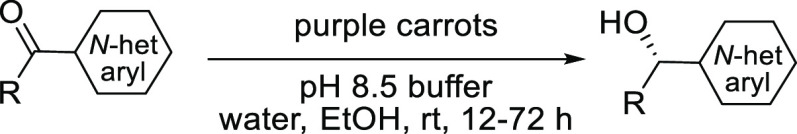
Abstract
We herein report an enantioselective bioreduction of ketones that bear the most frequently used nitrogen-heteroaromatics in FDA-approved drugs. Ten varieties of these nitrogen-containing heterocycles were systematically investigated. Eight categories were studied for the first time and seven types were tolerated, significantly expanding the substrate scope of plant-mediated reduction. By use of purple carrots in buffered aqueous media with a simplified reaction setup, this biocatalytic transformation was achieved within 48 h at ambient temperature, offering medicinal chemists a pragmatic and scalable tool to access a broad variety of nitrogen-heteroaryl-containing chiral alcohols. With multiple reactive sites, the structurally diverse set of chiral alcohols can be used for library compound preparation, early route-scouting activities, and synthesis of other pharmaceutical molecules, favorably accelerating medicinal chemistry campaigns.
Keywords: medicinally relevant, nitrogen-heteroaromatic, bioreduction, synthetic toolbox, practicality
Chiral secondary alcohols are prevalent in natural products and medicinal agents. Fused with nitrogen-heteroaromatics, they create marvelous structural complexity and diverse biological activity (Figure 1a). (+)-Bacillamide B (1), originally isolated in the Bahamas in 2007, displays antimicrobial and anti-HIV activities.1 Non-naturally occurring alcohols alongside their ether and amino derivatives that bear a nitrogen-heteroaromatic ring, such as thiazole (2),2 benzimidazole (3),3 pyrimidine (4, 5),4,5 pyrazine (6),6 quinoline (7),7 pyridine (8–10),8−12 and imidazole,13−15 are also commonly seen in medicinal chemistry studies, serving as important analogues for treatment of various diseases. For example, JAK2 (Janus kinase 2) inhibitor 5 and PI3Kδ (phosphoinositide-3-kinase δ) inhibitors 3 and 7 can be used to cure inflammatory diseases and cancers, whereas β3AR (β3 adrenergic receptor) agonist 9 is a potential therapeutic agent for obesity. Popular methods to prepare these alcohols from the corresponding ketones include chiral resolution of racemic alcohols and asymmetric ketone reduction (Figure 1b). Hydrolytic kinetic resolution of non-chiral epoxides and asymmetric addition of aryl nucleophiles to aldehydes are other common ways to prepare them. Chiral chromatography and kinetic resolution of racemic alcohols,16−18 likely the techniques most frequently used by medicinal chemists, discard half of the racemic materials. Therefore, enantioselective methods, such as Corey–Itsuno reduction19,20 and metal-mediated asymmetric (transfer) hydrogenation of heteroaryl ketones,21,22 represent better approaches, although they are sometimes problematic when applied to heteroaromatic ketones due to the chelating effect.23 Moreover, handling hydrogen gas21 requires stringent safety protocols, and removal of transition-metal-associated impurities can be practically challenging and costly.24 While enzymatic reduction of ketones with ketoreductases provides an alternative approach,25 expensive cofactors [NADH/NADPH (reduced form of nicotinamide adenine dinucleotide phosphate)] and the need for a system to recycle them create obstacles for scalable synthesis. Hence, developing practical protocols for asymmetric reduction of heteroaromatic ketones is desirable.
Figure 1.

Bioactive nitrogen-heteroaromatic-containing chiral alcohols, methods of preparation, and their popularity in FDA-approved drugs.
Motivated by early projects that required chiral alcohols bearing a structurally diverse set of nitrogen-heteroaromatics, we looked for pragmatic methods and eventually turned our attention to plant-mediated ketone reduction, given that neither a cofactor nor its recycling system is needed for this biocatalytic approach.26 However, this bioreduction often suffers from low conversion, poor enantioselectivity, and narrow substrate scope,26−28 for which reasons it does not meet our needs. Among previously tested plants, carrots present a better option, represented by the most studied reduction of acetophenone-type substrates;29,30 nevertheless, nitrogen-heteroaromatic ketone substrates have not been well studied. For instance, among ketones that bear the top 10 most commonly seen 5- and 6-membered N-heteroaromatics in FDA-approved drugs (Figure 1c),31 only 2-acetylthiazole and acetylpyridines were examined at higher incubation temperatures (37 °C)32 or long reaction times (7 days).33 Hence, we were curious to see if ketones bearing the other 8 types of nitrogen-heteroaromatics would be amenable to carrot-mediated reduction. To make this reaction practical for medicinal chemists, accomplishing this transformation within reasonable time (e.g., 48 h) at ambient temperature was equally crucial. Furthermore, previously reported plant-involved reactions often utilize an orbital shaker,26,30 which is rarely found in synthetic laboratories. Accordingly, a simplified setup using a stir plate would be ideal. Herein we report our findings.
