Abstract
Background
Sepsis-induced endothelial dysfunction is proposed to cause angiotensin-converting enzyme (ACE) dysfunction and renin–angiotensin–aldosterone system (RAAS) derangement, exacerbating vasodilatory shock and acute kidney injury (AKI). Few studies test this hypothesis directly, including none in children. We measured serum ACE concentrations and activity, and assessed their association with adverse kidney outcomes in pediatric septic shock.
Methods
A pilot study of 72 subjects aged 1 week–18 years from an existing multicenter, observational study. Serum ACE concentrations and activity were measured on Day 1; renin + prorenin concentrations were available from a previous study. The associations between individual RAAS components and a composite outcome (Day 1–7 severe persistent AKI, kidney replacement therapy use, or mortality) were assessed.
Results
50/72 subjects (69%) had undetectable ACE activity (< 2.41 U/L) on Day 1 and 27/72 (38%) developed the composite outcome. Subjects with undetectable ACE activity had higher Day 1 renin + prorenin compared to those with activity (4533 vs. 2227 pg/ml, p = 0.017); ACE concentrations were no different between groups. Children with the composite outcome more commonly had undetectable ACE activity (85% vs. 65%, p = 0.025), and had higher Day 1 renin + prorenin (16,774 pg/ml vs. 3037 pg/ml, p < 0.001) and ACE concentrations (149 vs. 96 pg/ml, p = 0.019). On multivariable regression, increasing ACE concentrations (aOR 1.01, 95%CI 1.002–1.03, p = 0.015) and undetectable ACE activity (aOR 6.6, 95%CI 1.2–36.1, p = 0.031) retained associations with the composite outcome.
Conclusions
ACE activity is diminished in pediatric septic shock, appears uncoupled from ACE concentrations, and is associated with adverse kidney outcomes. Further study is needed to validate these findings in larger cohorts.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13054-023-04518-2.
Keywords: Sepsis, Shock, Pediatrics, Acute kidney injury, Renin–angiotensin–aldosterone system, Angiotensin-converting enzyme, Renin, Angiotensin II, Biomarkers
Introduction:
Septic shock is common in the pediatric intensive care unit and associated with increased morbidity and mortality [1, 2]. Children with septic shock often develop acute kidney injury (AKI), which confers increased risk of poor outcomes [3]. Unfortunately, the treatment of septic shock and sepsis-associated AKI remains limited to supportive care, as no specific disease-modifying therapies exist [4]. A better understanding of the mechanisms of organ dysfunction in septic shock is necessary to improve outcomes.
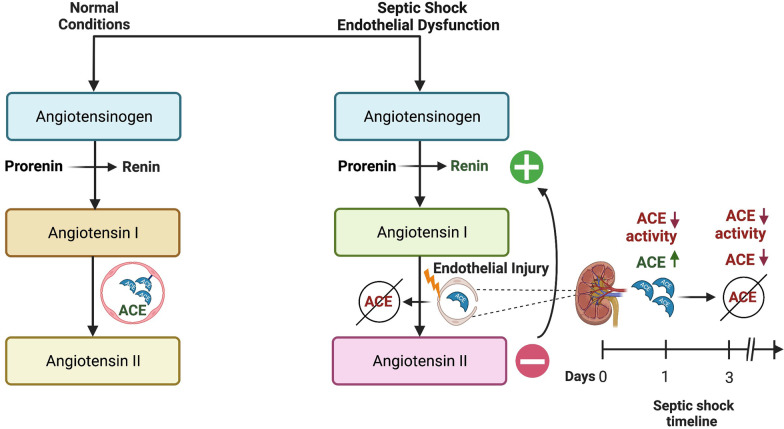
There is increasing evidence that the renin–angiotensin–aldosterone system (RAAS) is dysregulated in critical illness [5–9], and may represent a modifiable target for treatment of patients with septic shock and AKI [6, 9, 10]. Specifically, it is postulated that endothelial injury results in ACE dysfunction with subsequent decreased conversion of angiotensin I (AngI) to angiotensin II (AngII) (a potent vasoconstrictor), exacerbating vasodilatory shock (Fig. 1) [6, 9]. AngII also has direct effects on intrarenal circulation, causing preferential vasoconstriction of efferent arterioles to increase glomerular perfusion pressure, and thus decreased levels may also exacerbate AKI [10]. This hypothesis is supported by evidence demonstrating increased AngI/AngII ratios in adults with catecholamine resistant vasodilatory shock (CRVS) [6, 9], however, few studies have directly measured ACE concentrations [11] and none have measured ACE activity. The importance of testing this hypothesis directly is underscored by the availability of synthetic AngII for treatment of CRVS.
