Abstract
Objectives
Dyspnoea is a common persistent symptom post-coronavirus disease 2019 (COVID-19) illness. However, the mechanisms underlying dyspnoea in the post-COVID-19 syndrome remain unclear. The aim of our study was to examine dyspnoea quality and intensity, burden of mental health symptoms, and differences in exercise responses in people with and without persistent dyspnoea following COVID-19.
Methods
49 participants with mild-to-critical COVID-19 were included in this cross-sectional study 4 months after acute illness. Between-group comparisons were made in those with and without persistent dyspnoea (defined as modified Medical Research Council dyspnoea score ≥1). Participants completed standardised dyspnoea and mental health symptom questionnaires, pulmonary function tests, and incremental cardiopulmonary exercise testing.
Results
Exertional dyspnoea intensity and unpleasantness were increased in the dyspnoea group. The dyspnoea group described dyspnoea qualities of suffocating and tightness at peak exercise (p<0.05). Ventilatory equivalent for carbon dioxide (VʹE/VʹCO2) nadir was higher (32±5 versus 28±3, p<0.001) and anaerobic threshold was lower (41±12 versus 49±11% predicted maximum oxygen uptake, p=0.04) in the dyspnoea group, indicating ventilatory inefficiency and deconditioning in this group. The dyspnoea group experienced greater symptoms of anxiety, depression and post-traumatic stress (all p<0.05). A subset of participants demonstrated gas-exchange and breathing pattern abnormalities suggestive of dysfunctional breathing.
Conclusions
People with persistent dyspnoea following COVID-19 experience a specific dyspnoea quality phenotype. Dyspnoea post-COVID-19 is related to abnormal pulmonary gas exchange and deconditioning and is linked to increased symptoms of anxiety, depression and post-traumatic stress.
Short abstract
COVID-19 survivors experience specific qualitative dyspnoea sensations. Dyspnoea post-COVID-19 is related to abnormal pulmonary gas exchange and deconditioning, and is linked to increased symptoms of anxiety, depression and post-traumatic stress. https://bit.ly/3AM0ulm
Introduction
Persistent symptoms following coronavirus disease 2019 (COVID-19), termed post-COVID syndrome, represent an important clinical challenge [1]. Studies examining persistent symptoms following acute COVID-19 have used varying symptom and temporal definitions, resulting in large reported ranges (4.7–80%) of individuals developing this syndrome [2]. Dyspnoea is common post-COVID-19 infection, estimated to occur in ∼40% of people [2]. Lasting dyspnoea is variably associated with severity of acute illness, age, female sex and number of comorbidities [2]. Moreover, abnormal resting lung function, imaging abnormalities and reduced functional capacity are not consistently associated with persistent dyspnoea [3]. Recent studies have described heterogenous clusters within the broader post-COVID syndrome, raising the possibility that mechanisms underlying dyspnoea in this population may be diverse [3, 4].
Cardiopulmonary exercise testing (CPET) has proved to be a valuable tool for characterising the pathophysiology of exertional dyspnoea across cardiopulmonary, neurologic and metabolic diseases. Most studies that have performed CPET in COVID-19 survivors have focused on comparing individuals with and without exercise intolerance [5], and stratified participants by severity of acute illness [6–8], lung function impairment [9] or presence of imaging abnormalities [10]. Mechanisms of reduced exercise capacity described in available studies include deconditioning [6, 9], ventilatory limitation [6, 7, 9], cardiac limitation [6], ventilatory inefficiency [6] and hyperventilation [4, 9, 11]. Importantly, to our knowledge, no studies have examined dyspnoea intensity and quality, mental health symptoms, and exercise responses in combination. Therefore, the aim of our study was to examine dyspnoea quality and intensity, burden of mental health symptoms, and differences in exercise responses in participants with and without persistent dyspnoea following COVID-19. We hypothesised that people with persistent dyspnoea following COVID-19 infection would have a greater burden of mental health symptoms as well as abnormal gas exchange during exercise. This study is a sub-group analysis of the larger Long-term Effect of severe acute respiratory syndrome (SARS) coronavirus 2 infection (LEFT) study on physiological and psychological health [12] and describes results related to persistent dyspnoea and mental health symptoms not previously reported.
Methods
Study participants
Included participants were ≥18 years of age and were diagnosed with SARS coronavirus 2 infection causing COVID-19 illness by positive nasopharyngeal swab reverse-transcription PCR prior to study participation. People who were unable to perform CPET on a cycle ergometer, had a diagnosis of current malignancy or had self-reported pre-existing cardiopulmonary disease that could contribute to exertional dyspnoea (e.g. asthma, interstitial lung disease, pulmonary hypertension, heart failure), had a contraindication to exercise testing or were treated with ambulatory oxygen were excluded from analysis. The study was approved by the Ottawa Health Science Network Research Ethics Board (20200371-01H). All participants provided written informed consent.
Study design
This is a cross-sectional sub-group analysis of the LEFT study on physiological and psychological health [12]. Pulmonary function testing and incremental CPET were performed in hospitalised individuals ∼3 months post-discharge and nonhospitalised individuals 3 months after a positive SARS coronavirus 2 test. Participants were stratified into those with and without persistent dyspnoea, defined as a modified Medical Research Council (mMRC) dyspnoea score ≥1.
Study procedures
Pulmonary function testing
Spirometry, body plethysmography, diffusing capacity of the lungs for carbon monoxide (DLCO) and maximum inspiratory (MIP) and expiratory mouth pressures were performed in accordance with international guidelines using automated equipment (Vmax 229d with Vs62j body plethysmograph; SensorMedics; California, USA) [13–16]. Results are reported as absolute values and % predicted normal values [17–19].
CPET
CPET was performed on an upright cycle ergometer (Ergoline 800s; SensorMedics; California, USA) using a breath-by-breath CPET system (Vmax229d; SensorMedics; California, USA) [20]. Results are expressed as absolute values and % predicted [21]. CPET consisted of a 3-min resting period, followed by a 1-min warm-up of unloaded pedalling, then 15 W·min−1 stepwise increases in work rate to symptom limitation. Results were assessed for meeting maximal test criteria [22]. Standard cardiorespiratory variables were averaged every 30 s during exercise. Operating lung volumes were derived from inspiratory capacity (IC) manoeuvres collected every 2 min. Highest equivalent submaximal work rate (HEWR) was defined as the 30 s increment achieved by all participants during CPET. Peak exercise was defined as the highest work rate maintained for 30 s. Ventilatory equivalent for carbon dioxide (VʹE/VʹCO2) nadir was the lowest value measured during a 30 s increment during exercise. Anaerobic threshold (AT) was identified using the dual criteria method and expressed as a percentage of predicted maximum oxygen uptake (VʹO2) [20]. Possible dysfunctional breathing was identified if a participant met any one of three criteria including gas exchange (end-tidal carbon dioxide tension (PETCO2) <30 mmHg at rest and exercise; VʹE/VʹCO2 >35 at submaximal work rate (60 W)) or erratic breathing pattern abnormalities [23].
