Abstract
Introduction:
Parkinson disease (PD) results from the destruction of dopaminergic neurons in the brain. This study aimed to investigate the protective effects of natural antioxidants such as caffeic acid phenethyl ester (CAPE) to maintain these neurons.
Methods:
CAPE is one of the main ingredients of propolis. Intranasal administration of 1-methyl-4-phenyl-2;3;4;6-tetrahydropyridine (MPTP) was used to generate a PD model in rats. A total of 2×bone marrow stem cells (BMSCs) were injected from the tail vein. Behavioral tests, immunohistochemistry, DiI, cresyl fast violet, and TUNEL staining were used to evaluate the rats 2 weeks after treatment.
Results:
In all treatment groups with stem cells, the DiI staining method revealed that the cells migrated to the substantia nigra pars compacta after injection. Treatment with CAPE significantly protects dopaminergic neurons from MPTP. The highest number of tyrosine hydroxylase (TH) positive neurons was seen in the pre-CAPE+PD+stem cell (administration of CAPE, then the creation of PD, finally injection of stem cells) group. The number of TH+cells in all groups that received CAPE was significant compared to groups that received the stem cells only (P<0.001). Intranasal administration of MPTP significantly increases the number of apoptotic cells. The lowest number of apoptotic cells was in the CAPE+PD+stem cell group.
Conclusion:
The results showed that the use of CAPE and stem cells in Parkinson rats caused a significant reduction in the apoptotic cells.
Keywords: Parkinson disease, MPTP, Antioxidant, Caffeic acid phenethyl ester, TUNNEL staining
Highlights
Caffeic acid phenethyl ester (CAPE) is a natural antioxidant and a powerful neuronal protector due to its anti-inflammatory properties.
CAPE plus bone marrow stem cells can reduce apoptotic cells in the striatum and sustantia nigra.
Pretreatment with CAPE protects dopaminergic neurons from degeneration.
Plain Language Summary
The Parkinson model caused by the injection of 1-methyl-4-phenyl-2;3;4;6-tetrahydropyridine (MPTP) is used as a standard model. MPTP causes a significant reduction of dopaminergic neurons in the substantia nigra. Caffeic acid phenethyl ester (CAPE) has an anti-inflammatory effect, effectively contributing to the collection of free radicals and removing inflammatory agents, and subsequently improving the disease. Stem cells can regulate immune responses and differentiate into special cells to replace injured cells. By inhibiting apoptotic pathways, these cells create tropical factors to protect and repair cells.
1. Introduction
Parkinson Disease (PD) is caused by central nervous system destruction (Badban et al., 2015; Dauer & Przedborski, 2003). PD results from the destruction or malfunction of dopaminergic secretory neurons in the substantia Nigra Pars Compacta (SNpc) in the midbrain. The loss of dopamine-secreting cells in the corpus luteum depends on several factors (Safari et al., 2016). Symptoms of Parkinson disease appear when at least 80% of dopaminergic neurons are destroyed (Braak et al., 2004). The goal of treatment should be to protect the remaining neurons or to replace the damaged neurons with stem cells (Hald et al., 2007). There are several treatments for Parkinson disease that are used to relieve symptoms. The most commonly used drug is levodopa. In the early stages of the disease, this drug improves symptoms, but it gradually causes memory, learning, and sleep disorders (Cools et al., 2003). Studies have shown that the use of antioxidants such as propolis to protect neurons and the use of stem cells to replace damaged cells is helpful (Dantuma et al., 2010; Pellegrini et al., 2003).
1-Methyl-4-phenyl-2;3;4;6-tetrahydropyridine (MPTP) is widely used to create an animal model of Parkinson disease. Intranasal injection of this toxin effectively and selectively destroys the dopaminergic neurons (Prediger et al., 2006). Propolis is a waxy substance and a bee product, it has strong antibacterial, antifungal, anti-inflammatory, anti-parasitic, and antioxidant properties, and the most important of its active antioxidant is caffeic acid phenethyl ester. CAPE inhibits lipid peroxidation and lipoxygenase activities (Sud'Ina et al., 1993). Stem cell use is a promising treatment for neurodegenerative diseases. Mesenchymal stem cells have advantages over other stem cells because of their high proliferative power (Jadidi et al., 2016), easy preparation, no moral problems, and no transplant rejection (Jäger et al., 2010). Stem cells can self-renew and differentiate into all types of cells, including blood, nervous, and cartilage cells (Glavaski-Joksimovic & Bohn, 2013). Bone marrow stem cells (BMSCs) transplanted into the adult brain exhibit characteristics of microglia, astrocytes, and neuronal-like cells (Li et al., 2001). The purpose of this study was to examine the neuroprotective effect of CAPE as pre-treatment and co-treatment with BMSCs on a dopaminergic neuron in the midbrain in a rat model of Parkinson disease.
