Abstract
This study was carried out to evaluate the effects of vitamin A (Vit A) and probiotic co-supplementation with rabies vaccine on humoral immune response in New Zealand white (NZW) rabbits. For this experiment, 54 rabbits were randomized into six experimental and three control groups. Mixed cultures of commercial probiotics supplements and a dose of Vit A were administered to each animal. Results were compared with the control group fed with only basal diet. Animals in different treatment groups showed significantly higher sero-conversions against rabies vaccine. There was a significant increase (p < 0.001) in the titers of rabies antibodies in all treatment groups on 14th and 35th days than control C3 group. Both commercial probiotics irrespective of brand increase the humoral immune response of rabbits against rabies vaccine. The mean titer values of all groups G1–G6 and sub-controls (C1, C2) were generally above 3.6 EU/ml on day 14th and between 3.7 and 3.9 EU/ml, showing highest sero-conversion on 35th day than mean titer of C3 control = 3.091 and 3.505 EU/ml respectively on both days. The maximum titer values were obtained with the addition of organic carrots to the daily diet. These results suggest that simple dietary interventions using probiotics and Vit A in natural form may enhance the efficacy of rabies vaccine in the host. These cost-effective strategies can be applied for getting higher yields of polyclonal antibody production in animal models, thus providing promising means of improving the final product yield and can be adopted easily by the manufacturers.
Keywords: Rabies vaccine, Probiotics, Vitamin A, Humoral immune response, Rabbit
Introduction
Rabies is a fatal viral encephalitis that kills approximately 59,000 people each year globally, yet it is included in the list of neglected tropical diseases (Rimal et al. 2020). According to global estimates, almost 95% cases of human rabies are reported from Asia and Africa alone where 99% of human rabies deaths are caused by stray dogs (Anderson and Shwiff 2015; Gan et al. 2023). Pakistan is situated in an important geographical region of South Asia, where the rabies disease burden is quite higher than rest of the world. Although, it is reported that each year 2000–5000 people die from dog bites in Pakistan, this may be an underestimation of the true disease burden (Iqbal et al. 2020).
Rabies is fortuitously a vaccine preventable disease and effective pre- or post-exposure prophylaxis (PEP) is one of the most important preventive measures in areas where dog bites are quite common (Yousaf et al. 2012; Schwartz and Caplan 2021). Rabies vaccine is among a few human vaccines that can protect against disease after an exposure to a pathogen has occurred. Rabies PEP includes immunization with multiple doses of vaccine, administered over a specific time period for effective disease protection (Briggs and Moore 2021). Cell culture rabies vaccine (CCRV) is among the most effective human rabies vaccine ever developed for four decades and has changed the way for successful implementation of rabies prevention programs worldwide (Shuman and Hirsh 2000; Abedi et al. 2022).
The high mortality due to this zoonotic disease is mainly attributed to the lack of access to an affordable treatment referred to as PEP that includes timely rabies vaccination and immunoglobulin (antiserum) therapy in severe bite cases. Few high income countries provide expensive rabies vaccine free of cost to the victims of poor high risk countries. Moreover, rabies immunoglobulin (RIGs) are unavailable in rural areas of Africa and Asia having 90% of the rabies disease burden. The overall treatment cost of dog bite cases is too high and beyond the reach of the common public, thus further imposing a financial burden on the high risk poor population already suffering from various communicable diseases (Radhakrishnan et al. 2020).
World Health Organization (WHO) has recently revised its recommendations for rabies prophylaxis after a scientific review by a SAGE (Strategic Advisory Group of Experts) group. The immunization strategies are published in scientific literature comparing different rabies vaccine regimes including intramuscular (IM) and recently recommended intradermal regimens based on immunogenicity, cost efficacy, vaccine requirements to get desired humoral response in humans and animals (Warrell 2019; Quiambao et al. 2022). For category III injuries, the immediate treatment also includes rabies immunoglobulin (RIGs) along with a vaccine for complete protection (Quiambao et al. 2019).
Rabies prevention remains a challenge for successful control in low and middle income countries of the world including Pakistan due to the shortage of rabies vaccine, anti-sera and overall high total treatment costs, thus leaving most dog bite victims untreated. Therefore, simple and practical measures are required to improve the efficacy of vaccines for the vulnerable population with weak immunity. Moreover, using simpler and cost-effective strategies to get higher antibody yield required for antiserum production provides better outcomes. This results in enhanced production of locally manufactured animal derived products to cater the shortage of antisera, hence providing better access for needy patients to immunoglobulin therapy against deadly diseases. Many reviews and studies have intensified the need to continuously improve the vaccine efficacy with affordable dietary interventions such as probiotics, vitamins and other nutraceuticals but further research is required on parenteral vaccines like rabies (Alagawany et al. 2021). It is well recognized that gut microbiota plays a key role in shaping immune system maturation and activity.
Probiotics are gram positive bacteria, also termed as functional foods or nutritional supplements that play a major role in host health. Probiotics have the ability to alter, modify, and reinstate the pre-existing intestinal flora of the host, hence facilitating the smooth functioning of the intestinal environment. The most commonly used probiotics include Lactobacilli, Bifidobacterium, Saccharomyces boulardii and Bacillus coagulans. Lactic acid bacteria (LAB) are the most studied group of gram positive bacteria which includes Lactobacilli, Streptococci, and Enterococci. These microorganisms are called GRAS (Generally Recognized as Safe) by intentional health authorities. During the last two decades, these beneficial bacteria have been widely used as an important research tool, especially in the development of mucosal vaccines (Wang et al. 2020).
Probiotics are defined as “live microorganisms that confer a health benefit when administered in adequate amounts” and act as biological response modifiers, thus influencing the microbiota composition of the host (Abid et al. 2022a, b; Idrees et al. 2022). Probiotics gained attention as potential vaccine adjuvants for boosting humoral immunity, having the capability to modulate systemic and mucosal immune functions of the vulnerable population including children and elderly individuals (Taghinezhad-S et al. 2021).
