Abstract
Gastrointestinal stromal tumors (GISTs) are considered rare, yet they represent the most common sarcomas of the gastrointestinal tract. The development of tyrosine kinase inhibitors (TKI) for the treatment of GISTs changed the way we treat patients and has greatly impacted outcomes. However, most patients who initially benefit from TKIs eventually develop disease progression and require subsequent therapies. Ripretinib is a switch-control TKI approved for the treatment of adult patients with advanced GIST who received prior treatment with three or more TKIs, including imatinib. Our objective was to review existing treatment options for patients with advanced GIST, focusing on management optimization for heavily pretreated patients receiving ripretinib. With the integration of ripretinib as a fourth-line therapy, the GIST treatment landscape continues to evolve. As the treatment paradigm becomes increasingly complex, successful management of adverse events and individualized supportive care remain crucial to maintaining effective treatment and patient quality of life. Additionally, we present a detailed case study of a heavily pretreated patient with advanced GIST who received ripretinib as fourth-line therapy. The information provided here should help inform advanced practitioners on the effective management of patients with GIST who have progressed on multiple therapies. Advanced practitioners are well positioned to provide the necessary supportive care to achieve optimal outcomes and drug compliance.
CASE STUDY
A 65-year-old female who was working full time and caring for her special needs child presented to her primary care physician with long-term abdominal pain and weight loss (Figure 1). She was referred for imaging where an abdominal/pelvic CT scan revealed a 4-cm mass at the gastroesophageal junction and multiple liver metastases. After being referred to interventional radiology, an endoscopic biopsy confirmed the diagnosis of GIST; mutational testing was positive for a KIT exon 11 mutation. The patient was diagnosed with advanced/metastatic disease and surgical intervention was not pursued. After discussions with her oncologist, the patient initiated imatinib 400 mg once daily.
Figure 1.
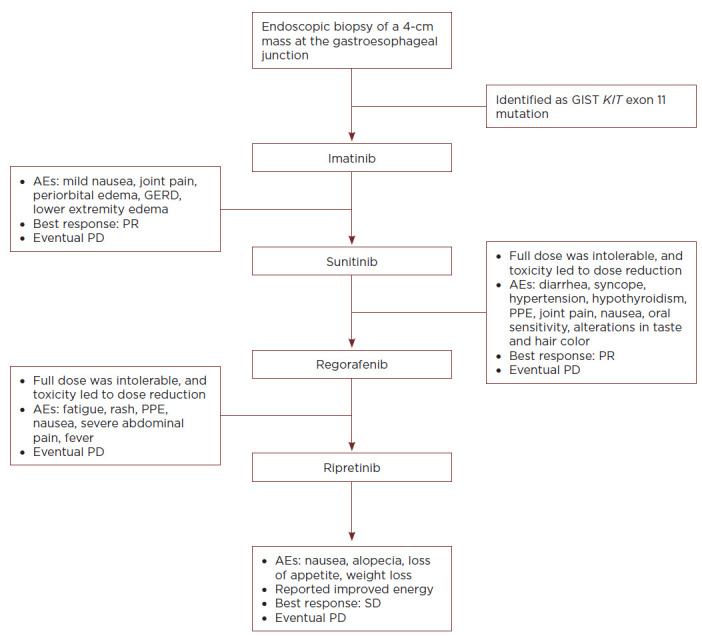
Case study of a patient with advanced GIST. AE = adverse event; GERD = gastroesophageal reflux disease; GIST = gastrointestinal stromal tumor; PD = progressive disease; PPE = palmar-plantar erythrodysesthesia; PR = partial response; SD = stable disease.
The patient experienced adverse events (AEs) that included nausea, joint pain, periorbital edema, gastroesophageal reflux disease (GERD), and lower extremity edema; these were all categorized by the patient as mild (all grade 1), and she continued to live with a high quality of life for 2 years on treatment, with her best response being a partial response (PR). The patient did not require an antiemetic for her nausea and was given a proton pump inhibitor to relieve symptoms associated with GERD. She did not require a diuretic or dose modification for her edema; her lower extremity edema was managed conservatively with a low sodium diet, elevation of the lower extremities, and compression stockings. The swelling was not unilateral and did not increase while being managed with the aforementioned conservative measures; therefore, a duplex ultrasound and an echocardiogram for concerns of heart failure were not necessary. The patient experienced disease progression (increased liver metastases) and was escalated to imatinib 400 mg twice daily. On the twice-daily dose, the patient experienced slowed disease progression and a slight increase in AEs (but still remained mild at a grade 1 severity).
After 7 months on the imatinib 400 mg twice-daily regimen, the patient experienced progressive disease and transitioned to sunitinib 50 mg (4 weeks on, 2 weeks off). Initial assessments of side effects were complicated by a concomitant flu diagnosis; the patient experienced grade 3 diarrhea resulting in a syncopal episode due to dehydration. The patient was admitted to the hospital and sunitinib was withheld until the patient recovered to mild (grade 1) toxicities. Sunitinib was restarted at 25 mg (4 weeks on, 2 weeks off), and an attempt was made to escalate the dose back up to 50 mg. The care team implemented a bland diet with the use of imodium/lomotil to manage diarrhea. The patient developed grade 3 hypertension and palmar-plantar erythrodysesthesia (PPE); grade 2 joint pain, nausea, and oral sensitivity; and grade 1 altered taste and hair depigmentation; and was diagnosed with hypothyroidism. She was started on antihypertensive medication and thyroid hormone supplementation; she also used a series of oral rinses, gentle oral hygiene measures, and PPE creams and soaks to manage the other toxicities. However, the patient still had difficulty tolerating the 50-mg dose, and the regimen was modified to 37.5 mg (2 weeks on, 1 week off). Her best response was a PR, but the patient ultimately experienced disease progression and an increase in liver metastases; one liver lesion required treatment with an ablation procedure.
The patient initiated regorafenib 160 mg once daily (3 weeks on, 1 week off). She experienced grade 3 PPE and abdominal pain. The patient's abdominal pain required hospitalization and was likely disease related. Focus was placed on the management of PPE with topical creams, soaks, moisture-wicking socks, and comfortable/supportive shoes. The patient also experienced grade 2 fatigue, rash, and nausea. Regorafenib was decreased to 120 mg once daily (3 weeks on, 1 week off); the patient experienced disease progression on the max tolerated dose, and regorafenib was discontinued.
