Abstract
Endoscopic endonasal skull base surgery is the preferred surgical approach for the management of pituitary adenomas. Perioperative management of pituitary lesions requires multidisciplinary care and typically includes a dual surgeon team consisting of a neurosurgeon and an otolaryngologist. The involvement of the otolaryngologist allows for a safe surgical approach with excellent intraoperative visualization of the tumor to enable an effective resection of the tumor by the neurosurgeon. Detection and treatment of sinonasal pathology is essential prior to surgery. Patients may experience sinonasal complaints following endoscopic transsphenoidal surgery, although this is typically temporary. Sinonasal care in the postoperative period can expedite recovery to baseline. Here we discuss the perioperative factors of endoscopic pituitary surgery that endocrinologists should be aware of, ranging from preoperative patient selection and optimization to postoperative care, with a particular emphasis on anatomic and surgical factors.
Keywords: pituitary adenoma, nasal surgical procedure, endoscopic surgical procedure, skull base surgery
Case Report
A 43-year-old male was referred to the neurosurgery and endocrinology clinics for a chief complaint of gradual-onset left peripheral visual loss developing over a few months. He also complained of significant 50 pound weight gain over a year but denied increase in hand or shoe size, galactorrhea, or heat intolerance. He had a past medical history of hypertension, asthma, obstructive sleep apnea, gout, and allergic rhinitis with primary manifestation as nasal congestion for which he previously received subcutaneous immunotherapy. He underwent preoperative evaluation by the endocrinology, neurosurgery, and otolaryngology teams. Physical examination revealed left temporal hemianopsia. Computed tomography (CT) and magnetic resonance imaging (MRI) with contrast revealed a sellar mass with suprasellar extension and elevation of the optic chiasm, as well as extension into the right cavernous sinus with incomplete encasement of the right internal carotid artery (Fig. 1). Preoperative evaluation of pituitary function was remarkable for elevated prolactin (35 ng/mL, reference 2-18), low total testosterone (59 ng/dL, reference 250-1100), and low free T4 (0.75 ng/dL, reference 0.8-1.5) with normal TSH (1.54 mcIU/L, reference 0.36-3.74). Other endocrinologic lab values were normal, including random serum cortisol (6.7 mcg/dL, reference 2.7-23.4). He was referred to otolaryngology for preoperative evaluation at which time nasal polyps were identified on nasal endoscopy. He was started on intranasal fluticasone preoperatively to decrease the nasal inflammatory burden.
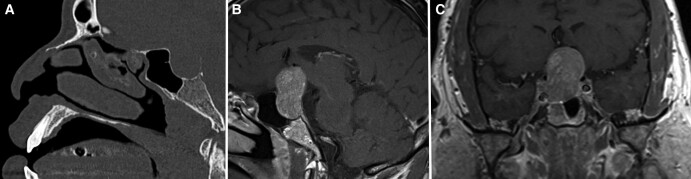
Figure 1.
Preoperative imaging of pituitary adenoma. (A) Computed tomography without contrast in sagittal view demonstrating relation of the pituitary adenoma to the posterior sphenoid sinus. (B) T1 weighted MRI sequence with contrast in sagittal view demonstrating suprasellar extension of the pituitary adenoma. (C) T1 weighted MRI sequence with contrast in coronal view demonstrating extension of the pituitary adenoma to the right cavernous sinus with 180-degree encasement of the cavernous segment of the right internal carotid artery.
Abbreviations: MRI, magnetic resonance imaging.
He underwent surgical resection of the pituitary mass via an endoscopic endonasal approach. His nasal cavity was narrow, so the right middle turbinate was resected to allow for wider surgical access to the face of the sphenoid sinus. A wide bilateral sphenoidotomy with posterior septectomy was performed for broad visualization of the sella. The intersinus septation of the sphenoid sinus was removed carefully to avoid transmission of force to its attachment to the internal carotid artery. The sphenoid mucosa overlying the face of the sella turcica was removed, and the bone of the sella was removed to encounter the tumor. A gross total resection of the mass was performed, and no cerebrospinal fluid leak was seen. The sellar defect was closed with a free mucosal graft harvested from the resected middle turbinate, and absorbable packing was placed to bolster the graft against the defect. Surgical pathology was consistent with gonadotroph adenoma with immunohistochemistry staining positive for steroidogenic factor 1 and negative for adrenocorticotropic hormone, growth hormone, and prolactin.
His postoperative admission course was within expectations. He had immediate improvement in his vision upon waking from anesthesia. He was started on a regimen of prednisone and levothyroxine at the guidance of the endocrinology team and was discharged to home on the second postoperative day. At home he reported intermittent nasal drainage and self-limited nasal bleeding that resolved without intervention. Around 2 weeks after surgery, his steroids were discontinued. However, on his initial postoperative visit with otolaryngology, he complained of worsened nasal obstruction and nasal drainage and was noted to have greater than anticipated mucosal edema and mucopurulent secretions in his sinonasal cavity. He was prescribed amoxicillin with clavulanate and prednisone (30 mg daily for 5 days) and recommended to perform sinonasal irrigations with large-volume saline twice a day. At his most recent visit at 2 months after surgery he reported a return to baseline in terms of vision, nasal congestion, and smell function and was without sinonasal complaints. Nasal endoscopy at that time revealed normalization of nasal mucosa without scarring or synechiae. Repeat laboratory evaluation demonstrated persistently low testosterone (77 ng/dL) but normalization of prolactin (14.6 ng/mL) and free T4 (1.1 ng/dL).
Discussion
Pituitary adenomas are a very common clinical entity, with prevalence increasing with greater availability of brain MRI resulting in incidental detection of more asymptomatic lesions [1, 2]. Successful evaluation and treatment of these lesions require close collaboration within a multidisciplinary team with expertise spanning endocrinology, surgery, anesthesiology, radiology, pathology, and ophthalmology [3, 4]. This review largely focuses on the anatomic and surgical aspects of endoscopic endonasal skull base surgery (EESBS). Specific endocrinologic considerations of the treatment of pituitary adenomas are beyond the scope of this discussion and are well reviewed in other guidelines (Table 1) [5-7]. Surgical resection is indicated for pituitary adenomas that secrete hormones other than prolactin and those causing symptoms by mass effect, such as visual symptoms, ophthalmoplegia, endocrinologic dysfunction, refractory headaches, or apoplexy [5-7]. There are no strict contraindications to surgical resection of pituitary adenomas. Acute bacterial rhinosinusitis is a relative contraindication against elective resection for the duration of the infection, which can be treated with routine antibiotic therapy [8]. Extension of the pituitary lesion into the cavernous sinus with encasement of the carotid artery is not a contraindication for surgical resection but is associated with increased risk of tumor progression or recurrence as gross total resection may not be feasible in such cases [9].
