Abstract
The beneficial glycemic and food intake-suppressive effects of glucagon-like peptide-1 (GLP-1) have made this neuroendocrine system a leading target for pharmacological approaches to the treatment of diabetes and obesity. One strategy to increase the activity of endogenous GLP-1 is to prevent the rapid degradation of the hormone by the enzyme dipeptidyl peptidase-IV (DPP-IV). However, despite the expression of both DPP-IV and GLP-1 in the brain, and the clear importance of central GLP-1 receptor (GLP-1R) signaling for glycemic and energy balance control, the metabolic effects of central inhibition of DPP-IV activity are unclear. To test whether hindbrain DPP-IV inhibition suppresses blood glucose, feeding, and body weight gain, the effects of 4th intracerebroventricular (ICV) administration of the FDA-approved DPP-IV inhibitor sitagliptin were evaluated. Results indicate that hindbrain delivery of sitagliptin improves glycemic control in a GLP-1R-dependent manner, suggesting that this effect is due at least in part to increased endogenous brainstem GLP-1 activity after sitagliptin administration. Furthermore, 4th ICV injection of sitagliptin reduced 24h body weight gain and energy intake, with a selective suppression of high-fat diet, but not chow, intake. These data reveal a novel role for hindbrain GLP-1R activation in glycemic control and also demonstrate that DPP-IV inhibition in the caudal brainstem promotes negative energy balance.
Keywords: sitagliptin, Januvia, blood glucose, body weight, food intake
Introduction
Metabolic disorders such as type 2 diabetes mellitus (T2DM) and obesity represent major health and economic challenges in modern society that affect millions of individuals worldwide [1–3]. Pharmacological approaches to treat T2DM and obesity have focused in part on targeting the glucagon-like peptide-1 (GLP-1) system [4–7]. GLP-1 is an incretin hormone produced in the L-cells of the intestine and in neurons of the nucleus tractus solitarius (NTS) of the brainstem that acts at the GLP-1 receptor (GLP-1R) to improve glycemic control, reduce food intake, and suppress body weight gain [8, 9]. Because native GLP-1 is rapidly degraded by the enzyme dipeptidyl peptidase-IV (DPP-IV) [10, 11], pharmacological strategies include inhibiting DPP-IV activity and/or creating a GLP-1R agonist resistant to enzymatic degradation. Indeed, a variety of DPP-IV inhibitors including sitagliptin and saxagliptin are FDA-approved for the treatment of T2DM [12–14]. These drugs are taken orally by humans to improve glycemic control, and accordingly, much of the research on DPP-IV inhibitors has focused on the peripheral (e.g., pancreatic, gastrointestinal, and vascular) actions of these small molecule inhibitors. DPP-IV is expressed ubiquitously throughout the body [15–17] and DPP-IV activity has been demonstrated in capillaries of the brain and in whole brain tissue [18, 19]. However, very little research has investigated the behavioral and physiological effects of central DPP-IV inhibition.
Here, we test the hypothesis that DPP-IV inhibition in the caudal brainstem improves glycemic control and promotes negative energy balance. Previous reports show that activation of hindbrain GLP-1Rs suppresses feeding and body weight [20, 21], but it is unknown if DPP-IV inhibition in the caudal brainstem is sufficient to produce these outcomes. In contrast, although hindbrain GLP-1R-mediated regulation of glycemic control is likely [see [22] for review], this has not been investigated in detail. Furthermore, as DPP-IV degrades not only GLP-1 but also other substrates such as glucose-dependent insulinotropic polypeptide (GIP) and peptide-YY (PYY) [23–26], we assess whether the glycemic effects of hindbrain DPP-IV inhibition are mediated by GLP-1R activation. The results show that delivery of the DPP-IV inhibitor sitagliptin into the 4th cerebroventricle reduces blood glucose levels in a GLP-1R-dependent manner, and also suppresses feeding and body weight gain in rats.
Materials and Methods
Animals
Adult, male Sprague Dawley rats (Charles River; 455.0±9.8g at beginning of testing) were maintained on a reverse 12h:12h light:dark cycle in a temperature- and humidity-controlled environment. Animals had 60% high-fat diet (HFD; Research Diets), chow (Purina 5001), and water available ad libitum except where noted. Procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Pennsylvania. All experiments were conducted using counterbalanced, within-subject designs.
Surgery and verification of cannula placement
Rats were anesthetized by intramuscular (IM) injection of a cocktail containing ketamine (90mg/kg), xylazine (2.7mg/kg), and acepromazine (0.64mg/kg). Using a stereotaxic apparatus, each rat was surgically implanted with a cannula (Plastics One) aimed at the fourth ventricle (4V; guide cannula coordinates: on midline, 2.5mm anterior to occipital suture, 5.2mm ventral to skull; internal cannula aimed 1.5–2.5mm ventral to termination of guide cannula, based on results of functional verification procedures). Cannula were anchored to the skull with jeweler’s screws and dental cement. Analgesic (2mg/kg meloxicam, subQ) was provided.
Correct placement of the cannula was functionally verified prior to the start of experimental testing by assessing the hyperglycemic response after 4V injection of 5-thio-D-glucose (210µg/2µl) as described previously [21, 27]. Rats were considered to have correct cannula placement if at least a doubling of blood glucose levels was observed after 5-thio-D-glucose injection. Only data from rats with verified cannula placements were included in statistical analyses.
