Abstract
Objective
Binge eating is characterized by repeated intermittent bouts of compulsive overconsumption of food. Treatment is challenging given limited understanding of the mechanisms underlying this type of disordered eating. The hypothesis that dysregulation of mesocortical dopaminergic and GABAergic systems underlie binge eating was tested.
Methods
Analysis of gene expression within the ventral tegmental area and its terminal mesocortical regions was examined in bingeing rats before and after bingeing occurred. In addition, alterations in binge-type behavior induced by pharmacological inactivation of subnuclei of the prefrontal cortex (PFC) and by pharmacological activation and inhibition of cortical D1 and D2 receptors were examined.
Results
Correlative and functional evidence demonstrates dysregulated neurotransmitter processing by the PFC and ventral tegmental area, but not the amygdala or nucleus accumbens, in bingeing rats. Either GABAergic inactivation or D2-like receptor activation within the PFC increased consumption in bingeing rats, but not controls, suggesting that the PFC, and D2 receptors in particular, functions as a behavioral brake to limit bingeing.
Conclusions
The act of bingeing resolved some gene expression differences that preceded binge onset, further suggesting that bingeing may partially serve to self-medicate a system driving this maladaptive behavior. However, the failure of bingeing to resolve other dopaminergic/GABAergic differences may render individuals vulnerable to future binge episodes.
Introduction
Binge eating involves eating more in a discrete time period than normally would be consumed under similar circumstances within the same time period, accompanied by a sense of loss of control (1). Binge eating characterizes several eating disorders and is associated with psychiatric comorbidities. About 5% of Americans binge, but only ~35% of those who binge have obesity, due to behavioral compensation for the excess consumed during binge episodes (2). Limited understanding of mechanistic underpinnings has made treatment particularly challenging.
In humans who binge eat, imaging studies have revealed dysfunction within dopaminergic pathways and terminal regions thought to be involved in reward processing and cognitive control (3). Some of this dysfunction may reflect genetic contributions to binge vulnerability. However, some of the dysfunction may result from bingeing itself (4).
Midbrain dopamine neurons project from the ventral tegmental area (VTA) to the nucleus accumbens (NA) and prefrontal cortex (PFC). The PFC includes several subnuclei, which collectively are involved in attention, decision-making, and inhibitory control (5). Both D1 and D2 receptors are expressed on PFC pyramidal cells as well as interneurons, which allows for complex neurochemical actions of dopamine in the PFC (5,6), thereby influencing executive function, as well as the activity of downstream projection sites involved in ingestive behavior and motivation.
The midbrain and its terminal areas are key components of integrated neural circuitry controlling ingestive behavior (7). Among the many nuclei that modulate food-motivated behaviors and integrate input from the VTA and PFC are the NA and central nucleus of the amygdala (8). However, the function of this circuitry during a binge versus the normal consumption of palatable food has not been characterized. Nonhuman animal studies of binge-type eating have reported alterations in dopamine receptor and transporter binding, mRNA, and extracellular dopamine in food-deprived rats consuming sugar (4,9). In addition, elevations of dopamine and its metabolites have been reported in food- and space-restricted rats consuming chow (10). While these reports have relevance to bingeing that occurs after a period of energy restriction, an energy deficit is not necessary for bingeing to occur (1). Thus, bingeing models that do not depend upon food restriction are needed. Such models have the advantage of determining the influence of binge-type eating on neurobiology without the confounding effects that food deprivation itself can have (11).
We previously developed a rat model of binge-type eating in which non-food-deprived rats with brief intermittent (3 days/week) access to an optional source of dietary fat binge on the fat relative to rats with brief daily access to the same fat (12). This allows for intakes in bingeing rats to be compared to intakes in control rats that are consuming the same food under similar circumstances, thereby fulfilling one of the defining characteristics of binge eating (1).
In the present investigations, gene expression within the VTA and its terminal mesocortical regions was examined in bingeing rats before and after bingeing occurred. In addition, alterations in binge-type behavior induced by pharmacological inactivation of discrete subnuclei of the PFC, and by pharmacological activation and inhibition of cortical D1 and D2 receptors are reported.
Methods
Male Sprague-Dawley rats (Harlan, Indianapolis, IN) (60 days of age upon arrival) were used in all studies and housed individually in hanging stainless steel wire cages in a temperature- and humidity-controlled vivarium maintained on a 12:12 light:dark cycle. Unless stated otherwise, rats had continuous access to tap water and nutritionally complete chow (Laboratory Rodent Diet 5001, PMI Feeds, Richmond IN; percent energy as protein: 28.05%, fat: 12.14%, carbohydrate: 59.81%; 3.3 kcal/g). All procedures were approved by the Pennsylvania State University Institutional Animal Care and Use Committee.
Feeding protocols
After 5 days of adaptation, body weights were recorded and vegetable shortening (CriscoV® All-Vegetable shortening, J.M Smucker Co., Orrville, OH) was provided during a single overnight period. Rats were then matched for body weight and shortening intake and assigned to two different groups. In one group, shortening was provided for 1 h starting ~3 h before lights out on an intermittent (INT) basis (Mondays, Wednesdays, and Fridays); in the other group, shortening was provided for 1 h daily (D) at the same time. Chow and water were available at all times. These feeding protocols have been used extensively by this laboratory to model binge-type eating in rats, as the INT rats come to consume significantly more shortening during the limited access period than do the D rats (e.g., Ref. 12). The shortening-access protocols were in effect for 5 to 8 weeks such that bingeing was clearly established before testing began.
