Abstract
Targeted protein degradation (TPD), using chimeric molecules such as proteolysis-targeting chimeras (PROTACs), has attracted attention as a strategy for selective degradation of intracellular proteins by hijacking the ubiquitin-proteasome system (UPS). However, it is often difficult to develop such degraders due to the absence of appropriate ligands for target proteins. In targeting proteins for degradation, the application of nucleic acid aptamers is considered to be effective because these can be explored using systematic evolution of ligand by exponential enrichment (SELEX) methods. In this study, we constructed chimeric molecules in which nucleic acid aptamers capable of binding to the estrogen receptor α (ERα) and E3 ubiquitin ligase ligands were linked via a linker. ERα aptamer-based PROTACs were found to degrade ERα via the UPS. These findings represent the development of novel aptamer-based PROTACs that target intracellular proteins and are potentially applicable to other proteins.
Keywords: ubiquitin-proteasome system, PROTAC, aptamer, estrogen receptor
A number of drug discovery technologies have been developed to target a multitude of proteins involved in the pathogenesis of various diseases. Recently, protein degraders based on the ubiquitin-proteasome system (UPS), have attracted significant attention; proteolysis-targeting chimeras (PROTACs)1 and specific and nongenetic inhibitor of apoptosis protein [IAP]-dependent protein erasers (SNIPERs)2,3 are typical examples. These degraders were part of an innovative drug discovery aimed at treating diseases refractory to existing therapies.
These molecules consist of a ligand that binds to the target protein (protein of interest, POI) and a ubiquitin ligase (E3 ligase), that catalyzes its ubiquitination. PROTAC acts as a molecular mediator, binding both the POI and E3 ligase in close proximity, thereby promoting ubiquitination and subsequent degradation of the POI. Numerous degradation inducers have been developed, demonstrating that a variety of targets can be degraded using this system.4,5 ARVINAS Inc. has recently conducted phase 2 clinical trials of their newly developed PROTACs (ARV-110 and ARV-471),6−8 and it is expected that, in the future, further degraders will be formulated to act as novel therapeutic agents. Thus, PROTACs have been developed primarily using small molecule compounds as binding ligands for target proteins; several small molecule PROTACs with multiple targets have successfully entered clinical trials. However, some disadvantages of PROTAC-induced targeted protein degradation (TPD) include insufficiency of available ligand against a POI and difficulty in synthesis. To address these shortcomings, non-small molecule PROTACs such as peptide PROTACs, nucleic acid PROTACs, and antibody PROTACs have emerged in recent years.9 PROTACs using peptide-based ligands that bind to the surfaces of target proteins are easier to design than small molecule-based PROTACs;10 in fact, many proteins that cannot be targeted by small molecules can be degraded by peptide PROTACs.11−13
An alternative approach is to use nucleic acids as warheads for binding a POI.14 The emergence of nucleic acid PROTACs provides the possibility of direct targeting of elusive POIs, that are difficult to target with small molecules such as transcription factors (TFs) and nonenzymatic proteins. For example, TF-targeting PROTACs accomplish direct targeting for aberrant TF-related diseases. Some TFs can be selectively degraded by employing double-stranded decoys as POI ligands.15,16 Recently, our group also developed a decoy nucleic acid–based PROTAC against the estrogen receptor α (ERα) and demonstrated that this PROTAC was capable of selectively degrading the ERα.17 Thus, selective PROTACs can be readily designed by utilizing known nucleic acid sequences targeting TFs. However, this type of PROTAC has limited potential targets.