Symptom evaluation
Diverse domains of dyspnoea including symptom intensity, associated affective distress and sensory-perceptual experience of the participants were collected by validated questionnaires. Dyspnoea intensity and unpleasantness as well as leg discomfort were rated at rest, every 2 min throughout exercise and at peak exercise using the modified 0–10 category ratio Borg scale [24]. Dyspnoea intensity and unpleasantness were described to participants using a standardised script frequently used in dyspnoea research [25]. Individuals verbalised their main reasons for stopping exercise and, if applicable, described the proportion to which dyspnoea or leg discomfort contributed to exercise limitation. Qualitative descriptors of respiratory discomfort were collected by questionnaire (supplementary table 1) [26]. Dyspnoea/ventilation (VʹE) and dyspnoea unpleasantness/VʹE slopes were calculated using linear regression from rest to peak exercise excluding zero values.
Validated questionnaires were used to assess: dyspnoea using the Dyspnoea-12 and mMRC [27, 28], stress using the Perceived Stress Scale [29], anxiety sensitivity using the Anxiety Sensitivity Index-Revised [30], symptoms associated with post-traumatic stress disorder (PTSD) using the Impact of Event Scale – Revised [31], anxiety and depression using the Hospital Anxiety and Depression scale [32], and quality of life using the St George's Respiratory Questionnaire [33].
Statistical analysis
Independent t-tests with Bonferroni correction for multiple comparisons at a priori defined exercise work rates were used to make between-group comparisons in exercise responses. Independent t-tests and Fisher's exact tests were used to compare post-COVID-19 dyspnoea and nondyspnoea groups for continuous and categorical variables, respectively. Spearman's correlation was used to characterise bivariate relationships between dyspnoea intensity and unpleasantness at HEWR with mental health symptom scores and physiologic variables. Best subset multivariable linear regression was used to explore associations between variables of interest and dyspnoea intensity and unpleasantness measured at HEWR using the 0–10 category ratio Borg scale. Statistical significance was defined as p<0.05. Data are presented as mean±standard deviation unless otherwise indicated. Statistical analyses were performed in SPSS (SPSS v27, IBM) and RStudio.
Results
Study participants
Recruitment for the LEFT study took place between June and October 2020 as previously described [12]. A total of 62 participants with complete CPET data were available for sub-study inclusion. After excluding participants with pre-existing cardiopulmonary disease or current malignancy, a total of 49 participants were included in the study and final analysis. There were 26 participants in the dyspnoea group and 23 participants in the nondyspnoea group. There was no age difference between included and excluded participants, although more males were excluded based on exclusion criteria. Included participants were diagnosed with COVID-19 between March to June 2020. Participants performed CPET 125±18 days following acute COVID-19 diagnosis. Participant demographic, anthropomorphic, COVID-19 illness and questionnaire results are summarised in table 1.
TABLE 1.
Participant demographic, anthropomorphic, acute coronavirus disease 2019 (COVID-19) and pulmonary function characteristics comparing those with and without persistent dyspnoea
| Variable | Dyspnoea group | Nondyspnoea group |
| Demographic and anthropomorphic | ||
| Participants, n | 26 | 23 |
| Age, years | 50±15 | 46±14 |
| Sex, male:female | 12:14 | 14:9 |
| BMI, kg·m−2 | 29±7 | 28±6 |
| Ever-smoker, n (%) | 7 (27) | 5 (22) |
| Smoking, pack-years | 10±12 | 4±3 |
| Comorbidities: | ||
| Hypertension, n (%) | 6 (23) | 3 (13) |
| Atrial fibrillation, n (%) | 2 (8) | 2 (9) |
| Coronary artery disease, n (%) | 1 (4) | 1 (4) |
| Diabetes mellitus, n (%) | 4 (15) | 3 (13) |
| Dyslipidaemia, n (%) | 3 (12) | 4 (17) |
| Hypothyroidism, n (%) | 1 (4) | 1 (4) |
| Arthritis, n (%) | 1 (4) | 2 (9) |
| OSA, n (%) | 0 (0) | 3 (13) |
| GERD, n (%) | 3 (12) | 0 (0) |
| Acute COVID-19 illness | ||
| COVID-19 severity: | ||
| Outpatient#, n (%) | 15 (58) | 17 (74) |
| Hospitalised, n (%) | 11 (42) | 6 (26) |
| Hospitalised without supplemental oxygen, n | 1 | 1 |
| Supplemental oxygen in hospital, n | 8 | 2 |
| Invasive ventilation, n | 2 | 3 |
| Duration of hospitalisation, days | 7±14 | 6±15 |
| Intensive care unit stay, n (%) | 4 (15) | 4 (17) |
| Duration of intensive care unit stay, days | 12±5 | 19±18 |
| Inpatient rehabilitation¶, n (%) | 3 (12) | 2 (9) |
| Symptoms and quality of life | ||
| mMRC, 0–4 scale | 1±1*** | 0±0 |
