Abstract
5-Aminolevulinic acid (ALA) is an intraoperative molecular probe approved for fluorescence-guided resection (FGR) of high-grade gliomas to achieve maximal safe tumor resection. Although ALA has no fluorescence on its own, it is metabolized in the heme biosynthesis pathway to produce protoporphyrin IX (PpIX) with red fluorescence for tumor detection and photosensitizing activity for photodynamic therapy (PDT). The preferential tumor accumulation of PpIX following ALA administration enables the use of ALA as a prodrug for PpIX FGR and PDT of gliomas. Since intracellular PpIX in tumor cells after ALA treatment is influenced by biological processes including PpIX bioconversion catalyzed by ferrochelatase (FECH) and PpIX efflux by ATP-binding cassette subfamily G member 2 (ABCG2), we determined the activity of FECH and ABCG2 in a panel of human glioma cell lines and correlated with intracellular and extracellular PpIX levels and PDT response. We found that glioma cell lines with ABCG2 activity exhibited the trend of low intracellular PpIX, high extracellular PpIX and low PDT response, whereas no particular correlation was seen with FECH activity. Inhibition of PpIX efflux with ABCG2 inhibitors was more effective in enhancing ALA-PpIX fluorescence and PDT response than blocking PpIX bioconversion with iron chelator deferoxamine. We also showed that a clinically used kinase inhibitor lapatinib could be repurposed for therapeutic enhancement of ALA due to its potent ABCG2 inhibitory activity. Our study reveals ABCG2 as an important biological determinant of PpIX fluorescence in glioma cells and suggests ABCG2 inhibition with lapatinib as a promising therapeutic enhancement approach.
Keywords: 5-aminolevulinic acid (ALA), protoporphyrin IX (PpIX), photodynamic therapy (PDT), ABCG2, lapatinib, gliomas, deferoxamine, isocitrate dehydrogenase (IDH) mutation
1. Introduction
Glioma derived from the glial cells in the central nervous system is the most common type of primary brain tumor [1]. About half of all diagnosed glioma cases are classified as glioblastoma, the highest grade of gliomas and the most malignant type of brain tumor. The standard therapy for high-grade gliomas includes surgical resection of the malignant tissue followed by radiation therapy and chemotherapy [2]. As the first choice of therapy, glioma surgery aims to maximally dissect tumor tissues while preserving normal function. It has been well established that the extent of glioma resection is highly associated with the patient survival [3]. However, the infiltrating growth of glioma cells and the similarity in appearance to surrounding normal tissues, particularly at the tumor margin, make maximal safe resection of gliomas a great challenge [4, 5].
5-aminolevulinic acid (ALA, Gleolan) has been approved by the U.S. FDA as an intraoperative probe to aid neurosurgeons to achieve maximal safe resection of high-grade gliomas [6]. As an endogenous metabolite, ALA is synthesized from glycine and succinyl-CoA by ALA synthase (ALAS) in mitochondria, and further metabolized in the heme biosynthesis pathway composed of multiple cytosolic and mitochondrial steps to produce the pathway end product heme [7]. Exogenously administered ALA bypasses ALA synthesis, the rate-limiting step of the heme biosynthesis pathway, which overwhelms the pathway activity and causes the accumulation of intermediate metabolites. A predominate one is protoporphyrin IX (PpIX), the penultimate metabolite in the pathway. PpIX is not only a fluorophore emitting red fluorescence upon light excitation but also a photosensitizer for photodynamic therapy (PDT), whereas ALA, heme, and all other metabolites in the pathway lack these properties.
The preferential accumulation of PpIX in glioma tumor tissues enables neurosurgeons to dissect tumor tissues under the guidance of PpIX fluorescence, thereby increasing surgical precision and accuracy. A pivotal Phase III clinical trial which led to the approval of ALA in Europe demonstrates that ALA-PpIX fluorescence-guided surgery increases progression-free survival of high-grade glioma patients by dissecting more tumor tissues than conventional white light tumor surgery [8]. A recent multicenter clinical study in the U.S. confirmed the usefulness and safety of ALA for enhanced surgical visualization and resection of high-grade gliomas [9].
Despite the clinical success of ALA as a molecular imaging probe for cancer surgery, the molecular mechanism underlying the preferential accumulation of ALA-PpIX in gliomas remains elusive. Furthermore, although ALA-PpIX fluorescence has over 95% positive predictive value (PPV) as a diagnostic tool for high-grade gliomas, the negative predicative value (NPV) is typically below 50% [9–11], suggesting that tumors may have been missed during surgery due to the absence of visible PpIX fluorescence. To overcome this limitation, it is necessary to determine the cause for such PpIX fluorescence heterogeneity in tumor tissues and develop therapeutic strategies to enhance tumor PpIX fluorescence.
PpIX in tumor cells is cleared predominantly by two mechanisms, PpIX to heme bioconversion and PpIX efflux transport [7]. While PpIX bioconversion is catalyzed by ferrochelatase (FECH) that uses ferrous iron (Fe2+) as the other substrate, PpIX efflux is mainly through ATP-binding cassette subfamily G member 2 (ABCG2) transporter. Inhibition of these two PpIX-reduction processes has been explored to enhance ALA-PpIX fluorescence with positive outcomes. By chelating Fe2+, iron chelators such as deferoxamine (DFO) block PpIX bioconversion and increase ALA-PpIX fluorescence in glioma cell lines [12, 13] and in a tumor model [14]. Similarly, ABCG2 inhibition has been shown to enhance ALA-PpIX fluorescence and PDT effects in glioma cell lines [15, 16]. Although these results are encouraging, studies carried out in different conditions by different groups make it difficult to compare the efficacy of PpIX enhancement induced by PpIX bioconversion blockade versus PpIX efflux inhibition. In addition, no ABCG2 inhibitor is currently available for the clinical use [17], which hampers the clinical evaluation of ABCG2-targeted PpIX enhancement approach.
To address these issues, we performed the present study with the following objectives/hypotheses. 1. Evaluate the activity of FECH and ABCG2 in a panel of human glioma cell lines and correlate with ALA-PpIX fluorescence and PDT response. 2. Assess the effects of iron chelator DFO versus ABCG2 inhibitors on the enhancement of ALA-PpIX fluorescence and PDT. 3. Test the hypothesis that lapatinib (Lap), a FDA-approved receptor tyrosine kinase inhibitor with ABCG2 inhibitory activity [18], enhances ALA-PpIX fluorescence and PDT in glioma cell lines. 4. Compare the efficacy and potency of Lap versus Ko143, a pharmacological tool compound for ABCG2 inhibition [19], for ALA-PpIX enhancement. 5. Determine the effects of isocitrate dehydrogenase (IDH) mutation on ALA-PpIX fluorescence and PDT, given its importance in the glioma genetics [1].
