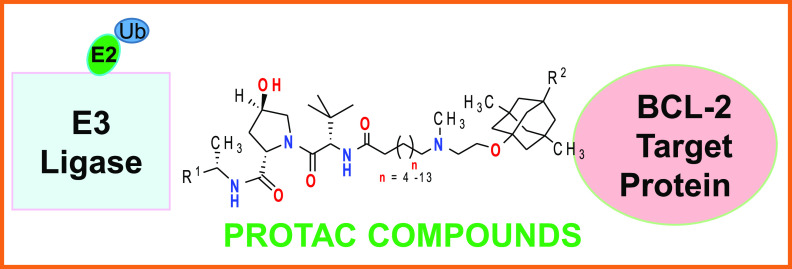
Abstract
The B-cell lymphoma 2 (BCL-2) protein is the most extensively studied anti-apoptotic member within the BCL-2 protein family. It functions to inhibit programmed cell death by forming a heterodimer with BAX, thereby promoting cellular survival through the extension of tumor cell lifespan and facilitating malignant transformation. This Patent Highlight reveals the development of small molecule degraders that consist of a ligand targeting the protein of interest, BCL-2, an E3 ubiquitin ligase recruitment ligand (such as Cereblon or Von Hippel–Lindau ligands), and a chemical linker that connects the two ligands. The proteolysis-targeting chimera (PROTAC)-mediated heterodimerization of the bound proteins leads to the ubiquitination of the target protein, which is subsequently degraded by the proteasome. This strategy offers innovative therapeutic options for cancer, immunology, and autoimmune disease management.
Important Compound Classes
Titles
Pro-Apoptotic Construct and Use Thereof; and Modulation of BCL-2 to Enhance Chimeric Antigen Receptor Cancer Immunotherapy Efficacy
Patent Publication Numbers
WO 2023/031215 A2 (URL: https://patents.google.com/patent/WO2023031215A2/en?oq=WO+2023%2f031215+A2); WO 2023/019165 A1 (URL: https://patents.google.com/patent/WO2023019165A1/en?oq=WO2023019165)
Publication Dates
March 9, 2023; and February 16, 2023.
Priority Applications
EP21194538.1; and US 63/232,051
Priority Dates
September 2, 2021; and August 11, 2021
Inventors
Peperzak, V.; Sebestyen, Z.; Kimman, T. (WO 2023/031215 A2); and Ruella, M.; Lee, Y. G. (WO 2023/019165 A1)
Assignee Companies
UMC Utrecht Holding B.V. [NL/NL]; 8 Heidelberglaan, 3584 CS Utrecht, The Netherlands (WO 2023/031215 A2); and The Trustees of the University of Pennsylvania [US/US]; 3600 Civic Center Boulevard, 9th Floor, Philadelphia, PA 19104, United States (WO 2023/019165 A1)
Disease Areas
Cancer and immunotherapy
Biological Target
B-cell lymphoma 2 (BCL-2) protein
Summary
Apoptosis, a form of genetically regulated cell death, is an evolutionarily conserved process essential for maintaining tissue homeostasis in eukaryotes, supporting development, and eliminating damaged cells. This process occurs not only in response to cellular damage or external stress but also during normal cell development and morphogenesis. Consequently, dysregulation of apoptosis contributes to various human diseases, including cancer, immune system disorders, neurodegenerative conditions, and autoimmune diseases.
Two primary apoptotic pathways have been identified: the exogenous (cell death) pathway, which is activated by pro-apoptotic stimuli external to the cell, and the endogenous (mitochondrial) pathway, triggered by the cell’s intrinsic mechanisms. Evasion of apoptosis is a key factor in cancer pathogenesis, playing a critical role in tumor development, continued growth, and resistance to anti-cancer therapies.
The B-cell lymphoma 2 (BCL-2) protein family comprises key regulators involved in both pro-apoptotic and anti-apoptotic activities. These proteins possess four conserved BCL-2 homology (BH) domains, namely BH1, BH2, BH3, and BH4. The function of BCL-2 proteins is contingent upon the highly conserved BH domain, and in mammals, these proteins are classified into three subfamilies: anti-apoptotic proteins (e.g., BCL-2 and BCL-XL), pro-apoptotic proteins (e.g., BAD and BAK), and BH3-domain-only proteins (e.g., BAD and BID).
In response to stress stimuli, the initiation of either apoptosis or cell survival depends on the extent of interactions between BCL-2 family members that promote cell death and those that support cell survival. These interactions primarily involve the docking of the pro-apoptotic family members’ BH3 domain into a groove on the surface of pro-survival members. Overexpression of pro-survival BCL-2 family members is a characteristic feature of cancer, and these proteins have been demonstrated to play a crucial role in tumor development, maintenance, and resistance to anti-cancer therapy.
In patient samples, high or persistent expression of anti-apoptotic BCL-2 protein family members has been observed. Notably, T-cells isolated from the joints of rheumatoid arthritis patients exhibit increased BCL-xL expression and demonstrate resistance to spontaneous apoptosis.
