Abstract
Although informed consent is critical for all research, there is increased ethical responsibility as individuals with intellectual or developmental disabilities (IDD) become the focus of more clinical trials. This study examined decisional capacity for informed consent to clinical trials in individuals with fragile X syndrome (FXS). Participants were 152 adolescent and adults (80 males, 72 females) with FXS who completed a measure of decisional capacity and a comprehensive battery of neurocognitive and psychiatric measures. Females outperformed males on all aspects of decisional capacity. The ability to understand aspects of the clinical trial was most predictive of the ability to appreciate and reason about the decision. Scaffolding improved understanding, suggesting researchers can take steps to improve decisional capacity and the informed consent process.
Keywords: Fragile X syndrome, Decisional Capacity, Informed Consent, Clinical Trials
INTRODUCTION
Most researchers would agree in principle that it is never ethical to enroll an individual in a study when they cannot provide informed consent. But in the case of research investigations involving participants with intellectual or developmental disabilities (IDD), guidance has been lacking on how to appropriately assess understanding and capacity to consent. There is considerable disagreement as to how to ensure consent or assent in people with IDD (McDonald and Kidney 2012). Some argue for assuming capacity unless a judge has determined that the person lacks capacity (Becker et al. 2004; Dalton and McVilly 2004), whereas others suggest that assessment of capacity is necessary in every case prior to consent (Iacono and Murray 2003), and still others reject any assessment of capacity in favor of a process of consent focused on shared decision-making (Dye et al. 2004; Dye, Hare, and Hendy 2007).
The informed consent and assent processes for people with IDD may involve efforts to increase the accessibility of information (e.g., simplifying language, or having the researcher read the information aloud or provide highlights); however, in many cases researchers engage primarily with the individual’s parent or guardian, and the individual with IDD is given minimal information or told that he or she will be participating with little or no direct involvement in the consent process. But, with appropriate supports, those with IDD may be able to make decisions about participation in research studies, and researchers have a moral and ethical obligation to maximize their participation in the process. This study explored variables associated with the capacity to make decisions about clinical trial participation in a sample of adolescents and young adults with the leading inherited form of IDD: fragile X syndrome (FXS).
Assessing Decisional Capacity.
Well-accepted theory and ethical guidelines (Grisso and Appelbaum 1998; Appelbaum 2007) suggest that decisional capacity involves four components: (1) understanding—perceiving and retaining information; (2) appreciation—linking the decision to one’s own situation; (3) reasoning—considering all information and weighing the consequences and choices; and (4) making and communicating a choice—reaching and communicating the decision. Making an informed choice first requires comprehending what is involved in a decision. How much an individual understands depends on how information is presented (written, verbal, visual) and the individual’s ability to comprehend and link information with prior knowledge. Next, an individual must be able to appreciate how the decision about participation may affect his/her life. Then, the person must engage in a process of reasoning about the decision. Effective reasoning requires processing information in a timely manner (processing speed), attending to and retaining key information while considering options (attention/working memory), and applying forward and flexible thinking to determine and compare consequences (planning and cognitive flexibility). These future-oriented skills can be significant weaknesses in individuals with IDD, suggesting that the ability to link a current decision with a future outcome may be challenging without additional support. Finally, unless an individual can express a logical choice, it is impossible to know his/her intended decision. The ability to communicate and maintain consistency in one’s expressed choice is crucial for consent.
The concept of decisional capacity has been given considerable attention in psychiatric research—especially in schizophrenia and other psychotic disorders (see Wang et al. 2017a for a review), severe mood disorders such as depression (Nugent et al. 2017; Wang et al. 2017b), anorexia (Grisso and Appelbaum 2006), and progressive cognitive disorders such as Alzheimer’s disease (Palmer et al. 2017). However, less focus has been placed on understanding variability in decisional capacity in individuals with childhood-onset IDD. Qualitative studies suggest that individuals with IDD want to participate in research and in the decision-making process (McDonald and Kidney 2012; McDonald 2012) and would like accommodations to help maximize their participation (McDonald 2012; McDonald, Kidney and Patka 2013), making this an issue of importance for primary stakeholders that deserves focused attention.
Clinical Trials and FXS.
Although it is always important to address decisional capacity for research, there are special considerations when determining the capacity to consent to clinical trials. In addition to requiring understanding of the general purpose of the research and procedural elements of a study, participants in a clinical trial also may need to understand (depending on the study’s methods) abstract concepts such as placebo, randomization, and the concept that the neither the participant nor the doctors doing the study will know which treatment the participant will receive (double-blind). In a recent meta-analysis of participants’ understanding of specific elements in clinical trials, nearly half of the presumably cognitively intact participants were not able to understand the concepts of placebo and randomization (Tam et al., 2015). These concepts are therefore likely to be significantly harder to understand in individuals with impaired capacity for abstract thinking, such as is often the case for individuals with FXS.
FXS is the leading hereditary cause of intellectual disability, highly co-morbid with autism, and one of the most studied neurogenetic disorders. It is an X-linked disorder, caused by an expansion of the CGG trinucleotide repeat on the 5’ untranslated region of the FMR1 gene. When this expansion reaches more than 200 repeats, methylation of the gene occurs, resulting in significantly reduced or absent fragile X mental retardation protein (FMRP), which is necessary for normal brain development. Because it is an X-linked disorder, there are significantly different outcomes based on sex—males with the expansion are almost uniformly affected, with cognitive functioning in the mild to severe range of intellectual disability. In contrast, depending on their X inactivation ratio, females have a much more variable profile, with some relatively unaffected, showing mild to no intellectual impairment, whereas others present with more severe outcomes similar to males (Loesch, Huggins, and Hagerman 2004).
Recently, an increasing number of clinical trials have investigated pharmaceuticals specifically for FXS, elevating the need to address decisional capacity for informed consent in this population. The potential for substantial benefit exists, assuming that a medication targets the core mechanism for FXS (Bear et al., 2004), rather than symptoms such as anxiety. These medications could have a major impact on functioning, as evidenced by research touting the “rescue” of FXS in mouse and Drosophila models (Burket et al. 2011; de Vrij et al. 2008; Michalon et al. 2012; Thomas et al. 2012; Yan, Rammal, Tranfaglia and Bauchwitz, 2005). If true, finding a “cure” becomes closer to reality; however, the potential therapeutic benefit could be unsettling for some individuals and raises important ethical questions. What does a cure mean for an individual who has lived their entire lives with the brain wiring produced by the gene change that caused their IDD? These drugs, if they work as purported, could have the potential to change an individual’s personhood, upping the ante considerably for ensuring informed consent and assent for clinical trials relative to most observational studies with fewer potential adverse outcomes. An additional complication is that most clinical trials in FXS to date have not shown benefit (Jonch and Jacquemont 2017), which has only increased the need and challenge for researchers involved in future trials to convey the likelihood of the potential risks and benefits of participation.
This study examined the extent to which males and females with FXS display decisional capacity for informed consent to clinical trials and sought to identify factors associated with decisional capacity. The study goal was to identify ways to improve how researchers convey information about studies to this population. Three primary research questions guided this work:
What are the strengths and weaknesses in the components of decisional capacity (understanding, appreciation, reasoning, expressing a choice) in individuals with FXS?
Which aspects of participation in clinical trials are more or less difficult for individuals with FXS to understand?
To what extent does variability in neurocognitive, affective, familial, and experiential factors account for variability in decisional capacity?
We hypothesized that females would outperform males on all areas assessed, but that we would find variability in profiles which would help in identifying subgroups of participants who might benefit from different types of support. We expected that more specific information about clinical trial participation would be easier than abstract concepts such as placebo and randomization, but that understanding could be improved with scaffolding (e.g., visual cues, repetition). Finally, we expected that, in males, overall cognitive level and presence of comorbid autism would be the strongest predictor of variability in decisional capacity, and for females we expected that variables such as anxiety and executive function would have a greater impact.
METHODS
Participants.
Participants in this study were 152 individuals with a confirmed diagnosis of FXS. Recruitment was conducted through multiple means, including outreach to families enrolled in FXS research registries, postings on webpages of national advocacy groups, and direct enrollment of participants at the National Fragile X Foundation family conference. The sample was roughly equal between males (80) and females (72) with a mean age of 20.45 (SD = 7.05; range = 12–40). The sample was mostly white (89%) and relatively wealthy (mean family income = $140K, SD = $109K; range $7–450K). See Table 1 for more details about the sample.
Table 1.
