Abstract
Prostaglandin E2 (PGE2) receptor 4 (EP4) is one of four EP receptors commonly upregulated in the tumor microenvironment and plays vital roles in stimulating cell proliferation, invasion, and metastasis. Biochemical blockade of the PGE2–EP4 signaling pathway is a promising strategy for controlling inflammatory and immune related disorders. Recently combination therapies of EP4 antagonists with anti-PD-1 or chemotherapy agents have emerged in clinical studies for lung, breast, colon, and pancreatic cancers. Herein, a novel series of indole-2-carboxamide derivatives were identified as selective EP4 antagonists, and SAR studies led to the discovery of the potent compound 36. Due to favorable pharmacokinetics properties and good oral bioavailability (F = 76%), compound 36 was chosen for in vivo efficacy studies. Compound 36 inhibited tumor growth in a CT-26 colon cancer xenograft better than E7046 and a combination of 36 with capecitabine significantly suppressed tumor growth (TGI up to 94.26%) in mouse models.
Keywords: Prostaglandin, PGE2, EP4, antagonist, capecitabine, CT-26, colon cancer, xenografts
Prostaglandin E2 receptor 4 (EP4), one of four EP receptors (EP1, EP2, EP3 and EP4), is most widely distributed in human body.1 EP receptors belong to the family of G-protein-coupled receptors (GPCRs) which are cell surface signaling receptors and control several cellular functions. Under inflammatory conditions, prostaglandin E2 (PGE2) is generated from arachidonic acid by cyclooxygenase isoenzymes (COX1 and COX2), peroxidase, and PGE syntheses pathway.2,3 PGE2 activates the EP receptors that modulate the function of myeloid immune cells and maintain body homeostasis. In the tumor microenvironment (TME), EP4 receptor produces PGE2-elicited immunosuppressive activity that causes tumor development and disease progression.4 The EP4 receptor stimulates cyclic adenosine monophosphate (cAMP) production and increases intracellular cAMP levels to initiate multiple downstream signaling pathways. Up-regulation of EP4 receptor is observed for breast cancer,5 prostate cancer cells,6 and squamous cell carcinomas7 of uterus and cervix.
The PGE2–EP2 and PGE2–EP4 signaling pathways are considered as key regulatory modes for inflammation and immune suppression in the TME.8 Biochemical blockade of PGE2–EP4 signaling by EP4 antagonists is one of the promising approaches for controlling tumor growth and metastasis. The modulators of EP4 receptor can be used as the drugs for treatment of cancer and immune related diseases. In recent years, several pharmaceutical and biotechnology companies have put significant efforts into identifying effective, structurally diverse EP4 antagonists and demonstrated therapeutic effects in preclinical research and clinical studies for pain, inflammatory diseases, and cancers.9
Colorectal cancer (CRC) is known as the third most common malignancy and the second most common deadly cancer in recent years. In 2020, more than 1.9 million new cases and 0.9 million deaths were estimated due to CRC worldwide.10 Therefore, the development of innovative treatments is an urgent requirement to improve the clinical benefits for patients with advanced or metastatic CRC. The non-steroidal anti-inflammatory drugs (NSAIDs) or selective COX2 inhibitors are commonly used and beneficial for the treatment of the disease.11 However, long-term administration of NSAIDs in patients caused gastrointestinal (GI) bleeding and increased the risk of heart attack.12 Instead of NSAIDs, EP4 antagonists may be used for better safety and tolerability. In this Letter, we report a novel series of orally bioavailable indole-2-carboxamide derivatives (8) as selective EP4 receptor antagonists and in vivo efficacy studies in colon cancer animal models.
The EP4 antagonists are broadly classified into two major categories: first- and second-generation EP4 antagonists. The first-generation EP4 antagonists contain sulfonyl urea/acylsulfonamide units. The FDA approved grapiprant/CJ-023423 (1, Figure 1) as the first selective EP4 antagonist for the treatment of osteoarthritis pain and inflammation in dogs.13 Due to poor pharmacokinetic (PK) profiles and low blood–brain–barrier (BBB) permeability, many first-generation EP4 antagonists, except grapiprant, were unsuccessful for clinical use. To improve the metabolic stability, the acylsulfonamide group was replaced by a bioisosteric nonacidic amide group in the second-generation antagonist structures.14,15 EP4 antagonist CJ-042794 (2) was demonstrated in pain and inflammation models.14 Thiophen-3-carboximide MK-2894 (3) was reported for pain/inflammation in arthritis.15 With favorable immune-modulator properties, CR6086 (4) was shown to be effective in rodents for inflammatory diseases.16 The EP4 antagonist ONO-4578/BMS-986310 (5) promoted T-cell activation and myeloid cell differentiation, and it was pushed to clinical trials phase 1 for advanced or metastatic solid tumors.17 Orally bioavailable EP4 antagonist E7046 (6) showed antitumor efficacy and prolonged survival of animals in a CT-26 colon cancer mouse model.18 E7046 diminished myeloid immunosuppression and synergized with Treg-reducing IL-2–diphtheria toxin fusion protein in restoring antitumor immunity. A few EP4 antagonists, including LY3127760, were reported for cancer models.19,20 A scaffold hopping strategy was adopted to identify EP4 antagonists and applied for cancer immunotherapy.21 EP4 antagonist L001 (7) was demonstrated to suppress pancreatic cancer in animal models.22 A few recent studies indicated that the combination of an EP4 inhibitor with anti-PD-1 or chemotherapy significantly produced synergistic effect and improved efficacy for checkpoint-based immunotherapy23,24 and immuno-oncology.25,26 Oncology and immuno-oncology are focused areas of our research interests, and specific targets such as lung, breast, stomach, and colorectal cancer are being explored at our company.27,28 We are also developing orally bioavailable EP4 antagonists for cancer treatment.29
Figure 1.
(a) Grapiprant (1), an EP4 receptor antagonist approved by the FDA. (b) Representative amide derivatives as EP4 receptor antagonists 2–7.