2. Materials and Methods
Study animals
Adult male Wistar albino rats weighing 200–250 g were prepared from the stem cells research center of Semnan University of Medical Sciences, Semnan, Iran. All rats were kept in separate cages. They also had free access to water and food. The room temperature and humidity were constant, and the light was on for 12 h. All principles of working with animals were followed per the National Institutes of Health Guide for Care and Use of Laboratory Animals authorized by the Ethics Committee (ethical code: IR.SEMUMS.REC.1395.153) of Semnan University of Medical Sciences, Semnan City, Iran. Rats were randomly assigned to 7 groups (n=7 for each group). The first group received normal saline as the control group or the sham-control group. The second group received intranasal MPTP (2 mg, 12 μL) as the Parkinson group without receiving treatment. The third group received MPTP, and 1 week after the creation of the PD model, received intravenous stem cells (from the caudal vein,). The fourth group received intraperitoneal CAPE (10 μM) as a pre-treatment 2 weeks before PD. The fifth group received CAPE as a pre-treatment 2 weeks before PD induction and 1 week after PD received intravenous stem cells. The sixth group received stem cells 1 week after PD and 2 weeks later CAPE as a co-treatment. The seventh group received CAPE 1 week before PD and 1 week after PD as a co-treatment.
Study methods
Intranasal administration of MPTP
The intranasal (IN) injection procedure was done according to the method described by Prediger (Prediger et al., 2006). In summary, rats were lightly anesthetized, and from a PE-50 tube, a 10-mm piece was prepared and inserted into the nostril. The pipe was connected to a manual injection pump, and 12 μL of neurotoxin was injected into the nostrils. The MPTP HCL (Sigma Chemical Co., USA) was dissolved in 0.9% NaCl (saline). After which it was infused for 8 minutes, animals were given 1-minute time to get normal breathing function, and then the operation was performed by injection using the opposite nostril. To receive a 2 mg dose, 12 μL of the toxin was injected into each nostril.
Behavioral test
The pole test was originally presented by Ogawa (Ogata et al., 1997; Sedelis et al., 2001) to appraise MPTP-induced bradykinesia in an animal model of PD. The test includes an approximately 50 cm vertical pole (1 cm diameter) with a small sphere on the above. The animals are placed on the sphere while their heads are up. In this test, the time it takes until the animals descend to the floor is checked. This test consists of 6 degrees. Grade 1: The animal cannot maintain balance and falls. Grade 2: The animal is hardly balanced at the pole, takes less than ten centimeters, and falls. Grade 3: The animal retains its balance on the pole but cannot walk more than 10 cm. Grade 4: The animal moves through the pole using a paw and jumps on its hind legs. Grade 5: The animal passes naturally from the pole but has a 3- or 4-foot slip. Grade 6: The animal naturally extends from the pole and has less than a 3- or 4-foot slip. Animals in grades 5 or 6 are healthy. Tests were taken in two stages. The first stage is one week after the creation of the PD model as pretreatment, and the second stage is 2 weeks after the creation of the model and the end of the treatment as a post-treatment test.
TH-immunohistochemistry
We used the immunohistochemical staining method to study the number of dopaminergic neurons of SNpc. Animals were killed 28 days after creating the Parkinson model, the rat brain was removed, and after the tissue processing, the middle cerebral area was evaluated by immunohistochemistry. In tissue sections, after washing with PBS buffer and retrieving the antigen, 2N hydrochloric acid was poured on the samples over minutes. Then, to penetrate the cell membrane, 0.3% Triton was used. Samples were washed with PBS, and to block the secondary antibody response, 10% goat serum was added for 30 minutes. The diluted primary antibody (anti-tyrosine hydroxylase [1:100, Abcam] monoclonal to tyrosine hydroxylase) was added to the sample and placed at a temperature of 2°C to 8°C for one night. The next day, the secondary antibody (FITC Anti-Mouse IgG2a-gamma chain [1:200, Abcam]) was added to samples and then incubated at 37°C for 1.5 h in the darkness. Then, DAPI (4′,6-diamidino-2-phenylindole) staining was used. Finally, the samples were examined by the Olympus fluorescent microscope (400×magnification).