Quantitative real-time PCR studies
INT and D rats were maintained on their respective protocols for 8 weeks and assigned to subgroups for sacrifice on the Monday of week 9 either 20 min before, or 20 min after, the start of the shortening access period. Brains were flash frozen in isopentane and stored at −80°C until processing. Micropunched tissue from the VTA, NA core and shell, central nucleus of the amygdala, and PFC were collected and analyzed for relative mRNA expression using the comparative threshold cycle method as previously described (13). Details provided in Supporting Information Methods.
GABA-A/B (muscimol/baclofen) PFC—Motor cortex 2 (M2) inactivation
To determine whether inactivation of dorsomedial PFC (dmPFC) GABA receptors would affect binge-type behavior, a cocktail of the GABA-A agonist muscimol (0.1 mM; Tocris Bioscience, Ellisville MO) and GABA-B agonist (RS)-baclofen (1 mM; Tocris Bioscience, Ellisville MO) or the phosphate buffered saline (PBS) vehicle (total volume 0.3 µL) was infused bilaterally targeting the M2 region of the PFC (AP +2.7, L ±1.8, DV −2.0 using a Flat Skull and 34 degree lateral angle; see Supporting Information Methods for surgical and infusion procedures).
GABA-A/B (muscimol/baclofen) PFC—Prelimbic (PL) inactivation
To determine whether inactivation of GABA receptors in a more ventral region of the PFC would affect binge-type behavior, the muscimol/baclofen cocktail, or PBS was infused bilaterally targeting the PL region of the PFC (AP +3.0, L ±1.7, DV −3.5; flat skull, 14 degree lateral angle).
D1 agonist (SKF 81297) targeting PFC—M2 region
The effects of manipulating dopamine receptor activation in the PFC on fat intake were also evaluated. SKF 81297 (0.03, 0.1, 0.3 µg/infusion; Tocris Bioscience, Minneapolis, MN) or PBS was infused bilaterally targeting the M2 region.
D1 antagonist (SCH 23390) targeting PFC—M2 region
SCH 23390(0.1, 0.3, 1.0 µg/infusion; Tocris Bioscience, Minneapolis, MN) or PBS was infused bilaterally targeting the M2 region.
D2 agonist (quinpirole) targeting PFC—M2 region
Quinpirole (1, 3, 10 µg/infusion; R&D Systems (Tocris), Minneapolis, MN) or PBS was infused bilaterally, targeting the M2 region.
D2 antagonist (eticlopride) targeting the Cg1/M2
Eticlopride (0.1, 0.3 µg/infusion; 0.5 µL/infusion; Sigma-Aldrich, Allentown, PA) or PBS was infused bilaterally, targeting the M2/Cg1 area (AP +2.2, L ±1.4, DV −1.3; 15 degree lateral angle).
Statistical analyses
Results
Binge-type eating alters dopaminergic and GABAergic gene expression
INT rats consumed significantly more shortening than D rats in the week before sacrifice [average ± SE INT: 4.4 ± 0.5g; D: 2.9± 0.4 g; main effect of diet F(1,26) = 7.20, P < 0.02] but not during week 1 [average ± SE INT: 2.1 ± 0.5g; D: 1.5± 0.2 g; F(1,26) = 1.41, P = NS]. There was no effect of pre/post designation nor was there an interaction at either time point. Thus, although intakes in the groups were matched at the start of the study, they increased across time such that INT rats were bingeing relative to D rats before sacrifice. In addition, body weights did not differ between INT and D groups by the end of the study [main effect of diet: F(1,26) = 3.40, P = NS; no main effect of pre/post designation, no interaction].
Significant differences in gene expression between INT and D rats were seen only in the VTA and PFC. There were no significant differences between groups or time points in the NA shell, NA core, or central nucleus of the amygdala.
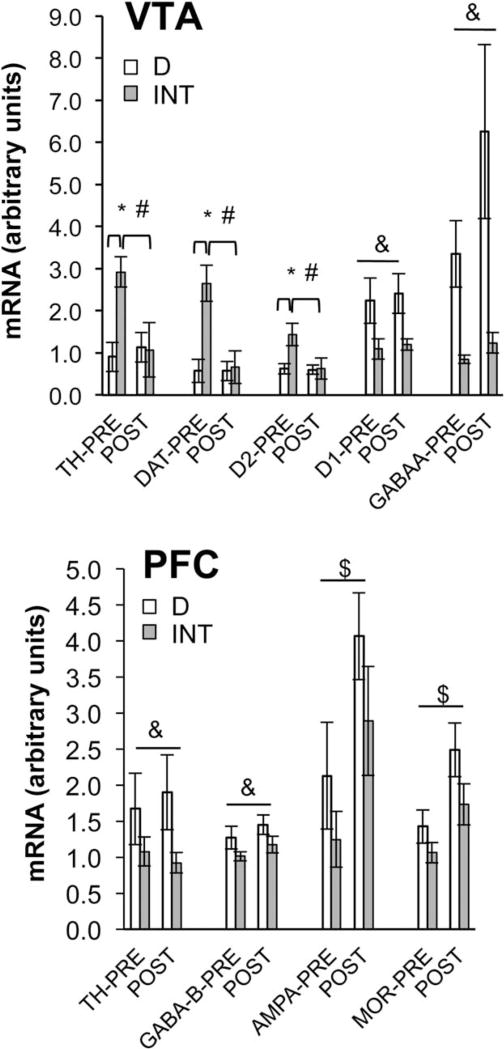
Within the VTA, binge-related alterations in gene expression were observed primarily for the dopamine system (Figure 1, top). Within the INT rats, mRNA for tyrosine hydroxylase, the dopamine transporter, and the D2-like receptor was higher before the binge than after. In contrast, consuming fat had no effect on the expression of these genes in the D rats. Furthermore, relative to the D rats, gene expression for these three proteins was higher in the INT rats before the binge, but no different from D after the binge (LS means P ≤ 0.025 for all). Thus, the act of binge eating appeared to “normalize” aspects of dopaminergic signaling within this brain area. However, gene expression for the D1-like receptor and for the GABA-A receptor was significantly lower in the INT rats relative to D, and was not “normalized” by binge-type eating.