Effect of hindbrain sitagliptin on glycemic control
To evaluate the effect of hindbrain DPP-IV inhibition on blood glucose levels, an oral glucose tolerance test (OGTT) was conducted. Rats (n=7) were deprived of food overnight during the rats’ light cycle prior to OGTT testing to ensure that the gastrointestinal tract was empty. Experimental testing began approximately 1h after the onset of the dark cycle. First, at time=0 min, a baseline blood glucose (BG) reading was taken from each rat by collecting a small sample of blood from the tail tip with a standard glucometer (Accucheck) and each rat was given a 4V injection of either the GLP-1R antagonist exendin-(9–39) (Ex-9, 50µg; American Peptide) or its vehicle (artificial cerebrospinal fluid [aCSF], 1µl). Fifteen minutes later (time=15 min), BG was measured again and each rat received a second injection into the 4V containing the DPP-IV inhibitor sitagliptin (150µg; BioVision) or its vehicle (3µl aCSF). After another 15min had elapsed (time=30 min), another BG reading was taken and each rat was given an oral gavage of glucose solution (2g/kg). Subsequent BG readings were taken every 20min after the gavage for one additional hour (times=50, 70, 90min).
Effect of hindbrain sitagliptin on energy balance
Shortly before the onset of the dark phase, rats (n=8) were given a 4V injection of sitagliptin (150µg) or vehicle (3µl aCSF), and preweighed HFD and chow were simultaneously returned to the home cage. Subsequent intakes of each food were recorded at 1, 3, 6, and 24h after injection. Spillage was accounted for in food intake measurements. All food intake data were converted to kcal to account for the different caloric densities of the foods (HFD = 5.24 kcal/g, chow = 3.35 kcal/g), allowing for accurate comparisons. The change in body weight during the 24h post-injection was also monitored.
Statistical analyses
Statistical analyses were conducted using Statistica (StatSoft), with an α-level of p<0.05 for all tests. BG data from OGTT were analyzed by ANOVA, with pretreatment (vehicle or Ex-9) and treatment (vehicle or sitagliptin) as within-subjects factors, and time as an additional within-subjects factor when applicable. Area under the curve (AUC) from 0–90min was calculated in Excel (Microsoft) using the trapezoidal method. For feeding studies, separate ANOVAs were run for chow intake, HFD intake, total energy intake, and 24h body weight change, with drug (and time, where applicable) as a within-subjects factor. Significant results from the ANOVA were probed using Student Newman Keuls post-hoc analyses. All data are presented as mean ± SEM.
Results
Hindbrain DPP-IV inhibition improves glycemic control via a GLP-1R-dependent mechanism
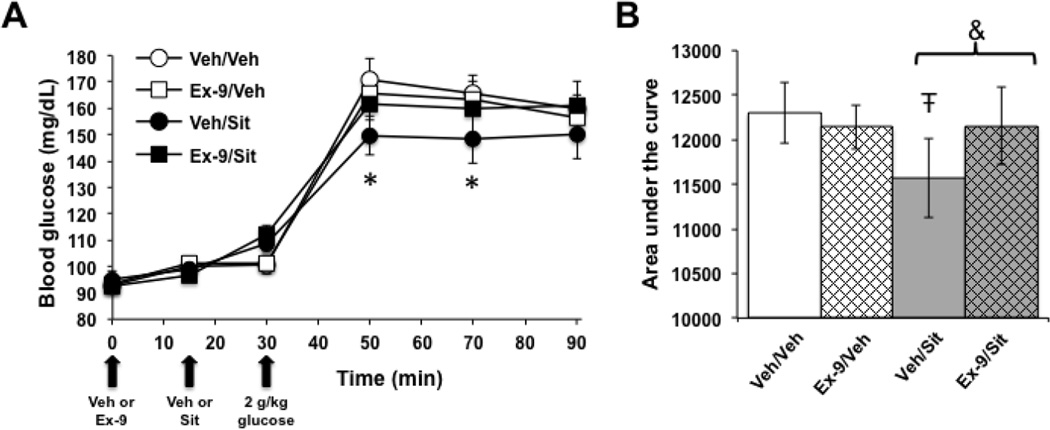
The effects of hindbrain DPP-IV inhibition on glycemic control were assessed by conducting an oral glucose tolerance test (OGTT) after 4V administration of sitagliptin (150µg) or vehicle (veh). Additionally, to evaluate whether any glycemic effects of 4V sitagliptin are mediated by GLP-1R signaling, these injections were combined with a 4V pretreatment of the competitive GLP-1R antagonist Ex-9 (50µg) or vehicle. Importantly, the dose of Ex-9 was subthreshold for an effect on blood glucose on its own. As shown in Figure 1A, a significant interaction of Ex-9 and sitagliptin on blood glucose levels was observed in the OGTT (F1,6=24.39, p=0.003). Post hoc analysis demonstrated that sitagliptin alone significantly suppresses blood glucose levels after the oral glucose load is delivered (Fig. 1A; veh/veh versus veh/sitagliptin, p<0.05 at 50 and 70 min). When the area under the curve (AUC) was analyzed for all treatment groups, a significant Ex-9 × sitagliptin interaction was found (F1,6=40.72, p=0.0007), such that the AUC for rats given veh/sitagliptin was less than the AUC for any other treatment (Fig. 1B; post hoc analyses, veh/sitagliptin versus all other groups p<0.05). These data indicate that hindbrain inhibition of DPP-IV lowers blood glucose levels via a GLP-1R-dependent mechanism.