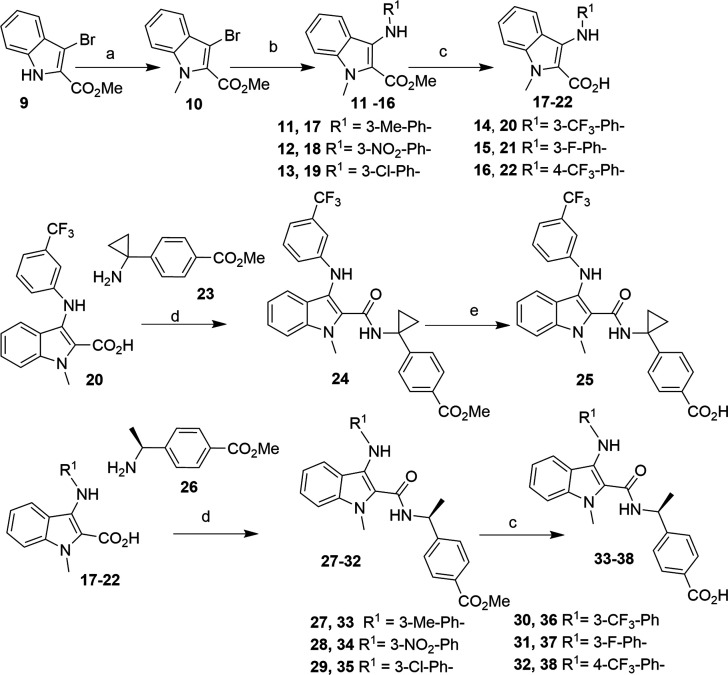
Structure-based drug design (SBDD) is a powerful technique and is widely utilized to identify lead molecules in drug discovery processes.30 The cocrystal structure of human EP4 receptor and its antagonist ONO-AE3-208 (PDB: 5YWY) was disclosed recently.31 Using the template and adopting a computer based drug design approach, we designed novel amide derivatives of general formula Gen-1 and Gen-2 where A represented six-membered aryl or heteroaryl rings or their saturated analogs (Figure 2). Interactions of 4-(1-(1-methyl-3-((3-(trifluoromethyl)phenyl) amino)-1H-indole-2-carboxamido)cyclopropyl)benzoic acid (S1) and 4-(1-(3-methyl-1-(3-(trifluoromethyl)benzyl)-1H-indole-2-carboxamido)cyclopropyl)benzoic acid (S2) with the EP4 receptor are illustrated in Figure 2a–d. Molecular docking results revealed that polar interactions (H-bonding) of the antagonists with the EP4 receptor were observed. The carboxylic acid (−COOH) and the carbonyl (CO) functional groups of S1 or S2 formed H-bonds with Thr168, Arg316, and Thr76 residues of EP4, respectively. It was expected that the indole-2-carboxylic acid derivatives might improve efficacy and drug-like properties. In vitro assay results of indole-2-carboxamide derivatives (8) indicated that the new series of compounds were selective EP4 receptor antagonists (Table 1). One of the best compounds, 36, exhibited excellent in vitro activity and good bioavailability. In vivo efficacy of compound 36 was evaluated in a mouse CT-26 colon carcinoma model combined with capecitabine.
Figure 2.
Newly designed chemical structures (Gen-1 and Gen-2) and novel indole-2-carboxamide derivatives (8) as EP4 antagonists. Molecular docking: (a) compound S1 with EP4; (c) compound S2 with EP4. Ligand–protein interactions: (b) S1 with EP4; (d) S2 with EP4.
Scheme 1. Synthesis of Indole-2-carboxamide Derivatives.
Reagents and conditions: (a) CH3I, K2CO3, DMF, 80–85%; (b) R1NH2, Pd(OAc)2, BINAP, tBuONa, PhMe, 55–65%; (c) LiOH, THF/H2O (4:1), 85–95%; (d) HATU, DIPEA, DMF, 60–75%; (e) NaOH, THF/H2O (4:1), 85–95%.
The reaction sequences for making the new indole-2-carboxamide derivatives are illustrated in Scheme 1 and Scheme 2. The synthetic routes were started with methyl 3-bromoindole-2-carboxylate (9), a commercially available material, and intermediate 10 was made by an N-methylation reaction in good yield, 80–85% (Scheme 1). Under Buchwald reaction conditions, different aromatic amines were allowed to react with 10 to give the desired methyl 3-N-aryl-indole-2-carboxylates 11–16 in moderate yield (55–65%). Hydrolysis of the esters under basic conditions led to the 3-N-aryl-indole-2-carboxylic acids 17–22 in good yield (85–95%). Intermediate indole-2-carboxylic acid 20 was coupled with methyl 4-(1-aminocyclopropyl)benzoate (23) under HATU/DIPEA coupling reaction conditions and subsequent hydrolysis of methyl ester gave the desired indole-2-carboxamide derivative 25 in good yield. Similarly, the intermediates 17–22 were separately coupled with methyl-4-[(1S)-1-aminoethyl]benzoate (25) under standard HATU/DIPEA reaction conditions, followed by ester hydrolysis leading to the desired products 33–38.
Scheme 2. Synthesis of Indole-2-carboxamide Derivatives 41 and 42.
Reagents and conditions: (a) HATU, DIPEA, DMF; (b) NaOH, THF/H2O, 85–95%; (c) TsCl, TEA, DMAP, DCM, NH4Cl, H2O, 50–65%.
Similarly, indole-2-carboxylic acid 20 was coupled with methyl-4-[(1R)-1-aminoethyl]benzoate (39), and subsequent methyl ester hydrolysis of 39 led to compound 41 in moderate yield (Scheme 2). Compound 42 was prepared from compound 36 in moderate yield (50–65%) under functional group transformation reaction conditions.