Aiming to enhance reactivity, we suspected that using small/thin pieces of carrots would increase the substrate–catalyst contact surface and therefore benefit the conversion. Carrot cultivars34 and additives could be further considerations for accelerating the reaction. To avoid accumulation of organic substrates on the top surface of the reaction vessel, an Erlenmeyer flask was selected to perform the reaction for its reduced top-surface area. A stir plate was designated to replace the previously used orbital shaker for further streamlining the reaction setup. Table 1 summarizes our optimization of the reaction conditions.
Table 1. Optimization of the Reaction Conditionsa.
| entry | carrot cultivar | additive | time (h) | conv (%)b | yield, erc |
|---|---|---|---|---|---|
| 1 | orange | – | 96 | 46 | – |
| 2 | yellow | – | 96 | 38 | – |
| 3 | purple | – | 96 | 66 | – |
| 4d | purple | TPGS-750-M | 96 | 35 | – |
| 5d | purple | Triton X-100 | 96 | 18 | – |
| 6e | purple | buffer pH 6.0, 6.5, 7.0, 7.5 | 96 | 16, 31, 30, 41 | – |
| 7e | purple | buffer pH 8.0, 8.5, 9.0, 9.5 | 96 | 53, 90, 87, 85 | – |
| 8 | purple | buffer pH 8.5 | 48 | 62 | – |
| 9f | purple | buffer pH 8.5 | 48 | 87 | 78%, >99:1 |
General conditions: 14 (50 mg), water (35 mL), EtOH (1 mL), precut carrots (5 g), in Erlenmeyer flask, rt, stirred at 250 rpm.
Conversion was determined by LCMS under 254 nm wavelength.
Enantiomeric ratio was determined by chiral SFC.
2 wt% of additive (0.7 g) was used.
20 mM of 1 M buffer solution (0.7 mL) was applied.
Purple carrots (10 g), isolated yield.
To test our ideas, heteroaryl ketone 14 was stirred in water with carrot cubes [orange color, 2–3 mm, B/S (ratio of biocatalyst to substrate by weight) = 100] in an Erlenmeyer flask at 250 rpm on a stir plate. In 4 days, 46% conversion was observed (entry 1). Screening carrot cultivars using a pack of organic carrots of many colors from a local market (Trader Joe’s, 907 g, $2.49) revealed that purple carrots displayed the best reactivity, increasing the conversion to 66% (entries 1–3). Previous studies in certain enzyme activities showed that added surfactant might be beneficial to biocatalytic conversion as it serves as a reservoir for substrates, products, and presumably enzymes in aqueous media to moderate the degree of enzyme saturation, thereby enhancing reactivity.35,36 Unfortunately, introducing surfactant TPGS-750-M or Triton X-100 to the reaction markedly inhibited the enzyme activity in this case (entries 4 and 5). Noting that the pH of the aqueous reaction media changed from 7 to 5 after 96 h, we scrutinized the effect of buffer. Assessment of buffer solutions at pH 6.0 to 9.5 indicated that use of pH 8.5 buffer gave a remarkable 90% conversion (entries 6 and 7). Given the fast pace of early-stage drug discovery, we assessed shortening the reaction time to 48 h and were encouraged to obtain a 62% conversion (entry 8). While increasing the reaction temperature might further facilitate the reaction, we opted for the more energy-efficient and cost-effective option by doubling the biocatalyst. This proved beneficial, affording us 87% conversion in 48 h (B/S = 200) and delivering chiral alcohol 15 in 78% isolated yield and >99:1 er (entry 9).
Next, the scope of carrot-mediated reduction of ketones with nitrogen-heteroaromatics was systematically investigated (Tables 2 and 3). The top 10 most common 5- and 6-membered nitrogen-heteroaromatics in all FDA-approved small-molecule drugs were selected for evaluation. To guide our substrate design, we paid particular attention to the substitution patterns of these heterocycles in approved drugs (Table 2, left).31 For simplicity, a methyl group was substituted for more elaborate ketone substituents, as shown in the basic substrate scope (Table 2, right). Since thiazole-containing drugs predominantly bear two substituents, at the C2- and C4-positions of the thiazole ring, substrates with such patterns were subjected to testing. Pleasingly, alcohols 15 and 16 were collected in 78–95% yields and >99:1 er. However, no reactivity was identified for ketones decorated with imidazoles or indoles (17, 18) (for specific substrates, see SI, Table S3), while tetrazole-containing ketone 19 gave trace conversion. Following the substitution pattern of benzimidazole-containing drugs, substrates both mono- and di-substituted at the corresponding most frequently observed position(s) were analyzed. They performed well in this bioreduction, giving alcohols 20–22 in 73–89% yields and >99:1 er. The tri-substituted substrate with a C5-acetyl motif (23) was also tolerated, albeit with a moderate yield.