Figure 1.
Gene expression within the VTA (top) and medial PFC (bottom) in bingeing (INT) and control (D) rats 20 min before and 20 min after fat presentation. TH, tyrosine hydroxylase; DAT, dopamine transporter; MOR, mu opioid receptor. Top graph (VTA): *INT > D before shortening presentation for TH, DAT, and D2 (LS means comparisons P < 0.025 for all). Diet × time interactions TH: F(1,15) = 8.56, P < 0.02; DAT: F(1,15) = 10.35, P < 0.01; D2: F(1,15) = 5.74, P < 0.02. #INT pre > INT post for TH, DAT, and D2 (LS means comparisons P < 0.025 for all). &Main effect of diet (INT < D) for D1 [F(1,15) = 6.75, P < 0.05] and GABA-A [F(1,15) = 6.58, P < 0.05]. INTpre n = 5; INTpost n = 3; Dpre n = 6; Dpost n = 5. Bottom graph (PFC): &Main effect of diet (INT < D) for TH F(1,19) = 4.39, P < 0.05 and GABA-B F(1,21) = 4.58, P < 0.05. $Main effect of time (pre < post) for AMPA [F(1,18) = 7.88, P < 0.02] and MOR [F(1,21) = 8.38, P < 0.01]. INTpre n = 7; INTpost n = 5; Dpre n = 5; Dpost n = 8.
In the PFC, no “normalization” of gene expression occurred as a function of bingeing (Figure 1, bottom). mRNA for tyrosine hydroxylase and the GABA-B receptor was significantly lower in INT rats relative to D (main effect of diet P ≤ 0.05 for both), but was unaffected by time. In contrast, mRNA for AMPA and for the mu-opioid receptor (MOR) was not significantly different between INT and D rats, although INT gene expression was somewhat lower than D (main effect of diet P = 0.1240, 0.0750, for AMPA and MOR, respectively). However, gene expression for AMPA and MOR was significantly higher after consuming fat than before in both the INT and D groups (main effect of time P ≤ 0.05 for both AMPA andMOR).
PFC pharmacological GABA-A/B inactivation with muscimol/baclofen cocktail
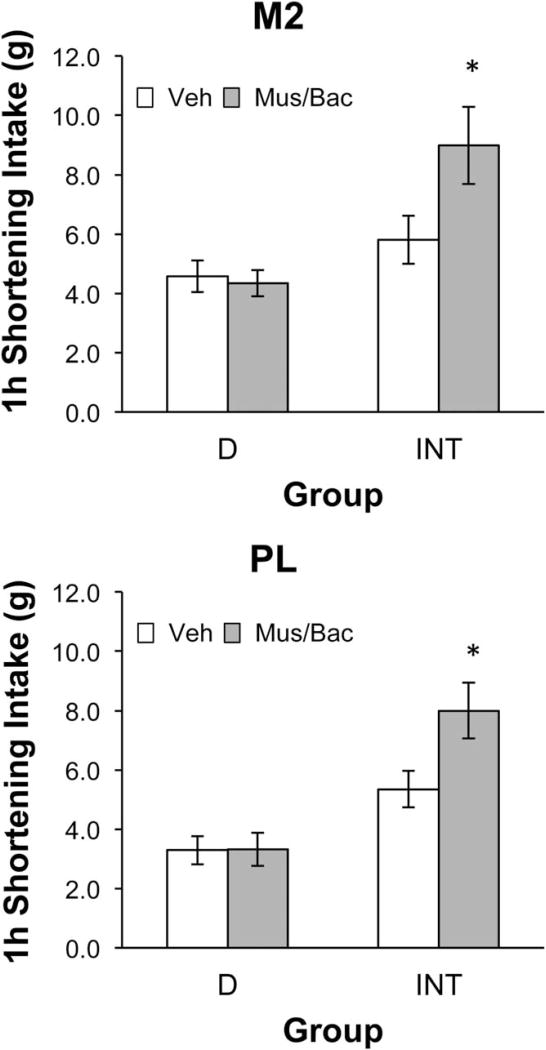
There was no significant effect of muscimol/baclofen injections on fat intake when all rats were included in the analysis (see Table 1). However, due to unusually high intakes in some of the INT rats and the statistical trend toward interaction after the M2 infusions when all rats were included, there was concern that ceiling effects on intake might mask possible stimulatory effects of inactivation in the INT rats. Therefore, we analyzed data for those rats that consumed 8 g or less of fat after receiving vehicle. Eight grams was selected as an amount that would allow a stimulation of intake to be determined, based upon previous experience with this protocol and the estimated gastric capacity of rats of this size (14), while still maintaining a sufficient number of animals for the analyses. For the M2 analyses, 62% of the INT rats (8 out of 13 rats) and 91% of the D rats (10 out of 11 rats) consumed ≤8 g after vehicle. For the PL analyses, 64% of the INT rats (7 out of 11) and 88% of the D rats (7 out of 8 rats) consumed ≤8 g after vehicle. Inactivation of either the M2 (Figure 2, top) or the PL region (Figure 2, bottom) of the PFC significantly stimulated binge intake in the INT rats (paired t-tests P ≤ 0.05 for both regions) but was without effect in the D rats. Body weight did not influence the results obtained in the M2 or PL that are shown in Figure 2 or Table 1 (see Supporting Information Results).
