Abstract
Background
The carbonic anhydrases (CAs) which are found in most living organisms is a member of the zinc-containing metalloenzyme family. The abnormal levels and activities are frequently associated with various diseases therefore CAs have become an attractive target for the design of inhibitors or activators that can be used in the treatment of those diseases.
Methods
Herein, we have designed and synthesized new benzimidazole-hydrazone derivatives to investigate the effects of these synthesized compounds on CA isoenzymes. Chemical structures of synthesized compounds were confirmed by 1H NMR, 13C NMR, and HRMS. The synthetic derivatives were screened for their inhibitory potential against carbonic anhydrase I and II by in vitro assay.
Results
These compounds have IC50 values of 5.156-1.684 µM (hCA I) and 4.334-2.188 µM (hCA II). Inhibition types and Ki values of the compounds were determined. The Ki values of the compounds were 5.44 ± 0.14 µM-0.299 ± 0.01 µM (hCA I) and 3.699 ± 0.041 µM-1.507 ± 0.01 µM (hCA II). The synthetic compounds displayed inhibitory action comparable to that of the clinically utilized reference substance, acetazolamide. According to this, compound 3p was the most effective molecule with an IC50 value of 1.684 µM. Accordingly, the type of inhibition was noncompetitive and the Ki value was 0.299 ± 0.01 µM.
Conclusion
According to the in vitro test results, detailed protein-ligand interactions of the compound 3p, which is more active against hCA I than standard azithromycin (AZM), were analyzed. In addition, the cytotoxic effects of the compounds on the L929 healthy cell line were evaluated.
Keywords: Hydrazone, benzimidazole, carbonic anhydrase I, carbonic anhydrase II, MTT, molecular docking
1. INTRODUCTION
Benzimidazole scaffold, which is an N-heterocyclic compound, is the most common in medicinal chemistry. Since heterocycles are used in pharmaceutical, bioinformatics, and drug design, regularly, such scaffolds are often called ‘privileged’ [1]. This heterocyclic is formed by the fusion of a benzene ring to the 4 and 5 positions of an imidazole ring [2]. Some drugs containing benzimidazole ring are marketed like anti-cancer drugs nocodazole and velipralib, anti-protozoal albendazole, phosphodiesterase inhibitor adibenden, analgesic benzitramide, hypotensive diabezole, antiviral maribavir and antihistamine lerisetron [1, 3]. More-over, new benzimidazole analogues continue to be synthe-sized and their biological activities are tested worldwide these biological activities contain antileukemic [4, 5], antimicrobial [6, 7], antiulcer [8, 9], diuretic [10], analgesic [11], calcium channel blocker [12], anti-Alzheimer [13], anti-cancer [14] activities.
The n-Acylhydrazone skeleton which is composed of an amide and an imine group has the ability to interact with hydrogen-bond acceptor and donor sites and could be interacted with a wide range of amino-acid residues. Although there are not many drugs with the N-acyl hydrazone group, [15] it is considered to have potential chemical, therapeutic, biological, and industrial properties [16]. In the search for biological activities of compounds containing N-acyl hydrazone groups, they have been found to exhibit antiprotozoal [17], anti-inflammatory [18, 19], antitrypanosomal [20], antiviral [21], antituberculosis [22], antitumoral [23], antileishmanial [24], and antihypertensive [25] activities.
The carbonic anhydrases (CAs) which are found in most living organisms are a member of the zinc-containing metalloenzyme family. Their duty is to catalyze the conversion of CO2 and H2O to HCO3- and H+ [26, 27]. The type of CAs found in mammals is α-class. Many biochemical processes such as respiration, calcification, pH and bicarbonate homeostasis, signal transduction, lipogenesis, and ureagenesis can be counted among the pivotal physiological events in which these enzymes participate [28, 29]. Moreover, abnormal levels and activities are frequently associated with various diseases so CAs have become an attractive target for the design of inhibitors or activators that can be used in the treatment of those diseases [30-32]. Now, CA inhibitors have been used as anticonvulsants [33], diuretics [34], antiglaucoma [35], or anti-obesity drugs [36]. Among the CAs, CA II, CA IV, and CA XII are CA isoenzymes that are antiglaucoma drug targets [37, 38]. Glaucoma is an optic neuropathy and one of the leading causes of global irreversible blindness in the world [39, 40]. Laser treatment, incisional surgery, and drug therapy are treatment options for glaucoma [41, 42]. Although drug therapy is an essential part of the treatment for glaucoma, systemic side effects such as neurological, psychiatric, and gastrointestinal side effects with currently used drugs create a big problem [43], so there is an urgent need for innovative drug development for glaucoma therapy. Acetazolamide, ethoxzolamide, and dichlorphenamide are clinically important CA inhibitors (Fig. 1).
Fig. (1).
General structure of acetazolamide, ethoxzolamide and dichlorphenamide and synthesized compounds.
Considering above mentioned problems and the logic explained, the design and synthesis of novel N-acyl hydrazones containing benzimidazole ring were considered to develop new hCA I (human carbonic anhydrase) and hCA II inhibitor agents. Antimicrobial effects of compounds 3a, 3b, 3e, 3g, 3k, 3m, 3n, and 3p have been reported in previous studies [44]. In this study, the effects of carbonic anhydrase were investigated.
2. MATERIALS AND METHODS
2.1. Chemistry
Synthesis of sodium metabisulfite salt of benzaldehyde derivative:
Ethanol was used to dissolve 5g (0.03 mol) of methyl 4-formyl benzoate. Drop by drop, ethanol-dissolved sodium metabisulfite (6.84 g, 0.036 mol) was added to the benzaldehyde solution. The reaction's components were mixed for an hour at room temperature once the dripping process was finished. It was filtered to obtain the precipitated product.
Synthesis of methyl 4-(1H-benzimidazole-2-yl)benzoate (1):
The sodium metabisulfite salt of the benzaldehyde derivative (7.09 g, 0.026 mol) was added after the benzene-1,2-diamine (0.022 mol) had been dissolved in dimethylformamide (DMF). The reaction was completed, and the result was precipitated by adding the reaction's components to iced water. The precipitated product was filtered off and crystallized from ethanol.
Synthesis of 4-(1H-benzimidazole-2-yl)-benzohydrazide derivatives (2):
Compound 1 (0.018 mol), excess of hydrazine hydrate (5 mL), and ethanol (15 mL) were all put into the same vial. Refluxing the mixture for 12 hours. Following the completion of the reaction, the mixture was poured into iced water, the product was filtered.
Synthesis of target compounds 3a-3r:
The compound 2 and appropriate benzaldehyde derivatives in ethanol were refluxed. The precipitated product is filtered off.
2.1.1. N-(1H-benzimidazole-2-yl)-N’-benzylidenebenzo hydrazide (3a)
Yield: 74%. M.p. 279.5oC. 1H NMR (300 MHz, DMSO-d6): δ = 7.22-7.27 (2H, m, Aromatic CH), 7.46 (3H, s, Aromatic CH), 7.57 (1H, d, J= 6.06 Hz, Aromatic CH), 7.70-7.77 (3H, m, Aromatic CH), 8.11 (2H, d, J=7.50 Hz, 1,4-disubstituebenzene), 8.33 (2H, d, J=7.59 Hz, 1,4-disubstituebenzene), 8.50 (1H, s, Aromatic CH), 12.00 (1H, s, NH), 13.12 (1H, s, NH). 13C NMR (75 MHz, DMSO-d6): δ(ppm): 110.99, 113.04, 118.58, 121.36, 123.57, 125.72, 127.77, 128.34, 128.68, 130.43, 133.48, 134.68, 135.54, 147.40, 149.56, 150.70, 162.98. [M+H]+ calcd for C21H16N4O: 341.1383; found: 341.1397.