In view of these problems, we aimed to develop a new type of nucleic acid-based PROTAC, which uses nucleic acid aptamers as POI ligands, thereby broadening the range of target proteins for TPD strategies. Nucleic acid aptamers are single strands of oligonucleotides around 20–100 bases in length, which can be chemically synthesized.18,19 Nucleic acid aptamers can adopt certain conformations, binding to various target molecules including proteins, with high affinity and specificity. Aptamers for various target proteins can be explored by SELEX (Systematic Evolution of Ligands by Exponential Enrichment).20 SELEX finds nucleic acid aptamers that strongly bind to specific molecular targets from a single stranded DNA and/or RNA library of random sequences. This technology makes it possible to obtain suitable aptamers for almost any target protein,21−23 and permits discrimination between wild-type and mutant proteins.24 Nucleic acid aptamer-based PROTACs have recently been reported25 by Zhang et al. and other groups who have used AS1411,26,27 an aptamer against nucleolin, to achieve tumor selective degradation.28−30 Nucleolin is highly expressed on the surface of tumor cells31 and this aptamer-based approach using AS1411 enables tumor cell-selective intracellular delivery of PROTAC molecules. Their approach was developed for improving the tumor-specific targeting ability and in vivo antitumor potency of conventional PROTACs.
In contrast to published reports, we postulated that nucleic acid aptamer-based PROTACs against intracellular proteins could be used as a strategy to expand the range of target molecules in TPD. In the present study, we designed and synthesized aptamer-based PROTACs against the ERα as a model target (Figure 1).
Figure 1.

Design of nucleic acid aptamer-based PROTACs against ERα.
The ERα-binding aptamer ER(apt)D1 (40 nucleotides) is a known sequence found using the SELEX method.32 The 5′-end of the aptamer (5′-CCCGGCATGGTTGCGGAGCAGGAGTATAACACTACCATTG-3′) was modified with a hexynyl group. For construction of the E3 ligase ligands, a PEG3 linker with an azide group was attached to cIAP ligand, LCL161 (LCL), VHL ligand, VH032 (VH), and CRBN ligand, pomalidomide (POM), respectively.17 The 5′-alkyne-modified ER(apt)D1 was bound to each azide-containing E3 ligase ligand by copper-catalyzed click reactions to obtain the desired chimeric molecules, LCL-ER(apt)D1, VH-ER(apt)D1 and POM-ER(apt)D1 (Figure 2). Fluorescein (FAM)-labeled 3′ ends of each aptamer, ER(apt)D1-FAM, LCL-ER(apt)D1-FAM, VH-ER(apt)D1-FAM, POM-ER(apt)D1-FAM, and POM-Scr(apt)D1-FAM, were synthesized for use in fluorescence polarization (FP) assays. The detailed synthetic protocols of these molecules are described in the Supporting Information (Figure S1).
Figure 2.
Chimeric molecules LCL-ER(apt)D1, VH-ER(apt)D1, POM-ER(apt)D1, and POM-Scr(apt)D1 synthesized in this study.
The preferred higher order structures of the synthesized ER(apt)D1 and the PROTACs, LCL-ER(apt)D1, VH-ER(apt)D1, and POM-ER(apt)D1, were analyzed using CD spectra. Each PROTAC showed a negative maximum at around 250 nm and a positive maximum at around 280 nm. This was similar to ER(apt)D1, indicating that the linkage of aptamer to the E3 ligase ligand did not significantly affect the formation of the higher-order aptamer structure (Figure S2).
The binding affinities of ER(apt)D1 and each PROTAC (LCL-ER(apt)D1, VH-ER(apt)D1, and POM-ER(apt)D1) to the ERα were evaluated by FP assays. The dissociation constant Kd values were calculated by mixing and incubating a fixed concentration of ER(apt)D1 with different concentrations of ERα. The Kd values were 7.8 nM for ER(apt)D1, 36.4 nM for LCL-ER(apt)D1, 21.2 nM for VH-ER(apt)D1, and 10.4 nM for POM-ER(apt)D1 (Figure S3 and Table 1). These results indicate that the binding activity of aptamer ER(apt)D1 to the ERα is retained to the extent that when ER(apt)D1 is linked to E3 ligase ligands, the compound is sufficiently able to bind to ERα, regardless of E3 ligase ligand type.