Demographics of Sample
| Full Sample N=152 |
Males N=80 |
Females N=72 |
|
|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | |
| Age | 20.4 (7.0) | 20.3 (7.1) | 20.6 (6.9) |
| IQ | 57.3 (19.7) | 45.9 (13.3) | 70.1 (17.7) |
| N (%) | N (%) | N (%) | |
| Race/Ethnicity | |||
| Asian, Hawaiian/Pacific Islander | 3 (2.0) | 1 (1.3) | 2 (2.8) |
| Non-Hispanic Black | 6 (4.0) | 4 (5.0) | 2 (2.8) |
| Non-Hispanic White | 130 (85.5) | 65 (81.3) | 65 (90.3) |
| Hispanic/Latino | 5 (3.3) | 4 (5.0) | 1 (1.4) |
| Multiple | 2 (1.3) | 1 (1.3) | 1 (1.4) |
| Missing | 6 (4.0) | 5 (6.3) | 1 (1.4) |
| Autism Status | |||
| No | 108 (71.1) | 45 (56.3) | 63 (87.5) |
| Yes | 35 (23.0) | 30 (37.5) | 5 (6.9) |
| Missing | 9 (5.9) | 5 (6.3) | 4 (4.6) |
| Family Income Category | |||
| <$50,000 | 11 (7.2) | 8 (10.0) | 3 (4.2) |
| $50,001–$75,000 | 13 (8.9) | 9 (11.3) | 4 (5.6) |
| $75,001–$100,000 | 21 (13.8) | 11 (13.8) | 10 (13.9) |
| >$100,000 | 38 (25.0) | 23 (28.8) | 15 (20.8) |
| Missing | 69 (45.4) | 29 (36.3) | 40 (55.6) |
| Highest Parental Education | |||
| High school or less | 5 (3.3) | 2 (2.5) | 3 (4.2) |
| Some College or Associates Degree | 22 (14.5) | 13 (16.3) | 9 (12.5) |
| College Degree | 59 (38.8) | 26 (32.5) | 33 (45.8) |
| Master’s Degree | 41 (27.0) | 24 (30.0) | 17 (23.6) |
| Professional Degree | 9 (5.9) | 6 (7.5) | 3 (4.2) |
| Missing | 16 (10.5) | 9 (11.3) | 7 (9.7) |
| Mother’s Marital Status | |||
| Single, Never Married | 8 (5.3) | 4 (5.0) | 4 (5.6) |
| Married | 118 (77.6) | 65 (81.3) | 53 (73.6) |
| Divorced, Separated, Widowed | 16 (10.5) | 5 (6.2) | 11 (15.3) |
| Missing | 10 (6.6) | 6 (7.5) | 4 (4.6) |
Instruments and Procedures.
All study procedures were approved by the investigators’ institutional review board (IRB). Each participant and their parent or legal guardian were provided with a thorough verbal and written review of all study requirements. Participants who were minors or who had a legal guardian provided assent and their guardian provided consent to participate. Those adult participants who did not have a legal guardian provided their own consent to participate.
A comprehensive assessment battery was used to collect measures of decisional capacity, cognitive functioning, learning and memory, comprehension, and executive functioning. Participants also completed a gold standard autism evaluation. Measures of social-emotional functioning (e.g., anxiety), social communication, and adaptive behavior were also collected through caregiver report.
Decisional Capacity.
The MacArthur Competence Assessment Tool for Clinical Research (MacCAT-CR; Appelbaum and Grisso 2001) was used as the measure of decisional capacity. The MacCAT-CR is widely recognized as a gold standard for measuring decisional capacity (Dunn et al. 2006). The MacCAT-CR is traditionally administered in a semi-structured interview format and with a standard rating scale for each item in four domains: Understanding, Appreciation, Reasoning, and Expressing a Choice. The number of items varies by domain, with each item assigned a score of 0, 1, or 2. The Understanding domain contains 13 items, with total scores ranging from 0 to 26. The Appreciation domain contains 3 items, with total scores ranging from 0 to 6. The Reasoning domain contains 4 items, with total scores ranging from 0 to 8. The Expressing a Choice domain contains a single item scored from 0 to 2. Scoring guidelines are provided for each item. For example, the Reasoning domain contains an item on “logical consistency,” with ratings of 2 (subject’s final choice follows logically from the subject’s own reasoning, as explained by the subject in response to the three previous subparts), 1 (it is not clear whether the choice follows logically from the subject’s own reasoning), or 0 (subject’s choice clearly does not follow logically from subject’s own reasoning).
For the current study, the format and administration of the MacCAT-CR content were modified to increase accessibility and support participant engagement for individuals with FXS. The adapted version was developed with consultation from experts in the field to ensure that the assessment protocol was consistent with prior work (Appelbaum, 2007; Cea and Fisher 2003). Similar to previous studies using the MacCAT-CR in populations with IDD (Fisher and Cea 2003), a hypothetical scenario asked study participants to consider a clinical trial to test the efficacy of a new medication.
Administration and Scoring.
The hypothetical scenario was presented in written text and read aloud by the research assistant. In addition, all concepts were paired with simple graphics to support individuals with FXS with reduced literacy. At the beginning of the scenario, participants were introduced to a main character consistent with their gender (Joe or Jane) and told that the main character also had FXS. The scenario depicted the main character being invited to participate in a research study by his or her doctor. Domain-specific questions were asked at the end of each section of the hypothetical scenario. In the Understanding section, information was presented and queried about the focus of the study (a new medication), how the study would be conducted (e.g., with a placebo control), what participation entails (e.g., taking a pill daily; blood draws), and possible risks and benefits (e.g., feeling better, feeling sick, getting blood taken). In the Appreciation section, participants were asked to consider why the character in the disclosure was asked to participate (i.e., because the person has FXS), whether the character would get the medication or placebo (i.e., they won’t know), and the consequences of not participating (i.e., the doctor will still take care of them). Participants were then asked, in the Reasoning domain, to consider whether the character should participate and to provide reasons why or why not. Last, the study participant was asked in the Expressing a Choice domain whether he or she thought the main character should participate in the study. Questions were worded to maintain the intent of the item from the original MacCAT-CR, but with simplified language.
MacCAT-CR administration was standardized and administered by trained research assistants. Additionally, each administration was video-recorded for the purpose of reliability and consensus scoring. Administration consisted of a maximum of two trials for the Understanding domain. Trials 1 and 2 each consisted of domain-specific content and open-ended questions, with the second trial occurring when full credit was not earned on all questions in the first trial for that domain. Trial 2 was included primarily to assess the effects of repeated exposure of the material (e.g., improved understanding). When full credit was not earned on either of the first two trials, a third “Recognition” trial was also administered after completion of trials for all domains, specifically for Understanding and Appreciation items on which participants did not earn full credit in either trial. In this trial, items were presented as multiple-choice questions. It was included to (1) minimize the possible effects of individual characteristics (e.g., limited expressive language, social communication impairments, anxiety) on a participant’s ability to demonstrate aspects of decisional capacity, and (2) assess the utility of a modified format (i.e., multiple-choice) for individuals with FXS.
To standardize scores on the MacCAT-CR items, the performance of participants was recoded using the following coding scheme for Understanding items: 4 = correct response on Trial 1; 3 = correct response on Trial 2; 2 = partial scores on Trials 1 and 2; 1 = correct response on recognition; 0 = never correct. For Appreciation and Reasoning items a second trial was not offered and for the Reasoning items, multiple choice items were not appropriate; therefore coding of scores was slightly different for items in these domains (See Table 2 for items, scoring scheme, and recognition item options; note that this scoring system differs from the usual MacCAT-CR scoring, limiting the comparability of these findings to other studies).
Table 2.
Adapted MacCAT-CR Items
| Question | Scoring | Recognition options | |
|---|---|---|---|
| Understanding | |||
| Purpose of Study | What is the study about? | 4 points: Says both on 1st attempt 3 points: Says both on 2nd attempt
2 points: Says one on 1st or 2nd try
1 point: Answers recognition correctly 0 points: None of the above |
|
| Purpose is for research not individualized care | Why are the doctors doing the study? | 4 points: Says one on 1st attempt 3 points: Says one on 2nd attempt
2 points: (e.g., partial response)
0 points: None of the above |
|
| Duration of project | How many times a day would Joe/Jane need to take the medicine? | 4 points: Says one on 1st attempt 3 points: Says one on 2nd attempt
0 points: None of the above |
|
| Procedural element #1 | What is one thing that will happen when Joe/Jane sees the doctor? | 4 points: Says one on 1st attempt 3 points: Says one on 2nd attempt
2 points:
0 points: None of the above |
|
| Procedural element #2 | What is another thing that will happen when Joe/Jane sees the doctor? | 4 points: Says one on 1st attempt 3 points: Says one on 2nd attempt
2 points:
0 points: None of the above |
|
| Placebo | Why are there two kinds of pills – one with the new medicine and one with no medicine? | 4 points: Says on 1st attempt 3 points: Says on 2nd attempt
2 points:
0 points: None of the above |
|
| Randomization | What will decide which pill Joe/Jane will take? | 4 points: Says one on 1st attempt 3 points: Says one on 2nd attempt
0 points: None of the above |
|
| Double blind | Who will know which pill Joe/Jane is taking? | 4 points: Says one on 1st attempt 3 points: Says one on 2nd attempt
0 points: None of the above |
|
| Societal benefit | What will the doctors learn from the study? | 4 points: Says one on 1st attempt 3 points: Says one on 2nd attempt
2 points:
1 point: Answers recognition correctly 0 points: None of the above |
|
| Personal benefit | What is one good thing that might happen to Joe/Jane if she gets the pill with the new medicine? | 4 points: Says one on 1st attempt 3 points: Says one on 2nd attempt
1 point: Answers recognition correctly 0 points: None of the above |
|
| Risk/Dislike | What is one thing that Joe/Jane might not like if she is part of the study? | 4 points: Says one on 1st attempt 3 points: Says one on 2nd attempt
0 points: None of the above |
|
| Risk/Hurt | What is one thing that would happen to Joe/Jane that might hurt? | 4 points: Says one on 1st attempt 3 points: Says one on 2nd attempt
2 points:
1 point: Answers recognition correctly 0 points: None of the above |
|
| Ability to withdraw | What should Joe/Jane do if she does not want to be in the study? | 4 points: Says one on 1st attempt 3 points: Says one on 2nd attempt
2 points:
1 points: Answers recognition correctly 0 points: None of the above |
|
| Appreciation | |||
| Recruitment not for personal benefit | Why do you think Joe/Jane’s doctor asked him/her to be part of the study? | 3 points: States that his/her personal benefit is not the primary objective of the study on the 1st attempt.
