Abstract
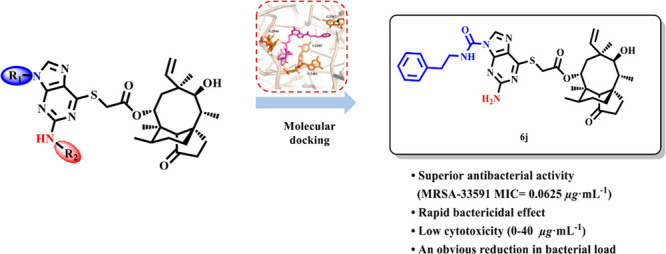
Antibiotic overuse has caused the increasingly serious problem of bacterial drug resistance, with numerous marketed antibiotics exhibiting significantly reduced activity against drug-resistant bacteria. Therefore, there is urgent demand for the development of novel antibiotics. Pleuromutilin is a tricyclic diterpene exhibiting antibacterial activity against Gram-positive bacteria and is currently considered the most promising natural antibiotic. In this study, novel pleuromutilin derivatives were designed and synthesized by introducing thioguanine units, and their antibacterial activities against drug-resistant strains were evaluated in vitro and in vivo. Compound 6j was observed to have a rapid bactericidal effect, low cytotoxicity, and potent antibacterial activity. The in vitro results suggest that 6j has a significant therapeutic effect on local infections, and its activity is equal to that of retapamulin, an anti-Staphylococcus aureus pleuromutilin derivative.
Keywords: Pleuromutilin analogues, Thioguanine, Synthesis, In vitro and in vivo assays, Structure-activity relationship studies
Staphylococcus aureus (S. aureus) is a widespread and highly infectious Gram-positive bacterium that causes various diseases such as soft tissue infections, pneumonia,1 septicemia,2 osteomyelitis,3 and mastitis.4 Antibiotics are important for preventing and treating bacterial infections, but due to long-term antibiotic overuse, bacterial resistance has become even more prevalent. In 2019, the number of deaths from drug-resistant infections reached 920 000 globally, including more than 100 000 deaths from methicillin-resistant S. aureus.5−8 These staggering numbers draw attention to the urgent need to develop new antibacterial drugs.
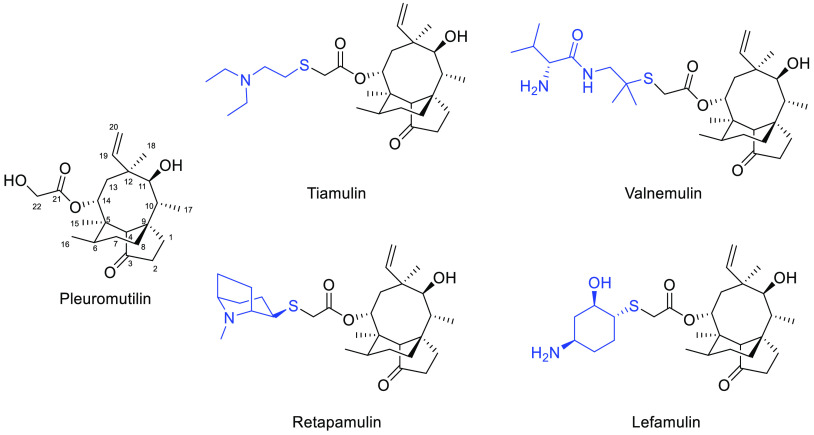
In 1951, pleuromutilin was isolated from Pleurotus mutilus and P. passeckerianus.9 Pleuromutilin and its derivatives inhibit bacterial protein synthesis, primarily by binding to the central part of domain V of the 50s subunit at the bacterial ribosome.10 Over 40 years have passed since a derivative of pleuromutilin, tiamulin,11 was first marketed (Figure 1). Subsequently, valnemulin12 was approved in 1999 for use in poultry as well, and in 2007, retapamulin13 was developed as the first pleuromutilin-derived drug for human topical use. In 2019, lefamulin14 was approved as a systemic drug for humans. According to the differences in the modification sites, semisynthetic pleuromutilin derivatives can be classified into parent ring modifications15−17 and C-14 side chain modifications.18−21 Possible locations for modifications of the parent ring include the tricyclic structure,22 C-2 methylene,15 C-3 carbonyl,23 C-8 methylene,17 C-13 methylene,24 and C-19 double bond.25 Disadvantages of parent ring modifications include the complexity of the reaction, difficulty of synthesis, and a decline in antibacterial activity. Due to these disadvantages, most researchers worldwide have shifted their attention to C-14 side chain modifications. Previous studies indicate that the introduction of thioether structures at the C-14 position is conducive to increasing the antimicrobial activity of compounds.26,27 Additionally, all four approved pleuromutilin drugs use thioether structures as linkages; thus, this linkage structure was used in our compound design. Furthermore, purines are key functional groups that are frequently used in drug modification because of their high heteroatom content and good water solubility. Existing research suggests that the presence of purine groups in the C-14 side chain of pleuromutilin derivatives can increase their antibacterial activity.28,29 Thus, a combination of thioether and purine (such as thioguanine units) can be introduced into the pleuromutilin skeleton to develop new drugs with potent antidrug-resistant bacterial activity. In this study, we synthesized plueromutilin derivatives by introducing thioguanine units and evaluated their antibacterial activities in vitro and in vivo against drug-resistant strains.
Figure 1.
Approved drugs with pleuromutilin.