| D-12 total | 10.7±9.3*** | 0.5±1.6 |
| D-12 physical | 7.6±5.8*** | 0.5±1.5 |
| D-12 affective | 3.1±3.8*** | 0.0±0.2 |
| HADS total | 15.9±8.2* | 8.3±7.1 |
| HADS anxiety | 9.4±4.9* | 6.0±4.6 |
| HADS depression | 6.5±4.3*** | 2.3±2.8 |
| SGRQ total | 32.3±19.3*** | 8.6±8.7 |
| SGRQ symptom | 40.2±24.9*** | 14.5±14.5 |
| SGRQ activity | 53.7±26.3*** | 15.4±17.9 |
| SGRQ impact | 22.3±19.7*** | 2.8±5.5 |
| PSS, score out of 40 | 18.6±6.5* | 13.4±9.2 |
| ASI-R, score out of 144 | 44.7±36.0* | 21.6±20.3 |
| IES-R total, 0–4 scale | 1.5±1.1*** | 0.4±0.6 |
| IES-R intrusion, 0–4 scale | 1.6±1.2*** | 0.4±0.6 |
| IES-R avoidance, 0–4 scale | 1.5±1.0*** | 0.4±0.5 |
| IES-R hyperarousal, 0–4 scale | 1.4±1.2* | 0.4±0.8 |
| Pulmonary function | ||
| FEV1, % pred | 96±16 | 95±10 |
| FVC, % pred | 98±17 | 101±13 |
| FEV1/FVC | 0.79±0.06 | 0.76±0.05 |
| TLC, % pred | 90±16 | 95±12 |
| VC, % pred | 92±18 | 99±15 |
| IC, % pred | 92±23 | 94±17 |
| RV, % pred | 87±20 | 83±16 |
| DLCO, % pred | 87±18 | 92±18 |
| MIP, cmH2O | 76±29* | 95±32 |
| MEP, cmH2O | 81±27 | 100±41 |
Presented values are mean±standard deviation, unless otherwise specified. *p<0.05, ***p<0.001. ASI-R: revised Anxiety Sensitivity Index; BMI: body mass index; D-12: Dyspnoea-12 dyspnoea score; DLCO: diffusing capacity of the lungs for carbon monoxide; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; GERD: gastroesophageal reflux disease; HADS: Hospital Anxiety and Depression Scale; IC: inspiratory capacity; IES-R: Revised Impact of Event Scale; MEP: maximum expiratory mouth pressure; MIP: maximum inspiratory mouth pressure; mMRC: modified Medical Research Council dyspnoea score; OSA: obstructive sleep apnoea; PSS: Perceived Stress Scale; RV: residual volume; SGRQ: St. George Respiratory Questionnaire; TLC: total lung capacity; VC: vital capacity. #: Outpatient cases included participants that required no medical intervention or had an isolated visit to the emergency department without admission to hospital. ¶: Inpatient rehabilitation consisted of admission to a rehabilitation focused hospital while undergoing intensive physical and occupational therapy rehabilitation.
Most study participants self-identified as Caucasian. Both groups were overweight and the proportion of female and males was similar between groups (table 1). There were no current cigarette smokers in either group. The number of participants with a self-reported psychiatric diagnosis was similar between groups (3/26 dyspnoea group, 1/23 nondyspnoea group). Numbers of participants taking an anti-anxiety or antidepressant medication were not available. Frequencies of common medical comorbidities were similar (table 1). Many people in both groups were treated as outpatients. There was no difference in the number of participants that received therapeutic anticoagulation during their hospitalisation between groups (12% dyspnoea group versus 17% nondyspnoea group, p=0.69). Only three participants in each group received glucocorticoid steroid.
Resting pulmonary function tests
Pulmonary function test results are presented in table 1. One participant in the nondyspnoea group had a forced expiratory volume in 1 s/forced vital capacity (FEV1/FVC) below the lower limit of normal (LLN). Total lung capacity (TLC) was below the LLN in 27% of the dyspnoea participants and 9% of the nondyspnoea participants (p=0.15). People in each group had DLCO below the LLN (32% dyspnoea group versus 22% nondyspnoea group, p=0.52). The proportion of participants with MIP below thresholds associated with higher likelihood of respiratory muscle weakness [34] was greater in the dyspnoea group (19% dyspnoea group versus 9% nondyspnoea group), although this was not significant (p=0.42).
Symptom evaluation
Dyspnoea
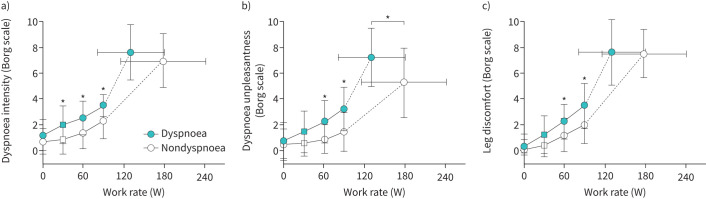
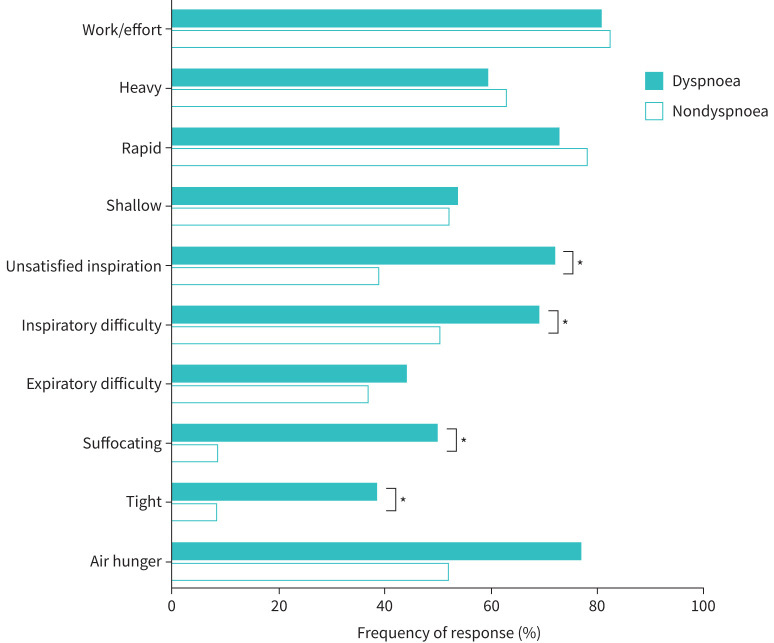
During CPET, submaximal dyspnoea intensity (figure 1a) and unpleasantness (figure 1b) were higher in the dyspnoea group. Dyspnoea unpleasantness was higher at peak exercise in the dyspnoea group, at peak VʹO2 37% lower than that achieved by the nondyspnoea group. Submaximal leg discomfort was also elevated in the dyspnoea group (figure 1c). The dyspnoea intensity/VʹE slope tended to be higher in the dyspnoea group (0.16±0.16 versus 0.10±0.05 Borg 0–10/L·min−1) but this did not reach statistical significance (p=0.11). The dyspnoea unpleasantness/VʹE slope was greater in the dyspnoea group (0.17±0.16 versus 0.09±0.07 Borg 0–10/L·min−1, p=0.03). Qualitative dyspnoea descriptive clusters were different between the dyspnoea and nondyspnoea groups (figure 2), with unsatisfied inspiration, inspiratory difficulty, suffocating and tightness sensations more frequently described in the dyspnoea group.
FIGURE 1.