DiI staining
DiI staining has been developed to help the morphological recognition of neurons. In this method, for cells suspended in the culture medium (DMEM), add 5 μL/mL DiI (1, 1′-dioctadecyl-3, 3, 3′, 3′-tetramethylenedocarbocyanine perchlorate) and incubate for 20 minutes. Then cells were centrifuged at 1200 rpm for 5 min. Remove the supernatant and rinse the pellet slowly with PBS and dissolve with 15 μL of culture medium. The solution should be injected as soon as possible in this study, 2×cells were injected into the dorsal caudal vein.
TUNEL staining
For this staining, we used the detection kit of POD from (Roche Company). At first, the samples were incubated in proteinase K for 15–30 minutes at 37°C. For the permeabilization of the samples, we used 0.1% Triton x-100 and 0.1% sodium citrate. After that, the sections were incubated in the main TUNEL solution (containing 450 μL of label solution with 50 μL of enzyme solution) for 1 hour at 37°C. Finally, after washing, the samples were observed under a fluorescent microscope (400×magnification). In negative control groups, the sections were incubated only with label solution instead of the TUNEL reaction mixture.
Cell culture
To produce mesenchymal stem cells, the tibias and femur rat bone marrow were first removed under anesthesia. The cells were incubated in culture media of Dulbecco's Modified Eagle Medium (DMEM, 5 mL, Invitrogen), 5% CO2 at 37°C in an incubator that was supplemented with fetal bovine serum (FBS, 10%, 1 mL) with penicillin-streptomycin (50 λ). After 72 h, the supernatant was removed, and adherent cells were cultured to produce BMSCs (Badban et al., 2015). After the third passage, fat and fibroblast were removed, and only the BMSCs could survive (Badban et al., 2015). Finally, 2× cells were used for injection after counting.
Statistical analysis
All data were reported as mean±SEM. Statistical analysis was done using multiple-comparison post hoc tests in SPSS. P values of less than 0.05 were considered significant. Each tissue was analyzed by 1-way ANOVA and followed by the Tukey post hoc test.
3. Results
Behavioral examination
Results of the behavioral test show that the grade of the test after treatment was increased in all treatment groups (F2,12=15.36, P≤0.05; Figure 1). Post-treatment grade on the MPTP group was 2.4±0.2, the smallest amount among all groups. The highest grade belonged to the group of pre-CAPE+PD+stem cells (administration of CAPE, then the creation of PD, finally injection of stem cells) therapy (4.6±1.2). In all groups, the animals could hardly maintain their balance at the top of the rod. In groups using only stem cells or CAPE, the results were not significant compared to the Parkinson group (2.9±0.6 vs 2.4±0.2) (P≤0.01). In groups using CAPE as a pre-treatment, the results were significant compared to the Parkinson group (3.5±0.4 vs 2.4±0.2) (P≤0.001). All treatment groups were compared with the PD group. In all treatment groups except BMSCs, after treatment have a significant difference with the PD group (P≤0.05).
Figure 1.
Improving behavioral pole test before and after treatment with Caffeic Acid Phenethyl Ester (CAPE)
One-way ANOVA and Tukey post hoc test (n=7) showed that behavioral improvement was significantly higher in pre-treatment with CAPE groups than in other treatment groups. All values are Mean±SEM. *P≤0.001compared with the MPTP (1-methyl-4-phenyl-2;3;4;6-tetrahydropyridine) (PD) group.
DiI staining
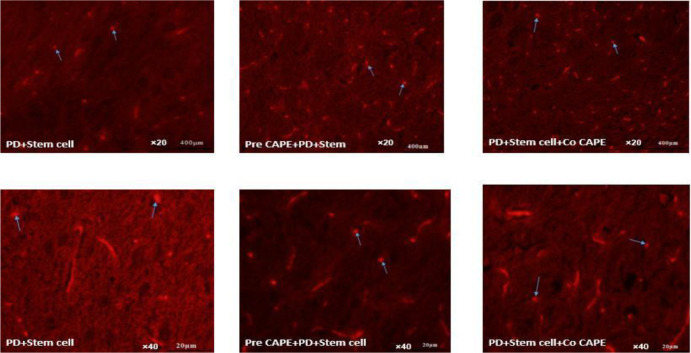
DiI labeling was carried out to evaluate the site of injected cells. The results showed that the injected cells were replaced in different areas, but most were located in the affected areas (Figure 2). Using stem cells along with CAPE has increased the number of injected cells in the affected area. The average injected cells in the SNpc treatment groups that used CAPE compared to stem cells were higher and more significant (P≤0.05).