TABLE 1.
Effects of PFC pharmacological GABA-A/B inactivation with muscimol/baclofen cocktail on fat intake with all rats
| Group | Vehicle (g ± SE) |
Muscimol/baclofen (g ± SE) |
|---|---|---|
| M2 region | ||
| D (n = 11) | 4.9 ± 0.6 | 4.5 ± 0.4 |
| INT (n = 13) | 7.2 ± 0.7 | 8.8 ± 0.8 |
| PL region | ||
| D (n = 8) | 3.9 ± 0.7 | 4.0 ± 0.8 |
| INT (n = 11) | 7.0 ± 0.8 | 7.4 ± 0.6 |
In M2 region, ANOVA main effect of group F(1,22) = 16.68, P < 0.001. Main effect of dose F(1,22) = 1.08, P = 0.31. Interaction F(1,22) = 3.58, P = 0.07.
In PL region, ANOVA main effect of group F(1,17) = 15.47, P < 0.01. Main effect of dose F(1,17) = 0.11, P = 0.74. Interaction F(1,17) = 0.05, P = 0.82.
Figure 2.
Effects of pharmacological inactivation using a cocktail of the GABA-A agonist muscimol and the GABA-B agonist baclofen (Mus/Bac) delivered into either the M2 (top) or PL (bottom) region of the mPFC on shortening consumption in bingeing (INT) or control (D) rats during the 1-h shortening access period. Data are presented for those rats consuming ≤8 g of shortening after vehicle. Inactivation significantly stimulated shortening intake in INT, but not D, rats. *Intake after Mus/Bac significantly greater than intake after vehicle (P ≤ 0.05). Top graph (M2): dose × group interaction F(1,16) = 8.69, P < 0.01; main effect of group F(1,16) = 9.55, P < 0.01; main effect of dose F(1,16) = 6.42, P < 0.05. INT paired t-test t (veh vs. Mus/Bac) = −2.59, P < 0.05 (n = 8). D paired t-test (veh vs. Mus/Bac) t = 0.67, P = NS (n = 10). Bottom graph (PL): dose × group interaction F(1,12) = 5.22, P < 0.05. Main effect of group F(1,12) = 19.63, P < 0.001; main effect of dose F(1,12) = 5.45, P < 0.05. INT paired t-test (veh vs. Mus/Bac) t = −3.40, P < 0.02 (n = 7); D paired t-test (veh vs. Mus/Bac) t = −0.03, P = NS (n = 7).
D1/D2 PFC pharmacological activation and blockade
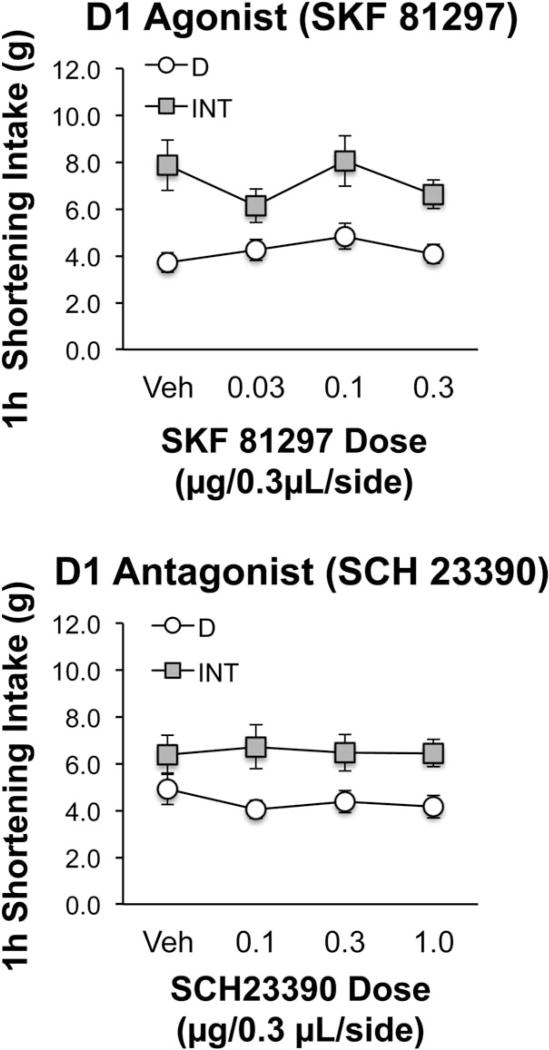
Neither the D1 agonist (SKF 81297) nor the D1 antagonist (SCH 23390) infused into the M2 region of the PFC affected shortening intake (Figure 3). Similar results were obtained when data from only those rats consuming ≤8 g after vehicle were analyzed (data not shown).
Figure 3.
Neither the D1 agonist SKF 81297 (top) nor the D1 antagonist SCH 23390 (bottom) significantly affected shortening intake in bingeing (INT) or control (D) rats when infused into the M2 region of the mPFC. SKF 81297 n = 10 (D group), n = 8 (INT group); SCH 23390 n = 14 (D group), n = 16 (INT group).
The lack of effect after the D1 antagonist was probably not due to the apparent lack of bingeing in INT rats after vehicle administration. The INT rats in this study consumed significantly more shortening than the D rats after all other doses of SCH 23390 [main effect of group F(1,28) = 6.83, P < 0.02; P < 0.05 for comparisons between groups at each dose except vehicle] as well as in the week immediately preceding the initiation of infusion tests (t = −3.65, P < 0.01). INT rats weighed more than D rats by the end of the SCH 23390 study (INT: 393.0 ± 3.4 g; D: 376.6 ± 3.3 g; t = −3.43; P < 0.01); therefore, data were normalized to body weight and reanalyzed. Similar results were still obtained, i.e., there was no effect of the D1 antagonist for all rats or for the subset that consumed ≤8 g after vehicle. Thus, body weight differences did not contribute to the negative drug results. Body weight also did not contribute to the binge-type behavior of the INT rats relative to D. Even when normalized to body weight, INT rats consumed significantly more shortening than D rats during the SCH 23390 tests (main effect of group F(1,28) = 6.23; P < 0.02). See also Supporting Information Results.