Table 2. Basic Substrate Scope: Evaluating the Most Common 5- and 6-Membered Nitrogen-Heteroaromatics in FDA-Approved Drugsa.
Reaction conditions: substrate (50 mg), water (35 mL), EtOH (1 mL), pH 8.5 buffer (0.7 mL), pre-cut purple carrots (10 g), in an Erlenmeyer flask, stirred at 250 rpm, rt, 48 h. Isolated yields are provided. The er was determined by chiral SFC or HPLC. Reaction times: a12 h, b24 h, c72 h. N.R.: no reaction. The substitution patterns of nitrogen-heteroaromatics with a frequency of over 10 in FDA-approved drugs are highlighted in the boxes (marked with the “club” symbol), whereas the corresponding patterns of quinazoline, quinoline, and pyrazine are not listed as not enough statistical data has been reached yet (frequency <10). E.g., for thiazole, “28/30” means that among 30 FDA-approved thiazole-containing drugs, 28 bear a substituent at this site.
Table 3. Extended Substrate Scope 3a.
Reaction conditions: substrate (50 mg), water (35 mL), EtOH (1 mL), pH 8.5 buffer (0.7 mL), pre-cut purple carrots (10 g), in an Erlenmeyer flask, stirred at 250 rpm. The er was determined by chiral SFC or HPLC. For compound 46: 1 g scale, 69% yield, 97:3 er.
Then, we examined ketones bearing 6-membered nitrogen-heteroaromatics. Pyridine substrates with di- and tri-substituents were first assessed, both displaying great reactivity and enantioselectivity (24, 25). For pyrimidine ketones, the position of the acetyl moiety did not affect the reduction (26–28), and di-substituted pyrimidines also delivered comparable results (29, 30). The tri-substituted substrate gave the desired product 31 in excellent enantioselectivity but low yield, likely due to the steric hindrance of this ketone arising from the neighboring methyl group. For ketones with quinazoline, quinoline, and pyrazine motifs, mono-substituted substrates were explored. The acetyl moiety could be placed at either the pyrimidine or the benzene ring of the quinazoline core, producing alcohols 32 and 33 in fair yields and great enantioselectivity. As to quinoline-containing substrates, the position of the acetyl group had little impact on reactivity, and outstanding enantioselective results were reached in all cases (34–37). Lastly, 2-acetylpyrazine was also smoothly reduced to chiral alcohol 38 in a single enantiomer with good yield. Overall, 7 out of the 10 categories of the most commonly used nitrogen-heteroaromatics tolerated this enantioselective reduction.
To further scrutinize the scope and limitations of this biocatalytic transformation, we studied additional alkyl nitrogen-heteroaromatic ketones with a focus on pyridine-type substrates (Table 3), considering that pyridine represents the most frequently used nitrogen-heteroaromatic in approved drugs.31 Ethyl ketone was well tolerated (39), whereas a CF3-containing substrate gave good yield yet diminished enantioselectivity (40). The ketone with an iPr motif suffered from low conversion albeit with a decent 93:7 er (41). These results indicate that both electronic and steric factors influence the reactivity and enantioselective outcome. Cyclic ketones were also well suited for this biocatalytic transformation, delivering alcohols 42 and 43, signifying potential application of this bioreduction in the synthesis of 5HT2c receptor agonist 8 (Figure 1). Swapping pyridine with pyrimidine barely affected the result (39 vs 44), suggesting that the trend observed in the pyridine ketone series would be applicable to ketones bearing other nitrogen-heteroaromatics.