2 points: Gives reasons both related and unrelated to personal benefit/plausible explanation for recruitment only for personal benefit
1 point: Answers recognition correctly 0 points: No plausible explanation for recruitment/only for personal benefit Doesn’t know or something unrelated |
|
| Research over individualized care | Do you think Joe/Jane will get the pill with the new medicine or the pill with no medicine? Why? | 3 points: Research protocol takes precedence. Says on 1st attempt.
2 points: Uncertain if research/personal needs dictate which medicine or personal needs will determine which medicine w/plausible explanation
1 point: Answers recognition correctly 0 point: Personal needs will dictate which medicine with no plausible explanation
Doesn’t know or something unrelated |
|
| Ability to decline or withdraw | What will happen to Joe/Jane if he/she does not want to be part of the study? | 3 points: No adverse effects for decline/withdraw. Says on 1st attempt.
2 points: Uncertain if decline/withdraw will have adverse effect or decline/withdraw will have adverse effect but plausible explanation
1 point: Answers recognition correctly 0 points: Decline/withdraw will have adverse effect with no plausible explanation
|
|
| REASONING | |||
| Preliminary choice | Do you think that Joe/Jane should say YES and be part of the study or should Joe/Jane say NO and not be part of the study? | No score for this | |
| Consequential reasoning | Why do you think Joe/Jane [should say YES/should say NO] to being part of the study? | 3 points: Mentions at least 2 consequences
2 points: Mentions 1 consequence
0 point: Mentions no consequences |
Do you think Joe/Jane should say YES and be part of the study because
Do you think Joe/Jane should say NO and not be part of the study because
|
| Comparative reasoning | Why is it better for Joe/Jane to [be part of/not be part of] the study? | 2 points: Compares two options with a reason that the two choices are different
1 point: Compares two options but does not include a reason
Does not know or something unrelated |
|
| Generating consequences | How might these things change what Joe/Jane does every day? | 2 points: Gives two reasonable consequences that relate to everyday activities or social relationships
1 point: Gives one reasonable consequence that relates to everyday activities or social relationships
0 points: Makes no comparison
|
|
| Logical consistency | No question | 2 points: Subject’s final choice (in Expressing a Choice) follows logically from their reasoning 1 point: It is not clear whether subject’s choice follows logically from their reasoning 0 points: Choice clearly does not follow from reasons given |
|
| Expressing a Choice | |||
| Now that we’ve talked about everything, what do you think Joe/Jane should do? | 3 points: Subject states choice 2 points: Subjects states more than one choice, seems ambivalent 1 point: Subject states choice from recognition item 0 points: Subject does not state choice |
|
|
Cognitive Functioning.
The Stanford Binet Intelligence Scales 5th edition (SB5; Roid 2003) was used to measure cognitive functioning. The SB5 provides scores for verbal and nonverbal ability across five domains: Fluid Reasoning, Knowledge, Quantitative Reasoning, Visual-Spatial Reasoning, and Working Memory. Standardized IQ tests, including the SB5, have limited range and precision for those with IDD, including people with FXS. As a result, we used a previously published method (Sansone et al. 2014) of z-score transformation based on the norm sample from the SB5 to correct for floor effects.
Comprehension.
Subtests from the Woodcock-Johnson Tests of Achievement 3rd edition (WJIII-Ach; Woodcock, McGrew, and Mather 2001, 2007), Reading and Oral Comprehension domains were administered to assess comprehension.
Executive Function.
Select subtests from the Delis-Kaplan Executive Function System (DKEFS; Delis, Kaplan, and Kramer 2001) were used as measures of cognitive flexibility (Twenty Questions and Color-Word subtests), inhibitory control (Color-Word subtest), and planning and problem solving (Tower subtest).
Visual and Verbal Memory.
Select subtests from the Wide Range Assessment of Memory and Learning 2nd edition (WRAML-2, Sheslow and Adams 2003) were used as measures of verbal and visual memory and learning.
Adaptive Behavior.
The Scales of Independent Behavior, revised (SIB-R; Bruininks, et al. 1996) composite score was used as a measure of adaptive behavior. The composite comprises several subdomains, including motor (fine and gross), social communication, personal living, and community living.
Social-Behavioral Skills.
The Anxiety, Depression, and Mood Scale (ADAMS; Esbensen et al. 2003) is a parent-report questionnaire consisting of 28 items that serves as a screen for psychiatric disorders in individuals with IDD. The scale’s psychometric properties were evaluated and normed with 265 individuals with IDD and validated with a total of 129 psychiatric patients with IDD (Esbensen et al. 2003). Three scales of the ADAMS were used as measures of General Anxiety (seven items), Social Avoidance (seven items), and Hyperactivity (five items).
Autism Spectrum Disorder (ASD).
The Social Communication Questionnaire Lifetime Form (SCQ; Rutter, Bailey, and Lord 2003) was used as a measure of developmental history based on caregiver report and the Autism Diagnostic Observation System 2nd edition (ADOS-2; Lord et al. 2012) was used as a direct assessment of ASD symptoms. The ADOS was administered by research reliable assessors. Only those who met criteria for ASD on both the SCQ (Rutter, Bailey, and Lord 2003) and the ADOS-2 (Lord et al. 2012) were considered to meet criteria for ASD for this study.
Data Analysis.
Data were analyzed using SAS Enterprise Guide, v. 7.2 (Cary, NC). To answer research questions 1 and 2, descriptive statistics were used to explore participant performance in Trials 1, 2, and recognition for each domain and by item in the full sample and by gender. Among those that did not answer questions correctly during the first trial, we describe the percentage of remaining participants that received credit for questions after one repetition of information, and the percentage of participants that received full credit when presented with the recognition trial; this is described in the full sample and by gender.
To answer research question 3, multiple linear regressions in four steps were used to identify predictors (e.g., cognitive ability, comorbid ASD, anxiety) of three domains of decisional capacity: (A) Understanding, (B) Appreciation, and (C) Reasoning. Due to minimal variability in Expressing a Choice, we did not model findings for that domain, but we do report correlations among the observed sample for males only between the Expressing a Choice domain and continuous predictors of interest, and the chi-square test statistic for Expressing a Choice and comorbid ASD. For the former analysis, we dichotomized the Expressing a Choice variable into two groups: those receiving full credit on the first trial vs. not. Because we hypothesized that the factors predicting the domains of decision capacity may differ between males and females, we first created interaction terms to characterize the possible differential effect of demographic, functional, and cognitive variables by gender. Next, multiple imputation procedures (25 imputations) were used to generate complete data for individual scores for all predictors (% missing ranged from 0%–7% for all variables of interest except for sequential processing [16%], and inhibitory control [22%]). To ease interpretability, all predictor and outcome variables except for gender and comorbid ASD (both dichotomous) were standardized so that mean = 0 and standard deviation = 1.
Within each step for the models of Understanding, Appreciation, and Reasoning, interaction terms that were not significant at an alpha of .05 were removed and the regression was rerun. Gender was included as a main effect in all models testing the significance of interactions with gender. Main effects and interaction terms that continued to be significantly associated with the outcome variable within each step were carried into all subsequent steps.
In Step 1, age, gender, autism status, age*gender, and autism status*gender were regressed onto each outcome variable; in Step 2, measures of IQ, broad independence, oral comprehension, passage comprehension, and corresponding interaction terms were entered into the models. In Step 3, social avoidance, general anxiety, hyperactivity and corresponding interaction terms were entered; in Step 4, measures of inhibitory control, cognitive flexibility, planning and problem solving, verbal memory, visual memory, working memory, communication, and corresponding interaction terms were entered. As a secondary analysis, we explored the extent to which the addition of the individual’s Understanding score to the models predicting Appreciation and Reasoning contributed to the explanation of those domains, and how this addition altered the effect sizes for other predictor variables. All regression models were analyzed using PROC REG with estimates pooled across imputations using PROC MI ANALYZE.
RESULTS
Decisional Capacity Strengths and Weaknesses.