2. Materials & Methods
2.1. Chemicals
ALA hydrochloride, PpIX and pheophorbide a (Pha) were obtained from Frontier Scientific Inc. (Logan, UT) and dissolved in PBS (for ALA) or DMSO (for PpIX and Pha). Lapatinib was obtained from LC Laboratories (Woburn, MA) and dissolved in DMSO. Ko143, DFO and palmitic acid were purchased from Sigma (St. Louis, MO) and dissolved in DMSO. Ethylenediaminetetraacetic acid (EDTA), zinc acetate, and Triton X-100 were from Sigma and dissolved in deionized water. Protease inhibitor cocktail (1× working solution) includes aprotinin (2 μg/mL), leupeptin (2 μg/mL), pepstatin A (1 μg/mL) and phenylmethanesulfonyl fluoride (PMSF, 1 mM), all from Sigma. All chemicals were sterilized through filtration using 0.22-μm pore size filters and stored in a −20°C freezer.
2.2. Cell culture
Human high-grade glioma cell lines A172, H4, SW1088, U-118, U-87 and U-87-IDH1 (R132H) mutant were obtained from American Type Culture Collection (ATCC, Manassas, VA) and cultured in DMEM medium (Corning, Manassas, VA) supplemented with 9% fetal bovine serum (Atlanta Biologicals, Flowery Branch, GA) and 1% antibiotics and antimycotics (Corning). Cells were maintained at 37°C in a humidified incubator with 5% CO2. Cells less than 25 passages were used for experiments.
2.3. Intracellular and extracellular PpIX measurement by spectrofluorometry
Cells were implanted in 6-well cell culture plates and allowed to grow for 24 h before treatment. Cells were treated with ALA alone or in combination with other chemicals (Ko143, Lap, DFO) in complete RPMI 1640 medium for 4 h. Cell culture medium was collected after treatment and centrifuged (200 g) for 5 min. The supernatants were collected for PpIX measurement to indicate the extracellular PpIX. Cells were rinsed twice with PBS and lysed with the lysis buffer containing 0.1% Triton X-100, 100 μM EDTA and protease inhibitor cocktail in PBS. Cell lysates were centrifuged (3000 g) for 10 min to obtain the supernatants for PpIX measurement to indicate the intracellular PpIX. PpIX fluorescence was measured with Fluoromax-3 fluorescence spectrometer (Horiba JY, Edison, NJ) with the excitation wavelength at 400 ± 2.5 nm. The protein concentration of cell lysate extracts was measured using the Bio-Rad Bradford protein assay reagents. The intracellular and extracellular PpIX was normalized to the amount of protein and reported as nmol PpIX per gram cell protein.
2.4. PpIX fluorescence measurement by flow cytometry
Cells were implanted in 60-mm cell culture dishes and allowed to grow for 2 days. Cells were treated with ALA alone or in combination with other chemicals (Ko143, Lap, DFO) in complete RPMI 1640 medium for 4 h. After medium was removed, cells were rinsed twice with PBS, trypsinized and re-suspended in ice-cold PBS. Cell fluorescence was measured with either FACScalibur or Accuri C6 Plus flow cytometer (both from BD Biosciences) in the FL3 channel (488 nm excitation, 650 nm long-pass emission in FACScalibur or 670 nm long-pass emission in Accuri C6). About 20,000 cells were measured and recorded for each experiment.
2.5. ABCG2 activity assay
Glioma cell ABCG2 activity was assessed as described previously [20]. Briefly, cells in 12-well plates were incubated with ABCG2 substrate pheophorbide a (Pha, 500 nM) alone or in combination with Ko143 (1 μM) for 2 h. Then cells treated with Pha alone were incubated with fresh culture medium and cells treated with Pha in combination with Ko143 were incubated with medium containing 1 μM Ko143. After 20 min incubation, cells were lysed in 0.1% sodium dodecyl sulfate (SDS) in PBS. Cell lysates were centrifuged and the supernatants were collected for Pha fluorescence measurement by Fluoromax-3 fluorescence spectrometer (excitation: 400 ± 2.5 nm, emission: 677 nm). The percent change of Pha fluorescence in cells treated with Pha in combination with Ko143 to the fluorescence of cells treated with Pha alone was used to indicate ABCG2 activity.
2.6. FECH activity assay
FECH activity was determined as described previously [21]. Briefly, cells in 60-mm dishes were lysed in PBS-based lysis buffer containing 0.1% Triton X-100, 200 μM palmitic acid and protease inhibitors. Cell lysates were centrifuged. The supernatants were collected and incubated with zinc acetate (5 μM) and PpIX (1 μM) for 10 min at 37°C. The amount of Zn-PpIX produced was measured using Fluoromax-3 fluorescence spectrometer (excitation: 400 ± 2.5 nm, emission: 590 nm) and normalized to the protein level to indicate FECH activity as nmol Zn-PpIX per gram cell protein per minute.
2.7. PDT treatment & cytotoxicity assay
Cells were implanted in 96-well plates and allowed to grow for 24 h. Cells were treated with each individual chemical (including ALA, Lap, Ko143 and DFO) or combination treatments for 4 h. Then cells were treated with 5 mW/cm2 irradiance of 633 nm light for 10 min, resulting in a light fluence of 3 J/cm2. Light illumination was provided by a diode laser system (High Power Devices Inc., North Brunswick, NJ) coupled to a 600 μm core diameter optical fiber fitted with a microlens at the end of fiber to achieve homogeneous irradiation. Light intensity was measured with an optical power meter (Thorlabs, Inc., North Newton, NJ). Immediately after light treatment, drug-containing medium was replaced with the fresh medium. Cell viability was determined at 48 h after treatment with Alamar blue (resazurin from Sigma) assay.
2.8. Statistical analysis
One-way or Two-way ANOVA test followed by multiple group comparisons was used to determine the statistical difference. Data correlation was evaluated with Pearson correlation analysis. Statistical significance was accepted at P < 0.05.