Figure 2.
Migration of injected cells to the damaged area of the Substantia Nigra Pars Compacta (SNpc)
Arrows represent Bone Marrow Stem Cells (BMSCs) (2×106) labeled with DiI in the substantia nigra of the mid-brain in rats (×20: Scale bar 400 μm, 40× magnification: scale bar 20 μm). The results show that in the pre-CAPE+PD+stem cells treatment group, the number of stained cells with DiI is higher than in other treatment groups. The images were at 20× and 40× magnifications.
TH-immunohistochemistry
TH-immunohistochemistry staining was done for the evaluation of the dopamine-secreting neurons. In the normal group, the number of TH-positive neurons in the SNpc was significantly higher than in the striatum area (F5,24=55.08, 28±2.3 vs F5,24=41.03, 23±1.9; P≤0.001). Intranasal administration of MPTP significantly reduced the number of TH-positive cells in SNpc and striatum of all groups (P≤0.001) (Figures 3 and 4). The highest number of neuronal reductions was seen in the Parkinson group (11±1.94; P≤0.001). This neuronal decline was seen in all groups, but the number of neurons in all therapeutic groups was higher than that in the PD group. The highest number of TH + neurons was seen in the group of pre-CAPE+PD+stem cells (SNpc: 23.40±1.14, striatum: 21±1.19; P≤0.001) and pre-CAPE (SNpc: 23±2.1 striatum 19±1.1; P≤0.001). In the groups that used stem cells with CAPE, the number of TH +neurons was more than those using just stem cells (15.80±1.64; P≤0.001). There was a significant difference in the number of TH+ cells in therapeutic groups that received pre-CAPE compared to the stem cells only (P<0.001). In the treatment group that received only CAPE, the number of TH+ neurons was also higher than the group of stem cells only (15.1±1.13vs 14±1.64; P≤0.001). This result can indicate that CAPE has a great protective role for tyrosine hydroxylase neurons against MPTP.
Figure 3.
Caffeic Acid Phenethyl Ester (CAPE) increasing the number of dopamine-positive cells in the Substantia Nigra Pars Compacta (SNpc)
One-way ANOVA and Tukey post hoc test (n=7) showed that the number of tyrosine hydroxylase positive neurons in the pretreatment treatment group increased significantly and decreased significantly in the Parkinson group compared to the other groups. All values are Mean±SEM. * P≤ 0.001 compared with the MPTP (1-methyl-4-phenyl-2;3;4;6-tetrahydropyridine) (PD) group.
Figure 4.
Caffeic acid phenethyl ester (CAPE) and bone marrow stem cells (BMSCs) increasing the number of dopamine-positive cells in the Substantia Nigra Pars Compacta (SNpc)
Immunohistochemical images showed that using CAPE before PD could significantly protect dopamine-secreting neurons. Also, in the groups that received CAPE or stem cells separately after PD, there was no significant difference in the number of positive dopamine-secreting neurons. The squares in all the pictures show the border of the SNpc. Groups in figures included A: PD group, B: PD plus stem cell, C: Pre-CAPE plus PD, D: Pre-CAPE+PD+stem cell, E: PD+Stem cell+CAPE, and F: PD+CAPE. The images were at a 20×magnification. The results were evaluated using the 1-way ANOVA and the Tukey post hoc (n=7).
TUNEL staining
This protocol detects and quantifies apoptotic cells in SNpc and striatum. The intranasal administration of MPTP significantly increases the number of apoptotic cells in the SNpc and striatum (F5,24=62.75, SNpc: 24.8±2.9; F5,24=44.43 striatum: 22.8±3.1; P≤0.001). Apoptotic cells were observed in all groups (Figure 5). The highest number of apoptotic cells belonged to the Parkinson group (24.8±2.9; P≤0.001), and the lowest number to the group pre-CAPE+PD+stem cell (SNpc: 13.40±1.14, striatum: 16.8±2.6; P≤0.001). The results show that using CAPE as a pre-treatment will significantly reduce the number of apoptotic cells in the treatment groups (P≤0.001). In other treatment groups, a decrease in the number of apoptotic cells was observed, but the results were not significant.