Fig. 1.
Proposed Mechanism of Renin-Angiotensin System Derangement in Septic Shock. Under normal circumstances, inactive prorenin undergoes proteolytic activation to renin in the kidney, where active renin is stored and released immediately upon stimulation of the juxtaglomerular apparatus. In the setting of septic shock and associated endothelial damage, angiotensin-converting enzyme (ACE) function is proposed to be impaired, resulting in acutely decreased production of angiotensin II and resultant increase in serum renin concentrations secondary to release of active enzyme. While proteolytic activation of prorenin to renin is also ongoing, this process is influenced more by chronic stimuli as opposed to acute stimuli. Finally, the right lower panel demonstrates a putative etiology for the uncoupling of ACE activity and ACE levels at early stages of pediatric septic shock; at Day 1 following endothelial injury, ACE stored in kidney endothelia is released to the circulation, possibly resulting in transient increase of ACE levels with subsequent decrease when storage is exhausted
We performed a pilot study measuring serum ACE concentrations and activity in a cohort of children with septic shock. We hypothesized children with septic shock would have low ACE concentrations and activity, and that these values would be associated with increased renin concentrations and adverse kidney outcomes.
Methods
Further details are provided in the Additional file 2.
Study design and patient selection
We performed an exploratory analysis of subjects from a multicenter observational study of children with septic shock. The original study protocol, which has been published in detail [12], was approved by local Institutional Review Boards prior to enrollment. From an existing secondary analysis cohort examining serum renin + prorenin concentrations (n = 233) [13], 72 subjects were randomly selected for inclusion in our study based on residual serum availability, and ensuring representative incidences of severe persistent AKI, kidney replacement therapy (KRT) use, and mortality (Additional file 1: Fig. S1).
Measurements
Day 1 serum was analyzed for ACE concentrations using a human ACE Quantikine ELISA Kit (R&D Systems Inc., Minneapolis, MN, USA) and activity using a high sensitivity enzymatic assay (Bulhmann Diagnostics Corp, Amherst, NH, USA). Renin + prorenin concentrations were measured as part of a previous study [13]. In children, a normal ACE concentration is ~ 200 ng/ml [14], and ACE activity is expected to be 20–50% higher than adults (16–85 U/L) [15, 16]. A normal serum renin is 2–59 pg/ml [6]. All other clinical/laboratory data were measured as part of clinical care.
Outcomes and definitions
We assessed the values of the individual RAAS components and their associations with a composite outcome: Day 1–7 severe persistent AKI (Kidney Disease Improving Global Outcomes Stage 2 or higher lasting by serum creatinine [SCr] for ≥ 48 h [17]), and/or Day 1–7 KRT use, and/or 28-day mortality. Baseline SCr values were unknown, and were imputed using calculated body surface area (m2) and an eGFR of 120 ml/min per 1.73 m2 [18]. Additional secondary outcomes are outlined in the Additional file 2.
Statistical analysis
Data were described using medians, interquartile ranges, frequencies and percentages. Comparisons between groups were performed using Wilcoxon rank-sum or Chi-square test. ACE and renin + prorenin concentrations were treated as continuous variables; ACE activity was treated as a dichotomous variable (detectable: > 2.41 U/L or undetectable: < 2.41 U/L). Multivariable logistic regression was utilized to assess for associations between RAAS components and the composite outcome, after adjustment for illness severity by PRISM III score and vasoactive burden by vasoactive-inotropic score (VIS). A p-value of < 0.05 was considered statistically significant. Analyses were performed using Sigmaplot 14.0 (Systat Software Inc., San Jose, CA, USA).
Results
ACE activity is low in children with septic shock
Fifty of 72 subjects (69%) had undetectable ACE activity (< 2.41 U/L) on Day 1. When compared to subjects with detectable activity, those without had higher PRISM III scores; there were no other demographic differences (Table 1). Subjects with undetectable ACE activity had higher Day 1 renin + prorenin concentrations (4533 vs. 2227 pg/ml, p = 0.017). There was no difference in ACE concentration between those with and without detectable ACE activity (122 vs. 102 ng/ml, p = 0.094).