3. Results
3.1. Some human glioma cell lines exhibited low intracellular ALA-PpIX fluorescence and little response to ALA-PDT.
The intracellular and extracellular PpIX in human glioma cell lines was measured by spectrometry after 4 h incubation with ALA (Fig. 1a). It was striking to note that the majority of PpIX produced in glioma cell lines such as H4, SW1088, U-87 and U-87-IDH1 was released into the medium, resulting in low intracellular PpIX. In contrast, U-118 cells were able to retain most of the PpIX produced, showing the highest intracellular PpIX in the panel. There was no significant difference between U-87 and U-87-IDH1 mutant cells in either intracellular or extracellular PpIX. Glioma cells were treated with 3 J/cm2 light after 4 h incubation with ALA and cell viability was measured at 48 h after PDT (Fig. 1b). PDT induced significant cell viability decrease in A172, U-118 and U-87 cells, but no significant reduction in cell viability was detected in H4, SW1088 and U-87-IDH1 cells. Such a disparity in PDT response was associated with the difference in intracellular PpIX (Pearson r: −0.813, P = 0.049). The difference in cell viability between U-87 and U-87-IDH1 mutant cells was not statistically significant (P > 0.05).
Figure 1.
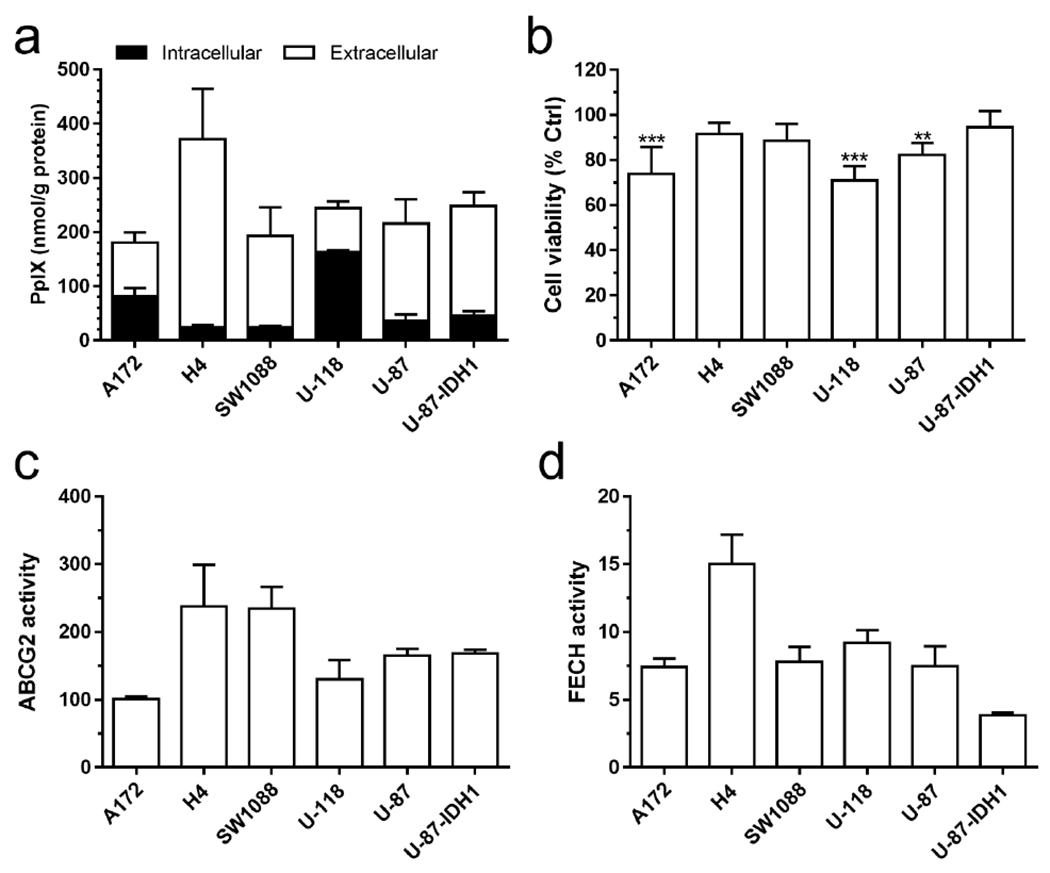
(a) The intracellular (in cell lysates) and extracellular (in cell culture medium) PpIX after 4 h incubation with ALA (1 mM) in a panel of human glioma cell lines (n = 4-7). (b) Cell viability at 48 h after ALA-PDT (1 mM ALA for 4 h incubation, 3 J/cm2 633 nm light irradiation, n = 5). ** P < 0.01, *** P < 0.001, compared with light only control (100%, not shown) by one-way ANOVA. (c, d) The activity ABCG2 (c) and FECH (d) was determined as described in Materials & Methods (n = 3 or 4). All error bars indicate the standard deviation (SD).
The activity of ABCG2 and FECH was measured and shown in Fig. 1c and Fig. 1d, respectively. H4 and SW1088 cells exhibited the highest ABCG2 activity, whereas A172 showed the lowest ABCG2 activity. Although no statistical difference in ABCG2 activity was found between U-87 and U-87-IDH1 mutant cells, mutant cells exhibited significantly lower FECH activity than the wildtype U-87 cells (P < 0.01). ABCG2 activity showed a trend of positive correlation with extracellular PpIX (Pearson r: 0.773, P = 0.072) and negative correlation with intracellular PpIX (Pearson r: −0.691, P = 0.128). No particular correlation was found between FECH activity and intracellular/extracellular PpIX in glioma cell lines.
3.2. ABCG2 inhibitor Ko143 was more effective than iron chelator DFO in enhancing ALA-PpIX fluorescence in glioma cell lines.
To enhance ALA-PpIX fluorescence, glioma cells were treated with ALA in combination with ABCG2 inhibitor Ko143 or iron chelator DFO. Fig. 2a shows the mean cell fluorescence measured with flow cytometry after 4 h incubation with ALA alone or in combination with Ko143 or DFO. Two-way ANOVA analysis indicates that ALA in combination with Ko143 (1 μM) caused significant increase of PpIX fluorescence in all glioma cell lines compared with ALA alone (P < 0.001). In contrast, PpIX fluorescence increase induced by DFO (1 mM) was much less in extent and statistically significant only in U-118 cells (P < 0.05). The fold change of fluorescence after the combination treatments to ALA treatment alone was shown in Fig. 2b. Ko143 induced several folds increase of PpIX fluorescence in glioma cell lines with high ABCG2 activity (H4, SW1088, U-87 and IDH1 mutant), whereas DFO treatment led to only slight increases of PpIX fluorescence in all glioma cell lines. The fluorescence fold increase induced by Ko143 was significantly higher than DFO-induced increase in all glioma cell lines except A172 cells, indicating that inhibition of ABCG2 by Ko143 was more effective than iron chelation by DFO in enhancing ALA-PpIX fluorescence in glioma cell lines.
Figure 2.