Table 1.
Table of relevant citations for review
| Year | Author | Title |
|---|---|---|
| 2006 | Hadad et al | A novel reconstructive technique after endoscopic expanded endonasal approaches: vascular pedicle nasoseptal flap |
| 2011 | Freda et al | Pituitary incidentaloma: an Endocrine Society clinical practice guideline |
| 2016 | Aghi et al | Congress of Neurological Surgeons systematic review and evidence-based guidelines on the management of patients with nonfunctioning pituitary adenomas |
| 2019 | Wang et al | International consensus statement on allergy and rhinology: endoscopic skull-base surgery |
| 2019 | Bhenswala et al | Sinonasal quality-of-life outcomes after endoscopic endonasal skull base surgery |
| 2019 | Yin et al | Olfactory outcomes after endoscopic skull base surgery: a systematic review and meta-analysis |
| 2020 | Goshtasbi et al | Endoscopic vs nonendoscopic surgery for resection of pituitary adenomas: a national database study |
| 2020 | Rowan et al | Prospective characterization of postoperative nasal deformities in patients undergoing endoscopic endonasal skull-base surgery |
| 2022 | Melmed et al | Clinical biology of the pituitary adenoma |
Preoperative evaluation and optimization of the nasal cavity is crucial to the success of surgical resection. Information obtained in the preoperative period allows for appropriate patient selection for EESBS, patient optimization for major intracranial surgery, improved visualization, safe navigation of critical structures, and decreased blood loss during the surgery, all leading to a better outcome for the patient. Evaluation begins with a thorough history and physical examination in the preoperative clinic visit. The history for a patient considering EESBS for a pituitary lesion should include (1) preoperative sinonasal disease, (2) prior sinonasal surgeries or procedures, (3) patient goals of care and quality of life (QOL) considerations, and (4) patient overall health and frailty. The common element in the key history points for EESBS candidates are factors that affect the health and integrity of the sinonasal mucosa. Any perturbance affecting its vascular supply can later lead to reconstructive failures [10]. Chronic rhinosinusitis (CRS) is the most common preoperative issue, found in about half of EESBS candidates with preoperative sinonasal inflammation [11]. About 60% of EESBS patients have radiographic evidence of inflammatory sinus disease at presentation [12]. If detected preoperatively, rhinosinusitis is treated with intranasal topical steroids such as fluticasone or mometasone. These second-generation intranasal corticosteroids are known to have low systemic absorption and minimal effect on the hypothalamic-pituitary-adrenal axis when used for a short duration [13, 14]. These medications have a low risk of causing adrenal insufficiency, with a recent meta-analysis finding a 0.58% incidence of adrenal insufficiency in usage of second-generation intranasal corticosteroids [15]. Attention to the usage of these medications is critical in the postoperative period, when additional perturbations to endocrinologic function may be expected from surgery on the pituitary sella. While CRS can usually be managed at the time of surgery without increasing infection risk, patients with acute bacterial rhinosinusitis or fungal balls discovered on the day of surgery should have their resection deferred to avoid the heightened risk of intracranial infection [11]. Other important history items to note include prior nasal trauma, granulomatous disease, intranasal drug use, and history of sinonasal malignancy. Prior sinonasal surgeries or procedures are also very important to elucidate before undertaking any EESBS operation. This can affect both the availability of tissue for a skull base reconstruction (eg, a patient with a history of internal maxillary artery embolization for epistaxis would not be a candidate for a reconstructive nasoseptal flap on that side since the arterial supply was embolized) and the ease of an operation (eg, a patient with a prior septoplasty could make for a very challenging nasoseptal flap on that side to due to scarring). Next, even though pituitary surgery is by and large very safe, preoperative goals of care conversations are very important to undertake. Rare complications during EESBS can result in significant adverse consequences for patients, and knowing patients’ wishes prior to such challenging situations is critical. Finally, assessment of patient frailty can be very useful, as this has been shown to be an independent predictor of complications, increased length of stay, and mortality [16, 17]. The modified frailty index-5 is the index used by the American College of Surgeons and incorporates in its model (1) functional status, (2) diabetes, (3) history of chronic obstructive pulmonary disease, (4) history of congestive heart failure, and (5) hypertension requiring medication [18]. In addition to these factors, increasing evidence suggests that multiple hormonal deficiencies may be a significant contributor to the frailty phenotype, and this should be taken into consideration in patients with pituitary lesions [19].
Preoperative physical examination of the patient preparing for EESBS is also crucial. The prime objective of the examination is to identify major anatomic or inflammatory barriers to an endonasal endoscopic approach. Examination is performed with a combination of anterior rhinoscopy using a speculum to visualize the anterior nasal cavity and rigid nasal endoscopy to visualize the posterior nasal cavity and nasopharynx. Obstructive anatomy such as a deviated septum or enlarged inferior turbinates are noted, and these may require surgical correction to facilitate operative access to the sphenoid sinus and sella. Evidence of infection such as purulent drainage is cultured and treated with antibiotic therapy. Exam findings of chronic rhinosinusitis are noted and treated appropriately with topical and oral steroids with or without a course of antibiotics. Some conditions require surgical therapy as part of the treatment course, which can also be incorporated into the surgical plan for EESBS as mentioned previously. Decreasing inflammation and noting anatomic obstructions can allow surgeons to improve visualization of critical structures and minimize bleeding, allowing for an efficient and safe approach to the pituitary sella.