Figure 5.
Caffeic acid phenethyl ester (CAPE) reducing the number of apoptotic cells in substantia nigra pars compacta (SNpc)
The results of 1-way ANOVA and Tukey post hoc test (n=7). The results showed that in the pretreatment groups with CAPE alone or with stem cells, the number of apoptotic cells will be significantly reduced. In other treatment groups, although there is a decrease in apoptotic cells, there is no significant difference with the Parkinson group. * P≤0.001 compared with the MPTP (1-methyl-4-phenyl-2;3;4;6-tetrahydropyridine) (PD) group.
4. Discussion
Numerous studies have shown that the main cause of Parkinson disease is the destruction of dopamine-secreting neurons in the substantia nigra. Dopamine plays a role as a neurotransmitter in motion, so motor disorders occur in Parkinson disease (Braak et al., 2004). One of our goals in this study is to show whether the CAPE as a powerful antioxidant can protect dopaminergic neurons against the MPTP neurotoxin. MPTP neurotoxin decreases dopaminergic neurons and creates behavioral and non-behavioral defects. The present study shows that the intranasal administration of MPTP causes a significant reduction of dopaminergic neurons in the substantia nigra. In the brain, MPTP is rapidly converted to MPP+(1-methyl-4-phenylpyridinium) by the enzyme MAO-B, the majority in glial cells. This ion does not cross the cytoplasmic membrane freely. This toxin has a strong tendency to bind to the dopamine transporter (DAT). Therefore, they are captured in dopaminergic terminals. In dopamine-secreting neurons, MPP+ cumulate in mitochondria and then inhibit complex-I of the electron transport chain, and lead to reduce the level of ATP and an increase in the level of reactive oxygen species (ROS), particularly superoxide. ROS production appears to be one of the first incidents of MPP+neural toxicity. Although it will not cause cell death directly, it can cause cell death by stimulating some mechanisms (Prediger et al., 2010). In this study, results showed that CAPE could protect neurons against MPTP. This neuroprotection is due to its strong antioxidant properties. Oxidative stress plays a role in the pathogenesis of Parkinson disease. Therefore, CAPE probably protects neurons by removing free radicals (Song et al., 2012).
In a study conducted in 2007 (McGeer & McGeer, 2007) on the pathogenesis of Parkinson disease, chronic inflammation occurs in the basal ganglia. In inflammatory conditions, activated glial cells produce large amounts of free radicals and toxins for neurons. Since the dopaminergic neurons are very sensitive to free radicals. Due to the release of free radicals by active glial, dopaminergic neurons can be seriously damaged. CAPE has an anti-inflammatory effect, effectively contributing to the collection of free radicals and removing inflammatory agents, subsequently improving the disease. CAPE has a different mechanism of action; for example, CAPE prevents the formation of oxygen free radicals (Russo et al., 2002). Blocks MPTP-increase the amount of mid-brain iNOS and caspase 1. CAPE can block the MPTP-induced loss of striatal DA, MPP+ induced neurotoxicity and the MPP+ induced cytochrome C release (Fontanilla et al., 2011).
In this study, CAPE could improve pathological and behavioral symptoms in PD groups. This study showed that CAPE has better efficacy in pre-treatment. The other aim of this project is to use stem cells to transfer them to the damaged black body and replace them with damaged dopaminergic neurons. However, the simultaneous use of CAPE and stem cells can be used to protect stem cells using the different mechanisms mentioned. Stem cells have significant properties. Stem cells are proliferative cells that can be divided into various cells, and these cells also can self-renew (Elbana et al., 2015). Stem cells play a role in the treatment of various diseases, including neurodegenerative diseases such as Parkinson disease (Dantuma et al., 2010; Maltman et al., 2011). Stem cells can regulate immune responses (Zhou et al., 2014) and differentiate into special cells to replace injured cells (Zeng et al., 2011). By inhibiting apoptotic pathways, these cells create tropical factors to protect and repair cells (Jäger et al., 2010). We observed that the labeled cells have entered the midbrain and are located in different areas. Most cells were located in the substantia nigra. The results indicate that these cells can cross the blood-brain barrier (Jadidi et al., 2016) and be located in damaged areas. It is not well-known that these cells can differentiate into other cells or produce dopamine. Probably, the secretion of chemical factors such as cytokines from damaged cells drags the stem cells toward the affected area. Migrated cells under the influence of chemical factors are differentiated into dopaminergic neurons and produce dopamine. Due to the above characteristics, stem cells will be able to replace the damaged dopaminergic neurons of the substantia nigra in the brainstem. The use of CAPE with these cells can play an important role in repairing the damaged area in Parkinson disease.