Table 1. ERα Binding Affinity (Kd) of ER(apt)D1, LCL-ER(apt)D1, VH-ER(apt)D1, POM-ER(apt)D1, and POM-Scr(apt)D1a.
| entry | compound | Kd (nM) |
|---|---|---|
| 1 | ER(apt)D1-FAM | 7.8 ± 0.6 |
| 2 | LCL-ER(apt)D1-FAM | 36.4 ± 0.6 |
| 3 | VH-ER(apt)D1-FAM | 21.2 ± 3.2 |
| 4 | POM-ER(apt)D1-FAM | 10.4 ± 2.5 |
| 5 | POM-Scr(apt)D1-FAM | >400 |
Data represent the mean ± SD (n = 3).
To investigate whether POM-ER(apt)D1, VH-ER(apt)D1, and LCL-ER(apt)D1 degrade the ERα, the effects of these E3 ligand-aptamer conjugates on ERα protein expression were evaluated by Western blotting using MCF-7 breast cancer cells. All aptamer conjugates reduced ERα protein levels after 24 h of transfection; POM-ER(apt)D1 had the greatest effect compared with other aptamer conjugates (Figure 3A). In addition, we confirmed POM-ER(apt)D1 was efficiently taken up by MCF-7 cells by treatment with the transfection reagent (Figure S4). We next investigated the mechanism of ERα reduction by focusing on POM-ER(apt)D1, which showed the highest activity. To investigate the selectivity for the target protein, the effects of POM-ER(apt)D1 on the levels of different proteins were examined (Figure 3B). POM-ER(apt)D1 did not effectively reduce the levels of other transcription factors (AR, AhR, and NF-kB p65), transcription-related factors (p300 and BRD4), or proteins unrelated to transcription (CRABP2, GAPDH, and β-actin). These findings indicated that POM-ER(apt)D1 selectively reduced protein levels of ERα. The POM-ER(apt)D1- and VH-ER(apt)D1-induced reductions of ERα were abrogated by cotreatment with the proteasome inhibitor, MG132 and the ubiquitin-activating inhibitor, MLN7243 (Figure 3C). This suggested that both aptamer conjugates induced ubiquitin-proteasome system (UPS)-dependent degradation of the ERα. Unlike POM-ER(apt)D1, ER(apt)D1 did not induce ERα protein degradation (Figure 3D), indicating that conjugation of E3 ligands is important for degradation. In addition, the nondegradable control NMePOM-ER(apt)D1, which contains an N-methylated analog of the POM ligand, did not induce ERα degradation as effectively as POM-ER(apt)D1 (Figure S5), suggesting that the ability to bind CRBN is critical for the degradation activity. To examine whether target recognition by aptamer ligands was DNA-sequence dependent, we synthesized a POM ligand-aptamer chimera using a different aptamer composed of a scrambled DNA sequence as the target ligand. This aptamer chimera, POM-Scr(apt)D1, did not show significant ERα-degradation activity (Figure 3E), which correlated with its weak ERα binding affinity (Table 1). These results suggest that aptamer conjugates are useful strategies for the development of chimeric molecules capable of inducing targeted protein degradation.
Figure 3.

Degradation of the ERα by the synthesized aptamer-based PROTACs. (A, B) Each aptamer-based PROTAC selectively induced a reduction in ERα protein. MCF-7 cells were transiently transfected with indicated concentrations of POM-ER(apt)D1, VH-ER(apt)D1, and LCL-ER(apt)D1 for 24 h. (C) Effect of UPS inhibitors on aptamer-based PROTAC-induced reduction of ERα levels. MCF-7 cells were transiently transfected with indicated concentrations of PROTACs in the presence or absence of 10 μM MG132 or MLN7243 for 24 h. (D) ER(apt)D1 did not induce degradation of ERα protein. (E) Degradation of ERα by POM-ER(apt)D1 is DNA-sequence-dependent. MCF-7 cells were transiently transfected with indicated concentrations of POM-Scr(apt)D1 for 24 h. Whole-cell lysates were analyzed by Western blotting with the indicated antibodies; representative data are shown. The numbers below the ERα panels represent the ER/actin ratios, normalized by designating expression of the vehicle control (condition without a decoy) as 100%. The changes in protein levels were reproducible between two independent experiments. The data in the bar graph are the means of two experiments. Abbreviations: AR, androgen receptor; AhR, aryl hydrocarbon receptor; BRD4, bromodomain-containing protein 4; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; CRABP2, cellular retinoic acid binding protein 2.