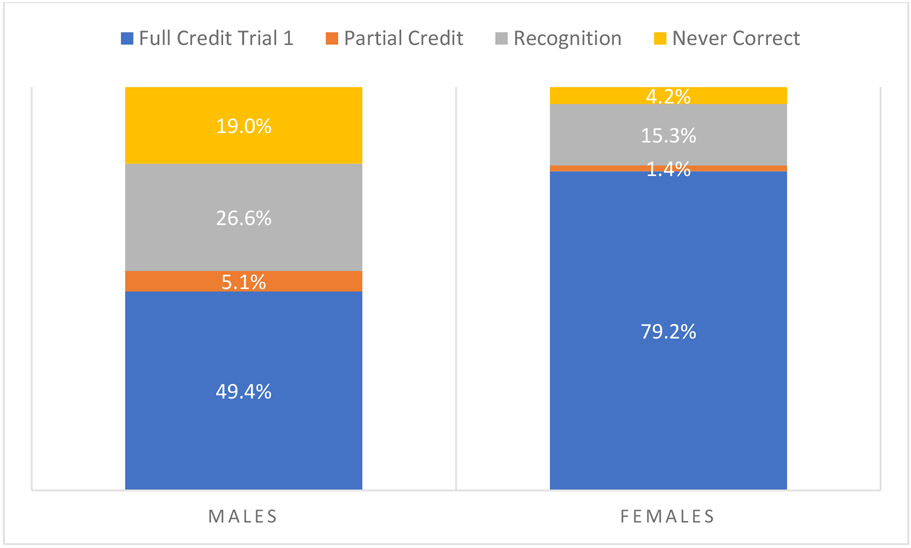
As expected, females with FXS performed better than males on all domains of decisional capacity, but the profiles of strengths and weaknesses were similar. The domain in which both males and females performed best was the Understanding domain (females X = 82.6, SD = 22.82, range = 7.69–100; males X = 48.73, SD = 26.01, range = 0–96.15). The domains for Appreciation (females X = 64.04, SD = 29.57, range = 0–100; males X = 26.53, SD = 27.53, range = 0–88.89) and Reasoning (females X = 59.74, SD = 31.10, range = 0–100; males X = 18.70, SD = 22.14, range = 0–100) were more difficult. Most females (79%) received full credit on Expressing a Choice on the first trial, and all but three (4%) received credit with repetition or recognition cues. In contrast, less than half (49.4%) of males received full credit on Expressing a Choice on the first trial. The majority (90%) of those who did not get full credit on the first trial were able to receive credit with recognition (27% of full sample). Nineteen percent of males were unable to obtain any credit on Expressing a Choice even with additional support. See Figure 1.
Figure 1.
Percent of Males and Females Receiving Credit for Expressing a Choice
Clinical Trial Understanding.
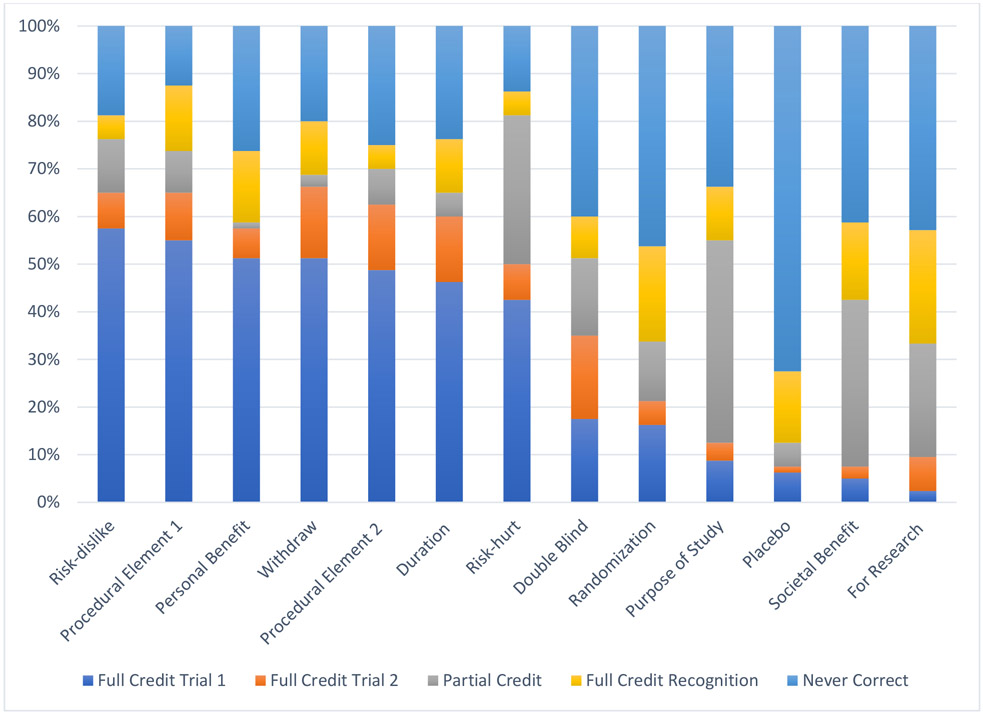
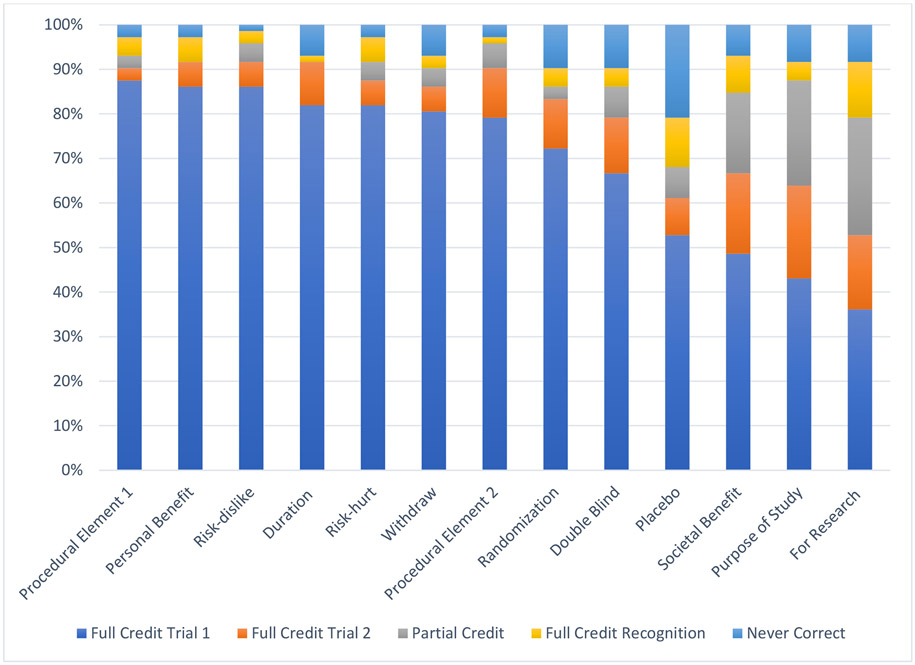
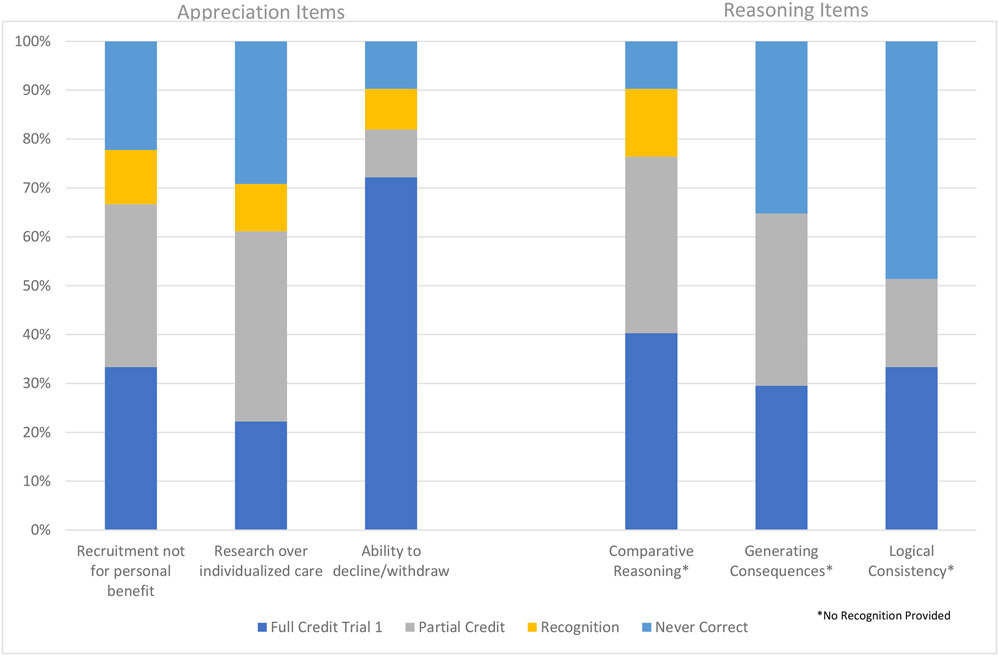
Almost all females and about half of males received full credit on understanding items related to technical details of clinical trials, such as the duration of the study (82% females; 49% males); procedural elements (88% females; 55% males); benefits (86% females; 51% males), risks (86% females; 58% males), and ability to withdraw (81% females; 51% males). As hypothesized, more abstract concepts were more difficult to understand, such as the purpose of the trial being for research rather than clinical care (36% females; 3% males), placebo (53% females; 6% males), and societal benefit (49% females; 5% males).