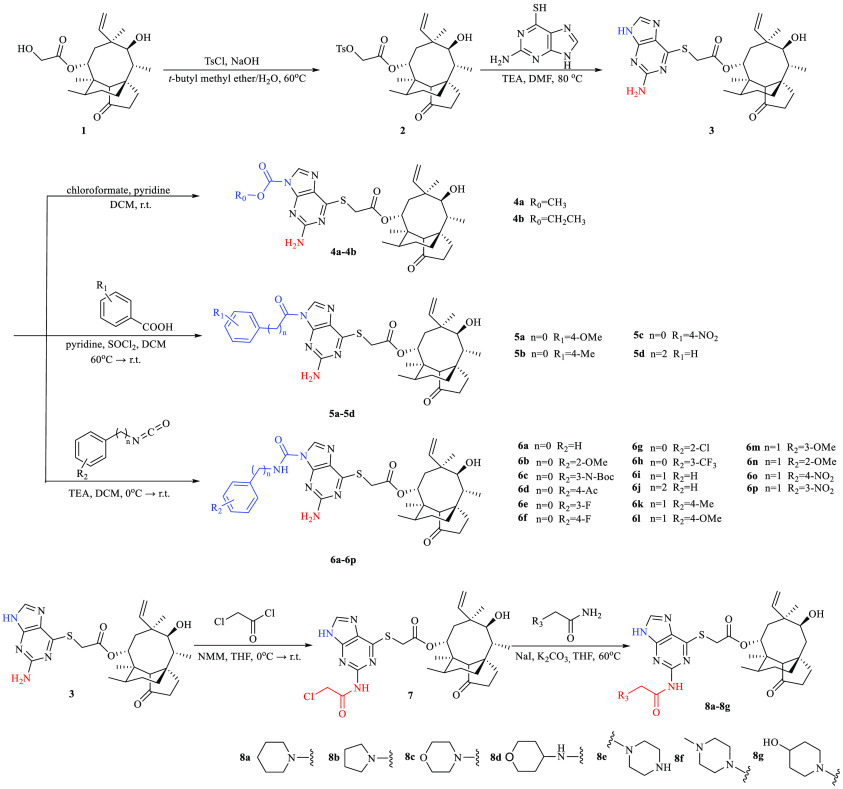
Thioguanine-modified pleuromutilin derivatives were synthesized via four strategies (Scheme 1). Hydroxyl at the C-22 position of pleuromutilin was activated with p-toluenesulfonyl chloride30 to obtain intermediate 2 via nucleophilic substitution. Intermediate 2 was then reacted with 6-thioguanine and triethylamine (TEA) in dimethylformamide (DMF) to form intermediate 3 in an 85% yield, which was then reacted with chloroformates and pyridine in dichloromethane (DCM) to form carbamate derivatives 4a–4b in 51–52% yields. Intermediate 3 was used to react with the benzoyl or acyl chloride derivatives (R = H, Me, OMe, and NO2) to synthesize amide derivatives 5a–5d in 45–60% yields. For the urea structure, intermediate 3 was reacted with the isocyanate derivatives (R = H, Me, and OMe) to synthesize urea derivatives 6a–6p in 43–62% yields. The crystal structures of 6b and 6i were obtained from a solution of ethyl acetate/petroleum ether by using the slow evaporation method at ambient temperature (Figures S32 and S33).
Scheme 1. Synthesis Route of Target Compounds.
Intermediate 3 was also reacted with chloroacetyl chloride and N-methylmorpholine in tetrahydrofuran (THF) via substitution to generate the amide intermediate 7, followed by a substitution reaction with K2CO3 in the presence of NaI in THF to give the secondary amine derivatives (8a–8g) in 40–50% yields.
The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) values are important indicators for determining antibacterial activity. In this study, the antibacterial activities of our synthesized compounds were evaluated against six Staphylococcus strains: S. aureus ATCC 25923, MSSA ATCC 29213, MSSE ATCC 12228, MRSA ATCC 33591, MRSA ATCC 43300, and MRSE ATCC 51625. Tiamulin, valnemulin, and retapamulin were used as control drugs. The results showed that most of the compounds exhibited potent antibacterial activity against the tested strains.
Among carbamate derivatives 4a–4b and amide derivatives 5a–5d, almost all compounds showed superior antibacterial activity to the approved drug tiamulin, with MIC values of 0.0625–1 μg·mL–1. Compound 5c, with an electron-withdrawing group (NO2), exhibited optimal antibacterial activity.
The antibacterial activities of urea derivatives 6a–6p were significantly enhanced and similar to or higher than those of the carbamate and amide derivatives (4a–4b and 5a–5d, respectively). This result confirms that the introduction of the urea group is conducive to increasing the antibacterial activity. Some compounds (e.g., 6d, 6i–6l, and 6p) exhibited significant antibacterial activity, with MIC values as low as 0.0625 μg·mL–1. Compared with 6a, 6i and 6j achieved lower MIC values, suggesting that the antibacterial activity of the compounds in which the urea group and benzene ring are linked by carbon atoms may be higher than that of the compounds in which they are directly linked. Thus, antibacterial activity can presumably be enhanced by introducing appropriate hydrophobic chains and extending the distance between the urea bond and the aryl group. A similar phenomenon was reported in a previous study.31 Substituents on the benzene ring also affected the antibacterial activity. Using methoxy substitution as an example, the para substitution derivative (6l) exhibited higher activity than ortho and meta substitution derivatives (6b and 6m, respectively), suggesting that the sites of substituents on the benzene ring also affect the antibacterial activity.
Compared to the other compounds, pleuromutilin derivatives 8a–8g, linked by a chloroacetyl group, exhibited reduced antibacterial activity. Among N5-substituted derivatives 8a–8g, 8a exhibited the optimal antibacterial activity with an MIC value of 0.125 μg·mL–1, whereas its activity was not significant compared with that of the N1-substituted derivatives. The activity of most of the N1-substituted derivatives was superior to that of the N5-substituted derivatives, suggesting that substitution at different sites on the purine ring significantly affects the activity of the derivatives.
The MBC values of the targets were examined to determine whether they were bacteriostatic or bactericidal. As shown in Table 1, most of the compounds exhibited MBC/MIC values higher than 4, indicating bacteriostatic effects, and individual compounds exhibited bactericidal effects at low concentrations. Notably, 6j showed bactericidal effects against MRSA ATCC 33591 at a concentration of 1 × MIC.