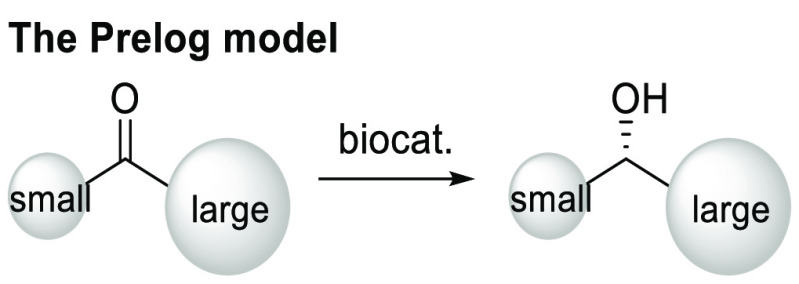
Moreover, we assessed ketones bearing functional groups that could serve as synthetic handles for other chemical transformations. Analysis of our internal construction of compound libraries over the past decade showed that the top five most applied chemistries included amide formation, Suzuki coupling, reductive amination, nucleophilic substitution, and Buchwald–Hartwig coupling.37 Therefore, substrates with functionalities that might participate in such transformations were tested (45–50). Pleasingly, all afforded outstanding enantioselectivity, and low reactivity was observed in only one case (48). Compounds 45 and 47 might serve as starting materials for amide libraries, while product 46 was suitable for nucleophilic substitution and Suzuki/Buchwald–Hartwig couplings. Functional groups were also tolerated on the alkyl chain (49, 50). Likewise, compound 49 might be used for click chemistry or reductive amination upon azide reduction. It is worth noting that the free 2-aminopyrimidine motif, found in many approved drugs,31 was also tolerated (47). The easy preparation of alcohols 47, 49, and 50 suggested that this biocatalytic reaction could be used to build bioactive molecules like HCN channel blocker 4, β3AR agonist 9, and Nav1.7 inhibitor 10 (Figure 1). During preparation of racemic alcohols 32, 33, and 46 using NaBH4, partial over-reduction of nitrogen-heteroaromatics was found. In contrast, no such side reaction was observed in our system, demonstrating the mild reaction conditions of the carrot-mediated reduction. The absolute stereochemistry of chiral alcohols 15, 20, 21, 34, 36, 38, 39, 41, and 43 was determined as (S) by reference to known optical rotations, and the remaining products were assigned in analogy, all following Prelog’s rule.38
Afterward, we tested the robustness of the bioreduction by first checking the carrot source. Purple carrots purchased in assorted packs from local stores within the largest grocery chains in the United States (for details, see SI) were subjected to the reaction using 3-acetylquinoline as the substrate (Table 4). To our delight, no difference in reactivity or enantioselectivity was discovered among carrots from Trader Joe’s, Whole Foods, Jewel-Osco, Mariano’s, and Target (entries 1–5). As previous reports always mentioned employing freshly cut carrots,30 we explored the robustness of the biocatalyst by placing freshly cut purple carrots in a sealed container and storing at 4 °C in a refrigerator for a week before use. No obvious change in enzymatic outcome was detected (entry 6). Lastly, to demonstrate the scalability and practicality of this transformation, reaction of 3-acetylquinoline was performed at 1.5 g scale using half of the biocatalyst and water (B/S = 100), delivering 1.41 g of optically pure alcohol 35 in 93% yield (eq 1). Product 46 could also be easily scaled up at 1 g scale (see Table 3 footnote), offering another interesting synthetic intermediate. It is worth noting that 1 g scale would satisfy the quantity requirement of starting materials for early route-scouting activities.
 |
1 |
Table 4. Studies of the Robustness of the Bioreductiona.
| entry | carrot vendorb | notes | conv (%)c | erd |
|---|---|---|---|---|
| 1 | Trader Joe’s (Aldi) | 907 g pack, $2.49, | 100 | >99:1 |
| carrots of many colors | ||||
| 2 | Whole Foods (Amazon) | 340 g pack, $2.49, | 100 | >99:1 |
| baby rainbow carrots | ||||
| 3 | Jewel-Osco (Albertsons) | 340 g pack, $2.49, | 100 | >99:1 |
| baby rainbow carrots | ||||
| 4 | Mariano’s (Kroger) | 680 g pack, $1.99, | 100 | >99:1 |
| rainbow carrots | ||||
| 5 | Target | 340 g pack, $2.19, | 100 | >99:1 |
| baby rainbow carrots | ||||
| 6 | Trader Joe’s (Aldi) | precut and stored at 4 °C for 7 days | 100 | >99:1 |
Reaction conditions: substrate (50 mg), water (35 mL), EtOH (1 mL), pH 8.5 buffer (0.7 mL), pre-cut purple carrots (10 g), in an Erlenmeyer flask, stirred at 250 rpm.
Parent company, if applicable, is listed in parentheses.
Conversion was determined by LCMS.
er was determined by chiral SFC.
With multiple reactive sites, the obtained chiral alcohols could serve as valuable intermediates for further chemical transformations (Figure 2). For example, the newly created chiral alcohol was readily converted from (S) to (R) using the Mitsunobu reaction (35 → 51). Conversions of alcohol 35 to its amino and ether derivatives 52 and 53 also proceeded smoothly. In addition, the functional group on the nitrogen-heteroaromatics might act as another handle for structural modification, as showed in the SNAr reaction of compound 46 with 1-methylpiperazine and Suzuki coupling with PhB(OH)2 (46 → 54, 55). Furthermore, upon Staudinger reaction the alkyl side chain of 49 was converted to an amine intermediate, which was used as a third site to edit the molecule via amide formation (49 → 56). Generally, these transformations mimicked the key fragments or stitching strategies in the synthesis of a variety of valuable pharmaceutical molecules 57–61,7,39−42 further demonstrating the synthetic application of our newly developed bioreduction.