2.12. 4-((2-(4-(1H-benzimidazole-2-yl)benzoyl)hydrazine ylidene)methyl)benzoic acid (3b)
Yield: 78%. M.p. 338.3oC. 1H NMR (300 MHz, DMSO-d6): δ = 7.23-7.26 (2H, m, Aromatic CH), 7.61-7.66 (2H, m, Aromatic CH), 7.87 (2H, d, J= 8.37 Hz, Aromatic CH), 8.03 (2H, d, J= 7.83 Hz, Aromatic CH), 8.10 (2H, d, J= 8.64 Hz, Aromatic CH), 8.33 (2H, d, J=8.16 Hz, Aromatic CH), 8.54 (1H, s, Aromatic CH), 12.15 (1H, s, NH). 13C NMR (75 MHz, DMSO-d6): δ(ppm): 125.76, 126.63, 127.83, 127.98, 128.74, 129.18, 129.28, 131.33, 131.41, 132.19, 133.59, 134.38, 138.84, 146.13, 148.33, 150.66, 163.12, 167.38. [M+H]+ calcd for C22H16N4O3: 385.1285; found: 385.1295.
2.1.3. 4-(1H-benzimidazole-2-yl)-N’-(4-(diethylamino) benzylidene)benzohydrazide (3c)
Yield: 72%. M.p. 175.5oC. 1H NMR (300 MHz, DMSO-d6): δ = 1.12 (6H, s, -CH3), 3.36 (4H, s, -CH2), 6.72 (2H, s, Aromatic CH), 7.24 (2H, s, Aromatic CH), 7.54 (3H, s, Aromatic CH), 7.70 (1H, s, Aromatic CH), 8.07 (2H, s, Aromatic CH), 8.30 (3H, s, Aromatic CH), 11.64 (1H, s, NH), 13.10 (1H, s, NH). 13C NMR (75 MHz, DMSO-d6): δ(ppm): 12.92, 44.20, 111.52, 111.99, 119.53, 120.93, 121.74, 122.52, 123.49, 126.75, 128.64, 128.95, 129.35, 133.20, 135.50, 135.63, 149.38, 150.64, 162.38. [M+H]+ calcd for C25H25N5O: 412.2140; found: 412.2132.
2.1.4. 4-(1H-benzimidazole-2-yl)-N’-(4-isopropyl benzylidene)benzohydrazide (3d)
Yield: 76%. M.p. 280.9oC. 1H NMR (300 MHz, DMSO-d6): δ = 1.22 (6H, d, J= 6.81 Hz, -CH3), 2.89-2.98 (1H, m, -CH), 7.22-7.27 (2H, m, Aromatic CH), 7.35 (2H, d, J=7.95 Hz, Aromatic CH), 7.57 (1H, dd, J1= 6.39 Hz, J2=1.29 Hz, Aromatic CH), 7.66-7.72 (3H, m, Aromatic CH), 8.09 (2H, d, J=8.37 Hz, Aromatic CH), 8.32 (2H, d, J=8.43 Hz, Aromatic CH), 8.45 (1H, s, Aromatic CH), 11.93 (1H, s, NH), 13.11 (1H, s, NH). 13C NMR (75 MHz, DMSO-d6): δ(ppm): 24.15, 33.87, 112.01, 119.58, 122.45, 123.50, 126.80, 127.33, 127.72, 128.78, 132.43, 133.41, 134.65, 135.53, 144.25, 148.49, 150.70, 151.26, 162.88. [M+H]+ calcd for C24H22N4O: 383.1863; found: 383.1866.
2.1.5. 4-(1H-benzimidazole-2-yl)-N’-(4-chlorobenzylidene) benzohydrazide (3e)
Yield: 76%. M.p. 322.4oC. 1H NMR (300 MHz, DMSO-d6): δ = 7.07-7.10 (1H, m, Aromatic CH), 7.20 (1H, d, J=8.31 Hz, Aromaric CH), 7.45 (2H, d, J=8.28 Hz, Aromaric CH), 7.78-7.81 (3H, m, Aromatic C-H), 7.89-7.90 (3H, m, Aromatic C-H), 7.98-8.00 (3H, m, Aromatic C-H). 13C NMR (75 MHz, DMSO-d6): δ(ppm):112.68, 114.05, 118.19, 118.43, 119.12, 121.11, 123.14, 128.38, 130.92, 131.80, 132.38, 134.83, 149.62, 151.71, 153.68, 156.20, 193.17. [M+H]+ calcd for C21H15N4OCl: 375.1005; found: 375.1007.
2.1.6. 4-(1H-benzimidazole-2-yl)-N’-(4-methylthio benzylidene)benzohydrazide (3f)
Yield: 78%. M.p. 290.4oC. 1H NMR (300 MHz, DMSO-d6): δ = 2.52 (3H, s, -CH3), 7.25-7.27 (2H, m, Aromatic CH), 7.33 (2H, d, J=8.37 Hz, 1,4-disubstituebenzene), 7.53-7.58 (1H, m, Aromatic CH), 7.68 (3H, d, J=8.37 Hz, Aromatic CH), 8.09 (2H, d, J=8.37 Hz, 1,4-disubstituebenzene), 8.32 (2H, d, J=8.40 Hz, 1,4-disubstituebenzene), 8.44 (1H, s, Aromatic CH), 11.95 (1H, s, NH), 13.12 (1H, s, NH). 13C NMR (75 MHz, DMSO-d6): δ(ppm): 13.71, 113.14, 118.56, 121.39, 125.05, 125.71, 127.10, 127.74, 127.89, 129.12, 131.07, 133.33, 133.43, 133.53, 134.58, 144.19, 147.01, 150.70, 162.87. [M+H]+ calcd for C22H18N4OS: 387.1263; found: 387.1274.
2.1.7. 4-(1H-benzimidazole-2-yl)-N’-(4-trifluoromethyl benzylidene)benzohydrazide (3g)
Yield: 81%. M.p. 335.5oC. 1H NMR (300 MHz, DMSO-d6): δ = 7.23-7.25 (2H, m, Aromatic CH), 7.64-7.66 (2H, m, Aromatic CH), 7.83-7.85 (2H, m, Aromatic CH), 7.97-7.99 (2H, m, Aromatic CH), 8.10-8.12 (2H, m, Aromatic CH), 8.32-8.35 (2H, m, Aromatic CH), 8.55 (1H, s, Aromatic CH), 12.16 (1H, s, NH). 13C NMR (75 MHz, DMSO-d6): δ(ppm): 105.78, 113.20, 114.05, 118.24, 119.31, 120.99, 122.61, 123.41, 133.31, 135.23, 136.28, 144.02, 149.57, 150.78, 151.69, 153.66, 190.35. [M+H]+ calcd for C22H15N4OF3: 409.1279; found: 409.1271.
2.1.8. N’-([1,1’-biphenyl]-4-ylmethylene)-4-(1H-benzimidazole-2-yl) benzohydrazide (3h)
Yield: 69%. M.p. 307.4oC. 1H NMR (300 MHz, DMSO-d6): δ = 7.09 (2H, dd, J1=3.30 Hz, J2=9.06 Hz, Aromatic CH), 7.19 (1H, s, Aromatic CH), 7.22 (1H, s, Aromatic CH), 7.36-7.38 (4H, m, Aromatic CH), 7.44 (2H, dd, J1=1.59 Hz, J2=8.34 Hz, Aromatic CH), 7.78 (1H, s, Aromatic CH), 7.81 (1H, s, Aromatic CH), 7.87-7.90 (4H, m, Aromatic CH), 7.93 (2H, d, J=8.25 Hz, Aromatic CH).13C NMR (75 MHz, DMSO-d6): δ(ppm): 125.76, 126.11, 126.54, 127.17, 127.78, 127.93, 128.40, 128.50, 128.58, 128.66, 129.28, 129.88, 130.63, 133.47, 133.90, 134.57, 139.79, 146.92, 149.09, 150.66, 162.98. [M+H]+ calcd for C27H20N4O: 417.1727; found: 417.1710.
