve and area under the curve (AUC) were conducted to estimate the discriminatory ability of the established model and the AJCC 8th staging system. Next, the concordance of predicted probability and actual rate of CSS was further evaluated by calibration plots. Decision curve analysis (DCA) was carried out to illustrate the clinical utility of the nomogram. Eventually, by using the X-tile software (version 3.6.1), a risk stratification system using the Kaplan–Meier method with the log-rank test was developed to present the survival distinction between risk groups.
2.4. Statistical analysis
R software (version 4.1.0, www.R-project.org) was applied to perform all analyses with the R packages “rms”, “cmprsk”, “surviminer”, “mstate”, “riskRegression”, and “dcurves”. All categorical variables are described as frequency and percentage, which were compared through the χ2 test and Fisher's exact test. The incidence of CSD was assessed by the Gray's test and plotted as the CIF curves. Factors with statistical significance in the multivariate competing risk regression were incorporated to establish a nomogram for predicting 1-, 3-, and 5-year CSS. All calculated P values were bilateral with significance set at p < 0.05.
3. Results
3.1. Patient characteristics and cancer-specific death of RLMS
The general demographic and clinical variables are summarized in Table 1. Overall, 788 patients histologically diagnosed with RLMS were enrolled in the study and randomly divided into the training set (n = 551) and the validation set (n = 237). No significant difference was found between the two cohorts. Among all patients, their mean age was 61 years old at diagnosis (interquartile range: 52–72 years old) with females accounting for 70.2%. Most patients received surgery treatment (83.5%). The median follow-up time was 44 months (interquartile range: 18–90 months). By the last follow-up, 578 deaths (73.4%) were observed, with 412 CSD (52.3%) and 166 NCSD (21.1%) from RLMS, indicating that NCSD also contributed a great part to the outcome of RLMS. In addition, a distinction in the overall cumulative incidence between CSD and NCSD was displayed in a CIF plot, which showed a greater difference with a longer survival time (Fig. S1A). During the follow-up period, the 5-year CIF for CSD and NCSD were 56.6% and 20.2%, respectively; the 10-year CIF were 67.6% and 26.1%, respectively.
Table 1.
Demographic and clinicopathologic characteristics of RLMS patients.
| Characteristic | Category | Training set (N = 551) | Validation set (N = 237) | Overall (N = 788) | P-value |
|---|---|---|---|---|---|
| Age, years | Mean (SD) | 62.1 (13.7) | 60.8 (14.1) | 61.7 (13.8) | 0.274 |