As part of a clinical trial, the patient initiated ripretinib 150 mg once daily; the patient received a thorough baseline assessment and detailed information regarding potential risks and side effects. Her best overall response on ripretinib was stable disease (Figure 1). She experienced nausea, alopecia, loss of appetite, and weight loss (all grade 1). However, despite these AEs, she continued to work full time and care for her child throughout treatment. She reported improved energy and quality of life (per patient-reported outcome assessments) and followed up with the necessary members of her care team to discuss and manage her side effects. The patient regularly saw her dermatologist to check for any skin abnormalities and worked with a nutritionist/dietician to manage her appetite and weight loss. The patient experienced disease progression after six cycles with ripretinib and was dose escalated to 150 mg twice daily, per the trial protocol, which permitted dose escalation at the patient's election. She continued on the twice-daily regimen for seven cycles, with no significant increase in the AEs listed above, before ultimate disease progression.
This case study illustrates the value of support from a multidisciplinary team. Although dermatologic symptoms are generally not life threatening, they have been shown to negatively affect patient health-related quality of life (Nardone et al., 2012). Educating the patient about potential side effects, encouraging discussion about skin concerns early in treatment, and including a dermatologist in the care team may improve outcomes and optimize patient quality of life. Many of these patients experience loss of appetite and subsequent weight loss and may benefit from consultation with a nutritionist. While not addressed in this specific case, orthopedists and massage therapists are beneficial for patients who experience joint pain and muscle cramping. Similarly, some patients may benefit from consultation with social workers and palliative care specialists. We encourage patients to maintain a drug diary to help keep track of any potential side effects; this often helps practitioners implement early intervention from a multidisciplinary team. Finally, this case study also demonstrates that heavily pretreated patients can derive not only clinical benefit, but also quality of life improvement from ripretinib therapy. However, this is representative of a single patient and individual results may vary.
Gastrointestinal stromal tumors (GISTs) are considered a rare type of cancer but represent the most common sarcoma of the gastrointestinal (GI) tract (Ma et al., 2015; Søreide et al., 2016). While they may occur anywhere in the GI tract, GISTs are most frequently located in the stomach (55%–61%) and small intestine (30%); they are less commonly seen in the duodenum (4%–5%), rectum (4%), colon/appendix (1%–2%), and esophagus (< 1%–2%; Miettinen & Lasota, 2006; Søreide et al., 2016). Estimates of the annual incidence of GISTs worldwide range between 4.3 and 15 cases per million (Søreide et al., 2016). While they have been reported to occur in patients from 10 to 100 years of age, GISTs are rarely seen in patients under the age of 18 (1%–2%; Call et al., 2012); the median age at presentation is approximately 65 years old (Søreide et al., 2016).
A diagnosis of GIST requires histologic analysis of biopsied tissue for specific cellular morphology, and molecular genetic testing for mutations is recommended (National Comprehensive Cancer Network [NCCN], 2022). Most GISTs harbor activating mutations in the genes encoding one of two receptor tyrosine kinases: approximately 69% to 83% of GISTs have mutations in KIT (NCCN, 2022; Szucs et al., 2017), while approximately 5% to 10% have mutations in the gene for the related kinase platelet-derived growth factor α (PDGFRA; Martin-Broto et al., 2017; NCCN, 2022). Approximately 15% of GISTs in adult patients do not have a mutation in either of these two genes; these are referred to as “wild-type” with respect to KIT/PDGFRA mutations (Szucs et al., 2017). All GISTs lacking a KIT/PDGFRA mutation should be tested for other potential driver mutations, such as succinate dehydrogenase (SDH), B-Raf proto-oncogene (BRAF), and neurotrophic tyrosine receptor kinase (NTRK), in order to determine appropriate options for targeted therapy (NCCN, 2022). During treatment, patients may develop secondary mutations, most often in KIT or PDGFRA, that confer resistance to drug therapy (Szucs et al., 2017). Risk stratification (assessment of malignant potential based on mitotic rate, size, and location) is an important prognostic factor in the postsurgical setting and as patients progress on different lines of therapy (Joensuu, 2008; NCCN, 2022). However, prognostic assessment has been historically more accurate in KIT- or PDGFRA-positive GIST than SDH-deficient GIST (NCCN, 2022).
Gastrointestinal stromal tumor is a heterogeneous disease with a complex mutational landscape that presents with significant treatment challenges. In this review, we discuss treatment options for patients with GIST, focusing on the management of patients in the advanced stages of the disease who are undergoing treatment with ripretinib (Qinlock). We also present a case study of a patient with advanced GIST that exemplifies treatment with multiple lines of therapy and the associated challenges.
TREATMENT OPTIONS
For localized GIST, the first line of treatment is surgical resection with the goal of complete gross resection of the tumor with histologically negative margins (NCCN, 2022; Rubin et al., 2007). Neoadjuvant targeted therapy may be used to reduce tumor size in cases where the tumor is resectable, but the surgery would result in significant morbidity (Ishikawa et al., 2018; NCCN, 2022). Even when resection is complete, however, the disease may recur; almost all patients with advanced disease who are treated with resection experience subsequent recurrence, irrespective of the quality of the surgical procedure (Rubin et al., 2007; Rutkowski et al., 2007). Therefore, adjuvant targeted therapy may be used to control any micrometastases left after surgery with the goal of reducing recurrence and improving survival compared with surgery alone (Ishikawa et al., 2018).
The discovery of receptor tyrosine kinase mutations in patients with GIST led to the development of oral tyrosine kinase inhibitors (TKIs) as targeted systemic therapy for the treatment of GIST, particularly for patients with resectable GIST with significant morbidity and for patients with unresectable/progressive disease (NCCN, 2022). Tyrosine kinase inhibitors have become the standard of care for patients with unresectable, recurrent, or metastatic GIST (NCCN, 2022). Although the prognosis for patients with advanced GIST has been greatly improved by the implementation of TKI therapy, resistance to these inhibitors remains a challenge. Each TKI differs in its efficacy against specific mutations; thus, a tumor may show primary resistance to a given agent. Additionally, the development of subsequent mutations often leads to secondary drug resistance, in which the tumor progresses after an initial period of stabilization or response to the inhibitor (Li & Raut, 2019).
