Abstract
The esophagus undergoes shrinkage after resection and fixation. The surgical in situ margin is greater than the specimen margin, measured by the pathologist. The length of disease‐free margins is critical to therapeutic planning. We propose specimen fixing to avoid discrepancies between the operative finding and the pathological result.
Keywords: esophagogastric junction adenocarcinoma, free margins, shrinkage, specimen
Short abstract
Esophagus undergoes shrinkage after resection and fixation. The surgical in situ margin is greater than the specimen margin, measured by the pathologist. The length of disease‐free margins is critical to therapeutic planning. We propose specimen fixing.
1. INTRODUCTION
Gastric cancer incidence has changed over the past few decades. The incidence of distal gastric cancer decreased, while the incidence of esophageal–gastric junction cancer increased. 1 , 2
In 1998, Siewert and Stain proposed a classification of esophagogastric junction tumors (GOJ), dividing them into three groups based on the tumor location.
Siewert III cancers are gastric cancers with some peculiarities and require dedicated studies and deserve more consideration in the current literature, mainly because their treatment is particularly challenging.
In Siewert III, the standard surgical treatment involves total gastrectomy and resection of the abdominal esophagus. In the esophagus, cancer spreads longitudinally in the submucosal lymphatics. The incidence of positive resection margins reported in the literature is high. 3
In common with the rest of the gastrointestinal tract, the esophagus undergoes considerable shrinkage after resection and, more so, after fixation.
The original in situ length cannot restore the fresh specimen by stretching it.
Invariably, the in situ margin, as estimated by the surgeon, is greater than the specimen margin measured by the pathologist. In contrast to the resection margins, the tumor itself shows slight shrinkage. 4
2. MATERIALS AND METHODS
At the National Cancer Institute of Naples, we treated a 41 years old man with Siewert III cancers with perioperative chemotherapy with a FLOT regimen (four preoperative and four postoperative every 2‐week cycles of FLOT‐docetaxel 50 mg/m2, oxaliplatin 85 mg/m2, leucovorin 200 mg/m2, and fluorouracil 2600 mg/m2 as a 24 h infusion on day 1) 5 , 6 and total gastrectomy and resection of the abdominal esophagus + D2 lymphadenectomy 1 month after the last cycle of chemotherapy.
The surgical procedure starts with the patient in the supine position and in endotracheal intubation. We perform an upper midline incision and an intraoperative staging. At the beginning of dissection, we separate the greater omentum from the transverse colon along a bloodless line about 1 cm from the bowel. On the right side, this dissection line leads onto the right gastroepiploic vessels and subpyloric nodes (station 6). We have swept up the vessels bared and ligated at their origins. We prolonged the left side dissection upward with the opening of the gastrosplenic ligament and the lymphadenectomy of station 4. We divided the lesser omentum along the line of the reflection on the liver capsule. The dissection line continued upward proximally over the esophagogastric junction to include the esophagophrenic ligament, with lymphadenectomy of station 1; dissection of the Bertelli line and section of the left esophagophrenic ligament with station 2 lymphadenectomy. Distally, the line of dissection passes down the peritoneum over the hepatoduodenal ligament to the upper border of the duodenum, with the dissection of the lymphatic tissue in the hepatoduodenal ligament (station 12a) and the ligation of the right gastric artery to its origin with lymphadenectomy (station 5). In this phase, we performed the lymphadenectomy of station 3 too. The first part of the duodenum is now freed from the head of the pancreas, and the duodenal stump is closed by a stapler. We ligated the left gastric artery by lifting the distal stomach, and to the left, at its origin, and we performed the lymphadenectomy of the 7, 8, 9, and 11p stations. We divided both vagi so that only the esophagus attaches to the stomach. The length of the esophagus mobilized depends on the resection margins required. In our case, we have 5 cm of margins intraoperatively (Figure 1).
FIGURE 1.
