Abstract
Currently transgenic frog embryos are generated using restriction-enzyme-mediated integration (REMI) on decondensed sperm nuclei followed by nuclear transplantation into unfertilized eggs. We have developed a simplified version of this protocol that has the potential to increase the numbers of normally developing transgenic embryos.
INTRODUCTION
The development of a reliable method of generating transgenic frog embryos (1) has transformed the potential of Xenopus laevis and the related diploid species Xenopus (Silurana) tropicalis, offering for the first time the possibility of applying powerful genetic tools to studying developmental biology in amphibians. Central to this method is the use of restriction-enzyme-mediated integration (REMI) of DNA in partially chromatin decondensed sperm nuclei prior to transplantation into unfertilized eggs. A major concern of REMI is the generation of aneuploid embryos which do not develop normally beyond neurula stages. This is likely to be due, at least in part, to the fragility of the decondensed sperm nuclei during the transfer process. Recently a simplified method of generating transgenic mice has been reported that involves intra-cytoplasmic sperm injection (ICSI), using membrane-disrupted sperm heads that are briefly incubated with linearised DNA prior to injection (2). This method bears similarities to the REMI technique, but does not require any sperm decondensation or treatment with restriction enzymes. We have tested whether such an approach is successful in X.laevis and compared the viability of injected eggs and the frequency of transgenesis with results obtained using the REMI technique.
MATERIALS AND METHODS
Transgenesis
A synthetic green fluorescent protein (GFP) reporter gene containing the GFP open reading frame and the SV40 polyadenylation signal was cloned downstream of the 580 bp Xenopus laevis cardiac α-actin promoter (3). NotI sites were added to either end by linker addition. DNA for transgenesis was perpared by NotI digestion, followed by gel purification using Qiagen QIAquick gel extraction kit. DNA concentration was estimated by OD260/280. Sperm nuclei were prepared by the method of Kroll and Amaya (1), except that sperm were permeabilised with digitonin in preference to lysolecithin (4). Sperm nuclei were either used fresh, or snap frozen in liquid nitrogen and stored at –80°C as noted in the text. The standard method of REMI followed by sperm transplantation was as described (1). For the simplified method, 250 000 sperm nuclei in 2.5 µl were combined with 125 ng of carGFP in 2.5 µl water. The reaction was incubated at room temperature for 10–15 min, then 2.1 µl was diluted into 200 µl of sperm dilution buffer (1), mixed by pipetting up-and-down with a clipped tip 30 times to ensure an even suspension, and injected into unfertilized eggs using a Harvard Instruments constant flow injector with a flow rate of 0.6 µl/min. Embryo and tadpole husbandry was as described (1). For control experiments, fertilized embryos were injected with 100 pg of either linear carGFP or circular carGFP in pKSII+ vector at the 1–4 cell stage. GFP expression was visualised using a Leica fluorescent dissecting microscope. Bright field and GFP images were captured using a JVC KY-F55B 3 chip CCD colour video camera and images overlayed using Adobe Photoshop 5.0.
Genomic DNA extraction and Southern blotting
To extract genomic DNA, tadpoles were sacrificed and incubated for 4–5 h at 55°C in 10 mM Tris, pH 8.0, 0.5% SDS, 0.1 M EDTA and 100 µg/ml proteinase K. After three phenol extractions, DNA was precipitated by adding 0.2 vol 10 M ammonium acetate and 2 vol of ethanol. The DNA pellets were washed twice in 70% ethanol, air-dried, and resuspended in TE. Genomic DNA was digested with the appropriate restriction enzyme for 2–3 h, separated on a 0.7% agarose gel and blotted onto Hybond nylon membrane (Amersham). The filter was hybridised with an oligo-labelled probe consisting of a 499 bp EcoRV–BamHI fragment from carGFP and washed to a stringency of 0.1× SSC, 0.1% SDS at 42°C. A Molecular Dynamics phosphorimager was used for quantitation. Plasmid copy number and number of copies per integration site were determined as described (1).