Figure 2.
Synthetic applications. Abbreviations: TPGS-750-M, dl-α-tocopherol methoxypolyethylene glycol succinate solution; DMEAD, di-2-methoxyethyl azodicarboxylate; T3P, propanephosphonic acid anhydride.
In summary, ketones bearing the most common 5- and 6-membered nitrogen-heteroaromatics were systematically studied in carrot-mediated reduction, with a close focus on their substitution patterns encompassed in FDA-approved small-molecule drugs. Eight varieties of these nitrogen-heterocycles were studied for the first time and seven types were tolerated: thiazole, benzimidazole, pyridine, pyrimidine, quinazoline, quinoline, and pyrazine. The biocatalytic transformation took advantage of cheap purple carrots in a simplified setup, and most reactions finished within 48 h at room temperature. Both the vendor and the freshness of carrots had little impact on the biocatalytic outcome. Compared to prior plant-involved reactions, the substrate scope was considerably expanded and the practicality was largely improved, ultimately satisfying our desire to access optically pure alcohols with a structurally diverse set of nitrogen-heteroaromatics. This robust reaction could be performed at gram scale, thereby meeting the need of material supply for early route scouting activities in medicinal chemistry. The obtained chiral alcohols with multiple reactive sites provide medicinal chemists synthetic handles to build libraries of compounds and synthesize other bioactive molecules, favorably advancing medicinal chemistry campaigns.
Acknowledgments
This paper is dedicated to Prof. Barry Trost on the occasion of his 82nd birthday. We thank the following AbbVie scientists for assistance with buffer selection (Luke Welk and Ben Danna), enantioselectivity determination (Vivian Juarez, Matthew Natschke, Lisandra Santiago-Capeles, and Emily Rouse), NMR experiments (Jan Waters, Leena Bhatt, and Rick Yarbrough), and HRMS analysis (Amanda Wall).
Glossary
Abbreviations
- FDA
U.S. Food & Drug Administration
- JAK2
Janus kinase 2
- PI3Kδ
phosphoinositide-3-kinase δ
- β3AR
β3 adrenergic receptor
- B/S
ratio of biocatalyst to substrate by weight
- SFC
supercritical fluid chromatography
- NADPH
reduced form of nicotinamide adenine dinucleotide phosphate
- TPGS-750-M
dl-α-tocopherol methoxypolyethylene glycol succinate solution
- DMEAD
di-2-methoxyethyl azodicarboxylate
- T3P
propanephosphonic acid anhydride
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.3c00114.
General information, experimental procedures, characterization data, NMR spectra, and HPLC traces (PDF)
Author Contributions
W.-J.B. conceived and directed the project. W.-J.B. and A.S.J. designed the extended substrate scope and robustness studies. W.-J.B., M.A.E., and J.A.G. performed the experiments and analyzed the data. W.-J.B., M.A.E., J.A.G., and A.J. wrote the manuscript. All authors have given approval to the final version of the manuscript.
The authors declare the following competing financial interest(s): All authors are employees of AbbVie. The design, study conduct, and financial support for this research were provided by AbbVie. AbbVie participated in the interpretation of data, review, and approval of the publication.
Supplementary Material
References
- Socha A. M.; Long R. A.; Rowley D. C. Bacillamides from a Hypersaline Microbial Mat Bacterium. J. Nat. Prod. 2007, 70, 1793–1795. 10.1021/np070126a. [DOI] [PubMed] [Google Scholar]
- Johnson G.; Colabuono P.; Pearson P. G.; Manning D. D.. Chiral indole compounds and their use. WO 2020214740 A1, 2020.
- Castanedo G.; Chan B.; Lucas M. C.; Safina B.; Sutherlin D. P.; Sweeney Z. K.. Pyrido[3,2-d]pyrimidine pi3k delta inhibitor compounds and methods of use. WO 2011101429 A1, 2011.
- Grove S. J. A.; Morrison A. J.; Jamieson C.; Palin R.; Maclean J. K. F.. Amino-heteroaryl derivatives as HCN blockers. WO 2011076723 A1, 2011.
- Guan H.; Lamb M. L.; Peng B.; Huang S.; DeGrace N.; Read J.; Hussain S.; Wu J.; Rivard C.; Alimzhanov M.; Bebernitz G.; Bell K.; Ye M.; Zinda M.; Ioannidis S. Discovery of novel JAK2–STAT pathway inhibitors with extended residence time on target. Bioorg. Med. Chem. Lett. 2013, 23, 3105–3110. 10.1016/j.bmcl.2013.02.111. [DOI] [PubMed] [Google Scholar]
- Ogawa A. K.; Sinz C. J.; Hicks J. D.; Cheng A. C.; Yang S.; Bao J.; Hayes D. A. A. W.; Lang S. B.; Tian M.; Jabri S.; Shearn-Nance G. P.; Kuang R.; Zhao Z.; Wu Z.. Plasma kallikrein inhibitors. WO 2022056051 A1, 2022.