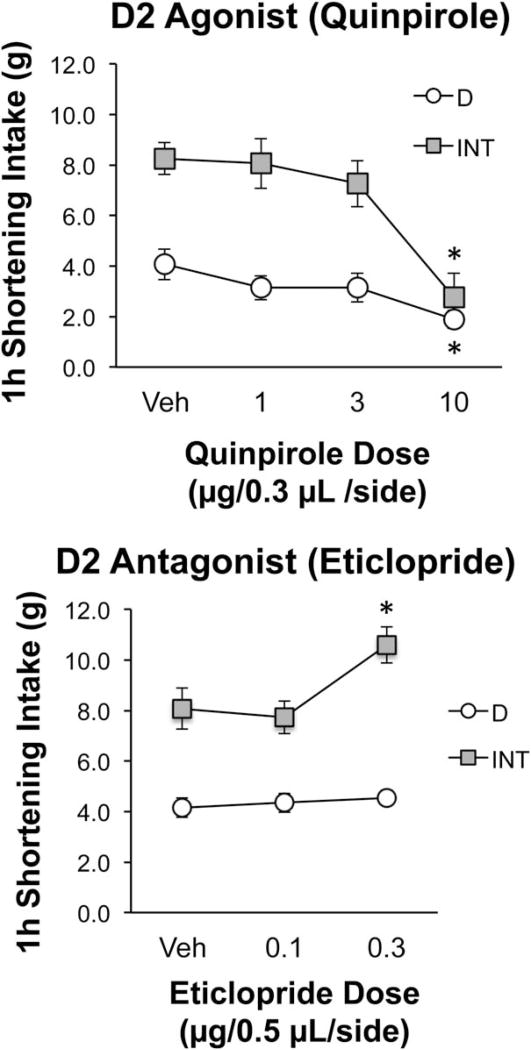
When the D2 agonist (quinpirole) was infused into the M2 region, shortening intake was significantly reduced after the highest dose in both INT and D rats (Tukey’s HSD, P ≤ 0.05; Figure 4, top). Since intakes were reduced, there was concern that this may have been due to nonspecific motoric deficits. Therefore, consumption of the simultaneously available chow was also analyzed. Quinpirole had no significant effect on chow intake indicating that the capacity to eat was still intact (see Table 2). See also Supporting Information Results.
Figure 4.
Effects of the D2 agonist quinpirole (top) and the D2 antagonist eticlopride (bottom) on shortening intake in bingeing (INT) and control (D) rats when infused into the M2 region of the mPFC. Top graph: The D2 agonist quinpirole reduced shortening intake in INT and D rats when infused into the M2 region of the mPFC. Dose × group interaction F(3,51) = 5.21, P < 0.01. *Significantly less than vehicle (Tukey’s HSD P ≤ 0.05). n = 9 (INT group); n = 10 (D group). Bottom graph: The D2 antagonist eticlopride stimulated shortening intake in INT rats but had no effect in D when infused into the M2/Cg1 region of the mPFC. Dose × group interaction F(2,86) = 7.55, P = 0.0010. *Significantly greater than vehicle (Tukey’s HSD P ≤ 0.05). n = 27 (D group); n = 18 (INT group).
TABLE 2.
Effects of the D2 agonist quinpirole infused into the M2 region of the PFC on chow intakea
| Group | Vehicle (g ± SE) |
1 µg (g ± SE) |
3 µg (g ± SE) |
10 µg (g ± SE) |
|---|---|---|---|---|
| D | 0.9 ± 0.3 | 0.6 ± 0.3 | 0.5 ± 0.2 | 1.4 ± 0.3 |
| INT | 1.4 ± 0.4 | 1.1 ± 0.4 | 1.1 ± 0.4 | 1.3 ± 0.3 |
ANOVA main effect of group F(1,17) = 1.18, P = 0.29. Main effect of dose F(3,51) = 1.76, P = 0.17. Interaction F(3,51) = 0.87, P = 0.46.
Chow and fat were simultaneously available during the 1-h fat access period.
In contrast to the results obtained with D2 stimulation, D2 blockade with eticlopride stimulated intake in the INT rats, but was without effect in the D rats (Tukey’s HSD, P ≤ 0.05; Figure 4, bottom). Eticlopride also stimulated intake in the subset of INT rats consuming ≤8 g after vehicle (50% of the rats; n = 9) [dose × group interaction F(2,86) = 8.00, P < 0.001; Tukey’s post hoc P ≤ 0.05 for 0.3 µg vs. vehicle]. There was no effect of eticlopride in D rats consuming ≤8 g after vehicle (100% of the rats; n = 27). See also Supporting Information Results.
Discussion
Dysregulated neural processing by the VTA and its mesocortical projection targets has been proposed to underlie maladaptive eating (3), but few studies have directly investigated the contribution of classic mesocorticolimbic neurotransmitter systems to binge eating. We provide direct correlative and functional evidence for dysregulated neurotransmitter processing by the VTA and dmPFC in bingeing rats. In addition, we report differential responsiveness of bingeing and control rats to temporary PFC inactivation and D2-like receptor blockade within the PFC. Interestingly, bingeing resolved some gene expression differences that preceded binge onset, suggesting that bingeing may normalize a dysregulated system. However, some differences were not resolved by bingeing, which may render individuals vulnerable to future binge events. The present studies indicate that repeated binge episodes, even in the absence of food-deprivation, may provoke neuronal adaptations that serve to perpetuate aberrant eating behavior.