Imatinib Mesylate
Imatinib mesylate (Gleevec) was granted approval by the US Food and Drug Administration (FDA) in 2002 for the first-line treatment of malignant metastatic and/or unresectable GISTs (Dagher et al., 2002). Imatinib inhibits the tyrosine kinase activity of KIT and PDGFRA by binding close to the ATP-binding site, locking it in an inactive conformation and preventing downstream signal transduction (Iqbal & Iqbal, 2014). The recommended dose of imatinib is 400 mg once daily for adult patients, with the possibility of escalation to 400 mg twice daily for patients showing disease progression at the lower dose (Novartis Pharmaceuticals Corporation, 2022). Response rates are 90% for tumors with a KIT exon 11 mutation and 50% for tumors that have a mutation in KIT exon 9 (NCCN, 2022). The use of imatinib in metastatic GIST was associated with an approximately 73% 2-year survival (Gold et al., 2007; Verweij et al., 2004). Patients treated with imatinib demonstrated a median overall survival (OS) of approximately 4 to 5 years (Blanke et al., 2008; Kim et al., 2019). Higher-dose imatinib (400 mg twice daily) demonstrated a progression-free survival (PFS) advantage in patients with GIST harboring a KIT exon 9 mutation (Gastrointestinal Stromal Tumor Meta-Analysis Group, 2010). Therefore, the recommended dose for patients with KIT exon 9 mutations receiving first-line imatinib is 400 mg twice daily (Kelly et al., 2021; NCCN, 2022). A recent retrospective analysis demonstrated that the higher-dose imatinib was not associated with better survival outcomes in an adjuvant setting compared with the standard dose (400 mg daily) in patients with a KIT exon 9 mutation (Vincenzi et al., 2021), suggesting a difference in treatment efficacy in the adjuvant vs. the first-line metastatic setting.
Avapritinib
Avapritinib (Ayvakit) was approved by the FDA in 2020 as first-line therapy for patients with advanced GISTs having PDGFRA exon 18 mutations that are insensitive to imatinib (Blueprint Medicines Corporation, 2020; NCCN, 2022). A selective inhibitor of KIT and PDGFRA, avapritinib shows high potency for the KIT exon 17 mutation D816V and the PDGFRA exon 18 D842V mutation, which are associated with resistance to imatinib, sunitinib (Sutent), and regorafenib (Stivarga; Evans et al., 2017). A phase I study identified 300 mg once daily as the recommended phase II dose of avapritinib. In that study, the overall response rate for patients with PDGFRA D842V mutations receiving 300 mg once daily was 93% (95% confidence interval [CI] = 77%–99%; 4% complete response, 89% PR, 7% stable disease; Heinrich et al., 2020).
Sunitinib
Sunitinib was approved by the FDA in 2006 as second-line treatment for adult patients with advanced GIST after disease progression on or intolerance to imatinib (NCCN, 2022; Pfizer Inc, 2020). Sunitinib is a multitargeted inhibitor of several receptor tyrosine kinases, including KIT and PDGFRA (Li & Raut, 2019; Mendel et al., 2003). In a phase III study of patients with progressive disease or intolerance to therapy while receiving imatinib, the median time to tumor progression was 27.3 weeks for patients receiving sunitinib compared with 6.4 weeks for patients receiving placebo (Demetri et al., 2006).
Regorafenib
Regorafenib was approved by the FDA in 2012 as third-line therapy for patients with advanced GIST who have been previously treated with imatinib and sunitinib (Bayer HealthCare Pharmaceuticals Inc., 2020; NCCN, 2022). Regorafenib has broad activity against a number of receptor protein kinases, including those involved in regulating oncogenesis (KIT, RET, RAF-1, BRAF, and BRAF V600E), the tumor microenvironment (PDGFR and FGFR), and tumor angiogenesis (VEGFR-1, -2, and -3, and TIE2; Demetri et al., 2013; Wilhelm et al., 2011). A phase III trial evaluating regorafenib in patients with metastatic and/or unresectable GIST who progressed after the failure of at least imatinib and sunitinib found that the median PFS was 4.8 months for patients receiving regorafenib and 0.9 months for patients receiving placebo (Demetri et al., 2013).
Ripretinib
Ripretinib was approved by the FDA in 2020 for the treatment of adult patients with advanced GIST who have received prior treatment with three or more kinase inhibitors, including imatinib (Deciphera Pharmaceuticals LLC, 2022); this treatment is also approved in Canada, Australia, China, Hong Kong, Taiwan, Switzerland, the UK, and the EU (Deciphera Pharmaceuticals LLC, 2020, 2021; Deciphera Pharmaceuticals Netherlands B.V. NL, 2021; Deciphera Pharmaceuticals Switzerland AG Zug, 2021; Medicines & Healthcare products Regulatory Agency, 2022; Zai Lab Limited, 2021a, 2021b). Ripretinib is a switch-control TKI that broadly inhibits KIT and PDGFRA kinase signaling through a dual mechanism of action. It was designed to bind to both the switch pocket and the activation switch to lock the kinase in the inactive state, preventing downstream signaling and cell proliferation. This dual mechanism of action provides broad inhibition of KIT and PDGFRA kinase activity, including wild-type and multiple primary and secondary mutations (Smith et al., 2019).
In the pivotal phase III INVICTUS study (NCT03353753), an international, multicenter, randomized, double-blind, placebo-controlled trial, 129 patients with progressive disease who had received three or more prior TKIs for advanced GIST (at least imatinib, sunitinib, and regorafenib) were randomized 2:1 to receive ripretinib 150 mg daily (n = 85) or placebo (n = 44) until disease progression or unacceptable toxicity (Blay et al., 2020). The median PFS of patients receiving ripretinib was 6.3 months (95% CI = 4.6–6.9), compared with 1.0 month (95% CI = 0.9–1.7) in patients receiving placebo (hazard ratio [HR], 0.15; 95% CI = 0.09–0.25; p < .0001). The median OS was 15.1 months (95% CI = 12.3–15.1) in the ripretinib group vs. 6.6 (95% CI = 4.1–11.6) months in the placebo group (HR, 0.36; 95% CI = 0.21–0.62). Eight of 85 patients (9.4%; 95% CI = 4.2–17.7; p = .0504 compared with placebo) receiving ripretinib had a confirmed objective response, all of whom had a PR; none of the patients receiving placebo had a confirmed objective response. In a long-term update to the primary INVICTUS analysis, the median PFS for patients receiving ripretinib was still 6.3 months vs. 1.0 month for placebo; however, the median OS was increased to 18.2 months in the ripretinib arm but remained unchanged at 6.3 months in the placebo arm (von Mehren et al., 2021).