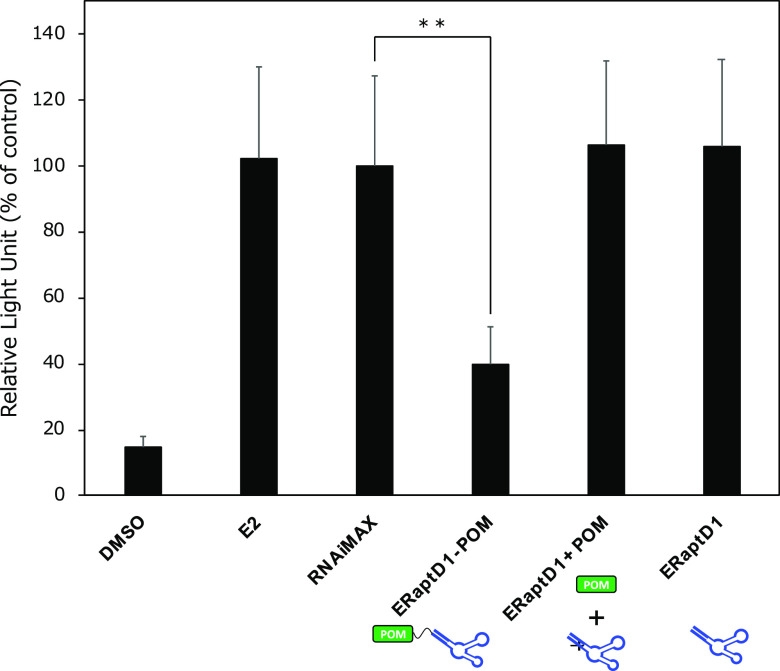
Finally, we utilized a luciferase gene reporter assay to evaluate the effect of POM-ER(apt)D1 on the estrogen-dependent transcriptional activity of ERα, given its essential role in estrogen signaling (Figure 4). Although ER(apt)D1 bound to the ERα (Table 1), ER(apt)D1 did not inhibit ERα-dependent transcriptional activation in this luciferase assay using an estrogen response element (ERE) reporter. This result suggested that ER(apt)D1, the nucleic acid aptamer used in this study, binds to ERα but does not inhibit transcriptional activity. We hypothesize that ER(apt)D1 might bind to a region distinct from the DNA-binding and ligand-binding domains of ERα. On the other hand, a decrease of transcriptional activity was observed in the presence of a chimeric molecule, POM-ER(apt)D1. This decrease in transcriptional activity might be due to degradation of intracellular ERα by POM-ER(apt)D1. Furthermore, a mixture of unconjugated molecules, ER(apt)D1 and POM–PEG3-N3 did not effectively decrease transcriptional activity, indicating that conjugation of these two ligands is essential for inhibition of ERα-dependent transcriptional activation. In addition, POM-ER(apt)D1, but not ER(apt)D1, effectively suppressed the proliferation of ERα-positive breast cancer MCF7 cells (Figure S6). These results demonstrated that POM-ER(apt)D1 induced ERα degradation leading to effective suppression of estrogen signaling.
Figure 4.
Inhibition of the estrogen-dependent transcriptional activity of ERα by POM-ER(apt)D1. MCF-7 cells were transiently transfected with a luciferase reporter plasmid containing three tandem copies of the ERE and control Renilla luciferase plasmid-SV40. After 24 h, the cells were further transfected with 30 μM concentrations of the indicated compounds in the presence of 3 nM β-estradiol (E2), and incubated for 24 h. The ERα-dependent transcriptional activity was evaluated by a luciferase assay, and the relative luciferase activity was normalized by designating the activity of the nontreated control (column 3; RNAiMAX) as 100%. The data represent the mean ± SD (n = 5). p-Values were determined using the unpaired two-tailed Student’s t-test. **p < 0.01.