Figure 1.
Hindbrain DPP-IV inhibition improves glycemic control through a GLP-1R-dependent mechanism. In an oral glucose tolerance test, 4V administration of sitagliptin (Sit) alone (Veh/Sit) suppressed blood glucose levels at the two timepoints after oral glucose gavage (A). When area under the curve was evaluated, Veh/Sit produced a significant reduction in AUC compared to all other treatment conditions (B). *, different from Veh/Veh, p<0.05; Ŧ, different from all other conditions, p<0.05; &, Veh/Sit versus Ex-9/Sit, p<0.05. Data are mean ± SEM.
Hindbrain DPP-IV inhibition reduces food intake and body weight by selectively suppressing HFD intake
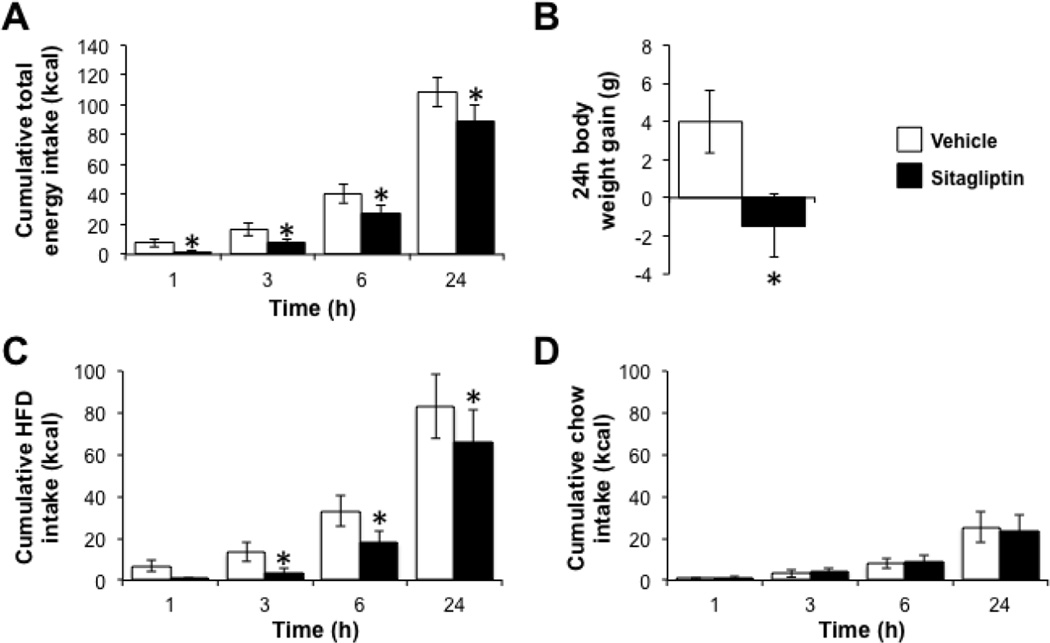
GLP-1R activation in the caudal brainstem, in particular in the NTS, suppresses food intake and promotes negative energy balance [27]. To test whether hindbrain DPP-IV inhibition elicits similar effects, rats were given 4V injection of either sitagliptin (150µg) or vehicle just prior to the onset of the dark cycle, and subsequent food intake and body weight were monitored. Total energy intake was decreased by 4V administration of sitagliptin (Fig. 2A; main effect of drug, F1,7=20.54, p=0.003; drug × time interaction, F3,21=5.34, p=0.007; post hoc comparisons of vehicle versus sitagliptin, p<0.05 at all times), as was 24h body weight gain (Fig. 2B; F1,7=11.98, p=0.01). To determine whether these effects were driven by a selective reduction in the intake of one food, energy intakes from chow and from HFD were examined. Indeed, energy intake from HFD was potently suppressed by sitagliptin (Fig. 2C; main effect of drug, F1,7=12.72, p=0.009; drug × time interaction, F3,21=2.30, p=0.11; planned comparisons of vehicle versus sitagliptin, p<0.05 from 3–24h). In contrast, hindbrain sitagliptin had no effect on chow intake (Fig. 2D; main effect of drug, F1,7=0.04, p=0.84; drug × time interaction, F3,21=1.21, p=0.33).
Figure 2.
Hindbrain administration of a DPP-IV inhibitor promotes negative energy balance and selectively reduces intake of a high-fat diet. Rats given a 4V injection of sitagliptin consume fewer kilocalories (A) and gain less body weight (B) over the 24h post-injection compared to vehicle-treated animals. The reduction in energy intake is driven by a suppression of HFD intake (C), with no change in chow intake (D). Key in (B) applies to all panels. *, p<0.05 compared to vehicle. Data are mean ± SEM.
Discussion
Given the existence of DPP-IV activity in the brain [18, 19], along with the interest in GLP-1-based pharmacotherapies for the treatment of diabetes and obesity [4, 5], the effects of central DPP-IV inhibition on control of glycemia and energy balance are surprisingly understudied. As several small molecule DPP-IV inhibitors are FDA-approved for the treatment of T2DM, this represents an important question with possible therapeutic implications. Due to the critical role of the caudal brainstem in mediating the physiological and behavioral effects of GLP-1R activation [27, 28], the present studies focused on testing the ability of hindbrain DPP-IV inhibition to impact glycemia, feeding, and body weight gain. The results demonstrate clearly that hindbrain DPP-IV inhibition by sitagliptin can suppress blood glucose levels and promote negative energy balance.