Imaging is also critical in evaluating patients prior to EESBS and has 3 main objectives: (1) assessing patient anatomy, (2) assessing tumor anatomy, and (3) allowing for intraoperative navigation. Each patient's nasal cavity and anterior skull base is unique with a broad range of anatomic variations documented in the literature. High-resolution CT of the sinuses allows for assessment of the bony sinonasal anatomy beyond what is visible on physical examination, and inclusion of an arterial-phase contrast CT angiogram in this imaging protocol more clearly delineates critical surrounding vascular structures. CT imaging is excellent for assessing the height and slope of the anterior skull base (to avoid inadvertent violation of the intracranial space) as well as the orbit and integrity of the lamina papyracea. Neurovascular structures such as the anterior ethmoidal arteries, the internal carotid arteries, and the optic nerves are evaluated for dehiscences in their bony covering that place them at greater risk for intraoperative injury [20]. The structure of the sphenoid sinus is carefully evaluated, as it is a highly variable region in close proximity to critical structures. The intersinus septum, a bony partition separating the 2 sides of the sphenoid sinus, can vary in quantity and attachment, with attachment to the internal carotid artery being a common variation [21]. The pneumatization pattern of the sphenoid sinus determines the optimal amount of bony removal of the pituitary sella necessary to maximize exposure of the pituitary while limiting the size of the resulting defect that requires repair [21]. Tumor anatomy is also critical to assess, and bony destruction caused by an expansile lesion is best evaluated on CT imaging. In addition to CT, MRI with intravenous contrast is used to characterize soft tissue structures. In particular, MRI is needed to evaluate the anatomic extent of the lesion, such as those with cavernous sinus invasion or suprasellar extension, found to occur in nearly 70% of pituitary macroadenomas [22, 23]. Consistency of the lesion (ie, soft vs fibrous), and therefore the expected challenge in removing it from surrounding delicate structures, can be estimated on MRI as well [24, 25]. This information guides the determination of the optimal technique and end point of the resection [26]. Pituitary MRI protocols typically consist of pre- and post-contrast T1-weighted images and a fat-suppressed T2-weighted sequence. These are often completed with a smaller field of view for higher anatomical resolution [27]. Finally, in the modern era, it is also common practice to register the patient's physical anatomy to the scan to enable intraoperative navigation using electromagnetic or optical navigation technology [28, 29], the accuracy of which can be augmented in skull base surgery by fixating the patient's head using a Mayfield skull clamp [30]. In short, the anatomic complexity and wide variation between patients makes imaging a crucial part of preoperative preparation.
Regarding the surgical approach, EESBS has become favored over traditional transcranial approaches due to decreased morbidity, quicker recovery, and lower risk of injury to critical neurovascular structures [31]. Endoscopic surgery also allows for panoramic field of view, improved illumination, and a minimally-invasive approach in comparison to microscopic approaches. While rates of gross tumor removal and remission of hormone hypersecretion remain equivalent between both approaches in a meta-analysis, endoscopic surgery is associated with reduced incidence of diabetes insipidus, hypothyroidism, and septal perforation [32]. As its name implies, EESBS is performed with the use of endoscopes and endoscopic instruments through the nasal cavities to access the sphenoid sinus, the epicenter of EESBS. From the sphenoid sinus, the sella, tuberculum, and planum of the sphenoid bone can be accessed to expose the pituitary gland. Surrounding critical neurovascular structures like the internal carotid arteries, optic nerves, and cavernous sinus are accessible from the sphenoid as well. External incisions are unnecessary for routine approaches. EESBS is often performed by a multidisciplinary team of an otolaryngologist and a neurosurgeon to enable a 3 or even 4-handed approach to resection of the pituitary lesion. The operation is feasible for a single neurosurgeon to perform, but surgeons are increasingly preferring a team approach to the surgery, with a recent international survey indicating that neurosurgeon preference for independent completion of the intranasal portion of EESBS dropped from 65% to 49% between 2010 and 2020. This trend was especially pronounced in the United States [33]. In order to facilitate the simultaneous use of the endoscope and multiple instruments in this approach, broad surgical access is critical. The surgery begins with nasal decongestion by topical oxymetazoline, epinephrine, or cocaine. If a deviated nasal septum is encountered, a septoplasty is performed to bypass this obstruction. Depending on the size and extent of the pituitary lesion and surgeon preference, unilateral or bilateral ethmoidectomies may be performed to allow for further access. A nasoseptal flap and its vascular pedicle, the posterior septal artery, are raised from their tissue beds and protected in order to preserve them as a potential reconstructive option for closure of the skull base defect should a high-flow cerebrospinal fluid (CSF) leak occur during tumor resection. The anterior wall of the sphenoid sinus is broadly removed, exposing the bony landmarks of the sphenoid sinus. A sellotomy, or removal of the bony sellar floor, is performed to expose the dura, which is also opened to access the pituitary gland and associated tumor.
Following resection of the tumor, a number of reconstructive methods can be utilized to close the sellar defect depending on the extent of dissection, exposed neurovascular structures, and whether or not there is an active CSF leak. Reconstruction options include single or multilayered reconstruction with free mucosal grafts, synthetic grafts, fascial or fat grafts, or vascularized tissue (eg, nasoseptal flap). The rates of successful primary repair are typically greater than 95%, and there is no consensus within the literature regarding the best treatment option or a specific reconstructive ladder [34]. The choice of reconstructive method often depends on the presence and magnitude of the CSF leak. In cases where there is no CSF leak and the diaphragma sella is not patulous, the defect can be closed by simply covering the diaphragma sella with material ranging from oxidized cellulose to a free mucosal graft harvested from other nasal donor sites. In cases with a small CSF leak, synthetic collagen-based dural substitutes or other tissue substitutes are layered within the defect for additional closure of the leak. If a high-flow CSF leak is encountered or the patient is at high risk for a CSF leak as in revision cases or cases with large tumor size, a vascular pedicled mucosal flap such as the nasoseptal flap is utilized.12 This provides coverage of the defect with well-vascularized tissue with its native blood supply intact and has been demonstrated to significantly reduce the incidence of CSF leaks following endoscopic endonasal skull base surgery [35, 36]. Provocative testing with a Valsalva maneuver by the anesthesiologist can also aid in identifying small CSF leaks intraoperatively and assess the integrity of the skull base repair. Most reconstructions are bolstered with dissolvable packing material and/or nondissolvable nasal tampons to prevent disruption and facilitate integration of the reconstruction material into the wound bed [37]. This packing also provides pressure hemostasis of the nasal cavity. If a septoplasty was performed, silicone Doyle splints are inserted along both sides of the septum in order to keep the compress the layers of the septum together and prevent a hematoma, which may result in a perforation (Fig. 2A). These silicone splints can also be inserted to separate tissue surfaces in cases where there is concern for postoperative scarring or synechiae formation, which may ultimately obstruct the sinonasal outflow tracts [38]. In the authors’ experience, patients with secreting pituitary adenomas are at greater risk for scar formation and benefit from utilization of silicone splints. Nondissolvable packing and splints can serve as a persistent nidus of infection by native sinonasal bacteria, and it is standard practice to provide antibiotic prophylaxis for as long as the packing remains in place to prevent staphylococcal toxic shock syndrome [39]. Nondissolvable packing is typically removed 7 to 10 days following surgery, depending on surgeon preference and the nature of the skull base reconstruction.