The application of BH3 mimetics has demonstrated potential in preclinical models for immune system disorders and autoimmune diseases, such as treatment with ABT-737 (an inhibitor of BCL-2, BCL-xL, and BCL-w). Mice treated with ABT-737 in animal models of arthritis and lupus exhibited a significant decrease in disease severity. These findings have driven the discovery and development of a new class of drugs known as BH3 mimetics (inhibitors of BCL-2, BCL-xL, BCL-w, and MCL-1), which are potent apoptosis inducers that disrupt the interaction between pro-apoptotic and anti-apoptotic members of the BCL-2 family.
The selective BCL-2 inhibitor ABT-199 has been approved for the treatment of patients with chronic lymphocytic leukemia (CLL) and acute myeloid leukemia (AML) in combination therapy. Meanwhile, MCL-1 selective inhibitors (S64315, AMG176, and AZD5991) have demonstrated promise in various types of hematological malignancies in preclinical models and are currently under investigation in clinical trials. As a result, BH3 mimetics represent a highly attractive approach for developing novel therapies in the fields of oncology, immunology, and autoimmune diseases.
The disclosures in patents WO 2023019165 A1 and WO 2023031215 A2 show pro-apoptotic molecules with a BCL-2 homology 3 (BH3) effector domain. These molecules are associated with constructs containing a granule-localizing domain and can be transferred from effector cells to target cells to induce apoptosis. Nucleic acid molecules encoding these pro-apoptotic proteins can be used in medical therapies such as cancer treatments, including chimeric antigen receptor (CAR) cell therapy.
The inventors found that tumor cell resistance to engineered cytotoxic lymphocytes (CLs) is likely due to the expression of Serpin B9, a protein that inhibits the activity of Granzyme B (GzmB) and makes target cells resistant to killing. Serpin B9 is expressed in various cancers, including hematological and solid tumors. This resistance may explain relapses after initial responses using engineered CLs and why gene-engineered T cells have had limited success in treating solid tumors. To bypass this resistance, the inventors developed modified variants of BCL-2 BH3-only proteins, like NOXA, which inactivate pro-survival BCL-2 family proteins. NOXA is induced during genotoxic stress through activation of p53 and can be translocated from a CL effector cell to a tumor target cell. By replacing NOXA’s BH3 effector domain with the BH3 domain of pro-apoptotic BCL-2 interaction mediator of cell death (BIM), the modified NOXA molecule can inhibit multiple or even all pro-survival BCL-2 family proteins, resulting in maximal induction of apoptosis. The inventors expect that all tumor types will be sensitive to this modified version of NOXA, which they demonstrated with NOXA(BIM) constructs in various tumor types. Furthermore, the inventors found that it is possible to replace the BH3 domain in BH3-only proteins other than NOXA, which may lead to enhanced pro-apoptotic effects depending on the type of cancer and the patient.
Moreover, the patent disclosure WO 2022169780 A1 shows a novel approach in the development of alternative BH3 mimetics involving their use as both protein binders and inhibitors. The mechanism of action for BCL-xL inhibitors can be transformed from inhibition to degradation via proteolysis-targeting chimeras (PROTACs). This Patent Highlight discloses small molecule degraders that include (i) a ligand targeting a protein of interest (BH3) for degradation, (ii) an E3 ubiquitin ligase recruitment ligand (primarily Cereblon or Von Hippel–Lindau ligands), and (iii) a chemical linker connecting the two ligands. PROTAC-mediated heterodimerization of the two bound proteins results in the target protein becoming ubiquitinated, followed by degradation via the proteasome.