Oral glucose tolerance testing revealed that hindbrain administration of sitagliptin potently suppressed blood glucose levels. This effect is consistent in direction with the glucose-lowering effects of peripheral sitagliptin [29, 30]. Furthermore, this experiment demonstrated that 4V pretreatment with the competitive GLP-1R antagonist Ex-9 attenuated the glycemic effects of hindbrain sitagliptin. Because DPP-IV can act on a number of bioactive peptides [23–26], this was an important step to establish that the ability of 4V sitagliptin to lower blood glucose was mediated, at least in part, by GLP-1R signaling. These findings suggest that hindbrain DPP-IV inhibition may prolong the ability of endogenous GLP-1 to activate GLP-1Rs in the caudal brainstem, highlighting a previously unidentified role of hindbrain GLP-1R signaling in glycemic control.
The effects of central GLP-1R signaling on blood glucose levels remain an open area of research. Our results suggest an important role of hindbrain GLP-1R signaling in blood glucose control. However, previously published glycemic data after lateral or third intracerebroventricular administration of GLP-1R agonists to bathe the brain have produced varying results depending on the particular drug used. For example, acute 3rd ICV delivery of GLP-1 had no effect on blood glucose levels in an intravenous glucose tolerance test, although it did increase insulin [31]. In contrast, acute lateral ventricle administration of the GLP-1R agonist exendin-4 actually had hyperglycemic effects via sympathetic activation [32]. It is possible that the use of a DPP-IV inhibitor in our studies may have been a more physiologically relevant manipulation to evaluate GLP-1R-mediated glycemic effects than administration of an exogenous GLP-1R ligand. Alternatively, a hindbrain-targeted manipulation may be more potent than more widespread delivery of drug via lateral or 3rd ICV injection. Indeed, site-specific administration of GLP-1 can produce robust glycemic benefits, for example after direct injection in the arcuate nucleus of the hypothalamus (Arc) [31], suggesting the potential importance of a more targeted application of drug.
The endogenous source of GLP-1 to the central GLP-1R populations impacting glycemia is uncertain. As circulating GLP-1 has a very short half-life [9] and is unlikely to reach the brain in large amounts, specific GLP-1R-expressing populations such as those in the Arc and the hindbrain are likely activated by GLP-1 from direct projections of preproglucagon neurons in the NTS [33]. However, the specific GLP-1R-expressing nuclei in the hindbrain mediating the glycemic effects of 4V sitagliptin are unclear. GLP-1 can activate vagal motoneurons in the dorsal motor nucleus of the vagus (DMV) that project to the pancreas [34]. Therefore, reducing DPP-IV enzymatic activity throughout the entire hindbrain might theoretically increase GLP-1R signaling in the DMV, potentially in sufficient magnitude to alter the activity of the DMV-vagal efferent-pancreatic pathway and in turn influence pancreatic islets to control blood glucose levels [35]. Beyond the DMV, GLP-1Rs are expressed in other metabolically relevant caudal brainstem nuclei such as the NTS and area postrema [36], highlighting the possibility that GLP-1 may additionally or alternatively activate receptors in these nuclei to influence glycemic control via indirect input to the DMV. It is also possible that DPP-IV inhibition in the brainstem disrupts the glucosensing processes involved in hypoglycemic counter-regulation, which in turn disrupts brainstem-to-hypothalamic signaling involved in modulating counter-regulatory endocrine responses from the hypothalamic-pituitary axis (e.g. alterations in epinephrine, glucocorticoid, glucagon signaling) [35]. Each of these possibilities remains to be empirically tested in future studies.
Previous studies examining the role of oral or systemically-delivered DPP-IV inhibitors have shown little or no effect on food intake or energy balance control [29, 30, 37–39]. However, the role of central DPP-IV enzymatic activity in energy balance regulation has remained largely unexplored. Therefore, the finding that 4V sitagliptin significantly reduced total food intake and body weight is novel and suggests that central / hindbrain manipulation of DPP-IV activity may have more potent effects on energy balance than a systemic DPP-IV inhibitor. One caveat in this conclusion, however, is that the current findings may be the result of a higher concentration of sitagliptin being present in the hindbrain compared with the amount of the small molecule that might access the brainstem following peripheral delivery. Nevertheless, current data reveal a unique and previously unidentified role of brainstem DPP-IV activity in energy balance control.
Many of the published studies evaluating the role of sitagliptin in glycemic and energy balance control have examined the effects of the drug after chronic peripheral administration. It is important to note that the vast majority of these studies use much higher doses of sitagliptin (e.g. in the mg/kg or mg/day range) than those used in the current experiments [40–43]. Fewer studies have assessed the intake- and glycemic-suppressive effects of acute sitagliptin administration. A previous publication from our laboratory demonstrated that an intraperitoneal injection of sitagliptin (6 mg/kg) had no effect on chow intake in fasted rats, but was effective to reduce blood glucose levels in an OGTT [29]. Another paper by Tahara et al. showed that in a diabetic rat model, AUC in a glucose tolerance test was decreased by oral administration of sitagliptin at doses of 1 mg/kg and higher, but not at 0.3 mg/kg and lower [44]. Many methodological differences exist between these previously published studies and the current experiments, limiting direct comparison, but this information suggests that the 150µg dose of sitagliptin used here is unlikely to produce feeding or glycemic effects when administered peripherally. Clearly, further pharmacokinetic and pharmacological analyses are warranted to evaluate the effects of both peripheral and central DPP-IV inhibitor administration in dose-response studies.