Table 1.
Demographic, clinical and outcome data by patients by the presence or absence of detectable (> 2.41 U/L) angiotensin-converting enzyme activity and for patients with angiotensin converting enzyme concentrations above and below the cohort median
| All | ACE activity < 2.41 U/L |
ACE activity > 2.41 U/L |
Comparison | ACE level < 104 ng/ml |
ACE level > 104 ng/ml |
Comparison | |
|---|---|---|---|---|---|---|---|
| N (% cohort) | 72 | 50 (69) | 22 (31) | – | 36 (50) | 36 (50) | – |
| Age, years | 12.1 (3.2, 17.7) | 12.1 (4.1, 7.6) | 13.1 (0.9, 20.4) | 0.91 | 12.7 (3.6, 17.9) | 11 (2.3, 17.1) | 0.67 |
| Sex, male (%) | 27 (38) | 16 (32) | 11 (50) | 0.15 | 16 (44) | 11 (31) | 0.22 |
| PRISM III | 11.5 (8.3, 15) | 13 (9.8, 16) | 10 (6.5, 12) | 0.012 | 10.7 (8.3, 15) | 12 (8.3, 15.8) | 0.86 |
| D1 Vasoactives, yes (%) | 63 (88) | 44 (88) | 19 (86) | 1.0 | 32 (89) | 31 (86) | 1.0 |
| Epinephrine (low) | 5 (7.9) | 3 (6.8) | 2 (10.5) | 0.17 | 4 (12.5) | 1 (3.2) | 0.57 |
| Epinephrine (high) | 11 (17.5) | 10 (22.7) | 1 (5.3) | 6 (18.8) | 5 (16.1) | ||
| Norepinephrine | 25 (39.7) | 19 (43.2) | 6 (31.5) | 12 (37.5) | 13 (42) | ||
| Combination | 18 (28.6) | 9 (20.5) | 9 (47.4) | 9 (28.1) | 9 (29) | ||
| Other | 4 (6.3) | 3 (6.8) | 1 (5.3) | 1 (3.1) | 3 (9.7) | ||
| D1 VIS | 12.5 (5, 30) | 11.5 (5, 30) | 12.5 (5, 36) | 0.82 | 10 (5, 25) | 18.6 (8.3, 45) | 0.11 |
| D1 MV, yes (%) | 48 (67) | 35 (70) | 13 (59) | 0.37 | 25 (69) | 23 (64) | 0.62 |
| Organism, n (%) | 0.92 | 0.40 | |||||
| Gram positive | 13 (18.1) | 9 (18) | 4 (18.2) | 4 (11.1) | 9 (25) | ||
| Gram negative | 19 (26.4) | 13 (26) | 6 (27.3) | 12 (33.3) | 7 (19.4) | ||
| Viral | 2 (2.8) | 1 (2) | 1 (4.5) | 1 (2.8) | 1 (2.8) | ||
| Fungal | 6 (8.3) | 5 (10) | 1 (4.5) | 4 (11.1) | 2 (5.6) | ||
| None | 32 (44.4) | 22 (44) | 10 (45.5) | 15 (41.7) | 17 (47.2) | ||
| D1 Renin + Prorenin, pg/ml | 3940 (1749, 11,671) |
4533 (2782, 21,834) |
2227 (1053, 6284) |
0.017 |
3288 (1267, 7557) |
6096 (2539, 19,903) | 0.092 |
| D1 ACE, ng/ml | 104 (78, 149) | 102 (71, 144) | 122 (92, 166) | 0.094 | – | – | – |
| D1-7 Severe Persistent AKI, n (%) | 18 (25) | 15 (30) | 3 (14) | OR 2.7 (95% CI 0.70–10.6, p = 0.14) | 3 (8) | 15 (42) | OR 7.9 (95%CI 2.0–30.5, p = 0.001) |
| D1-7 KRT, n (%) | 12 (17) | 10 (20) | 2 (9) | OR 2.5 (95% CI 0.50–12.5, p = 0.32) | 2 (6) | 10 (28) | OR 6.5 (95%CI 1.3–32.4, p = 0.011) |
| 28-day PICU-Free Days | 13.5 (0, 24) | 13 (0, 24) | 20.5 (0.75, 25) | 0.27 | 17.5 (0, 24) | 12 (0, 24) | 0.36 |
| 7-day Vasoactive-Free Days | 4 (2, 5) | 4 (1.8, 5) | 4.5 (2.75, 6) | 0.40 | 4 (3.25, 5) | 3.5 (1, 5) | 0.17 |
| 28-day Mortality, n (%) | 19 (26) | 16 (32) | 3 (14) | OR 3.0 (95%CI 0.77–11.6, p = 0.10) | 7 (19) | 12 (33) | OR 2.1 (95%CI 0.71–6.1, p = 0.18) |
| Composite Outcome | 27 (38) | 23 (46) | 4 (18) | OR 3.8 (95%CI 1.1–13.0, p = 0.025) | 9 (25) | 18 (50) | OR 3.0 (95%CI 1.1–8.1, p = 0.028) |
Continuous data reported as median (IQR)
PRISM III, Pediatric Risk of Mortality Score III; D, day; Low dose epinephrine, < 0.1 mcg/kg/min; High dose epinephrine, ≥ 0.1 mcg/kg/min; Combination, epinephrine ± vasopressin ± norepinephrine; Other, dopamine, vasopressin or milrinone monotherapy; VIS, vasoactive-inotrope score; MV, mechanical ventilation; ACE, angiotensin-converting enzyme; AKI, acute kidney injury; KRT, kidney replacement therapy; Composite Outcome, Day 1–7 severe persistent AKI, Day 1–7 KRT, and/or 28-day mortality