ABCG2 inhibitor Ko143 was more effective than iron chelator DFO in enhancing ALA-PpIX fluorescence in glioma cell lines. (a) Mean cell fluorescence (FL3) measured by a flow cytometer after 4 h treatment with ALA alone or combination treatments (n = 3. Bars, SD). * P < 0.05, *** P < 0.001, compared with ALA (1 mM) alone in the corresponding cell line by two-way ANOVA. (b) The fluorescence fold change after the combination treatments to ALA treatment alone (n = 3. Bars, SD). * P < 0.05, *** P < 0.001, compared with ALA in combination with DFO (1 mM) in the corresponding cell line by two-way ANOVA.
3.3. Comparison between kinase inhibitor Lap and ABCG2 inhibitor Ko143 for the enhancement of ALA-PpIX fluorescence.
Effects of Lap versus Ko143 on ALA-PpIX fluorescence were determined with flow cytometry in H4 cells with robust ABCG2 activity (as shown in Fig. 1c). Both Ko143 (Fig. 3a) and Lap (Fig. 3b) increased ALA-PpIX fluorescence in a dose-dependent manner with similar potency (EC50 at ~71 nM), although Ko143 was about 15% higher than Lap in the efficacy (the maximum enhancement effect). However, in the presence of Lap (50 nM), both the potency and efficacy of Ko143 for enhancing ALA-PpIX was substantially reduced as indicated by the EC50 increased from 71.52 nM to 141.30 nM and the maximum enhancement effect decreased by about 30%. In contrast, the presence of Ko143 (50 nM) significantly increased the potency of Lap for ALA-PpIX enhancement (EC50 decreased from 71.48 nM to 46.70 nM) with no significant effect on the efficacy. These results suggest that Lap and Ko143 competed for the same binding sites in ABCG2 transporter, although Lap was less efficacious than Ko143 for enhancing ALA-PpIX.
Figure 3.

Effects of ABCG2 inhibitor Ko143 (a) and kinase inhibitor Lap (b) on the enhancement of ALA-PpIX fluorescence. H4 cells were incubated with ALA (1 mM) alone or in combination with Ko143 and/or Lap as indicated for 4 h. Fluorescence (FL3) was measured with a flow cytometer. The increase of fluorescence after different combination treatments was normalized to Ko143 (1 μM)-induced fluorescence increase and fitted with Log (drug concentration) vs normalized response (variable slope) non-linear regression (n = 3. Bars, SD).
3.4. Lap significantly enhanced ALA-PpIX fluorescence and reduced PpIX fluorescence heterogeneity in glioma cell lines.
Effects of Lap on ALA-PpIX fluorescence were evaluated with flow cytometry in human glioma cell lines. Fig. 4a shows the representative fluorescence histograms of untreated control, ALA alone and ALA in combination with Lap. The mean fluorescence intensity after different treatments was shown in Fig. 4b. Compared to ALA treatment alone, ALA in combination with Lap resulted in significantly higher PpIX fluorescence in all glioma cell lines. It was noted that PpIX fluorescence increase induced by Lap in H4, SW1088, U-87 and IDH1 mutant cells (all with high ABCG2 activity) exhibited good dose-dependence and was much greater in extent than in A172 and U-118 cells (with low ABCG2 activity), resulting in higher fluorescence fold change compared to ALA treatment alone (Fig. 4c). Furthermore, the coefficient of variation of fluorescence, an indicator of tumor cell heterogeneity of ALA-PpIX fluorescence, was significantly reduced by the combination treatments (Fig. 4d).
Figure 4.

Lap significantly enhanced ALA-PpIX fluorescence and reduced PpIX fluorescence heterogeneity in glioma cell lines. (a) Representative flow cytometer FL3 fluorescence histograms of glioma cells receiving no treatment (blue), ALA (1 mM) alone (black), and ALA in combination with Lap (100 nM, red). (b) Mean FL3 fluorescence measured by a flow cytometer after 4 h treatment with ALA alone, Lap alone or combination treatments (n = 3 or 4). (c) The fold change of FL3 fluorescence after the combination treatments to ALA treatment alone (n = 3 or 4). (d) FL3 fluorescence coefficient of variation (CV) after 4 h treatment with ALA alone or in combination with Lap (n = 3 or 4). * P < 0.05, ** P < 0.01, *** P < 0.001, compared with ALA (1 mM) alone in the corresponding cell line by two-way ANOVA. All error bars indicate the SD.
3.5. Lap, similar to Ko143, significantly increased the intracellular ALA-PpIX by reducing PpIX efflux in glioma cell lines.
To determine how Lap and Ko143 affected the intracellular and extracellular PpIX in glioma cell lines, cells were treated with ALA (1 mM) alone or ALA in combination with Lap or Ko143 for 4 h and PpIX in the cell lysates (intracellular PpIX) and medium (extracellular PpIX) was measured with spectrometry (Fig. 5). Compared to ALA alone, combination treatments with Lap or Ko143 significantly increased the intracellular PpIX and/or reduced the extracellular PpIX in all glioma cell lines, particularly in cell lines with high ABCG2 activity (H4, SW1088, U-87 and U-87-IDH1). No significant effect on the total amount of PpIX (the intracellular and extracellular PpIX combined) was found after the combination treatments.
Figure 5.

Lap significantly increased intracellular ALA-PpIX by reducing PpIX efflux in glioma cell lines. Cells were treated with ALA (1 mM) alone or the combination treatments as indicated for 4 h. PpIX in the cell lysates and medium was measured with spectrometry to indicate the intracellular and extracellular PpIX, respectively (n = 3-7. Bars, SD). * P < 0.05, ** P < 0.01, *** P < 0.001, compared with the intracellular PpIX of ALA alone in the corresponding cell line by two-way ANOVA. ^ P < 0.05, ^^ P < 0.01, ^^^ P < 0.001, compared with the extracellular PpIX of ALA alone in the corresponding cell line by two-way ANOVA.
3.6. Increase of intracellular PpIX induced by Lap or Ko143 showed a significant correlation with the ABCG2 activity across the glioma cell lines.
The fold change of intracellular PpIX after ALA in combination with Lap or Ko143 to ALA treatment alone was shown in Fig. 6a. In agreement with the flow cytometry data (Fig. 4c), increase of intracellular PpIX induced by Lap or Ko143 was much greater in glioma cell lines with high ABCG2 activity (H4, SW1088, U-87 and U-87 IDH1) than in cell lines with low ABCG2 activity (A172 and U-118). There was a significant correlation between Lap- or Ko143-induced intracellular PpIX increase and the ABCG2 activity (Fig. 6b).
Figure 6.