For understanding items, 49% of questions were answered correctly on the first trial, with a range of 18% to 71% answered correctly by item. For males, 31% of questions were answered correctly on the first trial, with a range of 3% to 58% by item. For females, 69% of questions were answered correctly on the first trial, with a range of 36% to 88% by item. Among those questions that were not answered correctly on the first trial, 19% of missed questions were given credit after one repetition of information, with a range between 6% to 34% by item. Within males, 12% of missed questions were given credit after one repetition of information, with a range from 1% to 31% by item, and for females, 34% of missed questions received credit, with a range between 18% and 54% by item. Among those that did not receive full or partial credit after one repetition of information, 30% of missed questions were given credit during the recognition trial, with a range from 19% to 54% by item; within males, 27% of questions were given credit during the recognition trial, with a range from 17% to 52% by item. For females, 43% of missed questions were credit during the recognition trial, with a range between 17% and 67% by item.
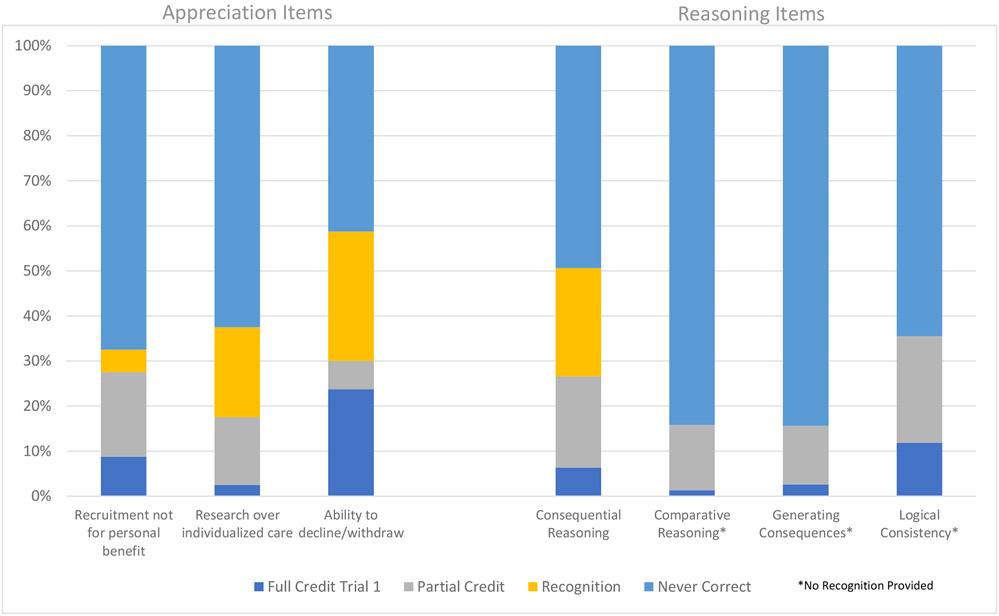
For appreciating items, 26% of all questions received credit during the first trial, with a range of 12% to 47% by item. In males, 12% of all questions received full credit during the first trial, with a range of 3% to 24% by item. In females, 43% of questions were answered correctly during the first trial, with a range of 22% to 72% by item. Among those that did not receive full or partial credit on the first trial and were thus directed to the recognition items, 26% of missed questions received credit, with a range of 15% to 42% by item. Within males, 24% of missed questions received credit during the recognition items, with a range from 7% to 41% by item, and for females, 32% of missed questions received credit for recognition items, with a range between 25% and 46% by item.
Predictors of Decisional Capacity
Understanding.
When all predictors and interaction terms were entered into the model in Step 1, the interaction of age*gender was significantly associated with poorer understanding and was thus retained. After step 1 was rerun removing non-significant interaction terms, gender (female; β = 1.70, p < .001), age (β = 0.24, p = .007), comorbid ASD (β = −0.24, p = <.001) and age*gender (β = −0.41, p = 0.05) continued to be related to understanding in a model that explained approximately 41% of its variance. No interaction terms in Steps 2, 3, or 4 were significantly related to understanding. In the simplified (i.e., removing interaction terms) Step 2, IQ (β = 0.28, p = .002) and oral comprehension (β = 0.51, p < .001) were related to better understanding in a model that explained 80% of its variance, while in the simplified Step 3, higher general anxiety (β = −0.14, p = .01) and lower social avoidance (β = 0.09, p = .04) was related to poorer understanding in a model that predicted 81% of its variance. Better verbal memory (β = 0.16, p =.01) and poorer working memory (β = −0.24, p = <.001) were associated with higher understanding in the simplified Step 4. At the conclusion of this regression built over four steps, the interaction of age*gender was no longer significantly related to understanding and was thus removed for the final model. In the final model of understanding, higher IQ (β = 0.49, p < .001), oral comprehension (β = 0.48, p < .001), social avoidance (β = 0.08, p = .04) and verbal memory (β = 0.17, p = .006), and lower general anxiety (β = −0.08, p = .05) and working memory (β = −0.27, p < .001) continued to be related to better understanding in a model that explained approximately 84% of its variance. See Table 3 for full results of the Understanding models.
Table 3.
Predictors of the Understanding Decisional Capacity Domain in the Total Sample and by Gender
| Understanding | ||
|---|---|---|
| Regression in Four Steps | Final Model | |
| β (95% CI) | β (95% CI) | |
| Step 1 | ||
| Gender (Female) | 1.70 (0.91, 2.49)*** | 0.15 (−0.03, 0.32) |
| Age | 0.24 (0.07, 0.41)** | 0.01 (−0.07, 0.08) |
| Autism (Yes) | −0.24 (−0.37, −0.10)*** | −0.06 (−0.14, 0.02) |
| Age*Gender | −0.41 (−0.81, −0.001)* | |
| R2 | 0.41 | |
| Step 2 | ||
| IQ | 0.28 (0.10, 0.45)** | 0.49 (0.30, 0.68)*** |
| Independence | 0.06 (−0.07, 0.19) | |
| Oral Comprehension | 0.51 (0.36, 0.66)*** | 0.48 (0.35, 0.60)*** |
| Passage Comprehension | 0.05 (−0.12, 0.23) | |
| R2 | 0.80 | |
| Step 3 | ||
| Social Avoidance | 0.09 (0.002, 0.17)* | 0.08 (0.003, 0.16)* |
| General Anxiety | −0.14 (−0.24, −0.03)* | −0.08 (−0.16, −0.001)* |
| Hyperactivity | 0.05 (−0.06, 0.15) | |
| R2 | 0.81 | |
| Step 4 | ||
| Cognitive Flexibility | 0.003 (−0.09, 0.10) | |
| Inhibitory Control | −0.04 (−0.13, 0.05) | |
| Planning & Problem Solving | −0.07 (−0.17, 0.04) | |
| Visual Memory | 0.05 (−0.03, 0.12) | |
| Verbal Memory | 0.16 (0.04, 0.28)* | 0.17 (0.05, 0.29)** |
| Working Memory | −0.24 (−0.39, −0.10)*** | −0.27 (−0.41, −0.14)*** |
| R2 | 0.84 | |
| Final Model R2 | 0.84 | |
Within each step, first, interactions with gender were tested; non-significant interaction terms were removed from the step and the step was rerun. No other interactions with gender were significant at an alpha = 0.05 level (data not shown).
For all variables except for gender and autism, beta parameters represent the change in the understanding score in standard deviation units per one standard deviation increase in the predictor variable. Beta parameters for female gender and autism represent the change in the understanding score in standard deviation units among females and those with autism, respectively.
p < .05
p < .01
p < .001.
Appreciation.
There were no interaction terms that were significantly related to Appreciation in any of Steps 1 through 4. In the simplified Step 1, gender (female; β = 0.93, p < .001) and comorbid ASD (β = −0.23, p = .001) were significantly associated with appreciation and were thus retained. In Step 2, higher IQ (β = 0.33, p = .002) and oral comprehension (β = 0.36, p < .001) predicted better appreciation scores; the addition of the predictors in Step 2 improved the explanation of the model from 37% (Step 1 R2) to 66% (Step 2 R2). There were no variables from Steps 3 or 4 that significantly predicted appreciation. In the final model, higher IQ (β = 0.38, p < .001) and oral comprehension (β = 0.37, p < .001) continued to be related to higher appreciation in a model that explained 66% of the variance of Appreciation scores. When the Understanding score was added to the model, oral comprehension was no longer significantly related to Appreciation; however, understanding was strongly positively related to the appreciation score (β = 0.49, p < .001). Introducing the Understanding score increased the total variability explained by the model from 66% in the final model to 71% in the secondary model. See Table 4 for full results of the Appreciation models.
Table 4.