Table 1. MIC and MBC/MIC Valuesa.
| MIC
(μg·mL–1)/(MBC/MIC) |
||||||
|---|---|---|---|---|---|---|
| Cpd | MRSA ATCC 33591 | MRSA ATCC 43300 | MRSE ATCC 51625 | MSSA ATCC 29213 | MSSE ATCC 12228 | S. aureus ATCC 25923 |
| 3 | 0.125(4) | 0.125(>4) | 0.125(2) | 0.125(>4) | 0.125(>4) | 0.25(>4) |
| 4a | 0.125(>4) | 0.125(>4) | 0.125(>4) | 0.5(>4) | 0.0625(>4) | 0.125(>4) |
| 4b | 0.25(4) | 0.125(>4) | 0.25(>4) | 0.125(>4) | <0.0078(>4) | 0.25(4) |
| 5a | 0.5(>4) | 0.125(>4) | 0.25(4) | 0.5(>4) | <0.0078(>4) | 0.25(>4) |
| 5b | 0.125(>4) | 0.25(>4) | 0.125(>4) | 0.125(>4) | 0.0078(>4) | 0.125(>4) |
| 5c | 0.125(>4) | 0.0625(>4) | 0.0625(>4) | 0.0625(>4) | <0.0078(>4) | 0.125(>4) |
| 5d | 0.125(4) | 0.125(4) | 0.125(>4) | 0.125(>4) | 0.0156(>4) | 0.25(4) |
| 6a | 0.125(>4) | 0.0625(>4) | 0.125(>4) | 0.125(4) | 0.0156(>4) | 0.125(>4) |
| 6b | 0.125(>4) | 0.25(>4) | 0.125(>4) | 0.125(2) | 0.0156(>4) | 0.25(>4) |
| 6c | 0.25(4) | 0.25(>4) | 0.125(>4) | 0.25(2) | 0.0156(>4) | 0.25(>4) |
| 6d | 0.0625(>4) | 0.125(>4) | 0.125(>4) | 0.125(4) | 0.0156(>4) | 0.0625(>4) |
| 6e | 0.25(>4) | 0.25(>4) | 0.125(>4) | 0.0625(>4) | 0.0156(>4) | 0.25(>4) |
| 6f | 0.125(>4) | 0.125(>4) | 0.125(4) | 0.25(2) | 0.0156(>4) | 0.125(>4) |
| 6g | 0.125(>4) | 0.125(>4) | 0.0625(>4) | 0.25(4) | 0.0078(>4) | 0.125(>4) |
| 6h | 0.25(>4) | 0.25(>4) | 0.125(4) | 0.125(>4) | 0.0156(>4) | 0.25(>4) |
| 6i | 0.03125(>4) | 0.0625(>4) | 0.0625(>4) | 0.0625(>4) | <0.0078(>4) | 0.03125(>4) |
| 6j | 0.0625(4) | 0.0625(4) | 0.0625(>4) | 0.125(>4) | <0.0078(>4) | 0.0625(>4) |
| 6k | 0.0625(>4) | 0.0625(>4) | 0.0625(>4) | 0.125(4) | <0.0312(>4) | 0.125(>4) |
| 6l | 0.0625(>4) | 0.0625(>4) | 0.03125(>4) | 0.0625(>4) | <0.0312(>4) | 0.125(>4) |
| 6m | 0.125(4) | 0.125(>4) | 0.0625(2) | 0.0625(4) | 0.0156(>4) | 0.125(>4) |
| 6n | 0.125(4) | 0.0625(>4) | 0.0625(>4) | 0.0625(2) | 0.0156(>4) | 0.125(>4) |
| 6o | 0.25(>4) | 0.25(>4) | 0.0625(>4) | 0.0625(>4) | <0.0078(>4) | 0.125(>4) |
| 6p | 0.03125(>4) | 0.0625(>4) | 0.0625(>4) | 0.0625(4) | <0.0078(>4) | 0.0625(>4) |
| 7 | 0.5(>4) | 0.5(>4) | 0.25(>4) | 0.5(>4) | 0.0156(>4) | 0.5(>4) |
| 8a | 0.125(>4) | 0.125(>4) | 0.5(>4) | 0.25(>4) | 0.5(>4) | 0.0625(>4) |
| 8b | 2(4) | 1(>4) | 1(4) | 2(4) | 2(>4) | 2(>4) |
| 8c | 1(>4) | 1(>4) | 0.125(>4) | 1(>4) | 0.0156(>4) | 0.5(Nd) |
| 8d | 0.5(>4) | 16(>4) | 1(>4) | 8(>4) | 2(>4) | 0.5(>4) |
| 8e | 32(>4) | 64(>4) | 16(>4) | 32(>4) | 4(>4) | 32(>4) |
| 8f | 16(>4) | 32(>4) | 1(>4) | 8(4) | 4(>4) | 16(>4) |
| 8g | 16(>4) | 32(>4) | 2(>4) | 16(>4) | 2(>4) | 16(>4) |
| T | 1(4) | 2(4) | 1(>4) | 1(>4) | 0.5(4) | 2(4) |
| V | 0.25(1) | 0.125(4) | 0.125(>4) | 0.125(>4) | 2(>4) | 0.25(1) |
| R | 0.0625(2) | 0.0625(4) | 0.03125(4) | 0.125(4) | 0.01562(>4) | 0.0625(4) |
T is tiamulin and V is valnemulin; Nd is not detected.
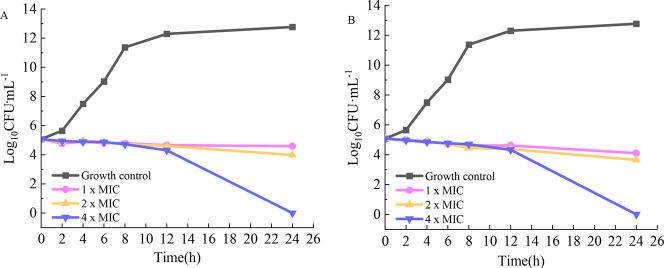
Time-kill curves of tiamulin (Figure 2A) and 6j (Figure 2B) against MRSA ATCC 33591 were obtained to study the correlation between the compound concentration and bactericidal rate. Three experimental concentrations (1×, 2×, and 4 × MIC) were assayed using tiamulin as the positive control. Figure 2 shows that the bacteria in the growth control group grew rapidly, particularly during 2–8 h, with a growth rate of 106. The number of colonies at 8 h was approximately 1012 CFU·mL–1. At concentrations of 1× and 2 × MIC, the results indicated that both 6j and tiamulin inhibited bacterial growth and reduced the bacterial colony count to a certain extent but did not have any significant bactericidal effect. In the 4 × MIC group, 6j exerted a significant bactericidal effect at 12 h, similar to that of tiamulin, both of which exhibited time dependency. Although 6j showed bactericidal activity similar to that of tiamulin, the concentration of 6j was 0.25 μg·mL–1, which is 16 times lower than the bactericidal concentration of tiamulin, suggesting that 6j performs more effectively at a low concentration.