5. Conclusion
The results of this study showed that the use of CAPE could protect dopamine-secreting neurons in the SNpc of the brain. This effect can be achieved by inhibiting inflammatory factors.
Acknowledgments
Thanks to the staff of the neural Stem Cell Research Center and vice chancellor of research of Semnan University of Medical Sciences.
Ethical Considerations
Compliance with ethical guidelines
All ethical principles were considered in this article.
Funding
This project was supported by Grant number 1142 from the Semnan University of Medical Sciences and Health Services, and the (Grant No.: 93033267) from the Iran National Science Foundation (INSF), Tehran, Iran.
Authors' contributions
All authors equally contributed to preparing all parts of the research.
Conflict of interest
The authors declared no competing interest.
References
- Badban L., Safari M., Sameni H. R., Bandegi A. R., Vafaei A. A., Rashidy-Pour A., et al. (2015). Protective effects of water extract of propolis on dopaminergic neurons, brain derived neurotrophic factor and stress oxidative factors in the rat model of parkinson's disease. International Journal of Pharmacology, 11(4), 300–308. [DOI: 10.3923/ijp.2015.300.308] [DOI] [Google Scholar]
- Braak H., Ghebremedhin E., Rüb U., Bratzke H., Del Tredici K. (2004). Stages in the development of Parkinson's disease-related pathology. Cell and Tissue Research, 318(1), 121–134. [PMID] [DOI] [PubMed] [Google Scholar]
- Cools R., Barker R. A., Sahakian B. J., Robbins T. W. (2003). L-Dopa medication remediates cognitive inflexibility, but increases impulsivity in patients with Parkinson's disease. Neuropsychologia, 41(11), 1431–1441. [PMID] [DOI] [PubMed] [Google Scholar]
- Dantuma E., Merchant S., Sugaya K. (2010). Stem cells for the treatment of neurodegenerative diseases. Stem Cell Research & Therapy, 1(5), 37. [PMID] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauer W., Przedborski S. (2003). Parkinson's disease: Mechanisms and models. Neuron, 39(6), 889–909. [PMID] [DOI] [PubMed] [Google Scholar]
- Elbana A. M., Abdel-Salam S., Morad G. M., Ibrahim M., Omran A. A. (2015). Endogenous bone marrow stem cell mobilization in rats: Its potential role in homing and repair of damaged inner ear. Egyptian Journal of Ear, Nose, Throat and Allied Sciences, 16(1), 55–67. [DOI: 10.1016/j.ejenta.2013.01.003] [DOI] [Google Scholar]
- Fontanilla C. V., Ma Z., Wei X., Klotsche J., Zhao L., Wisniowski P., et al. (2011). Caffeic acid phenethyl ester prevents 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-induced neurodegeneration. Neuroscience, 188, 135–141. [PMID] [DOI] [PubMed] [Google Scholar]
- Glavaski-Joksimovic A., Bohn M. C. (2013). Mesenchymal stem cells and neuroregeneration in Parkinson's disease. Experimental Neurology, 247, 25–38. [PMID] [DOI] [PubMed] [Google Scholar]
- Hald A., Beek J. V., Lotharius J. (2007). Inflammation in parkinson's disease. In Harris R. E., Bittman R., Dasgupta D., Engelhardt H., Flohe L., Herrmann H., et al. (Eds.), Inflammation in the pathogenesis of chronic diseases (pp. 249–279). Dordrecht: Springer. [Link] [Google Scholar]
- Jadidi M., Biat S. M., Sameni H. R., Safari M., Vafaei A. A., Ghahari L. (2016). Mesenchymal stem cells that located in the electromagnetic fields improves rat model of Parkinson's disease. Iranian Journal of Basic Medical Sciences, 19(7), 741–748. [PMID] [PMC free article] [PubMed] [Google Scholar]
- Jäger M., Hernigou P., Zilkens C., Herten M., Li X., Fischer J., et al. (2010). Cell therapy in bone healing disorders. Orthopedic reviews, 2(2), e20. [PMID] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Chen J., Wang L., Zhang L., Lu M., Chopp M. (2001). Intracerebral transplantation of bone marrow stromal cells in a 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyri-dine mouse model of Parkinson's disease. Neuroscience Letters, 316(2), 67–70. [PMID] [DOI] [PubMed] [Google Scholar]