2.1.9. 4-(1H-benzimidazole-2-yl)-N’-(4-ethoxybenzylidene) benzohydrazide (3i)
Yield: 79%. M.p. 270.1oC. 1H NMR (300 MHz, DMSO-d6): δ = 1.36 (3H, t, J=6.90 Hz, CH3), 4.09-4.12 (2H, m, -CH2), 7.13 (3H, d, J=8.88 Hz, Aromatic C-H), 7.43-7.47 (2H, m, Aromatic C-H), 7.81 (2H, s, Aromatic CH), 8.04-8.07 (3H, m, Aromatic C-H), 8.15 (3H, d, J=8.79 Hz, Aromatic C-H). 13C NMR (75 MHz, DMSO-d6): δ(ppm): 15.53, 64.36, 115.87, 117.31, 119.49, 121.84, 122.20, 123.70, 129.42, 129.82, 134.75, 136.27, 138.81, 145.57, 150.31, 153.74, 156.50, 161.45, 194.29. [M+H]+ calcd for C23H20N4O2: 385.1670; found: 385.1659.
2.1.10. 4-(1H-benzimidazole-2-yl)-N’-(4-(benzyloxy) benzylidene)benzohydrazide (3j)
Yield: 70%. M.p. 287.0oC. 1H NMR (300 MHz, DMSO-d6): δ= 5.18 (2H, s, -CH3), 7.12 (2H, d, J=8.64 Hz, Aromatic CH), 7.30-7.41 (4H, m, Aromatic CH), 7.47 (2H, d, J=7.35 Hz, Aromatic CH), 7.67-7.72 (4H, m, Aromatic CH), 8.11 (2H, d, J=8.13 Hz, Aromatic CH), 8.32 (2H, d, J=7.95 Hz, Aromatic CH), 8.43 (1H, s, Aromatic CH), 11.91 (1H, s, NH).13C NMR (75 MHz, DMSO-d6): δ(ppm): 69.81, 115.66, 115.99, 116.25, 122.55, 122.93, 123.66, 127.11, 127.45, 128.28, 128.43, 128.83, 128.96, 129.26, 132.31, 135.36, 137.18, 138.26, 139.32, 148.55, 160.48, 162.66. [M+H]+ calcd for C28H22N4O2: 447.1803; found: 447.1816.
2.1.11. 4-(1H-benzimidazole-2-yl)-N’-(4-methoxy benzylidene)benzohydrazide (3k)
Yield: 71%. M.p. 276.6oC. 1H NMR (300 MHz, DMSO-d6): δ = 3.81 (3H, s, -OCH3), 7.03 (2H, d, J=8.67 Hz, Aromatic CH), 7.28-7.31 (2H, m, Aromatic CH), 7.66-7.69 (4H, m, Aromatic CH), 8.10 (2H, d, J=8.43 Hz, 1,4-disubstituebenzene), 8.33 (2H, d, J=8.34 Hz, 1,4-disubstituebenzene), 8.44 (1H, s, Aromatic CH), 11.91 (1H, s, NH). 13C NMR (75 MHz, DMSO-d6): δ(ppm): 55.75, 114.84, 115.43, 115.66, 123.51, 127.06, 127.25, 128.38, 128.82, 129.25, 132.31, 135.17, 138.76, 145.41, 148.45, 150.38, 161.37, 162.68. [M+H]+ calcd for C22H18N4O2: 371.1504; found: 371.1503.
2.1.12. 4-(1H-benzimidazole-2-yl)-N’-(4-fluoro benzylidene)benzohydrazide (3l)
Yield: 68%. M.p. 303.9oC. 1H NMR (300 MHz, DMSO-d6): δ = 7.22-7.27 (2H, m, Aromatic CH), 7.30-7.36 (2H, m, Aromatic CH), 7.57 (1H, dd, J1= 6.54 Hz, J2=1.29 Hz, Aromatic CH), 7.71 (1H, dd, J1= 7.02 Hz, J2=1.50 Hz, Aromatic CH), 7.80-7.85 (2H, m, Aromatic C-H), 8.09 (2H, d, J=8.31 Hz, 1,4-disubstituebenzene), 8.32 (2H, d, J=8.31 Hz, 1,4-disubstituebenzene), 8.49 (1H, s, Aromatic CH), 12.01 (1H, s, NH), 13.12 (1H, s, NH). 13C NMR (75 MHz, DMSO-d6): δ(ppm): 112.01, 116.30, 116.59, 119.58, 122.46, 123.51, 126.82, 127.75, 128.81, 129.76, 133.48, 134.53, 135.53, 144.26, 147.32, 150.68, 162.97. [M+H]+ calcd for C21H15N4OF: 359.1301; found: 359.1303.
2.1.13. 4-(1H-benzimidazole-2-yl)-N’-(4-cyano benzylidene)benzohydrazide (3m)
Yield: 66%. M.p. 297.1oC. 1H NMR (300 MHz, DMSO-d6): δ = 7.23-7.26 (2H, m, Aromatic CH), 7.64-7.66 (2H, m, Aromatic CH), 7.94 (4H, s, Aromatic CH), 8.11 (2H, d, J=8.01 Hz, 1,4-disubstituebenzene), 8.32 (2H, d, J=8.43 Hz, 1,4-disubstituebenzene), 8.53 (1H, s, Aromatic CH), 12.23 (1H, s, NH). 13C NMR (75 MHz, DMSO-d6): δ(ppm): 112.35, 119.14, 122.06, 124.48, 125.76, 127.09, 127.87, 129.26, 129.74, 132.19, 133.55, 134.38, 138.95, 139.17, 145.38, 147.71, 150.62, 163.33. [M+H]+ calcd for C22H15N5O: 366.1335; found: 366.1349.
2.1.14. 4-(1H-benzimidazole-2-yl)-N’-(4-nitrobenzylidene) benzohydrazide (3n)
Yield: 78%. M.p. 333.2oC. 1H NMR (300 MHz, DMSO-d6): δ = 7.23-7.26 (2H, m, Aromatic CH), 7.64 (1H, m, Aromatic CH), 8.01 (2H, d, J=9.03 Hz, 1,4-disubstituebenzene), 8.11 (2H, d, J=8.31 Hz, 1,4-disubstituebenzene), 8.30-8.35 (5H, m, Aromatic CH), 8.58 (1H, s, Aromatic CH), 12.29 (1H, s, NH). 13C NMR (75 MHz, DMSO-d6): δ(ppm): 123.48, 125.72, 127.43, 127.92, 129.44, 130.14, 133.61, 134.16, 136.11, 136.23, 140.99, 144.81, 147.05, 148.32, 150.62, 155.13, 163.25.