- Cushing T. D.; Hao X.; Shin Y.; Andrews K.; Brown M.; Cardozo M.; Chen Y.; Duquette J.; Fisher B.; Gonzalez-Lopez de Turiso F.; He X.; Henne K. R.; Hu Y.-L.; Hungate R.; Johnson M. G.; Kelly R. C.; Lucas B.; McCarter J. D.; McGee L. R.; Medina J. C.; San Miguel T.; Mohn D.; Pattaropong V.; Pettus L. H.; Reichelt A.; Rzasa R. M.; Seganish J.; Tasker A. S.; Wahl R. C.; Wannberg S.; Whittington D. A.; Whoriskey J.; Yu G.; Zalameda L.; Zhang D.; Metz D. P. Discovery and in Vivo Evaluation of (S)-N-(1-(7-Fluoro-2-(Pyridin-2-Yl)Quinolin-3-Yl)Ethyl)-9H-Purin-6-Amine (AMG319) and Related PI3Kδ Inhibitors for Inflammation and Autoimmune Disease. J. Med. Chem. 2015, 58, 480–511. 10.1021/jm501624r. [DOI] [PubMed] [Google Scholar]
- Chen H.; Coffey S. B.; Lefker B. A.; Liu K. K.-C.. Cyclopentapyridine and tetrahydroquinoline derivatives. WO 2006103511 A1, 2006.
- Stearns R. A.; Miller R. R.; Tang W.; Kwei G. Y.; Tang F. S.; Mathvink R. J.; Naylor E. M.; Chitty D.; Colandrea V. J.; Weber A. E.; Colletti A. E.; Strauss J. R.; Keohane C. A.; Feeney W. P.; Iliff S. A.; Chiu S.-H. L. The Pharmacokinetics of a thiazole benzenesulfonomide β3-adrenergic receptor agonist and its analogys in rats, dogs and monkeys: improving oral bioavailbility. Drug Metab. Dispos. 2002, 30, 771–777. 10.1124/dmd.30.7.771. [DOI] [PubMed] [Google Scholar]
- Ni C.; Shao B.; Tafesse L.; Yao J.; Yu J.; Zhou X.. Pyridine compounds and the uses thereof. WO 2012035421 A2, 2012.
- Norman M. H.; Andrews K. L.; Bo Y. Y.; Booker S. K.; Caenepeel S.; Cee V. J.; D’Angelo N. D.; Freeman D. J.; Herberich B. J.; Hong F. T.; Jackson C. L.; Jiang J.; Lanman B. A.; Liu L.; McCarter J. D.; Mullady E. L.; Nishimura N.; Pettus L. H.; Reed A. B.; Miguel T. S.; Smith A. L.; Stec M. M.; Tadesse S.; Tasker A.; Aidasani D.; Zhu X.; Subramanian R.; Tamayo N. A.; Wang L.; Whittington D. A.; Wu B.; Wu T.; Wurz R. P.; Yang K.; Zalameda L.; Zhang N.; Hughes P. E. Selective class I phosphoinositide 3-kinase inhibitors: optimization of a series of pyridyltriazines leading to the identification of a clinical candidate, AMG 511. J. Med. Chem. 2012, 55, 7796–7816. 10.1021/jm300846z. [DOI] [PubMed] [Google Scholar]
- Lanman B. A.; Reed A. B.; Cee V. J.; Hong F.-T.; Pettus L. H.; Wurz R. P.; Andrews K. L.; Jiang J.; McCarter J. D.; Mullady E. L.; San Miguel T.; Subramanian R.; Wang L.; Whittington D. A.; Wu T.; Zalameda L.; Zhang N.; Tasker A. S.; Hughes P. E.; Norman M. H. Phosphoinositide-3-Kinase Inhibitors: Evaluation of Substituted Alcohols as Replacements for the Piperazine Sulfonamide Portion of AMG 511. Bioorg. Med. Chem. Lett. 2014, 24, 5630–5634. 10.1016/j.bmcl.2014.10.085. [DOI] [PubMed] [Google Scholar]
- Clausen D. J.; Liu J.; Yu W.; Duffy J. L.; Chung C. C.; Myers R. W.; Klein D. J.; Fells J.; Holloway K.; Wu J.; Wu G.; Howell B. J.; Barnard R. J.O.; Kozlowski J. Development of a selective HDAC inhibitor aimed at reactivating the HIV latent reservoir. Bioorg. Med. Chem. Lett. 2020, 30, 127367–127373. 10.1016/j.bmcl.2020.127367. [DOI] [PubMed] [Google Scholar]