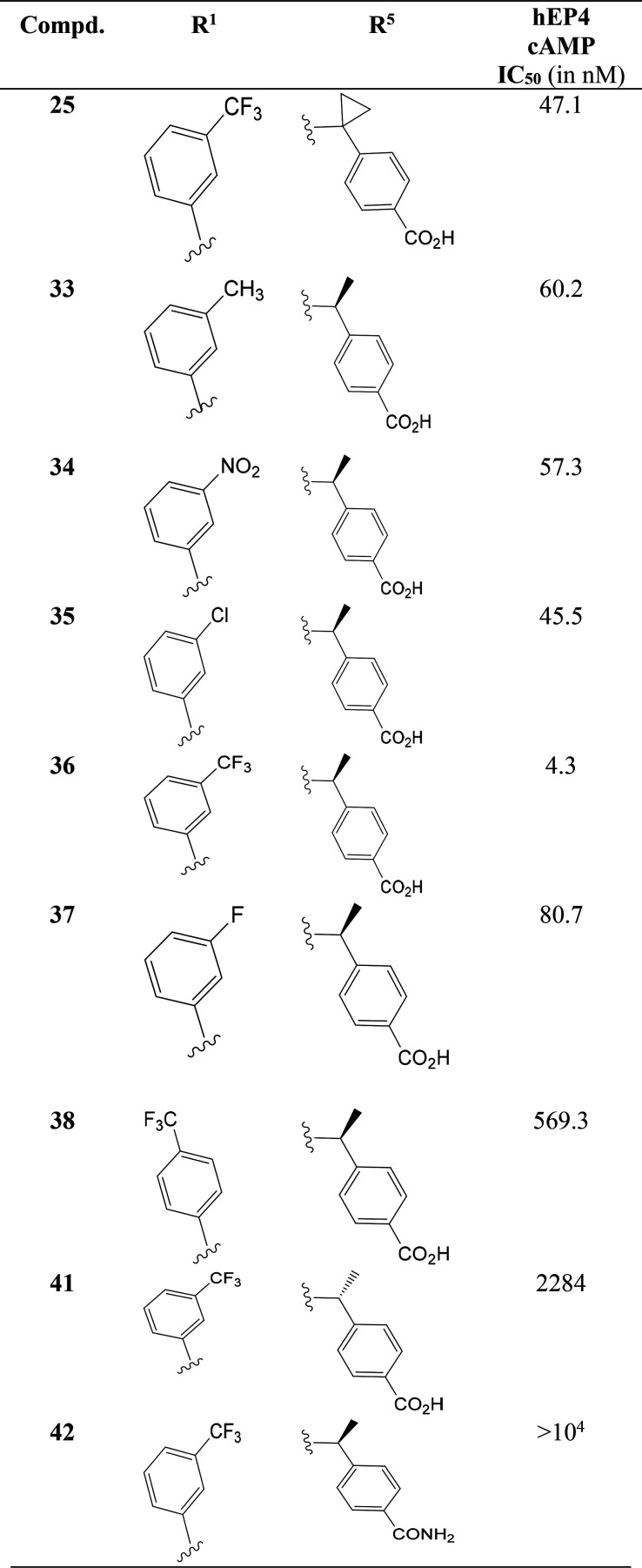
A number of studies indicated that EP4 receptor stimulation can increase intracellular cAMP levels, through the activation of the Gα S pathway.32 The indole-2-caroboxamide derivatives (Table 1) were tested in the human EP4 functional assay measuring PGE2-induced cAMP formation in HEK293-hEP4 cells that overexpress the human EP4 receptor. Compound 25 exhibited potent inhibitory activity (IC50 = 47.1 nM) in the cAMP functional assay. Introducing chirality in the molecule and adding different substituents on the phenyl ring resulted in a good change of potency in the range 4.3–60.2 nM. The introduction of an electron-withdrawing substituent at the meta-position of the benzene ring, such as nitro (34, IC50 = 57.3 nM), chloro (35, IC50 = 45.5 nM), or cyano groups, improved potency over an electron-donating methyl group (33, IC50 = 60.2 nM). A sharp improvement of the potency was observed for the trifluoromethyl group in 36 (IC50 = 4.3 nM).
Table 1. Antagonistic Effect of Compounds against Human EP4 Receptora.

IC50 values represent the antagonistic activity at the human EP4 receptor. The data were taken as the average of two experimental values.
The antagonism of compound 36 against EP4 was better than a reference standard E7046 (IC50 = 13.5 nM).18 Introduction of (S)-absolute configuration in 36 with (1S)-1-aminoethyl]benzoic acid improved EP4 functional activity about 10-fold compared to achiral compound 25. Substitution with fluorine 37 did not increase the activity (IC50 = 80.7 nM), perhaps due to the small size and poor interaction with the hydrophobic pocket. The CF3-group at the para-position of the benzene ring in 38 decreased the functional activity (IC50 = 569.3 nM) as compared to the meta-isomer 36. Hydrophilic functionality including carboxamides, sulfones, and sulfonamide did not produce any EP4 antagonistic activity, and the data are not discussed here. The (R)-methyl isomer 41 was less active (IC50 = 2284 nM) than the corresponding antipode (S)-methyl isomer 36. Hence, the (S)-absolute configuration was essential for gaining potency. An amide functional group bearing compound, 42, was inactive (IC50 > 104 nM). We presumed that the change of electronic environment (carboxylic acid to amide functionality) weakened the affinity of binding 42 to Arg316 of EP4 receptor and caused the huge difference of activity.
The antagonism selectivity of compound 36 against EP1, EP2, EP3, and EP4 receptors is summarized in Table 2. Observed data were compared with known EP3 and EP4 receptor inhibitors L-798106 and E7046, respectively. Compound 36 appeared to be a selective EP4 antagonist (IC50 = 4.3 nM) over other EP1–EP3 receptors (IC50 > 104 nM). The compounds did not show any significant antagonism against EP1–EP3 receptors at >10 μM. In the same experiment, L-798106 inhibited EP3 at IC50 = 33 nM and E7046 showed EP4 antagonism at IC50 = 10.19 nM. E7046 (previously known as ER-886046) was demonstrated in a cellular PGE2 induced cAMP reporter assay to have an IC50 of 13.5 nM.18 So, compound 36 was a selective antagonist of EP4 over other EP receptors.
