Abstract
Barrett’s esophagus (BE) is a common condition associated with chronic gastroesophageal reflux disease. BE is the only known precursor to esophageal adenocarcinoma, a highly lethal cancer with an increasing incidence over the last 5 decades. These revised guidelines implement Grading of Recommendations, Assessment, Development, and Evaluation methodology to propose recommendations for the definition and diagnosis of BE, screening for BE and esophageal adenocarcinoma, surveillance of patients with known BE, and the medical and endoscopic treatment of BE and its associated early neoplasia. Important changes since the previous iteration of this guideline include a broadening of acceptable screening modalities for BE to include nonendoscopic methods, liberalized intervals for surveillance of short-segment BE, and volume criteria for endoscopic therapy centers for BE. Were commend endoscopic eradication therapy for patients with BE and high-grade dysplasia and those with BE and low-grade dysplasia. We propose structured surveillance intervals for patients with dysplastic BE after successful ablation based on the baseline degree of dysplasia. We could not make recommendations regarding chemoprevention or use of biomarkers in routine practice due to insufficient data.
INTRODUCTION
Barrett’s esophagus (BE) is a metaplastic change of the distal esophagus, whereby the normal squamous epithelium is replaced by specialized columnar epithelium with goblet cells (1). This metaplastic change is associated with chronic gastroesophageal reflux disease (GERD), such that 5%–12% of patients with chronic GERD symptoms will harbor BE (2,3). BE is the only known precursor lesion of esophageal adenocarcinoma (EAC), a cancer with a rapidly increasing incidence over the last 40 years in the United States and other Western countries (4).
In this revised guideline, the American College of Gastroenterology (ACG) offers recommendations for the diagnosis, screening, surveillance, and endoscopic and medical therapy of BE. Although BE may be considered as a severe manifestation of GERD, this guideline makes no recommendations as to care of GERD, and we call to the reader’s attention a recent ACG guideline for care of patients with GERD (5). Below we briefly review the methodology for the creation of these guidelines. Following that, the guidelines are broken into 5 sections, titled diagnosis, screening, surveillance, medical therapy of BE, and endoscopic therapy of BE.
These guidelines are established to support clinical practice and suggest preferable approaches to a typical patient with a particular medical problem based on the currently available published literature. When exercising clinical judgment, particularly when treatments pose significant risks, health care providers should incorporate this guideline in addition to patient-specific medical comorbidities, health status, and preferences to arrive at a patient-centered care approach.
METHODS
The guideline is structured in the format of statements that were considered to be clinically important by the authors. Twenty-one clinically relevant questions were developed and refined by 5 content experts who focus their clinical and research efforts on the care of patients with BE, who composed the authoring panel (panel) for this statement. Questions were formatted in the PICO structure (Population, Intervention, Comparator, and Outcome). The Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) process (Table 1) was used to assess the quality of evidence for each question by 2 formally trained GRADE methodologists (B.G.S. and R.H.Y.) (6). The quality of evidence is expressed as high (we are confident in the effect estimate to support a particular recommendation), moderate, low, or very low (we have very little confidence in the effect estimate to support a particular recommendation) based on the risk of bias of the studies, evidence of publication bias, heterogeneity among studies, directness of the evidence, and precision of the estimate of effect (7). A strength of recommendation is given as either strong (noted as recommendations, and meaning that most patients should receive the recommended course of action) or conditional (noted as suggestions, and meaning that many patients should have this recommended course of action, but different choices may be appropriate for some patients) based on the quality of evidence, risks vs benefits, feasibility, and costs, taking into account perceived patient and population-based factors (8). Furthermore, a narrative evidence summary for each section provides important details for the data supporting the statements. It should be noted that the strengths of recommendation are meant to apply to the average or typical patient with BE. Individual patients with BE may benefit from diagnostic or therapeutic strategies not endorsed for the average patient.
Table 1.