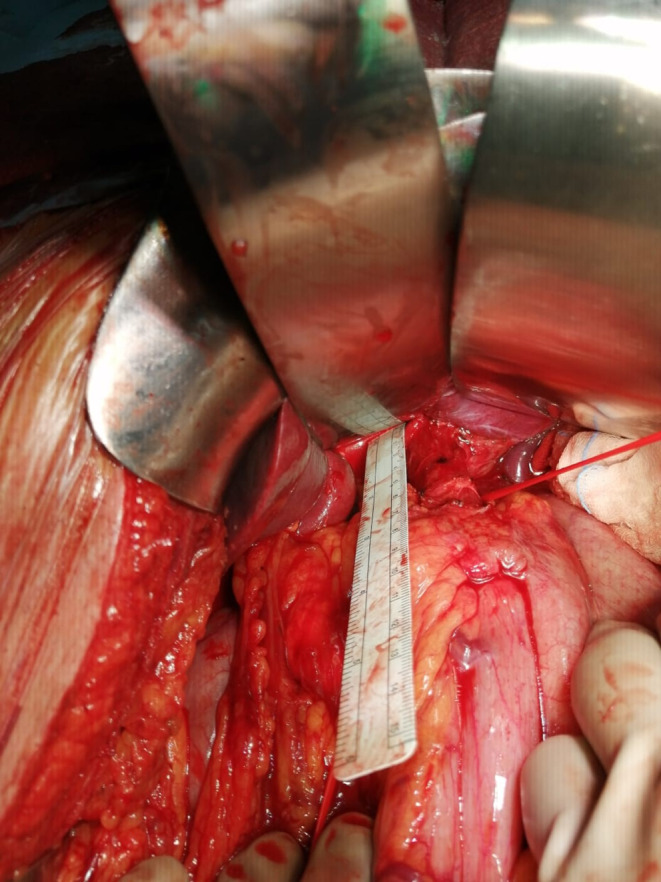
Intraoperative measurement.
We used the proximal jejunum to provide continuity according to Roux—the en‐Y technique.
At the operating field, we measured the free margin from cancer, and after the resection, we fixed the specimen on a cork sheet with punes in order to avoid shrinkage, maintaining the proximal free margin of 5 cm (Figure 2).
FIGURE 2.
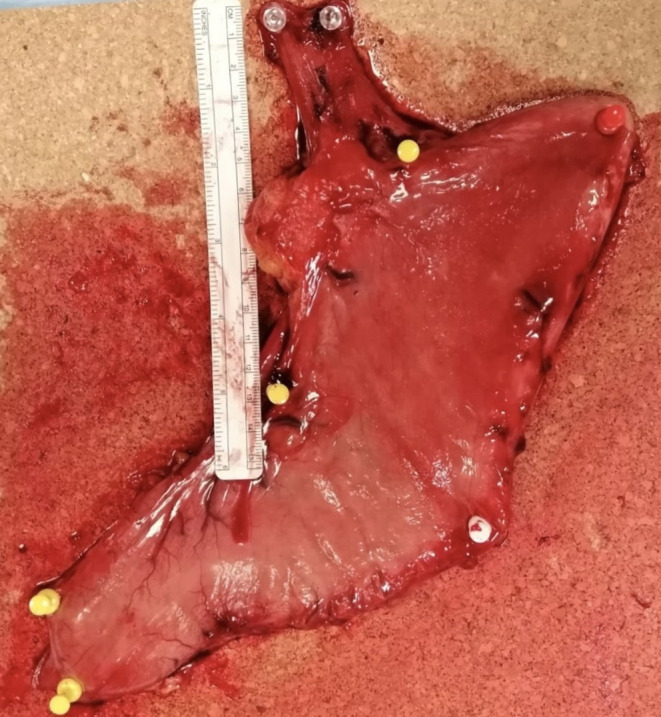
The specimen on a cork sheet with punes.