Figure 2.
Time-kill curves of tiamulin (A) and 6j (B) against MRSA ATCC 33591.
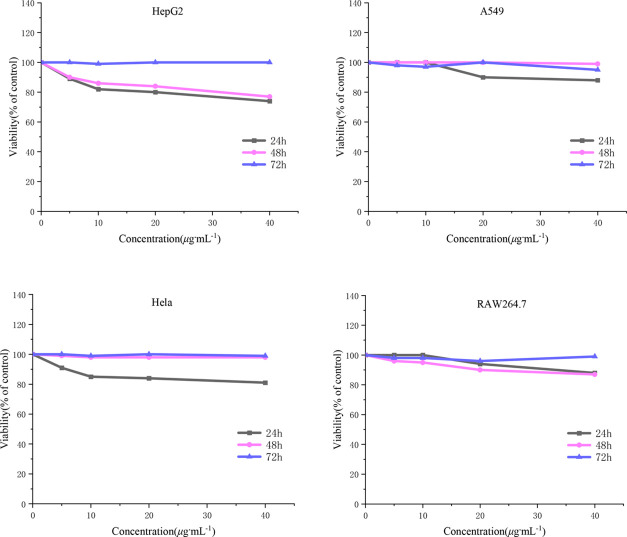
Cell survival and growth were examined at 24, 48, and 72 h through the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay. The specific tetrazole salt is transformed into insoluble formazan via enzymatic activity in the mitochondria of living cells.32 The cytotoxicity of 6j was examined in HepG2, HeLa, and A549 cancer cells as well as RAW264.7 normal cells. The results indicated that the viability of the above cells was not significantly affected, and cell survival was more than 75% in the concentration range of 0–40 μg·mL–1 (Figure 3). These results suggest that 6j exhibits low cytotoxicity against both tested cancer and normal cell lines. Moreover, at a prolonged incubation time of 72 h, 6j was shown to slightly affected cell proliferation.
Figure 3.
Toxicity of compound 6j to the different cell lines.
Thigh infection models were used to examine MRSA bacterial load and histopathological changes to determine the antimicrobial effect of 6jin vivo. The results indicated that 6j exhibited a better antibacterial effect than tiamulin and was comparable to that of retapamulin. As shown in Figure 4, the bacterial load in the model group is approximately 108 CFU·g–1, which was reduced to approximately 107 CFU·g–1 after treatment with 40 mg·kg–1 of tiamulin. This is comparable to the effect of using 6j or retapamulin at a lower dose of 20 mg·kg–1, and the bacterial load can be reduced to as low as 105 CFU·g–1 at a dose of 40 mg·kg–1.
Figure 4.
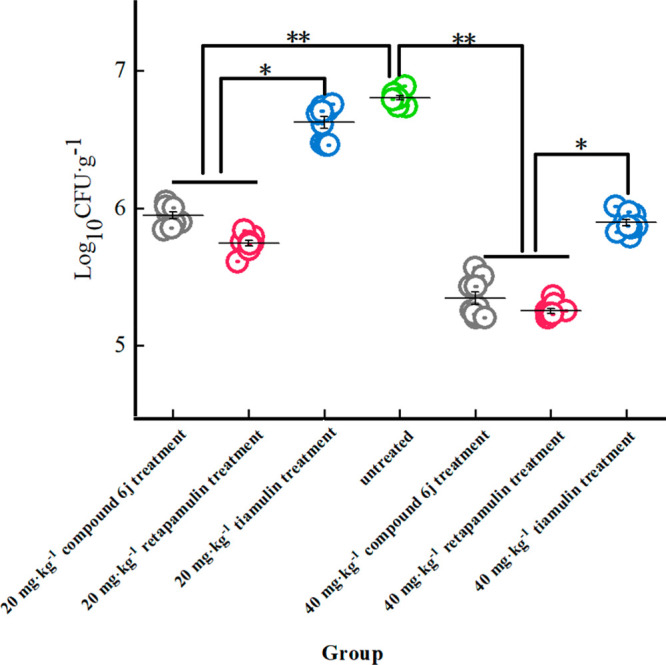
Bacterial load of different groups in thigh infection models of MRSA. * represents significant difference (vs the control group). ** represents extremely significant difference (vs the control group).
Pathological changes were observed under a light microscope by hematoxylin and eosin (H&E) staining. Compared to the blank control group (Figure 5A), inflammatory cell infiltration and accumulation are observed in the MRSA-infected group (Figure 5B), whereas a significant reduction in inflammation is observed in the experimental group administered with 6j or retapamulin Figure 5C–F). Notably, the inhibitory effect of 6j on inflammation was dose-dependent. The thigh muscle in the group treated with 40 mg·kg–1 of 6j (Figure 5D) was histologically normal, without a significant number of inflammatory cells in the blank control group and an effect comparable to that of retapamulin (Figure 5F).
Figure 5.
Effect of 6j on pathological changes in MRSA-infected skin (HE: 200×). Blank control (A) group, untreated (B) group, 20 mg·kg–16j treatment (C) group, 40 mg·kg–16j treatment (D) group, 20 mg·kg–1 retapamulin treatment (E) group, 40 mg·kg–1 retapamulin treatment (F) group in thigh after challenge of MRSA.