Perceptual responses throughout exercise comparing dyspnoea and nondyspnoea groups. Values are mean±standard deviation. *: p<0.05 difference between groups following Bonferroni correction for multiple comparisons. Square symbols represent highest equivalent work rate. Submaximal exercise responses are presented up to 90 W achieved by 73% of dyspnoea group and 91% of nondyspnoea group participants.
FIGURE 2.
Selection of qualitative dyspnoea descriptors at peak exercise comparing dyspnoea and nondyspnoea groups. *: p<0.05.
Mental health associated symptoms and quality of life
Participants with persistent dyspnoea had higher anxiety and depression scores and lower quality of life in comparison to their counterparts without dyspnoea (table 1). The dyspnoea group exhibited higher levels of perceived stress and experienced greater panic-like anxiety in comparison to those without dyspnoea and reported reference values [29, 30, 35]. Participants with dyspnoea additionally experienced symptoms of PTSD similar to those reported in people recovering from motor vehicle accidents [31].
Exercise responses
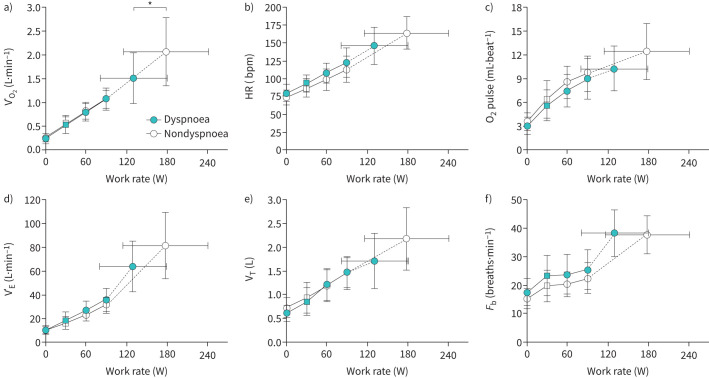
All participants met at least one criterion for a maximal CPET [22]. Peak exercise and HEWR results are presented in table 2. Peak VʹO2 (figure 3a) and work rate were lower in the dyspnoea group. Dyspnoea and leg fatigue contributed to exercise limitation in both groups. Dyspnoea was rated as contributing to exercise limitation to a greater extent in the dyspnoea group (51±35% dyspnoea group versus 39±33% nondyspnoea group), although it was not statistically significant (p=0.26).
TABLE 2.
Select variables at highest equivalent work rate (HEWR) (30 W) and peak exercise measured during incremental cardiopulmonary exercise test comparing coronavirus disease survivors with and without persistent dyspnoea
| Variable | HEWR | Peak exercise | ||
| Dyspnoea group | Nondyspnoea group | Dyspnoea group | Nondyspnoea group | |
| VʹO2, L·min−1 | 0.52±0.18 | 0.54±0.19 | 1.51±0.53* | 2.07±0.71 |
| VʹO2, % pred | 25±10 | 24±9 | 71±22* | 86±16 |
| VʹO2, mL·kg−1·min−1 | 6.5±2.5 | 6.5±2.1 | 18.5±5.9* | 24.7±7.4 |
| Work rate, W | 30±0 | 30±0 | 130±49* | 179±63 |
| Work rate, % pred | 20±5 | 18±5 | 85±29 | 102±19 |
| VʹE, L·min−1 | 18.5±6.6 | 16.7±5.0 | 63.9±21.3 | 81.3±27.7 |
| VʹE/MVC, % | 19±9 | 15±6 | 60±14 | 67±14 |
| VT, L | 0.86±0.29 | 0.93±0.32 | 1.71±0.57 | 2.18±0.66 |
| Fb, breath·min−1 | 23±7 | 20±6 | 38±8 | 38±7 |
| RER | 0.91±0.10 | 0.86±0.09 | 1.25±0.10 | 1.24±0.08 |
| PETCO2, mmHg | 37±3* | 39±2 | 35±5 | 36±3 |
| SpO2, % | 97±1 | 97±1 | 97±2 | 97±1 |
| VD/VT | 0.27±0.06 | 0.26±0.06 | 0.17±0.06* | 0.13±0.04 |
| IC, L | 2.81±0.75 | 3.14±0.73 | 2.75±0.81 | 3.18±0.93 |
| IC, % pred | 85±23 | 88±14 | 86±20 | 88±18 |
| IRV, L | 1.95±0.72 | 2.21±0.67 | 1.04±0.53 | 1.01±0.51 |
| EILV/TLC, % | 64±11 | 65±7 | 81±8 | 84±8 |
| EELV/TLC, % | 48±10 | 49±6 | 49±9 | 49±7 |
| HR, bpm | 93±12 | 87±12 | 146±26 | 164±23 |
| HRR, bpm | 24±20* | 10±14 | ||
| HR, % pred | 58±10 | 52±9 | 90±13 | 98±9 |
| O2 pulse, mL·beat−1 | 5.6±1.9 | 6.4±2.3 | 10.3±2.8 | 12.5±3.6 |
| O2 pulse, % pred | 41±15 | 44±16 | 74±17 | 83±16 |
| SBP, mmHg | 164±18 | 174±21 | ||
| DBP, mmHg | 85±4 | 86±6 | ||
| Dyspnoea intensity, 0–10 Borg scale | 2.0±1.4* | 0.9±1.1 | 7.6±2.1 | 7.0±2.1 |
| Dyspnoea unpleasantness, 0–10 Borg scale | 1.5±1.6 | 0.6±1.0 | 7.3±2.3* | 5.3±2.7 |
| Leg fatigue, 0–10 Borg scale | 1.3±1.5 | 0.4±0.8 | 7.6±2.5 | 7.5±1.9 |
Presented values are mean±standard deviation, unless otherwise specified. *: p<0.05 difference between groups following Bonferroni correction at the respective exercise intensity (HEWR or peak exercise).
DBP: diastolic blood pressure; EELV: end-expiratory lung volume; EILV: end-inspiratory lung volume; Fb: breathing frequency; HR: heart rate; HRR: heart rate reserve; IC: inspiratory capacity; IRV: inspiratory reserve volume; MVC: maximum ventilatory capacity (forced expiratory volume in 1 s×35); PETCO2: partial pressure of end-tidal carbon dioxide; RER: respiratory exchange ratio; SBP: systolic blood pressure; SpO2: peripheral oxygen saturation measured by finger pulse oximetry; TLC: total lung capacity; VD/VT: noninvasive estimation of dead space tidal volume ratio; VʹE: minute ventilation; VʹO2: oxygen uptake; VT: tidal volume.