Table 2. Selectivity of 36 and Human cAMP EP4 Receptor Antagonism IC50a.
The data were taken as the average of two experimental values and are reported in nM.
Observed and reported values.18
Not determined.
The binding affinity of compounds 25 and 36 (Ki values) is summarized in Table 3. The Ki values of compound 36 for prostanoid receptors were >9.6 μM, >8.8 μM, >6.4 μM, and 65.9 ± 20.4 nM for EP1, EP2, EP3, and EP4, respectively. The Ki value of compound 25 (Ki= 143.9 ± 55.8 nM) was slightly weaker than that of compound 36 for the EP4 receptor.
Table 3. EP Receptor Binding Affinity of 25 and 36a.
| compd | EP1 (μM) | EP2 (μM) | EP3 (μM) | EP4 (nM) |
|---|---|---|---|---|
| 25 | >9.6 | >8.8 | >6.4 | 143.9 ± 55.8 |
| 36 | >9.6 | >8.8 | >6.4 | 65.9 ± 20.4 |
Data (Ki values) are presented as the mean ± standard error of three independent experiments.
EP4 antagonists are effective for the treatment of colon, pancreatic, lung, and breast cancers.9 To evaluate the cytotoxic effect, compounds 25, 33, 36, and 37, compared with E7046 (6), were screened against different cancer cell lines: breast cancer (MCF-7, 4T1), colon cancer (HCA-7, CT-26 WT), and lung cancer (LLC) (Table 4). Compound 36 inhibited the cancer cells at IC50 = 46.73 and 79.47 μM in breast cancer cell lines MCF-7 and 4T1 respectively, at IC50 = 41.39 and >100 μM in colon cancer cell lines CT-26 WT and HCA-7, and at IC50 > 100 μM in lung cancer cell lines. The reference compound 6 inhibited at IC50 > 100 μM concentration under the testing conditions. Another three compounds, 25, 33, and 37, were tested in CT-26 WT cell lines, and all the compounds had IC50 > 100 μM. In these in vitro assays, compound 36 exerted prominent cytotoxic activity against breast and colon cancer cell lines.
Table 4. Cytotoxicity Study of Selected Compounds in Breast, Colon, and Lung Cancer Cell Linesa.
| cell
lines (IC50 in μM) |
|||||
|---|---|---|---|---|---|
| compd | MCF-7 (breast cancer) | 4T1 (breast cancer) | HCA-7 (colon cancer) | CT-26 WT (colon cancer) | LLC (lung cancer) |
| 25 | b | b | b | >100 | b |
| 33 | b | b | b | >100 | b |
| 36 | 46.73 | 79.47 | >100 | 41.39 | >100 |
| 37 | b | b | b | >100 | b |
| E7046 | >100 | >100 | >100 | >100 | >100 |
Data are presented as the mean of at least two independent experiments.
Not determined.
Encouraged by the favorable in vitro biological data, compound 36 was selected for testing drug-like properties and submitted for preliminary ADME tests. Compound 36 showed good stability in rat, mouse, and human liver microsomes with half-lives of 260, 98.6, and 24 min, respectively. The data for plasma protein binding of 36 to rat, mouse, and human plasma were 99.73%, 99.66%, and 99.68%, respectively, and % remaining after 6 h was 88.79%, 70.47%, and 83.02% (Supporting Information, Tables S1 and S2). In vivo preclinical pharmacokinetics (PK) properties of 36 were evaluated in Sprague–Dawley male rats. The results are summarized in Table 5. After intravenous injection of 36 in rats at a dose of 1 mg/kg, the plasma elimination half-life (T1/2) values were measured. Compound 36 demonstrated a good PK profile with low clearance (4.95 ± 0.77 mL/min/kg) and moderate volume distributions (0.41 ± 0.04 L/kg).
Table 5. Pharmacokinetic Parameters of Compound 36 in Ratsa.
| route
and dose |
||
|---|---|---|
| iv 1 mg/kg | po, 10 mg/kg | |
| Tmax (h) | 0.5 | |
| Cmax (ng/mL) | 4627 ± 304 | 8243 ± 370 |
| AUC0–t (h·ng/mL) | 3375 ± 477 | 25672 ± 5668 |
| AUC0–∞ (h·ng/mL) | 3416 ± 495 | 25707 ± 5682 |
| T1/2 (h) | 1.4 ± 0.3 | 2.7 ± 0.2 |
| Cl (mL/min/kg) | 4.95 ± 0.77 | |
| Vdss (L/kg) | 0.41 ± 0.04 | |
| F (%) | 76.1 | |
Sprague–Dawley, n = 3.
Compound 36 showed good oral bioavailability (F = 76.1%) in rats and AUC0–∞ = 25707 ± 5682 h·ng/mL. Comparative results of the PK profile of 36 to reference compound E7046 was promising. Oral bioavailability of E7046/ER-886046 was reported to be greater than 31% with a half-life of 3 h in mice.18 Observed oral bioavailability of compound 36 was better than that of E7046.
Recent studies and clinical trials revealed that PGE2–EP4 activation plays an important role in the development of colorectal cancer (CRC) and COX2–PGE2 signaling influences the progression of CRC. Non-steroidal anti-inflammatory drugs (NSAIDs) or selective COX2 inhibitors are commonly used for the treatment of CRC. The EP4 antagonists were used for blocking the PGE2–EP4 signaling pathway and utilized as aq potential therapeutic method for colon cancer treatment.18,33 Toward that direction, we were interested to check the efficacy of EP4 antagonist 36 in colon cancer animal models. The CT-26 colon cancer subcutaneous mouse models have become popular for evaluating drugs. The inhibitory effect of E7046 was demonstrated in CT-26 colon cancer animal models. HCA-7 human cancer cells cannot grow on conventional immune cells easily. So, the CT-26 mouse model was chosen for efficacy study. The antitumor efficacy studies were done with a single drug (36) and a drug-combination formulation of 36 with capecitabine.