In summary, we have developed aptamer-based PROTACs to expand the range of target proteins in TPD technologies. We selected the ERα as a model intracellular target protein. The chimeric molecules, LCL-ER(apt)D1, VH-ER(apt)D1, and POM-ER(apt)D1, were synthesized by conjugating a known ERα aptamer with E3 ligase ligands, LCL161, VH032, and POM; E3 ligase ligands commonly used in TPD. Among these chimeric molecules, POM-ER(apt)D1 showed the highest degradation-inducing activity against ERα via the UPS. In addition, POM-ER(apt)D1 selectively degraded the ERα without impacting levels of other TFs (AR, AhR, and NF-κB, p65), transcription-related factors (p300 and BRD4), and proteins (CRABP2, GAPDH, and β-actin). However, ERα was not degraded by chimeric compound POM-Scr(apt)D1 with scrambled DNA sequences. This result suggested that POM-ER(apt)D1 binds to its target, ERα, using the aptamer sequence as a warhead to induce degradation. Recently, an RNA aptamer-based PROTAC, which can distinguish and degrade mutant p53, has been reported.33 Although, the aptamers used in this study still require transfection reagents for introduction into cells due to their limited molecular size; using aptamers as POI ligands would be a useful strategy to expand the range of intracellular target proteins in TPD. In recent years, several modalities34 that can efficiently deliver nucleic acids into cells using drug delivery system (DDS) technologies, such as lipid nanoparticles, have been put into practical use, and many clinical trials are in progress. The combination of DDS technologies and aptamer-based PROTACs will be one of useful tools for degrading various intracellular proteins in the future.
Acknowledgments
We thank Edanz (https://www.jp.edanz.com/ac) for editing a draft of this manuscript.
Glossary
Abbreviations
- PROTACs
proteolysis targeting chimeras
- IAP
inhibitor of apoptosis protein
- SNIPERs
specific and nongenetic IAP-dependent protein erasers
- UPS
ubiquitin proteasome system
- TF
transcription factor
- ERα
estrogen receptor α
- POI
protein of interest
- TPD
targeted protein degradation
- SELEX
systematic evolution of ligands by exponential enrichment
- VHL
von Hippel-Lindau protein
- FAM
fluorescein
- FP
fluorescence polarization
- CD
circular dichroism
- DDS
drug delivery system
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.3c00126.
Synthetic procedures for all compounds listed in this manuscript and the protocols for the in vitro assays (binding and protein degradation assays) (PDF)
Author Contributions
¶ H.T. and M. Naganuma contributed equally to this work. H.T., N.O., Miyako Naganuma, and G.T. performed the experiments and analyzed results. G.T., N.O., T.I., M. Naito, and Y.D. designed the research and wrote the paper. All authors discussed the results and commented on the manuscript.
This study was supported in part by grants from AMED under grant numbers JP21mk0101197 (to Y.D. and N.O.), JP22ak0101186 (to N.O. and T.I.), 21mk0101187 (to N.O. and T.I.), JP21fk0108564 (to N.O.), JP22fk0310504 (to N.O.) and JP21am0401003 (to T.I.). The study also received support from the Japan Society for the Promotion of Science (KAKENHI, grants JP21K05320 to Y.D.; JP23H04926 to Y.D.; JP18H05502 to M.N. and Y.D.; JP18K06567 and JP21K06490 to N.O.; JP19K16333 to G.T.; and JP21H02777 and 22F22107 to M.N.; JSPS Fellows grant 21J23036 to Miyako Naganuma).
The authors declare no competing financial interest.