Additionally, patient-reported outcome measures of quality of life were assessed as part of the INVICTUS trial. Role and physical functioning were assessed by the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire for Cancer 30-item (EORTC QLQ-C30), and overall health was assessed by the EuroQol 5-Dimension 5-Level visual analogue scale (EQ-5D-5L VAS). Patients receiving ripretinib showed stable scores for both measures, indicating that they were able to maintain quality of life, while the scores declined for patients receiving placebo; the results indicated a clinically relevant difference between the groups (Blay et al., 2020).
ADVERSE EVENTS ASSOCIATED WITH TKI THERAPY
Tyrosine kinase inhibitors, particularly those in the second and third line of treatment, are associated with adverse events (AEs) that may necessitate dose reductions, treatment interruptions, or permanent discontinuation of treatment, limiting their use in certain patients (Demetri et al., 2012; Rizzo et al., 2020). In a phase II study, the most commonly seen AEs of any grade for patients receiving imatinib 400 mg once daily included edema (most frequently periorbital), nausea, diarrhea, myalgia, and fatigue (all ≥ 30%). The most serious AEs reported were gastrointestinal or intraabdominal hemorrhages in patients with large tumors; these were considered likely to be due to the degeneration of tumors by imatinib (Demetri et al., 2002). Serious AEs, dose modifications, and grade 3 to 5 toxicities were more common in patients receiving the 400-mg twice-daily imatinib dose compared with patients receiving the 400-mg once-daily dose (Blanke et al., 2008).
In a phase I study, the most common treatment-related AEs of grade 1 or 2 for patients taking avapritinib 300 mg once daily were nausea, diarrhea, decreased appetite, and fatigue (Heinrich et al., 2020). The most common serious AEs related to treatment with avapritinib were anemia, pleural effusion, diarrhea, and vertigo. Across all doses of avapritinib, there were two AEs considered to be of special interest: cognitive effects and intracranial bleeding. Cognitive effects were seen in 40% (out of 82 patients); these included memory impairment, cognitive disorder, confused state, and encephalopathy. Intracranial bleeding was found in 2% of patients (Heinrich et al., 2020).
For patients receiving sunitinib in a phase III trial, AEs of grade 3 or higher that appeared more frequently than in patients receiving placebo included but were not limited to fatigue, palmar-plantar erythrodysesthesia (PPE, hand-foot syndrome), diarrhea, hypertension, leucopenia, neutropenia, lymphopenia, and thrombocytopenia. Additionally, 4% of patients receiving sunitinib developed hypothyroidism (Demetri et al., 2006). In a phase III trial of regorafenib, the most common AEs of grade 3 or higher for patients taking regorafenib were hypertension, PPE, and diarrhea. There were two grade 5 AEs of cardiac arrest and hepatic failure that were considered to be regorafenib-related (Demetri et al., 2013). The FDA-approved dosing information for both sunitinib and regorafenib carry warnings regarding severe hepatotoxicity; monitoring of liver function at baseline and during treatment is recommended (Bayer HealthCare Pharmaceuticals Inc., 2020; Pfizer Inc, 2020). Table 1 summarizes the most common treatment-related AEs observed for the first- through third-line therapies approved for advanced GIST.
Table 1. Treatment-Related Adverse Events Reported in Pivotal Trials of TKIs Approved For First- Through Third-Line Treatment of GIST.
| Imatinib 400 mg (N = 73) | Avapritinib 300 mg (N = 32) | Sunitinib (N = 202) | Regorafenib (N = 132) | |
|---|---|---|---|---|
| Common AEsa | Edema/fluid retention, nausea, diarrhea, myalgia/musculoskeletal pain, fatigue, abdominal pain, dermatitis/rash | Nausea, diarrhea, fatigue, decreased appetite, periorbital edema, anemia, memory impairment, hair color changes, increased lacrimation, peripheral edema, increased blood bilirubin, face edema, dysgeusia, neutropenia | Fatigue, diarrhea, skin discoloration, nausea, anemiab, leucopenia, neutropenia, lymphopenia, thrombocytopenia | PPE, hypertension, diarrhea, fatigue, oral mucositis, alopecia, hoarseness, anorexia |
| Common grade 3/4 AEsc | Neutropenia | Diarrhea, anemia, neutropenia, decreased neutrophil count | Fatigue, neutropenia, lymphopenia, thrombocytopenia | Hypertension, PPE, diarrhea |
| Serious AEs (%) | Gastrointestinal or intraabdominal hemorrhages (5% total regardless of dose) | Anemia, pleural effusion, diarrhea, vertigo (26% across all doses); cognitive effectsd (40% across all doses); intracranial bleedingd (2% across all doses) | Fatigue, PPE, diarrhea, hypertension, hematological AEs | 29 |
| Deaths (%) | NR | 0 | NR | NR |
| Dose interruptions (%) | NR | NRe | 28 | NR |
| Dose modifications (%) | NR | NRe | 11 | 72 |
| Treatment discontinuations due to AEs (%) | NR | 12% across all doses | 9 | 6 |
Note. AE = adverse event; GIST = gastrointestinal stromal tumor; NR = not reported; PPE = palmar-plantar erythrodysesthesia; TKI = tyrosine kinase inhibitor. Information from Demetri et al. (2002, 2006, 2013); Heinrich et al. (2020).
Reported in ≥ 20% of patients receiving study drug.
Anemia was included despite a difference of less than 5% between the sunitinib and placebo groups because of its frequency and clinical relevance in GIST.
Reported in ≥ 5% of patients receiving study drug.
Considered by the study authors to be an AE of special interest.
84% of patients receiving study drug across all doses required at least one dose reduction or dose interruption.
Ripretinib 150 mg once daily is generally well tolerated and associated with an acceptable safety profile. In the phase III INVICTUS trial, the most common treatment-emergent AEs (TEAEs) related to treatment in patients receiving ripretinib (≥ 20%) were alopecia, myalgia, nausea, fatigue, PPE, and diarrhea. Constipation, decreased appetite, and weight loss occurred in ≥ 15% of patients. Most TEAEs were grades 1 or 2. Serious TEAEs were reported in 9% of patients taking ripretinib and 7% of patients taking placebo.