| Age ≤ 60 | 247 (44.8%) | 117 (49.4%) | 364 (46.2%) | ||
| Age > 60 | 304 (55.2%) | 120 (50.6%) | 424 (53.8%) | ||
| Sex | 0.485 | ||||
| Female | 387 (70.2%) | 173 (73.0%) | 560 (71.1%) | ||
| Male | 164 (29.8%) | 64 (27.0%) | 228 (28.9%) | ||
| Race | 0.975 | ||||
| Black | 95 (17.2%) | 41 (17.3%) | 136 (17.3%) | ||
| White | 402 (73.0%) | 174 (73.4%) | 576 (73.1%) | ||
| Other | 54 (9.8%) | 22 (9.3%) | 76 (9.6%) | ||
| Marital status | 0.332 | ||||
| No/Unknown | 245 (44.5%) | 115 (48.5%) | 360 (45.7%) | ||
| Yes | 306 (55.5%) | 122 (51.5%) | 428 (54.3%) | ||
| Tumor grade | 0.931 | ||||
| I/II | 161 (29.2%) | 72 (30.4%) | 233 (29.6%) | ||
| III/IV | 275 (49.9%) | 115 (48.5%) | 390 (49.5%) | ||
| Unknown | 115 (20.9%) | 50 (21.1%) | 165 (20.9%) | ||
| Tumor size, cm | 0.090 | ||||
| ≤10 | 262 (47.6%) | 129 (54.4%) | 391 (49.6%) | ||
| >10 | 289 (52.5%) | 108 (45.6%) | 397 (50.4%) | ||
| AJCC.8th. T stage | 0.308 | ||||
| T1 | 65 (11.8%) | 36 (15.2%) | 101 (12.8%) | ||
| T2 | 197 (35.8%) | 93 (39.2%) | 290 (36.8%) | ||
| T3 | 146 (26.5%) | 55 (23.2%) | 201 (25.5%) | ||
| T4 | 143 (26.0%) | 53 (22.4%) | 196 (24.9%) | ||
| Lymph node metastasis | 0.091 | ||||
| No | 517 (93.8%) | 230 (97.0%) | 747 (94.8%) | ||
| Yes | 34 (6.2%) | 7 (3.0%) | 41 (5.2%) | ||
| Tumor range | 0.477 | ||||
| Localized | 235 (42.6%) | 110 (46.4%) | 345 (43.8%) | ||
| Regional | 210 (38.1%) | 89 (37.6%) | 299 (37.9%) | ||
| Distant | 106 (19.2%) | 38 (16.0%) | 144 (18.3%) | ||
| AJCC.8th. Tumor stage | 0.059 | ||||
| I | 116 (21.1%) | 54 (22.8%) | 170 (21.6%) | ||
| II | 28 (5.1%) | 23 (9.7%) | 51 (6.5%) | ||
| III | 319 (57.9%) | 131 (55.3%) | 450 (57.1%) | ||
| IV | 88 (16.0%) | 29 (12.2%) | 117 (14.8%) | ||
| Surgery | 0.336 | ||||
| No | 96 (17.4%) | 34 (14.3%) | 130 (16.5%) | ||
| Yes | 455 (82.6%) | 203 (85.7%) | 658 (83.5%) | ||
| Radiation | 0.431 | ||||
| No/Unknown | 355 (64.4%) | 145 (61.2%) | 500 (63.5%) | ||
| Yes | 196 (35.6%) | 92 (38.8%) | 288 (36.5%) | ||
| Chemotherapy | 0.0612 | ||||
| No/Unknown | 388 (70.4%) | 182 (77.2%) | 571 (72.5%) | ||
| Yes | 163 (29.6%) | 54 (22.8%) | 217 (27.5%) |
3.2. Screening of independent prognostic factors
Subsequently, the CIF curves were plotted according to different patient characteristics (Figure S1B–K). Five factors, including higher grade (III/IV), larger tumor size (>10 cm), distant tumor range, chemotherapy, and no surgery status, were found significantly correlated with increased incidence of CSD through univariate analysis. Only age and race were found related to the growing incidence of NCSD. Finally, after adjustment for potential confounders and consideration for clinical practice, five factors significant in univariate analysis were included in the multivariable analysis of CSS (Table 2). Regarding the AJCC 8th T stage, the analysis showed no difference between T1 (tumor size ≤ 5 cm) and T2 (5 cm < tumor size ≤ 10 cm). Meanwhile, no distinction was observed between AJCC 8th tumor stage I and II.