FIGURE 3.
Cardiopulmonary exercise test results comparing cardiovascular and ventilatory responses between dyspnoea and nondyspnoea groups. Values are mean±standard deviation. *: p<0.05 difference between groups following Bonferroni correction for multiple comparisons. Square symbols represent highest equivalent work rate. Submaximal exercise responses are presented up to 90 W achieved by 73% of dyspnoea group and 91% of nondyspnoea group participants. bpm: beats per minute; Fb: breathing frequency; HR: heart rate; VʹO2: oxygen uptake; VʹE: minute ventilation; VT: tidal volume.
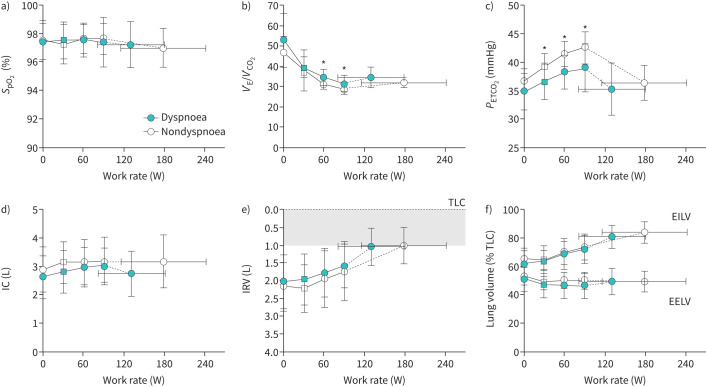
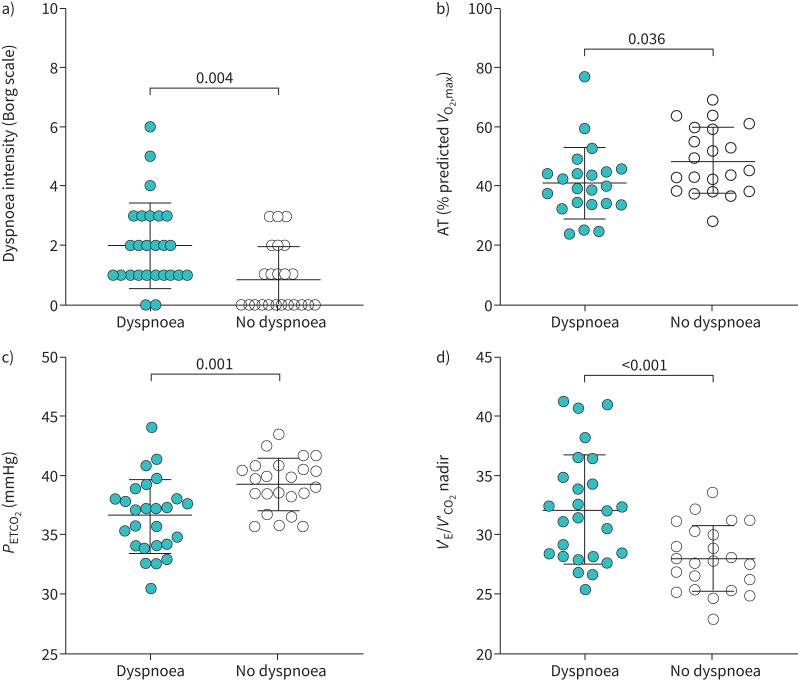
The dyspnoea group had evidence of abnormal pulmonary gas exchange during exercise with ventilatory inefficiency (higher VʹE/VʹCO2) and lower PETCO2 during exercise (figure 4b and c, figure 5c and d). At HEWR, dyspnoea intensity was higher in the dyspnoea group (figure 5a). VʹE/VʹCO2 nadir was higher in the dyspnoea group (figure 5d), which occurred in the absence of significant desaturation (figure 4a) during exercise. There was no significant difference between groups in the frequency of possible dysfunctional breathing; however, there were more possible cases in the dyspnoea group (38% dyspnoea group versus 22% nondyspnoea group, p=0.23).
FIGURE 4.
Cardiopulmonary exercise test results comparing gas exchange and noninvasive respiratory mechanical responses between dyspnoea and nondyspnoea groups. Values are mean±standard deviation. *: p<0.05 difference between groups following Bonferroni correction for multiple comparisons. Square symbols represent highest equivalent work rate (HEWR). Submaximal exercise responses are presented up to 90 W achieved by 73% of dyspnoea group and 91% of nondyspnoea group participants. EELV: end-expiratory lung volume; EILV: end-inspiratory lung volume; IC: inspiratory capacity; IRV: inspiratory reserve volume; PETCO2: partial pressure of end-tidal carbon dioxide; SpO2: oxygen saturation measured by finger pulse oximetry; TLC: total lung capacity; VʹE/VʹCO2: ventilatory equivalent for carbon dioxide.
FIGURE 5.
Gas-exchange, metabolic and dyspnoea intensity responses for individual participants with and without dyspnoea. Bars represent mean±standard deviation. Dyspnoea intensity and PETCO2 values are presented at highest equivalent work rate. AT: anaerobic threshold; PETCO2: partial pressure of end-tidal carbon dioxide; VʹE/VʹCO2: ventilatory equivalent for carbon dioxide; VʹO2max: maximum oxygen uptake.
There were no overall differences in ventilation or breathing pattern (figure 3d–f). Despite a greater proportion of individuals in the dyspnoea group with TLC<LLN, there was no evidence of abnormal dynamic respiratory mechanics or development of critical respiratory constraint throughout (figure 4d–f) or at peak exercise (table 2). Cardiovascular responses were similar (figure 3b and c, table 2) between groups, although O2 pulse tended to be lower and heart rate reserve was greater in the dyspnoea group (table 2). The AT occurred at a lower percentage of predicted maximal oxygen uptake in the dyspnoea group (figure 5b).
Correlates and predictors of dyspnoea
When all participants were pooled together, dyspnoea unpleasantness at HEWR (r=−0.370, p=0.010), but not intensity (r=−0.223, p=0.128), was correlated with lower DLCO. Both dyspnoea intensity (r=−0.346, p=0.015) and unpleasantness (r=−0.366, p<0.001) were associated with ventilatory inefficiency. Dyspnoea unpleasantness was additionally associated with a lower anaerobic threshold (r=−0.162, p=0.007). Both dyspnoea intensity and unpleasantness were associated with a greater burden of mental health symptoms.