Grading of Recommendations, Assessment, Development, and Evaluation quality assessment criteria (6)
| Study design | Quality of evidence | Factors lowering quality of evidence | Factors increasing quality of evidence |
|---|---|---|---|
|
| |||
| Randomized trial | High | Imprecision of
estimate Risk of bias Inconsistency of data Indirectness of evidence Likely publication bias |
Dose-response
gradient Large effect of intervention If plausible confounding would reduce effector suggest a spurious effect when reported results show no effect |
| Moderate | |||
| Observational study | Low | ||
| Very low | |||
The panel used literature searches of MEDLINE and PubMed since inception to provide pertinent literature on each of the 21 PICO questions to the GRADE methodologists. The strongest evidence pertaining to each question was selected, with an emphasis on well-executed meta-analyses and randomized controlled trials, when available. Abstracts and case reports were not included. These PICO questions formed the basis of the 21 recommendations accompanying this statement (Table).
The panel has additionally highlighted key concepts that were not included in the GRADE assessment (Table 3). Key concepts are statements to which the GRADE process has not been applied and often include definitions and epidemiological statements rather than diagnostic or management recommendations.
Table 3.
Barrett’s esophagus key concepts
| Key concept |
|---|
|
|
| 1. Consider cessation of endoscopic surveillance when a patient is no longer a candidate for endoscopic eradication therapy |
| 2. Consider utilization of published quality indicators to benchmark your unit’s performance against published standards |
| 3. Endoscopic cryotherapy may be considered as an alternative modality in patients unresponsive to radiofrequency ablation |
| 4. Patients with Barrett’s esophagus embarking on endoscopic eradication therapy should have a clear understanding of the risks and benefits associated with these therapies before the initiation of therapy |
| 5. Gastroenterologists and centers performing endoscopic eradication therapy should monitor their rates of complete eradication of dysplasia, complete eradication of intestinal metaplasia, and adverse events |
DIAGNOSIS OF BE
Recommendation.
-
1
We suggest that a diagnosis of BE require the finding of intestinal metaplasia (IM) in the tubular esophagus (conditional recommendation, very-low-quality evidence).
Summary of evidence.
BE develops when metaplastic columnar mucosa replaces the normal esophageal squamous epithelium of the esophagus in response to damage caused by gastroesophageal reflux (9). The columnar-lined esophagus contains a mosaic of 3 different cell types: gastric fundic type epithelium, junctional cardiac epithelium, and specialized columnar epithelium with intestinal type goblet cells (10). Most professional guidelines from around the world agree that a diagnosis of BE requires the presence of IM because of an increased risk of EAC associated with IM, although guidelines from the British Society of Gastroenterology and the Asia Pacific region do not require this (11).
Support for the increased risk of EAC with metaplastic IM emerges from several lines of evidence. The strongest evidence comes from a large population-based study of 8,522 patients with BE from the Northern Ireland Cancer Registry (12). The risk for EAC was elevated in patients with IM at index endoscopy compared with those without IM (0.38% vs 0.07%/year; hazard ratio [HR] 3.54; 95% confidence interval [CI] 2.09–6.00). In addition, in a case series of 45 patients with BE or EAC, frequent copy number alterations targeting cancer-associated genes were found in tissue with IM, but no such changes were encountered in columnar metaplasia without goblet cells (13). On the other hand, other studies suggest no difference in cancer risk of columnar epithelium with or without IM. A single-center UK study of 688 patients with a median follow-up of 12 years found no difference in cancer risk for those with a columnar-lined esophagus with or without IM: 0.37% vs 0.30%/year (14). Similarly, a multicenter UK study of 1,751 patients found a similar cancer risk in patients with and without IM (HR 1.36; 95% CI 0.63–2.96) (15). Other data also support these observations. DNA content abnormalities have been observed in equal frequency from metaplastic columnar epithelium with and without goblet cells (16).
Any effort to delete goblet cells from the diagnostic criteria for BE is problematic, as it would dramatically increase the pool of patients undergoing surveillance with concomitant cost and quality of life implications (17,18). For example, work from the University of Chicago suggested that eliminating the requirement of IM would increase the frequency of diagnosis of BE in that center by 147% (19). The inability to find IM in a given patient may reflect biopsies obtained from the proximal stomach or inadequate sampling of the Barrett’s segment. Studies have shown that the yield of IM increases with both the number of biopsies obtained and the length of the Barrett’s segment (20). The implications of a potential non-IM EAC phenotype remain to be determined (21,22).