Moreover, the role of vitamin A supplementation (VAS) on immune responses has been well documented. In many countries VAS has been routinely integrated with childhood vaccination campaigns especially in low and middle income countries to improve immune status and reduce disease burden among children with generally weak health and immunity (Oresanya et al. 2022).
Keeping in view all above stated advantages of probiotics and vitamin A, this study was designed to explore new affordable strategies with rabies vaccines to get better humoral immune outcomes. The study aims to evaluate the potential immuno-modulatory effects of probiotics and vitamin A (Vit A) on rabies vaccine humoral response in animal models. Further studies are still needed on the optimization of strains, dosage and duration of probiotics administration on large donor animals for the production of animal derived anti-sera and clinical trials on humans regarding the immuno-modulatory potential of probiotics for better immune outcomes, especially for children and the elderly population with weak immunity.
Materials and methods
Study design and experimental animals
A 6-week experiment was designed on 54 New Zealand white (NZW) rabbits, weighing 1.5–2.0 kg and approximately 5–6 weeks of age. Six animals were included in each group (n = 6). The study protocol was approved by the ethics committee of the National Institute of Health (NIH), Islamabad (Ethical Approval No. F.1–5/ERC/2020). The animals were vaccinated with a cell culture rabies vaccine (CCRV) purchased from NIH. The animals showing any signs of sluggishness were not included in this study.
Study procedures
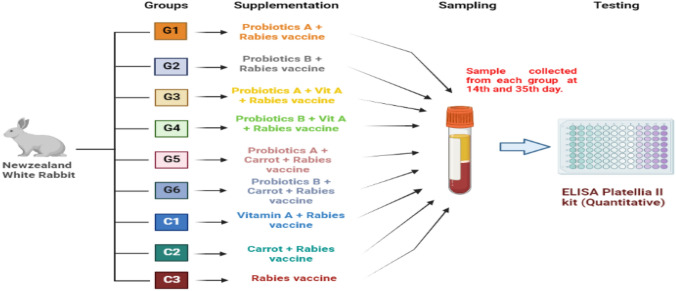
All 54 animals were randomized into six experimental and three control groups as (a) PBA/G1, (b) PBB/G2, (c) PBA/Vit A (G3), (d) PBB/Vit A (G4), (e) PBA/carrot (G5), (f) PBB/carrot (G6), (g) C1, (h) C2, and (i) C3 groups. Commercial probiotics supplements, mostly containing mixed cultures of Lactobacilli and Bifidobacteria strains were administered for a total of 8 weeks. The dose of 10,000 IU Vit A for each animal in the designated groups was provided for 6 weeks along with probiotics. Organic carrots were included only in the diet of specific groups to check the effect of natural source of Vit A in the diet on general health and immunity. In addition, physical fitness, mortality ratio and clinical parameters were also observed to find out the correlation of interventions on the general health performance of rabbits. Blood was drawn from the marginal ear vein of rabbits to study overall blood parameters. The rabies antibodies were evaluated to study the immune outcome of rabies vaccination on days 14th and 35th using ELISA kit Platellia II specially designed test for rabies antibody detection (Fig. 1).
Fig. 1.
Flow chart of study design describing animal groupings according to different dietary treatments, blood sampling and ELISA testing for titer determination. G1 and G2 groups were supplemented with two different probiotics, PBA and PBB, G3 and G4 groups received Vit A along with both types of probiotics. Similarly G5 and G6 were fed with carrots along with PBA and PBB respectively. C1 and C2 groups were only administered VAS and carrots without probiotics. C3 is the main control group without any added supplementation. All groups were immunized with the same rabies vaccine schedule of 2-site doses on days 0, 3rd, 7th and 28th using the TRC-ID regimen for rabies vaccine
Vaccine and immunization of animals
All the animals in experimental and control groups were immunized with CCRV, prepared by the Biological Production Division of NIH, Pakistan. Each vaccine dose contains purified inactivated rabies virus > 2.5 IU as per WHO recommendations. An Intradermal Thai Red Cross (TRC-ID) immunization regimen was used with 2-site (2–2–2–0–2) injections for 0, 3rd, 7th, 14th and 28th days. This intradermal regimen was selected to achieve the desired humoral response in the form of an antibody titer with fewer vaccine dose requirements and reduced immunization duration. The dose of vaccine was 0.1 ml at each site, therefore, for 2 sites total dose of 0.1 × 2 ml was injected at a time to get a better immune response as compared to the previously described IM schedule.
Housing and diet of experimental animals
Animal care and husbandry were provided as per institutional guidelines for the care and use of laboratory animals. Each rabbit was placed in a separate compartment to ensure the consumption of a proper diet and was properly caged with proper provision of heating and cooling arrangements. Animals were fed on a standardized diet of animal houses formulated on current industry nutrient specifications. Moreover, in specific carrot groups (G5, G6 and C2), 18 g of organic carrots were fed daily to each animal.
Probiotics selection for the experiment
Each animal in the experimental groups daily received a 2 ml suspension containing the mixed commercial probiotic as per the manufacturer’s instructions. Two freeze-dried commercial probiotics were purchased from the market and were used for food supplementation in this study. Animals were fed with probiotics as per daily recommended doses in daily feed for 2 weeks before day zero till the 28th day and then kept only on a maintenance diet from day 29th till completion of the experiment.
Composition of probiotic A/PBA; (proprietary blend, Equate™)
Each animal was fed one capsule daily, in a liquid suspension containing approximately 30 billion CFU (colony-forming units) with 18 billion Bifidobacteria and 12 billion CFU Lactobacilli cultures.
Composition of probiotic B/PBB; (proprietary blend, Top formula™)
Animals consumed two capsules in suspension as a daily serving according to the manufacturer’s daily dose recommendation of the product. Each capsule contained seven bacterial strains with a total of 10 billion CFU daily doses. The control groups received only a standard diet with defined diet composition as C1 was supplemented with only Vit A, C2 group was fed with organic carrots and C3 was without any diet supplementation. However, all nine groups were immunized with the same vaccination schedule and immunization protocol.