Best subset multivariable linear regression models were constructed for dyspnoea intensity and unpleasantness (supplementary table 2). Physiologic, mental health and quality of life variables were included in the model. Sex, age, smoking pack years and body mass index were included in the model as potential confounders. The model did not predict dyspnoea intensity, adjusted R2=0.357, F(13,8)=1.897, p=0.184, despite VʹE/VʹCO2 nadir and perceived stress being related to dyspnoea intensity. Although VʹE/VʹCO2 nadir was related to dyspnoea unpleasantness (supplementary table 2), the overall model did not significantly predict dyspnoea unpleasantness, adjusted R2=0.463, F(13,8)=2.394, p=0.110.
Discussion
To our knowledge, this is the first study to simultaneously examine differences in both the intensity and qualitative dimensions of exertional dyspnoea, burden of mental health symptoms and exercise responses in people with and without persistent breathlessness following COVID-19. Participants with persistent dyspnoea had a higher burden of mental health symptoms related to anxiety, depression and PTSD, and lower quality of life compared to their counterparts without dyspnoea. Importantly, dyspnoea quality in this group was characterised by increased unpleasantness and sensations of suffocating and tightness. Participants with persistent dyspnoea demonstrated reduced peak exercise VʹO2 and work rate, abnormalities of gas exchange with increased ventilatory inefficiency, as well as a lower AT and increased heart rate reserve in comparison to their counterparts without dyspnoea. Altered exercise responses were uncovered during CPET in these people with preserved resting lung function. Collectively, our results demonstrate that persistent dyspnoea post-COVID-19 is related to abnormal pulmonary gas exchange and deconditioning, and is associated with symptoms of anxiety, depression and PTSD.
We demonstrate that dyspnoea unpleasantness is increased and not explained as a function of relatively greater ventilation during exercise in people with chronic breathlessness and significant mental health symptoms. Dyspnoea was described as suffocating in over 50% and tightness in approximately 40% of participants in the dyspnoea group. These are not common descriptors of breathlessness at peak exercise in health or other chronic respiratory diseases such as chronic obstructive pulmonary disease or interstitial lung disease where dyspnoea is more frequently described as inspiratory difficulty and unsatisfied inspiration [26, 36, 37]. Although not significant, there were more participants that met criteria for possible dysfunctional breathing in the dyspnoea group. The interaction between afferent signals of impaired pulmonary gas exchange, breathing pattern and increased mental health symptoms in the integrated experience of dyspnoea is not completely understood. Our novel results demonstrate that the dyspnoea qualities in COVID-19 survivors are different from those in some chronic respiratory diseases and underscore the importance of a multidimensional assessment that considers both dyspnoea intensity, unpleasantness and quality.
A minority of studies have examined exercise responses in COVID-19 survivors and fewer still have included measurement of exertional dyspnoea during CPET. Baratto et al. [38] demonstrated that COVID-19 survivors had reduced VʹO2 peak, impaired peripheral oxygen extraction and ventilatory inefficiency. Ventilatory inefficiency was attributed by the authors to altered chemosensitivity in most subjects with a minority having increased dead space [38]. Increased VʹE/VʹCO2 has been described in people hospitalised for COVID-19 with dyspnoea 3–6 months following acute illness, consistent with our results [6, 8]. Operating lung volumes during CPET showed no evidence of significant respiratory mechanical constraints in another study [38]. This is also demonstrated by our participants studied at 4 months into recovery, despite reduced TLC in a proportion of participants.
Both dyspnoea intensity and unpleasantness were correlated with ventilatory inefficiency on bivariate analysis. Ventilatory inefficiency may be related to either increased physiological dead space or increased chemosensitivity and alveolar hyperventilation, alone or in variable combination [39]. Hyperventilation during exercise in COVID-19 survivors has been variably defined in available studies to date [9], with a paucity of studies employing arterial blood gas measurements [4, 7, 11, 38]. A study by Singh et al. [11] using invasive CPET in 10 people recovering from mild acute COVID-19 illness with persistent symptoms at 11 months found that an increased VʹE/VʹCO2 slope during exercise was not associated with increased physiological dead space and was related to hyperventilation and increased chemosensitivity. Hyperventilation as a consequence of an alteration in the arterial carbon dioxide set point and increased chemosensitivity may occur in the setting of altered pH balance, hypoxemia, stimulation of baroreceptors within the pulmonary vasculature or autonomic nervous system activation [39]. Hyperventilation has additionally been described in people with dysfunctional breathing syndromes associated with anxiety [40]. We did not employ arterial blood gas measurements, but the association of increased dyspnoea intensity and unpleasantness with greater burden of mental health symptoms raises the possibility that multiple mechanisms may account for increased VʹE/VʹCO2 in post-COVID syndrome, including dysfunctional breathing syndromes.
Although we present unique data incorporating dyspnoea intensity and quality with exercise responses in COVID-19 survivors, our study has some limitations. Assessment of mental health symptoms and physical activity prior to COVID-19 infection was not possible. We also did not have access to a matched healthy control population. As previously mentioned, we did not employ arterial blood gas measurements and conclusions regarding the mechanisms of increased VʹE/VʹCO2 in our participants are therefore limited. Results of chest imaging were not available as a part of this study. Our moderate sample size limits further exploring heterogeneous physiological mechanisms contributing to dyspnoea between sub-groups (i.e. people with lower MIP, TLC<LLN or suspected hyperventilation). Larger studies using CPET in addition to chest imaging will be needed to fully characterise phenotypes within post-COVID syndrome.
We describe unique insights into persistent dyspnoea after acute COVID-19 illness in people with and without chronic breathlessness. Our work highlights the increased burden of mental health symptoms in COVID-19 survivors with persistent dyspnoea and associated dyspnoea qualities and unpleasantness experienced by these participants. People with dyspnoea and normal resting lung function exhibit gas-exchange abnormalities and reduced AT during CPET. Ventilatory inefficiency was more frequently observed in the dyspnoea group in our study and the relationship between ventilatory inefficiency and dyspnoea, as well as its multiple potential causes, requires further detailed physiologic study. Comprehensive care of the COVID-19 survivor will undoubtedly require attention and targeted interventions, which may include physiotherapy for dysfunctional breathing, pulmonary rehabilitation, occupational therapy and medical therapies focused on both abnormal physiology contributing to dyspnoea as well as the increased burden of mental health comorbidity.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00606-2022.SUPPLEMENT (90.5KB, pdf)
Acknowledgements
We thank our participants for enthusiastically volunteering for this study.