2.1.15. 4-(1H-benzimidazole-2-yl)-N’-(3-nitrobenzylidene) benzohydrazide (3o)
Yield: 73%. M.p. 310.1oC. 1H NMR (300 MHz, DMSO-d6): δ = 7.23-7.26 (2H, m, Aromatic CH), 7.64 (2H, s, Aromatic CH), 7.74-7.79 (1H, m, Aromatic CH), 8.11 (2H, d, J=8.16 Hz, 1,4-disubstituebenzene), 8.17 (1H, d, J=7.50 Hz, Aromatic CH), 8.27 (1H, d, J=7.92 Hz, Aromatic CH), 8.33 (2H, d, J=8.19 Hz, 1,4-disubstituebenzene), 8.59 (2H, s, Aromatic CH), 12.26 (1H, s, NH). 13C NMR (75 MHz, DMSO-d6): δ(ppm): 121.43, 122.83, 122.99, 123.40, 124.83, 126.85, 127.93, 128.58, 128.92, 130.98, 133.65, 133.93, 134.23, 136.59, 139.24, 146.01, 148.69, 150.63, 163.23.
2.1.16. 4-(1H-benzimidazole-2-yl)-N’-(4-methylbenzyli-dene) benzohydrazide (3p)
Yield: 72%. M.p. 325.8oC. 1H NMR (300 MHz, DMSO-d6): δ = 2.36 (3H, s, -CH3), 7.22-7.25 (2H, m, Aromatic CH), 7.29 (2H, d, J=7.98 Hz, Aromatic CH), 7.57 (1H, dd, J1= 6.48 Hz, J2=1.35 Hz, Aromatic CH), 7.65 (2H, d, J=7.95 Hz, Aromatic CH), 7.70 (1H, dd, J1= 7.23 Hz, J2=1.98 Hz, Aromatic CH), 8.08 (2H, d, J=8.34 Hz, 1,4-disubstituebenzene), 8.31 (2H, d, J=8.43 Hz, 1,4-disubstituebenzene), 8.45 (1H, s, Aromatic CH), 11.92 (1H, s, NH), 13.11 (1H, s, NH). 13C NMR (75 MHz, DMSO-d6): δ(ppm): 15.54, 115.35, 116.29, 117.31, 120.72, 121.04, 122.86, 128.36, 128.97, 130.92, 132.37, 133.52, 134.89, 149.59, 153.79, 156.18, 160.15, 193.17. [M+H]+ calcd for C22H18N4O: 355.1545; found: 355.1553.
2.1.17. 4-(1H-benzimidazole-2-yl)-N’-(2-methylbenzyli-dene) benzohydrazide (3r)
Yield: 81%. M.p. 282.3oC. 1H NMR (300 MHz, DMSO-d6): δ = 2.36 (3H, s, -CH3), 7.20-7.31 (4H, m, Aromatic CH), 7.57 (1H, dd, J1= 6.48 Hz, J2=1.35 Hz, Aromatic CH), 7.64-7.71 (3H, m, Aromatic CH), 8.08 (2H, d, J=8.34 Hz, 1,4-disubstituebenzene), 8.31 (2H, d, J=8.43 Hz, 1,4-disubstituebenzene), 8.45 (1H, s, Aromatic CH), 11.92 (1H, s, NH), 13.11 (1H, s, NH). 13C NMR (75 MHz, DMSO-d6): δ(ppm): 20.35, 123.79, 125.63, 125.72, 125.84, 127.26, 127.63, 127.72, 127.86, 127.94, 132.78, 133.37, 133.46, 134.56, 137.46, 146.07, 147.64, 148.14, 150.99, 163.73. [M+H]+ calcd for C22H18N4O: 355.1544; found: 355.1553.
2.2. hCA Inhibition Assay
Purification of hCA I and hCA II by affinity chromatography was performed as described in the previous work [45-47].
2.3. Hydratase Activity
The Wilbur-Anderson method, as modified by Wilber et al. [47, 48], was used to calculate CA activity. With the help of a bromothymol blue indicator and a measurement of the passing time, the pH changes were calculated using this approach, which causes the hydration of CO2 to release H+ ions. The equation (to-tc/tc), where to and tc are the times for pH change of the enzymatic and nonenzymatic processes, respectively, was used to compute the enzyme unit (EU).
2.4. Inhibition Assay
Investigated were the inhibitory effects of compounds 3a-3r and AZM on the hydratase activity of the isoenzymes hCA I and hCA II. While keeping the concentration of the substrate constant, IC50 values for the various compounds were computed. Enzyme activities in the absence of inhibitors in the medium were taken as 100% activity. By assessing the hydratase activity in the presence of various inhibitor concentrations, the activity % values of enzymes were calculated. Utilizing graphs of activity %-[I] for each inhibitor, the IC50 value was determined [48-50]. The Cheng-Prusoff equation was used to derive inhibition constants using the nonlinear least squares method [51-53].
2.5. Cytotoxicity Assay
The effect of the compounds between 3a-3r on the viability of the L929 cell line was analyzed by MTT assay as described in the previous work [54-56]. Cell L929 was obtained from ATCC and multiplied in Hepokur Lab.
2.6. Molecular Docking
All stages of molecular docking studies protein preparation, ligand preparation, active site grid generation, and ligand docking were carried out using Schrödinger Suite software 2021.1 version. 3D structures of target proteins hCA I (PDB ID: 3W6H, Resolution: 2.96 Å) [54] and hCA II (PDB ID: 4G0C, Resolution: 2.00 Å) [55] were obtained from the protein data bank (PDB). Target proteins were created using the 'Protein Preparation Wizard' default parameters after water and other heteroatoms other from Zn2+ were eliminated. The “LigPrep” tool was used to create the 3D minimizing structures of the compounds 3a-3r at pH:7.2 With the “Receptor Grid Generation” module based on the cocrystal ligand AZM, the active site coordinates file for both target proteins hCA I (x: 33.6, y: -1.33, z: 9.01) and hCA II (x: -4.98, y: 3.81, z: 14.7) were generated as 20*20*20 Å3. To validate the molecular docking work, re-docking was performed with Glide SP and the cocrystal ligand AZM [56]. Then, molecular docking of all compounds was performed with Glide SP ligand docking tools. 2D protein-ligand interactions diagram ‘Ligand Interaction' module and 3D interactions were made in Maestro v12.8 interface.
3. RESULTS AND DISCUSSION
3.1. Chemistry
As shown in Scheme 1, the target molecules were synthesized in four steps. First, the aldehyde part of the methyl 4-formylbenzoate compound was treated with sodium metabisulfite in ethanol to obtain the sodium disulfide addition product of the aldehyde. In the second step, as a result of the condensation reaction of benzaldehyde sodium metabisulfite product and o-phenylenediamine under reflux and methyl 4-(1H- benzimidazol-2-yl)benzoate (1) was obtained. In the next step, compound 1 was treated with hydrazine hydrate in ethanol to obtain the 4-(1H-benzimidazol-2-yl)benzohydra-zide (2). The hydrazide derivative compound (2) and appropriate benzaldehyde derivatives in ethanol were refluxed and obtained target compounds 3a-3r. The structures of the target compounds were confirmed via 1H NMR, 13C NMR, and HRMS spectroscopy.
Scheme 1.
General procedure for synthesis of the final compounds 3a-3r.
Methyl protons from the -C2H5 protons of compound 3c were observed at 1.12 ppm, and -CH2 protons at 3.36 ppm. Methyl protons from the -isopropyl group of compound 3d were observed at 1.22 ppm as duplet, and -CH protons at 2.89-2.98 ppm as a multiplet. The protons of the -CH3 group of thiomethyl substituent of compound 3f were observed at 2.52 ppm as a singlet. The methoxy group in the 4th position of the phenyl ring of compound 3k was observed as a singlet at 3.81 ppm. The signals belonging to aromatic protons were found at 6.72-8.59 ppm. The 13C NMR spectra showed peaks around 165 ppm due to the carbonyl group (C=O). All of the derivatives' 13C NMR spectra revealed carbon values in the expected locations, and the HRMS analysis supported the mass with the target compounds' estimated values.