Predictors of the Appreciation Decisional Capacity Domain in the Total Sample and by Gender
| Appreciation | |||
|---|---|---|---|
| Regression in Four Steps β (95% CI) |
Full Model β (95% CI) |
Secondary Analysis β (95% CI) |
|
| Step 1 | |||
| Gender (Female) | 0.93 (0.65, 1.21)*** | 0.22 (−0.03, 0.47) | 0.14 (−0.10, 0.37) |
| Age | 0.10 (−0.03, 0.23) | ||
| Autism (Yes) | −0.23 (−0.38, −0.09)** | −0.08 (−0.20, 0.03) | −0.05 (−0.16, 0.05) |
| R2 | 0.37 | ||
| Step 2 | |||
| IQ | 0.33 (0.12, 0.54)** | 0.38 (0.22, 0.55)*** | 0.20 (0.03, 0.37)* |
| Independence | 0.07 (−0.09, 0.23) | ||
| Oral Comprehension | 0.36 (0.18, 0.55)*** | 0.37 (0.22, 0.52)*** | 0.12 (−0.05, 0.29) |
| Passage Comprehension | 0.001 (−0.21, 0.21) | ||
| R2 | 0.66 | ||
| Step 3 | |||
| Social Avoidance | 0.08 (−0.03, 0.19) | ||
| General Anxiety | 0.02 (−0.12, 0.16) | ||
| Hyperactivity | −0.08 (−0.22, 0.06) | ||
| R2 | 0.67 | ||
| Step 4 | |||
| Cognitive Flexibility | 0.002 (−0.12, 0.12) | ||
| Inhibitory Control | −0.01 (−0.13, 0.11) | ||
| Planning & Problem Solving | −0.002 (−0.15, 0.14) | ||
| Visual Memory | −0.01 (−0.11, 0.09) | ||
| Verbal Memory | 0.12 (−0.04, 0.28) | ||
| Working Memory | 0.19 (−0.01, 0.39) | ||
| R2 | 0.68 | ||
| Step 5 | |||
| Understanding | 0.49 (0.29, 0.69)*** | ||
| R2 | 0.66 | 0.71 | |
Within each step, first, interactions with gender were tested; non-significant interaction terms were removed from the step and the step was rerun. No other interactions with gender were significant at an alpha = 0.05 level (data not shown).
For all variables except for gender and autism, beta parameters represent the change in the understanding score in standard deviation units per one standard deviation increase in the predictor variable. Beta parameters for female gender and autism represent the change in the understanding score in standard deviation units among females and those with autism, respectively.
p < .05
p < .01
p < .001.
Reasoning.
There were no interaction terms that were significantly related to reasoning in Steps 1 or 2. Female gender (β = 1.09, p < .001), age (β = 0.15, p = .01) and comorbid ASD (β = −0.18, p = .01) were significant predictors of reasoning in a model that explained 43% of its variance and were carried into subsequent steps. In step 2, higher IQ (β = 0.26, p = .02) and independence (β = 0.17, p = .02), predicted better reasoning (R2 = 0.71). There were no variables in Step 3 that significant predicted reasoning. There were no significant interaction terms in Step 4, though lower inhibitory control (β = −0.13, p = .02) was associated with higher reasoning scores in the simplified step. In the final model, female gender (β = 0.35, p = .004), older age (β = 0.12, p = .01), higher IQ (β = 0.46, p < .001), higher independence (β = 0.27, p < .001), and lower inhibitory control (β = −0.13, p = .02) predicted higher reasoning; this model represented 71% of the variance in reasoning. Understanding was positively related to reasoning in the secondary analysis (β = 0.26, p = .004). After introducing the Understanding score, the main effect of inhibitory control was no longer statistically significant, the total variability explained by the model minimally increased from 71% to 73%. See Table 5 for full results of the Reasoning models.
Table 5.
Predictors of the Reasoning Decisional Capacity Domain in the Total Sample and by Gender
| Reasoning | |||
|---|---|---|---|
| Regression in Four Steps β (95% CI) |
Full Model β (95% CI) |
Secondary Analysis β (95% CI) |
|
| Step 1 | |||
| Gender (Female) | 1.09 (0.83, 1.35)*** | 0.35 (0.11, 0.58)** | 0.32 (0.09, 0.54)** |
| Age | 0.15 (0.03, 0.28)* | 0.12 (0.03, 0.21)** | 0.10 (0.01, 0.19)* |
| Autism (Yes) | −0.18 (−0.32, −0.04)* | −0.08 (−0.18, 0.02) | −0.05 (−0.15, 0.06) |
| R2 | 0.43 | ||
| Step 2 | |||
| IQ | 0.26 (0.05, 0.48)* | 0.46 (0.30, 0.63)*** | 0.30 (0.10, 0.50)** |
| Independence | 0.17 (0.02, 0.32)* | 0.27 (0.11, 0.43)*** | 0.23 (0.07, 0.38)** |
| Oral Comprehension | 0.14 (−0.04, 0.32) | ||
| Passage Comprehension | 0.19 (−0.02, 0.40) | ||
| R2 | 0.71 | ||
| Step 3 | |||
| Social Avoidance | 0.04 (−0.07, 0.15) | ||
| General Anxiety | −0.01 (−0.14, 0.13) | ||
| Hyperactivity | 0.03 (−0.11, 0.17) | ||
| R2 | 0.70 | ||
| Step 4 | |||
| Cognitive Flexibility | 0.02 (−0.11, 0.16) | ||
| Inhibitory Control | −0.13 (−0.24, −0.02)* | −0.13 (−0.23, −0.02)* | −0.09 (−0.19, 0.02) |
| Planning & Problem Solving | −0.03 (−0.17, 0.11) | ||
| Visual Memory | 0.03 (−0.07, 0.13) | ||
| Verbal Memory | 0.09 (−0.06, 0.25) | ||
| Working Memory | 0.10 (−0.09, 0.28) | ||
| R2 | 0.72 | ||
| Secondary Analysis (Step 5) | |||
| Understanding | 0.26 (0.08, 0.44)** | ||
| R2 | 0.71 | 0.73 | |
Within each step, first, interactions with gender were tested; non-significant interaction terms were removed from the step and the step was rerun. No other interactions with gender were significant at an alpha = 0.05 level (data not shown).
For all variables except for gender and autism, beta parameters represent the change in the understanding score in standard deviation units per one standard deviation increase in the predictor variable. Beta parameters for female gender and autism represent the change in the understanding score in standard deviation units among females and those with autism, respectively.
p < .05
p < .01
p < .001.
Expressing a Choice.
Finally, we examined correlations between the Expressing a Choice score and the continuous demographic, cognitive, and functioning variables tested in Models 1–3 in the male sample only. Higher IQ (r = 0.38, p = < .001), independence (r = 0.27, p = .02), oral comprehension (r = 0.36, p = .001), passage comprehension (r = 0.35, p = .002), and verbal memory (r = .37, p = .001) were significantly correlated with higher scores on Expressing a choice. High Understanding (r = 0.48, p < .001), Appreciation (r = 0.38, p < .001), and Reasoning (r = 0.48, p < .001) were also significantly related to higher scores in Expressing a choice. See Table 6 for the correlation matrix. Those with comorbid ASD (χ2 = 7.4, p = .005; data not shown) were less likely to receive full credit on the first trial for the Expressing a choice item; 60% of those without comorbid ASD received full credit on the first trial compared with 23% of those with comorbid ASD.
Table 6.
Correlations among Males between Expressing a Choice Score and Predictors of Interest
| Expressing a Choice r |
|
|---|---|
| Age | 0.15 |
| IQ | 0.38*** |
| Broad Independence | 0.27* |
| Oral Comprehension | 0.36** |
| Passage Comprehension | 0.35** |
| Social Avoidance | −0.10 |
| General Anxiety | 0.05 |
| Hyperactivity | −0.03 |
| Cognitive Flexibility | 0.04 |
| Inhibitory Control | −0.11 |
| Planning & Problem Solving | 0.18 |
| Visual Memory | 0.13 |
| Verbal Memory | 0.37** |
| Working Memory | 0.21 |
| Understanding | 0.48*** |
| Appreciation | 0.38*** |
| Reasoning | 0.48*** |
p < .05
p < .01
p < .001.
DISCUSSION
Over the last half century, the disability rights movement has made great headway in promoting autonomy and empowerment of individuals with IDD. Increasing independence and opportunities are key goals for students with IDD in schools, and principles such as self-advocacy, self-determination, normalization, and opportunity are now critical concepts in transition plans for young adults with IDD (Powers, Dinerstein, and Holmes 2005). Simultaneously, a movement toward shared and collaborative decision making in health care has worked to foster respect for the autonomy, quality of life, and well-being of patients with IDD.
Commensurate with these social movements has been a movement toward more inclusive research. This concept posits that research involving people with IDD should view participants not just as subjects or respondents (Walmsley 2001). As the popular disability advocacy slogan says, “nothing about me, without me;” inclusive research seeks to ensure that the goals and implementation of any study are in line with the priorities and needs of the population being studied. Researchers who aspire to be truly inclusive must, at minimum, ensure that each participant is adequately informed about what the research is about and what will be asked of them should they agree to participate. At the most basic level, this means that the assent or consent process ensures that each person understands and appreciates what they are being asked to do and what the risks and benefits will be.