Provenance: Submitted article, peer reviewed.
Author contributions: K.M. Milne, S.J. Abdallah and J.A. Guenette conceived the study. J. Cowan, N. Voduc, V. Corrales-Medina, K.L. Lavoie, J.A. Chirinos and S.J. Abdallah participated in data collection. K.M. Milne performed statistical analysis and wrote the first draft of the manuscript. K.M. Milne and J.A. Guenette wrote the final draft of the manuscript. All authors contributed significantly to revision of the manuscript.
Support statement: The study was funded by the Ottawa Hospital COVID-19 Emergency Response Fund. K.M. Milne was supported by a Michael Smith Health Research BC Postdoctoral Fellowship and a Transition to Faculty Award from the Faculty of Medicine Academic Enhancement Fund at The University of British Columbia. Funding information for this article has been deposited with the Crossref Funder Registry.
Conflict of interest: K.L. Lavoie reports consulting fees from AbbVie, Takeda, Astellas, Boehringer Ingelheim, AstraZeneca, Janssen, Novartis, GSK, Bausch and Sojecci Inc., outside the submitted work; payment or honoraria from AbbVie, Boehringer Ingelheim, Takeda, Pfizer, Merck, GSK, Astra-Zeneca, Novartis, Janssen, Bayer, Mundi Pharma, Bayer, Air Liquide, Astellas and Xfacto, outside the submitted work; and participation on a Data Safety Monitoring Board or Advisory Board for Astra-Zeneca, GSK and Bausch, outside the submitted work.
Conflict of interest: J. Cowan reports support for the present manuscript from The Ottawa Hospital Foundation; grants or contracts from Octapharma and Takeda, outside the submitted work; payment or honoraria from GSK, Sanofi, EMD Serono, Alexion and Takeda, outside the submitted work; and support for attending meetings and/or travel from Octapharma, outside the submitted work.
Conflict of interest: J.A. Chirinos reports grants or contracts from University of Pennsylvania research grants from National Institutes of Health, Fukuda-Denshi, Bristol-Myers Squibb, Microsoft and Abbott, outside the submitted work; consulting fees from Bayer, Sanifit, Fukuda-Denshi, Bristol-Myers Squibb, JNJ, Edwards Life Sciences, Merck, NGM Biopharmaceuticals and the Galway-Mayo Institute of Technology, outside the submitted work; patents planned, issued or pending: inventor in a University of Pennsylvania patent for the use of inorganic nitrates/nitrites for the treatment of Heart Failure and Preserved Ejection Fraction and for the use of biomarkers in heart failure with preserved ejection fraction, outside the submitted work; participant on advisory board for BMS, outside the submitted work; Vice President of North American Artery Society, outside the submitted work; received research device loans from Atcor Medical, Fukuda-Denshi, Uscom, NDD Medical Technologies, Microsoft and MicroVision Medical, outside the submitted work; received payments for editorial roles from the American Heart Association, the American College of Cardiology and Wiley, outside the submitted work.
Conflict of interest: J.A. Guenette is an associate editor of this journal.
Conflict of interest: The remaining authors have nothing to disclose.
References
- 1.World Health Organization . Post COVID-19 condition (long COVID). Date last accessed: 13 January 2023. Date last updated: 7 December 2022. www.who.int/europe/news-room/fact-sheets/item/post-covid-19-condition
- 2.Iqbal FM, Lam K, Sounderajah V, et al. Characteristics and predictors of acute and chronic post-COVID syndrome: a systematic review and meta-analysis. EClinicalMedicine 2021; 36: 100899. doi: 10.1016/j.eclinm.2021.100899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evans RA, McAuley H, Harrison EM, et al. Physical, cognitive, and mental health impacts of COVID-19 after hospitalisation (PHOSP-COVID): a UK multicentre, prospective cohort study. Lancet Respir Med 2021; 9: 1275–1287. doi: 10.1016/S2213-2600(21)00383-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Motiejunaite J, Balagny P, Arnoult F, et al. Hyperventilation: a possible explanation for long-lasting exercise intolerance in mild COVID-19 survivors? Front Physiol 2020; 11: 614590. doi: 10.3389/fphys.2020.614590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rinaldo RF, Mondoni M, Parazzini EM, et al. Deconditioning as main mechanism of impaired exercise response in COVID-19 survivors. Eur Respir J 2021; 58: 2100870. doi: 10.1183/13993003.00870-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skjorten I, Ankerstjerne OAW, Trebinjac D, et al. Cardiopulmonary exercise capacity and limitations 3 months after COVID-19 hospitalisation. Eur Respir J 2021; 58: 2100996. doi: 10.1183/13993003.00996-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Debeaumont D, Boujibar F, Ferrand-Devouge E, et al. Cardiopulmonary exercise testing to assess persistent symptoms at 6 months in people with COVID-19 who survived hospitalization: a pilot study. Phys Ther 2021; 101: pzab099. doi: 10.1093/ptj/pzab099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aparisi A, Ybarra-Falcon C, Garcia-Gomez M, et al. Exercise ventilatory inefficiency in post-COVID-19 syndrome: insights from a prospective evaluation. J Clin Med 2021; 10: 2591. doi: 10.3390/jcm10122591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Motiejunaite J, Balagny P, Arnoult F, et al. Hyperventilation as one of the mechanisms of persistent dyspnoea in SARS-CoV-2 survivors. Eur Respir J 2021; 58: 2101578. doi: 10.1183/13993003.01578-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu M, Lv F, Huang Y, et al. Follow-up study of the chest CT characteristics of COVID-19 survivors seven months after recovery. Front Med 2021; 8: 636298. doi: 10.3389/fmed.2021.636298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh I, Joseph P, Heerdt PM, et al. Persistent exertional intolerance after COVID-19: insights from invasive cardiopulmonary exercise testing. Chest 2022; 161: 54–63. doi: 10.1016/j.chest.2021.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdallah SJ, Voduc N, Corrales-Medina VF, et al. Symptoms, pulmonary function, and functional capacity four months after COVID-19. Ann Am Thorac Soc 2021; 18: 1912–1917. doi: 10.1513/AnnalsATS.202012-1489RL [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graham BL, Brusasco V, Burgos F, et al. 2017 ERS/ATS standards for single-breath carbon monoxide uptake in the lung. Eur Respir J 2017; 49: 1600016. doi: 10.1183/13993003.00016-2016 [DOI] [PubMed] [Google Scholar]