RESULTS AND DISCUSSION
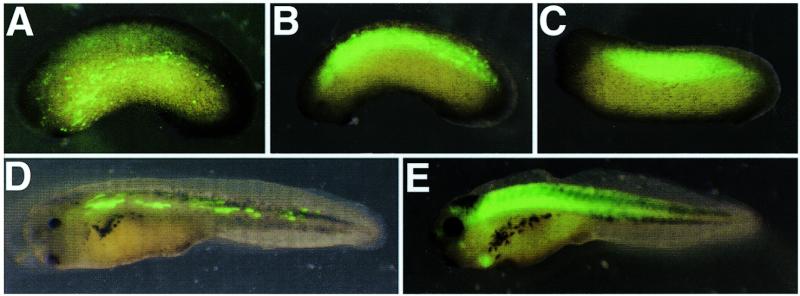
We tested a simplified version of the standard method of generating transgenic X.laevis in which partial sperm decondensation by interphase egg extract was omitted, along with restriction enzyme treatment. Sperm nuclei were injected into unfertilized eggs after a 10–15 min incubation with linear DNA containing a 600 bp promoter from the Xenopus laevis cardiac actin gene (3) upstream of a GFP coding sequence as a reporter (carGFP). In this experiment, 59 surviving embryos were scored for GFP expression at stage 34–35. Nineteen (32%) showed uniform expression restricted to the somites and heart (Fig. 1C and E), and six (10%) showed varying degrees of non-uniform expression (mosaicism) within the somites (Fig. 1B). In no case was expression of GFP seen outside the somitic or heart tissue. The standard transgenesis method also generates similar proportions of embryos expressing transgenes in mosaic patterns (1). When sperm nuclei and linear DNA were incubated together for shorter periods (1–2 min) we found a marked increase in muscle-restricted mosaicism (11% non-mosaic; 43% mosaic; n = 142). This may be due to a lack of time for the linear transgene and genomic DNA to interact prior to transplantation. This observation may ultimately be useful in the study of the effects of ectopic expression of a gene product in vivo, providing an easy method of controlling the degree of mosaic expression of transgenes in a similar manner to that used extensively in Drosophila melanogaster. We also tested longer incubation times to determine whether this would increase the percentage of transgenics. When DNA and sperm nuclei were incubated at room temperature for 30–180 min before injection, similar numbers of correctly cleaving embryos were observed at the four cell stage (10–20% of total eggs injected). However, these embryos showed a dramatic decrease in survival beyond gastrulation, when compared to a 15 min incubation, and no obvious difference in percentage of transgenics was observed.
Figure 1.
Comparison of GFP expression in transgenic and DNA injected embryos. (A) Naturally fertilized embryos were injected with 100 pg of circular DNA containing carGFP plus the pKSII+ vector backbone. (B) Mosaic and (C,E) non-mosaic transgene expression in embryos generated from uncondensed sperm nuclei. (D) Highly mosaic GFP expression in normally fertilized embryos injected with 100 pg of linear carGFP.
Since it has been demonstrated that sperm nuclei snap-frozen in liquid nitrogen and stored at –80°C retain their capacity for successful transgenesis (4), we compared the efficacy of fresh and frozen sperm nuclei in the simplified procedure. In this experiment, frozen sperm nuclei consistently gave the same or better overall transgenic percentage when compared to fresh. These results are in agreement with mouse studies (2) where transgenic numbers produced by ICSI were maximised by disruption of the sperm membrane, and for this purpose freeze–thawing was found to be superior to use of detergent.
One of the major problems with the standard transgenesis procedure is the large size of the wound produced by injection with a wide bored needle. This type of needle is required to avoid disrupting the decondensed nuclei. We reasoned that since we were transplanting condensed nuclei, then a narrower needle would be as efficient in delivering nuclei, as well as improving the health of the embryos. Comparison of 80–100 µm (standard) and 30–40 µm (narrow) needles revealed no significant difference in the number of normally cleaving embryos produced at the four cell stage. However, by gastrulation, the narrow needles produced a mild increase in the proportion of healthy embryos.
Thus we standardised the conditions as follows. Sperm nuclei were snap frozen in liquid nitrogen and stored at –80°C prior to use. Linear DNA was incubated with thawed sperm nuclei for 15 min at room temperature. Nuclei were diluted to a concentration of 1–2 nuclei per nl, mixed extensively to ensure an even suspension and injected into unfertilised eggs using a 30–40 µm needle.
In agreement with Kroll and Amaya (1), we found that the quality of unfertilized eggs was the major determinant of the success of the standard transgenic procedure. To remove this variable from a direct comparison of standard and simplified reactions, we performed parallel nuclei transplantations using equal numbers of unfertilized eggs from one batch of eggs from a single Xenopus female. In five separate parallel injection experiments the simplified reaction was at least as efficient at producing normally expressing transgenic embryos, with a similar percentage of mosaics. In addition, the number of embryos produced that survived to late tailbud stages was improved over the standard technique.
Trangenic embryos generated using the modified procedure were successfully raised to metamorphosis, and continued to express GFP in both skeletal and heart muscle, suggesting that the transgene had been successfully integrated into the Xenopus genome. To confirm this, month-old tadpoles were sacrificed and analysed by genomic Southern blotting using a probe to the GFP coding region (Fig. 2). Embryos were raised for this length of time since little or no non-integrated plasmid DNA can be detected in single embryos by Southern blotting at these stages (5). Non-expressing embryos showed no hybridisation to the GFP probe (Fig. 2B, lanes 7–9), however a 1.7 kb fragment was common to all GFP-expressing tadpoles, representing head-to-tail concatemers of the transgene (Fig. 2B, lanes 1–3). In addition, 1.3 kb bands were seen in these tadpoles, most likely representing head-to-head concatemers (Fig. 2A). Each GFP-expressing tadpole also contained several other fragments, probably representing junction sites between the transgene and chromosomal sequences. By comparison with plasmid standards (1), we estimated that each transgenic tadpole contained 1–15 copies of the transgene, present in short concatemers (1–8 copies) at 1–6 sites within the genome. These results are similar to those reported in the original Xenopus transgenesis method (1), although copy numbers are slightly lower. Embryos expressing GFP in a non-uniform manner in the somites generally showed weak hybridisation to the probe (Fig. 2B, lanes 4–6). This is consistent with the assumption that these embryos contain integrated transgene in only a small subset of cells.