Table 2.
Univariate and multivariate competing risk analysis of cancer-specific mortality for patients with RLMS.
| Characteristic | Category | Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|---|
| SHR† (95% CI*) | P-value | SHR† (95% CI*) | P-value | ||
| Age, years | Mean (SD) | ||||
| Age ≤ 60 | Reference | ||||
| Age > 60 | 0.974 (0.777–1.220) | 0.820 | |||
| Sex | |||||
| Female | Reference | ||||
| Male | 0.905 (0.697–1.180) | 0.450 | |||
| Race | |||||
| Black | Reference | ||||
| White | 0.775 (0.574–1.050) | 0.096 | |||
| Other | 1.061 (0.698–1.610) | 0.780 | |||
| Marital status | |||||
| No/Unknown | Reference | ||||
| Yes | 0.855 (0.680–1.070) | 0.180 | |||
| Tumor grade | |||||
| I/II | Reference | Reference | |||
| III/IV | 1.830 (1.398–2.410) | <0.001*** | 1.766 (1.525–2.045) | <0.001*** | |
| Unknown | 1.270 (0.898–1.790) | 0.180 | 1.040 (0.861–1.258) | 0.834 | |
| Tumor size, cm | |||||
| ≤10 | Reference | Reference | |||
| >10 | 1.910 (1.510–2.410) | <0.001*** | 1.745 (1.541–1.977) | <0.001*** | |
| AJCC.8th.T stage | |||||
| T1 | Reference | ||||
| T2 | 1.330 (0.848–2.090) | 0.210 | |||
| T3 | 2.240 (1.427–3.520) | <0.001*** | |||
| T4 | 2.530 (1.611–3.980) | <0.001*** | |||
| Lymph node metastasis | |||||
| No | Reference | ||||
| Yes | 1.530 (0.961–2.440) | 0.073 | |||
| Tumor range | |||||
| Localized | Reference | Reference | |||
| Regional | 1.470 (1.130–1.920) | 0.004 ** | 1.392 (1.211–1.600) | 0.018* | |
| Distant | 3.000 (2.210–4.070) | <0.001*** | 2.488 (2.077–2.979) | <0.001*** | |
| AJCC.8th. Tumor stage | |||||
| I | Reference | ||||
| II | 1.02 (0.525–1.990) | 0.950 | |||
| III | 1.76 (1.263–2.450) | <0.001*** | |||
| IV | 4.04 (2.709–6.030) | <0.001*** | |||
| Surgery | |||||
| No | Reference | Reference | |||
| Yes | 0.490 (0.360–0.667) | <0.001*** | 0.443 (0.374–0.524) | <0.001*** | |
| Radiation | |||||
| No/Unknown | Reference | ||||
| Yes | 0.901 (0.715–1.130) | 0.370 | |||
| Chemotherapy | |||||
| No/Unknown | Reference | Reference | |||
| Yes | 1.730 (1.370–2.190) | <0.001*** | 1.100 (0.9543–1.269) | 0.502 | |
†SHR: subdistribution hazard ratio.
*CI: confidence interval.
3.3. Development and validation of a prognostic nomogram for RLMS
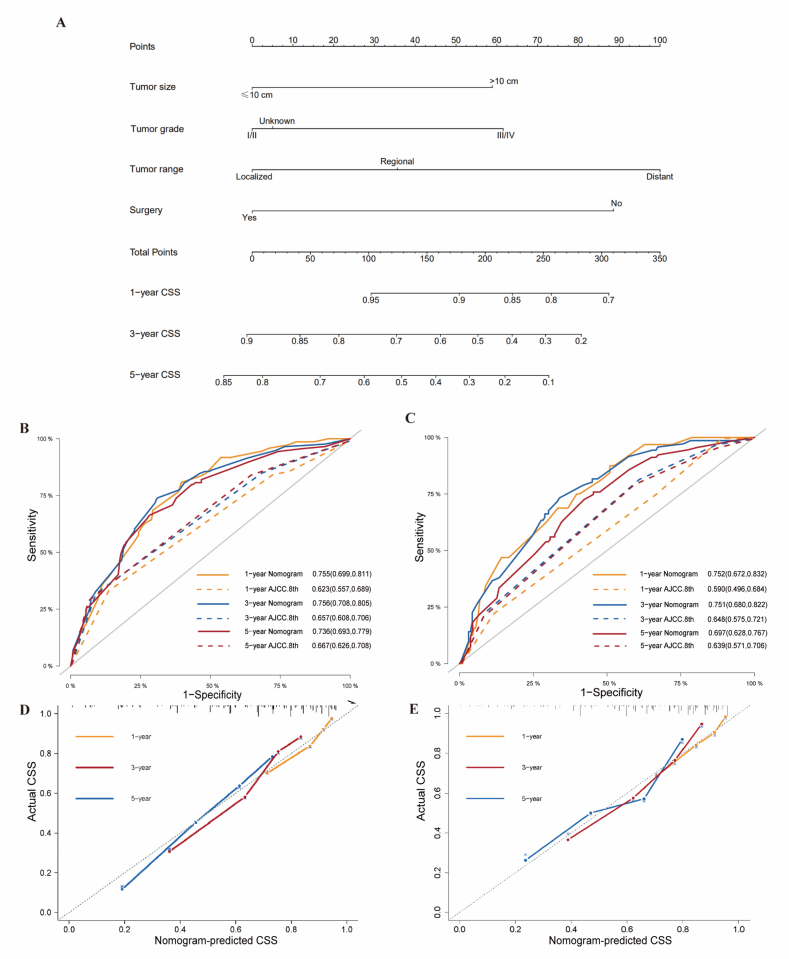
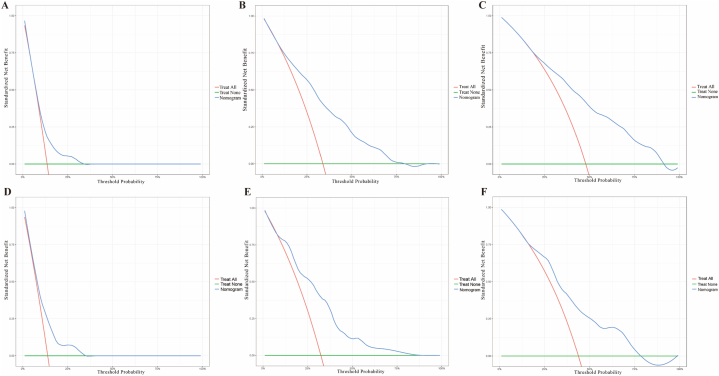