An interesting discovery of the food intake study described here was the demonstration that hindbrain sitagliptin administration reduced caloric intake by a selective suppression of palatable HFD intake, with no significant effects observed for chow intake. This is similar to the effects of mesolimbic GLP-1R activation on food intake. Mesolimbic structures such as the ventral tegmental area (VTA) and nucleus accumbens are often considered to be involved in “hedonic” feeding and processes related to food reward. GLP-1R activation in the ventral tegmental area or nucleus accumbens core seems to more potently suppress intake of palatable food (HFD or sucrose) with little or no effect on intake of chow when a choice of foods is available [8]. However, when only chow is available, GLP-1R activation in the nucleus accumbens can still suppress feeding [45, 46]. Therefore, the ability of hindbrain sitagliptin to selectively suppress HFD intake in the current studies may be related to the opportunity to choose between foods and/or the increased palatability of the HFD relative to the chow.
In contrast to the role of these mesolimbic structures, other areas of the brain such as the hindbrain and hypothalamus are often considered to be responsible for “homeostatic” feeding (e.g., energy intake to meet metabolic need) [47, 48]. However, a growing body of literature indicates that hindbrain GLP-1R activation can control aspects of food reward [8, 20, 49]. One intriguing study demonstrated that activation of GLP-1R in the NTS specifically reduced palatable food intake when rats had a choice of peanut butter or chow [49]. More recently, we have shown that virogenetic knockdown of GLP-1R in the NTS increases the motivation of an animal to lever press for sucrose reinforcement [20]. These data suggest that neuroanatomical substrates of “hedonic” versus “homeostatic” feeding, particularly in relation to the effects of GLP-1R signaling, are not as clearly delineated as previously hypothesized. Indeed, it will be interesting to determine whether hindbrain DPP-IV inhibition affects motivated feeding behaviors.
Another potential mechanism that could explain the effects of hindbrain DPP-IV inhibition on both feeding and glycemic control is a GLP-1R-mediated reduction in gastric emptying. Previous studies demonstrate that GLP-1R agonists suppress gastric emptying [50–53], which could promote satiety [54] and improve blood glucose levels [55]. Furthermore, neuroanatomical substrates localized to the hindbrain are sufficient to mediate the gastric emptying effects of a peripheral GLP-1R agonist [50]. Direct hindbrain delivery of a DPP-IV inhibitor such as sitagliptin may increase local GLP-1 levels in the hindbrain to impinge on this neural circuit. However, this remains to be tested; it will also be important to confirm an increased concentration of active GLP-1 in the caudal brainstem after central administration of the DPP-IV inhibitor and to determine the time course of such changes.
Collectively, the studies presented here demonstrate that inhibition of hindbrain DPP-IV reduces blood glucose levels, food intake, and body weight gain. The finding that the glycemic effects of hindbrain sitagliptin administration are GLP-1R-dependent indicates not only that DPP-IV inhibition in the caudal brainstem likely prolongs the activity of local hindbrain GLP-1, but also provides novel evidence that hindbrain GLP-1 / GLP-1R activation controls glycemia. Moreover, the food intake data highlight a specific role for DPP-IV activity in regulating palatable food intake when a choice of foods is available. In contrast to the effects of peripheral DPP-IV inhibition, which improves glycemic control but has little effect on food intake or body weight [29, 30, 37– 39], these experiments suggest that central DPP-IV may be a novel therapeutic target for the simultaneous treatment of obesity and T2DM.
Highlights.
-
-
Hindbrain injection of the DPP-IV inhibitor sitagliptin improves glycemic control.
-
-
The glycemic effects of hindbrain sitagliptin are GLP-1 receptor-mediated.
-
-
Hindbrain sitagliptin suppresses palatable food intake and body weight gain.