The healing effect of 6j on MRSA-infected mice was examined using a murine skin wound model. Compared to the uninfected group, the MRSA-infected control group exhibited skin deterioration, local skin blackening, and wound suppuration. Comparative analysis of the control group and the treatment group indicated that 6j and retapamulin ointment facilitated wound repair. In particular, the groups that were administered 6j and retapamulin ointment began to scab at the edge of the wound after 2–3 days and gradually healed. At 5–6 days, the wound healing status of the 1% and 2% 6j ointment treatment groups was similar to that of the uninfected group, and the treatment effect of the 3% 6j and 2% retapamulin ointment treatment groups was significant (Figure 6).
Figure 6.
Observation of the wound healing process in each mice group at different times.
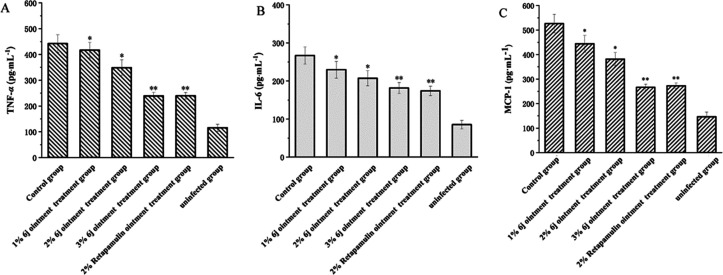
The expression of proinflammatory factors (for example, TNF-α and IL-6) is significantly correlated with wound healing. High expression of proinflammatory factors is a feature of systemic autoimmune and metabolic disorders, which causes abnormal inflammation of the wound, inhibits healing, and hinders wound repair.33,34 MCP-1 induces the expression of CC chemokine receptor 2 (CCR2) in various cells during tissue injury or infection. CCR2 causes cells to move to the site of tissue injury or infection with high MCP-1 concentrations, facilitating the development of an inflammatory response.35−38 In this study, the levels of TNF-α, IL-6, and MCP-1 in murine serum were measured using the ELISA method. Compared to the control group, the secretion levels of TNF-α, IL-6, and MCP-1 in the 1, 2, and 3% 6j and 2% retapamulin ointment groups were significantly lower than those in the control group. This result suggests that 6j and retapamulin can improve the inflammatory response to MRSA ATCC 43300. In particular, both 3% 6j and 2% retapamulin showed similar therapeutic effects (Figure 7). The effects on the downstream pathway signals will be explored in further detail.
Figure 7.
Effect of compounds on secretions of TNF-α (A), IL-6 (B), and MCP-1 (C).
The docking model was based on the crystal structures of Deinococcus radiodurans and tiamulin (PDB ID: 1XBP).39 The binding mode of the target compound was studied by deleting the original ligand to obtain the receptor and using target compounds 5c, 6j, and 8a as ligands.
As shown in Figure 8, the oxygen atom in the nitro group of 5c formed a hydrogen bond with N–H on residue U2564, and the carbonyl group at position C-21 formed a hydrogen bond with residue G2044. Moreover, residue G2484 formed a hydrogen bond with the carbonyl group on the parent ring of pleuromutilin. Compound 6j formed two hydrogen bonds with residue G2044 via the carbonyl group at the C-21 side chain, whereas residue G2484 formed a hydrogen bond with the carbonyl group at the C-3 position of the pleuromutilin parent ring. Residue U2046 also formed a hydrogen bond with NH2 in the purine. The carbonyl groups at the C-3 and C-21 positions of 8a formed hydrogen bonds with the residues G2044 and G2484, respectively. Furthermore, U2564 interacted with the purine ring via a hydrogen bond.
Figure 8.
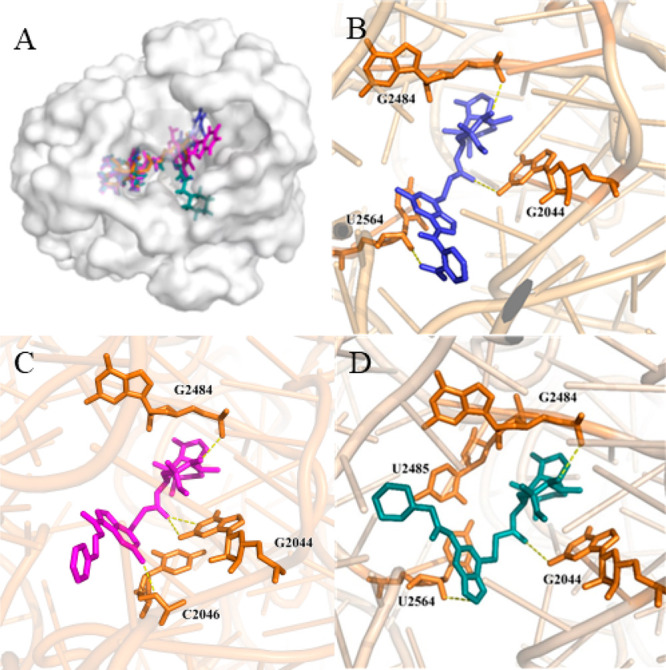
Interactions between ligands with PTC residues. Superimposed poses of selected compounds 5c (purple), 6j (magenta), and 8a (cyan) in the PTC of the 50s ribosomal (A). Interactions between compound 5c with residues (B). Interactions between compound 6j with residues (C). Interactions between compound 8a with residues (D).
A series of thioguanine-modified pleuromutilin derivatives were prepared, and their antibacterial activities against Gram-positive bacterial strains were examined. Almost all the synthesized compounds showed satisfactory antibacterial activity, and 6j exhibited significant antimicrobial activity against MRSA. The time-killing curve results indicated that 6j exhibited bactericidal activity similar to that of tiamulin and had more advantages and prospects at low concentrations. In addition, 6j exhibited satisfactory antibacterial activity in vivo, and its effect was similar to that of the approved drug retapamulin, which can facilitate wound healing and significantly downregulate the expression of inflammatory factors in serum. These results revealed that 6j is a potential drug candidate against MRSA infection, thus providing a reference for the development of anti-MRSA drugs.