Supplementary Material
References
- Békés M.; Langley D. R.; Crews C. M. PROTAC targeted protein degraders: the past is prologue. Nat. Rev. Drug. Discovery 2022, 21 (3), 181–200. 10.1038/s41573-021-00371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohoka N.; Okuhira K.; Ito M.; Nagai K.; Shibata N.; Hattori T.; Ujikawa O.; Shimokawa K.; Sano O.; Koyama R.; Fujita H.; Teratani M.; Matsumoto H.; Imaeda Y.; Nara H.; Cho N.; Naito M. In Vivo Knockdown of Pathogenic Proteins via Specific and Nongenetic Inhibitor of Apoptosis Protein (IAP)-dependent Protein Erasers (SNIPER). J. Biol. Chem. 2017, 292 (11), 4556–4570. 10.1074/jbc.M116.768853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohoka N.; Morita Y.; Nagai K.; Shimokawa K.; Ujikawa O.; Fujimori I.; Ito M.; Hayase Y.; Okuhira K.; Shibata N.; Hattori T.; Sameshima T.; Sano O.; Koyama R.; Imaeda Y.; Nara H.; Cho N.; Naito M. Derivatization of inhibitor of apoptosis protein (IAP) ligands yields improved inducers of estrogen receptor α degradation. J. Biol. Chem. 2018, 293 (18), 6776–6790. 10.1074/jbc.RA117.001091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K.; Crews C. M. PROTACs: past, present and future. Chem. Soc. Rev. 2022, 51 (12), 5214–5236. 10.1039/D2CS00193D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng S.; Huang W.; Zheng X.; Cheng L.; Zhang Z.; Wang J.; Shen Z. Proteolysis targeting chimera (PROTAC) in drug discovery paradigm: Recent progress and future challenges. Eur. J. Med. Chem. 2021, 210 (15), 112981. 10.1016/j.ejmech.2020.112981. [DOI] [PubMed] [Google Scholar]
- Mullard A. First targeted protein degrader hits the clinic. Nat. Rev. Drug Discovery 2019, 18, 237–239. 10.1038/d41573-019-00043-6. [DOI] [PubMed] [Google Scholar]
- Gao X.; Burris H. A.; Vuky J.; Dreicer R.; Sartor A. O.; Sternberg C. N.; Percent I. J.; Hussain M. H. A.; Kalebasty A. R.; Shen J.; Heath E. I.; Abesada-Terk G.; Gandhi S. G.; McKean M.; Lu H.; Berghorn E.; Gedrich R.; Chirnomas S. D.; Vogelzang N. J.; Petrylak D. P. Phase 1/2 study of ARV-110, an androgen receptor (AR) PROTAC degrader, in metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 2020, 40 (6), 17. 10.1200/JCO.2022.40.6_suppl.017. [DOI] [Google Scholar]
- Hamilton E. P.; Schott A. F.; Nanda R.; Lu H.; Keung C. F.; Gedrich R.; Parameswaran J.; Han H. S.; Hurvitz S. A. ARV-471, an estrogen receptor (ER) PROTAC degrader, combined with palbociclib in advanced ER+/human epidermal growth factor receptor 2–negative (HER2-) breast cancer: Phase 1b cohort (part C) of a phase 1/2 study. J. Clin. Oncol. 2022, 40 (16), TPS1120. 10.1200/JCO.2022.40.16_suppl.TPS1120. [DOI] [Google Scholar]
- Ma S.; Ji J.; Tong Y.; Zhu J.; Dou J.; Zhang X.; Xu S.; Zhu T.; Xu X.; Qidong You Q.; Jiang Z. Non-small molecule PROTACs (NSM-PROTACs): Protein degradation kaleidoscope. Acta Pharm. Sin. B 2022, 12 (7), 2990–3005. 10.1016/j.apsb.2022.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J.; Wu Y.; Chen J.; Shen Y.; Zhang L.; Zhang H.; Chen L.; Yuan H.; Chen H.; Zhang W.; Luan K. The peptide PROTAC modality: a novel strategy for targeted protein ubiquitination. Theranostics 2020, 10 (22), 10141–10153. 