Multivariate Fine & Gray's model was employed to identify predictors of CSS in the training cohort. Cause-specific mortality was found to correlate with tumor characteristics (grade, tumor range, tumor size), and surgery status, which were incorporated to develop a prognostic nomogram for predicting the 1-, 3-, and 5-year probability of CSS (Fig. 2A). Chemotherapy was finally ruled out after multivariate analysis. The ROC curves and AUCs for the prediction of 1-, 3- and 5-year CSS of our nomogram in the training cohort (0.755, 0.756, 0.736) and in the validation cohort (0.752, 0.751, 0.697) were shown in Fig. 2B and C, demonstrating the robustness of our model. Compared with the AUCs of the AJCC 8th staging system at 1-, 3-, 5-year (0.623, 0.657, 0.667 in the training cohort; 0.590, 0.648, 0.639 in the validation cohort), our nomogram showed higher AUCs. Calibration plots in Fig. 2D and E displayed good coherence between the predicted probability and the actual incidence of CSS. Hence, our prognostic nomogram showed good net benefits at different time points both in training cohort [Fig. 3(A–C)] and validation cohort [Fig. 3D–F] according to the DCA curves.
Fig. 2.
Nomogram to predict 1-, 3- and 5-year cancer-specific survival in patients with RLMS (A). Receiver-operating characteristic curves (ROCs) and area under the curves (AUCs) of the nomogram and the AJCC 8th Staging system for predicting cancer-specific survival at 1-, 3-, and 5-year in training (B) and validation (C) cohorts respectively. Calibration plots at 1-, 3-, and 5- year (D) in the training cohort and 1-, 3-, and 5- year (E) in the validation cohort.
Fig. 3.
Decision curve analysis for (A) 1-year net benefit in training cohort; (B) 3-year net benefit in training cohort; (C) 5-year net benefit in training cohort; (D) 1-year net benefit in validation cohort; (E) 3-year net benefit in validation cohort; (F) 5-year net benefit in validation cohort.
3.4. Risk stratification for CSS in patients with RLMS
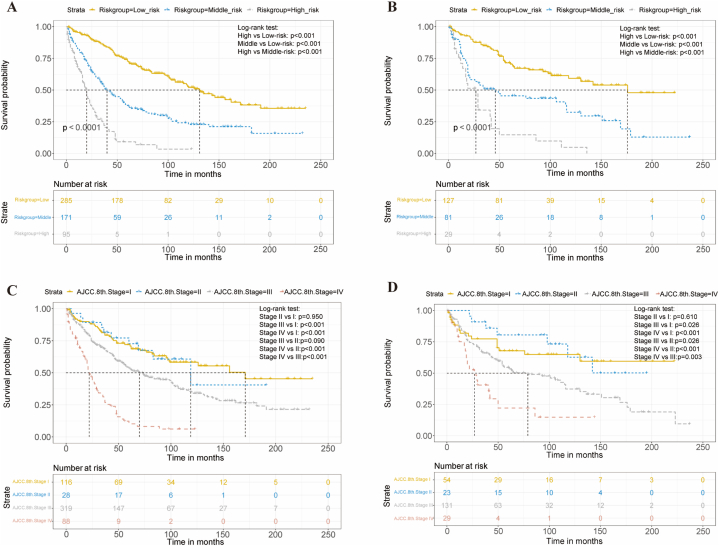
The nomogram score for each patient was calculated by summing the corresponding point of the four predictors (Table S1) and cut by 100 and 184 using the X-tile software (Fig. S2). According to the cancer-specific mortality risk, RLMS patients were then assigned to three risk subgroups: low (<100), middle (100–184), and high (>184) risk groups. In both the training and validation cohorts, Kaplan-Meier survival analysis showed significant discrimination between these subgroups. (Fig. 4A and B, p < 0.001). However, as shown in Fig. 4C and D, the AJCC 8th staging system had limited ability to distinguish patients with different tumor stages, particularly stage I and II.