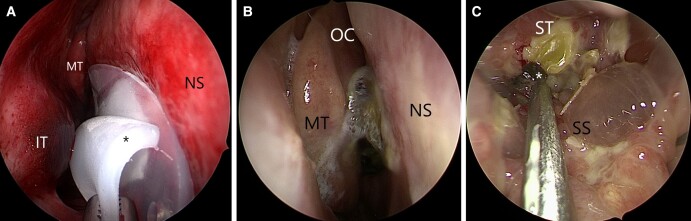
Figure 2.
Postoperative endoscopic view of right nasal cavity after resection of prolactinoma. (A) Doyle splint at time of removal. While the splint has a channel to allow for airflow, its obstruction of the nasal cavity is a common cause of postoperative nasal congestion until it is removed. * Doyle splint (B) Crusting is seen medial to the middle turbinate, obstructing airflow to the olfactory cleft. OC olfactory cleft. (C) Endoscopic view of the sphenoid sinus. Diffuse mucosal edema is seen as well as crusting and secretions over the sellar reconstruction. Here, gentle debridement of crusting is performed with a rigid suction tip. * postoperative crust. Abbreviations: NS, nasal septum; MT, middle turbinate; IT, inferior turbinate; SS, sphenoid sinus; ST, sella turcica.
Intraoperative complications of EESBS are possible given the proximity of the pituitary gland to critical neurovascular structures but are fortunately rare. Specifically, the internal carotid arteries, optic nerves, abducens, and oculomotor nerves are at risk, but reviews have found that injury of these structures occur in less than 1% of cases [5]. Ophthalmoplegia and vision changes from these injuries may occur in the immediate postoperative period or in a delayed fashion 12 to 72 hours after surgery [40]. Meningitis and death as complications are also rare and occur at a rate of .5% and .2%, respectively [5]. CSF leak is possible but rare, with a rate of .6% to 5% in large-review series of endoscopic pituitary adenoma resections [5, 34]. CSF leaks can be detected by testing collected fluid from the nasal cavity for Beta-2 transferrin with high sensitivity and specificity [41]. A CSF leak following EESBS increases the risk of ascending meningitis, and the presence of one would warrant expedited reoperation and closure of the leak [42]. There are no guidelines regarding positioning or mobilization of patients following EESBS to minimize the risk of postoperative CSF leak. In our practice, if there was no CSF leak noted intraoperatively, patients are typically allowed to mobilize to a seated position on the first postoperative day and then ambulate thereafter.
Nosebleeding is another potential complication following EESBS, occurring in .6% to 3.3% of cases [43]. In such cases, the bleeding can be controlled by an endoscopic procedure by an otolaryngologist or by an endovascular embolization procedure by an interventional radiologist or neurosurgeon. Endoscopic options range from placement of packing material for tamponade, which can be performed without anesthesia, to sphenopalatine artery ligation, which must be performed under general anesthesia. The endovascular embolization procedure can be performed under sedation but requires cannulation of the carotid artery system and carries a 4% risk of complications such as stroke or blindness, a risk that is not seen in the endoscopic procedures [44]. As such, the presence of the otolaryngologist on the team is crucial to provide the option of endoscopic bleeding control.
A possible delayed complication following EESBS is the formation of a sinonasal mucocele, which is a cystic collection of mucinous secretions that occurs when sinonasal mucosa is entrapped without an outflow tract for produced secretions [45]. There is potential for this to occur in EESBS if a nasoseptal flap is used to cover a defect without ensuring that all mucosa underneath the flap is removed. A large case series found the incidence of iatrogenic mucocele to be 2% following EESBS with nasoseptal flap reconstruction, and these cases are generally detected several months following surgery [46]. Mucoceles can be detected and differentiated from adenoma recurrence with MRI, which typically reveals the mucocele to have hyperintense contents on T2 sequence with contrast enhancement of the entrapped mucosa on T1 sequence [45].
Overall EESBS for pituitary surgery is safe in the modern era. Improvements in endoscope and camera technology combined with a wide array of endoscopic instrumentation allow for meticulous surgical technique and dissection to avoid inadvertent injury. There are a number of techniques available to surgeons to reduce mucosal edema and bleeding in order to improve surgical visualization, ranging from simple measures like positioning the patient in reverse-Trendelenburg to promote venous drainage of the head, to topical decongestants to promote mucosal vasoconstriction [47]. As mentioned previously, intraoperative navigation based on image guidance can allow for identification and preservation of critical structures prior to resection.
The importance of postoperative nasal care following EESBS cannot be overstated. The principles of nasal care following EESBS are largely derived from data of sinus surgery performed for other indications, such as chronic rhinosinusitis. Lack of proper postoperative care may lead to synechiae that obstruct natural sinus outflow tracts, which can result in sinonasal symptoms and impact postoperative QOL [48]. This obstruction of nasal drainage may also lead to chronic rhinosinusitis, recurrent acute bacterial rhinosinusitis, or mucocele formation. Many of these issues would necessitate future revision sinus surgery. Rinsing the nasal cavities with large volumes of saline is critical to mechanically remove crusts and other debris, as well as to thin secretions to facilitate natural drainage and removal. Multiple randomized control trials have demonstrated improvement in QOL scores and outcomes with postoperative saline rinses [49]. Timing of the initiation of rinses varies according to surgeon preference, as rinsing in the immediate postoperative period may risk disrupting the pituitary sellar closure. It may also confound the detection of CSF leaks, which commonly present as drainage of clear and salty or metallic-tasting fluid. Debridement of the sinonasal cavity with instrumentation in postoperative office visits is commonly performed to remove crusts and debris that cannot be removed with rinsing (Fig. 2). It is also critical to remove any packing that was placed during the surgery in a controlled fashion. This reduces the foreign material burden in the sinonasal cavity that can serve as a nidus for inflammation or infection, which may then lead to scarring and synechiae formation. To date there is still limited evidence demonstrating long-term objective benefits to postoperative debridements, but it is a common practice among otolaryngologists [49-51]. This is most often performed around 1 week after the surgery, with further evaluations and repeat debridements performed at increasing intervals until the sinonasal mucosa has healed (Fig. 3).