3.2. In vitro hCA Activity
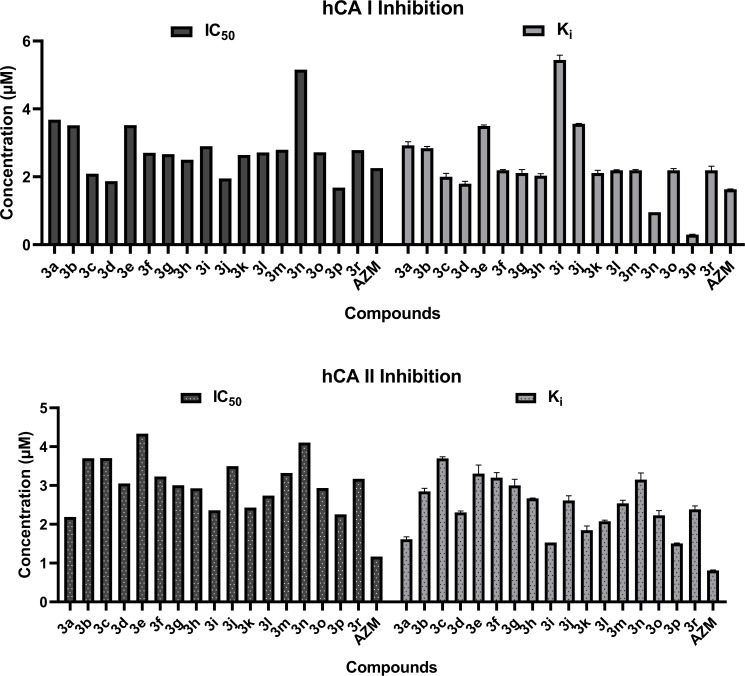
The compounds 3a-3r were tested for their in vitro inhibitory effects on hCA I and hCA II isoenzymes and the results are presented in (Fig. 1). In this work, acetazolamide was used as a reference compound. Compounds 3a-3r showed hCA I inhibitory activity with IC50 values ranging from 1.684 to 5.156 µM. In these series, compounds 3p, 3d, 3j, and 3c were the only compounds that showed better inhibitory activity against hCA I isoenzyme than the reference compound AZM with the IC50 values of 1.684, 1.87, 1.952, and 2.093 µM, respectively.
These series of N-acyl hydrazones exhibited inhibitory effects on hCA II isoenzyme with IC50 values ranging from 2.188 to 4.334 µM and none of the compounds had better inhibitory effects compared to AZM (IC50 = 1.17 µM). Compounds 3a, 3i, 3k, and 3n showed more inhibitory activity on hCA II than hCA I, but the other tested compounds showed more inhibitory effects on hCA I than hCA II, except compound 3l. 4-Fluoro derivative 3l indicated fairly close inhibitory properties on hCA I and hCA II isozymes with the IC50 values of 2.716 and 2.738 µM, respectively.
The inhibition constants (Ki) of compounds 3a-3r for hCA I were established between 0.299 and 5.44 µM and are shown in (Fig. 2). In these compounds, 4-methylphenyl derivative 3p (Ki = 0.299 µM) and a 4-nitrophenyl derivative 3n (Ki = 0.956 µM) had lower Ki values than that of AZM (Ki = 1.63 µM) representing their inhibitory activity against hCA I isoenzyme. On the other hand, all the tested compounds had greater Ki values ranging between 1.507 and 3.699 µM compared to AZM (Ki = 0.812 µM) on hCA II isoenzyme.
Fig. (2).
IC50 and Ki values (µM) of the new N-acyl hydrazones compounds 3a-3r and standard acetazolamide (AZM) with hCA I and hCA II.
The IC50 and Ki values obtained from activity tests revealed that all the compounds tested towards hCA I and II isoforms showed noncompetitive type enzyme inhibition. According to in vitro assay, compounds 3p, 3d, 3j, and 3c indicate significant hCA I inhibitory activity, even though the sulfonamide group, which is an important pharmacophore for hCA inhibitory activity, is not available in their structures. 4-methyl substituents on the phenyl ring at the 4th position were found a generally useful modification in increasing hCA I inhibitory activity. In compound 3r, where the methyl group is in the 2nd position, it is seen that the activity decreases significantly. It was determined that compound 3d, which has an isopropyl structure instead of methyl in the 4th position, also showed similar activity to compound 3p.
3.3. Cytotoxicity Assay
The cytotoxic bioactivity of synthetic compounds was assessed in vitro using the MTT test against the L929 cell line for preliminary screening. The target compounds were administered to the fibroblast cells at a constant dose of 100 M to assess their cytotoxic potential. After the cells had been treated for 48 hours, cell viability percentages were calculated. Preliminary anti ınflammatory effect results of compounds 3a-3r against L929 fibroblast are presented in (Fig. 3). As a result of the maximum dose applied, all compounds showed 75% more viability. So as a result of this IC50 values of compounds were not calculated because they were greater than 100 µM.
Fig. (3).
Cell Viability (%) of L929 fibroblast cell line against compounds (3a-3r) for 48h.
3.4. Molecular Docking
A molecular docking study was carried out to detect and show the interaction of the synthesized compounds with hCA I and II. In order to compare the synthesized compounds and to validate the molecular docking study, self-docking was performed on the cocrystal ligand AZM, which is located in the hCA I (PDB ID: 3W6H) [54] and II (PDB ID: 4G0C) [55] crystal three-dimensional structures. The RMSD value of AZM between the natural interaction pose and docking pose was measured as 1.36 Å for 3W6H and 0.17 Å for 4G0C. As given in (Table 1), the compounds gave docking scores between -3.369 and -6.713 kcal/mol, and glide emodel docking scores of -38.264 and -71.940 kcal/mol against the hCA I enzyme. Glide docking scores between -3.121 and -6.547 kcal/mol, and glide emodel binding energies of -39.199 and -70.942 kcal/mol against the CA II enzyme were formed. The standard compound and cocrystal ligand AZM gave -7.893 kcal/mol Glide binding energy and -70.865 Glide emodel binding energy to hCA I enzyme, while they gave -7.097 kcal/mol and -67.269 kcal/mol binding energies to hCA II enzyme, respectively.
Table 1.
Molecular docking binding energies (kcal/mol) of compounds 3a-3r and reference acetazolamide (AZM) with hCA I and hCA II.