Fig. 4.
Kaplan–Meier cancer-specific survival analysis of RLMS patients in low, middle, and high-risk groups according to the nomogram score in the training (A) and validation (B) cohorts. Kaplan–Meier cancer-specific survival analysis of RLMS patients stratified by the AJCC 8th Staging system in the training (C) and validation (D) cohorts.
4. Discussion
Previous studies preferred to mix different histological subtypes of RPS due to their rarity. Nevertheless, RPS should not be treated as a single homogeneous disease because of its diverse histology [1,2,18]. Increasing evidence supports that nomograms outperform traditional AJCC staging system in survival prediction for RPS [23,24]. The limitation of AJCC staging system mainly results from the neglect of histological differences and treatment regimens, which could be overcome by constructing a RLMS-specific nomogram and risk stratification.
Among all 578 deaths in this study, 28.7% occurred due to causes other than RLMS, which accounts for a great part of total deaths. To avoid overestimating the risk of disease-related deaths, Fine & Gray's method was employed to develop our nomogram for predicting CSS of RLMS, which was also applied by Nazzani et al. for surgically treated RPS [4] and Li et al. for retroperitoneal liposarcoma [20]. After univariate analysis of CSS, five predictors with significance were identified, including grade, tumor size, tumor range, chemotherapy and surgery status. However, chemotherapy was excluded after multivariate analysis.
Consistent with previous studies, RLMS patients with larger tumor size (>10 cm) and higher tumor grade (III/IV) had poor survival outcomes [25,26]. Results showed that the threshold 5-cm of the AJCC 8th T1/T2 has limited predictive value for RLMS patients, while 10-cm is more applicable. Surgical resection has been widely regarded as the mainstay therapy for all subtypes of RPS, which is also proven as a favorable factor for the CSS of RLMS in the study. Regardless of whether the tumor was primary or recurrent, patients with RLMS who underwent surgery had better outcomes than those who did not [27].
Since the occurrence and organ specificity of metastasis depends highly on tumor histology, RLMS preferred metastasis to distant organs (especially liver and lung), which largely contributes to decreased cancer-specific survival [28]. Likewise, patients in our study with distant tumor range were exposed to the highest risk of CSD, indicating a great importance of early diagnosis and treatment of RLMS. Although surgical margin status is not available in the SEER database, patients who have regional tumor range (invasion of adjacent structures) are more likely to have residual disease after resection, which has been emphasized in previous studies [29,30]. Lymph node metastasis (6.2%) is a rare-seen event and has no effect on CSS in RLMS as a result.
Several studies including randomized trials—STRASS-I (NCT01344018) and STREXIT have denied the benefit of preoperative radiotherapy for RLMS [14,15]. Likewise, radiotherapy was not a prognostic factor for RLMS in our study. Although showed certain efficacy in a group of RLMS patients, chemotherapy was found to have no influence on CSS after multivariate analysis. Further investigation showed the baseline difference that RLMS patients under 60 with advanced (Tumor grade: III/IV, Stage IV), metastatic (Tumor range: distant), and unresectable disease are more likely to receive chemotherapy (Table S2), which is consistent with clinical practice [9,10]. Thus, the univariate outcome of chemotherapy could be well explained by the late stage of the disease itself. Besides, to further investigate the role of preoperative chemotherapy in RLMS, a clinical trial-STRASS-II (NCT04031677) is now ongoing.
Despite the strong predictive power of the nomogram, our research has certain limitations. Firstly, although our study contains the largest population of RLMS patients, selection bias may exist due to the exclusion of patients with incomplete information and the retrospective nature of the SEER database. Secondly, there is room to further improve the nomogram due to limited variables in the SEER database. Lack of variables like recurrence, surgical margin, and multifocality of tumor deposits may result in the neglect of potential predictive value of these factors [29,31,32]. Moreover, adjuvant treatment regimens vary across centers and detailed information on them is not available, such as the dose and modality of adjuvant therapy. Thus, we only provide general information on the effect of chemotherapy and radiotherapy on RLMS patients. Lastly, it is necessary to estimate the dependability of our nomogram in an external cohort and further compare its efficacy with well-established nomograms. Given the rarity of RLMS, we will validate and optimize our nomogram in prospective clinical studies. Thus, this nomogram is only a guide for clinical decision-making and patient consultation on survival information.