Figure 3.
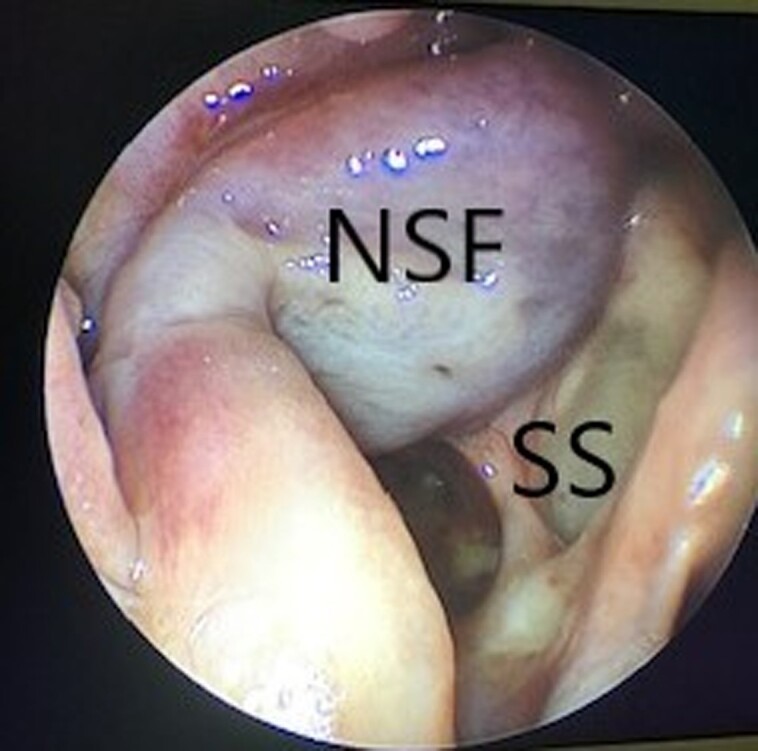
Endoscopic view of a sphenoid sinus with nasoseptal flap reconstruction of a pituitary sella defect following complete healing.
Abbreviations: NSF nasoseptal flap, SS sphenoid sinus.
Symptoms that patients of EESBS may notice beyond the immediate postoperative period include nasal crusting, nasal obstruction, or reduction in smell function as the sinonasal mucosa is disrupted as part of the approach to the pituitary sella. In fact, 3.4% of patients undergoing an endoscopic resection of a sinus or skull base neoplasm were noted in a large retrospective review to develop CRS [52]. Endonasal symptoms may cause a significant detriment to patients’ subjective QOL. It should be noted that studies evaluating the impact of EESBS typically include other pathologies aside from pituitary adenomas, although pituitary adenomas comprise most of the pathology in these data sets. A meta-analysis of 19 studies with 1025 patients undergoing EESBS demonstrated temporary worsening of sinonasal QOL within 4 weeks of surgery but improvement in QOL thereafter to either baseline or improved status. This temporary worsening aligns with observed symptomatology following EESBS and reflects the crusting, draining, and other postsurgical changes associated with the healing process. Patients without preoperative sinonasal symptoms returned to baseline sinonasal QOL, whereas patients with preoperative sinonasal symptoms actually had significant improvement in QOL at 6 months post-surgery and beyond [53]. The fact that patients with preoperative sinonasal symptoms experience improvement following EESBS is reflective of the added benefit that the endoscopic endonasal approach provides in addressing concurrent sinonasal pathology or anatomic obstructions. This improves the postoperative ventilation of the paranasal sinuses and allows access for delivery of topical therapies such as saline irrigations or topical corticosteroids. Decrease in olfaction can be a particularly bothersome complication that substantially impacts QOL, diminishes the experience of eating, and may be a harbinger of depression, declines in cognitive function, and frailty status [39, 54, 55]. The literature regarding the impact of EESBS on olfaction is mixed, with a recent meta-analysis finding high heterogeneity among studies evaluating olfactory outcomes [56]. However, recent reports demonstrate impacts to olfaction that may be long lasting after EESBS [57, 58]. Olfaction is known to be a significant contributor to the perception of taste and flavor as well, and emerging studies similarly demonstrate taste dysfunction following endoscopic skull base surgery [57]. Generally, efforts are made during EESBS to preserve the superior olfactory strip, a region of the nasal cavity known to house a majority of olfactory nerve fibers, but a recent analysis found an incidence of objective hyposmia of 5.5% in patients undergoing EESBS [58].
External nasal deformity, such as nasal dorsum collapse, is a complication that is unique to the EESBS approach for pituitary surgery. This is relatively rare, with 1 case series noting a rate of 5.8% in 328 patients, but is cosmetically noticeable and may require a revision reconstructive surgery to correct [59]. This complication almost exclusively occurs in cases that require a nasoseptal flap for skull base reconstruction and is more likely in patients with malignancies of the skull base rather than benign tumors [60].
Conclusion
The case presented here demonstrates the efficacy and safety of endoscopic transsphenoidal surgery, which is the first-line treatment for pituitary adenomas that secrete hormones other than prolactin or cause compressive symptoms. Thorough preoperative evaluation of the patient's history and anatomy is crucial to the success and safety of the surgery in identifying high-risk areas and allowing for surgical planning. Similarly, sinonasal comorbidities can be identified and treated medically in advance of the surgery, and surgical treatment can be incorporated into the pituitary surgery. Endoscopic endonasal surgical resection of pituitary adenomas is only technically feasible with the simultaneous use of multiple instruments and an endoscope operated by 2 surgeons, and the need for space to accommodate this determines the extent of resection of normal anatomic structures. There are a variety of methods and materials to close the resulting skull base defect, with low rates of CSF leak in the modern era. Patients will experience a temporary negative impact in sinonasal quality of life after EESBS, but most will return to their baseline status. Strict postoperative sinonasal care in the form of nasal rinses and debridements can expedite sinonasal healing, but there are still no concrete guidelines regarding the optimal postoperative regimen. Overall otolaryngologists play a critical role in the preoperative evaluation, surgery, and postoperative care of patients with pituitary adenomas and their involvement in all phases of care is critical to the success of treatment.