Correlation between Lap- or Ko143-induced intracellular PpIX increase with ABCG2 activity in glioma cell lines. Cells were treated with ALA (1 mM) alone or the combination treatments as indicated for 4 h. PpIX in the cell lysates was measured with spectrometry to indicate the intracellular PpIX (n = 3-7. Bars, SD). The fold change of intracellular PpIX after ALA in combination with Lap or Ko143 to ALA treatment alone was shown (a) and correlated with the ABCG2 activity of glioma cell lines (b).
3.7. Lap and Ko143 significantly enhanced ALA-PDT in glioma cell lines, whereas DFO showed less enhancement.
Effects of ALA-PDT alone and combination treatments with DFO, Lap or Ko143 were assessed in glioma cell lines and shown in Fig. 7. Overall, glioma cells showed little response to PDT alone at lower doses of ALA. While PDT at higher doses of ALA induced a moderate response in most cell lines, H4 and U-87-IDH1 were still resistant to PDT even at the highest dose of ALA of 4 mM. DFO (10 μM), Lap (100 nM) or Ko143 (1 μM) alone had no significant effect on cell viability in all glioma cell lines tested (bars with 0 mM ALA). Compared to ALA-PDT alone, PDT in combination with DFO caused significant cell viability reduction only in A172 cells. In contrast, Lap significantly increased PDT-induced cell viability reduction in all glioma cell lines except SW1088. In H4, U-118, U-87 and U-87-IDH1 cells, Lap in combination with PDT was significantly more effective than DFO combined with PDT in reducing cell viability. The greatest reduction of cell viability was caused by ALA in combination with Ko143, resulting in the lowest cell survival after treatments in all glioma cell lines.
Figure 7.

Effects of ALA-PDT alone and combination treatments on cell viability. Glioma cells were treated with ALA (0.25 - 4.00 mM) alone or the combination treatments as indicated for 4 h and irradiated with 3 J/cm2 light of 633 nm. Cell viability was determined by Alamar blue assay at 48 h after treatment (n = 4 or 5. Bars, SD). * P < 0.05, ** P < 0.01, *** P < 0.001, compared with ALA alone in the corresponding cell line by two-way ANOVA. ^ P < 0.05, ^^ P < 0.01, ^^^ P < 0.001, compared with ALA + DFO (10 μM) in the corresponding cell line by two-way ANOVA.
4. Discussion
As ALA is becoming widely used for PpIX fluorescence-guided glioma resection, it is necessary to identify important biological determinants of tumor PpIX fluorescence and develop corresponding strategies for therapeutic optimization. Analysis of intracellular and extracellular PpIX in a panel of human glioma cell lines showed that glioma cell lines with high ABCG2 activity exhibited low intracellular PpIX, high extracellular PpIX, and low response to ALA-PDT (Fig. 1). Inhibition of ABCG2 increased intracellular PpIX, reduced extracellular PpIX, and sensitized glioma cells to ALA-PDT (Fig. 5 & 7). Furthermore, ABCG2 inhibition was more effective than the inhibition of PpIX bioconversion with iron chelator DFO for enhancing ALA-PpIX fluorescence and PDT response (Fig. 2 & 7). These results reveal ABCG2 transporter as a critical biological determinant for reducing ALA-PpIX fluorescence in glioma cells and highlight it as an important and sensitive target for therapeutic enhancement.
Previous studies in glioma cell lines also showed that inhibition of ABCG2 led to enhanced ALA-PpIX fluorescence and PDT response. For instance, inducible expression of ABCG2 in U251 glioblastoma cells reduced PpIX fluorescence and PDT effects, which could be reversed by ABCG2 inhibitor Ko143 [15]. Not only in glioma cells, Ko143 has also been shown to increase ALA-PpIX fluorescence and PDT in other types of cancer cells [22–24]. Although Ko143 is a potent ABCG2 inhibitor commonly used in vitro, its chemical instability prevents its use in humans [19]. Despite the fact that ABCG2 has been known to play an important role in multidrug resistance to cancer therapy and considerable effort has been made to develop ABCG2 inhibitors, safe and effective inhibitors suitable for clinical application remain unavailable [17].
Since some approved drugs including kinase inhibitors are known to inhibit ABCG2 activity [25], repurposing some of these existing drugs as potential ABCG2 inhibitors is an efficient and practical approach. Gefitinib, a tyrosine kinase inhibitor of epidermal growth factor receptor (EGFR), was shown to increase intracellular PpIX and PDT after ALA in glioma cell lines [16]. Interestingly, the study also showed that gefitinib was able to reduce ABCG2 expression. We have evaluated over a dozen small molecular inhibitors and identified Lap as the most effective at enhancing intracellular PpIX in tumor cells [20]. In triple negative breast cancer cell lines with ABCG2 activity, Lap increases ALA-PpIX fluorescence and potentiates PDT for inducing apoptotic cell death in tumor cells that are otherwise resistant to ALA-PDT [26].
The present study demonstrates the effectiveness of Lap in enhancing ALA-PpIX and PDT in glioma cell lines. We showed that Lap, similar to Ko143, induced a dose-dependent increase of ALA-PpIX fluorescence in H4 cells with strong ABCG2 activity (Fig. 3). Both Lap and Ko143 increased intracellular PpIX and reduced extracellular PpIX without changing the total PpIX levels (Fig. 5). Importantly, PpIX fluorescence increase induced by Lap or Ko143 correlated well with the ABCG2 activity of glioma cell lines (Fig. 6). All these results indicate that Lap, like Ko143, enhanced ALA-PpIX via inhibiting ABCG2-mediated PpIX efflux. The dose-response study further suggests that Lap competed with Ko143 for the same binding sites in ABCG2, albeit less efficacious than Ko143 for enhancing ALA-PpIX and PDT (Fig. 3). This finding is in agreement with the notion that all ABCG2 substrates and inhibitors are required to bind to the highly conserved phenylalanine residue (F439) in the ligand-binding cavity of ABCG2 [27]. However, what determines a ligand as a substrate or an inhibitor and why Lap was less efficacious than Ko143 despite sharing similar binding sites in ABCG2 are not clear. One notable difference between Lap and Ko143 is that Lap activates the ATPase activity of ABCG2 [28], whereas Ko143 strongly inhibits the ATPase activity [19]. Inhibition of ATPase activity may lock ABCG2 in the ATP-bound conformation and prevent its switch to the substrate-bound conformation [29]. Because the dynamic cycling between the substrate-bound and ATP-bound conformation is necessary for ABCG2 transport function, inhibition ATPase activity by Ko143, but not Lap, may explain why Ko143 is a more effective ABCG2 inhibitor.