In summary, the largest retrospective study supports an increased risk for EAC in those with specialized IM with goblet cells compared with those with nongoblet columnar epithelium in the esophagus; however, other retrospective studies have not uniformly supported this finding, leading to inconsistency of the data. Based on the divided nature of the literature, and the retrospective nature of the studies, the quality of the evidence was considered very low.
Recommendation.
-
2We suggest that columnar mucosa of at least 1 cm in length be necessary for a diagnosis of BE, and that:
- Patients with a normal-appearing Z line should not undergo routine endoscopic biopsies.
- In the absence of any visible lesions, patients with a Z line demonstrating <1 cm of proximal displacement from the top of the gastric folds should not undergo routine endoscopic biopsies (quality of evidence: low; strength of recommendation: conditional).
Summary of evidence.
BE is best described by using the validated Prague criteria that includes both the circumferential and maximal extent of the columnar epithelium in the esophagus and the location of the proximal margin of the gastric folds and the diaphragmatic hiatus (Figure 1) (23). The Prague classification has been further validated in both gastroenterology trainees and in community practice (24,25). The Prague classification offers a standardized terminology, which demonstrates excellent reliability coefficients for both the circumferential (0.95) and maximal (0.94) extent of the Barrett’s mucosa, representing an almost perfect level of reliability for both measures. However, the reliability coefficient of the Prague criteria for segments <1 cm is only fair at 0.22. It is this finding that has led to the recommendation, among most professional societies, to require a threshold of 1 cm for the diagnosis of BE (11). Despite this recommendation, biopsies of an irregular or normal Z line remain common in clinical practice in North America.
Figure 1.
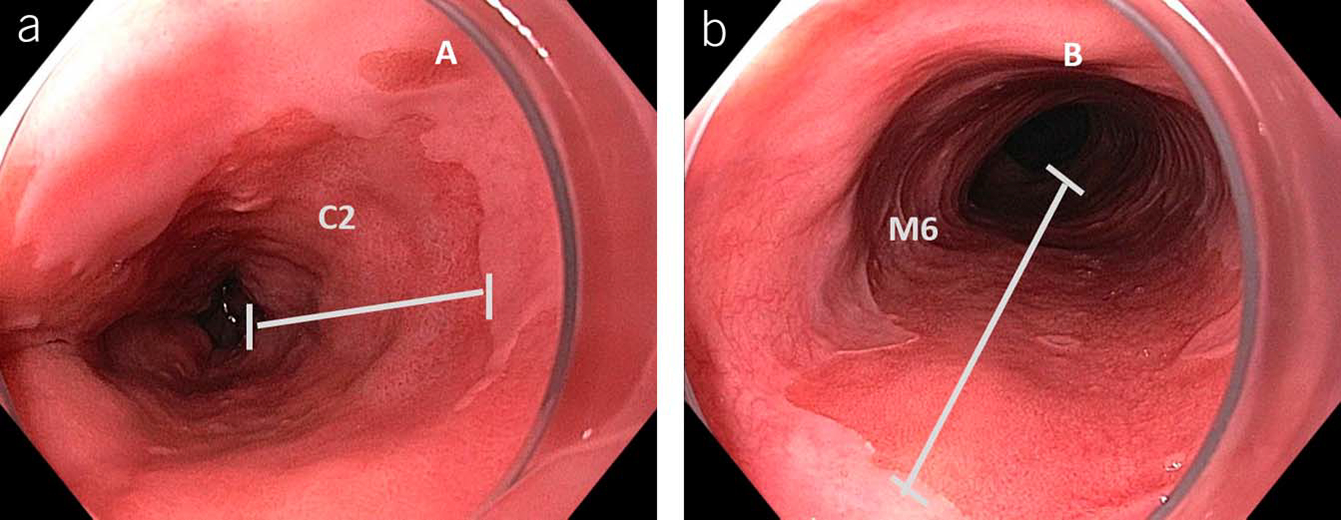
Grading of Barrett’s esophagus using Prague criteria: (a) defining the circumferential extent and (b) maximal extent of the columnar-lined esophagus.