Contributor Information
Michael Z Cheng, Department of Otolaryngology-Head and Neck Surgery, Johns Hopkins Medicine, Baltimore, MD 21287, USA.
Anirudh Saraswathula, Department of Otolaryngology-Head and Neck Surgery, Johns Hopkins Medicine, Baltimore, MD 21287, USA.
Hannan A Qureshi, Department of Otolaryngology-Head and Neck Surgery, Johns Hopkins Medicine, Baltimore, MD 21287, USA.
Debraj Mukherjee, Department of Neurosurgery, Johns Hopkins Medicine, Baltimore, MD 21287, USA.
Nicholas R Rowan, Email: nrowan1@jhmi.edu, Department of Otolaryngology-Head and Neck Surgery, Johns Hopkins Medicine, Baltimore, MD 21287, USA.
Funding
None.
Disclosures
None of the authors have a conflicted of interest that is relevant to the subject matter or materials included in this work.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1. Melmed S, Kaiser UB, Lopes MB, et al. Clinical biology of the pituitary adenoma. Endocr Rev. 2022;43(6):1003‐1037. Doi: 10.1210/endrev/bnac010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Daly AF, Beckers A. The epidemiology of pituitary adenomas. Endocrinol Metab Clin North Am. 2020;49(3):347‐355. Doi: 10.1016/j.ecl.2020.04.002 [DOI] [PubMed] [Google Scholar]
- 3. Barbot M, Ceccato F, Lizzul L, et al. Perioperative multidisciplinary management of endoscopic transsphenoidal surgery for sellar lesions: practical suggestions from the Padova model. Neurosurg Rev. 2020;43(4):1109‐1116. Doi: 10.1007/s10143-019-01132-1 [DOI] [PubMed] [Google Scholar]
- 4. Casanueva FF, Barkan AL, Buchfelder M, et al. Criteria for the definition of Pituitary Tumor Centers of Excellence (PTCOE): a Pituitary Society statement. Pituitary. 2017;20(5):489‐498. Doi: 10.1007/s11102-017-0838-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Molitch ME. Diagnosis and treatment of pituitary adenomas: a review. JAMA. 2017;317(5):516‐524. Doi: 10.1001/jama.2016.19699 [DOI] [PubMed] [Google Scholar]
- 6. Aghi MK, Chen CC, Fleseriu M, et al. Congress of Neurological Surgeons systematic review and evidence-based guidelines on the management of patients with nonfunctioning pituitary adenomas: executive summary. Neurosurgery. 2016;79(4):521‐523. Doi: 10.1227/NEU.0000000000001386 [DOI] [PubMed] [Google Scholar]
- 7. Freda PU, Beckers AM, Katznelson L, et al. Pituitary incidentaloma: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(4):894‐904. Doi: 10.1210/jc.2010-1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kasemsiri P, Carrau RL, Ditzel Filho LFS, et al. Advantages and limitations of endoscopic endonasal approaches to the skull base. World Neurosurg. 2014;82(6):S12‐S21. Doi: 10.1016/j.wneu.2014.07.022 [DOI] [PubMed] [Google Scholar]
- 9. Kuo JS, Barkhoudarian G, Farrell CJ, et al. Congress of Neurological Surgeons systematic review and evidence-based guideline on surgical techniques and technologies for the management of patients with nonfunctioning pituitary adenomas. Neurosurgery. 2016;79(4):E536. Doi: 10.1227/NEU.0000000000001390 [DOI] [PubMed] [Google Scholar]
- 10. Sansoni ER, Harvey RJ. Large skull base defect reconstruction with and without pedicled slaps. Atlas of endoscopic sinus and skull base surgery. 2nd ed. Elsevier; 2019. [Google Scholar]
- 11. Nyquist GG, Friedel ME, Singhal S, et al. Surgical management of rhinosinusitis in endoscopic-endonasal skull-base surgery. Int Forum Allergy Rhinol. 2015;5(4):339‐343. Doi: 10.1002/alr.21476 [DOI] [PubMed] [Google Scholar]
- 12. Shah J, Cappello ZJ, Roxbury C, et al. Prevalence and clinical significance of radiographic sinus disease on preoperative computed tomography imaging in the endoscopic skull base surgery population. Am J Rhinol Allergy. 2021;35(2):239‐244. Doi: 10.1177/1945892420949130 [DOI] [PubMed] [Google Scholar]
- 13. Howland WC III. Fluticasone propionate: topical or systemic effects? Clin Exp Allergy. 1996;26(s3):18‐22. [DOI] [PubMed] [Google Scholar]
- 14. Harding SM. The human pharmacology of fluticasone propionate. Respir Med. 1990;84(sA):25‐29. [DOI] [PubMed] [Google Scholar]
- 15. Sampieri G, Namavarian A, Lee JJW, Hamour AF, Lee JM. Hypothalamic-pituitary-adrenal axis suppression and intranasal corticosteroid use: a systematic review and meta-analysis. Int Forum Allergy Rhinol. 2022;12(1):11‐27. Doi: 10.1002/alr.22863 [DOI] [PubMed] [Google Scholar]
- 16. Henry RK, Reeves RA, Wackym PA, Ahmed OH, Hanft SJ, Kwong KM. Frailty as a predictor of postoperative complications following skull base surgery. Laryngoscope. 2021;131(9):1977‐1984. Doi: 10.1002/lary.29485 [DOI] [PubMed] [Google Scholar]
- 17. Goshtasbi K, Abiri A, Lehrich BM, et al. Association between modified frailty index and surgical outcomes in intradural skull base surgery. J Clin Neurosci. 2021;91:255‐259. Doi: 10.1016/j.jocn.2021.07.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Subramaniam S, Aalberg JJ, Soriano RP, Divino CM. New 5-factor modified frailty index using American College of Surgeons NSQIP data. J Am Coll Surg. 2018;226(2):173‐181.e8. Doi: 10.1016/j.jamcollsurg.2017.11.005 [DOI] [PubMed] [Google Scholar]
- 19. Clegg A, Hassan-Smith Z. Frailty and the endocrine system. Lancet Diabetes Endocrinol. 2018;6(9):743‐752. Doi: 10.1016/S2213-8587(18)30110-4 [DOI] [PubMed] [Google Scholar]