The histological examination described a total gastrectomy sample with an ulcerated lesion of a maximum diameter of 3 cm at 3 cm from the esophageal margin + esophageal resection margin of 2 × 1.8 cm on the resection ring of the stapler. The pathologist also revealed 56 lymph nodes.
According to the TNM classification, the pathology classified it as Adenocarcinoma ypT3 ypN0 ypR0 G3 Her2 negative. Currently, the patient completed the FLOT regimen of postoperative chemotherapy, and he is disease free.
3. DISCUSSION
Gastric adenocarcinoma is the fourth most common cause of cancer and the second most common cause of cancer‐related deaths worldwide. 1 , 2
The tumors of the esophagogastric junction share standard epidemiological features; having a significant male predominance, they are more frequently associated with hiatus hernia, reflux, and peptic strictures. 7
In 1998, Siewert and Stain proposed a classification of esophagogastric junction tumors, dividing them into three groups:
Type 1: Adenocarcinoma of the distal esophagus that usually arises from an area of specialized intestinal metaplasia of the esophagus (Barret's esophagus) and which may infiltrate the esophagogastric junction from above.
Type 2: True cardia carcinoma arising from the cardiac epithelium or short segments with intestinal metaplasia at the esophagogastric junction; this entity is also called “junctional carcinoma.”
Type 3: Subcardial gastric carcinoma infiltrates the esophagogastric junction and distal esophagus from below. 8
The majority of patients with gastric cancer present with the disease at an advanced stage: in most western countries, at least 80% have “late” (>T1) cancers. While the treatment of early gastric cancer provides a very high chance of cure, the outcome for patients with the later stages of the disease is much less predictable; the chance of cure for patients with cancer that involves the serosal surface (T3/T4) of the stomach is slight.
Surgical resection for curative intent remains the standard of care for gastric adenocarcinoma, and recent randomized control trials have demonstrated a survival benefit for perioperative chemotherapy and radiation therapy. 9 , 10 , 11
The Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) Trial and Intergroup Study 0116 provided Level 1 evidence supporting the use of perioperative chemotherapy and postoperative chemoradiation therapy. 10 , 11 , 12
The FLOT four preoperative and four postoperative 2‐week cycles of FLOT (docetaxel 50 mg/m2, oxaliplatin 85 mg/m2, leucovorin 200 mg/m2, and fluorouracil 2600 mg/m2 as a 24 h infusion on day 1) regimen without radiation therapy is the new standard treatment for preoperative chemotherapy in the West. 5 , 6
In Siewert III, the standard surgical treatment involves total gastrectomy and resection of the abdominal esophagus. In the esophagus, cancer spreads longitudinally in the submucosal lymphatics. The incidence of positive resection margins reported in the literature is high. 3
Since the free margins of resected specimens, mainly in the gastrointestinal tract, quickly shrink when removed from the patient, and further shrinkage occurs when the specimen is fixed in formalin or other fixatives, pathologist's measurements of resection margins are always much shorter than what surgeons do.
Since the most significant mucosa retraction over the muscular propria occurs at the cut margins, we should measure in correspondence with the ends of the mucosa in the fresh state and then, at least 48 h later, could be taken after complete fixation of the specimen in 10% buffered formalin or similar. This way, it is possible to accurately calculate the percentage of retraction of the specimen after fixation.
Generally, the degree of shrinkage is more remarkable for the upper margin than for the lower one.
The original in situ length is restored, even partially, by stretching the fresh specimen.
The resected specimen, opened and laid free, showed shrinkage of the upper and lower margins to 44% and 54%, respectively, of the in situ lengths, with slight tumor shrinkage of 8%. Stretching at maximum the resected specimen did not result in restoration of the in situ length, and there was a shortfall of 27% and 11%, respectively, for the upper and lower margins. A minor change occurred in the tumor lengths.