RAAS derangement is associated with adverse kidney outcomes
Among 72 subjects, 27 (38%) developed the composite outcome (Table 2). There were no demographic differences between those who developed the composite outcome and those who did not; however, those with the composite outcome had higher VIS (Table 2). Additionally, undetectable ACE activity occurred more frequently (85% vs. 60%, p = 0.025), and Day 1 renin + prorenin (16,774 pg/ml vs. 3037 pg/ml, p < 0.001) and ACE concentrations (149 vs. 96 pg/ml, p = 0.019) were higher in children with the composite outcome.
Table 2.
Bivariate analysis and multivariable logistic regression analysis examining the associations with the composite outcome of Day 1–7 severe persistent acute kidney injury, Day 1–7 kidney replacement therapy use, and/or 28-day mortality
| Variable | Bivariate analysis | Multivariable logistic regression | |||
|---|---|---|---|---|---|
| Composite outcome | No composite outcome | p-value | Adjusted odds ratio (95% CI) | p-value | |
| N (%) cohort | 27 (38) | 45 (62) | – | – | – |
| Age, years | 6.1 (2.5, 20.2) | 14 (4.8, 17.2) | 0.45 | – | – |
| Sex, male (%) | 10 (37) | 17 (38) | 0.95 | – | – |
| PRISM III | 13 (9, 19) | 10.4 (8, 13.5) | 0.071* | 1.09 (0.98–1.2) | 0.11 |
| D1 VIS | 25 (10, 44) | 10 (2, 23.5) | 0.004* | 1.03 (0.99–1.07) | 0.10 |
| D1 ACE Level, ng/ml | 149 (70, 177) | 96 (78, 126) | 0.019* | 1.01 (1.02–1.03) | 0.025 |
| D1 ACE activity (U/L) | |||||
| Undetectable, n (%) | 23 (85) | 27 (60) | 0.025* | 6.6 (1.2–36.1) | 0.031 |
| D1 Renin + Prorenin, pg/ml | 16,774 (3484, 28,016) | 3037 (1231, 5111) | < 0.001* | 1.0 (1.0–1.0) | 0.07 |
Continuous data reported as median (IQR)
PRISM III Pediatric Risk of Mortality Score III, D day, VIS vasoactive-inotrope score, ACE angiotensin-converting enzyme
*Variables with alpha level < 0.15 on bivariate analysis were included in the
multivariate logistic regression model
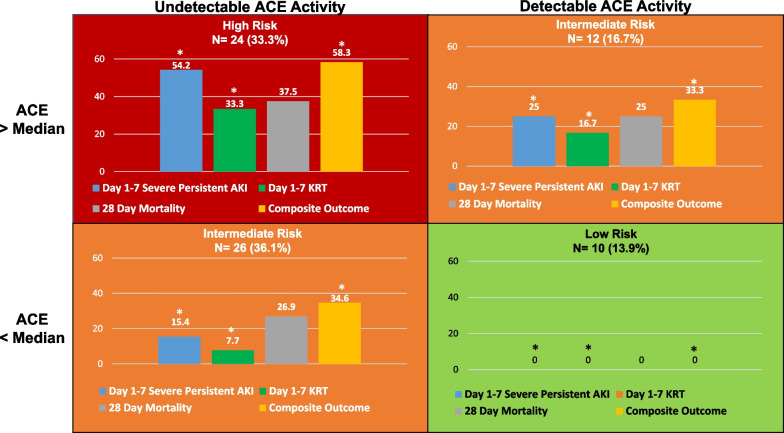
On multivariate regression (Table 2), increasing ACE concentrations (aOR 1.01, 95%CI 1.002–1.03, p = 0.015) and undetectable ACE activity (aOR 6.6, 95%CI 1.2–36.1, p = 0.031) retained associations with the composite outcome. ACE concentration above the median (104 ng/ml) was also associated with greater odds of severe persistent AKI (OR 7.9, 95%CI 2.0–31, p = 0.001) and KRT use (OR 6.5, 95%CI 1.3–32, p = 0.011) (Table 1). To better visualize the interplay between ACE concentrations and activity, four phenotypes were derived for comparison: (1) absent ACE activity/ACE above median (n = 24), (2) absent ACE activity/ACE below median (n = 26), (3) detectable ACE activity/ACE above median (n = 12), and (4) detectable ACE activity/ACE below median (n = 10) (Fig. 2, Additional file 1: Table S1). Notably, subjects with absent ACE activity/ACE above the median (Fig. 2, upper left) had uniformly worse outcomes, while those with detectable ACE activity/ACE below the median (Fig. 2, bottom right) suffered no adverse outcomes.
Fig. 2.