Focusing on decisional capacity and the informed consent process is critical, as clinical trials testing targeted therapeutics become more common in neurogenetic conditions like FXS. Although a variety of ethical frameworks, guidelines, and some legal requirements exist for researchers who study individuals with IDD (e.g., the Belmont Report, Department of Health 2014), the resulting regulations simply require legally effective informed consent from subjects or their legally authorized representatives (LAR). Researchers have generally been left with the difficult challenge of balancing autonomy and respect for the individual with IDD with the responsibility to protect their vulnerability, with the additional complexity that institutional review board (IRB) requirements may reflect significant differences across institutions (Freedman 2001). Most researchers acknowledge the wide range of decisional capacity among people with IDD and assume that many can participate in the consent process; however, the decisional capacity of people with IDD has not been widely studied. Thus, there is limited information available to help investigators determine how to maximize the participation of subjects in their studies (Cleaver, Ouellette-Kuntz, and Sakar 2010; Dunn et al. 2006; Goldsmith, Skirton, and Webb 2008).
To our knowledge, this is the first study to document the extent of decisional capacity in individuals with any specific type of neurodevelopmental disorder. This study explored the extent to which individuals with FXS understand the elements of a clinical trial and how well they can appreciate, reason, and express a consistent choice about participation. We also examined what factors might contribute to better or worse decisional capacity within this population. Our hypotheses were partially supported: females outperformed males, more abstract concepts were difficult, but performance improved with scaffolding, and overall IQ, anxiety, and executive functioning skills were significantly predictive of decisional capacity. However, we did not find differential relationships between our predictor variables and our outcomes for males and females. This suggests that although females outperformed males generally, their pattern of performance and variables associated with their decisional capacity are similar and not related to their sex per se. Rather, how they process information, including their overall ability to retain information and problem-solve are the main drivers of their decisional capacity. We summarize the major conclusions drawn from our findings in the sections below.
Understanding the details is critical.
Fisher et al. (2006), in one of the first studies to examine decisional capacity in individuals with IDD, found that cognitive status influenced consent capacity and that capacity varied in part as a function of the material to be understood. Adults with IDD were more likely to be able to make a choice about participation and understand research methods but were less able to understand the purpose of research and demonstrate their reasoning about participation. The current study found similar results—the ability to understand the material was the most significant predictor of the ability to appreciate and reason about the decision. In other words, regardless of one’s cognitive or social-emotional capacity, if the information about the study is not well understood, it will be very difficult to demonstrate appreciation and reasoning. This intuitive finding underscores the importance of ensuring that information about the research trial is conveyed to participants in ways that maximize understanding.
Abstract concepts are hard to grasp, which may put participants at risk for therapeutic misconception.
As expected, both males and females in this study demonstrated understanding of more concrete elements of clinical trials (e.g., what they would need to do, what the benefit to them might be, how often they would need to take the study medicine), but struggled with more abstract concepts like placebo and randomization, and the concept that they would be participating in research, not clinical care. These concepts were difficult even with additional scaffolding such as repetition and multiple-choice options. This pattern suggests that individuals with FXS (and IDD more generally) may be vulnerable to the misconception that the study is equivalent to clinical treatment, including mistaken perceptions about its therapeutic benefits (Appelbaum & Lidz, 2008). This risk should be carefully considered by any researcher working with individuals with IDD.
Simplified language, repetition, and recognition cues help.
In this study, we found that working memory, verbal memory, and oral presentation of information were significant predictors of understanding scores. This finding suggests that the ability to understand and retain orally presented information is critical, even in a situation in which the information was presented in multiple formats (with pictures, words to read, and read aloud to them). We also found that repeating the information and providing alternative ways to convey knowledge (e.g., multiple choice/recognition) improved understanding and appreciation, suggesting that scaffolding can improve retention and ultimately understanding. Other researchers have had similar results (Cameron & Murphy, 2006), suggesting that strategies that take into account the participant’s strengths and weaknesses can increase decisional capacity for informed consent. Researchers working with individuals with FXS and other IDD groups should consider a multimethod approach to providing information about what will be expected of the individual during the trial—this should include written as well as oral information and use of visual cues whenever possible. Researchers may also want to embed questions or other means to assess how well the individual is processing and retaining the information, and tailor the information appropriately.
Consent and assent should be considered an ongoing process of shared decision making.
This study did not seek to identify a specific cut-off by which to determine decisional capacity in people with FXS. Although the idea of a clear cut-off score for decisional capacity is appealing, it does not take into account the nuances inherent in the decision-making process. Decisional capacity is not a static trait—whether an individual has decisional capacity depends on the type and characteristics of decision to be made. One’s ability to make an informed decision may also change over time or in response to support or scaffolding provided. Decisional capacity has been defined as a clinical determination that a person is able to understand the consequences for health decisions and that they are able to make and take responsibility for those decisions (Mitty, 2012). Therefore, Fisher (2003) has argued for a shift away from an exclusive focus on individual decisional capacity to a more nuanced consideration of the goodness-of-fit between the individual with IDD and the consent process for each specific decision. Others go further, suggesting that decisional capacity and informed consent are dynamic, ongoing processes and that providing information about a study at one time point is not sufficient (Dye et al., 2004). In addition to ensuring ongoing understanding about the process of a given study, repeating the information and checking in with participants can also help build trust, another important factor in supporting decision making in individuals with IDD (Cameron & Murphy, 2006; Carey & Griffiths, 2017; McDonald & Kidney, 2012)
These factors are also important when considering proxy decision making. Most people with FXS, especially males, have guardians, and therefore do not have legal decision-making power when it comes to participation in research studies. However, even if an individual is not providing consent to participate, increasing their understanding in the assent process should be considered an equally important goal. Most individuals, regardless of cognitive ability, do not make important decisions, such as whether to participate in a clinical trial, without consultation and/or support from people close to them. Proper assent protocols not only allow the person to have some say about participation, but also provide them with information about what they will be asked to do if they participate in the study. Improving even partial participation through more accessible assent procedures can enable even the most severely affected individuals to play a greater role in making decisions about their lives (Shogren et al., 2017). This model of shared and collaborative decision making seeks to help an individual make a decision in partnership with another person, be that a parent, doctor, or researcher. In this model, when someone makes the decision to participate in a clinical trial, a researcher or physician provides information about the study; the person with IDD, together with a legal guardian, then provides information about his/her life, goals, and values. From within this larger context, the decision is made together (Peisah, Sorinmade, Mitchell, & Hertogh, 2013). Not only does the model provide a better way to obtain assent but it can also increase buy-in and therefore reduce attrition, an important goal especially for researchers who work with rare conditions.
Limitations and Future Directions.
This study asked participants to consider a hypothetical scenario in which an individual described as similar to the participant needed to decide whether to participate in a clinical trial. For those with IDD, the idea of a hypothetical clinical trial may be too abstract, and therefore their responses may not reflect how they would make decisions about their own participation in a clinical trial. Other factors not assessed, such as previous experience with clinical trials and family values about medication or participation in research, can also influence decision making in this context. Moreover, the population of this study was mostly white and relatively wealthy, and most parents of the participants were married and well-educated; therefore, results from this study may not be generalizable to a more diverse population. Finally, this study would have benefited from the inclusion of a comparison group of non-affected persons, which could have helped with interpreting the extent to which decisional capacity is impaired in individuals with FXS.
Despite these limitations, this study provides important information about the capacity of individuals with FXS to understand and make decisions about participation in clinical trials. Future studies should be focused on promoting better understanding and increased decisional capacity for individuals with FXS and other IDDs, with the ultimate goals of improving integrated research practices and enabling those with IDD to be more informed about their health care decisions.
Figure 2.
Scores on Understanding Items for Males
Figure 3.
Scores on Appreciation and Reasoning Items for Males
Figure 4.
Scores on Understanding Items for Females
Figure 5.
Scores on Appreciation and Reasoning Items for Females
Acknowledgements:
This study was funded by a grant from the Eunice Kennedy Shriver National Institute for Child Health and Human Development (R01HD071987-01A1). The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the National Institutes of Health.
Footnotes
Conflict of Interest: None of the authors have any conflict of interests to declare
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent: Informed consent and/or assent were obtained from all individual participants included in the study.