Although our data indicates that Lap-induced PpIX fluorescence enhancement correlated well with the ABCG2 activity of glioma cell lines, results of ALA-PDT enhancement by Lap showed two noticeable outliers. Lap significantly enhanced ALA-PpIX fluorescence in SW1088 cells with strong ABCG2 activity, but did not significantly enhance PDT response. U-118 cells were sensitive to the enhancement of PDT by Lap, despite showing relatively low ABCG2 activity and low response to Lap-induced PpIX enhancement. The cause for the mismatch between PpIX enhancement and PDT response across the glioma cell lines remains unclear, although the disparity in intrinsic sensitivity to ALA-PDT due to the differences in PpIX intracellular localization and the tolerance for PDT-induced oxidative stress cannot be ruled out.
It is important to find that inhibition of ABCG2 by Lap significantly reduced intra-tumor heterogeneity of ALA-PpIX in glioma cell lines, as indicated by the reduction of coefficient of variation of PpIX fluorescence (Fig. 4d). Similar findings were also reported in triple negative breast cancer cell lines [24] and pancreatic cancer cells [30]. As a cancer stem cell marker, ABCG2 is highly expressed in glioma stem cells in both cultured human glioma cell lines [31] and human glioma tissue samples [32]. Although glioma stem cells account for only a small fraction of tumor cell population, they play an important role in driving tumor self-renewal and promoting drug resistance [32, 33]. Increased ABCG2 expression in glioma stem cells compared to mature glioma cells may reduce ALA-PpIX fluorescence in glioma stem cells, causing PpIX fluorescence heterogeneity. If so, enhanced visualization/resection and PDT of ABCG2-overexpressing glioma stem cells as result of Lap-mediated ABCG2 suppression may result in more sustained therapeutic response. In addition to variation in ABCG2 expression, alterations in other biological parameters such as oligopeptide transporter 1 (PEPT1) involved in ALA uptake may also cause tumor PpIX heterogeneity [34]. However, whether modulating PEPT1 changes ALA-PpIX and fluorescence heterogeneity in tumor cells is yet to be determined.
A major genetic alteration in human gliomas is the mutation of IDH, a Krebs cycle enzyme catalyzing the decarboxylation of isocitrate to α-ketoglutarate (α-KG) [1]. IDH mutants, the most common of which is the IDH1-R132H missense mutation (involved in about 90% of IDH-mutated gliomas), acquire a neomorphic enzyme activity that converts α-KG to an oncometabolite D-2-hydroxyglutarate (D-2-HG). Different from its normal counterpart, D-2-HG inhibits a variety of dioxygenases that use α-KG as a substrate, causing aberrant DNA/histone methylation and driving oncogenesis. Interestingly, although ALA-PpIX fluorescence shows over 95% positivity in high-grade gliomas (often without IDH mutation), fluorescence-positive rate is only about 20% in low-grade gliomas that typically carry IDH mutations [35]. This raises the question of whether IDH mutations reduce ALA-PpIX fluorescence. Clinical evaluation on how IDH mutation status is associated with ALA-PpIX fluorescence in low-grade glioma patients yields controversial results, from being positively associated [36], not associated [35], to being negatively associated [37].
Using U-87 and its isogenic U-87-IDH1-R132H mutant cells, we found that introducing IDH1-R132H mutation had no significant effect on ALA-PpIX fluorescence and PDT response. It is interesting to find that IDH1-R132H mutation significantly reduced the FECH activity, but counterintuitive to observe that reduction of FECH activity did not result in increased PpIX fluorescence in U-87-IDH1-R132H mutant cells. Although we still do not understand how IDH1-R132H mutation reduced FECH activity and why it did not change ALA-PpIX fluorescence, this unexpected finding suggests that, in addition to FECH, other components of heme biosynthesis pathway including the enzymes, transporters and the availability of substrates may be affected by the mutation. We also did not see a correlation between FECH activity and ALA-PpIX fluorescence across the glioma cell lines in the present study and renal cell carcinoma cell lines previously [20], which is contradictory to the common assumption that enhanced PpIX fluorescence is due to reduced FECH expression/activity in tumor cells. Similarly, high-grade glioma tumors, which almost always show the positive ALA-PpIX fluorescence, were found to exhibit significantly higher FECH mRNA levels than low-grade gliomas often with the negative PpIX fluorescence [38]. All these results appear to indicate that FECH alone is not a reliable predictor of ALA-PpIX fluorescence.
Our present data strongly support the enhancement of ALA-PpIX fluorescence and PDT with ABCG2 inhibitors. Since all glioma cell lines used in this study are from high-grade human gliomas, our findings may translate to the clinical improvement of ALA for guiding the resection of high-grade gliomas by further enhancing PpIX fluorescence. This enhancement approach is particularly appealing when considering that gliomas, high-grade gliomas in particular, tend to show high ABCG2 expression [17, 39], although there is also evidence indicating no significant difference between low- and high-grade gliomas in ABCG2 expression [38]. Our results do not contradict the clinical observation that low-grade gliomas often do not display positive PpIX fluorescence because this fluorescence negativity is most likely due to the inability of ALA to cross the intact blood-brain barrier (BBB) in the majority of low-grade gliomas [35]. It can be argued that this ABCG2-targeted enhancement strategy may be well suited for the enhancement of ALA in low-grade gliomas with compromised BBB (showing contrast enhancement).
In summary, our results demonstrate that ABCG2 is an important biological determinant of ALA-PpIX fluorescence in human glioma cell lines. Inhibition of ABCG2 with clinically used kinase inhibitor lapatinib significantly increased intracellular PpIX fluorescence in tumor cells, reduced PpIX fluorescence heterogeneity, and potentiated PDT response. ABCG2 inhibition was more effective than the iron chelator DFO in enhancing ALA-PpIX and PDT. This study paves the way for using lapatinib to enhance the clinical application of ALA as a tumor diagnostic and PDT agent in gliomas.
Acknowledgements
This work was supported in part by the National Institutes of Health R15CA268200, R15EB026208. We thank Jordyn Olsen and Lolwah Alsalamah for helpful discussions and assistance in the lab.
Footnotes
Declarations of interest: none.