Also supporting a 1-cm threshold for a diagnosis of BE is evidence suggesting that the risk of progression to high-grade dysplasia (HGD) or EAC is extremely low for individuals with a normal or irregular Z line (<1 cm). A population-based cohort study from Olmsted County, MN, examined the natural history of 401 patients with BE (>1 cm) and 86 patients with IM of the gastroesophageal junction (GEJ) followed for a median of 7 and 8 years, respectively (26). None of the patients with IM of the GEJ progressed to HGD or EAC in comparison to a progression rate of 7.9/1,000 person-years in the BE group. A multicenter cohort study of 1,791 patients undergoing surveillance of BE defined as a columnar-lined esophagus with IM on biopsies and followed for a median of 5.9 years found that none of the 167 patients with an irregular Z line (<1 cm) developed HGD or cancer compared with 71 of 1,624 patients with BE ≥1 cm. Furthermore, IM was not found on follow-up biopsies in 53% of individuals with an irregular Z line (27). Neither of these studies demonstrated progression of an irregular Z line to HGD or EAC.
That being said, routine biopsy of the normal or irregular Z line in the absence of mucosal abnormalities has real-life consequences for patients. For example, approximately 80% of patients with IM found on such biopsies are recommended to undergo further surveillance endoscopy with the costs and risks encumbered with such an approach (28). Furthermore, mislabeling an individual with BE has other consequences including higher life insurance premiums and impaired quality of life (17,18).
For all these reasons, we continue to recommend that individuals with a normal- or irregular-appearing Z line should not undergo biopsies in the absence of a clear mucosal abnormality. However, we acknowledge indirectness in the studies, with the most supportive study (27) considered to be low-quality evidence.
Recommendation.
-
3
We suggest at least 8 endoscopic biopsies be obtained in screening examinations with endoscopic findings consistent with possible BE, with the Seattle protocol followed for segments of longer than 4 cm (quality of evidence: low; strength of recommendation: conditional).
Summary of evidence.
The distribution of goblet cells within a segment of BE may be patchy, and sometimes, the mucosa is only sparsely populated with these cells. For these reasons, sampling error may lead to a false-negative examination for IM, especially in those with short segments of columnar-lined esophagus in whom few samples are taken. The likelihood that IM is present increases as the segment length of the columnar epithelium in the esophagus increases (19).
When evaluating the GEJ for the presence of columnar epithelium, it is important to partially de insufflate the esophagus, as over insufflation may flatten gastric folds, making a hiatal hernia resemble a segment of columnar-lined esophagus. When, after careful inspection, a segment of columnar epithelium is identified in the tubular esophagus, enough biopsies must be taken to confidently exclude the presence of IM. Few data exist to document the appropriate number of biopsies to ascertain a diagnosis of BE. Harrison and colleagues analyzed 1,646 biopsies taken from 296 endoscopies in 125 patients with endoscopic evidence of columnar-lined esophagus. These investigators found that any given biopsy in these patients demonstrated IM only 34% of the time. However, if 8 biopsies were analyzed from any given endoscopy, the yield for IM in this group increased to 94% (20). There was no significant increase in this yield if additional biopsies were analyzed. Therefore, these investigators suggested that at least 8 biopsies be taken to rule out the presence of IM when encountering columnar-lined esophagus.