Maximal shrinkage occurred after fixation. The upper and lower margins reduced to 32% and 39% of their in situ lengths, respectively, after fixation. The tumor shrinkage after fixation was only 10%. 4
The adequacy of the resection margin is mandatory to obtain an R0 surgical resection for carcinoma of the esophagus because of the apparent propensity of intramural spread, especially proximally.
Shrinkage of the surgical specimen can lead the patient to a postoperative treatment, increasing morbidity and mortality.
A preoperative/postoperative treatment and definitive radiotherapy treatment have been performed. 13 , 14 However, radiotherapy and chemoradiotherapy can cause various kinds of treatment‐related sequelae, for example, cardiac toxicities, pulmonary toxicities, and esophageal fistulas. 15 , 16 , 17 Furthermore, the mortality rate of patients with esophageal fistula is high. 18 , 19 , 20
Reducing the risk of esophageal fistula formation could have a significant impact on the outcomes of the patient.
4. CONCLUSION
Tumor location and the resection margin are considered primary when determining the treatment for GOJ cancer. The length of disease‐free margins is critical to postoperative therapeutic planning. We propose specimen fixing to avoid discrepancies between the operative finding and the pathological result.
The oncological radicality, understood as 5 cm of disease‐free margin, avoids postoperative treatments such as radiotherapy. These treatments could increase morbidity and mortality, and overtreatments could be avoided.
AUTHOR CONTRIBUTIONS
Maddalena Leongito: Conceptualization; investigation; writing – original draft. Raffaele Palaia: Supervision. Rossana Casaretti: Supervision. Fabiana Tatangelo: Supervision. Francesca Foschini: Data curation. Annabella Di Mauro: Data curation. Andrea Belli: Data curation. Vittorio Albino: Conceptualization; investigation; writing – original draft.
FUNDING INFORMATION
This research received no specific grant from any funding agency in the public, commercial, or not‐for‐profit sectors.
ACKNOWLEDGMENT
None.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to declare. All co‐authors have seen and agreed with the contents of the article and there is no financial interest to report. We certify that the submission is original work and is not under review at any other publication.
CONSENT
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
Leongito M, Palaia R, Casaretti R, et al. Role of shrinkage in esophageal–gastric junction cancer. Clin Case Rep. 2023;11:e6982. doi: 10.1002/ccr3.6982
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author, [M.L and V.A], upon reasonable request.
REFERENCES
- 1. Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12(3):354‐362. doi: 10.3748/wjg.v12.i3.354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rawla P, Barsuk A. Epidemiology of gastric cancer: global trends, risk factors, and prevention. Prz Gastroenterol. 2019;14(1):26‐38. doi: 10.5114/pg.2018.80001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hill S, Cahill J, Wastell C. The right approach to carcinoma of the cardia: preliminary results. Eur J Surg Oncol. 1992;18(3):282‐286. [PubMed] [Google Scholar]
- 4. Siu KF, Cheung HC, Wong J. Shrinkage of the esophagus after resection for carcinoma. Ann Surg. 1986;203(2):173‐176. doi: 10.1097/00000658-198602000-00011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Al‐Batran S‐E, Homann N, Schmalenberg H, et al. Perioperative chemotherapy with docetaxel, oxaliplatin, and fluorouracil/leucovorin (FLOT) versus epirubicin, cisplatin, and fluorouracil or capecitabine (ECF/ECX) for resectable gastric or gastroesophageal junction (GEJ) adenocarcinoma (FLOT4‐AIO): a multicenter, randomized phase 3 trial. J Clin Oncol. 2017;35(15):4004. [Google Scholar]