References
- [1].Reifenberger G, Wirsching HG, Knobbe-Thomsen CB, Weller M, Advances in the molecular genetics of gliomas - implications for classification and therapy, Nature reviews. Clinical oncology 14(7) (2017) 434–452. [DOI] [PubMed] [Google Scholar]
- [2].Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO, European Organisation for R, Treatment T of Cancer Brain, G. Radiotherapy, G. National Cancer Institute of Canada Clinical Trials, Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma, N Engl J Med 352(10) (2005) 987–96. [DOI] [PubMed] [Google Scholar]
- [3].Molinaro AM, Hervey-Jumper S, Morshed RA, Young J, Han SJ, Chunduru P, Zhang Y, Phillips JJ, Shai A, Lafontaine M, Crane J, Chandra A, Flanigan P, Jahangiri A, Cioffi G, Ostrom Q, Anderson JE, Badve C, Barnholtz-Sloan J, Sloan AE, Erickson BJ, Decker PA, Kosel ML, LaChance D, Eckel-Passow J, Jenkins R, Villanueva-Meyer J, Rice T, Wrensch M, Wiencke JK, Oberheim Bush NA, Taylor J, Butowski N, Prados M, Clarke J, Chang S, Chang E, Aghi M, Theodosopoulos P, McDermott M, Berger MS, Association of Maximal Extent of Resection of Contrast-Enhanced and Non-Contrast-Enhanced Tumor With Survival Within Molecular Subgroups of Patients With Newly Diagnosed Glioblastoma, JAMA Oncol 6(4) (2020) 495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Claes A, Idema AJ, Wesseling P, Diffuse glioma growth: a guerilla war, Acta Neuropathol 114(5) (2007) 443–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Orringer D, Lau D, Khatri S, Zamora-Berridi GJ, Zhang K, Wu C, Chaudhary N, Sagher O, Extent of resection in patients with glioblastoma: limiting factors, perception of resectability, and effect on survival, Journal of neurosurgery 117(5) (2012) 851–9. [DOI] [PubMed] [Google Scholar]
- [6].Hadjipanayis CG, Stummer W, 5-ALA and FDA approval for glioma surgery, J Neurooncol 141(3) (2019) 479–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Yang X, Palasuberniam P, Kraus D, Chen B, Aminolevulinic Acid-Based Tumor Detection and Therapy: Molecular Mechanisms and Strategies for Enhancement, International journal of molecular sciences 16(10) (2015) 25865–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ, Group AL-GS, Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial, The Lancet. Oncology 7(5) (2006) 392–401. [DOI] [PubMed] [Google Scholar]
- [9].Schupper AJ, Baron RB, Cheung W, Rodriguez J, Kalkanis SN, Chohan MO, Andersen BJ, Chamoun R, Nahed BV, Zacharia BE, Kennedy J, Moulding HD, Zucker L, Chicoine MR, Olson JJ, Jensen RL, Sherman JH, Zhang X, Price G, Fowkes M, Germano IM, Carter BS, Hadjipanayis CG, Yong RL, 5-Aminolevulinic acid for enhanced surgical visualization of high-grade gliomas: a prospective, multicenter study, Journal of neurosurgery (2021) 1–10. [DOI] [PubMed] [Google Scholar]
- [10].Roberts DW, Valdes PA, Harris BT, Fontaine KM, Hartov A, Fan X, Ji S, Lollis SS, Pogue BW, Leblond F, Tosteson TD, Wilson BC, Paulsen KD, Coregistered fluorescence-enhanced tumor resection of malignant glioma: relationships between delta-aminolevulinic acid-induced protoporphyrin IX fluorescence, magnetic resonance imaging enhancement, and neuropathological parameters. Clinical article, Journal of neurosurgery 114(3) (2011) 595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Stummer W, Tonn JC, Goetz C, Ullrich W, Stepp H, Bink A, Pietsch T, Pichlmeier U, 5-Aminolevulinic acid-derived tumor fluorescence: the diagnostic accuracy of visible fluorescence qualities as corroborated by spectrometry and histology and postoperative imaging, Neurosurgery 74(3) (2014) 310–9; discussion 319-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Piffaretti D, Burgio F, Thelen M, Kaelin-Lang A, Paganetti P, Reinert M, D’Angelo ML, Protoporphyrin IX tracer fluorescence modulation for improved brain tumor cell lines visualization, Journal of photochemistry and photobiology. B, Biology 201 (2019) 111640. [DOI] [PubMed] [Google Scholar]
- [13].Blake E, Allen J, Curnow A, An in vitro comparison of the effects of the iron-chelating agents, CP94 and dexrazoxane, on protoporphyrin IX accumulation for photodynamic therapy and/or fluorescence guided resection, Photochemistry and photobiology 87(6) (2011) 1419–26. [DOI] [PubMed] [Google Scholar]
- [14].Valdes PA, Samkoe K, O’Hara JA, Roberts DW, Paulsen KD, Pogue BW, Deferoxamine iron chelation increases delta-aminolevulinic acid induced protoporphyrin IX in xenograft glioma model, Photochemistry and photobiology 86(2) (2010) 471–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Muller P, Abdel Gaber SA, Zimmermann W, Wittig R, Stepp H, ABCG2 influence on the efficiency of photodynamic therapy in glioblastoma cells, Journal of photochemistry and photobiology. B, Biology 210 (2020) 111963. [DOI] [PubMed] [Google Scholar]
- [16].Sun W, Kajimoto Y, Inoue H, Miyatake S, Ishikawa T, Kuroiwa T, Gefitinib enhances the efficacy of photodynamic therapy using 5-aminolevulinic acid in malignant brain tumor cells, Photodiagnosis and photodynamic therapy 10(1) (2013) 42–50. [DOI] [PubMed] [Google Scholar]
- [17].Robey RW, Pluchino KM, Hall MD, Fojo AT, Bates SE, Gottesman MM, Revisiting the role of ABC transporters in multidrug-resistant cancer, Nat Rev Cancer 18(7) (2018) 452–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Polli JW, Humphreys JE, Harmon KA, Castellino S, O’Mara MJ, Olson KL, John-Williams LS, Koch KM, Serabjit-Singh CJ, The role of efflux and uptake transporters in [N-{3-chloro-4-[(3-fluorobenzyl)oxy]phenyl}-6-[5-({[2-(methylsulfonyl)ethyl]amino }methyl)-2-furyl]-4-quinazolinamine (GW572016, lapatinib) disposition and drug interactions, Drug metabolism and disposition: the biological fate of chemicals 36(4) (2008) 695–701. [DOI] [PubMed] [Google Scholar]