In vivo antitumor efficacy of 36 (AMX12006) was evaluated at different doses (75 mg/kg, 150 mg/kg) in a CT-26 colon cancer xenograft (BALB/c) mouse model.34 After the tumor volume reached 100–200 mm3, animals were randomly divided into groups and orally treated with compound 36 (75 mg/kg and 150 mg/kg) or control vehicle (0.5% carboxymethyl-cellulose sodium in phosphate-buffered saline (PBS)) or E7046 (150 mg/kg) as an active comparator, once daily for 11 days. At the end of the experiment, significant tumor growth inhibition was observed for the 36 treatment group, compared to the control group. As shown in Figure 3A,B, treatment with 36 significantly inhibited the tumor growth in a dose dependent manner. Tumor growth inhibition (TGI) of 32.0% at 75 mg/kg, and 51.78% at 150 mg/kg was observed (Table 6). No significant body weight loss was observed in any study group (Figure 3B), suggesting that 36 was well tolerated in mice at the tested dosage. The high-dose group of 36 (150 mg/kg) demonstrated better antitumor efficacy than the reference standard E7046 at the same dose (150 mg/kg, TGI 25.9%) (Figure 3C–F).
Figure 3.
In vivo efficacy study of compound 36 (AMX12006). (A) Mice bearing CT-26 tumors were treated with no drug (vehicle, 0.5% carboxymethyl-cellulose sodium), compound AMX12006 (75 mg/kg, 150 mg/kg; po; daily) for 11 days, and E7046 (150 mg/kg; po; daily). (B) Tumor weights were recorded on day 11. (C–F) Tumor growth curves of individual mice treated with vehicle, 36 (75 mg/kg, 150 mg/kg; po; daily), and E7046 (150 mg/kg; p.o.; daily). Data were presented as mean ± SEM. Dunnett’s multicomparison test tests were performed; *P < 0.05, **P < 0.01, ***P < 0.001 vs vehicle group; n = 6 per group.
Table 6. In Vivo Therapeutic Effect of 36 (AMX12006) in CT-26 BALB/c Mouse Xenograftsa.
| group (n) | treatment | mode of treatment | mean tumor vol., TV (mm3) | TGI (%) |
|---|---|---|---|---|
| G1 (6) | vehicle | po, qd | 2145.58 ± 152.9 | b |
| G2 (6) | E7046 150 mg/kg | po, qd | 1619.76 ± 276.89 | 25.9 |
| G3 (6) | AMX12006 75 mg/kg | po, qd | 1497.04 ± 282.39 | 32.0 |
| G4 (6) | AMX12006 150 mg/kg | po, qd | 1098.99 ± 247.15 | 51.78 |
po, oral dosing; qd, administered every day; vehicle, 0.5% carboxymethyl-cellulose sodium. The animals were administered the doses for 11 days and sacrificed on the same day; n = number of animals in each group.
Not applicable.
There is growing evidence that a combination of EP4 antagonists with chemotherapy or endocrine or immune based therapies is beneficial for treatment of cancers. Combination of EP4 antagonist MF-766 and anti-PD-1 demonstrated antitumor activity by differentially modulating myeloid cells, NK cells, and T-cells.26 L001 alone or combined with a chemotherapy drug, gemcitabine, exhibited antimetastatic activity in a pancreatic cancer.22 Capecitabine is extensively used as the first-line treatment option for colorectal cancer.35 It is relatively cheap and widely used in a large dose, 2500 mg/m2, in clinical use. Combination formulas of capecitabine were effective for breast cancer treatment.36 The combination of 36 with capecitabine was applied, and the effectiveness of the therapy was evaluated in the colon cancer model.
Tumor growth inhibition (TGI, %) was observed up to 68.85% for the combination of 36 (75 mg/kg) and capecitabine (300 mg/kg), higher than capecitabine alone (Figure 4 and Table 7). A very good improvement of TGI to 94.26% was observed for combination formula of 36 (150 mg/kg) and capecitabine (300 mg/kg). No significant body weight loss was observed in any study group (Figure 4B), suggesting that the drug combination was well tolerated in mice at the tested dosages.
Figure 4.
In vivo efficacy study of compound 36 (AMX12006) with capecitabine. (A) Mice bearing CT-26 tumors were treated with no drug (vehicle, 0.5% carboxymethyl-cellulose sodium), capecitabine (300 mg/kg), AMX12006 (75 mg/kg) + capecitabine (300 mg/kg), AMX12006 (150 mg/kg) + capecitabine (300 mg/kg), or E7046 (150 mg/kg). (B) Body weight. (C) Tumor weight. (D–G) Tumor growth curves of individual mice treated with vehicle (150 mg/kg), capecitabine, AMX12006 (75 mg/kg) + capecitabine (300 mg/kg), or AMX12006 (150 mg/kg) + capecitabine (300 mg/kg). Data were presented as mean ± SEM. Dunnett’s multicomparison tests were performed; *P < 0.05, **P < 0.01, ***P < 0.001 vs vehicle group; n = 6 per group.
Table 7. In Vivo Therapeutic Effect of 36 (AMX12006) in Combination with Capecitabine in CT-26 BALB/c Mouse Xenograftsa.
| group (n) | treatment | mode of treatment | mean tumor vol., TV (mm3) | TGI (%) |
|---|---|---|---|---|
| G1 (6) | vehicle | po, qd | 2036.95 ± 333.12 | b |
| G2 (6) | capecitabine (300 mg/kg) | po, qd | 707.13 ± 124.69 | 67.88 |
| G3 (6) | AMX12006 (75 mg/kg) + capecitabine (300 mg/kg) | po, qd | 688.04 ± 177.96 | 68.85 |
| G4 (6) | AMX12006 (150 mg/kg) + capecitabine (300 mg/kg) | po, qd | 190.24 ± 53.87 | 94.26 |
po, oral dosing; qd, administered every day; vehicle, 0.5% carboxymethyl-cellulose sodium. The animals were administered the doses starting from the 8th day after tumor implantation and continuing until 21 days and sacrificed on the same day; n = number of animals in each group.