Serum sample collection and rabies antibody titer determination
Approximately, 3 ml venous blood sample was obtained on the day 14th after vaccination and on the 35th day after the first dose in EDTA tubes to get maximum plasma immunoglobulin. Samples were collected just before vaccination on day zero, 14th day and 1 week after the last dose on the 35th day.
Reagent and supplies for ELISA test, (an indirect immune-enzymatic assay)
Antibody titer was determined using the Platellia II™ ELISA kit purchased from Bio-rad, which is a standardized kit, approved by (Office International des Epizooties) OIE, for testing rabies virus specific antibodies in animal sera.
Protocol for detection of antibodies by ELISA
Platellia II kit™ was used for the detection of rabies virus anti-glycoprotein antibodies in serum samples according to the manufacturer’s instructions, ELISA test has many advantages over the mouse neutralization test (MNT) used previously in NIH such as rapidity in obtaining results within 3 h, 99% sensitivity and 99.4% specificity, safety for handlers and provision of both qualitative and quantitative results (Norouzbabaei et al. 2022).
Briefly, serum samples were diluted by adding 10 µl of sample in 990 µl of dilution solution. The detection process involved the distribution of controls (negative and positive), serum samples and quantification standards into micro-plates which were kept in an incubator for 1 h at 37 °C.
After incubation, three washing steps were performed to ensure the removal of unbound antibodies along with other proteins from the samples. A total of 100 µl of conjugate-protein A labeled with peroxidase was added to each well. The micro-plate was kept for second incubation for an hour at 37 °C followed by five washing steps for removal of the unbound conjugate. Peroxidase substrate and Chromogen were added to each well and the plate was incubated at room temperature for 30 min followed by the addition of 100 µl solution of 1 N H2SO4. Then optical density (OD) was obtained at 450 and 620 nm by ELISA micro-plate reader (Diateck, China).
The standard curve was constructed with the dilution of quantification standards. The OD values for the unknown sample were compared with positive controls, which aided in the determination of serum titer values. A direct reading on the standard curve corresponded to the quantified sera titer represented by equivalent units per ml (EU/ml). Titer values were calculated with the provided conversion software tool. Results were categorized as; high sero-conversion, sufficient sero-conversion, insufficient sero-conversion and undetectable sero-conversion at titer values of 4, 0.5–4, 0.125–0.5 and 0.125 EU/ml, respectively.
Statistical analysis
The data were analyzed using Graph Pad Prism (version 9) computer software. One-way analysis of variance (ANOVA) was used to determine the main effects of treatments such as rabies vaccination and dietary supplementation with probiotics and Vit A as the main factors and their interactions in NZW rabbits. Tukey’s post hoc test was used to determine the differences between the treatments and control group, and the effects were considered significant at a probability level of p = < 0.001, CI 95%.
Results
The minimal protective antibody titer recommended by WHO for effective prevention against rabies is 0.50 IU/ml. The same criteria was adopted in this study keeping this neutralizing antibody titer at a minimal level required to protect experimental rabbits. On day 0, the results of serum samples from all groups were non-reactive, thus indicating that none of the animals had either prior contact with the rabies virus or had been immunized against the rabies vaccine. Therefore, the differences in rabies antibody titers found after 14th and 35th days were induced by rabies vaccination as well as by the dietary treatments provided to animals. Thus the overall results of the experiment carried out on NZW rabbits show that the mean rabies antibody titer of the experimental groups was significantly higher than the control group as shown in Table 1. The results demonstrate that dietary interventions such as probiotics and Vit A rich sources exert a better effect on the humoral immune response against rabies vaccine than immunization alone.
Table 1.
Distribution of subject animals based on rabies vaccination and dietary supplementation (Vit A and organic carrots) with probiotics in various sub-groups, each having n = 6 animals
| Group ID | Intervention details | Number of animals | Mean antibody titers by ELISA (EU/ml) on 14th day | Mean antibody titers by ELISA (EU/ml) on day 35th | Total vaccine required/animal |
|---|---|---|---|---|---|
| G1 | RV + PBA | n = 6 | 3.683 | 3.811 | 4 × 0.2 ml |
| G2 | RV + PBB | n = 6 | 3.529 | 3.737 | 4 × 0.2 ml |
| G3 | RV + PBA + Vit A | n = 6 | 3.634 | 3.790 | 4 × 0.2 ml |
| G4 | RV + PBB + Vit A | n = 6 | 3.616 | 3.828 | 4 × 0.2 ml |
| G5 | RV + PBA + organic carrot | n = 6 | 3.824 | 3.936 | 4 × 0.2 ml |
| G6 | RV + PBB + organic carrot | n = 6 | 3.795 | 3.934 | 4 × 0.2 ml |
| C1 | RV + Vit A (sub control) | n = 6 | 3.479 | 3.719 | 4 × 0.2 ml |
| C2 | RV + organic carrots (sub control) | n = 6 | 3.773 | 3.901 | 4 × 0.2 ml |
| C3 | RV only* (control group) | n = 6 | 3.091 | 3.506 | 4 × 0.2 ml |
The total duration of the intervention was 6 weeks. The selected vaccination regimen was a Thai-red cross (TRC), Intradermal (ID) regimen comprising 2-site 4 shots (0, 3, 7, 28) and to immunization, each animal total vaccine used was 0.8 ml. Rabies-specific antibody titer was calculated on days 14th and 35th to study the effect of dietary treatments on the humoral response of animals. Sub-control groups C1 and C2 were not given probiotics, to check the effect of VAS or natural sources on immune outcomes of vaccination. C3 was selected as the main control group and was only vaccinated with a routine schedule and no extra supplementation was carried out to compare the results
Effect of probiotics supplementation on the humoral immune response against rabies vaccine in G1 and G2 experimental groups
In G1 and G2 groups, probiotics A (PBA) and probiotics B (PBB) were administered respectively to animals along with the rabies vaccine for 6–8 weeks (probiotics were given 2 weeks before starting immunization). The titer results obtained after days 14th and 35th of the entire experimental period are presented in Fig. 2a, Table 2. The influence of both types of probiotics, and their effects on the humoral response of vaccination was significant after day 14th and increased gradually on day 35th. Thus dietary supplementation with probiotics significantly increased the humoral response in all animals of both probiotic groups regardless of its brand. ANOVA comparison of antibody titers in both study groups with control group C3 antibody titer showed a significant difference (p = < 0.001, CI 95%).