5. Conclusions
In this study, a competing risk-based nomogram was constructed to predict the 1-, 3-, and 5-year CSS for RLMS patients. This individualized nomogram performs better than the AJCC 8th Staging system and can assist in the clinical management of RLMS, while external validation is needed.
Author contribution statement
Qian Fang; Guojing Cai: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Guizeng Chen; Xiang Xu; Haifeng Zeng: Contributed reagents, materials, analysis tools or data; Analyzed and interpreted the data.
Yulong He; Shirong Cai; Hui Wu: Conceived and designed the experiments.
Data availability statement
All data of this study can be obtained through the Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov): Incidence - SEER Research Plus Data, 18 Registries, Nov 2020 Sub (2000–2018). The login account was 10243-Nov2021.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This research was funded by the Guangdong Science and Technology Special Support Plan [No. KPT2020331].
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e16867.
Contributor Information
Shirong Cai, Email: caishr@mail.sysu.edu.cn.
Hui Wu, Email: wuhui3@mail.sysu.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Gamboa A.C., Gronchi A., Cardona K. Soft-tissue sarcoma in adults: an update on the current state of histiotype-specific management in an era of personalized medicine. CA A Cancer J. Clin. 2020;70(3):200–229. doi: 10.3322/caac.21605. [DOI] [PubMed] [Google Scholar]
- 2.Tan M.C., Brennan M.F., Kuk D., Agaram N.P., Antonescu C.R., Qin L.X., Moraco N., Crago A.M., Singer S. Histology-based classification predicts pattern of recurrence and improves risk stratification in primary retroperitoneal sarcoma. Ann. Surg. 2016;263(3):593–600. doi: 10.1097/SLA.0000000000001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jo V.Y., Fletcher C.D. WHO classification of soft tissue tumours: an update based on the 2013 (4th) edition. Pathology. 2014;46(2):95–104. doi: 10.1097/PAT.0000000000000050. [DOI] [PubMed] [Google Scholar]
- 4.Nazzani S., Preisser F., Bandini M., Marchioni M., Tian Z., Soulieres D., Montanari E., Ratti D., Acquati P., Briganti A., et al. Surgically treated retroperitoneal sarcoma: a population-based competing risks analysis. Eur. Urol. Oncol. 2018;1(4):346–351. doi: 10.1016/j.euo.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 5.Bonvalot S., Raut C.P., Pollock R.E., Rutkowski P., Strauss D.C., Hayes A.J., Van Coevorden F., Fiore M., Stoeckle E., Hohenberger P., et al. Technical considerations in surgery for retroperitoneal sarcomas: position paper from E-Surge, a master class in sarcoma surgery, and EORTC-STBSG. Ann. Surg Oncol. 2012;19(9):2981–2991. doi: 10.1245/s10434-012-2342-2. [DOI] [PubMed] [Google Scholar]
- 6.Blay J.Y., Honore C., Stoeckle E., Meeus P., Jafari M., Gouin F., Anract P., Ferron G., Rochwerger A., Ropars M., et al. Surgery in reference centers improves survival of sarcoma patients: a nationwide study. Ann. Oncol. 2019;30(8):1407. doi: 10.1093/annonc/mdz170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miah A.B., Hannay J., Benson C., Thway K., Messiou C., Hayes A.J., Strauss D.C. Optimal management of primary retroperitoneal sarcoma: an update. Expert Rev. Anticancer Ther. 2014;14(5):565–579. doi: 10.1586/14737140.2014.883279. [DOI] [PubMed] [Google Scholar]
- 8.Gronchi A., Strauss D.C., Miceli R., Bonvalot S., Swallow C.J., Hohenberger P., Van Coevorden F., Rutkowski P., Callegaro D., Hayes A.J., et al. Variability in patterns of recurrence after resection of primary retroperitoneal sarcoma (RPS): a report on 1007 patients from the multi-institutional collaborative RPS working group. Ann. Surg. 2016;263(5):1002–1009. doi: 10.1097/SLA.0000000000001447. [DOI] [PubMed] [Google Scholar]