| - | hCA I | hCA II | ||
|---|---|---|---|---|
| Comp. | Glide gscore |
Glide
emodel |
Glide gscore |
Glide
emodel |
| 3a | -3.708 | -44.053 | -3.819 | -39.199 |
| 3b | -6.713 | -71.940 | -6.547 | -70.942 |
| 3c | -3.657 | -50.887 | -3.022 | -44.450 |
| 3d | -4.536 | -38.264 | -3.482 | -45.824 |
| 3e | -3.955 | -39.943 | -3.250 | -48.468 |
| 3f | -3.369 | -45.741 | -3.233 | -50.193 |
| 3g | -3.771 | -47.527 | -3.137 | -48.827 |
| 3h | -3.878 | -50.963 | -3.453 | -53.708 |
| 3i | -3.243 | -45.768 | -3.121 | -47.667 |
| 3j | -3.692 | -50.278 | -3.473 | -53.466 |
| 3k | -3.887 | -46.158 | -3.199 | -46.962 |
| 3l | -3.887 | -46.158 | -3.199 | -46.962 |
| 3m | -4.926 | -47.682 | -3.823 | -44.852 |
| 3n | -4.224 | -51.377 | -3.103 | -50.201 |
| 3o | -3.969 | -50.551 | -3.301 | -51.546 |
| 3p | -4.430 | -44.340 | -4.213 | -47.914 |
| 3r | -4.295 | -49.790 | -3.514 | -45.492 |
| AZM | -7.893 | -70.865 | -7.097 | -67.269 |
According to the in vitro test results, detailed protein-ligand interactions of the compound 3p, which is more active against hCA I than standard AZM, were analyzed. As given in (Fig. 4), compound 3p formed one H bond with His67 (2.22 Å), π-π stacking interactions with His67 (4.83 Å) and His94 (4.75 Å), and pi-cation interactions with Zn2+ (4.25 Å). In addition, hydrophobic interactions with Ile60, Val62, Phe91, Ala121, Val143, Leu198, Val207 and Trp209, positively charged interactions with Lys170, polar van der Waals interactions with His64, His67, Gln92, His94, Hie119, Ser197, Thr199, and His200. The other active compound 3d, two H bonds with Trp5 (2.66 Å) and His67 (2.49 Å), π-π stacking with His64 (5.01 Å) and His200 (4.86 Å and 5.08 Å), and residues His94 (5.86 Å) and Lys170. (4.49 Å) with π-cation interactions. Compound 3j formed π-π stacking with His94 (4.86 Å), hydrophobic interactions with Leu198, Pro202, Tyr204, Ala135, Tyr20 and Ala121, and polar van der Waals interactions with His64, Gln92, His94, His200 and Thr199.
Fig. (4).
Binding poses and protein-ligand interaction diagram of most active compounds 3p against hCA I obtained from Glide SP molecular docking.
CONCLUSION
In this paper, new N-acyl hydrazones containing benzimidazole ring compounds 3a-3r, were synthesized and evaluated for their ability to inhibit hCA I and hCA II isoforms. Despite the absence of a sulfonamide group, which is an important functional group for carbonic anhydrase enzyme inhibitory activity, in the structures of these compounds, it is attractive that enzyme inhibition activity is observed. In this study, we have shown that the sulfonamide group is not a must for carbonic anhydrase inhibiting activity. Among them, a 4-methylphenyl derivative 3p was the strongest compound on hCA I isozyme according to IC50 and Ki values. According to the result, we showed that built-in guanidine or =NNH-CO moieties may also play a crucial role in the process of inhibition. These compounds may serve as a promising candidate for further studies to develop new hCA inhibitory compounds. According to the in vitro test results, detailed protein-ligand interactions of the compound 3p, which is more active against hCA I than standard AZM, were analyzed by molecular docking.
ACKNOWLEDGEMENTS
The authors thank Ankara University-Scientific Research Unit for supplying the Schrödinger software.
LIST OF ABBREVIATIONS
- CAs
Carbonic Anhydrases
- DMF
Dimethylformamide
- EU
Enzyme Unit
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No animals and humans were used for studies that are the basis of this research.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The data that support the findings of this study are available within the article.
FUNDING
Ankara University-Scientific Research Unit supported this study by supplying the Schrödinger software purchased under grant project number BAP-21B0237004.
CONFLICT OF INTEREST
The author(s) declare no conflict of interest, financial or otherwise.
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publisher's website along with the published article.
REFERENCES
- 1.Tahlan S., Kumar S., Narasimhan B. Pharmacological significance of heterocyclic 1H-benzimidazole scaffolds: A review. BMC Chem. 2019;13(1):101. doi: 10.1186/s13065-019-0625-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kamanna K. Synthesis and pharmacological profile of benzimidazoles. Chemistry and applications of benzimidazole and its derivatives. London, UK: IntechOpen; 2019. pp. 51–69. [Google Scholar]
- 3.Alaqeel S.I. Synthetic approaches to benzimidazoles from o-phenylenediamine: A literature review. J. Saudi Chem. Soc. 2017;21(2):229–237. doi: 10.1016/j.jscs.2016.08.001. [DOI] [Google Scholar]
- 4.Kamal A., Praveen Kumar P., Sreekanth K., Seshadri B.N., Ramulu P. Synthesis of new benzimidazole linked pyrrolo[2,1- c][1,4]benzodiazepine conjugates with efficient DNA-binding affinity and potent cytotoxicity. Bioorg. Med. Chem. Lett. 2008;18(8):2594–2598. doi: 10.1016/j.bmcl.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 5.Garuti L., Roberti M., Malagoli M., Rossi T., Castelli M. Synthesis and antiproliferative activity of some benzimidazole-4,7-dione derivatives. Bioorg. Med. Chem. Lett. 2000;10(19):2193–2195. doi: 10.1016/S0960-894X(00)00429-7. [DOI] [PubMed] [Google Scholar]
- 6.Rajiv D., Sonwane S.K., Srivastava S.K., Srivastava S.D. Conventional and greener approach for the synthesis of some novel substituted-4-oxothiazolidine and their 5-arylidene derivatives of 2-methylbenzimidazole: Antimicrobial activities. J. Chem. Pharm. Res. 2010;2(1):415–423. [Google Scholar]
- 7.Hosamani K.M., Seetharamareddy H.R., Keri R.S., Hanamanthagouda M.S., Moloney M.G. Microwave assisted, one-pot synthesis of 5-nitro- 2-aryl substituted-1H-benzimidazole libraries: Screening in vitro for antimicrobial activity. J. Enzyme Inhib. Med. Chem. 2009;24(5):1095–1100. doi: 10.1080/14756360802632716. [DOI] [PubMed] [Google Scholar]
- 8.Patil A., Ganguly S., Surana S. Synthesis and antiulcer activity of 2-[5-substituted-1-H-benzo(d) imidazol-2-yl sulfinyl]methyl-3-substituted quinazoline-4-(3H) ones. J. Chem. Sci. 2010;122(3):443–450. doi: 10.1007/s12039-010-0052-5. [DOI] [Google Scholar]
- 9.Bariwal J.B., Shah A.K., Kathiravan M.K., Somani R.S., Jagtap J.R., Jain K.S. Synthesis and antiulcer activity of novel pyrimidylthiomethyl- and pyrimidylsulfinylmethyl benzimidazoles as potential reversible proton pump inhibitors. Indian J. Pharm. Educ. Res. 2008;42(3):225–231. [Google Scholar]