Acknowledgments
The authors thank Marissa Kamarck for technical assistance with these studies. Grants: This work was supported by NIH DK103804 (EGM-B), DK105858 (DJR), and DK096139 (MRH). Disclosures: Authors receive funding from Zealand Pharma (EGM-B and MRH) and Novo Nordisk (MRH) that was not used to support these studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arroyo-Johnson C, Mincey KD. Obesity Epidemiology Worldwide. Gastroenterol Clin North Am. 2016;45:571–579. doi: 10.1016/j.gtc.2016.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balakumar P, Maung UK, Jagadeesh G. Prevalence and prevention of cardiovascular disease and diabetes mellitus. Pharmacol Res. 2016;113:600–609. doi: 10.1016/j.phrs.2016.09.040. [DOI] [PubMed] [Google Scholar]
- 3.Bischoff SC, Boirie Y, Cederholm T, Chourdakis M, Cuerda C, Delzenne NM, et al. Towards a multidisciplinary approach to understand and manage obesity and related diseases. Clin Nutr. 2016 doi: 10.1016/j.clnu.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 4.Sadry SA, Drucker DJ. Emerging combinatorial hormone therapies for the treatment of obesity and T2DM. Nat Rev Endocrinol. 2013;9:425–433. doi: 10.1038/nrendo.2013.47. [DOI] [PubMed] [Google Scholar]
- 5.Chatterjee S, Ghosal S, Chatterjee S. Glucagon-like peptide-1 receptor agonists favorably address all components of metabolic syndrome. World J Diabetes. 2016;7:441–448. doi: 10.4239/wjd.v7.i18.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burcelin R, Gourdy P. Harnessing glucagon-like peptide-1 receptor agonists for the pharmacological treatment of overweight and obesity. Obes Rev. 2016 doi: 10.1111/obr.12465. [DOI] [PubMed] [Google Scholar]
- 7.Rajeev SP, Wilding J. GLP-1 as a target for therapeutic intervention. Curr Opin Pharmacol. 2016;31:44–49. doi: 10.1016/j.coph.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 8.Alhadeff AL, Rupprecht LE, Hayes MR. GLP-1 neurons in the nucleus of the solitary tract project directly to the ventral tegmental area and nucleus accumbens to control for food intake. Endocrinology. 2012;153:647–658. doi: 10.1210/en.2011-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 10.Mentlein R, Gallwitz B, Schmidt WE. Dipeptidyl-peptidase IV hydrolyses gastric inhibitory polypeptide, glucagon-like peptide-1(7–36)amide, peptide histidine methionine and is responsible for their degradation in human serum. Eur J Biochem. 1993;214:829–835. doi: 10.1111/j.1432-1033.1993.tb17986.x. [DOI] [PubMed] [Google Scholar]
- 11.Mentlein R. Dipeptidyl-peptidase IV (CD26)--role in the inactivation of regulatory peptides. Regul Pept. 1999;85:9–24. doi: 10.1016/s0167-0115(99)00089-0. [DOI] [PubMed] [Google Scholar]
- 12.Traynor K. FDA approves saxagliptin for type 2 diabetes. Am J Health Syst Pharm. 2009;66:1513. doi: 10.2146/news090069. [DOI] [PubMed] [Google Scholar]
- 13.Lee M, Rhee MK. Sitagliptin for Type 2 diabetes: a 2015 update. Expert Rev Cardiovasc Ther. 2015;13:597–610. doi: 10.1586/14779072.2015.1046840. [DOI] [PubMed] [Google Scholar]
- 14.Choy M, Lam S. Sitagliptin: a novel drug for the treatment of type 2 diabetes. Cardiol Rev. 2007;15:264–271. doi: 10.1097/CRD.0b013e318123f771. [DOI] [PubMed] [Google Scholar]
- 15.Abbott CA, Baker E, Sutherland GR, McCaughan GW. Genomic organization, exact localization, and tissue expression of the human CD26 (dipeptidyl peptidase IV) gene. Immunogenetics. 1994;40:331–338. doi: 10.1007/BF01246674. [DOI] [PubMed] [Google Scholar]
- 16.Ahren B, Schmitz O. GLP-1 receptor agonists and DPP-4 inhibitors in the treatment of type 2 diabetes. Horm Metab Res. 2004;36:867–876. doi: 10.1055/s-2004-826178. [DOI] [PubMed] [Google Scholar]
- 17.Drucker DJ. Dipeptidyl peptidase-4 inhibition and the treatment of type 2 diabetes: preclinical biology and mechanisms of action. Diabetes Care. 2007;30:1335–1343. doi: 10.2337/dc07-0228. [DOI] [PubMed] [Google Scholar]
- 18.Schnabel R, Bernstein HG, Luppa H, Lojda Z, Barth A. Aminopeptidases in the circumventricular organs of the mouse brain: a histochemical study. Neuroscience. 1992;47:431–438. doi: 10.1016/0306-4522(92)90257-3. [DOI] [PubMed] [Google Scholar]
- 19.Gault VA, Lennox R, Flatt PR. Sitagliptin, a dipeptidyl peptidase-4 inhibitor, improves recognition memory, oxidative stress and hippocampal neurogenesis and upregulates key genes involved in cognitive decline. Diabetes Obes Metab. 2015;17:403–413. doi: 10.1111/dom.12432. [DOI] [PubMed] [Google Scholar]