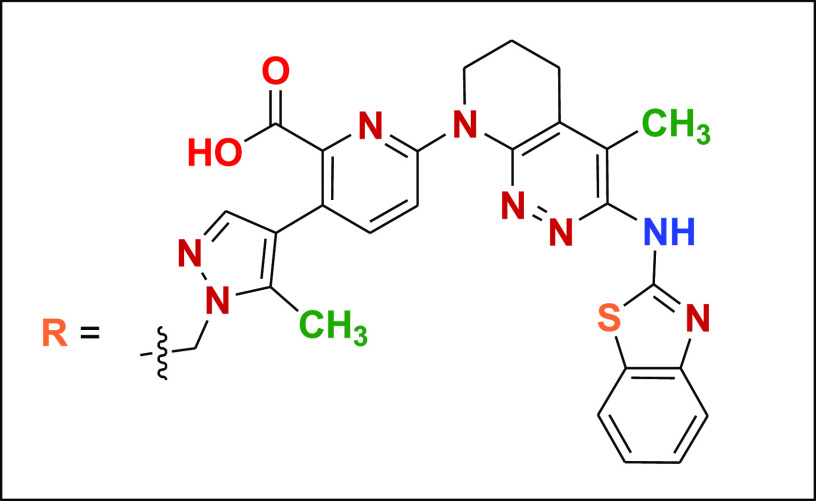
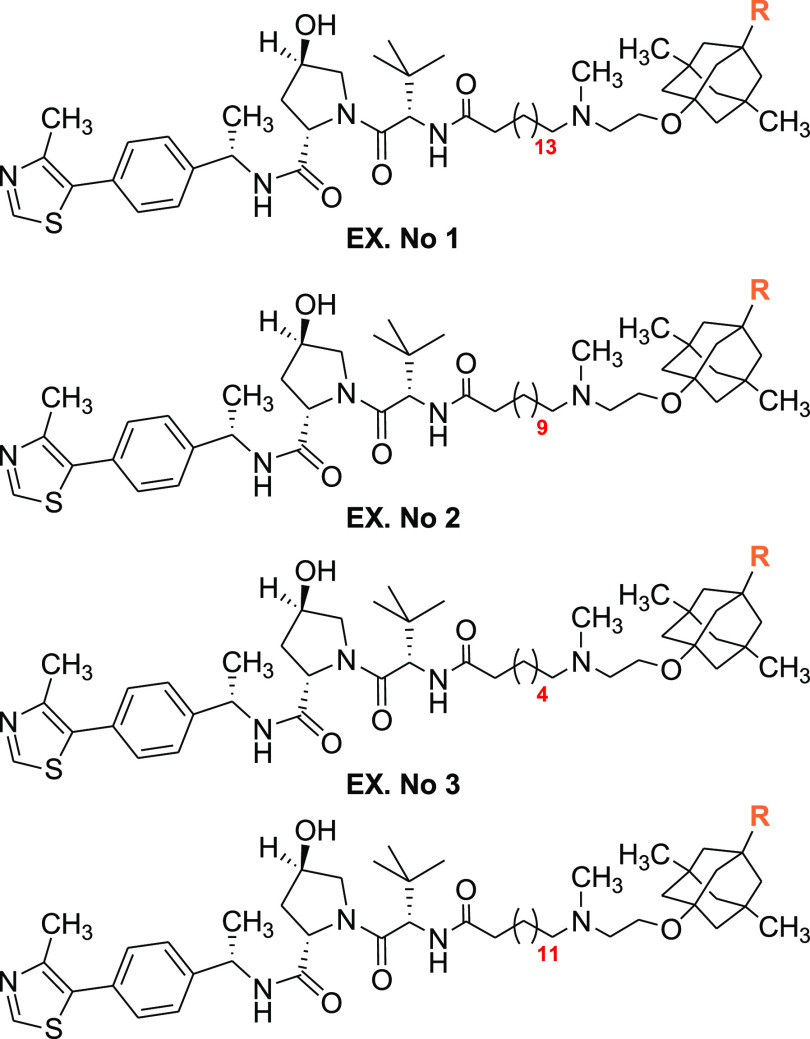
Key Structures
Biological Assays
CellTiter-Glo (CTG) proliferation assay, MTT assays, capillary-based immunoassay, Pierce BCA protein assay kit, WES assay, and Nano-Glo HiBiT assay technology
Biological Data
The table below shows the effect of
BCL-Xl degrader compounds on EBC-1 cell viability using CTG assay
and BCL-Xl protein degradation using HiBiT assay and VHL ligase.
Recent Review Articles
The author declares no competing financial interest.
References
- Sarkar A.; Paul A.; Banerjee T.; Maji A.; Saha S.; Bishayee A.; Maity T. K. Therapeutic advancements in targeting BCL-2 family proteins by epigenetic regulators, natural, and synthetic agents in cancer. Eur. J. Pharmacol. 2023, 944, 175588. 10.1016/j.ejphar.2023.175588. [DOI] [PubMed] [Google Scholar]
- Nieto-Jimenez C.; Morafraile E. C.; Alonso-Moreno C.; Ocana A. Clinical considerations for the design of PROTACs in cancer. Mol. Cancer 2022, 21, 67. 10.1186/s12943-022-01535-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhakal P.; Bates M.; Tomasson M. H.; Sutamtewagul G.; Dupuy A.; Bhatt V. R. Acute myeloid leukemia resistant to venetoclax-based therapy: What does the future hold?. Blood Rev. 2023, 59, 101036. 10.1016/j.blre.2022.101036. [DOI] [PubMed] [Google Scholar]
- Pal P.; Zhang P.; Poddar S. K.; Zheng G. Patent landscape of inhibitors and PROTACs of the antiapoptotic BCL-2 family proteins. Exp. Opin. Ther. Pat. 2022, 32, 1003. 10.1080/13543776.2022.2116311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.; Guo M.; Wei H.; Chen Y. Targeting MCL-1 in cancer: current status and perspectives. J. Hematol. Oncol. 2021, 14, 67. 10.1186/s13045-021-01079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X.; Dong R.; Zhang J.; Zheng X.; Sun L. PROTAC: A promising technology for cancer treatment. Eur. J. Med. Chem. 2020, 203, 112539. 10.1016/j.ejmech.2020.112539. [DOI] [PubMed] [Google Scholar]