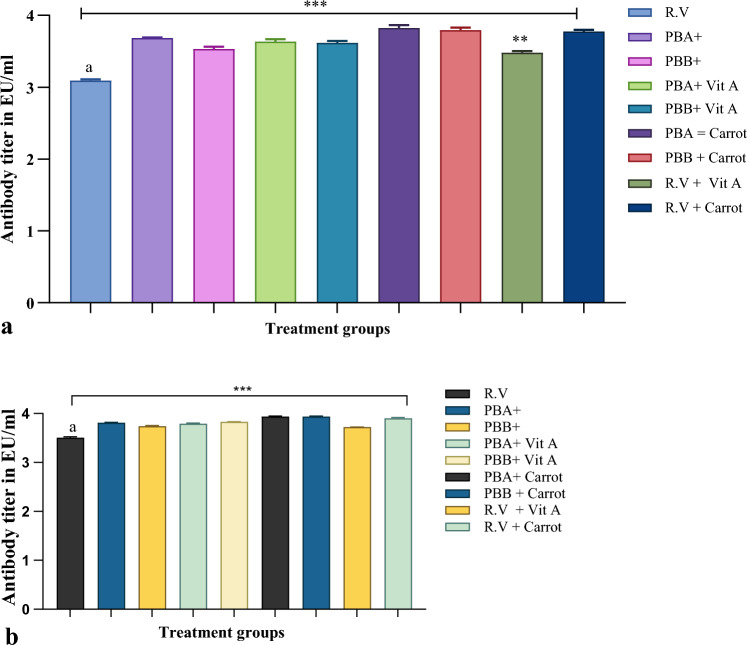
Fig. 2.
Mean antibody titer values against rabies vaccination with concomitant dietary interventions on the 14th and 35th days. Mean titers (± standard deviation) of rabies antibodies in first-time-vaccinated NZW rabbits receiving feed supplementation with a PBA (G1), PBB (G2), b PBA + VA (G3), PBB + VA (G4), c PBA + OC (G5), PBB + OC (G6), d C1, C2 and C3/animal/day for 6 weeks. Two-way ANOVA showed a significant difference among groups difference (p = < 0.001, CI 95%) on both sampling days 14th and 35th. The antibody titer continued to increase until 35 days later but with the same increasing pattern of titer values in experimental groups (G1–G6) than control group C3 (day 35th, mean range of experimental groups 3.7–3.9 IU/ml than mean control value 3.5 IU/ml. PBA probiotic A, PBB probiotic B, VA vitamin A, O.C organic carrots
Table 2.
Antibody titer of animals included in study groups on 14th and 35th day
| Groups | Day of sampling | Animal 1 | Animal 2 | Animal 3 | Animal 4 | Animal 5 | Animal 6 |
|---|---|---|---|---|---|---|---|
| G1 | 14th day | 3.666 | 3.65 | 3.681 | 3.685 | 3.703 | 3.714 |
| 35th day | 3.813 | 3.799 | 3.855 | 3.815 | 3.773 | 3.813 | |
| G2 | 14th day | 3.474 | 3.446 | 3.672 | 3.534 | 3.578 | 3.474 |
| 35th day | 3.745 | 3.754 | 3.723 | 3.815 | 3.694 | 3.69 | |
| G3 | 14th day | 3.481 | 3.723 | 3.602 | 3.685 | 3.635 | 3.679 |
| 35th day | 3.791 | 3.833 | 3.754 | 3.778 | 3.754 | 3.833 | |
| G4 | 14th day | 3.679 | 3.503 | 3.677 | 3.641 | 3.578 | 3.619 |
| 35th day | 3.809 | 3.817 | 3.817 | 3.855 | 3.837 | 3.833 | |
| G5 | 14th day | 3.699 | 3.705 | 3.905 | 3.853 | 3.899 | 3.883 |
| 35th day | 3.921 | 3.938 | 3.912 | 3.930 | > 4 | 3.98 | |
| G6 | 14th day | 3.707 | 3.729 | 3.901 | 3.872 | 3.725 | 3.837 |
| 35th day | 3.958 | 3.969 | 3.921 | > 4 | 3.899 | 3.923 | |
| C1 | 14th day | 3.413 | 3.448 | 3.452 | 3.460 | 3.527 | 3.575 |
| 35th day | 3.725 | 3.736 | 3.705 | 3.723 | 3.729 | 3.701 | |
| C2 | 14th day | 3.694 | 3.846 | 3.723 | 3.767 | 3.793 | 3.820 |
| 35th day | 3.899 | 3.925 | 3.914 | 3.817 | 3.916 | 3.936 | |
| C3 | 14th day | 3.076 | 3.065 | 3.094 | 3.034 | 3.157 | 3.122 |
| 35th day | 3.474 | 3.441 | 3.534 | 3.571 | 3.505 | 3.509 |
The titer values of all groups from G1 to G6, C1 and C2 are compared with the C3 control (without any supplements). A sufficient seroconversion level has been achieved on day 14th after 0, 3, and 7 vaccine shots, well above the protective titer of 0.5 EU/ml. The values gradually enhanced till day 35th with high seroconversion levels in all dietary intervention groups as compared to the control group (C3). Two-way ANOVA comparison of antibody titers in both study groups with control group C3 antibody titer showed a significant difference (p = < 0.001, CI 95%)
Effect of “probiotics and vitamin A” supplementation on the humoral immune response against rabies vaccine in G3 and G4 experimental groups
Animals in groups G3 and G4 were administered VAS along with PBA and PBB, respectively. On day 14th, visible antibody titer enhancement was achieved in both groups which further increased till day 35th in all animals as shown in Table 2. The results obtained are presented in Fig. 2b. The influence of both commercial brands of probiotics, and their effects on humoral response of vaccination was significant in all animals of G3 and G4. Two-way ANOVA comparison of antibody titers in both study groups with control group C3 antibody titer showed a significant difference (p = < 0.001, CI 95%).