Figure 2.

Tadpoles produced by sperm nuclear transplantation without decondensation contain integrated plasmid. (A) Schematic diagram of carGFP containing cardiac actin promoter sequences (black box) and GFP coding region followed by SV40 polyadenylation sequences (open box). Beneath are the products expected for head-to-tail, tail-to-tail and head-to-head concatemerisation respectively. N, NotI; R, EcoRV. (B) Southern blot of genomic DNA from 1-month-old tadpoles produced using carGFP nuclear transplantations. Genomic DNA from tadpoles expressing GFP (+), expressing GFP in a non-uniform pattern (m) and non-expressing GFP (–) were digested with EcoRV, and carGFP was detected using the probe shown in (A). The last two lanes contain the indicated amounts of linear carGFP as a control.
Direct injection of DNA into fertilized eggs is known to result in mosaic transgene expression (6). Since a proportion of embryos from the modified transgenesis procedure also showed mosaic transgene expression in the somites, we reasoned that this may have been due to co-injection of DNA with the sperm nuclei, or to the presence of circular DNA contaminating the linear DNA. To distinguish between these possibilities, we compared the effects of injecting fertilized Xenopus eggs with amounts of either linear DNA or circular plasmid DNA equivalent to the amount present in the sperm nuclei transplantation procedure on the resultant GFP expression patterns. In the fertilized egg, linear DNA is able to form concatemers and undergo DNA replication (7), and may also successfully integrate into the genome. However, circular DNA is a poor substrate for integration and is thus maintained extra-chromosomally. It is also unable to concatemerise or undergo DNA replication and thus is gradually lost as development proceeds (7).
In 13/21 embryos (61.9%) injected with circular DNA, GFP was expressed ectopically in scattered individual cells (Fig. 1A), with five of these embryos also having low levels of mosaic expression in the somites. The GFP expression in these embryos persisted to feeding tadpole stages, albeit at significantly lower levels than transgenic GFP expression, and levels were observed to decrease steadily with time. In contrast, injection of linear DNA produced mosaic GFP expression similar to that seen in transgenics in 17/79 embryos (21.5%; Fig. 1D). We conclude that whilst uniform transgene expression is due to integration of the reporter into sperm chromosomal DNA, mosaic expression is probably a direct result of linear DNA co-injected with the sperm nuclei.
In conclusion, we have modified the method of generating X.laevis transgenics that has several significant advantages. First the technique is much simplified, eliminating the need for interphase egg extract and REMI. Secondly the use of uncondensed sperm nuclei reduces the incidence of aneuploid embryos, and allows injection with a narrower gauge needle, which improves overall embryo health prior to gastrulation. We have demonstrated that this technique produces comparable or better numbers of viable embryos that correctly express the transgene, and that the DNA is integrated into the genome with approximately the same copy number and number of integration sites as the standard method. We hope that such simplification will encourage the use of Xenopus transgenesis as a convenient system for rapid mapping of promoter regulatory sequences, providing an alternative to the slow and costly methods required for mammalian embryos. In addition, although this method has been developed in X.laevis, we are currently attempting to apply it to the diploid sister species Xenopus (Silurana) tropicalis, where a simplifed technique that increases the health and viability of transgenic animals will be invaluable to the fledgling use of genetics in the amphibian model.
Acknowledgments
ACKNOWLEDGEMENTS
This work was supported by the Medical Research Council and the British Heart Foundation.
REFERENCES
- 1.Kroll K.L. and Amaya,E. (1996) Development, 122, 3173–3183. [DOI] [PubMed] [Google Scholar]
- 2.Perry A.C.F., Wakayama,T., Kishikawa,H., Kasai,T., Okabe,M., Toyoda,Y. and Yanagimachi,R. (1999) Science, 284, 1180–1183. [DOI] [PubMed] [Google Scholar]
- 3.Mohun T.J., Brennan,S., Dathan,N., Fairman,S. and Gurdon,J.B. (1986) EMBO J., 5, 3185–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang H., Marsh-Armstrong,N. and Brown,D.D. (1999) Proc. Natl Acad. Sci. USA, 96, 962–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kroll K.L. (1994) Transgenesis in Xenopus laevis. Ph.D. Thesis, University of California, Berkeley, CA.
- 6.Christian J.L. and Moon,R.T. (1993) Genes Dev., 7, 13–28. [DOI] [PubMed] [Google Scholar]
- 7.Marini N.J., Etkin,L.D. and Benbow,R.M. (1988) Dev. Biol., 127, 421–434. [DOI] [PubMed] [Google Scholar]