Not applicable.
In conclusion, a new series of indole-2-carboxamide derivatives were designed, synthesized, and demonstrated as potent and selective EP4 antagonists. Thorough SAR studies and PK analysis of the series was done, and compound 36 was identified as one of the best compounds and as highly selective over other EP receptors with nanomolar potency. On the basis of its favorable pharmacokinetic profile and good oral bioavailability (F = 76.1%), compound 36 was chosen for in vivo efficacy studies. In a CT-26 colon cancer mouse model, compound 36 demonstrated better antitumor efficacy at different doses than a reference standard, E7046. The combination formulation of 36 with capecitabine, a chemotherapy agent, produced synergistic effect and was more efficacious than the single drug. The drug combination was effective for controlling colorectal cancer and achieved TGI up to 94.26% in mouse models. The indole-2-carboxamide derivative 36 (AMX12006) is safe in a hERG assay (IC50 > 40 μM) and it is a suitable drug candidate for further development to treat cancer and inflammatory diseases.
Acknowledgments
The authors are thankful to Suzhou Medinoah Co. Ltd, Sundia, Shanghai Medicilon Inc., Peng Li Biomedical Technology (Shanghai) Co., Ltd. and ICE Bioscience Inc. for providing analytical and biological tests services.
Glossary
Abbreviations
- cAMP
cyclic adenosine monophosphate
- COX
cyclooxygenase
- EP4
EP4 receptor
- FDA
Food and Drug Administration
- NSCLC
non-small-cell lung cancer
- NSAID
non-steroidal anti-inflammatory drug
- PGE2
prostaglandin E2
- TME
tumor microenvironment
- TGI
tumor growth inhibition
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.2c00495.
Synthetic procedures, analytical data of 25, 33–38, 41, and 42, in vitro enzyme, cell assay, and ADME and in vivo efficacy of 36 and molecular modeling method (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Yokoyama U.; Iwatsubo K.; Umemura M.; Fujita T.; Ishikawa Y. The Prostanoid EP4 Receptor and Its Signaling Pathway. Pharmacol. Rev. 2013, 65 (3), 1010–1052. 10.1124/pr.112.007195. [DOI] [PubMed] [Google Scholar]
- Smith W. L.; Langenbach R. Why There Are Two Cyclooxygenase Isozymes. J. Clin. Invest. 2001, 107 (12), 1491–1495. 10.1172/JCI13271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvado M. D.; Alfranca A.; Haeggström J. Z.; Redondo J. M. Prostanoids in Tumor Angiogenesis: Therapeutic Intervention beyond COX-2. Trends in Molecular Medicine 2012, 18 (4), 233–243. 10.1016/j.molmed.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Take Y.; Koizumi S.; Nagahisa A. Prostaglandin E Receptor 4 Antagonist in Cancer Immunotherapy: Mechanisms of Action. Frontiers in Immunology 2020, 11, 324. 10.3389/fimmu.2020.00324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder M.; Nandi P.; Omar A.; Ugwuagbo K.; Lala P. EP4 as a Therapeutic Target for Aggressive Human Breast Cancer. International Journal of Molecular Sciences 2018, 19 (4), 1019. 10.3390/ijms19041019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada N.; Shimizu Y.; Kamba T.; Inoue T.; Maeno A.; Kobayashi T.; Nakamura E.; Kamoto T.; Kanaji T.; Maruyama T.; Mikami Y.; Toda Y.; Matsuoka T.; Okuno Y.; Tsujimoto G.; Narumiya S.; Ogawa O. Identification of EP4 as a Potential Target for the Treatment of Castration-Resistant Prostate Cancer Using a Novel Xenograft Model. Cancer Res. 2010, 70 (4), 1606–1615. 10.1158/0008-5472.CAN-09-2984. [DOI] [PubMed] [Google Scholar]
- Simper M. S.; Rundhaug J. E.; Mikulec C.; Bowen R.; Shen J.; Lu Y.; Lin K.; Surh I.; Fischer S. M. The Tumor Promoting Activity of the EP4 Receptor for Prostaglandin E 2 in Murine Skin. Molecular Oncology 2014, 8 (8), 1626–1639. 10.1016/j.molonc.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thumkeo D.; Punyawatthananukool S.; Prasongtanakij S.; Matsuura R.; Arima K.; Nie H.; Yamamoto R.; Aoyama N.; Hamaguchi H.; Sugahara S.; Takeda S.; Charoensawan V.; Tanaka A.; Sakaguchi S.; Narumiya S. PGE2-EP2/EP4 Signaling Elicits Immunosuppression by Driving the MregDC-Treg Axis in Inflammatory Tumor Microenvironment. Cell Reports 2022, 39 (10), 110914. 10.1016/j.celrep.2022.110914. [DOI] [PubMed] [Google Scholar]
- Das D.; Hong J. Prostaglandin E2 Receptor 4 (EP4): A Promising Therapeutic Target for the Treatment of Cancer and Inflammatory Diseases. Current Chemical Biology 2021, 15 (1), 50–68. 10.2174/2212796814999201222101310. [DOI] [Google Scholar]