Effects of “probiotics and organic carrots” feeding on the humoral immune response against rabies vaccine in G5 and G6 experimental groups
Animals in group G5 were administered probiotic A (PBA) and fresh organic carrots in the diet. The titer started to develop after 1 week and sampling on day the 14th showed sufficient sero-conversion and antibodies concentration as measured in each study animal as shown in Table 2, the results showed values ranging from 3.6 to 3.9 in experimental groups which are significantly higher among all experimental groups and also from C3 control. The mean titer values of G5 and G6 are shown in Fig. 2c. Similarly, on 35th day antibodies titer reached its highest value among all study groups showing high sero-conversion in all animals. Two-way ANOVA comparison of antibody titers in both study groups with control group C3 antibody titer shows a significant difference (p = < 0.001, CI 95%).
Humoral response observations in control and sub-control group (C1, C2 and C3)
Sub-control group C1 was only supplemented with Vit A dose along with rabies vaccination to study the effects of diet-improved changes in animals. The results of mean antibody titer values showed gradual enhancement from day 14th to 35th in all animals. Similarly, fresh organic carrots as natural vitamin sources were added to the diet of group C2 animals. Significant enhancement as observed in n = 6 with titer values of 3.694, 3.846, 3.723, 3.767, 3.793, and 3.820 on the 14th day and 3.899, 3.925, 3.914, 3.817, 3.916, and 3.936 on 35th day respectively. The control C3 group displayed the lowest antibodies concentration as compared with all study groups, showing antibodies level in n = 6 test animals from 3.076, 3.065, 3.094, 3.034, 3.157, and 3.122 EU/ml on 14th day and 3.474, 3.441, 3.534, 3.571, 3.505, and 3.509 EU/ml on 35th day, thus showing relatively slow and weak sero-conversion after 1 week 3 dose and even after 1 month of immunization as compared to experimental groups as shown in Table 2 and Fig. 2d.
Overall humoral response on rabies specific antibody titer values (IgG) in nine experimental groups after 14th and 35th days
The level of the anti-rabies antibody was determined as mean absorbance at 490 nm and was compared to that of the cutoff value (> 0.5 EU/ml). The serum samples above the cutoff value were considered positive. As a response to vaccination, all the vaccinated animals had antigen-specific antibodies in their serum well above the protective titer. As per the ELISA results, as depicted in Fig. 3 animals that received the dietary interventions such as probiotics, Vit A or organic carrots along with the vaccine had comparatively higher antibody titers on 14th day after 1 week of immunization (days 0, 3, and 7) mean values above 3.5–3.9 EU/ml as compared to control group C3 with only vaccination (3.09 EU/ml), which were well above the generally accepted protective threshold of 0.5 EU/ml for rabies vaccination. Titer values increased in all treatment groups on 35th day prominently.
Fig. 3.
Overall mean antibody titer comparison a after 14 days, b after 28 days post-vaccination in serum samples of different rabbit treatment groups, (n = 6/group). “a” represents a control group with only rabies vaccination, PBA and PBB were administered two different commercial probiotics, PBA + Vit A, PBB + Vit A with added Vit A supplements, PBA + carrots and PBB + carrots were fed with organic carrots with both probiotic brands respectively. Data were analyzed using Graph Pad Prism (version 9) computer software. Differences among experimental treatments were tested using Tukey’s test following ANOVA. There was a statistically significant difference between groups as determined (p < 0.001, CI 95%), RV Vit A (0.01) as compared to the RV control group
Discussion
A vaccine is defined as a biological response modifier (Speil and Rzepka 2011) that triggers the immune system against specific antigens. However, the world is facing vaccine efficacy issues in the development of effective adaptive immune responses in different societies due to various economic, social and biological factors (Siddiqui et al. 2012, 2020). The gut microbiota of the host (human or animal) is one of the key influential biological factors as there is a never ending cross-talk between gut microbes and the host immune system that directly controls the immune responses and protection against pathogen colonization. Dysbiosis is the term for defining imbalances in the gut microbiota composition that can trigger many immune disorders and impair the desired response to the vaccination (Kazemifard et al. 2022). The probiotics have recently been studied extensively as novel vaccine adjuvants having a potential role to enhance the humoral response of existing vaccines.
Jayawardena et al. (2020) reported, the combined effects of probiotics and VAS with vaccination on the humoral response to the rabies vaccine, evaluated on NZW rabbits. Two different brands of multi-strain commercial probiotics and VAS were used in different experimental groups which were divided based on dietary interventions as previously reported in many studies. Results showed that all animals in the probiotic treatment groups alone or in combination with Vit A rich diet (G1–G6) showed better antibody titers against the rabies vaccine as compared to the control group as observed in Fig. 3. Two-way ANOVA shows a significant difference among groups on both sampling days 14th and 35th (p = < 0.001, CI 95%). Our results are also supported by similar findings in previously published pre-clinical studies on various animal models like pigs, mice, chicken and fish, suggesting the immuno-modulatory role of specific probiotic strains on vaccine responses in host animals (Arczewska-Włosek et al. 2022). Parreno et al. (2022) demonstrated the immune-stimulating role of probiotic IgG as a vaccine adjuvant with the human rotavirus vaccine in the pig model.
Moradi-Kalbolandi et al. (2021) described the significance of probiotic-based mucosal vaccines as a promising protective strategy against respiratory and non-respiratory viruses as observed in the recent COVID-19 pandemic. This study also highlighted the advances in the modulation of immune responses using genetically modified probiotics (Licciardi and Tang 2011; Peroni and Morelli 2021).
Results of our experiment with dietary interventions with vaccination indicate that in all experimental groups, the immune response was higher than in control group C3. A visible increase in the titer values after the initial three doses on day 14th (from approximately 8–15% sero-conversion in both probiotic groups A and B (G1 and G2) vs. control group C3, p = 0. 001. However, it was more pronounced after a complete vaccination course of 4 doses and a total immunization period of 28 days. All treatment groups showed a significant increase after a month from a minimum of 18% to a maximum of 25.933% when compared to the C3 control group. The results of our study support the fact that probiotics and vitamins influence the gut microbiota which in turn activates innate and adaptive immunity by activating the pathways controlling it thereby influencing an overall increase in the humoral immune response against the rabies vaccine. Thus, our results are in correlation with previous findings with other vaccines on animal models as well as human trials. Probiotic supplementation with vaccination, therefore, enhances overall immunity, especially the adaptive immune response.