- 20. Chiu AG, Palmer JN, Adappa ND. Atlas of Endoscopic Sinus and Skull Base Surgery. 2nd ed. Elsevier; 2019:133‐140. [Google Scholar]
- 21. Wiebracht ND, Zimmer LA. Complex anatomy of the sphenoid sinus: a radiographic study and literature review. J Neurol Surg B Skull Base. 2014;75(6):378‐382. Doi: 10.1055/s-0034-1376195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Boxerman JL, Rogg JM, Donahue JE, Machan JT, Goldman MA, Doberstein CE. Preoperative MRI evaluation of pituitary macroadenoma: imaging features predictive of successful transsphenoidal surgery. Am J Roentgenol. 2010;195(3):720‐728. Doi: 10.2214/AJR.09.4128 [DOI] [PubMed] [Google Scholar]
- 23. Ramakrishnan VR, Suh JD, Lee JY, O’Malley BW, Grady MS, Palmer JN. Sphenoid sinus anatomy and suprasellar extension of pituitary tumors. J Neurosurg. 2013;119(3):669‐674. Doi: 10.3171/2013.3.JNS122113 [DOI] [PubMed] [Google Scholar]
- 24. Smith KA, Leever JD, Chamoun RB. Prediction of consistency of pituitary adenomas by magnetic resonance imaging. J Neurol Surg B Skull Base. 2015;76(5):340‐343. Doi: 10.1055/s-0035-1549005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yiping L, Ji X, Daoying G, Bo Y. Prediction of the consistency of pituitary adenoma: a comparative study on diffusion-weighted imaging and pathological results. J Neuroradiol. 2016;43(3):186‐194. Doi: 10.1016/j.neurad.2015.09.003 [DOI] [PubMed] [Google Scholar]
- 26. Chen CC, Carter BS, Wang R, et al. Congress of Neurological Surgeons systematic review and evidence-based guideline on preoperative imaging assessment of patients with suspected nonfunctioning pituitary adenomas. Neurosurgery. 2016;79(4):E524. Doi: 10.1227/NEU.0000000000001391 [DOI] [PubMed] [Google Scholar]
- 27. Chapman PR, Singhal A, Gaddamanugu S, Prattipati V. Neuroimaging of the pituitary gland: practical anatomy and pathology. Radiol Clin North Am. 2020;58(6):1115‐1133. Doi: 10.1016/j.rcl.2020.07.009 [DOI] [PubMed] [Google Scholar]
- 28. Fried MP, Moharir VM, Shin J, Taylor-Becker M, Morrison P, Kennedy DW. Comparison of endoscopic sinus surgery with and without image guidance. Am J Rhinol. 2002;16(4):193‐197. Doi: 10.1177/194589240201600403 [DOI] [PubMed] [Google Scholar]
- 29. Dalgorf DM, Sacks R, Wormald P-J, et al. Image-guided surgery influences perioperative morbidity from endoscopic sinus surgery: a systematic review and meta-analysis. Otolaryngol Head Neck Surg. 2013;149(1):17‐29. Doi: 10.1177/0194599813488519 [DOI] [PubMed] [Google Scholar]
- 30. Keschner D, Lee J. Use of surgical navigation during endoscopic skull base surgery. Oper Tech Otolaryngol-Head and Neck Surg. 2010;21(1):44‐50. Doi: 10.1016/j.otot.2009.06.003 [DOI] [Google Scholar]
- 31. Goshtasbi K, Lehrich BM, Abouzari M, et al. Endoscopic versus nonendoscopic surgery for resection of pituitary adenomas: a national database study. J Neurosurg. 2021;134(3):816‐824. Doi: 10.3171/2020.1.JNS193062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen J, Liu H, Man S, et al. Endoscopic vs. microscopic transsphenoidal surgery for the treatment of pituitary adenoma: a meta-analysis. Front Surg. 2022;8:806855. Doi: 10.3389/fsurg.2021.806855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Khalafallah AM, Liang AL, Jimenez AE, et al. Trends in endoscopic and microscopic transsphenoidal surgery: a survey of the International Society of Pituitary Surgeons between 2010 and 2020. Pituitary. 2020;23(5):526‐533. Doi: 10.1007/s11102-020-01054-y [DOI] [PubMed] [Google Scholar]
- 34. Wang EW, Zanation AM, Gardner PA, et al. ICAR: endoscopic skull-base surgery. Int Forum Allergy Rhinol. 2019;9(2):S145‐S365. Doi: 10.1002/alr.22326 [DOI] [PubMed] [Google Scholar]
- 35. Hadad G, Bassagasteguy L, Carrau RL, et al. A novel reconstructive technique after endoscopic expanded endonasal approaches: vascular pedicle nasoseptal flap. Laryngoscope. 2006;116(10):1882‐1886. Doi: 10.1097/01.mlg.0000234933.37779.e4 [DOI] [PubMed] [Google Scholar]
- 36. Kessler R, Garzon-Muvdi T, Kim E, Ramanathan M, Lim M. Utilization of the nasoseptal flap for repair of cerebrospinal fluid leak after endoscopic endonasal approach for resection of pituitary tumors. Brain Tumor Res Treat. 2019;7(1):10‐15. Doi: 10.14791/btrt.2019.7.e19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stamm A, Pignatari S, Balsalobre L, Dassi C. Transnasal endoscopic-assisted surgery of the anterior skull base. Cummings otolaryngology: head and neck surgery. 7th ed. Elsevier; 2021. [Google Scholar]
- 38. Lee JM, Grewal A. Middle meatal spacers for the prevention of synechiae following endoscopic sinus surgery: a systematic review and meta-analysis of randomized controlled trials. Int Forum Allergy Rhinol. 2012;2(6):477‐486. Doi: 10.1002/alr.21052 [DOI] [PubMed] [Google Scholar]
- 39. Kuan EC, Palmer JN.. Epistaxis. Cummings otolaryngology: head and neck surgery. 7th ed. Elsevier; 2021. [Google Scholar]
- 40. Florea SM, Graillon T, Cuny T, Gras R, Brue T, Dufour H. Ophthalmoplegic complications in transsphenoidal pituitary surgery. J Neurosurg. 2019;133(3):693‐701. Doi: 10.3171/2019.5.JNS19782 [DOI] [PubMed] [Google Scholar]