- [19].Weidner LD, Zoghbi SS, Lu S, Shukla S, Ambudkar SV, Pike VW, Mulder J, Gottesman MM, Innis RB, Hall MD, The Inhibitor Ko143 Is Not Specific for ABCG2, J Pharmacol Exp Ther 354(3) (2015) 384–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Howley R, Mansi M, Shinde J, Restrepo J, Chen B, Evaluation of aminolevulinic acid-mediated protoporphyrin IX fluorescence and enhancement by ABCG2 inhibitors in renal cell carcinoma cells, Journal of photochemistry and photobiology. B, Biology 211 (2020) 112017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Palasuberniam P, Kraus D, Mansi M, Braun A, Howley R, Myers KA, Chen B, Ferrochelatase Deficiency Abrogated the Enhancement of Aminolevulinic Acid-mediated Protoporphyrin IX by Iron Chelator Deferoxamine, Photochemistry and photobiology 95(4) (2019) 1052–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Barron GA, Moseley H, Woods JA, Differential sensitivity in cell lines to photodynamic therapy in combination with ABCG2 inhibition, Journal of photochemistry and photobiology. B, Biology 126 (2013) 87–96. [DOI] [PubMed] [Google Scholar]
- [23].Kobuchi H, Moriya K, Ogino T, Fujita H, Inoue K, Shuin T, Yasuda T, Utsumi K, Utsumi T, Mitochondrial localization of ABC transporter ABCG2 and its function in 5-aminolevulinic acid-mediated protoporphyrin IX accumulation, PloS one 7(11) (2012) e50082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Palasuberniam P, Yang X, Kraus D, Jones P, Myers KA, Chen B, ABCG2 transporter inhibitor restores the sensitivity of triple negative breast cancer cells to aminolevulinic acid-mediated photodynamic therapy, Sci Rep 5 (2015) 13298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Mao Q, Unadkat JD, Role of the breast cancer resistance protein (BCRP/ABCG2) in drug transport--an update, AAPS J 17(1) (2015) 65–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Palasuberniam P, Kraus D, Mansi M, Howley R, Braun A, Myers K, Chen B, Small molecule kinase inhibitors enhance aminolevulinic acid-mediated protoporphyrin IX fluorescence and PDT response in triple negative breast cancer cell lines, Journal of biomedical optics 26(9) (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gose T, Shafi T, Fukuda Y, Das S, Wang Y, Allcock A, Gavan McHarg A, Lynch J, Chen T, Tamai I, Shelat A, Ford RC, Schuetz JD, ABCG2 requires a single aromatic amino acid to “clamp” substrates and inhibitors into the binding pocket, FASEB journal : official publication of the Federation of American Societies for Experimental Biology 34(4) (2020) 4890–4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Dai TA, CL, Wu CP, Su XD, Wang SR, Liu DG, Ashby CR Jr, Huang Y, Robey RW, Liang YJ, Chen LM, Shi CJ, Ambudkar SV, Chen ZS, Fu LW, Lapatinib (Tykerb, GW572016) Reverses Multidrug Resistance in Cancer Cells by Inhibiting the Activity of ATP-Binding Cassette Subfamily B Member 1 and G Member 2, Cancer Res. 68(19) (2008) 7905–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Manolaridis I, Jackson SM, Taylor NMI, Kowal J, Stahlberg H, Locher KP, Cryo-EM structures of a human ABCG2 mutant trapped in ATP-bound and substrate-bound states, Nature 563(7731) (2018) 426–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kawai N, Hirohashi Y, Ebihara Y, Saito T, Murai A, Saito T, Shirosaki T, Kubo T, Nakatsugawa M, Kanaseki T, Tsukahara T, Shichinohe T, Li L, Hirano S, Torigoe T, ABCG2 expression is related to low 5-ALA photodynamic diagnosis (PDD) efficacy and cancer stem cell phenotype, and suppression of ABCG2 improves the efficacy of PDD, PloS one 14(5) (2019) e0216503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Weber K, Paulus W, Senner V, The side population of gliomas exhibits decreased cell migration, J Neuropathol Exp Neurol 69(6) (2010) 623–31. [DOI] [PubMed] [Google Scholar]
- [32].Emery IF, Gopalan A, Wood S, Chow KH, Battelli C, George J, Blaszyk H, Florman J, Yun K, Expression and function of ABCG2 and XIAP in glioblastomas, J Neurooncol 133(1) (2017) 47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Wee B, Pietras A, Ozawa T, Bazzoli E, Podlaha O, Antczak C, Westermark B, Nelander S, Uhrbom L, Forsberg-Nilsson K, Djaballah H, Michor F, Holland EC, ABCG2 regulates self-renewal and stem cell marker expression but not tumorigenicity or radiation resistance of glioma cells, Sci Rep 6 (2016) 25956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Suzuki T, Wada S, Eguchi H, Adachi J, Mishima K, Matsutani M, Nishikawa R, Nishiyama M, Cadherin 13 overexpression as an important factor related to the absence of tumor fluorescence in 5-aminolevulinic acid-guided resection of glioma, Journal of neurosurgery 119(5) (2013) 1331–9. [DOI] [PubMed] [Google Scholar]
- [35].Jaber M, Ewelt C, Wolfer J, Brokinkel B, Thomas C, Hasselblatt M, Grauer O, Stummer W, Is Visible Aminolevulinic Acid-Induced Fluorescence an Independent Biomarker for Prognosis in Histologically Confirmed (World Health Organization 2016) Low-Grade Gliomas?, Neurosurgery 84(6) (2019) 1214–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kim JE, Cho HR, Xu WJ, Kim JY, Kim SK, Kim SK, Park SH, Kim H, Lee SH, Choi SH, Park S, Park CK, Mechanism for enhanced 5-aminolevulinic acid fluorescence in isocitrate dehydrogenase 1 mutant malignant gliomas, Oncotarget (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ohba S, Murayama K, Kuwahara K, Pareira ES, Nakae S, Nishiyama Y, Adachi K, Yamada S, Sasaki H, Yamamoto N, Abe M, Mukherjee J, Hasegawa M, Pieper RO, Hirose Y, The Correlation of Fluorescence of Protoporphyrinogen IX and Status of Isocitrate Dehydrogenase in Gliomas, Neurosurgery 87(2) (2020) 408–417. [DOI] [PubMed] [Google Scholar]
- [38].Mischkulnig M, Kiesel B, Lotsch D, Roetzer T, Borkovec M, Wadiura LI, Mercea PA, Jaklin FJ, Hervey-Jumper S, Roessler K, Berger MS, Widhalm G, Erhart F, TCGA mRNA Expression Analysis of the Heme Biosynthesis Pathway in Diffusely Infiltrating Gliomas: A Comparison of Typically 5-ALA Fluorescent and Non-Fluorescent Gliomas, Cancers (Basel) 12(8) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Jin Y, Bin ZQ, Qiang H, Liang C, Hua C, Jun D, Dong WA, Qing L, ABCG2 is related with the grade of glioma and resistance to mitoxantone, a chemotherapeutic drug for glioma, Journal of cancer research and clinical oncology 135(10) (2009) 1369–76. [DOI] [PMC free article] [PubMed] [Google Scholar]