- Liu J.; Kelly J.; Yu W.; Clausen D. J.; Yu Y.; Kim H.; Duffy L. J.; Chang C. C.; Myers R. W.; Carroll S.; Klein D. J.; Fells J.; Holloway K.; Wu J.; Wu G.; Howell B. J.; Barnard J. O. R.; Kozlowski A. J. Selective class I HDAC inhibitors based on aryl ketone zinc binding induce HIV-1 protein for clearance. ACS Med. Chem. Lett. 2020, 11, 1476–1483. 10.1021/acsmedchemlett.0c00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W.; Liu J.; Clausen D.; Yu Y.; Duffy J. L.; Wang M.; Xu S.; Deng L.; Suzuki T.; Chung C. C.; Myers R. W.; Klein D. J.; Fells J. I.; Holloway M. K.; Wu J.; Wu G.; Howell B. J.; Barnard R. J. O.; Kozlowski J. Discovery of Ethyl Ketone-Based Highly Selective HDACs 1,2,3 Inhibitors for HIV Latency Reactivation with Minimum Cellular Potency Serum Shift and Reduced hERG Activity. J. Med. Chem. 2021, 64, 4709–4729. 10.1021/acs.jmedchem.0c02150. [DOI] [PubMed] [Google Scholar]
- Ruble J. C.; Latham H. A.; Fu G. C. Effective Kinetic Resolution of Secondary Alcohols with a Planar–Chiral Analogue of 4-(Dimethylamino)pyridine. Use of the Fe(C5Ph5) Group in Asymmetric Catalysis. J. Am. Chem. Soc. 1997, 119, 1492–1493. 10.1021/ja963835b. [DOI] [Google Scholar]
- Cheedrala R. K.; Sachwani R.; Radha Krishna P. Lipase mediated kinetic resolution of benzimidazolyl ethanols. Tetrahedron: Asymmetry 2008, 19, 901–905. 10.1016/j.tetasy.2008.03.021. [DOI] [Google Scholar]
- Kucher O. V.; Kolodyazhnaya A. O.; Smolii O. B.; Nazarenko N. K.; Kubyshkin V.; Mykhailiuk P. K.; Tolmachev A. A. Lipase kinetic enantiomeric resolution of 1-heteroarylethanols. Tetrahedron Asymmetry 2016, 27, 341–345. 10.1016/j.tetasy.2016.02.012. [DOI] [Google Scholar]
- Hirao A.; Itsuno S.; Nakahama S.; Yamazaki N. Asymmetric reduction of aromatic ketones with chiral alkoxy-amineborane complexes. J. Chem. Soc., Chem. Commun. 1981, 315–317. 10.1039/c39810000315. [DOI] [Google Scholar]
- Corey E. J.; Bakshi R. K.; Shibata S. Highly Enantioselective Borane Reduction of Ketones Catalyzed by Chiral Oxazaborolidines. Mechanism and Synthetic Implications. J. Am. Chem. Soc. 1987, 109, 5551–5553. 10.1021/ja00252a056. [DOI] [Google Scholar]
- Ohkuma T.; Koizumi M.; Yoshida M.; Noyori R. General asymmetric hydrogenation of hetero-aromatic ketones. Org. Lett. 2000, 2, 1749–1751. 10.1021/ol0000814. [DOI] [PubMed] [Google Scholar]
- Okano K.; Murata K.; Ikariya T. Stereoselective synthesis of optically active pyridyl alcohols via asymmetric transfer hydrogenation of pyridyl ketones. Tetrahedron Lett. 2000, 41, 9277–9280. 10.1016/S0040-4039(00)01695-6. [DOI] [Google Scholar]
- Liu Q.; Wang C.; Zhou H.; Wang B.; Lv J.; Cao L.; Fu Y. Iridium-Catalyzed Highly Enantioselective Transfer Hydrogenation of Aryl N-Heteroaryl Ketones with N-Oxide as a Removable ortho-Substituent. Org. Lett. 2018, 20, 971–974. 10.1021/acs.orglett.7b03878. [DOI] [PubMed] [Google Scholar]
- Thayer A. Removing Impurities. C&EN 2005, 83 (36), 55. [Google Scholar]; https://cen.acs.org/articles/83/i36/REMOVING-IMPURITIES.html
- Moore J. C.; Pollard D. J.; Kosjek B.; Devine P. N. Advances in the enzymatic reduction of ketones. Acc. Chem. Res. 2007, 40, 1412–1419. 10.1021/ar700167a. [DOI] [PubMed] [Google Scholar]
- Cordell G. A.; Lemos T. L. G.; Monte F. J. Q.; de Mattos M. C. Vegetables as Chemical Reagents. J. Nat. Prod. 2007, 70, 478–492. 10.1021/np0680634. [DOI] [PubMed] [Google Scholar]
- Andrade L. H.; Utsunomiya R. S.; Omori A. T.; Porto A. L. M.; Comasseto J. V. Edible catalysts for clean chemical reactions: Bioreduction of aromatic ketones and biooxidation of secondary alcohols using plants. J. Mol. Catal. B: Enzym. 2006, 38, 84–90. 10.1016/j.molcatb.2005.11.009. [DOI] [Google Scholar]