- 20.Alhadeff AL, Mergler BD, Zimmer DJ, Turner CA, Reiner DJ, Schmidt HD, et al. Endogenous Glucagon-Like Peptide-1 Receptor Signaling in the Nucleus Tractus Solitarius is Required for Food Intake Control. Neuropsychopharmacology. 2016 doi: 10.1038/npp.2016.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rupprecht LE, Mietlicki-Baase EG, Zimmer DJ, McGrath LE, Olivos DR, Hayes MR. Hindbrain GLP-1 receptor-mediated suppression of food intake requires a PI3K-dependent decrease in phosphorylation of membrane-bound Akt. Am J Physiol Endocrinol Metab. 2013;305:E751–E759. doi: 10.1152/ajpendo.00367.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayes MR, Mietlicki-Baase EG, Kanoski SE, De Jonghe BC. Incretins and amylin: neuroendocrine communication between the gut, pancreas, and brain in control of food intake and blood glucose. Annu Rev Nutr. 2014;34:237–260. doi: 10.1146/annurev-nutr-071812-161201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leiting B, Pryor KD, Wu JK, Marsilio F, Patel RA, Craik CS, et al. Catalytic properties and inhibition of proline-specific dipeptidyl peptidases II, IV and VII. Biochem J. 2003;371:525–532. doi: 10.1042/BJ20021643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kieffer TJ, McIntosh CH, Pederson RA. Degradation of glucose-dependent insulinotropic polypeptide and truncated glucagon-like peptide 1 in vitro and in vivo by dipeptidyl peptidase IV. Endocrinology. 1995;136:3585–3596. doi: 10.1210/endo.136.8.7628397. [DOI] [PubMed] [Google Scholar]
- 25.Ballantyne GH. Peptide YY(1–36) and peptide YY(3–36): Part I. Distribution, release and actions. Obes Surg. 2006;16:651–658. doi: 10.1381/096089206776944959. [DOI] [PubMed] [Google Scholar]
- 26.Medeiros MD, Turner AJ. Processing and metabolism of peptide-YY: pivotal roles of dipeptidylpeptidase-IV, aminopeptidase-P, and endopeptidase-24.11. Endocrinology. 1994;134:2088–2094. doi: 10.1210/endo.134.5.7908871. [DOI] [PubMed] [Google Scholar]
- 27.Hayes MR, Leichner TM, Zhao S, Lee GS, Chowansky A, Zimmer D, et al. Intracellular signals mediating the food intake-suppressive effects of hindbrain glucagon-like peptide-1 receptor activation. Cell Metab. 2011;13:320–330. doi: 10.1016/j.cmet.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grill HJ, Hayes MR. The nucleus tractus solitarius: a portal for visceral afferent signal processing, energy status assessment and integration of their combined effects on food intake. Int J Obes (Lond) 2009;33(Suppl 1):S11–S15. doi: 10.1038/ijo.2009.10. [DOI] [PubMed] [Google Scholar]
- 29.Olivos DR, McGrath LE, Turner CA, Montaubin O, Mietlicki-Baase EG, Hayes MR. Intraduodenal milk protein concentrate augments the glycemic and food intake suppressive effects of DPP-IV inhibition. Am J Physiol Regul Integr Comp Physiol. 2014;306:R157–R163. doi: 10.1152/ajpregu.00358.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herman GA, Bergman A, Stevens C, Kotey P, Yi B, Zhao P, et al. Effect of single oral doses of sitagliptin, a dipeptidyl peptidase-4 inhibitor, on incretin and plasma glucose levels after an oral glucose tolerance test in patients with type 2 diabetes. J Clin Endocrinol Metab. 2006;91:4612–4619. doi: 10.1210/jc.2006-1009. [DOI] [PubMed] [Google Scholar]
- 31.Sandoval DA, Bagnol D, Woods SC, D'Alessio DA, Seeley RJ. Arcuate glucagon-like peptide 1 receptors regulate glucose homeostasis but not food intake. Diabetes. 2008;57:2046–2054. doi: 10.2337/db07-1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perez-Tilve D, Gonzalez-Matias L, Aulinger BA, Alvarez-Crespo M, Gil-Lozano M, Alvarez E, et al. Exendin-4 increases blood glucose levels acutely in rats by activation of the sympathetic nervous system. Am J Physiol Endocrinol Metab. 2010;298:E1088–E1096. doi: 10.1152/ajpendo.00464.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Llewellyn-Smith IJ, Reimann F, Gribble FM, Trapp S. Preproglucagon neurons project widely to autonomic control areas in the mouse brain. Neuroscience. 2011;180:111–121. doi: 10.1016/j.neuroscience.2011.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wan S, Coleman FH, Travagli RA. Glucagon-like peptide-1 excites pancreas-projecting preganglionic vagal motoneurons. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1474–G1482. doi: 10.1152/ajpgi.00562.2006. [DOI] [PubMed] [Google Scholar]
- 35.Watts AG, Donovan CM. Sweet talk in the brain: glucosensing, neural networks, and hypoglycemic counterregulation. Front Neuroendocrinol. 2010;31:32–43. doi: 10.1016/j.yfrne.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Merchenthaler I, Lane M, Shughrue P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol. 1999;403:261–280. doi: 10.1002/(sici)1096-9861(19990111)403:2<261::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 37.Raz I, Hanefeld M, Xu L, Caria C, Williams-Herman D, Khatami H, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy in patients with type 2 diabetes mellitus. Diabetologia. 2006;49:2564–2571. doi: 10.1007/s00125-006-0416-z. [DOI] [PubMed] [Google Scholar]
- 38.Burkey BF, Li X, Bolognese L, Balkan B, Mone M, Russell M, et al. Acute and chronic effects of the incretin enhancer vildagliptin in insulin-resistant rats. J Pharmacol Exp Ther. 2005;315:688–695. doi: 10.1124/jpet.105.087064. [DOI] [PubMed] [Google Scholar]