10.7150/thno.46985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao H.; Li X.; Zhao L.; Wang Y.; Wang X.; Wu Y.; Zhou X.; Wei Fu W.; Liu L.; Hu H.-G.; Chen Y.-G. A PROTAC peptide induces durable β-catenin degradation and suppresses Wnt-dependent intestinal cancer. Cell Discovery 2020, 6, 35. 10.1038/s41421-020-0171-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D.; Zou Y.; Chu Y.; Liu Z.; Liu G.; Chu J.; Li M.; Wang J.; Sun S.-Y.; Chang Z. A cell-permeable peptide-based PROTAC against the oncoprotein CREPT proficiently inhibits pancreatic cancer. Theranostics 2020, 10 (8), 3708–3721. 10.7150/thno.41677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines J.; Gough J. D.; Corson T. W.; Crews C. M. Posttranslational protein knockdown coupled to receptor tyrosine kinase activation with phosphoPROTACs. Proc. Natl. Acad. Sci. U. S. A. 2013, 110 (22), 8942–8947. 10.1073/pnas.1217206110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W.; He S.; Dong G.; Sheng C. Nucleic-Acid-Based Targeted Degradation in Drug Discovery. J. Med. Chem. 2022, 65 (15), 10217–10232. 10.1021/acs.jmedchem.2c00875. [DOI] [PubMed] [Google Scholar]
- Liu J.; Chen H.; Kaniskan H. Ü.; Xie L.; Chen X.; Jin J.; Wenyi Wei W. TF PROTACs Enable Targeted Degradation of Transcription Factors. J. Am. Chem. Soc. 2021, 143 (23), 8902–8910. 10.1021/jacs.1c03852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao J.; Yan Y.; Ding D.; Wang D.; He Y.; Pan Y.; Yan W.; Kharbanda A.; Li H.; Huang H. Destruction of DNA-Binding Proteins by Programmable Oligonucleotide PROTAC (O’PROTAC): Effective Targeting of LEF1 and ERG. Adv. Sci. 2021, 8, 2102555. 10.1002/advs.202102555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naganuma M.; Ohoka N.; Tsuji G.; Tsujimura H.; Matsuno K.; Inoue T.; Naito M.; Demizu Y. Development of Chimeric Molecules That Degrade the Estrogen Receptor Using Decoy Oligonucleotide Ligands. ACS Med. Chem. Lett. 2022, 13 (1), 134–139. 10.1021/acsmedchemlett.1c00629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röthlisberger P.; Hollenstein M. Aptamer chemistry. Adv. Drug Delivery Rev. 2018, 134, 3–21. 10.1016/j.addr.2018.04.007. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Lai B. S.; Juhas M. Recent Advances in Aptamer Discovery and Applications. Molecules 2019, 24 (5), 941. 10.3390/molecules24050941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuerk C.; Gold L. Systematic Evolution of Ligands by Exponential Enrichment: RNA Ligands to Bacteriophage T4 DNA Polymerase. Science 1990, 249 (4968), 505–510. 10.1126/science.2200121. [DOI] [PubMed] [Google Scholar]
- Zhuo Z.; Yu Y.; Wang M.; Li J.; Zhang Z.; Liu J.; Wu X.; Lu A.; Zhang G.; Zhang B. Recent Advances in SELEX Technology and Aptamer Applications in Biomedicine. Int. J. Mol. Sci. 2017, 18 (10), 2142. 10.3390/ijms18102142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito S. SELEX-Based DNA Aptamer Selection: A Perspective from the Advancement of Separation Techniques. Anal. Sci. 2021, 37 (1), 17–26. 10.2116/analsci.20SAR18. [DOI] [PubMed] [Google Scholar]