- 9.Seddon B., Strauss S.J., Whelan J., Leahy M., Woll P.J., Cowie F., Rothermundt C., Wood Z., Benson C., Ali N., et al. Gemcitabine and docetaxel versus doxorubicin as first-line treatment in previously untreated advanced unresectable or metastatic soft-tissue sarcomas (GeDDiS): a randomised controlled phase 3 trial. Lancet Oncol. 2017;18(10):1397–1410. doi: 10.1016/S1470-2045(17)30622-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Management of metastatic retroperitoneal sarcoma: a consensus approach from the trans-atlantic retroperitoneal sarcoma working group (TARPSWG) Ann. Oncol. 2018;29(4):857–871. doi: 10.1093/annonc/mdy052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi Y., Yun M.S., Lim S.H., Lee J., Ahn J.-H., Kim Y.J., Park K.H., Park Y.S., Lim H.Y., An H., et al. Gemcitabine and docetaxel combination for advanced soft tissue sarcoma: a nationwide retrospective study. Cancer Res. Treat. 2018;50(1):175–182. doi: 10.4143/crt.2016.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gronchi A., Strauss D.C., Miceli R., Bonvalot S., Swallow C.J., Hohenberger P., Van Coevorden F., Rutkowski P., Callegaro D., Hayes A.J., et al. Variability in patterns of recurrence after resection of primary retroperitoneal sarcoma (RPS): a report on 1007 patients from the multi-institutional collaborative RPS working group. Ann. Surg. 2016;263(5):1002–1009. doi: 10.1097/SLA.0000000000001447. [DOI] [PubMed] [Google Scholar]
- 13.Miura J.T., Charlson J., Gamblin T.C., Eastwood D., Banerjee A., Johnston F.M., Turaga K.K. Impact of chemotherapy on survival in surgically resected retroperitoneal sarcoma. Eur. J. Surg. Oncol. 2015;41(10):1386–1392. doi: 10.1016/j.ejso.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 14.Bonvalot S., Gronchi A., Le Péchoux C., Swallow C.J., Strauss D., Meeus P., van Coevorden F., Stoldt S., Stoeckle E., Rutkowski P., et al. Preoperative radiotherapy plus surgery versus surgery alone for patients with primary retroperitoneal sarcoma (EORTC-62092: STRASS): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2020;21(10):1366–1377. doi: 10.1016/S1470-2045(20)30446-0. [DOI] [PubMed] [Google Scholar]
- 15.Callegaro D., Raut C.P., Ajayi T., Strauss D., Bonvalot S., Ng D., Stoeckle E., Fairweather M., Rutkowski P., van Houdt W.J., et al. Preoperative radiotherapy in patients with primary retroperitoneal sarcoma: EORTC-62092 trial (STRASS) versus off-trial (STREXIT) results. Ann. Surg. 2022 doi: 10.1097/SLA.0000000000005492. [DOI] [PubMed] [Google Scholar]
- 16.Tawbi H.A., Burgess M., Bolejack V., Van Tine B.A., Schuetze S.M., Hu J., D'Angelo S., Attia S., Riedel R.F., Priebat D.A., et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): a multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol. 2017;18(11):1493–1501. doi: 10.1016/S1470-2045(17)30624-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gronchi A., Miceli R., Shurell E., Eilber F.C., Eilber F.R., Anaya D.A., Kattan M.W., Honore C., Lev D.C., Colombo C., et al. Outcome prediction in primary resected retroperitoneal soft tissue sarcoma: histology-specific overall survival and disease-free survival nomograms built on major sarcoma center data sets. J. Clin. Oncol. 2013;31(13):1649–1655. doi: 10.1200/JCO.2012.44.3747. [DOI] [PubMed] [Google Scholar]
- 18.Ardoino I., Miceli R., Berselli M., Mariani L., Biganzoli E., Fiore M., Collini P., Stacchiotti S., Casali P.G., Gronchi A. Histology-specific nomogram for primary retroperitoneal soft tissue sarcoma. Cancer. 2010;116(10):2429–2436. doi: 10.1002/cncr.25057. [DOI] [PubMed] [Google Scholar]
- 19.Fine J.P., Gray R.J. A proportional hazards model for the subdistribution of a competing risk. J. Am. Stat. Assoc. 1999;94(446):496–509. [Google Scholar]