Although this approach is backed by evidence, it does present operational problems. For instance, the endoscopist may encounter a single tongue of a centimeter or 2, which will not support 8 biopsies. In patients with short (1–2 cm) segments of suspected BE in whom 8 biopsies may be unobtainable, at least 4 biopsies per centimeter of circumferential BE and 1 biopsy per centimeter in tongues of BE should be obtained. If any of these biopsies demonstrates IM, the patient is a candidate for inclusion in endoscopic surveillance. A second commonly faced issue is how to manage the patient with a previous endoscopy demonstrating columnar-lined epithelium, but biopsies negative for IM (29).Because endoscopists rarely document the exact number of biopsies taken in this situation, it may be reasonable to repeat the examination a single time because the yield of such an examination for IM may be 25% or more (30). Additional endoscopic examinations beyond this second endoscopy are unlikely to be of utility and are not recommended. Figure 2 demonstrates the recommended care pathway for patients with a columnar-lined esophagus.
Figure 2.
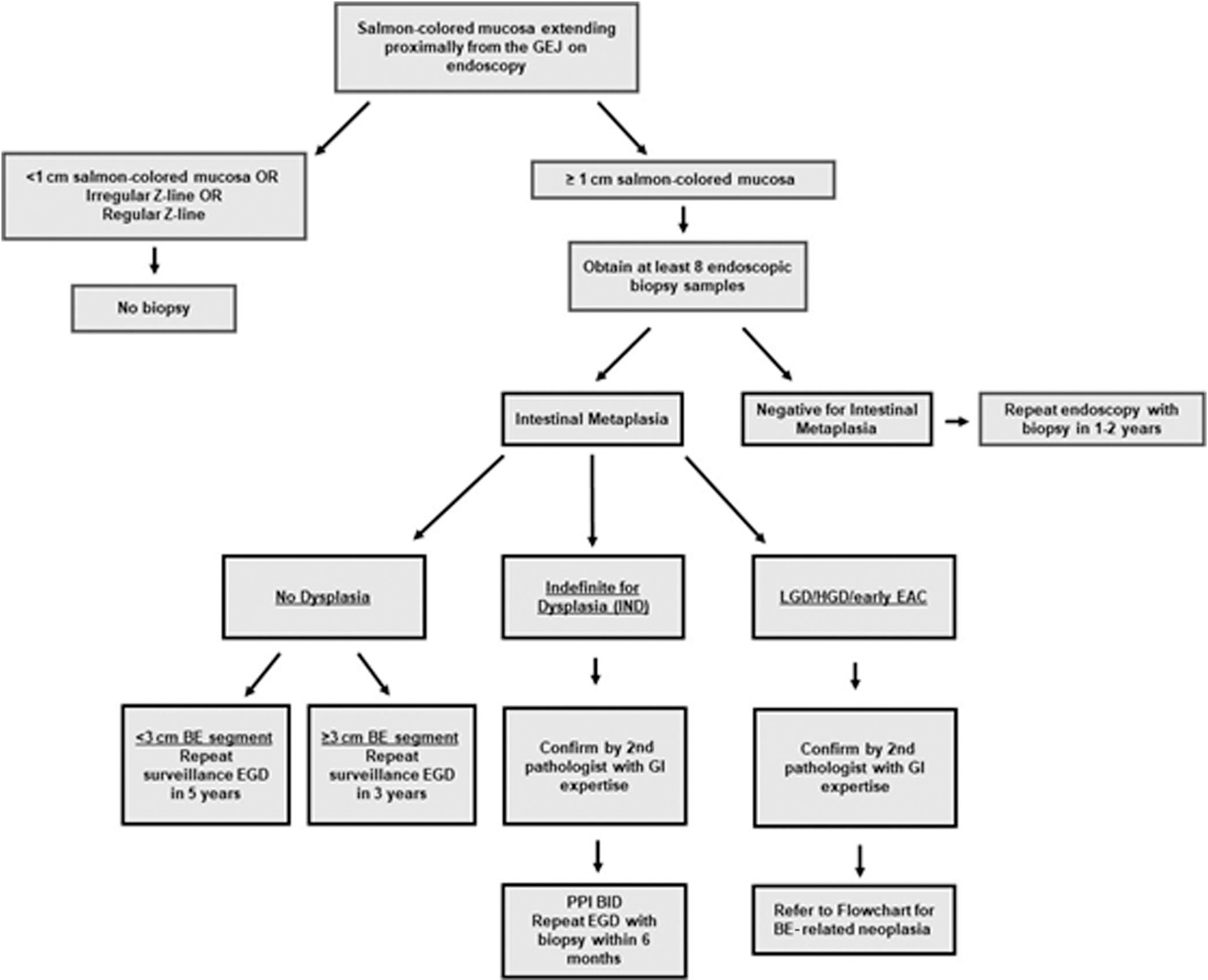
Care algorithm for patients noted to have columnar mucosa in the tubular esophagus. Note the stratification of surveillance interval by length of nondysplastic BE. BE, Barrett’s esophagus; EAC, esophageal adenocarcinoma; GEJ, gastroesophageal junction; GI, gastrointestinal; HGD, high-grade dysplasia; LGD, low-grade dysplasia.