Glossary
Abbreviations
- K2CO3
potassium carbonate
- MeCN
acetonitrile
- DCM
dichloromethane
- EDCI
N-(3-(dimethylamino)propyl)-N′-ethylcarbodiimide hydrochloride
- HOBt
1-hydroxybenzotriazole
- CDI
N,N-carbonyldiimidazole
- THF
tetrahydrofuran
- NMM
4-methylmorpholine
- NaI
sodium iodide
- r.t.
room temperature
- MIC
minimum inhibitory concentration
- MBC
minimum bactericidal concentration
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.3c00004.
Detailed experimental information: Synthetic experimental procedures, biology experimental methods, data of HPLC, 1H NMR, 13C NMR, and HRMS, molecular docking of some compounds, and the crystal data of some compounds (PDF)
This research was funded by Science and Technology Department of Sichuan Province (No. 2023JDRC0027).
The authors declare no competing financial interest.
Supplementary Material
References
- Francis J. S.; Doherty M. C.; Lopatin U.; Johnston C. P.; Sinha G.; Ross T.; Cai M.; Hansel N. N.; Perl T.; Ticehurst J. R.; et al. Severe Community-Onset Pneumonia in Healthy Adults Caused by Methicillin-Resistant Staphylococcus aureus Carrying the Panton-Valentine Leukocidin Genes. Clin. Infect. Dis. 2005, 40, 100–107. 10.1086/427148. [DOI] [PubMed] [Google Scholar]
- Keynan Y.; Rubinstein E. Staphylococcus aureus Bacteremia, Risk Factors, Complications, and Management. Crit. Care Clin. 2013, 29, 547–562. 10.1016/j.ccc.2013.03.008. [DOI] [PubMed] [Google Scholar]
- Belthur M. V.; Birchansky S. B.; Verdugo A. A.; Mason E. O.; Hulten K. G.; Kaplan S. L.; O’Brian Smith E.; Phillips W. A.; Weinberg J. Pathologic Fractures in Children with Acute Staphylococcus aureus Osteomyelitis. J. Bone Jt. Surg., Am. Vol. 2012, 94, 34–42. 10.2106/JBJS.J.01915. [DOI] [PubMed] [Google Scholar]
- Nataraj B. H.; Mallappa R. H. Antibiotic Resistance Crisis: An Update on Antagonistic Interactions between Probiotics and Methicillin-Resistant Staphylococcus aureus (MRSA). Curr. Microbiol. 2021, 78, 2194–2211. 10.1007/s00284-021-02442-8. [DOI] [PubMed] [Google Scholar]
- Murray C. J. L.; Ikuta K. S.; Sharara F.; Swetschinski L.; Aguilar G. R.; Gray A.; Han C.; Bisignano C.; Rao P.; Wool E.; Johnson S. C.; Browne A. J.; Chipeta M. G.; Fell F.; Hackett S.; Haines-Woodhouse G.; Hamadani B. H. K.; Kumaran E.a.P.; Mcmanigal B.; Agarwal R.; Akech S.; Albertson S.; Amuasi J.; Andrews J.; Aravkin A.; Ashley E.; Bailey F.; Baker S.; Basnyat B.; Bekker A.; Bender R.; Bethou A.; Bielicki J.; Boonkasidecha S.; Bukosia J.; Carvalheiro C.; Castaneda-Orjuela C.; Chansamouth V.; Chaurasia S.; Chiurchiu S.; Chowdhury F.; Cook A. J.; Cooper B.; Cressey T. R.; Criollo-Mora E.; Cunningham M.; Darboe S.; Day N. P. J.; De Luca M.; Dokova K.; Dramowski A.; Dunachie S. J.; Eckmanns T.; Eibach D.; Emami A.; Feasey N.; Fisher-Pearson N.; Forrest K.; Garrett D.; Gastmeier P.; Giref A. Z.; Greer R. C.; Gupta V.; Haller S.; Haselbeck A.; Hay S. I.; Holm M.; Hopkins S.; Iregbu K. C.; Jacobs J.; Jarovsky D.; Javanmardi F.; Khorana M.; Kissoon N.; Kobeissi E.; Kostyanev T.; Krapp F.; Krumkamp R.; Kumar A.; Kyu H. H.; Lim C.; Limmathurotsakul D.; Loftus M. J.; Lunn M.; Ma J.; Mturi N.; Munera-Huertas T.; Musicha P.; Mussi-Pinhata M. M.; Nakamura T.; Nanavati R.; Nangia S.; Newton P.; Ngoun C.; Novotney A.; Nwakanma D.; Obiero C. W.; Olivas-Martinez A.; Olliaro P.; Ooko E.; Ortiz-Brizuela E.; Peleg A. Y.; Perrone C.; Plakkal N.; Ponce-De-Leon A.; Raad M.; Ramdin T.; Riddell A.; Roberts T.; Victoriarobotham J.; Roca A.; Rudd K. E.; Russell N.; Schnall J.; Scott J.a.G.; Shivamallappa M.; Sifuentes-Osornio J.; Steenkeste N.; Stewardson A. J.; Stoeva T.; Tasak N.; Thaiprakong A.; Thwaites G.; Turner C.; Turner P.; Van Doorn H. R.; Velaphi S.; Vongpradith A.; Vu H.; Walsh T.; Waner S.; Wangrangsimakul T.; Wozniak T.; Zheng P.; Sartorius B.; Lopez A. D.; Stergachis A.; Moore C.; Dolecek C.; Naghavi M.; et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022, 399, 629–655. 10.1016/S0140-6736(21)02724-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rump B.; Timen A.; Verweij M.; Hulscher M. Experiences of carriers of multidrug-resistant organisms: a systematic review. Clin. Microbiol. Infect. 2019, 25, 274–279. 10.1016/j.cmi.2018.10.007. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y.; Izumikawa K.; Hashiguchi K.; Fukuda Y.; Kobayashi T.; Kondo A.; Inoue Y.; Morinaga Y.; Nakamura S.; Imamura Y.; Miyazaki T.; Kakeya H.; Yanagihara K.; Kohno S. The efficacy and safety of high-dose arbekacin sulfate therapy (once-daily treatment) in patients with MRSA infection. J. Infect. Chemother. 2012, 18, 241–246. 10.1007/s10156-012-0397-4. [DOI] [PubMed] [Google Scholar]