In binge rats, elevations in VTA dopaminergic gene expression before the binge returned to control levels after the binge. However, VTA gene expression in control rats was unaffected by a brief bout of fat consumption. The initially-elevated VTA dopaminergic gene expression may contribute to binge initiation and the drive for temporary relief of negative mood that occurs during a binge (15). Only in the VTA, and not in the other brain regions examined here, did we observe a restoration of the elevated gene expression to control levels by bingeing. This is consistent with reports suggesting the importance of VTA dopaminergic signaling to cue-induced learning, behavioral initiation, and mood (16).
Within the PFC, the collective data show a general state of reduced gene expression, suggesting long-term depression of neuronal signaling within a region critically involved in executive function, decision-making, and behavioral control (5). The present findings are consistent with reports of reduced PFC activity in subjects with binge-eating disorder (BED) (17), but offer new insights regarding candidate dysregulated neurotransmitter systems that may be contributing to the reduced activity in humans.
Together, the gene expression results strongly suggest that mesocortical circuitry is critically involved in the maintenance of binge-type behavior. Remarkably, there were no differences in gene expression between groups within other classic mesolimbic nuclei, such as the NA and central amygdala. It is possible that if gene expression had been analyzed in the basolateral amygdala, a nucleus implicated in modulating conditioned feeding (18), we may have observed some alterations in pre- vs. post-Crisco access. Such studies are certainly warranted in future experiments. While classical “reward” areas are known to be involved in the consumption of high-fat food (19), the present results indicate that the manner in which high-fat food is consumed is not associated with differential gene expression in those regions. Indeed, the involvement of the PFC, but not the accumbens, has also been reported in rats consuming sweetened fat and exposed to restraint stress and in rats consuming sweet chocolate-flavored pellets (20,21). Together, these findings strongly support a unique role for the PFC in binge-type consumption of palatable food. One limitation of this study was that only mRNA expression was analyzed, while protein expression and phosphorylation remain unknown. Importantly, however, the 40-min time frame between the pre- and within-binge sacrifices is sufficient to observe not only alterations in mRNA, but also protein translation (see for example Ref. 22). Thus, we offer cautious speculation here that these changes in mRNA may be driving similar alterations in protein signaling.
Restricted access to optional food and the associated uncertainty (23,24), as well as possible differential prediction error in INT and D groups (25,26), may be important for driving the binge behavior (see also Supporting Information Discussion for alternative hypotheses). Neuronal firing in the midbrain and dopaminergic signaling in the PFC and NA vary as a function of uncertainty associated with reward delivery (27). Manipulations that modulate the firing of midbrain (VTA) neurons can differentially influence dopamine release in the PFC and accumbens (28). Therefore, uncertainty associated with binge opportunities could explain why VTA gene expression was elevated in the INT rats before shortening access but not in the D rats, since cues associated with fat provision are unpredictable/uncertain in the INT rats, but highly predictable in the D rats. Since binge eating in humans is also associated with uncertainty, the present results may have relevance to bingeing-related disorders (see Ref. 23 for review).
In order to directly examine the role of the PFC in bingeing, we analyzed fat intake in rats following temporary GABAergic PFC inactivation. Inactivation of dorsomedial (M2/Cg1 region) and ventromedial (PL) regions increased binge size in the subset of INT rats consuming ≤8 g after vehicle infusions, but had no effect in D rats. While we originally examined this subset due to concerns regarding ceiling effects on intake, results with eticlopride argue against this concern, as even high INT intakes were stimulated further by D2 blockade. Instead, the results point to the distinction between generalized inhibition of the PFC via GABAergic activation versus targeted blockade of a specific receptor class (D2-like in this case) within that region. In this case, inactivation only stimulated intake in the subset of rats that did not respond as robustly to the INT protocol, i.e., to “binge-resistant” rats (29). This suggests the intriguing possibility that “binge resistance” may be dependent upon PFC circuitry. An important limitation to note is that while data were only included in analyses from rats with confirmed postmortem cannula placements in the PFC, we cannot completely rule out the possibility that a small amount of the intraparenchymal injection volume may have partially diffused to adjacent CNS nuclei.
Importantly, PFC inactivation did not provoke binge-type consumption in either the full cohort or the subset of D rats, indicating that reduced PFC activity alone does not cause binge-type eating. In human subjects with BED, reduced mPFC activity was reported during testing for impulse control (17) and when performing a monetary incentive delay task (30), but the response to food cues is mixed (3). Regardless, the ability to distinguish the cause of bingeing from its effects in humans has proven difficult. The present results strongly suggest that alterations within mesocortical regions are an adaptive consequence of bingeing, not a primary cause.
Although functional dissociation between the dorsal and ventral PFC has been shown (31), these regions are densely interconnected (32) and appear to work together in the coordinated control of behavior. The present report demonstrates the unique role these subregions appear to play in binge-type behavior (as represented by the INT rats) as opposed to ingestion mediated by palatability (as represented by the D rats). In short, the mPFC appears to function as a coordinated “behavioral brake” to inhibit maladaptive or dysfunctional consummatory behavior once that behavior has been established.
Targeted dmPFC infusions of the D2-like antagonist eticlopride produced effects that were similar to inactivation of this region, i.e., stimulation of fat intake in INT but not D rats. The fact that only the bingeing rats were affected by D2 blockade indicates that binge experience alters endogenous D2 receptor signaling within the pdmPFC, in a manner that is distinct from the effects of nonbinge consumption of fat. Furthermore, the fact that D2 blockade did not affect intake in the D rats indicates that cortical D2 receptor activation does not cause binge-type eating. Taken together, these results support the idea that D2-like receptors are a key component of a PFC behavioral brake (see also Supporting Information Discussion).