- Bennamane M.; Razi S.; Zeror S.; Aribi-Zouioueche L. Preparation of chiral phenylethanols using various vegetables grown in Algeria. Biocatal. Agric. Biotechnol. 2018, 14, 52–56. 10.1016/j.bcab.2018.02.003. [DOI] [Google Scholar]
- Yadav J. S.; Nanda S.; Reddy P. T.; Rao A. B. Efficient Enantioselective Reduction of Ketones with Daucus carota Root. J. Org. Chem. 2002, 67, 3900–3903. 10.1021/jo010399p. [DOI] [PubMed] [Google Scholar]
- Blanchard N.; Van de Weghe P. Daucus carota L. mediated bioreduction of prochiral ketones. Org. Biomol. Chem. 2006, 4, 2348–2353. 10.1039/b605233a. [DOI] [PubMed] [Google Scholar]
- Vitaku E.; Smith D. T.; Njardarson J. T. Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. 10.1021/jm501100b. [DOI] [PubMed] [Google Scholar]
- Lakshmi C. S.; Reddy G. R.; Rao A. B. Asymmetric Reduction of Heteroaryl Methyl Ketones Using Daucus carota. Green Sustain. Chem. 2011, 1, 117–122. 10.4236/gsc.2011.14019. [DOI] [Google Scholar]
- Caron D.; Coughlan A. P.; Simard M.; Bernier J.; Piche Y.; Chenevert R. Stereoselective reduction of ketones by Daucus carota hairy root cultures. Biotechnol. Lett. 2005, 27, 713–716. 10.1007/s10529-005-5187-y. [DOI] [PubMed] [Google Scholar]
- Omori A. T.; Lobo F. G.; Goncalves do Amaral A. C.; de Souza de Oliveira C. Purple carrots: Better biocatalysts for the enantioselective reduction of acetophenones than common orange carrots (D. carota). J. Mol. Catal. B: Enzym. 2016, 127, 93–97. 10.1016/j.molcatb.2016.02.009. [DOI] [Google Scholar]
- Cortes-Clerget M.; Akporji N.; Zhou J.; Gao F.; Guo P.; Parmentier M.; Gallou F.; Berthon J.-Y.; Lipshutz B. H. Bridging the Gap between Transition Metal- and Bio-Catalysis via Aqueous Micellar Catalysis. Nat. Commun. 2019, 10, 2169. 10.1038/s41467-019-09751-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie B.; Yang J.; Yang Q.; Yuan W. Enantioselective reduction of fluorenones in surfactant-aqueous solution by fruits and vegetables. J. Mol. Catal. B: Enzym. 2009, 61, 284–288. 10.1016/j.molcatb.2009.08.007. [DOI] [Google Scholar]
- Wang Y.; Haight I.; Gupta R.; Vasudevan A. What Is in Our Kit? An Analysis of Building Blocks Used in Medicinal Chemistry Parallel Libraries. J. Med. Chem. 2021, 64, 17115–17122. 10.1021/acs.jmedchem.1c01139. [DOI] [PubMed] [Google Scholar]
-
Prelog V.
Specification
of the stereospecificity of some oxido-reductases by diamond lattice
sections. Pure Appl. Chem.
1964, 9, 119–130. 10.1351/pac196409010119. [DOI] [Google Scholar];
- Thompson S. K.; Priestley T.; Kundu M.; Saha A.; Nath S.. P2x3 and/or p2x2/3 compounds and methods. WO 2018064135 A1, 2018.
- Quattropani A.; Kulkarni S. S.; Giri A. G.. Glycosidase inhibitors. WO 2016030443 A1, 2016.
- Sugiyama S.; Yokosaka T.; Minamizono K.; Kawana A.; Kaneko T.; Maruyama A.; Sasaki K.; Hosoda S.; Koshimizu M.; Takeuchi S.; Kato K.; Chakka N.; Johnson B. M.; White R. D.; Zhao W.. Aryl or heteroaryl derivative. WO 2021210650 A1, 2021.
- Larsen M.; Ritzen A.; Norremark B.; Greve D. R.. 5-(7H-Pyrrolo[2,3-d]pyrimidin-4-yl)-5-azaspiro[2.5]octane-8-carboxylic acid derivatives as novel JAK kinase inhibitors. WO 2018141842 A1, 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.