- 10.Radha Y., Manjula K.M., Reddy B.M., Rao B.V. Synthesis and biological activity of novel benzimidazoles. Indian J. Chem. 2011;50B:1762–1773. [Google Scholar]
- 11.Demirayak S., Karaburun A.C., Kayagil I., Uçucu U., Beis R. Synthesis and analgesic activities of some 2-(benzazolylacetyl) amino-3-ethoxycarbonylthiophene derivatives. Phosphorus Sulfur Silicon Relat. Elem. 2005;180(8):1841–1848. doi: 10.1080/104265090889503. [DOI] [Google Scholar]
- 12.Law C.S.W., Yeong K.Y. Benzimidazoles in drug discovery: A patent review. ChemMedChem. 2021;16(12):1861–1877. doi: 10.1002/cmdc.202100004. [DOI] [PubMed] [Google Scholar]
- 13.Acar Cevik U., Saglik B., Levent S., Osmaniye D. Kaya Cavuşoglu, B.; Ozkay, Y.; Kaplancikli, Z. Synthesis and AChE-inhibitory activity of new benzimidazole derivatives. Molecules. 2019;24(5):861. doi: 10.3390/molecules24050861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blagosklonny M.V. Analysis of FDA approved anticancer drugs reveals the future of cancer therapy. Cell Cycle. 2004;3(8):1033–1040. doi: 10.4161/cc.3.8.1023. [DOI] [PubMed] [Google Scholar]
- 15.Maia R.C., Tesch R., Fraga C.A.M. Acylhydrazone derivatives: A patent review. Expert Opin. Ther. Pat. 2014;24(11):1161–1170. doi: 10.1517/13543776.2014.959491. [DOI] [PubMed] [Google Scholar]
- 16.Carvalho S.A., Feitosa L.O., Soares M., Costa T.E.M.M., Henriques M.G., Salomão K., de Castro S.L., Kaiser M., Brun R., Wardell J.L., Wardell S.M.S.V., Trossini G.H.G., Andricopulo A.D., da Silva E.F., Fraga C.A.M. Design and synthesis of new (E)-cinnamic N-acylhydrazones as potent antitrypanosomal agents. Eur. J. Med. Chem. 2012;54:512–521. doi: 10.1016/j.ejmech.2012.05.041. [DOI] [PubMed] [Google Scholar]
- 17.Gorantla V., Gundla R., Jadav S.S., Anugu S.R., Chimakurthy J., Rao N.S.K., Korupolu R. New anti-inflammatory Hybrid N-acyl hydrazone-linked isoxazole derivatives as COX-2 inhibitors: rational design, synthesis and biological evaluation. Chem. Select. 2017;2(26):8091–8100. [Google Scholar]
- 18.Osmaniye D. Sağlık, B.N.; Levent, S.; Özkay, Y.; Kaplancıklı, Z.A. Design, synthesis and biological evaluation of new N -acyl hydrazones with a methyl sulfonyl moiety as selective COX-2 inhibitors. Chem. Biodivers. 2021;18(11):, e2100521. doi: 10.1002/cbdv.202100521. [DOI] [PubMed] [Google Scholar]
- 19.Hernandes M.Z., Rabello M.M., Leite A.C.L., Cardoso M.V.O., Moreira D.R.M., Brondani D.J., Simone C.A., Reis L.C., Souza M.A., Pereira V.R.A., Ferreira R.S., McKerrow J.H. Studies toward the structural optimization of novel thiazolylhydrazone-based potent antitrypanosomal agents. Bioorg. Med. Chem. 2010;18(22):7826–7835. doi: 10.1016/j.bmc.2010.09.056. [DOI] [PubMed] [Google Scholar]
- 20.Ali O., Abdel-Rahman A., Amer H. Synthesis and antiviral valuation of sugar uracil-1-ylmethylhydrazones and their oxadiazoline derivatives. Synthesis. 2007;2007(18):2823–2828. doi: 10.1055/s-2007-983878. [DOI] [Google Scholar]
- 21.Ferreira M.L., Gonçalves R.S.B., Cardoso L.N.F., Kaiser C.R., Candéa A.L.P., Henriques M.G.M.O., Lourenço M.C.S., Bezerra F.A.F.M., de Souza M.V.N. Synthesis and antitubercular activity of heteroaromatic isonicotinoyl and 7-chloro-4-quinolinyl hydrazone derivatives. ScientificWorldJournal. 2010;10:1347–1355. doi: 10.1100/tsw.2010.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang D., Ma Y., Liu Y., Liu Z.P. Synthesis of sulfonylhydrazone- and acylhydrazone-substituted 8-ethoxy-3-nitro-2H-chromenes as potent antiproliferative and apoptosis inducing agents. Arch. Pharm. 2014;347(8):576–588. doi: 10.1002/ardp.201400082. [DOI] [PubMed] [Google Scholar]
- 23.Coimbra E.S., Nora de Souza M.V., Terror M.S., Pinheiro A.C., da Trindade Granato J. Synthesis, biological activity, and mechanism of action of new 2-pyrimidinyl hydrazone and N-acylhydrazone derivatives, a potent and new classes of antileishmanial agents. Eur. J. Med. Chem. 2019;184:, 111742. doi: 10.1016/j.ejmech.2019.111742. [DOI] [PubMed] [Google Scholar]
- 24.Alencar A.K.N., Pereira S.L., Montagnoli T.L., Maia R.C., Kümmerle A.E., Landgraf S.S., Caruso-Neves C., Ferraz E.B., Tesch R., Nascimento J.H.M., de Sant’Anna C.M.R., Fraga C.A.M., Barreiro E.J., Sudo R.T., Zapata-Sudo G. Beneficial effects of a novel agonist of the adenosine A 2A receptor on monocrotaline-induced pulmonary hypertension in rats. Br. J. Pharmacol. 2013;169(5):953–962. doi: 10.1111/bph.12193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Supuran C.T., Scozzafava A. Carbonic anhydrases as targets for medicinal chemistry. Bioorg. Med. Chem. 2007;15(13):4336–4350. doi: 10.1016/j.bmc.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 26.Scozzafava A., Passaponti M., Supuran C.T. Gülçin, İ Carbonic anhydrase inhibitors: Guaiacol and catechol derivatives effectively inhibit certain human carbonic anhydrase isoenzymes (hCA I, II, IX and XII). J. Enzyme Inhib. Med. Chem. 2015;30(4):586–591. doi: 10.3109/14756366.2014.956310. [DOI] [PubMed] [Google Scholar]
- 27.Supuran C.T. Carbonic anhydrases: Novel therapeutic applications for inhibitors and activators. Nat. Rev. Drug Discov. 2008;7(2):168–181. doi: 10.1038/nrd2467. [DOI] [PubMed] [Google Scholar]
- 28.Thiry A., Dogné J.M., Supuran C., Masereel B. Carbonic anhydrase inhibitors as anticonvulsant agents. Curr. Top. Med. Chem. 2007;7(9):855–864. doi: 10.2174/156802607780636726. [DOI] [PubMed] [Google Scholar]
- 29.Supuran C.T., Capasso C. Biomedical applications of prokaryotic carbonic anhydrases. Expert Opin. Ther. Pat. 2018;28(10):745–754. doi: 10.1080/13543776.2018.1497161. [DOI] [PubMed] [Google Scholar]
- 30.Bozdag M., Altamimi A.S.A., Vullo D., Supuran C.T., Carta F. State of the art on carbonic anhydrase modulators for biomedical purposes. Curr. Med. Chem. 2019;26(15):2558–2573. doi: 10.2174/0929867325666180622120625. [DOI] [PubMed] [Google Scholar]
- 31.Angeli A., Vaiano F., Mari F., Bertol E., Supuran C.T. Psychoactive substances belonging to the amphetamine class potently activate brain carbonic anhydrase isoforms VA, VB, VII, and XII. J. Enzyme Inhib. Med. Chem. 2017;32(1):1253–1259. doi: 10.1080/14756366.2017.1375485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Di Fiore A., De Simone G., Alterio V., Riccio V., Winum J.Y., Carta F., Supuran C.T. The anticonvulsant sulfamide JNJ-26990990 and its S,S-dioxide analog strongly inhibit carbonic anhydrases: Solution and X-ray crystallographic studies. Org. Biomol. Chem. 2016;14(21):4853–4858. doi: 10.1039/C6OB00803H. [DOI] [PubMed] [Google Scholar]