- Arianpoor A.; Askari E.; Soleymani F.; Tabatabai S. M.; Rahbar M.; Naderi Nasab M. Updates on the global prevalence of MRSA: results from Iran. Int. J. Antimicrob. Agents. 2012, 40, 373. 10.1016/j.ijantimicag.2012.05.004. [DOI] [PubMed] [Google Scholar]
- Kavanagh F.; Hervey A.; Robbins W. J. Antibiotic Substances From Basidiomycetes: VIII. Pleurotus Multilus (Fr.) Sacc. and Pleurotus Passeckerianus Pilat. Proc. Natl. Acad. Sci. U. S. A. 1951, 37, 570–574. 10.1073/pnas.37.9.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paukner S.; Riedl R. Pleuromutilins: Potent Drugs for Resistant Bugs-Mode of Action and Resistance. Cold Spring Harbor Perspect. Med. 2017, 7, a027110. 10.1101/cshperspect.a027110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drews J.; Georgopoulos A.; Laber G.; Schutze E.; Unger J. Antimicrobial Activities of 81.723 hfu, a New Pleuromutilin Derivative. Antimicrob. Agents Chemother. 1975, 7, 507–516. 10.1128/AAC.7.5.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X.; Sun J.; Yang T.; Fang X.; Wu D.; Xiong Y. Q.; Cheng J.; Chen Y.; Shi W.; Liu Y. H. In vivo pharmacokinetic/pharmacodynamic profiles of valnemulin in an experimental intratracheal Mycoplasma gallisepticum infection model. Antimicrob. Agents Chemother. 2015, 59, 3754–3760. 10.1128/AAC.00200-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meydan S.; Marks J.; Klepacki D.; Sharma V.; Baranov P. V.; Firth A. E.; Margus T.; Kefi A.; Vázquez-Laslop N.; Mankin A. S. Retapamulin-Assisted Ribosome Profiling Reveals the Alternative Bacterial Proteome. Mol. Cell 2019, 74, 481–493. 10.1016/j.molcel.2019.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eraikhuemen N.; Julien D.; Kelly A.; Lindsay T.; Lazaridis D. Treatment of Community-Acquired Pneumonia: A Focus on Lefamulin. Infect. Dis. Ther. 2021, 10, 149–163. 10.1007/s40121-020-00378-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu L. Q.; Guo X. S.; Liu X.; He H. L.; Wang Y. L.; Yang Y. S. Synthesis and antibacterial activity of C-2(S)-substituted pleuromutilin derivatives. Chin. Chem. Lett. 2010, 21, 507–510. 10.1016/j.cclet.2010.01.001. [DOI] [Google Scholar]
- Llabani E.; Hicklin R. W.; Lee H. Y.; Motika S. E.; Crawford L. A.; Weerapana E.; Hergenrother P. J. Diverse compounds from pleuromutilin lead to a thioredoxin inhibitor and inducer of ferroptosis. Nat. Chem. 2019, 11, 521–532. 10.1038/s41557-019-0261-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer D. M.; Sorenson M. E.; Huang S.; Connolly T. P.; Bronson J. J.; Matson J. A.; Hanson R. L.; Brzozowski D. B.; Laporte T. L.; Patel R. N. Synthesis and Activity of a C-8 Keto Pleuromutilin Derivative. Bioorganic & Medicinal Chemistry Letters 2003, 13, 1751–1753. 10.1016/S0960-894X(03)00297-X. [DOI] [PubMed] [Google Scholar]
- Yi Y.; Xu X.; Liu Y.; Xu S.; Huang X.; Liang J.; Shang R. Synthesis and antibacterial activities of novel pleuromutilin derivatives with a substituted pyrimidine moiety. Eur. J. Med. Chem. 2017, 126, 687–695. 10.1016/j.ejmech.2016.11.054. [DOI] [PubMed] [Google Scholar]
- Ling C.; Fu L.; Gao S.; Chu W.; Wang H.; Huang Y.; Chen X.; Yang Y. Design, Synthesis, and Structure-Activity Relationship Studies of Novel Thioether Pleuromutilin Derivatives as Potent Antibacterial Agents. J. Med. Chem. 2014, 57, 4772–4795. 10.1021/jm500312x. [DOI] [PubMed] [Google Scholar]
- Ling Y.; Wang X.; Wang H.; Yu J.; Tang J.; Wang D.; Chen G.; Huang J.; Li Y.; Zheng H. Design, synthesis, and antibacterial activity of novel pleuromutilin derivatives bearing an amino thiazolyl ring. Arch. Pharm. 2012, 345, 638–646. 10.1002/ardp.201100430. [DOI] [PubMed] [Google Scholar]
- Liu H.; Ma D.; Cui G.; Zhang Y.; Xue F. Design, synthesis and antibacterial activities of pleuromutilin derivatives. J. Asian Nat. Prod. Res. 2021, 23, 123–137. 10.1080/10286020.2020.1713764. [DOI] [PubMed] [Google Scholar]
- Springer D. M.; Bunker A.; Luh B. Y.; Sorenson M. E.; Goodrich J. T.; Bronson J. J.; Denbleyker K.; Dougherty T. J.; Fung-Tomc J. Cyclopentanone ring-cleaved pleuromutilin derivatives. Eur. J. Med. Chem. 2007, 42, 109–113. 10.1016/j.ejmech.2006.07.018. [DOI] [PubMed] [Google Scholar]
- Siricilla S.; Mitachi K.; Yang J.; Eslamimehr S.; Lemieux M. R.; Meibohm B.; Ji Y.; Kurosu M. A New Combination of a Pleuromutilin Derivative and Doxycycline for Treatment of Multidrug-Resistant Acinetobacter baumannii. J. Med. Chem. 2017, 60, 2869–2878. 10.1021/acs.jmedchem.6b01805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uccello D. P.; Miller S. M.; Dieterich N. A.; Stepan A. F.; Chung S.; Farley K. A.; Samas B.; Chen J.; Montgomery J. I. The synthesis of C-13 functionalized pleuromutilins via C-H amidation and subsequent novel rearrangement product. Tetrahedron Lett. 2011, 52, 4247–4251. 10.1016/j.tetlet.2011.06.028. [DOI] [Google Scholar]