- 39.Ahren B, Landin-Olsson M, Jansson PA, Svensson M, Holmes D, Schweizer A. Inhibition of dipeptidyl peptidase-4 reduces glycemia, sustains insulin levels, and reduces glucagon levels in type 2 diabetes. J Clin Endocrinol Metab. 2004;89:2078–2084. doi: 10.1210/jc.2003-031907. [DOI] [PubMed] [Google Scholar]
- 40.Sakai M, Uchii M, Myojo K, Kitayama T, Kunori S. Critical role of renal dipeptidyl peptidase-4 in ameliorating kidney injury induced by saxagliptin in Dahl salt-sensitive hypertensive rats. Eur J Pharmacol. 2015;761:109–115. doi: 10.1016/j.ejphar.2015.04.023. [DOI] [PubMed] [Google Scholar]
- 41.Chen B, Moore A, Escobedo LV, Koletsky MS, Hou D, Koletsky RJ, et al. Sitagliptin lowers glucagon and improves glucose tolerance in prediabetic obese SHROB rats. Exp Biol Med (Maywood) 2011;236:309–314. doi: 10.1258/ebm.2010.010161. [DOI] [PubMed] [Google Scholar]
- 42.Badole SL, Mahamuni SP, Bagul PP, Khose RD, Joshi AC, Ghule AE, et al. Cycloart-23-ene-3beta, 25-diol stimulates GLP-1 (7–36) amide secretion in streptozotocin-nicotinamide induced diabetic Sprague Dawley rats: a mechanistic approach. Eur J Pharmacol. 2013;698:470–479. doi: 10.1016/j.ejphar.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 43.Maiztegui B, Borelli MI, Madrid VG, Del Zotto H, Raschia MA, Francini F, et al. Sitagliptin prevents the development of metabolic and hormonal disturbances, increased beta-cell apoptosis and liver steatosis induced by a fructose-rich diet in normal rats. Clin Sci (Lond) 2011;120:73–80. doi: 10.1042/CS20100372. [DOI] [PubMed] [Google Scholar]
- 44.Tahara A, Matsuyama-Yokono A, Nakano R, Someya Y, Hayakawa M, Shibasaki M. Antihyperglycemic effects of ASP8497 in streptozotocin-nicotinamide induced diabetic rats: comparison with other dipeptidyl peptidase-IV inhibitors. Pharmacol Rep. 2009;61:899–908. doi: 10.1016/s1734-1140(09)70147-1. [DOI] [PubMed] [Google Scholar]
- 45.Dossat AM, Lilly N, Kay K, Williams DL. Glucagon-like peptide 1 receptors in nucleus accumbens affect food intake. J Neurosci. 2011;31:14453–14457. doi: 10.1523/JNEUROSCI.3262-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dickson SL, Shirazi RH, Hansson C, Bergquist F, Nissbrandt H, Skibicka KP. The glucagon-like peptide 1 (GLP-1) analogue, exendin-4, decreases the rewarding value of food: a new role for mesolimbic GLP-1 receptors. J Neurosci. 2012;32:4812–4820. doi: 10.1523/JNEUROSCI.6326-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Faulconbridge LF, Hayes MR. Regulation of energy balance and body weight by the brain: a distributed system prone to disruption. Psychiatr Clin North Am. 2011;34:733–745. doi: 10.1016/j.psc.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Berthoud HR. Metabolic and hedonic drives in the neural control of appetite: who is the boss? Curr Opin Neurobiol. 2011;21:888–896. doi: 10.1016/j.conb.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Richard JE, Anderberg RH, Goteson A, Gribble FM, Reimann F, Skibicka KP. Activation of the GLP-1 receptors in the nucleus of the solitary tract reduces food reward behavior and targets the mesolimbic system. PLoS One. 2015;10:e0119034. doi: 10.1371/journal.pone.0119034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hayes MR, Skibicka KP, Grill HJ. Caudal brainstem processing is sufficient for behavioral, sympathetic, and parasympathetic responses driven by peripheral and hindbrain glucagon-like-peptide-1 receptor stimulation. Endocrinology. 2008;149:4059–4068. doi: 10.1210/en.2007-1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Imeryuz N, Yegen BC, Bozkurt A, Coskun T, Villanueva-Penacarrillo ML, Ulusoy NB. Glucagon-like peptide-1 inhibits gastric emptying via vagal afferent-mediated central mechanisms. Am J Physiol. 1997;273:G920–G927. doi: 10.1152/ajpgi.1997.273.4.G920. [DOI] [PubMed] [Google Scholar]
- 52.Naslund E, Gutniak M, Skogar S, Rossner S, Hellstrom PM. Glucagon-like peptide 1 increases the period of postprandial satiety and slows gastric emptying in obese men. Am J Clin Nutr. 1998;68:525–530. doi: 10.1093/ajcn/68.3.525. [DOI] [PubMed] [Google Scholar]
- 53.Young AA, Gedulin BR, Rink TJ. Dose-responses for the slowing of gastric emptying in a rodent model by glucagon-like peptide (7–36) NH2, amylin, cholecystokinin, and other possible regulators of nutrient uptake. Metabolism. 1996;45:1–3. doi: 10.1016/s0026-0495(96)90192-4. [DOI] [PubMed] [Google Scholar]
- 54.Hellstrom PM, Naslund E. Interactions between gastric emptying and satiety, with special reference to glucagon-like peptide-1. Physiol Behav. 2001;74:735–741. doi: 10.1016/s0031-9384(01)00618-7. [DOI] [PubMed] [Google Scholar]
- 55.Vella A, Camilleri M, Rizza RA. The gastrointestinal tract and glucose tolerance. Curr Opin Clin Nutr Metab Care. 2004;7:479–484. doi: 10.1097/01.mco.0000134375.01310.97. [DOI] [PubMed] [Google Scholar]