REFERENCES
- Appelbaum PS 2007. Assessment of patients' competence to consent to treatment. New England Journal of Medicine 357(18):1834–1840. [DOI] [PubMed] [Google Scholar]
- Appelbaum PS, and Grisso T. 2001. The MacArthur Competence Assessment Tool for Clinical Research (MacCAT-CR). Sarasota, FL: Professional Resource Press. [Google Scholar]
- Appelbaum PS, Grisso T, Frank E, O’Donnell S, and Kupfer DJ. 1999. Competence of depressed patients for consent to research. American Journal of Psychiatry 156:1380–1384. [DOI] [PubMed] [Google Scholar]
- Appelbaum PS, and Lidz C. 2008. The therapeutic misconception. In The Oxford textbook of clinical research ethics, eds. Emanuel EJ, Grady C, Crouch RA, Lie RK, Miller FG, and Wendler D. New York, Oxford University Press. [Google Scholar]
- Bear MF, Huber KM, and Warren ST. 2004. The mGluR theory of fragile X mental retardation. Trends in Neurosciences 27 (7):370–377. [DOI] [PubMed] [Google Scholar]
- Becker H, Roberts G, Morrison J, and Silver J. 2004. Recruiting people with disabilities as research participants: Challenges and strategies to address them. Mental Retardation 42 (6):471–475. [DOI] [PubMed] [Google Scholar]
- Bruininks RH, Woodcock RW, Weatherman RF, and Hill BK. 1996. Scales of independent behavior—revised comprehensive manual. Itasca, IL: Riverside Publishing. [Google Scholar]
- Burket JA, Herndon AL, Winebarger EE, Jacome LF, and Deutsch SI. 2011. Complex effects of mGluR5 antagonism on sociability and stereotypic behaviors in mice: possible implications for the pharmacotherapy of autism spectrum disorders. Brain Research Bulletin 86 (3–4):152–158. [DOI] [PubMed] [Google Scholar]
- Cea CD, and Fisher CB. 2003. Health care decision-making by adults with mental retardation. Mental Retardation 41:78–87. [DOI] [PubMed] [Google Scholar]
- Cleaver S, Ouellette-Kuntz H, and Sakar A. 2010. Participation in intellectual disability research: a review of 20 years of studies. Journal of Intellectual Disability Research 54 (3):187–193. [DOI] [PubMed] [Google Scholar]
- Dalton AJ, and McVilly KR. 2004. Ethics guidelines for international, multicenter research involving people with intellectual disabilities. Journal of Policy and Practice in Intellectual Disabilities 1 (2):57–70. [Google Scholar]
- de Vrij FM, Levenga J, van der Linde HC, et al. 2008. Rescue of behavioral phenotype and neuronal protrusion morphology in Fmr1 KO mice. Neurobiology Disease 31 (1):127–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, and Kramer JH. 2001. Delis-Kaplan Executive Function System™. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Department of Health. 2014. The Belmont Report. Ethical principles and guidelines for the protection of human subjects of research. The Journal of the American College of Dentists 81 (3):4. [PubMed] [Google Scholar]
- Dunn LB, Nowrangi MA, Palmer BW, Jeste DV, and Saks ER. 2006. Assessing decisional capacity for clinical research or treatment: A review of instruments. The American Journal of Psychiatry 163:1323–1334. [DOI] [PubMed] [Google Scholar]
- Dye L, Hare DJ, and Hendy S. 2007. Capacity of people with intellectual disabilities to consent to take part in a research study. Journal of Applied Research in Intellectual Disabilities 20 (2):168–174. [Google Scholar]
- Dye L, Hendy S, Hare DJ, and Burton M. 2004. Capacity to consent to participate in research–a recontextualization. British Journal of Learning Disabilities 32 (3):144–150. [Google Scholar]
- Esbensen AJ, Rojahn J, Aman MG, and Ruedrich S. 2003. Reliability and validity of an assessment instrument for anxiety, depression, and mood among individuals with mental retardation. Journal of Autism and Developmental Disorders 33:617–629. [DOI] [PubMed] [Google Scholar]
- Fisher CB 2003. Goodness-of-fit ethic for informed consent to research involving adults with mental retardation and developmental disabilities. Developmental Disabilities Research Review 9 (1):27–31. [DOI] [PubMed] [Google Scholar]
- Fisher CB, Cea CD, Davidson PW, and Fried AL. 2006. Capacity of persons with mental retardation to consent to participate in randomized clinical trials. American Journal of Psychiatry 163 (10):1813–1820. [DOI] [PubMed] [Google Scholar]
- Freedman RI 2001. Ethical challenges in the conduct of research involving persons with mental retardation. Mental Retardation 39 (2):130–141. [DOI] [PubMed] [Google Scholar]
- Goldsmith L, Skirton H, and Webb C. 2008. Informed consent to healthcare interventions in people with learning disabilities–an integrative review. Journal of Advanced Nursing 64 (6):549–563. [DOI] [PubMed] [Google Scholar]
- Grisso T, and Appelbaum PS. 2006. Appreciating anorexia: decisional capacity and the role of values. Philosophy, Psychiatry, & Psychology 13 (4):293–297. [Google Scholar]
- Grisso T, and Appelbaum PS. 1998. Assessing competence to consent to treatment: A guide for physicians and other health professionals. Oxford University Press, USA. [Google Scholar]
- Iacono T, and Murray V. 2003. Issues of informed consent in conducting medical research involving people with intellectual disability. Journal of Applied Research in Intellectual Disabilities 16 (1):41–51. [Google Scholar]
- Jønch AE, and Jacquemont S. 2017. Reflections on clinical trials in Fragile X Syndrome. In Fragile X Syndrome, 419–441. [Google Scholar]
- Loesch DZ, Huggins RM, and Hagerman RJ. 2004. Phenotypic variation and FMRP levels in fragile X. Mental Retardation and Developmental Disabilities Research Reviews 10 (1):31–41. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, Dilavore PC, Risi S, Gotham K, and Bishop SL. 2012. Autism Diagnostic Observation Schedule. 2nd ed. Los Angeles, CA: Western Psychological Services. [Google Scholar]
- McDonald KE 2012. “We want respect”: Adults with intellectual and developmental disabilities address respect in research. American Journal on Intellectual and Developmental Disabilities 117 (4):263–274. [DOI] [PubMed] [Google Scholar]
- McDonald KE, and Kidney CA. 2012. What is right? Ethics in intellectual disabilities research. Journal of Policy and Practice in Intellectual Disabilities 9 (1):27–39. [Google Scholar]
- McDonald KE, Kidney CA, and Patka M. 2013. ‘You need to let your voice be heard’: research participants' views on research. Journal of Intellectual Disability Research 57 (3):216–225. [DOI] [PubMed] [Google Scholar]
- Michalon A, Sidorov M, Ballard TM, et al. 2012. Chronic pharmacological mGlu5 inhibition corrects fragile X in adult mice. Neuron 74 (1):49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitty EL 2012. Decision-making and dementia. In Try this: Best practices in nursing care to older adults with dementia, D, 9. [Google Scholar]
- Nugent AC, Miller FG, Henter ID, and Zarate CA. 2017. The ethics of clinical trials research in severe mood disorders. Bioethics 31 (6):443–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer BW, Harmell AL, Pinto LL, Dunn LB, Kim SY, Golshan S, and Jeste DV. 2017. Determinants of capacity to consent to research on Alzheimer’s disease. Clinical Gerontologist 40 (1):24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peisah C, Sorinmade OA, Mitchell L, and Hertogh CM. 2013. Decisional capacity: toward an inclusionary approach. International Psychogeriatrics 25 (10):1571–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers L, Dinerstein R, and Holmes S. 2005. Self-advocacy, self-determination, social freedom, and opportunity. In National goals and research for people with intellectual and developmental disabilities, 257–287. [Google Scholar]
- Roid GH 2003. Stanford Binet Intelligence Scales (SB5), 5th ed. Itasca, IL: Riverside Publishing. [Google Scholar]
- Rutter M, Bailey A, and Lord C. 2003. Social communication questionnaire. Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Sansone SM, Schneider A, Bickel E, Berry-Kravis E, Prescott C, and Hessl D. 2014. Improving IQ measurement in intellectual disabilities using true deviation from population norms. Journal of Neurodevelopmental Disorders 16:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheslow D, and Adams W. 2003. Wide range assessment of memory and learning. 2nd ed. Lutz, FL: Psychological Assessment Resources, Inc. [Google Scholar]
- Shogren KA, Wehmeyer ML, Uyanik H, and Heidrich M. 2017. Development of the Supported Decision Making Inventory System. Intellectual and Developmental Disabilities 55 (6):432–439. [DOI] [PubMed] [Google Scholar]
- Tam NT, Huy NT, Thoa LTB, Long NP, Trang NTH, Hirayama K, & Karbwang J (2015). Participants’ understanding of informed consent in clinical trials over three decades: systematic review and meta-analysis. Bulletin of the World Health Organization, 93, 186–198H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas AM, Bui N, Perkins JR, Yuva-Paylor LA, and Paylor R. 2012. Group I metabotropic glutamate receptor antagonists alter select behaviors in a mouse model for fragile X syndrome. Psychopharmacology (Berl) 219 (1):47–58. [DOI] [PubMed] [Google Scholar]
- Walmsley J 2001. Normalisation, emancipatory research and inclusive research in learning disability. Disability & Society 16 (2):187–205. [Google Scholar]
- Wang SB, Wang YY, Ungvari GS, Ng CH, Wu RR, Wang J, and Xiang YT. 2017a. The MacArthur Competence Assessment Tools for assessing decision-making capacity in schizophrenia: A meta-analysis. Schizophrenia research 183, 56–63. [DOI] [PubMed] [Google Scholar]
- Wang YY, Wang SB, Ungvari GS, Yu X, Ng CH, and Xiang YT. 2017b. The assessment of decision-making competence in patients with depression using the MacArthur competence assessment tools: A systematic review. Perspectives in Psychiatric Care. [DOI] [PubMed] [Google Scholar]
- Woodcock RW, McGrew KS, and Mather N. (2001; 2007). Woodcock Johnson III Tests of Achievement. Rolling Meadows, IL: Riverside Publishing. [Google Scholar]
- Yan QJ, Rammal M, Tranfaglia M, and Bauchwitz RP. 2005. Suppression of two major Fragile X Syndrome mouse model phenotypes by the mGluR5 antagonist MPEP. Neuropharmacology 49 (7):1053–1066. [DOI] [PubMed] [Google Scholar]