- Thai Le S.; Páll D.; Rőth E.; Tran T.; Debreczeni N.; Bege M.; Bereczki I.; Ostorházi E.; Milánkovits M.; Herczegh P.; Borbás A.; Csávás M. The Very First Modification of Pleuromutilin and Lefamulin by Photoinitiated Radical Addition Reactions--Synthesis and Antibacterial Studies. Pharmaceutics. 2021, 13, 2028. 10.3390/pharmaceutics13122028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H. X.; Cui G.; Ma D. L.; Zhang Y.; Xue F. Q. Design, synthesis and antibacterial activity evaluation of pleuromutilin derivatives according to twin drug theory. J. Asian Nat. Prod. Res. 2022, 24, 371–387. 10.1080/10286020.2021.1920581. [DOI] [PubMed] [Google Scholar]
- Li Y. G.; Wang J. X.; Zhang G. N.; Zhu M.; You X. F.; Hu X. X.; Zhang F.; Wang Y. C. Antibacterial Activity and Structure Activity Relationship of a Series of Newly Synthesized Pleuromutilin Derivatives. Chem. Biodiversity. 2019, 16, e1800560 10.1002/cbdv.201800560. [DOI] [PubMed] [Google Scholar]
- Hirokawa Y.; Kinoshita H.; Tanaka T.; Nakamura T.; Fujimoto K.; Kashimoto S.; Kojima T.; Kato S. Pleuromutilin derivatives having a purine ring. Part 2: Influence of the central spacer on the antibacterial activity against Gram-positive pathogens. Bioorg. Med. Chem. Lett. 2009, 19, 170–174. 10.1016/j.bmcl.2008.10.123. [DOI] [PubMed] [Google Scholar]
- Hirokawa Y.; Kinoshita H.; Tanaka T.; Nakamura T.; Fujimoto K.; Kashimoto S.; Kojima T.; Kato S. Pleuromutilin derivatives having a purine ring. Part 3: Synthesis and antibacterial activity of novel compounds possessing a piperazine ring spacer. Bioorg. Med. Chem. Lett. 2009, 19, 175–179. 10.1016/j.bmcl.2008.10.127. [DOI] [PubMed] [Google Scholar]
- Gao M. L.; Zeng J.; Fang X.; Luo J.; Jin Z.; Liu Y. H.; Tang Y. Z. Design, synthesis and antibacterial evaluation of novel pleuromutilin derivatives possessing piperazine linker. Eur. J. Med. Chem. 2017, 127, 286–295. 10.1016/j.ejmech.2017.01.004. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Xie C.; Liu Y.; Shang F.; Shao R.; Yu J.; Wu C.; Yao X.; Liu D.; Wang Z. Synthesis, biological activities and docking studies of pleuromutilin derivatives with piperazinyl urea linkage. J. Enzyme Inhib. Med. Chem. 2021, 36, 764–775. 10.1080/14756366.2021.1900163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahuguna A.; Khan I.; Bajpai V. K.; Kang S. C. MTT assay to evaluate the cytotoxic potential of a drug. Bangl. J. Pharmacol. 2017, 12, 115–118. 10.3329/bjp.v12i2.30892. [DOI] [Google Scholar]
- Dayer J. M. Evidence for the biological modulation of IL-1 activity: The role of IL-1Ra. Clin. Exp. Rheumatol. 2002, 20, S14–S20. [PubMed] [Google Scholar]
- Goren I.; Muller E.; Schiefelbein D.; Christen U.; Pfeilschifter J.; Muhl H.; Frank S. Systemic anti-TNFalpha treatment restores diabetes-impaired skin repair in ob/ob mice by inactivation of macrophages. J. Invest. Dermatol. 2007, 127, 2259–2267. 10.1038/sj.jid.5700842. [DOI] [PubMed] [Google Scholar]
- Callewaere C.; Banisadr G.; Rostene W.; Parsadaniantz S. M. Chemokines and chemokine receptors in the brain: implication in neuroendocrine regulation. J. Mol. Endocrinol. 2007, 38, 355–363. 10.1677/JME-06-0035. [DOI] [PubMed] [Google Scholar]
- Thelen M. Dancing to the tune of chemokines. Nat. Immunol. 2001, 2, 129–134. 10.1038/84224. [DOI] [PubMed] [Google Scholar]
- Mellado M.; Rodriguez-Frade J. M.; Aragay A.; Del Real G.; Martin A. M.; Vila-Coro A. J.; Serrano A.; Mayor F.; Martínez-A. C. The chemokine monocyte chemotactic protein 1 triggers Janus kinase 2 activation and tyrosine phosphorylation of the CCR2B receptor. J. Immunol. 1998, 161, 805–813. 10.4049/jimmunol.161.2.805. [DOI] [PubMed] [Google Scholar]
- Han H. M.; Ko S.; Cheong M.-J.; Bang J. K.; Seo C. H.; Luchian T.; Park Y. Myxinidin2 and myxinidin3 suppress inflammatory responses through STAT3 and MAPKs to promote wound healing. OncoTargets Ther. 2017, 8, 87582–87597. 10.18632/oncotarget.20908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonath A.; Harms J. M.; Pyetan E.; Schlünzen F.; Fucini P. Inhibition of peptide bond formation by pleuromutilins: the structure of the 50S ribosomal subunit fromDeinococcus radioduransin complex with tiamulin. Mol. Microbiol. 2004, 54, 1287–1294. 10.1111/j.1365-2958.2004.04346.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.