The incidence of adverse kidney related outcomes of interest by unique ACE activity and ACE concentration-derived phenotype. Four unique phenotypes were derived by the presence or absence of detectable (> 2.41 U/L) ACE activity and ACE concentration above or below the cohort median (104 ng/ml). Statistically significant comparisons (p < 0.05) are denoted by *
Discussion
We report findings from a pilot study demonstrating serum ACE activity is low in children with septic shock, with over two-thirds having undetectable activity. Undetectable ACE activity was associated with upstream elevation in renin + prorenin, and with the composite outcome of severe persistent AKI, KRT use or 28-day mortality. Interestingly, though ACE concentrations were lower than expected in healthy children, increasing ACE concentrations were associated with poor outcomes, suggesting an uncoupling of ACE concentration and activity in this cohort.
The finding of diminished ACE activity in children with septic shock provides direct evidence in support of the hypothesis that ACE dysfunction occurs in these patients, presumably secondary to endothelial injury (Fig. 1) [6, 9]. While it is proposed that this dysfunction leads to reduced AngII production and exacerbation of vasodilatory shock and AKI, we were unable to measure AngI/AngII concentrations, which is a limitation of this study. Nevertheless, the association of absent ACE activity with poor outcomes is consistent with adult literature, which has shown similar associations in patients with CRVS, elevated renin concentrations (a proposed upstream surrogate for AngII deficiency that is more easily measured), and increased AngI/AngII ratios [6, 10]. Importantly, in these studies, investigators demonstrated that use of synthetic angiotensin II improved outcomes in patients with presumed AngII deficiency by elevated renin concentrations, including improved renal recovery [6, 10]. These data, combined with our recent data demonstrating extreme elevation of renin + prorenin concentrations in pediatric septic shock [13], suggest that further study is warranted to elucidate if AngII may be particularly beneficial in this population.
While the median ACE concentration for the cohort was lower than expected in healthy children, the finding that higher concentrations were associated with poor outcomes is unexpected. In this cohort, having an ACE concentration above the median was associated with severe persistent AKI and KRT use. This is contrary to a study in adults with sepsis, which demonstrated that lower ACE concentrations on Day 1 were associated with worse outcomes [11]. However, these values continued to decrease over the first 72 h [11], and thus we postulate that extensive endothelial injury may result in release of ACE reservoirs early in sepsis, with subsequent exhaustion over time (Fig. 1). Accordingly, data limited to Day 1 would prevent us from seeing this expected decrease in ACE concentrations. Additionally, it is possible that ACE activity and ACE levels may be uncoupled in this population, secondary to an intrinsic defect in ACE function [19] or circulating ACE inhibitors [20]. Further study is needed to examine these hypotheses.