- 41. Warnecke A, Averbeck T, Wurster U, Harmening M, Lenarz T, Stöver T. Diagnostic relevance of β2-transferrin for the detection of cerebrospinal fluid fistulas. Arch Otolaryngol–Head Neck Surg. 2004;130(10):1178‐1184. Doi: 10.1001/archotol.130.10.1178 [DOI] [PubMed] [Google Scholar]
- 42. Bernal-Sprekelsen M, Alobid I, Mullol J, Trobat F, Tomás-Barberán M. Closure of cerebrospinal fluid leaks prevents ascending bacterial meningitis. Rhinology. 2005;43(4):277‐281. [PubMed] [Google Scholar]
- 43. Alzhrani G, Sivakumar W, Park MS, Taussky P, Couldwell WT. Delayed complications after transsphenoidal surgery for pituitary adenomas. World Neurosurg. 2018;109:233‐241. Doi: 10.1016/j.wneu.2017.09.192 [DOI] [PubMed] [Google Scholar]
- 44. Schlosser RJ. Epistaxis. N Engl J Med. 2009;360(8):784‐789. Doi: 10.1056/NEJMcp0807078 [DOI] [PubMed] [Google Scholar]
- 45. Zada G, Lopes MBS, Mukundan S, Laws E. Mucocele and mucopyocele. In: Zada G, Lopes MBS, Mukundan S Jr, Laws ER Jr, editors. Atlas of sellar and parasellar lesions: clinical, radiologic, and pathologic correlations. Springer; 2016:411‐414. [Google Scholar]
- 46. McCoul ED, Anand VK, Singh A, Nyquist GG, Schaberg MR, Schwartz TH. Long-term effectiveness of a reconstructive protocol using the nasoseptal flap after endoscopic skull base surgery. World Neurosurg. 2014;81(1):136‐143. Doi: 10.1016/j.wneu.2012.08.011 [DOI] [PubMed] [Google Scholar]
- 47. Gan EC, Habib A-RR, Rajwani A, Five-degree JA. 10-degree, and 20-degree reverse Trendelenburg position during functional endoscopic sinus surgery: a double-blind randomized controlled trial. Int Forum Allergy Rhinol. 2014;4(1):61‐68. Doi: 10.1002/alr.21249 [DOI] [PubMed] [Google Scholar]
- 48. Henriquez OA, Schlosser RJ, Mace JC, Smith TL, Soler ZM. Impact of synechiae after endoscopic sinus surgery on long-term outcomes in chronic rhinosinusitis. Laryngoscope. 2013;123(11):2615‐2619. Doi: 10.1002/lary.24150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Eloy P, Andrews P, Poirrier A-L. Postoperative care in endoscopic sinus surgery: a critical review. Curr Opin Otolaryngol Head Neck Surg. 2017;25(1):35‐42. Doi: 10.1097/MOO.0000000000000332 [DOI] [PubMed] [Google Scholar]
- 50. Rudmik L, Soler ZM, Orlandi RR, et al. Early postoperative care following endoscopic sinus surgery: an evidence-based review with recommendations. Int Forum Allergy Rhinol. 2011;16(6):417‐430. Doi: 10.1002/alr.20072 [DOI] [PubMed] [Google Scholar]
- 51. Green R, Banigo A, Hathorn I. Postoperative nasal debridement following functional endoscopic sinus surgery, a systematic review of the literature. Clin Otolaryngol. 2015;40(1):2‐8. Doi: 10.1111/coa.12330 [DOI] [PubMed] [Google Scholar]
- 52. Naunheim MR, Sedaghat AR, Lin DT, et al. Immediate and delayed complications following endoscopic skull base surgery. J Neurol Surg B Skull Base. 2015;76(5):390‐396. Doi: 10.1055/s-0035-1549308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bhenswala PN, Schlosser RJ, Nguyen SA, Munawar S, Rowan NR. Sinonasal quality-of-life outcomes after endoscopic endonasal skull base surgery. Int Forum Allergy Rhinol. 2019;9(10):1105‐1118. Doi: 10.1002/alr.22398 [DOI] [PubMed] [Google Scholar]
- 54. Bernstein IA, Roxbury CR, Lin SY, Rowan NR. The association of frailty with olfactory and gustatory dysfunction in older adults: a nationally representative sample. Int Forum Allergy Rhinol. 2021;11(5):866‐876. Doi: 10.1002/alr.22718 [DOI] [PubMed] [Google Scholar]
- 55. Eliyan Y, Wroblewski KE, et al. Olfactory dysfunction predicts the development of depression in older us adults. Chem Senses. 2021;46:bjaa075. Doi: 10.1093/chemse/bjaa075/5983672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yin LX, Low CM, Puccinelli CL, et al. Olfactory outcomes after endoscopic skull base surgery: a systematic review and meta-analysis. Laryngoscope. 2019;129(9):1998‐2007. Doi: 10.1002/lary.28003 [DOI] [PubMed] [Google Scholar]
- 57. Hura N, Orlov CP, Khalafallah AM, Mukherjee D, Rowan NR. Impact of routine endoscopic skull base surgery on subjective olfaction and gustation outcomes. Oper Neurosurg (Hagerstown). 2021;21(3):137‐142. Doi: 10.1093/ons/opab137 [DOI] [PubMed] [Google Scholar]
- 58. Griffiths CF, Barkhoudarian G, Cutler A, et al. Analysis of olfaction after bilateral nasoseptal rescue flap transsphenoidal approach with olfactory mucosal preservation. Otolaryngol Head Neck Surg. 2019;161(5):881‐889. Doi: 10.1177/0194599819861340 [DOI] [PubMed] [Google Scholar]
- 59. Rowan NR, Wang EW, Gardner PA, Fernandez-Miranda JC, Snyderman CH. Nasal deformities following nasoseptal flap reconstruction of skull base defects. J Neurol Surg B Skull Base. 2016;77(1):14‐18. Doi: 10.1055/s-0035-1555136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rowan NR, Valappil B, Chen J, Wang EW, Gardner PA, Snyderman CH. Prospective characterization of postoperative nasal deformities in patients undergoing endoscopic endonasal skull-base surgery. Int Forum Allergy Rhinol. 2020;10(2):256‐264. Doi: 10.1002/alr.22466 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.