- Xu Y.; Xin J.; Zhou Y.; Ma M.; Wang M.; Ying B. Systematic Evolution of Ligands by Exponential Enrichment Technologies and Aptamer-Based Applications: Recent Progress and Challenges in Precision Medicine of Infectious Diseases. Front. Bioeng. Biotechnol. 2021, 9, 704077. 10.3389/fbioe.2021.704077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong S.; Han S. R.; Lee Y. J.; Kim J. H.; Lee S.-W. Identification of RNA aptamer specific to mutant KRAS protein. Oligonucleotides 2010, 20 (3), 155–161. 10.1089/oli.2010.0231. [DOI] [PubMed] [Google Scholar]
- Ni S.; Zhuo Z.; Pan Y.; Yu Y.; Li F.; Liu J.; Wang L.; Wu X.; Li D.; Wan Y.; Zhang L.; Yang Z.; Zhang B.-T.; Lu A.; Zhang G. Recent Progress in Aptamer Discoveries and Modifications for Therapeutic Applications. ACS Appl. Mater. Interfaces 2021, 13 (8), 9500–9519. 10.1021/acsami.0c05750. [DOI] [PubMed] [Google Scholar]
- Yazdian-Robati R.; Bayat P.; Oroojalian F.; Zargari M.; Ramezani M.; Taghdisi S. M.; Abnous K. Therapeutic applications of AS1411 aptamer, an update review. Int. J. Biol. Macromol. 2020, 155, 1420–1431. 10.1016/j.ijbiomac.2019.11.118. [DOI] [PubMed] [Google Scholar]
- Bie L.; Wang Y.; Jiang F.; Xiao Z.; Zhang L.; Wang J. Insights into the binding mode of AS1411 aptamer to nucleolin. Front. Mol. Biosci. 2022, 9, 1025313. 10.3389/fmolb.2022.1025313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L.; Li L.; Wang X.; Liu H.; Zhang Y.; Xie T.; Zhang H.; Li X.; Peng T.; Sun X.; Dai J.; Liu J.; Wu W.; Ye M.; Tan W. Development of a novel PROTAC using the nucleic acid aptamer as a targeting ligand for tumor selective degradation of nucleolin. Mol. Ther. Nucleic Acids. 2022, 30, 66–79. 10.1016/j.omtn.2022.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S.; Gao F.; Ma J.; Ma H.; Dong G.; Sheng C. Aptamer-PROTAC Conjugates (APCs) for Tumor-Specific Targeting in Breast Cancer. Angew. Chem., Int. Ed. 2021, 60 (43), 23299–23305. 10.1002/anie.202107347. [DOI] [PubMed] [Google Scholar]
- Chen M.; Zhou P.; Kong Y.; Li J.; Li Y.; Zhang Y.; Ran j.; Zhou J.; Chen Y.; Xie S. Inducible Degradation of Oncogenic Nucleolin Using an Aptamer-Based PROTAC. J. Med. Chem. 2023, 66 (2), 1339–1348. 10.1021/acs.jmedchem.2c01557. [DOI] [PubMed] [Google Scholar]
- Carvalho J.; Paiva A.; Cabral Campello M. P.; Paulo A.; Mergny J.-L.; Salgado G. F.; Queiroz J. A.; Cruz C. Aptamer-based Targeted Delivery of a G-quadruplex Ligand in Cervical Cancer Cells. Sci. Rep. 2019, 9, 7945. 10.1038/s41598-019-44388-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sett A.; Borthakur B. B.; Sharma J. D.; Kataki A. C.; Bora U. DNA aptamer probes for detection of estrogen receptor α positive carcinomas. Transl. Res. 2017, 183, 104–120. 10.1016/j.trsl.2016.12.008. [DOI] [PubMed] [Google Scholar]
- Kong L.; Meng F.; Wu S.; Zhou P.; Ge R.; Liu M.; Zhang L.; Zhou J.; Zhong D.; Xie S. Selective degradation of the p53-R175H oncogenic hotspot mutant by an RNA aptamer-based PROTAC. Clin. Transl. Med. 2023, 13, e1191. 10.1002/ctm2.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes B. B.; Conniot J.; Avital A.; Yao D.; Jiang X.; Zhou X.; Sharf-Pauker N.; Xiao Y.; Adir O.; Liang H.; Shi J.; Schroeder A.; Conde J. Nanodelivery of nucleic acids. Nat. Rev. Method Primers 2022, 2, 22. 10.1038/s43586-022-00104-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.