- 33.Carta F., Supuran C.T. Diuretics with carbonic anhydrase inhibitory action: A patent and literature review (2005 – 2013). Expert Opin. Ther. Pat. 2013;23(6):681–691. doi: 10.1517/13543776.2013.780598. [DOI] [PubMed] [Google Scholar]
- 34.Supuran C.T., Altamimi A.S.A., Carta F. Carbonic anhydrase inhibition and the management of glaucoma: A literature and patent review 2013-2019. Expert Opin. Ther. Pat. 2019;29(10):781–792. doi: 10.1080/13543776.2019.1679117. [DOI] [PubMed] [Google Scholar]
- 35.Scozzafava A., Supuran C.T., Carta F. Antiobesity carbonic anhydrase inhibitors: A literature and patent review. Expert Opin. Ther. Pat. 2013;23(6):725–735. doi: 10.1517/13543776.2013.790957. [DOI] [PubMed] [Google Scholar]
- 36.Alp C., Maresca A., Alp N.A., Gültekin M.S., Ekinci D., Scozzafava A., Supuran C.T. Secondary/tertiary benzenesulfonamides with inhibitory action against the cytosolic human carbonic anhydrase isoforms I and II. J. Enzyme Inhib. Med. Chem. 2013;28(2):294–298. doi: 10.3109/14756366.2012.658788. [DOI] [PubMed] [Google Scholar]
- 37.Masini E., Carta F., Scozzafava A., Supuran C.T. Antiglaucoma carbonic anhydrase inhibitors: A patent review. Expert Opin. Ther. Pat. 2013;23(6):705–716. doi: 10.1517/13543776.2013.794788. [DOI] [PubMed] [Google Scholar]
- 38.Kolko M., Horwitz A., Thygesen J., Jeppesen J., Torp-Pedersen C. The prevalence and incidence of glaucoma in Denmark in a fifteen year period: A nationwide study. PLOS One. 2015;10(7):, e0132048. doi: 10.1371/journal.pone.0132048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tham Y.C., Li X., Wong T.Y., Quigley H.A., Aung T., Cheng C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology. 2014;121(11):2081–2090. doi: 10.1016/j.ophtha.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 40.Storgaard L., Tran T.L., Freiberg J.C., Hauser A.S., Kolko M. Glaucoma clinical research: Trends in treatment strategies and drug development. Front. Med. 2021;8:, 733080. doi: 10.3389/fmed.2021.733080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.John M.E.C.C.D. Communications, S. Rockville, MD, US: Agency for Healthcare Research and Quality; 2007. [Google Scholar]
- 42.Arbabi A., Bao X., Shalaby W.S., Razeghinejad R. Systemic side effects of glaucoma medications. Clin. Exp. Optom. 2022;105(2):157–165. doi: 10.1080/08164622.2021.1964331. [DOI] [PubMed] [Google Scholar]
- 43.Arslan O., Nalbantoglu B., Demir N., Ozdemir H., Kufrevioglu O.I. A new method for the purification of carbonic anhydrase isozymes by affinity chromatography. Turk. J. Med. Sci. 1996;26(2):163–166. [Google Scholar]
- 44.Özkay Y. Tunalı, Y.; Karaca, H.; Işıkdağ, İ. Antimicrobial activity and a SAR study of some novel benzimidazole derivatives bearing hydrazone moiety. Eur. J. Med. Chem. 2010;45(8):3293–3298. doi: 10.1016/j.ejmech.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 45.Demir N., Demir Y., Nadaroglu H. Carbonic anhydrase from bovine bone. Prep. Biochem. Biotechnol. 2001;31(1):33–47. doi: 10.1081/PB-100103370. [DOI] [PubMed] [Google Scholar]
- 46.Demir Y., Demir N., Yildirim S. Nadaroğlu, H.; Karaosmanoğlu, M.; Bakan, E. The activities of carbonic anhydrase and alkaline phosphatase in ancient human bones. Purification and characterization of outer peripheral, cytosolic, inner peripheral, and integral CA. Prep. Biochem. Biotechnol. 2001;31(3):291–304. doi: 10.1081/PB-100104910. [DOI] [PubMed] [Google Scholar]
- 47.Wilbur K.M., Anderson N.G. Electrometric and colorimetric determination of carbonic anhydrase. J. Biol. Chem. 1948;176(1):147–154. doi: 10.1016/S0021-9258(18)51011-5. [DOI] [PubMed] [Google Scholar]
- 48.Rickli E.E., Ghazanfar S.A.S., Gibbons B.H., Edsall J.T. Carbonic anhydrases from human erythrocytes. Preparation and properties of two enzymes. J. Biol. Chem. 1964;239(4):1065–1078. doi: 10.1016/S0021-9258(18)91392-X. [DOI] [PubMed] [Google Scholar]
- 49.Altintop M.D., Ozdemir A., Kucukoglu K., Turan-Zitouni G., Nadaroglu H., Kaplancikli Z.A. Synthesis and evaluation of new thiadiazole derivatives as potential inhibitors of human carbonic anhydrase isozymes (hCA-I and hCA-II). J. Enzyme Inhib. Med. Chem. 2015;30(1):32–37. doi: 10.3109/14756366.2013.873038. [DOI] [PubMed] [Google Scholar]
- 50.Borras J., Scozzafava A., Menabuoni L., Mincione F., Briganti F., Mincione G., Supuran C.T. Carbonic anhydrase inhibitors. Bioorg. Med. Chem. 1999;7(11):2397–2406. doi: 10.1016/S0968-0896(99)00190-X. [DOI] [PubMed] [Google Scholar]
- 51.Akocak S., Lolak N., Vullo D., Durgun M., Supuran C.T. Synthesis and biological evaluation of histamine Schiff bases as carbonic anhydrase I, II, IV, VII, and IX activators. J. Enzyme Inhib. Med. Chem. 2017;32(1):1305–1312. doi: 10.1080/14756366.2017.1386660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Küçükbay H. Buğday, N.; Küçükbay, F.Z.; Berrino, E.; Bartolucci, G.; Del Prete, S.; Capasso, C.; Supuran, C.T. Synthesis and carbonic anhydrase inhibitory properties of novel 4-(2-aminoethyl) benzenesulfonamide-dipeptide conjugates. Bioorg. Chem. 2019;83:414–423. doi: 10.1016/j.bioorg.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 53.Takaoka Y., Kioi Y., Morito A., Otani J., Arita K., Ashihara E., Ariyoshi M., Tochio H., Shirakawa M., Hamachi I. Quantitative comparison of protein dynamics in live cells and in vitro by in-cell 19F-NMR. Chem. Commun. 2013;49(27):2801–2803. doi: 10.1039/c3cc39205h. [DOI] [PubMed] [Google Scholar]
- 54.Fisher S.Z., Aggarwal M., Kovalevsky A.Y., Silverman D.N., McKenna R. Neutron diffraction of acetazolamide-bound human carbonic anhydrase II reveals atomic details of drug binding. J. Am. Chem. Soc. 2012;134(36):14726–14729. doi: 10.1021/ja3068098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Friesner R.A., Murphy R.B., Repasky M.P., Frye L.L., Greenwood J.R., Halgren T.A., Sanschagrin P.C., Mainz D.T. Extra precision glide: Docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. J. Med. Chem. 2006;49(21):6177–6196. doi: 10.1021/jm051256o. [DOI] [PubMed] [Google Scholar]
- 56.Küçükoğlu, K.; Acar Çevik, U.; Nadaroglu, H.; Celik, I.; Işık, A.; Bostancı, H.E.; Özkay, Y.; Kaplancıklı, Z.A. Design, synthesis and molecular docking studies of novel benzimidazole-1,3,4-oxadiazole hybrids for their carbonic anhydrase inhibitory and antioxidant effects. Med. Chem. Res. 2022;31(10):1771–1782. doi: 10.1007/s00044-022-02943-6. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material is available on the publisher's website along with the published article.
Data Availability Statement
The data that support the findings of this study are available within the article.