- 6. Al‐Batran S‐E, Homann N, Pauligk C, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro‐oesophageal junction adenocarcinoma (FLOT4): a randomized, phase 2/3 trial. Lancet. 2019;393(10184):1948‐1957. [DOI] [PubMed] [Google Scholar]
- 7. Chow WH, Finkle WD, McLaughlin JK, Frankl H, Ziel HK, Fraumeni JF Jr. The relation of gastroesophageal reflux disease and its treatment to adenocarcinomas of the esophagus and gastric cardia. JAMA. 1995;274(6):474‐477. [PubMed] [Google Scholar]
- 8. Siewert JR, Stein HJ. Classification of adenocarcinoma of the oesophagogastric junction. Br J Surg. 1998;85(11):1457‐1459. doi: 10.1046/j.1365-2168.1998.00940.x [DOI] [PubMed] [Google Scholar]
- 9. Snyder RA, Penson DF, Ni S, Koyama T. Merchant NB trends in the use of evidence‐based therapy for resectable gastric cancer. J Surg Oncol. 2014;110(3):285‐290. doi: 10.1002/jso.23635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345(10):725‐730. doi: 10.1056/NEJMoa010187 [DOI] [PubMed] [Google Scholar]
- 11. Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355(1):11‐20. doi: 10.1056/NEJMoa055531 [DOI] [PubMed] [Google Scholar]
- 12. Choi AH, Kim J, Chao J. Perioperative chemotherapy for resectable gastric cancer: MAGIC and beyond. World J Gastroenterol. 2015;21(24):7343‐7348. doi: 10.3748/wjg.v21.i24.7343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nabavizadeh N, Shukla R, Elliott DA, et al. Pre‐operative carboplatin and paclitaxel‐based chemoradiotherapy for esophageal carcinoma: results of a modified CROSS regimen utilizing radiation doses greater than 41.4 Gy. Dis Esophagus. 2016;29(6):614‐620. doi: 10.1111/dote.12377 [DOI] [PubMed] [Google Scholar]
- 14. Burmeister BH. Role of radiotherapy in the pre‐operative management of carcinoma of the esophagus. World J Gastrointest Oncol. 2015;7(1):1‐5. doi: 10.4251/wjgo.v7.i1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tucker SL, Liu HH, Wang S, et al. Dose‐volume modeling of the risk of postoperative pulmonary complications among esophageal cancer patients treated with concurrent chemoradiotherapy followed by surgery. Int J Radiat Oncol Biol Phys. 2006;66(3):754‐761. doi: 10.1016/j.ijrobp.2006.06.002 [DOI] [PubMed] [Google Scholar]
- 16. Ogino I, Watanabe S, Iwahashi N, et al. Symptomatic radiation‐induced cardiac disease in long‐term survivors of esophageal cancer. Strahlenther Onkol. 2016;192(6):359‐367. doi: 10.1007/s00066-016-0956-1 [DOI] [PubMed] [Google Scholar]
- 17. Hihara J, Hamai Y, Emi M, et al. Role of definitive chemoradiotherapy using docetaxel and 5‐fluorouracil in patients with unresectable locally advanced esophageal squamous cell carcinoma: a phase II study. Dis Esophagus. 2016;29(8):1115‐1120. doi: 10.1111/dote.12433 [DOI] [PubMed] [Google Scholar]
- 18. Liu S‐Y, Xiao P, Li T‐X, et al. Predictor of massive bleeding following stent placement for malignant oesophageal stricture/fistulae: a multicentre study. Clin Radiol. 2016;71(5):471‐475. doi: 10.1016/j.crad.2016.02.001 [DOI] [PubMed] [Google Scholar]
- 19. Sdralis EIK, Petousis S, Rashid F, Lorenzi B, Charalabopoulos A. Epidemiology, diagnosis, and management of esophageal perforations: systematic review. Dis Esophagus. 2017;30(8):1‐6. doi: 10.1093/dote/dox013 [DOI] [PubMed] [Google Scholar]
- 20. Tajima T, Haruki S, Usui S, Ito K, Matsumoto A, Matsuhisa A. Takiguchi N Transcatheter arterial embolization for intercostal arterio‐esophageal fistula in esophageal cancer. Surg Case Rep. 2017;3(1):70. doi: 10.1186/s40792-017-0345-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, [M.L and V.A], upon reasonable request.