- Maltman D. J., Hardy S. A., Przyborski S. A. (2011). Role of mesenchymal stem cells in neurogenesis and nervous system repair. Neurochemistry International, 59(3), 347–356. [PMID] [DOI] [PubMed] [Google Scholar]
- McGeer E. G., McGeer P. L. (2007). The role of anti-inflammatory agents in Parkinson's disease. CNS Drugs, 21(10), 789–797. [PMID] [DOI] [PubMed] [Google Scholar]
- Ogata A., Tashiro K., Nukuzuma S., Nagashima K., Hall W. W. (1997). A rat model of Parkinson's disease induced by Japanese encephalitis virus. Journal of Neurovirology, 3(2), 141–147. [PMID] [DOI] [PubMed] [Google Scholar]
- Pellegrini N., Serafini M., Colombi B., Del Rio D., Salvatore S., Bianchi M., et al. (2003). Total antioxidant capacity of plant foods, beverages and oils consumed in Italy assessed by three different in vitro assays. The Journal of Nutrition, 133(9), 2812–2819. [PMID] [DOI] [PubMed] [Google Scholar]
- Prediger R. D., Aguiar A. S., Jr, Rojas-Mayorquin A. E., Figueiredo C. P., Matheus F. C., Ginestet L., et al. (2010). Single intranasal administration of 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine in C57BL/6 mice models early preclinical phase of Parkinson's disease. Neurotoxicity Research, 17(2), 114–129. [PMID] [DOI] [PubMed] [Google Scholar]
- Prediger R. D., Batista L. C., Medeiros R., Pandolfo P., Florio J. C., Takahashi R. N. (2006). The risk is in the air: Intranasal administration of MPTP to rats reproducing clinical features of Parkinson's disease. Experimental Neurology, 202(2), 391–403. [PMID] [DOI] [PubMed] [Google Scholar]
- Russo A., Longo R., Vanella A. (2002). Antioxidant activity of propolis: Role of caffeic acid phenethyl ester and galangin. Fitoterapia, 73(Suppl 1), S21–S29. [PMID] [DOI] [PubMed] [Google Scholar]
- Safari M., Jafari B., Zarbakhsh S., Sameni H., Vafaei A. A., Mohammadi N. K., et al. (2016). G-CSF for mobilizing transplanted bone marrow stem cells in rat model of Parkinson's disease. Iranian Journal of Basic Medical Sciences, 19(12), 1318–1324. [PMID] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedelis M., Schwarting R. K., Huston J. P. (2001). Behavioral phenotyping of the MPTP mouse model of Parkinson's disease. Behavioural Brain Research, 125(1–2), 109–125. [PMID] [DOI] [PubMed] [Google Scholar]
- Song J. J., Lim H. W., Kim K., Kim K. M., Cho S., Chae S. W. (2012). Effect of caffeic acid phenethyl ester (CAPE) on H2O2 induced oxidative and inflammatory responses in human middle ear epithelial cells. International Journal of Pediatric Otorhinolaryngology, 76(5), 675–679. [PMID] [DOI] [PubMed] [Google Scholar]
- Sud'ina G. F., Mirzoeva O. K., Pushkareva M. A., Korshunova G. A., Sumbatyan N. V., Varfolomeev S. D. (1993). Caffeic acid phenethyl ester as a lipoxygenase inhibitor with antioxidant properties. FEBS Letters, 329(1–2), 21–24. [PMID] [DOI] [PubMed] [Google Scholar]
- Zeng R., Wang L. W., Hu Z. B., Guo W. T., Wei J. S., Lin H., et al. (2011). Differentiation of human bone marrow mesenchymal stem cells into neuron-like cells in vitro. Spine, 36(13), 997–1005. [PMID] [DOI] [PubMed] [Google Scholar]
- Zhou Y., Singh A. K., Hoyt R. F., Jr, Wang S., Yu Z., Hunt T., et al. (2014). Regulatory T cells enhance mesenchymal stem cell survival and proliferation following autologous cotransplantation in ischemic myocardium. The Journal of Thoracic and Cardiovascular Surgery, 148(3), 1131–1137. [PMID] [DOI] [PMC free article] [PubMed] [Google Scholar]