The mucosal immune system is considered an active immunological site and has a crucial role in the defense mechanism against pathogens. As stated in many studies, probiotics can enhance innate and adaptive immunity. This has been demonstrated in a study on fish with probiotics supplementation, showing that probiotic administration through the oral route can influence both the systemic and the local immunity of the host animals (Guo et al. 2020; Simon et al. 2021).
Probiotic bacteria produce many effector molecules that interact with the host to stimulate their observed effects, such as host gut epithelial barrier functions, modulating the host immune system and gut microbiota, the host’s metabolic responses, and also affecting the central nervous system (Idrees et al. 2022). Probiotics communicate with their hosts through molecular signaling. Thus, the gut microbiota is largely being considered an attractive target to enhance vaccine effectiveness in vulnerable populations of lower and middle income countries.
Adjuvants play a key role in the ever-progressing field of vaccine development that helps to elicit very specific and potent immune responses for protection against deadly diseases. The role of probiotics in immuno-modulation and their possible health benefits on humans has been a widespread topic for nutritional and health research and researchers for the last few decades. Many other reviews and clinical studies on humans also demonstrate the potential adjuvant roles of probiotics, highlighting the possible benefits of probiotics including safety profiles, low cost, and ease of administration. Most studies on the role of probiotics as vaccine adjuvants have been carried out with oral vaccines (Rota vaccines) or mucosal vaccines such as the influenza vaccine (Sahoo et al. 2020). Moreover, they pointed out considerable variation in findings regarding the choice of probiotic, strain, dose, viability, purity, duration and timing of administration.
During the COVID-19 pandemic, many studies were carried out on viral infections. Interestingly, probiotics had been highlighted in most of these studies due to their potential adjuvant role on the inflammatory immune responses and have shown promising results, which may conclude the effectiveness of probiotics in respiratory virus infections (Lehtoranta et al. 2020). The immuno-modulatory role of probiotics has also been attributed to complex interactions in the gut and the release of cytokines that regulates the immune response involving various immune system components. These include interleukins (ILs), tumor necrosis factors (TNFs), interferons (IFNs), transforming growth factors (TGF), and chemokines released from immune cells such as dendritic cells (DCs), lymphocytes, granulocytes, macrophages, mast cells, epithelial cells. Lehtoranta et al. (2020) reported that fractions of probiotic bacteria, act as immunological adjuvants generate immuno-stimulatory effects mediated by cytokines. Thus, probiotics communicate with their hosts through molecular signaling. However, detailed studies and clinical trials may further be needed for future perspectives on probiotic research, since the interactions of the immune system and probiotic bacteria under in vitro conditions may differ from in vivo (Daliri et al. 2021).
Our results showed two different brands of commercial probiotics PBA and PBB. Such variations in the immune response of two different probiotics used in our study may be related to the above factors that may require further optimization studies with these influences. Thus probiotics and/or vitamin-rich diet may be associated with the modulation of the gut microbiota of the host animals as previously stated in many preclinical and clinical trials (Correa et al. 2022).
Animals that received combined supplementation with organic carrots and probiotics in G5 and G6 had the highest rates of sero-conversion to rabies vaccine (39.4% compared with 27.4% among animals receiving only probiotics with vaccination, p = 0.05). Carrots are considered one of the healthiest Vit A rich with high phytochemical content including phenolic, ascorbic acid, carotenoids and polyacetylenes. The addition of carrots to the diet boosts our immunity by affecting various aspects of the immune function (Vitetta et al. 2017).
The influence of Vit A as a supplement on vaccine humoral response has also been investigated in this study. A significant increase in antibody titer specific to the rabies vaccine was detected after the initial 3 doses of RV as observed in G3, G4, G5 and G6 that appeared to be associated with Vit A treatment even with or without probiotics. As previously reported, VAS has shown benefits when administered with different vaccines. Our results are also showing a sufficient increase in antibody titer value in rabbit groups G3, G4 and C1 that were provided VAS in daily feed. These results are in correlation with a previously published study on the role of VAS on rabies vaccine immunogenicity in a small clinical trial conducted at the National Institute of Health, Pakistan (Siddiqui et al. 2001). The study involved 40 human individuals, however, we have incorporated probiotics with Vit A in our study on animal models, keeping in view the importance of polyclonal antibody production using simple dietary interventions. The findings are quite encouraging toward the implementation of alternate strategies for rabies reduction in poor setups with Vit A and other nutrient deficiencies, especially for vulnerable populations including children and the elderly.
In our experimental study, a substantial increase was detected in sero-conversion rates after the initial three doses of RV with VAS, either with capsules or natural organic food source. The results of interventions with organic carrots in groups G5, G6 and sub-control group C2 also provide evidence of previously reported health benefits of carrot’s natural components on immunity. Many benefits of carotenoids have previously been reported (Eggersdorfer and Wyss 2018).
Thus probiotic, Vit A and carrot rich diets appeared to be associated with the modulation of gut microbiota of the host animals as previously stated in many clinical trials. Carrots are considered one of the healthiest vegetables because of their high phytochemical content including phenolic, ascorbic acid, carotenoids and polyacetylenes which exert anti-inflammatory activities.