Recommendation.
-
4
We recommend that dysplasia of any grade detected on biopsies of BE be confirmed by a second pathologist with expertise in gastrointestinal (GI) pathology (quality of evidence: low; strength of recommendation: strong).
Summary of evidence.
It has long been recognized that inter observer agreement among pathologists across the spectrum of BE from no dysplasia to HGD/EAC is problematic, especially for the diagnoses of indefinite for dysplasia (IND) and low-grade dysplasia (LGD) (31). This was recently confirmed in an international study of 51 pathologists who reviewed 55 digitized biopsies, where excellent concordance among pathologists was seen for nondysplastic BE (NDBE) (79%) and HGD (71%), but considerably less for LGD (42%) and IND (23%) (32). Furthermore, major underinterpretation or overinterpretation was found in 9% of the cases. Given the implications of a diagnosis of dysplasia regarding potential endoscopic eradication therapy (EET) or more intensive surveillance, it is clear that an accurate diagnosis of dysplasia is critical for clinical decision making.
A Dutch cohort study of 293 patients with BE with LGD diagnosed in a community setting, who had the slides reviewed by an expert panel of 6 pathologists, at least 2 of whom reviewed each case, led to downstaging of 73% and confirmation of LGD in 27% (33). In patients with confirmed LGD, risk of progression to HGD or cancer was 9.1%/patient-year of follow-up. In contrast, risk of progression was 0.9%/patient-year of follow-up for patients downstaged to IND and 0.6%/patient-year of follow-up for those downstaged to nondysplastic BE. A subsequent analysis by 3 pathologists of 255 patients with a community diagnosis of LGD found that there was a strong association between the number of pathologists agreeing on the diagnosis of LGD and progression (34). The annual rate of progression increased from 2.4% to 6.3%–20% when 1, 2, or 3 pathologists agreed on the diagnosis, respectively. Furthermore, pathologic confirmation was also associated with prevalent HGD or carcinoma. Increased risk of progression of LGD confirmed by 2 expert GI pathologists has been confirmed in a multicenter Mayo Clinic study, where expert confirmation led to an 8-fold increased risk of progression compared with those downstaged from LGD to NDBE (35).
Overall, there is very little risk in having a second pathologist review a diagnosis of dysplasia. This is accompanied by a reasonable cost and the potential for considerable benefit for risk stratification. The quality of the evidence supporting this recommendation is low, due in part to the lack of a consensus definition of expert in the literature. The authors acknowledge that a standardized definition for an expert pathologist does not exist. It has been suggested that an expert pathologist may be defined as a pathologist with a special interest in BE-related neoplasia who is recognized as an expert in this field by his/her peers (36). A recent study addressed this knowledge gap by assessing BE concordance rates among 51 pathologists across 20 countries and pathologist features predictive of diagnostic discordance. At least 5 years of professional experience was protective against major diagnostic errors (odds ratio [OR] 0.48, 95% CI 0.31–0.74), whereas working in a nonteaching hospital was associated with increased odds of major diagnostic errors (OR 1.76, 95% CI 1.15–2.69). Interestingly, neither case volume nor self-identifying as an expert predicted diagnostic proficiency (32).
SCREENING FOR BE
Recommendation.