Table 2 summarizes the AEs (≥ 10%) associated with ripretinib in patients with GIST according to the prescribing information. Additionally, the label includes warnings and precautions relating to PPE (grade 1–2), the development of new skin cancers (including cutaneous squamous cell carcinoma, keratoacanthoma, and melanoma), hypertension (grade 1–3), cardiac dysfunction (including cardiac failure, acute left ventricular failure, diastolic dysfunction, ventricular hypertrophy, and decreased ejection fraction), risk of impaired wound healing, photosensitivity, pregnancy risks (including embryo-fetal toxicity), and drug interactions (strong CYP3A inhibitors and inducers; Deciphera Pharmaceuticals LLC, 2022).
Table 2. Adverse Events (≥ 10%) in Patients With Advanced GIST Who Received Ripretinib in the INVICTUS Study (N = 85).
| Adverse event | All grades (1–4), (%) | Grades 3–4, (%) |
|---|---|---|
| Skin and subcutaneous tissue | ||
| Alopecia | 52 | 0 |
| Palmar-plantar erythrodysesthesia | 21 | 0 |
| Dry skin | 13 | 0 |
| Pruritus | 11 | 0 |
| General | ||
| Fatigue | 42 | 4 |
| Peripheral edema | 17 | 1 |
| Asthenia | 13 | 1 |
| Gastrointestinal | ||
| Nausea | 39 | 4 |
| Abdominal pain | 36 | 7 |
| Constipation | 34 | 1 |
| Diarrhea | 28 | 1 |
| Vomiting | 21 | 4 |
| Stomatitis | 11 | 0 |
| Musculoskeletal and connective tissue | ||
| Myalgia | 32 | 1 |
| Arthralgia | 18 | 0 |
| Muscle spasms | 15 | 0 |
| Metabolism and nutrition | ||
| Decreased appetite | 27 | 1 |
| Investigations | ||
| Decreased weight | 19 | 0 |
| Nervous system | ||
| Headache | 19 | 0 |
| Vascular | ||
| Hypertension | 14 | 7 |
| Respiratory, thoracic, and mediastinal | ||
| Dyspnea | 13 | 0 |
Note. GIST = gastrointestinal stromal tumor.
SUPPORTIVE CARE AND OPTIMIZING OUTCOMES
To optimize clinical outcomes in advanced GIST, it is important for patients receiving TKI therapy to maintain a regular dosing schedule for an extended period of time. In view of this, AEs that are severe enough to prompt lack of adherence to an otherwise effective drug regimen may result in suboptimal response or outcomes (Tetzlaff & Davey, 2013). Due to their involvement in ongoing patient care, oncology advanced practitioners, including nurse practitioners, physician assistants, and pharmacists, play an important role in promoting treatment adherence by promptly recognizing and effectively managing AEs. Strategies for managing AEs may include supportive care, dose modifications, and if necessary, treatment holds or discontinuations (Grothey et al., 2014; Joseph et al., 2021). Advanced practitioners can facilitate adherence to TKI treatment by educating patients about the risks and side effects prior to initiation of the therapy, including providing information about indicators of side effect severity and reduced quality of life, and addressing patient concerns.
Patients typically receive a thorough baseline assessment prior to starting a new treatment regimen; this includes both physical health and quality of life assessments. Advanced practitioners may facilitate increased communication in the form of frequent phone calls and appointments to review labs and give the patient opportunities to ask questions prior to starting a new therapy. Patients are encouraged to call or page their providers as needed to report any changes in symptoms and will receive regular check-in phone calls from an oncology nurse navigator. The patient's provider can also make referrals to other services, such as dermatology and nutrition, as needed. Similarly, advanced practitioners may also provide the patient with tools for support, such as recommendations for patient advocacy groups or support networks. This communication between patient and provider may enable the management of potential toxicity early in treatment, thus minimizing dose reduction or treatment discontinuation and maximizing clinical outcomes and patient quality of life.
FUTURE DIRECTIONS FOR RIPRETINIB
For patients with progressive GIST receiving ripretinib as fourth-line therapy, for whom treatment options are limited, intrapatient dose escalation (IPDE) of ripretinib may provide additional clinical benefit with an acceptable safety profile. In a phase I study (NCT02571036), dose escalation to ripretinib 150 mg twice daily after disease progression on ripretinib 150 mg once daily was evaluated for patients with GIST receiving second-, third-, or fourth-line therapy (George et al., 2021). A comparison of TEAEs reported in the ripretinib 150 mg once-daily and 150 mg twice-daily periods showed that ripretinib was similarly well tolerated at both doses. No new TEAEs were observed with the higher dose; TEAEs leading to dose interruption or dose reduction were comparable during the 150 mg once-daily period and IPDE periods. In an exploratory analysis of the INVICTUS phase III study, patients with advanced GIST randomized to ripretinib who were dose escalated to 150 mg twice daily (n = 43) after disease progression on ripretinib 150 mg once daily experienced meaningful additional clinical benefit (Zalcberg et al., 2021). The median PFS was 4.6 months (95% CI = 2.7–6.4) before and 3.7 months (95% CI = 3.1–5.3) after ripretinib IPDE. Ripretinib 150 mg twice daily was generally well tolerated; the most frequent new or worsening grade 3 to 4 TEAEs were anemia in 14% and abdominal pain in 7% of patients.
In addition, ripretinib is currently being investigated in the phase III INTRIGUE study (NCT03673501) as a potential second-line therapy in patients with advanced GIST following imatinib treatment, compared with standard second-line therapy with sunitinib (Nemunaitis et al., 2020). Primary results from the INTRIGUE study demonstrate that ripretinib did not meet the primary endpoint of superiority in PFS vs. sunitinib. However, the median PFS observed for patients with a KIT exon 11 mutation in either arm of this trial (ripretinib: 8.3 months; sunitinib: 7.0 months) was longer than the median PFS achieved by sunitinib in its pivotal phase III trial (5.6 months; Bauer et al., 2022; Demetri et al., 2006). There were fewer grade 3 to 4 TEAEs among patients receiving ripretinib vs. sunitinib (41.3% vs. 65.6%, respectively), suggesting that ripretinib has a more favorable safety profile than sunitinib as a second-line treatment for patients with advanced GIST (Bauer et al., 2022; Heinrich et al., 2022). Subanalyses are ongoing for the INTRIGUE trial.