Conclusions
Rabies is a fatal zoonotic disease with 100% mortality, but preventable with the timely and correct use of post-exposure prophylaxis (PEP) that includes vaccination and rabies immunoglobulins (RIGs) in case of severe bites. In developing countries, a shortage of vaccines, anti-sera and overall high total treatment costs leave most dog bite victims untreated, thus rabies prevention remains a challenge for successful control in developing countries like Pakistan. Simple and practical measures are required to improve the efficacy of vaccines for the vulnerable population of lower middle income countries with weak immunity. Moreover, enhancement of IgG production in donor animals is necessary to provide better and faster access to needy patients regarding immunoglobulin therapy. There is a dire need to strengthen the local manufacturing of rabies vaccines and animal derived rabies anti-sera for underprivileged populations. Our study aims to enhance rabies vaccine-specific immune outcomes with simple dietary strategies based on probiotics and Vit A to enhance the humoral immune response and hence the antibody titer. In conclusion, concomitant vaccines and probiotics/vitamins administration would be an extremely cost-effective approach that could substantially reduce the rabies disease burden with global efforts to eliminate dog-mediated rabies till 2030. Further studies are still needed on the optimization of strains, dosage and duration of probiotics administration on large donor animals for the production of animal derived anti-sera and clinical trials on humans regarding the immuno-modulatory potential of probiotics for better immune outcomes, especially for children and the elderly population with weak immunity.
Acknowledgements
Amina Najam extends her appreciation to the biological production division of National Institute of Health, Islamabad, Pakistan and National Institute for Genomics and Advanced Biotechnology (NIGAB), National Agricultural Research Centre, Park Road, Islamabad, Pakistan.
Funding
This research received no external funding.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants, however, animal model was used. The study protocol for the experimental use of the animals was approved by the ethics committee of National Institute of Health (NIH), Islamabad (Ethical Approval No. F.1–5/ERC/2020) according to the standard operating procedures (SOPs).
Contributor Information
Amina Najam, Email: ameenanajam@gmail.com.
Safia Ahmad, Email: sahmed@qau.edu.pk.
Rameesha Abid, Email: rameeshaabid@bs.qau.edu.pk.
Hussain Ali, Email: drali700@gmail.com.
Murtaza Husnain, Email: murtazahussnain678@gmail.com.
Tariq Aziz, Email: tariq.aziz@bs.qau.edu.pk.
Syeda Shazia Adeel, Email: syeda.shazia.adeel@gmail.com.
Naeil Muhammad, Email: mohammednaiel.1984@gmail.com.
Shakira Ghazanfar, Email: shakira_akmal@yahoo.com.
References
- Abedi M, Haftcheshmeh SM, Bashar R, et al. Rabies vaccine: recent update and comprehensive review of in vitro and in vivo studies. Process Biochem. 2022;124:201–220. doi: 10.1016/j.procbio.2022.11.011. [DOI] [Google Scholar]
- Abid R, Waseem H, Ali J, et al. Probiotic yeast saccharomyces: back to nature to improve human health. J Fungi. 2022;8:444. doi: 10.3390/jof8050444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abid S, Farid A, Abid R, et al. Identification, biochemical characterization, and safety attributes of locally isolated lactobacillus fermentum from Bubalus bubalis (Buffalo) milk as a probiotic. Microorganisms. 2022;10:954. doi: 10.3390/microorganisms10050954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alagawany M, Attia YA, Farag MR, et al. The strategy of boosting the immune system under the COVID-19 Pandemic. Front Vet Sci. 2021;7:570748. doi: 10.3389/fvets.2020.570748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson A, Shwiff SA. The cost of canine rabies on four continents. Transbound Emerg Dis. 2015;62:446–452. doi: 10.1111/tbed.12168. [DOI] [PubMed] [Google Scholar]
- Arczewska-Włosek A, Świ\katkiewicz S, Ognik K, Józefiak D, Effects of a dietary multi-strain probiotic and vaccination with a live anticoccidial vaccine on growth performance and haematological, biochemical and redox status indicators of broiler chickens. Animals. 2022;12:3489. doi: 10.3390/ani12243489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs DJ, Moore SM. The route of administration of rabies vaccines: comparing the data. Viruses. 2021;13:1252. doi: 10.3390/v13071252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa VA, Portilho AI, De Gaspari E. Vaccines, adjuvants and key factors for mucosal immune response. Immunology. 2022;167:124–138. doi: 10.1111/imm.13526. [DOI] [PubMed] [Google Scholar]
- Daliri EB-M, Ofosu FK, Xiuqin C, et al. Probiotic effector compounds: current knowledge and future perspectives. Front Microbiol. 2021;12:655705. doi: 10.3389/fmicb.2021.655705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggersdorfer M, Wyss A. Carotenoids in human nutrition and health. Arch Biochem Biophys. 2018;652:18–26. doi: 10.1016/j.abb.2018.06.001. [DOI] [PubMed] [Google Scholar]
- Gan H, Hou X, Wang Y, et al. Global burden of rabies in 204 countries and territories, from 1990 to 2019: results from the Global Burden of Disease Study 2019. Int J Infect Dis. 2023;126:136–144. doi: 10.1016/j.ijid.2022.10.046. [DOI] [PubMed] [Google Scholar]
- Guo S, Peng J, Xiao Y, et al. The construction and immunoadjuvant activities of the oral interleukin-17B expressed by lactobacillus plantarum NC8 strain in the infectious bronchitis virus vaccination of chickens. Vaccines. 2020;8:282. doi: 10.3390/vaccines8020282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idrees M, Imran M, Atiq N, et al. Probiotics, their action modality and the use of multi-omics in metamorphosis of commensal microbiota into target-based probiotics. Front Nutr. 2022;9:2133. doi: 10.3389/fnut.2022.959941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal M, Shahid B, Shaukat N. Rabies in Pakistan: roadmap for future. Pak J Public Health. 2020;9:130–131. doi: 10.32413/pjph.v9i3.431. [DOI] [Google Scholar]