- Xi Y.; Xu P. Global Colorectal Cancer Burden in 2020 and Projections to 2040. Translational Oncology 2021, 14 (10), 101174. 10.1016/j.tranon.2021.101174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazuelo E.; Lanas A. NSAIDS and Gastrointestinal Cancer. Prostaglandins & Other Lipid Mediators 2015, 120, 91–96. 10.1016/j.prostaglandins.2015.06.001. [DOI] [PubMed] [Google Scholar]
- Marsico F.; Paolillo S.; Filardi P. P. NSAIDs and Cardiovascular Risk:. Journal of Cardiovascular Medicine 2017, 18, e40 10.2459/JCM.0000000000000443. [DOI] [PubMed] [Google Scholar]
- Nakao K.; Murase A.; Ohshiro H.; Okumura T.; Taniguchi K.; Murata Y.; Masuda M.; Kato T.; Okumura Y.; Takada J. CJ-023,423, a Novel, Potent and Selective Prostaglandin EP 4 Receptor Antagonist with Antihyperalgesic Properties. Journal of Pharmacology and Experimental Therapeutics 2007, 322 (2), 686–694. 10.1124/jpet.107.122010. [DOI] [PubMed] [Google Scholar]
- Murase A.; Okumura T.; Sakakibara A.; Tonai-Kachi H.; Nakao K.; Takada J. Effect of Prostanoid EP4 Receptor Antagonist, CJ-042,794, in Rat Models of Pain and Inflammation. Eur. J. Pharmacol. 2008, 580 (1–2), 116–121. 10.1016/j.ejphar.2007.10.054. [DOI] [PubMed] [Google Scholar]
- Blouin M.; Han Y.; Burch J.; Farand J.; Mellon C.; Gaudreault M.; Wrona M.; Lévesque J.-F.; Denis D.; Mathieu M.-C.; Stocco R.; Vigneault E.; Therien A.; Clark P.; Rowland S.; Xu D.; O’Neill G.; Ducharme Y.; Friesen R. The Discovery of 4-{1-[({2,5-Dimethyl-4-[4-(Trifluoromethyl)Benzyl]-3-Thienyl}carbonyl)Amino]Cyclopropyl}benzoic Acid (MK-2894), A Potent and Selective Prostaglandin E 2 Subtype 4 Receptor Antagonist †. J. Med. Chem. 2010, 53 (5), 2227–2238. 10.1021/jm901771h. [DOI] [PubMed] [Google Scholar]
- Caselli G.; Bonazzi A.; Lanza M.; Ferrari F.; Maggioni D.; Ferioli C.; Giambelli R.; Comi E.; Zerbi S.; Perrella M.; Letari O.; Di Luccio E.; Colovic M.; Persiani S.; Zanelli T.; Mennuni L.; Piepoli T.; Rovati L. C. Pharmacological Characterisation of CR6086, a Potent Prostaglandin E2 Receptor 4 Antagonist, as a New Potential Disease-Modifying Anti-Rheumatic Drug. Arthritis Research Therapy 2018, 20 (1), 39. 10.1186/s13075-018-1537-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani T.; Takano H.; Yoshida T.; Hamasaki R.; Kohanbash G.; Takeda K.; Okada H. Abstract 4443: Inhibition of PGE2/EP4 Pathway by ONO-4578/BMS-986310, a Novel EP4 Antagonist, Promotes T Cell Activation and Myeloid Cell Differentiation to Dendritic Cells. Immunology 2020, 80, 4443. 10.1158/1538-7445.AM2020-4443. [DOI] [Google Scholar]
- Albu D. I.; Wang Z.; Huang K.-C.; Wu J.; Twine N.; Leacu S.; Ingersoll C.; Parent L.; Lee W.; Liu D.; Wright-Michaud R.; Kumar N.; Kuznetsov G.; Chen Q.; Zheng W.; Nomoto K.; Woodall-Jappe M.; Bao X. EP4 Antagonism by E7046 Diminishes Myeloid Immunosuppression and Synergizes with Treg-Reducing IL-2-Diphtheria Toxin Fusion Protein in Restoring Anti-Tumor Immunity. OncoImmunology 2017, 6 (8), e1338239 10.1080/2162402X.2017.1338239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco M.-J.; Vetman T.; Chandrasekhar S.; Fisher M. J.; Harvey A.; Kuklish S. L.; Chambers M.; Lin C.; Mudra D.; Oskins J.; Wang X.-S.; Yu X.-P.; Warshawsky A. M. Identification and Biological Activity of 6-Alkyl-Substituted 3-Methyl-Pyridine-2-Carbonyl Amino Dimethyl-Benzoic Acid EP4 Antagonists. Bioorg. Med. Chem. Lett. 2016, 26 (9), 2303–2307. 10.1016/j.bmcl.2016.03.041. [DOI] [PubMed] [Google Scholar]
- Cheng Z.; Wang Y.; Zhang Y.; Zhang C.; Wang M.; Wang W.; He J.; Wang Y.; Zhang H.; Zhang Q.; Ding C.; Wu D.; Yang L.; Liu M.; Lu W. Discovery of 2 H -Indazole-3-Carboxamide Derivatives as Novel Potent Prostanoid EP4 Receptor Antagonists for Colorectal Cancer Immunotherapy. J. Med. Chem. 2023, 66 (9), 6218–6238. 10.1021/acs.jmedchem.2c02058. [DOI] [PubMed] [Google Scholar]
- Wang W.; He J.; Yang J.; Zhang C.; Cheng Z.; Zhang Y.; Zhang Q.; Wang P.; Tang S.; Wang X.; Liu M.; Lu W.; Zhang H.-K. Scaffold Hopping Strategy to Identify Prostanoid EP4 Receptor Antagonists for Cancer Immunotherapy. J. Med. Chem. 2022, 65 (11), 7896–7917. 10.1021/acs.jmedchem.2c00448. [DOI] [PubMed] [Google Scholar]
- He J.; Lin X.; Meng F.; Zhao Y.; Wang W.; Zhang Y.; Chai X.; Zhang Y.; Yu W.; Yang J.; Li G.; Du X.; Zhang H.; Liu M.; Lu W. A Novel Small Molecular Prostaglandin Receptor EP4 Antagonist, L001, Suppresses Pancreatic Cancer Metastasis. Molecules 2022, 27 (4), 1209. 10.3390/molecules27041209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoos A. Development of Immuno-Oncology Drugs — from CTLA4 to PD1 to the next Generations. Nat. Rev. Drug Discov 2016, 15 (4), 235–247. 10.1038/nrd.2015.35. [DOI] [PubMed] [Google Scholar]