The specific neurological underpinnings of this proposed brake are not known, but the present data suggest that it is at least partially mediated by a shift in the balance of D1 and D2 receptor actions within the PFC. D1-like and D2-like receptors are localized both pre- and postsynaptically within the PFC. Presynaptically, D1-like receptors inhibit glutamate release, whereas D2 receptors inhibit GABA release. Postsynaptically, D1-like receptors can enhance, and D2-like receptors inhibit, excitatory currents (33). Others have proposed these combined effects are critical to normal associative learning, executive function, and the ability to engage in appropriate goal-directed behavior, and that disruptions in D1/D2 balance can result in psychiatric disturbances (e.g., Refs. 26 and 34). In particular, D2 receptors have been proposed to promote behavioral flexibility, allowing ongoing behavior to be disrupted by distractors, whereas D1 receptors are thought to promote more focused, goal-directed behavior that is not as readily interrupted (35). A D2 “brake” would conceivably permit a binge to be interrupted by internal (e.g., satiety signals) or external (e.g., sounds, lights, alternative reinforcers) stimuli. Administration of a D2 antagonist would remove the brake, thereby shifting the balance toward D1 actions, which would focus greater attention on the binge and make it less likely to be interrupted.
The involvement of cortical D2 receptors as an adaptive response to binge-type eating could be secondary to the uncertainty associated with bingeing mentioned earlier. That is, uncertainty regarding binge opportunities would promote differential firing patterns within VTA neurons in the INT and D rats, which would potentially result in increased DA release in the PFC, but not the accumbens, of the INT animals. Since higher DA concentrations have been shown to promote D2, but inhibit D1, signaling in the PFC (36), this transient surge in DA would activate the brake only in the INT rats.
In summary, we report alterations within mesocortical brain regions in rats bingeing on fat. The data presented here suggest that bingeing can temporarily return some, but not all, of the neurological alterations to control levels. Furthermore, D2 receptors within the mPFC appear to function as part of a behavioral brake to limit consummatory excess during a binge. Extensive experience with bingeing may cause the brakes to fail, making it difficult to stop eating once a binge has started and rendering individuals vulnerable to future binge episodes. In short, bingeing may “feed on itself,” fostering a cascading series of neurological events that then serves to perpetuate the behavior.
Supplementary Material
Acknowledgments
Thanks to Zhiping Yu and Mohammad Ebrahimzade Sarvestani for assistance with the infusion studies.
Funding agencies: MH67943 (RLWC), DK085435 (MRH), and DK096139 (MRH).
Footnotes
Disclosure: The authors declared no conflict of interest.
Additional Supporting Information may be found in the online version of this article.
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5. American Psychiatric Publishing; Washington, DC: 2013. (DSM-5). [Google Scholar]
- 2.Hudson JI, Hiripi E, Pope HG, Jr, Kessler RC. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol Psychiatry. 2007;61:348–358. doi: 10.1016/j.biopsych.2006.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garcia-Garcia I, Narberhaus A, Marques-Iturria I, Garolera M, et al. Neural responses to visual food cues: insights from functional magnetic resonance imaging. Eur Eat Disord Rev. 2013;21:89–98. doi: 10.1002/erv.2216. [DOI] [PubMed] [Google Scholar]
- 4.Bello NT, Hajnal A. Dopamine and binge eating behaviors. Pharmacol Biochem Behav. 2010;97:25–33. doi: 10.1016/j.pbb.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fuster JM. The Prefrontal Cortex. Academic Press; San Diego, CA: 2008. [Google Scholar]
- 6.Tritsch NX, Sabatini BL. Dopaminergic modulation of synaptic transmission in cortex and striatum. Neuron. 2012;76:33–50. doi: 10.1016/j.neuron.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berthoud HR. The neurobiology of food intake in an obesogenic environment. Proc Nutr Soc. 2012;71:478–487. doi: 10.1017/S0029665112000602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahn S, Phillips AG. Modulation by central and basolateral amygdalar nuclei of dopaminergic correlates of feeding to satiety in the rat nucleus accumbens and medial prefrontal cortex. J Neurosci. 2002;22:10958–10965. doi: 10.1523/JNEUROSCI.22-24-10958.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spangler R, Wittkowski KM, Goddard NL, Avena NM, Hoebel BG, Leibowitz SF. Opiate-like effects of sugar on gene expression in reward areas of the rat brain. Brain Res Mol Brain Res. 2004;124:134–142. doi: 10.1016/j.molbrainres.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Inoue K, Kiriike N, Okuno M, Fujisaki Y, et al. Prefrontal and striatal dopamine metabolism during enhanced rebound hyperphagia induced by space restriction–a rat model of binge eating. Biol Psychiatry. 1998;44:1329–1336. doi: 10.1016/s0006-3223(97)00518-0. [DOI] [PubMed] [Google Scholar]
- 11.Carr KD. Food scarcity, neuroadaptations, and the pathogenic potential of dieting in an unnatural ecology: binge eating and drug abuse. Physiol Behav. 2011;104:162–167. doi: 10.1016/j.physbeh.2011.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corwin RL, Wojnicki FHE. Binge-type eating induced by limited access to optional foods. In: Avena NM, editor. Animal Models of Eating Disorders. Vol. 74 Springer Science + Business Media, LLC; Totowa, NJ: 2013. pp. 51–68. Neuromethods . [Google Scholar]