- Jayawardena R, Sooriyaarachchi P, Chourdakis M, et al. Enhancing immunity in viral infections, with special emphasis on COVID-19: a review. Diabetes Metab Syndr Clin Res Rev. 2020;14:367–382. doi: 10.1016/j.dsx.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazemifard N, Dehkohneh A, Ghavami SB. Probiotics and probiotic-based vaccines: a novel approach for improving vaccine efficacy. Front Med. 2022 doi: 10.3389/fmed.2022.940454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtoranta L, Latvala S, Lehtinen MJ. Role of probiotics in stimulating the immune system in viral respiratory tract infections: a narrative review. Nutrients. 2020;12:3163. doi: 10.3390/nu12103163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licciardi PV, Tang MLK. Vaccine adjuvant properties of probiotic bacteria. Discov Med. 2011;12:525–533. [PubMed] [Google Scholar]
- Moradi-Kalbolandi S, Majidzadeh-A K, Abdolvahab MH, et al. The role of mucosal immunity and recombinant probiotics in SARS-CoV2 vaccine development. Probiot Antimicrob Proteins. 2021;13:1239–1253. doi: 10.1007/s12602-021-09773-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norouzbabaei Z, Jandaghi NZS, Foroushani AR et al (2022) Evaluation of the focus reduction neutralization and ELISA tests compared to the plaque reduction neutralization test for the detection of antibodies against measles virus
- Oresanya O, Phillips A, Okereke E, et al. Co-implementing vitamin A supplementation with seasonal malaria chemoprevention in Sokoto State, Nigeria: a feasibility and acceptability study. BMC Health Serv Res. 2022;22:871. doi: 10.1186/s12913-022-08264-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parreno V, Bai M, Liu F, et al. Probiotic as adjuvant significantly improves protection of the Lanzhou trivalent rotavirus vaccine against heterologous challenge in a gnotobiotic pig model of human rotavirus infection and disease. Vaccines. 2022;10:1529. doi: 10.3390/vaccines10091529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peroni DG, Morelli L. Probiotics as adjuvants in vaccine strategy: is there more room for improvement? Vaccines. 2021;9:811. doi: 10.3390/vaccines9080811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiambao BP, Ambas C, Diego S, et al. Intradermal post-exposure rabies vaccination with purified Vero cell rabies vaccine: comparison of a one-week, 4-site regimen versus updated Thai Red Cross regimen in a randomized non-inferiority trial in the Philippines. Vaccine. 2019;37:2268–2277. doi: 10.1016/j.vaccine.2019.02.083. [DOI] [PubMed] [Google Scholar]
- Quiambao BP, Lim JG, Bosch Castells V, et al. One-week intramuscular or intradermal pre-exposure prophylaxis with human diploid cell vaccine or Vero cell rabies vaccine, followed by simulated post-exposure prophylaxis at one year: a phase III, open-label, randomized, controlled trial to assess immunogenicity and safety. Vaccine. 2022;40:5347–5355. doi: 10.1016/j.vaccine.2022.07.037. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan S, Vanak AT, Nouvellet P, Donnelly CA. Rabies as a public health concern in India—a historical perspective. Trop Med Infect Dis. 2020;5:162. doi: 10.3390/tropicalmed5040162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimal S, Ojha KC, Chaisowwong W, et al. Detection of virus-neutralising antibodies and associated factors against rabies in the vaccinated household dogs of Kathmandu Valley Nepal. PLoS One. 2020;15:e0231967. doi: 10.1371/journal.pone.0231967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahoo A, Mandal AK, Dwivedi K, Kumar V. A cross talk between the immunization and edible vaccine: current challenges and future prospects. Life Sci. 2020;261:118343. doi: 10.1016/j.lfs.2020.118343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz JL, Caplan AL. Vaccination ethics and policy: an introduction with readings. Cambridge: MIT Press; 2021. [Google Scholar]
- Shuman LH, Hirsh HL. Animal bites. Trauma. 2000;41:65–90. [Google Scholar]
- Siddiqui FQ, Ahmad MM, Kakar F, et al. The role of vitamin A in enhancing humoral immunity produced by antirabies vaccine. East Mediterr Health J. 2001;7:799–804. doi: 10.26719/2001.7.4-5.799. [DOI] [PubMed] [Google Scholar]
- Siddiqui AJ, Bhardwaj J, Puri SK. mRNA expression of cytokines and its impact on outcomes after infection with lethal and nonlethal Plasmodium vinckei parasites. Parasitol Res. 2012;110:1517–1524. doi: 10.1007/s00436-011-2656-1. [DOI] [PubMed] [Google Scholar]
- Siddiqui AJ, Bhardwaj J, Goyal M, et al. Immune responses in liver and spleen against Plasmodium yoelii pre-erythrocytic stages in Swiss mice model. J Adv Res. 2020;24:29–41. doi: 10.1016/j.jare.2020.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon R, Docando F, Nuñez-Ortiz N, et al. Mechanisms used by probiotics to confer pathogen resistance to teleost fish. Front Immunol. 2021 doi: 10.3389/fimmu.2021.653025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speil C, Rzepka R. Vaccines and vaccine adjuvants as biological response modifiers. Infect Dis Clin. 2011;25:755–772. doi: 10.1016/j.idc.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Taghinezhad-S S, Mohseni AH, Bermúdez-Humarán LG, et al. Probiotic-based vaccines may provide effective protection against covid-19 acute respiratory disease. Vaccines. 2021;9:466. doi: 10.3390/vaccines9050466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitetta L, Saltzman ET, Thomsen M, et al. Adjuvant probiotics and the intestinal microbiome: enhancing vaccines and immunotherapy outcomes. Vaccines. 2017;5:50. doi: 10.3390/vaccines5040050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Jiang H, Yang R, et al. Construction and evaluation of recombinant Lactobacillus plantarum NC8 delivering one single or two copies of G protein fused with a DC-targeting peptide (DCpep) as novel oral rabies vaccine. Vet Microbiol. 2020;251:108906. doi: 10.1016/j.vetmic.2020.108906. [DOI] [PubMed] [Google Scholar]
- Warrell MJ. Rabies post-exposure vaccination in 2 visits within a week: a 4-site intradermal regimen. Vaccine. 2019;37:1131–1136. doi: 10.1016/j.vaccine.2019.01.019. [DOI] [PubMed] [Google Scholar]
- Yousaf MZ, Qasim M, Zia S, et al. Rabies molecular virology, diagnosis, prevention and treatment. Virol J. 2012;9:1–5. doi: 10.1186/1743-422X-9-5022348291. [DOI] [PMC free article] [PubMed] [Google Scholar]