Our study has several limitations. First, our sample size is small, and these findings need to be corroborated in a larger cohort. This was a secondary analysis of an existing study not designed to examine our hypotheses, introducing the possibility of bias. The type of shock at presentation (i.e., cold versus warm) was unknown, though 54/63 (86%) who required vasoactives received agents suggesting vasodilatory shock (high dose epinephrine, norepinephrine and/or vasopressin). We were unable to trend these concentrations over time and with changes in hemodynamics, an important limitation given the dynamic nature of critical illness. Finally, the lack of direct renin and AngI/AngII data makes direct comparison to the existing adult literature challenging. These issues should be addressed in future prospective studies.
Conclusions
We demonstrate ACE activity is low in children with septic shock, apparently uncoupled from ACE concentrations, and associated with poor outcomes. If validated in a larger cohort, these data provide clarity on the potentially modifiable mechanisms of RAAS derangement in these patients.
Supplementary Information
Acknowledgements
The Genomics of Septic Shock Investigators: Natalie Z. Cvijanovich, Julie C. Fitzgerald, Michael T. Bigham, Parag N. Jain, Adam J. Schwarz, Neal J. Thomas, Torrey Baines, Michael Quasney, Bereketeab Haileselassie.
Abbreviations
- PICU
Pediatric intensive care unit
- AKI
Acute kidney injury
- RAAS
Renin–angiotensin–aldosterone system
- ACE
Angiotensin-converting enzyme
Author contributions
NP-S, NLS and SLG conceptualized the work, performed the analysis and interpretation of the data, and drafted and revised the manuscript. GC acquired and analyzed data and drafted and revised the manuscript. JR performed all laboratory assays and revised the manuscript. The Genomics of Pediatric Septic Shock Investigators (see below) acquired data and revised the manuscript. All authors have read and approved the final manuscript.
Funding
This work was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health (KL2TR001426; PI: Natalja L. Stanski). The original study was funded by National Institute of General Medical Sciences, R35GM126943 (PI: Hector R. Wong). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The original study protocol was reviewed and approved by the Cincinnati Children’s Hospital Medical Center Institutional Review Board prior to patient enrollment (Study Number: 2008-0558; Study Title: Genomics of Septic Shock). Procedures were followed in accordance with the ethical standards of the responsible committee on human experimentation and with the 1964 Declaration of Helsinki and its later amendments. Importantly, this protocol includes approval for future secondary analyses of de-identified data.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Weiss SL, Fitzgerald JC, Pappachan J, Wheeler D, Jaramillo-Bustamante JC, Salloo A, et al. Global epidemiology of pediatric severe sepsis: the sepsis prevalence, outcomes, and therapies study. Am J Respir Crit Care Med. 2015;191:1147–1157. doi: 10.1164/rccm.201412-2323OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balamuth F, Weiss SL, Neuman MI, Scott H, Brady PW, Paul R, et al. Pediatric severe sepsis in US children’s hospitals. Pediatr Crit Care Med. 2014;15:798–805. doi: 10.1097/PCC.0000000000000225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fitzgerald JC, Basu RK, Akcan-Arikan A, Izquierdo LM, Piñeres Olave BE, Hassinger AB, et al. Acute kidney injury in pediatric severe sepsis: an independent risk factor for death and new disability. Crit Care Med. 2016;44:2241–2250. doi: 10.1097/CCM.0000000000002007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weiss SL, Peters MJ, Alhazzani W, Agus MSD, Flori HR, Inwald DP, et al. Surviving sepsis campaign international guidelines for the management of septic shock and sepsis-associated organ dysfunction in children. Intensive Care Med. 2020;46:10–67. doi: 10.1007/s00134-019-05878-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flannery AH, Ortiz-Soriano V, Li X, Gianella FG, Toto RD, Moe OW, et al. Serum renin and major adverse kidney events in critically ill patients: a multicenter