- 13.Bence KK, Delibegovic M, Xue B, et al. Neuronal PTP1B regulates body weight, adiposity and leptin action. Nat Med. 2006;12:917–924. doi: 10.1038/nm1435. [DOI] [PubMed] [Google Scholar]
- 14.Bull LS, Pitts GC. Gastric capacity and energy absorption in the force-fed rat. J Nutr. 1971;101:593–596. doi: 10.1093/jn/101.5.593. [DOI] [PubMed] [Google Scholar]
- 15.Deaver CM, Miltenberger RG, Smyth J, Meidinger A, Crosby R. An evaluation of affect and binge eating. Behav Modif. 2003;27:578–599. doi: 10.1177/0145445503255571. [DOI] [PubMed] [Google Scholar]
- 16.Pignatelli M, Bonci A. Role of dopamine neurons in reward and aversion: a synaptic plasticity perspective. Neuron. 2015;86:1145–1157. doi: 10.1016/j.neuron.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 17.Balodis IM, Molina ND, Kober H, et al. Divergent neural substrates of inhibitory control in binge eating disorder relative to other manifestations of obesity. Obesity (Silver Spring) 2013;21:367–377. doi: 10.1002/oby.20068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holland PC, Petrovich GD. A neural systems analysis of the potentiation of feeding by conditioned stimuli. Physiol Behav. 2005;86:747–761. doi: 10.1016/j.physbeh.2005.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rada P, Avena NM, Barson JR, Hoebel BG, Leibowitz SF. A high-fat meal, or intraperitoneal administration of a fat emulsion, increases extracellular dopamine in the nucleus accumbens. Brain Sci. 2012;2:242–253. doi: 10.3390/brainsci2020242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bello NT, Walters AL, Verpeut JL, Caverly J. Dietary-induced binge eating increases prefrontal cortex neural activation to restraint stress and increases binge food consumption following chronic guanfacine. Pharmacol Biochem Behav. 2014;125:21–28. doi: 10.1016/j.pbb.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 21.Blasio A, Steardo L, Sabino V, Cottone P. Opioid system in the medial prefrontal cortex mediates binge-like eating. Addict Biol. 2014;19:652–662. doi: 10.1111/adb.12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sie KC, Rubel EW. Rapid changes in protein synthesis and cell size in the cochlear nucleus following eighth nerve activity blockade or cochlea ablation. J Comp Neurol. 1992;320:501–508. doi: 10.1002/cne.903200407. [DOI] [PubMed] [Google Scholar]
- 23.Corwin RL. The face of uncertainty eats. Curr Drug Abuse Rev. 2011;4:174–181. doi: 10.2174/1874473711104030174. [DOI] [PubMed] [Google Scholar]
- 24.Wojnicki FH, Babbs RK, Corwin RL. Reinforcing efficacy of fat, as assessed by progressive ratio responding, depends upon availability not amount consumed. Physiol Behav. 2010;100:316–321. doi: 10.1016/j.physbeh.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- 26.Puig MV, Antzoulatos EG, Miller EK. Prefrontal dopamine in associative learning and memory. Neuroscience. 2014;282:217–229. doi: 10.1016/j.neuroscience.2014.09.026. C: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stefani MR, Moghaddam B. Rule learning and reward contingency are associated with dissociable patterns of dopamine activation in the rat prefrontal cortex, nucleus accumbens, and dorsal striatum. J Neurosci. 2006;26:8810–8818. doi: 10.1523/JNEUROSCI.1656-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vittoz NM, Berridge CW. Hypocretin/orexin selectively increases dopamine efflux within the prefrontal cortex: involvement of the ventral tegmental area. Neuropsychopharmacology. 2006;31:384–395. doi: 10.1038/sj.npp.1300807. [DOI] [PubMed] [Google Scholar]
- 29.Boggiano MM, Artiga AI, Pritchett CE, Chandler-Laney PC, Smith ML, Eldridge AJ. High intake of palatable food predicts binge-eating independent of susceptibility to obesity: an animal model of lean vs obese binge-eating and obesity with and without binge-eating. Int J Obes (Lond) 2007;31:1357–1367. doi: 10.1038/sj.ijo.0803614. [DOI] [PubMed] [Google Scholar]
- 30.Balodis IM, Grilo CM, Kober H, et al. A pilot study linking reduced fronto-striatal recruitment during reward processing to persistent bingeing following treatment for binge-eating disorder. Int J Eat Disord. 2014;47:376–384. doi: 10.1002/eat.22204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Passetti F, Chudasama Y, Robbins TW. The frontal cortex of the rat and visual attentional performance: dissociable functions of distinct medial prefrontal subregions. Cereb Cortex. 2002;12:1254–1268. doi: 10.1093/cercor/12.12.1254. [DOI] [PubMed] [Google Scholar]
- 32.Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct. 2007;212:149–179. doi: 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- 33.Xing B, Li YC, Gao WJ. Norepinephrine versus dopamine and their interaction in modulating synaptic function in the prefrontal cortex. Brain Res. 2016;1641(Pt B):217–233. doi: 10.1016/j.brainres.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lapish CC, Kroener S, Durstewitz D, Lavin A, Seamans JK. The ability of the mesocortical dopamine system to operate in distinct temporal modes. Psychopharmacology (Berl) 2007;191:609–625. doi: 10.1007/s00213-006-0527-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol. 2004;74:1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 36.Trantham-Davidson H, Neely LC, Lavin A, Seamans JK. Mechanisms underlying differential D1 versus D2 dopamine receptor regulation of inhibition in prefrontal cortex. J Neurosci. 2004;24:10652–10659. doi: 10.1523/JNEUROSCI.3179-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.