- 20.Li Y., Wu G., Zhang Y., Yang W., Wang X., Duan L., Niu L., Chen J., Zhou W., Liu J., et al. Development and validation of a prognostic model to predict the prognosis of patients with retroperitoneal liposarcoma: a large international population-based cohort study. Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.857827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luo X., Liu S., Chen Y., Qiu X., Huang D., Zhang D., Liang Q., Yang Y., Zeng X. Predicting cancer-specific mortality in patients with parotid gland carcinoma by competing risk nomogram. Head Neck. 2021;43(12):3888–3898. doi: 10.1002/hed.26890. [DOI] [PubMed] [Google Scholar]
- 22.Cronin K.A., Ries L.A.G., Edwards B.K. The surveillance, Epidemiology, and End results (SEER) Program of the national cancer institute. Cancer. 2014;120(Suppl 23):3755–3757. doi: 10.1002/cncr.29049. [DOI] [PubMed] [Google Scholar]
- 23.Cates J.M.M. Performance analysis of the American joint committee on cancer 8th edition staging system for retroperitoneal sarcoma and development of a new staging algorithm for sarcoma-specific survival. Ann. Surg Oncol. 2017;24(13):3880–3887. doi: 10.1245/s10434-017-6116-8. [DOI] [PubMed] [Google Scholar]
- 24.Huggett B.D., Cates J.M.M. The Vanderbilt staging system for retroperitoneal sarcoma: a validation study of 6857 patients from the National Cancer Database. Mod. Pathol. 2019;32(4):539–545. doi: 10.1038/s41379-018-0166-8. [DOI] [PubMed] [Google Scholar]
- 25.An J.Y., Heo J.S., Noh J.H., Sohn T.S., Nam S.J., Choi S.H., Joh J.W., Kim S.J. Primary malignant retroperitoneal tumors: analysis of a single institutional experience. Eur. J. Surg. Oncol. 2007;33(3):376–382. doi: 10.1016/j.ejso.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 26.Gladdy R.A., Qin L.X., Moraco N., Agaram N.P., Brennan M.F., Singer S. Predictors of survival and recurrence in primary leiomyosarcoma. Ann. Surg Oncol. 2013;20(6):1851–1857. doi: 10.1245/s10434-013-2876-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ikoma N., Torres K.E., Lin H.Y., Ravi V., Roland C.L., Mann G.N., Hunt K.K., Cormier J.N., Feig B.W. Recurrence patterns of retroperitoneal leiomyosarcoma and impact of salvage surgery. J. Surg. Oncol. 2017;116(3):313–319. doi: 10.1002/jso.24667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okamoto M., Matsuoka M., Soma T., Arai R., Kato H., Harabayashi T., Adachi H., Shinohara T., Sagawa T., Nishiyama N., et al. Metastases of soft tissue sarcoma to the liver: a historical cohort study from a hospital-based cancer registry. Cancer Med. 2020;9(17):6159–6165. doi: 10.1002/cam4.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singer S., Antonescu C.R., Riedel E., Brennan M.F. Histologic subtype and margin of resection predict pattern of recurrence and survival for retroperitoneal liposarcoma. Ann. Surg. 2003;238(3):358–370. doi: 10.1097/01.sla.0000086542.11899.38. ; discussion 370-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kirane A., Crago A.M. The importance of surgical margins in retroperitoneal sarcoma. J. Surg. Oncol. 2016;113(3):270–276. doi: 10.1002/jso.24135. [DOI] [PubMed] [Google Scholar]
- 31.Anaya D.A., Lahat G., Wang X., Xiao L., Pisters P.W., Cormier J.N., Hunt K.K., Feig B.W., Lev D.C., Pollock R.E. Postoperative nomogram for survival of patients with retroperitoneal sarcoma treated with curative intent. Ann. Oncol. 2010;21(2):397–402. doi: 10.1093/annonc/mdp298. [DOI] [PubMed] [Google Scholar]
- 32.Anaya D.A., Lahat G., Liu J., Xing Y., Cormier J.N., Pisters P.W., Lev D.C., Pollock R.E. Multifocality in retroperitoneal sarcoma: a prognostic factor critical to surgical decision-making. Ann. Surg. 2009;249(1):137–142. doi: 10.1097/SLA.0b013e3181928f2f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data of this study can be obtained through the Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov): Incidence - SEER Research Plus Data, 18 Registries, Nov 2020 Sub (2000–2018). The login account was 10243-Nov2021.