IMPLICATIONS FOR THE ADVANCED PRACTITIONER IN ONCOLOGY
Advanced practitioners are in a prime position to improve patient awareness of advanced GIST and support patients through each line of treatment to improve outcomes. Due to the development of secondary mutations, a patient with advanced GIST will progress through multiple lines of therapy, with each treatment having its own challenges and unique safety profile. It is extremely important that patients are educated prior to starting a new therapy. This includes providing information on possible side effects and how they would be treated, reviewing the known safety profile, and managing expectations regarding treatment efficacy and quality of life. We learn from the presented case study as we learn from all of our patients; by listening to their experiences, we gain valuable information about what interventions and resources are most likely to be helpful. As advanced practitioners in oncology, we may be the medical experts; however, the patient living with the diagnosis of GIST is the true expert.
Acknowledgment
Medical writing support was provided by Tara Rachinsky, PhD, and Lauren Hanlon, PhD, CMPP (AlphaBioCom, a Red Nucleus company, King of Prussia, Pennsylvania, USA). This support was funded by Deciphera Pharmaceuticals, LLC (Waltham, Massachusetts, USA).
Footnotes
Ms. Brackert reports fees from Deciphera for participation in their speaking program. Ms. Polson serves as a consultant on the patient advisory board for Deciphera. This work was supported by Deciphera Pharmaceuticals, LLC (Waltham, Massachusetts, USA).
References
- Bauer, S., Jones, R. L., Blay, J. Y., Gelderblom, H., George, S., Schoffski, P.,…Heinrich, M. C. (2022). Ripretinib versus sunitinib in patients with advanced gastrointestinal stromal tumor after treatment with imatinib (INTRIGUE): A randomized, open-label, phase III trial. Journal of Clinical Oncology, 40(34), 3918–3928. 10.1200/JCO.22.00294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer HealthCare Pharmaceuticals Inc. (2020). Stivarga (regorafenib) package insert. https://labeling.bayerhealthcare.com/html/products/pi/Stivarga_PI.pdf.
- Blanke, C. D., Rankin, C., Demetri, G. D., Ryan, C. W., von Mehren, M., Benjamin, R. S.,…Borden, E. C. (2008). Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. Journal of Clinical Oncology, 26(4), 626–632. 10.1200/jco.2007.13.4452 [DOI] [PubMed] [Google Scholar]
- Blay, J. Y., Serrano, C., Heinrich, M. C., Zalcberg, J., Bauer, S., Gelderblom, H.,…von Mehren, M. (2020). Ripretinib in patients with advanced gastrointestinal stromal tumours (INVICTUS): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncology, 21(7), 923–934. 10.1016/s1470-2045(20)30168-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blueprint Medicines Corporation. (2020). Ayvakit (avapritinib) tablets package insert. https://www.blueprintmedicines.com/wp-content/uploads/uspi/AYVAKIT.pdf
- Call, J., Walentas, C. D., Eickhoff, J. C., & Scherzer, N. (2012). Survival of gastrointestinal stromal tumor patients in the imatinib era: Life raft group observational registry. BMC Cancer, 12, 90. 10.1186/1471-2407-12-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagher, R., Cohen, M., Williams, G., Rothmann, M., Gobburu, J., Robbie, G.,…Pazdur, R. (2002). Approval summary: Imatinib mesylate in the treatment of metastatic and/or unresectable malignant gastrointestinal stromal tumors. Clinical Cancer Research, 8(10), 3034–3038. https://pubmed.ncbi.nlm.nih.gov/12374669/ [PubMed] [Google Scholar]
- Deciphera Pharmaceuticals LLC. (2020). Qinlock (ripretinib) tablets: Canadian product monograph. https://pdf.hres.ca/dpd_pm/00056679.PDF
- Deciphera Pharmaceuticals LLC. (2021). Deciphera announces approval of Qinlock in Switzerland for the treatment of fourth-line gastrointestinal stromal tumor. https://investors.deciphera.com/news-releases/news-release-details/deciphera-announces-approval-qinlockr-switzerland-treatment
- Deciphera Pharmaceuticals LLC. (2022). Qinlock (ripretinib) tablets package insert. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/213973s000lbl.pdf
- Deciphera Pharmaceuticals Netherlands B.V. NL. (2021). Qinlock (ripretinib). Summary of product characteristics. https://www.ema.europa.eu/en/documents/product-information/qinlock-epar-product-information_en.pdf
- Deciphera Pharmaceuticals Switzerland AG Zug. (2021). Deciphera Pharmaceuticals (Switzerland) AG, Zug. Qinlock (ripretinib) package insert. https://www.swissmedicinfo.ch/ShowText.aspx?textType=FI&lang=DE&authNr=68199
- Demetri, G. D., Garrett, C. R., Schöffski, P., Shah, M. H., Verweij, J., Leyvraz, S.,…Casali, P. G. (2012). Complete longitudinal analyses of the randomized, placebo-controlled, phase III trial of sunitinib in patients with gastrointestinal stromal tumor following imatinib failure. Clinical Cancer Research, 18(11), 3170–3179. 10.1158/1078-0432.Ccr-11-3005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demetri, G. D., Reichardt, P., Kang, Y. K., Blay, J. Y., Rutkowski, P., Gelderblom, H.,…Casali, P. G. (2013). Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): An international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet, 381(9863), 295–302. 10.1016/s0140-6736(12)61857-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demetri, G. D., von Mehren, M., Blanke, C. D., Van den Abbeele, A. D., Eisenberg, B., Roberts, P. J.,…Joensuu, H. (2002). Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. New England Journal of Medicine, 347(7), 472–480. 10.1056/NEJMoa020461 [DOI] [PubMed] [Google Scholar]