- Lu W.; Yu W.; He J.; Liu W.; Yang J.; Lin X.; Zhang Y.; Wang X.; Jiang W.; Luo J.; Zhang Q.; Yang H.; Peng S.; Yi Z.; Ren S.; Chen J.; Siwko S.; Nussinov R.; Cheng F.; Zhang H.; Liu M. Reprogramming Immunosuppressive Myeloid Cells Facilitates Immunotherapy for Colorectal Cancer. EMBO Mol. Med. 2021, 13 (1), e12798. 10.15252/emmm.202012798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J.-J.; Yu W.-W.; Hu L.-L.; Liu W.-J.; Lin X.-H.; Wang W.; Zhang Q.; Wang P.-L.; Tang S.-W.; Wang X.; Liu M.; Lu W.; Zhang H.-K. Discovery and Characterization of 1H-1,2,3-Triazole Derivatives as Novel Prostanoid EP4 Receptor Antagonists for Cancer Immunotherapy. J. Med. Chem. 2020, 63 (2), 569–590. 10.1021/acs.jmedchem.9b01269. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Cui L.; Georgiev P.; Singh L.; Zheng Y.; Yu Y.; Grein J.; Zhang C.; Muise E. S.; Sloman D. L.; Ferguson H.; Yu H.; Pierre C. St.; Dakle P. J.; Pucci V.; Baker J.; Loboda A.; Linn D.; Brynczka C.; Wilson D.; Haines B. B.; Long B.; Wnek R.; Sadekova S.; Rosenzweig M.; Haidle A.; Han Y.; Ranganath S. H. Combination of EP 4 Antagonist MF-766 and Anti-PD-1 Promotes Anti-Tumor Efficacy by Modulating Both Lymphocytes and Myeloid Cells. OncoImmunology 2021, 10 (1), 1896643. 10.1080/2162402X.2021.1896643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das D.; Xie L.; Wang J.; Shi J.; Hong J. In Vivo Efficacy Studies of Novel Quinazoline Derivatives as Irreversible Dual EGFR/HER2 Inhibitors, in Lung Cancer Xenografts (NCI-H1975) Mice Models. Bioorganic Chemistry 2020, 99, 103790. 10.1016/j.bioorg.2020.103790. [DOI] [PubMed] [Google Scholar]
- Das D.; Wang J.; Li Y.; Shi J.; Hong J. Design, Synthesis of Orally Bioavailable Novel Anaplastic Lymphoma Kinase (ALK) Inhibitor Diphenylaminopyrimidine Analogs and Efficacy Study on NCI-H2228 Xenografts Mice Model. Bioorg. Med. Chem. Lett. 2019, 29 (12), 1514–1517. 10.1016/j.bmcl.2019.04.012. [DOI] [PubMed] [Google Scholar]
- Hong J.; Das D.; Wang J.; Qiao D.. Amide Derivative Serving as Prostaglandin EP4 Receptor Antagonist and Use Thereof. WO2022111222A1, 2022.
- Brown D. G.; Boström J. Where Do Recent Small Molecule Clinical Development Candidates Come From?. J. Med. Chem. 2018, 61 (21), 9442–9468. 10.1021/acs.jmedchem.8b00675. [DOI] [PubMed] [Google Scholar]
- Toyoda Y.; Morimoto K.; Suno R.; Horita S.; Yamashita K.; Hirata K.; Sekiguchi Y.; Yasuda S.; Shiroishi M.; Shimizu T.; Urushibata Y.; Kajiwara Y.; Inazumi T.; Hotta Y.; Asada H.; Nakane T.; Shiimura Y.; Nakagita T.; Tsuge K.; Yoshida S.; Kuribara T.; Hosoya T.; Sugimoto Y.; Nomura N.; Sato M.; Hirokawa T.; Kinoshita M.; Murata T.; Takayama K.; Yamamoto M.; Narumiya S.; Iwata S.; Kobayashi T. Ligand Binding to Human Prostaglandin E Receptor EP4 at the Lipid-Bilayer Interface. Nat. Chem. Biol. 2019, 15 (1), 18–26. 10.1038/s41589-018-0131-3. [DOI] [PubMed] [Google Scholar]
- Emkey R.; Rankl N. B.. Screening G Protein-Coupled Receptors: Measurement of Intracellular Calcium Using the Fluorometric Imaging Plate Reader. In High Throughput Screening; Janzen W. P., Bernasconi P., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, 2009; Vol. 565, pp 145–158, 10.1007/978-1-60327-258-2_7. [DOI] [PubMed] [Google Scholar]
- Karpisheh V.; Joshi N.; Zekiy A. O.; Beyzai B.; Hojjat-Farsangi M.; Namdar A.; Edalati M.; Jadidi-Niaragh F. EP4 Receptor as a Novel Promising Therapeutic Target in Colon Cancer. Pathology - Research and Practice 2020, 216 (12), 153247. 10.1016/j.prp.2020.153247. [DOI] [PubMed] [Google Scholar]
- Pozzi A.; Yan X.; Macias-Perez I.; Wei S.; Hata A. N.; Breyer R. M.; Morrow J. D.; Capdevila J. H. Colon Carcinoma Cell Growth Is Associated with Prostaglandin E2/EP4 Receptor-Evoked ERK Activation. J. Biol. Chem. 2004, 279 (28), 29797–29804. 10.1074/jbc.M313989200. [DOI] [PubMed] [Google Scholar]
- Twelves C. Capecitabine as First-Line Treatment in Colorectal Cancer. Eur. J. Cancer 2002, 38, 15–20. 10.1016/S0959-8049(01)00415-4. [DOI] [PubMed] [Google Scholar]
- Rinnerthaler G.; Gampenrieder S. P.; Petzer A.; Hubalek M.; Petru E.; Sandholzer M.; Andel J.; Balic M.; Melchardt T.; Hauser-Kronberger C.; Schmitt C. A.; Ulmer H.; Greil R. Capecitabine in Combination with Bendamustine in Pretreated Women with HER2-Negative Metastatic Breast Cancer: Results of a Phase II Trial (AGMT MBC-6). Ther Adv. Med. Oncol 2021, 13, 175883592110423. 10.1177/17588359211042301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.