- 14.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005; 26: 319–338. doi: 10.1183/09031936.05.00034805 [DOI] [PubMed] [Google Scholar]
- 15.Wanger J, Clausen JL, Coates A, et al. Standardisation of the measurement of lung volumes. Eur Respir J 2005; 26: 511–522. doi: 10.1183/09031936.05.00035005 [DOI] [PubMed] [Google Scholar]
- 16.Laveneziana P, Albuquerque A, Aliverti A, et al. ERS statement on respiratory muscle testing at rest and during exercise. Eur Respir J 2019; 53: 1801214. doi: 10.1183/13993003.01214-2018 [DOI] [PubMed] [Google Scholar]
- 17.Gutierrez C, Ghezzo RH, Abboud RT, et al. Reference values of pulmonary function tests for Canadian Caucasians. Can Respir J 2004; 11: 414–424. doi: 10.1155/2004/857476 [DOI] [PubMed] [Google Scholar]
- 18.Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J 2012; 40: 1324–1343. doi: 10.1183/09031936.00080312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stanojevic S, Graham BL, Cooper BG, et al. Official ERS technical standards: Global Lung Function Initiative reference values for the carbon monoxide transfer factor for Caucasians. Eur Respir J 2017; 50: 1700010. doi: 10.1183/13993003.00010-2017 [DOI] [PubMed] [Google Scholar]
- 20.American Thoracic Society , American College of Chest Physicians . ATS/ACCP statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med 2003; 167: 211–277. doi: 10.1164/rccm.167.2.211 [DOI] [PubMed] [Google Scholar]
- 21.Lewthwaite H, Benedetti A, Stickland MK, et al. Normative peak cardiopulmonary exercise rest responses in Canadian adults aged ≥40 years. Chest 2020; 158: 2532–2545. doi: 10.1016/j.chest.2020.06.074 [DOI] [PubMed] [Google Scholar]
- 22.Radtke T, Crook S, Kaltsakas G, et al. ERS statement on standardisation of cardiopulmonary exercise testing in chronic lung diseases. Eur Respir Rev 2019; 28: 180101. doi: 10.1183/16000617.0101-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watson M, Ionescu MF, Sylvester K, et al. Minute ventilation/carbon dioxide production in patients with dysfunctional breathing. Eur Respir Rev 2021; 30: 200182. doi: 10.1183/16000617.0182-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 1982; 14: 377–381. [PubMed] [Google Scholar]
- 25.Banzett RB, Pedersen SH, Schwartzstein RM, et al. The affective dimension of laboratory dyspnea: air hunger is more unpleasant than work/effort. Am J Respir Crit Care Med 2008; 177: 1384–1390. doi: 10.1164/rccm.200711-1675OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simon PM, Schwartzstein RM, Weiss JW, et al. Distinguishable types of dyspnea in patients with shortness of breath. Am Rev Respir Dis 1990; 142: 1009–1014. doi: 10.1164/ajrccm/142.5.1009 [DOI] [PubMed] [Google Scholar]
- 27.Fletcher CM. Standardised questionnaire on respiratory symptoms: a statement prepared and approved by the MRC Committee on the Aetiology of Chronic Bronchitis (MRC breathlessness score). BMJ 1960; 2: 1662. [Google Scholar]
- 28.Yorke J, Moosavi SH, Shuldham C, et al. Quantification of dyspnoea using descriptors: development and initial testing of the Dyspnoea-12. Thorax 2010; 65: 21–26. doi: 10.1136/thx.2009.118521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav 1983; 24: 385–396. doi: 10.2307/2136404 [DOI] [PubMed] [Google Scholar]
- 30.Taylor S, Cox BJ. An expanded anxiety sensitivity index: evidence for a hierarchic structure in a clinical sample. J Anxiety Disord 1998; 12: 463–483. doi: 10.1016/S0887-6185(98)00028-0 [DOI] [PubMed] [Google Scholar]
- 31.Weiss D, CR M. The impact of event scale – revised. In: Wilson, JP, Keane, TM, eds. Assessing Psychological Trauma and PTSD. New York, Guilford Press, 1997. [Google Scholar]
- 32.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983; 67: 361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x [DOI] [PubMed] [Google Scholar]
- 33.Jones PW, Quirk FH, Baveystock CM. The St George's respiratory questionnaire. Respir Med 1991; 85: Suppl. B, 25–31; discussion 33–27. doi: 10.1016/S0954-6111(06)80166-6 [DOI] [PubMed] [Google Scholar]
- 34.Rodrigues A, Da Silva ML, Berton DC, et al. Maximal inspiratory pressure: does the choice of reference values actually matter? Chest 2017; 152: 32–39. doi: 10.1016/j.chest.2016.11.045 [DOI] [PubMed] [Google Scholar]
- 35.Deacon BJ, Abramowitz JS, Woods CM, et al. The Anxiety Sensitivity Index – Revised: psychometric properties and factor structure in two nonclinical samples. Behav Res Ther 2003; 41: 1427–1449. doi: 10.1016/S0005-7967(03)00065-2 [DOI] [PubMed] [Google Scholar]
- 36.O'Donnell DE, Bertley JC, Chau LK, et al. Qualitative aspects of exertional breathlessness in chronic airflow limitation: pathophysiologic mechanisms. Am J Respir Crit Care Med 1997; 155: 109–115. doi: 10.1164/ajrccm.155.1.9001298 [DOI] [PubMed] [Google Scholar]
- 37.O'Donnell DE, Chau LK, Webb KA. Qualitative aspects of exertional dyspnea in patients with interstitial lung disease. J Appl Physiol 1998; 84: 2000–2009. doi: 10.1152/jappl.1998.84.6.2000 [DOI] [PubMed] [Google Scholar]
- 38.Baratto C, Caravita S, Faini A, et al. Impact of COVID-19 on exercise pathophysiology: a combined cardiopulmonary and echocardiographic exercise study. J Appl Physiol 2021; 130: 1470–1478. doi: 10.1152/japplphysiol.00710.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Whipp BJ, Ward SA, Wasserman K. Ventilatory responses to exercise and their control in man. Am Rev Respir Dis 1984; 129: S17–S20. doi: 10.1164/arrd.1984.129.2P2.S17 [DOI] [PubMed] [Google Scholar]
- 40.Boulding R, Stacey R, Niven R, et al. Dysfunctional breathing: a review of the literature and proposal for classification. Eur Respir Rev 2016; 25: 287–294. doi: 10.1183/16000617.0088-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00606-2022.SUPPLEMENT (90.5KB, pdf)