- Demetri, G. D., van Oosterom, A. T., Garrett, C. R., Blackstein, M. E., Shah, M. H., Verweij, J.,…Casali, P. G. (2006). Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: A randomised controlled trial. Lancet, 368(9544), 1329–1338. 10.1016/s0140-6736(06)69446-4 [DOI] [PubMed] [Google Scholar]
- Evans, E. K., Gardino, A. K., Kim, J. L., Hodous, B. L., Shutes, A., Davis, A.,…Lengauer, C. (2017). A precision therapy against cancers driven by KIT/PDGFRA mutations. Science Translational Medicine, 9(414). 10.1126/scitranslmed.aao1690 [DOI] [PubMed] [Google Scholar]
- Gastrointestinal Stromal Tumor Meta-Analysis Group. (2010). Comparison of two doses of imatinib for the treatment of unresectable or metastatic gastrointestinal stromal tumors: A meta-analysis of 1,640 patients. Journal of Clinical Oncology, 28(7), 1247–1253. 10.1200/JCO.2009.24.2099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George, S., Chi, P., Heinrich, M. C., von Mehren, M., Jones, R. L., Ganjoo, K.,…Janku, F. (2021). Ripretinib intrapatient dose escalation after disease progression provides clinically meaningful outcomes in advanced gastrointestinal stromal tumour. European Journal of Cancer, 155, 236–244. 10.1016/j.ejca.2021.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold, J. S., van der Zwan, S. M., Gönen, M., Maki, R. G., Singer, S., Brennan, M. F.,…De Matteo, R. P. (2007). Outcome of metastatic GIST in the era before tyrosine kinase inhibitors. Annals of Surgical Oncology, 14(1), 134–142. 10.1245/s10434-006-9177-7 [DOI] [PubMed] [Google Scholar]
- Grothey, A., George, S., van Cutsem, E., Blay, J. Y., Sobrero, A., & Demetri, G. D. (2014). Optimizing treatment outcomes with regorafenib: Personalized dosing and other strategies to support patient care. Oncologist, 19(6), 669–680. 10.1634/theoncologist.2013-0059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich, M. C., Jones, R. L., Gelderblom, H., George, S., Schoffski, P., von Mehren, M.,…Bauer, S. (2022). INTRIGUE: A phase III, randomized, open-label study to evaluate the efficacy and safety of ripretinib versus sunitinib in patients with advanced gastrointestinal stromal tumor previously treated with imatinib. Journal of Clinical Oncology, 40(36_suppl), 359881–359881. 10.1200/JCO.2022.40.36_suppl.359881 [DOI] [Google Scholar]
- Heinrich, M. C., Jones, R. L., von Mehren, M., Schöffski, P., Serrano, C., Kang, Y. K.,…George, S. (2020). Avapritinib in advanced PDGFRA D842V-mutant gastrointestinal stromal tumour (NAVIGATOR): A multicentre, open-label, phase 1 trial. Lancet Oncology, 21(7), 935–946. 10.1016/s1470-2045(20)30269-2 [DOI] [PubMed] [Google Scholar]
- Iqbal, N., & Iqbal, N. (2014). Imatinib: A breakthrough of targeted therapy in cancer. Chemotherapy Research and Practice, 2014, 357027. 10.1155/2014/357027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa, T., Kanda, T., Kameyama, H., & Wakai, T. (2018). Neoadjuvant therapy for gastrointestinal stromal tumor. Translational Gastroenterology and Hepatology, 3(1), 3. 10.21037/tgh.2018.01.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joensuu, H. (2008). Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Human Pathology, 39(10), 1411–1419. 10.1016/j.humpath.2008.06.025 [DOI] [PubMed] [Google Scholar]
- Joseph, C. P., Abaricia, S. N., Angelis, M. A., Polson, K., Jones, R. L., Kang, Y. K.,…Havnaer, T. (2021). Optimal avapritinib treatment strategies for patients with metastatic or unresectable gastrointestinal stromal tumors. Oncologist, 26(4), e622–e631. 10.1002/onco.13632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly, C. M., Gutierrez Sainz, L., & Chi, P. (2021). The management of metastatic GIST: Current standard and investigational therapeutics. Journal of Hematology & Oncology, 14(1), 2. 10.1186/s13045-020-01026-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J. H., Ryu, M. H., Yoo, C., Chae, H., Na, H., Beck, M.,…Kang, Y. K. (2019). Long-term survival outcome with tyrosine kinase inhibitors and surgical intervention in patients with metastatic or recurrent gastrointestinal stromal tumors: A 14-year, single-center experience. Cancer Medicine, 8(3), 1034–1043. 10.1002/cam4.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, G. Z., & Raut, C. P. (2019). Targeted therapy and personalized medicine in gastrointestinal stromal tumors: Drug resistance, mechanisms, and treatment strategies. OncoTargets and Therapy, 12, 5123–5133. 10.2147/ott.S180763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, G. L., Murphy, J. D., Martinez, M. E., & Sicklick, J. K. (2015). Epidemiology of gastrointestinal stromal tumors in the era of histology codes: Results of a population-based study. Cancer Epidemiology, Biomarkers & Prevention, 24(1), 298–302. 10.1158/1055-9965.Epi-14-1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Broto, J., Martinez-Marín, V., Serrano, C., Hindi, N., López-Guerrero, J. A., Bisculoa, M.,…González-Cámpora, R. (2017). Gastrointestinal stromal tumors (GISTs): SEAP-SEOM consensus on pathologic and molecular diagnosis. Clinical Translational Oncology, 19(5), 536–545. 10.1007/s12094-016-1581-2 [DOI] [PubMed] [Google Scholar]
- Medicines & Healthcare Products Regulatory Agency. (2022). Public Assessment Report National Procedure Qinlock 50 mg tablets ripretinib. https://mhraproducts4853.blob.core.windows.net/docs/0d4b34210f1a10b98e56b08d5fa53b27a65ca8ab
- Mendel, D. B., Laird, A. D., Xin, X., Louie, S. G., Christensen, J. G., Li, G.,…Cherrington, J. M. (2003). In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: Determination of a pharmacokinetic/pharmacodynamic relationship. Clinical Cancer Research, 9(1), 327–337. [PubMed] [Google Scholar]