prospective study. Crit Care. 2021;25:294. doi: 10.1186/s13054-021-03725-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bellomo R, Forni LG, Busse LW, McCurdy MT, Ham KR, Boldt DW, et al. Renin and survival in patients given angiotensin II for catecholamine-resistant vasodilatory shock. Am J Respir Crit Care Med. 2020 doi: 10.1164/rccm.201911-2172OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gleeson PJ, Crippa IA, Mongkolpun W, Cavicchi FZ, Van Meerhaeghe T, Brimioulle S, et al. Renin as a marker of tissue-perfusion and prognosis in critically ill patients. Crit Care Med. 2019;47:152–158. doi: 10.1097/CCM.0000000000003544. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen M, Denimal D, Dargent A, Guinot P-G, Duvillard L, Quenot J-P, et al. Plasma renin concentration is associated with hemodynamic deficiency and adverse renal outcome in septic shock. Shock. 2019;52:e22–30. doi: 10.1097/SHK.0000000000001285. [DOI] [PubMed] [Google Scholar]
- 9.Bellomo R, Wunderink RG, Szerlip H, English SW, Busse LW, Deane AM, et al. Angiotensin I and angiotensin II concentrations and their ratio in catecholamine-resistant vasodilatory shock. Crit Care. 2020;24:43. doi: 10.1186/s13054-020-2733-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tumlin JA, Murugan R, Deane AM, Ostermann M, Busse LW, Ham KR, et al. Outcomes in patients with vasodilatory shock and renal replacement therapy treated with intravenous angiotensin II. Crit Care Med. 2018;46:949–957. doi: 10.1097/CCM.0000000000003092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang W, Chen X, Huang L, Lu N, Zhou L, Wu G, et al. Severe sepsis: low expression of the renin–angiotensin system is associated with poor prognosis. Exp Ther Med. 2014;7:1342–1348. doi: 10.3892/etm.2014.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong HR, Caldwell JT, Cvijanovich NZ, Weiss SL, Fitzgerald JC, Bigham MT, et al. Prospective clinical testing and experimental validation of the pediatric sepsis biomarker risk model. Sci Transl Med. 2019;11:eaax9000. doi: 10.1126/scitranslmed.aax9000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stanski NL, Shakked NP, Zhang B, Cvijanovich NZ, Fitzgerald JC, Jain PN, et al. Serum renin and prorenin concentrations predict severe persistent acute kidney injury and mortality in pediatric septic shock. Pediatr Nephrol. 2023 doi: 10.1007/s00467-023-05930-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kostadinova ES, Miteva LD, Stanilova SA. ACE serum level and I/D gene polymorphism in children with obstructive uropathies and other congenital anomalies of the kidney and urinary tract. Nephrology (Carlton) 2017;22:609–616. doi: 10.1111/nep.12824. [DOI] [PubMed] [Google Scholar]
- 15.Kruit A, Grutters JC, Gerritsen WBM, Kos S, Wodzig WKWH, van den Bosch JMM, et al. ACE I/D-corrected Z-scores to identify normal and elevated ACE activity in sarcoidosis. Respir Med. 2007;101:510–515. doi: 10.1016/j.rmed.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez GE, Shin BC, Abernathy RS, Kendig EL. Serum angiotensin-converting enzyme activity in normal children and in those with sarcoidosis. J Pediatr. 1981;99:68–72. doi: 10.1016/S0022-3476(81)80959-6. [DOI] [PubMed] [Google Scholar]
- 17.Chawla LS, Bellomo R, Bihorac A, Goldstein SL, Siew ED, Bagshaw SM, et al. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol. 2017;13:241. doi: 10.1038/nrneph.2017.2. [DOI] [PubMed] [Google Scholar]
- 18.Zappitelli M, Parikh CR, Akcan-Arikan A, Washburn KK, Moffett BS, Goldstein SL. Ascertainment and epidemiology of acute kidney injury varies with definition interpretation. Clin J Am Soc Nephrol. 2008;3:948–954. doi: 10.2215/CJN.05431207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chawla LS, Busse LW, Brasha-Mitchell E, Alotaibi Z. The use of angiotensin II in distributive shock. Crit Care. 2016;20:137. doi: 10.1186/s13054-016-1306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kistler E, Li J, Mazor R, Munoz D, Aletti F, Santamaria M. 170: Ace inhibition by circulating peptides formed de novo in experimental hemorrhagic shock. Crit Care Med. 2016;44:120. doi: 10.1097/01.ccm.0000508851.99369.fa. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.