- Miettinen, M., & Lasota, J. (2006). Gastrointestinal stromal tumors: Pathology and prognosis at different sites. Seminars in Diagnostic Pathology, 23(2), 70–83. 10.1053/j.semdp.2006.09.001 [DOI] [PubMed] [Google Scholar]
- Nardone, B., Hensley, J. R., Kulik, L., West, D. P., Mulcahy, M., Rademaker, A., & Lacouture, M. E. (2012). The effect of hand-foot skin reaction associated with the multikinase inhibitors sorafenib and sunitinib on health-related quality of life. Journal of Drugs in Dermatology, 11(11), e61–65. https://pubmed.ncbi.nlm.nih.gov/23135095/ [PubMed] [Google Scholar]
- National Comprehensive Cancer Network. (2022). NCCN Clinical Practice Guidelines in Oncology: Gastrointestinal Stromal Tumors (GISTs). V2.2022. https://www.nccn.org/professionals/physician_gls/pdf/gist.pdf
- Nemunaitis, J., Bauer, S., Blay, J. Y., Choucair, K., Gelderblom, H., George, S.,…Heinrich, M. C. (2020). Intrigue: Phase III study of ripretinib versus sunitinib in advanced gastrointestinal stromal tumor after imatinib. Future Oncology, 16(1), 4251–4264. 10.2217/fon-2019-0633 [DOI] [PubMed] [Google Scholar]
- Novartis Pharmaceuticals Corporation. (2022). Gleevec (imatinib mesylate) tablets package insert. https://www.novartis.us/sites/www.novartis.us/files/gleevec_tabs.pdf
- Pfizer Inc. (2020). Sutent (sunitinib malate) capsule package insert. https://labeling.pfizer.com/showlabeling.aspx?id=607
- Rizzo, A., Nannini, M., Novelli, M., Dalia Ricci, A., Scioscio, V. D., & Pantaleo, M. A. (2020). Dose reduction and discontinuation of standard-dose regorafenib associated with adverse drug events in cancer patients: A systematic review and meta-analysis. Therapeutic Advances in Medical Oncology, 12, 1758835920936932. 10.1177/1758835920936932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin, B. P., Heinrich, M. C., & Corless, C. L. (2007). Gastrointestinal stromal tumour. Lancet, 369(9574), 1731–1741. 10.1016/s0140-6736(07)60780-6 [DOI] [PubMed] [Google Scholar]
- Rutkowski, P., Nowecki, Z. I., Michej, W., Debiec-Rychter, M., Woźniak, A., Limon, J.,…Ruka, W. (2007). Risk criteria and prognostic factors for predicting recurrences after resection of primary gastrointestinal stromal tumor. Annals of Surgical Oncology, 14(7), 2018–2027. 10.1245/s10434-007-9377-9 [DOI] [PubMed] [Google Scholar]
- Smith, B. D., Kaufman, M. D., Lu, W. P., Gupta, A., Leary, C. B., Wise, S. C.,…Flynn, D. L. (2019). Ripretinib (DCC-2618) is a switch control kinase inhibitor of a broad spectrum of oncogenic and drug-resistant KIT and PDGFRA variants. Cancer Cell, 35(5), 738–751.e739. 10.1016/j.ccell.2019.04.006 [DOI] [PubMed] [Google Scholar]
- Søreide, K., Sandvik, O. M., Søreide, J. A., Giljaca, V., Jureckova, A., & Bulusu, V. R. (2016). Global epidemiology of gastrointestinal stromal tumours (GIST): A systematic review of population-based cohort studies. Cancer Epidemiology, 40, 39–46. 10.1016/j.canep.2015.10.031 [DOI] [PubMed] [Google Scholar]
- Szucs, Z., Thway, K., Fisher, C., Bulusu, R., Constantinidou, A., Benson, C.,…Jones, R. L. (2017). Molecular subtypes of gastrointestinal stromal tumors and their prognostic and therapeutic implications. Future Oncology, 13(1), 93–107. 10.2217/fon-2016-0192 [DOI] [PubMed] [Google Scholar]
- Tetzlaff, E. D., & Davey, M. P. (2013). Optimizing adherence to adjuvant imatinib in gastrointestinal stromal tumor. Journal of the Advanced Practitioner in Oncology, 4(4), 238–250. 10.6004/jadpro.2013.4.4.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verweij, J., Casali, P. G., Zalcberg, J., LeCesne, A., Reichardt, P., Blay, J. Y.,…Judson, I. (2004). Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: Randomised trial. Lancet, 364(9440), 1127–1134. 10.1016/s0140-6736(04)17098-0 [DOI] [PubMed] [Google Scholar]
- Vincenzi, B., Napolitano, A., Fiocco, M., Mir, O., Rutkowski, P., Blay, J. Y.,…Casali, P. G. (2021). Adjuvant imatinib in GIST patients harboring exon 9 KIT mutations: Results from a multi-institutional European retrospective study. Clinical Cancer Research, 28(8), 1672–1679. 10.1158/1078-0432.CCR-21-1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Mehren, M., Heinrich, M. C., George, S., Zalcberg, J., Bauer, S., Gelderblom, H.,…Blay, J. Y. (2021). Ripretinib as ≥4th-line treatment in patients with advanced gastrointestinal stromal tumor: Long-term update from the phase III INVICTUS study. Annals of Oncology, 32(Supplement 5), S1111–S1128. 10.1016/j.annonc.2021.08.870 [DOI] [Google Scholar]
- Wilhelm, S. M., Dumas, J., Adnane, L., Lynch, M., Carter, C. A., Schütz, G.,…Zopf, D. (2011). Regorafenib (BAY 73-4506): A new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. International Journal of Cancer, 129(1), 245–255. 10.1002/ijc.25864 [DOI] [PubMed] [Google Scholar]
- Zai Lab Limited. (2021a). China NMPA approves Qinlock (ripretinib) for treatment of advanced gastrointestinal stromal tumors (GIST). https://zailab.gcs-web.com/node/8936/pdf
- Zai Lab Limited. (2021b). Qinlock (ripretinib) approved in Taiwan for treatment of advanced gastrointestinal stromal tumors (GIST). http://ir.zailaboratory.com/news-releases/news-release-details/qinlockr-ripretinib-approved-taiwan-treatment-advanced
- Zalcberg, J. R., Heinrich, M. C., George, S., Bauer, S., Schoffski, P., Serrano, C.,…Blay, J. Y. (2021). Clinical benefit of ripretinib dose escalation after disease progression in advanced gastrointestinal stromal tumor: An analysis of the INVICTUS Study. Oncologist, 26(11), e2053–e2060. 10.1002/onco.13917 [DOI] [PMC free article] [PubMed] [Google Scholar]