-
5
We suggest a single screening endoscopy for patients with chronic GERD symptoms and 3 or more additional risk factors for BE, including male sex, age >50 years, White race, tobacco smoking, obesity, and family history of BE or EAC in a first-degree relative (strength of recommendation: conditional; quality of evidence: very low).
Summary of evidence.
Survival of patients diagnosed with EAC after the onset of symptoms remains dismal, at less than 20% at 5 years (37). The metaplasia-dysplasia-carcinoma progression paradigm in BE has led to the hypothesis that screening to detect BE, followed by endoscopic surveillance to detect prevalent or incident dysplasia or EAC, and subsequent EET to treat dysplasia or EAC, can lead to a decreased incidence of EAC (38,39). Unfortunately, there is no randomized controlled trial evidence demonstrating reduced EAC mortality with BE screening. Although the efficacy of endoscopic screening and surveillance in reducing EAC mortality is unknown, such programs seem to detect EAC at earlier stages (40).
A recent systematic review and meta-analysis reported visible or histological evidence of concurrent BE in almost 60% of all EACs (and in 91% of early-stage EAC) (41). In contrast, a prior diagnosis of BE was reported in only 12% of patients with EAC; hence, most EACs continue to be diagnosed outside of BE surveillance programs, despite arising in a background of BE, perhaps reflecting a substantial missed opportunity for cancer prevention, which might be afforded by BE screening followed by surveillance. Indeed, population-based studies have reported that more than 50% of prevalent BE in the community is undiagnosed, reducing the proportion of BE under surveillance and precluding the detection of incident dysplasia or early-stage EAC in these unscreened patients (26).
BE is associated with several risk factors. These include chronic reflux symptoms (defined as weekly symptoms for 5 or more years), male sex, age greater than 50 years, smoking, White race, central obesity, and family history. The prevalence of BE in those with these risk factors was recently assessed in a systematic review and meta-analysis (3). Although the prevalence of BE in those without GERD symptoms was low (0.8%), a higher prevalence was reported in those with known risk factors: age >50 years (6.1%), male sex (6.8%), obesity (1.9%), family history of BE/EAC (23%), and GERD (2.3%). The prevalence in those with GERD and 1 additional risk factor was, however, substantially higher than GERD alone (12.2%). In addition, a positive linear relationship was also shown between the number of risk factors and BE prevalence, with each additional risk factor increasing the prevalence of BE by 1.2%. These data support the concept of BE screening in those with multiple risk factors. The authoring panel acknowledges that the use of race to stratify risk is problematic, as it is a social construct not a biological variable and, in this situation, may reflect genomic variants associated with European descendancy (42). However, until such time as further research allows for more precise identification of important genetic variants, the strength and consistency of self-reported race as a risk factor for BE make it a logical variable with which to stratify risk.
There is substantial male predominance in patients with BE (67% male vs 33% female), which is further accentuated in EAC (89% male vs 11% female). Indeed, the risk of progression to EAC in patients with BE is markedly higher in men than in women (adjusted OR 2.2, 95% CI 1.8–2.5) (43), which likely accentuates this male predilection. In a modeling study (44), the incidence of EAC in women with weekly symptoms of GERD at age 60 years was markedly lower (3.9/100,000 person-years) compared with men (61/100,000 person-years). Hence, BE screening in women is likely low yield in terms of reducing EAC incidence. However, screening women with multiple risk factors for BE and EAC may be appropriate following discussion with the patient on the pros and cons of such an approach.
Conventional sedated per-oral endoscopy remains the gold standard for BE screening and is perhaps the most widely used method. However, it is invasive, expensive (45), and not ideal for wide scale application in the general population. This is likely one of the reasons why the utilization of sedated endoscopy for BE screening remains low despite the increasing volume of endoscopic procedures. Studies have shown that most patients with chronic GERD symptoms do not undergo endoscopic evaluation (46). Notably, in a Veterans Affairs population study, predictors of undergoing endoscopy in patients with uncomplicated GERD included female sex and younger age, which are not consistent with risk factors for BE (47).
Given the large number of patients with chronic weekly reflux in the United States who could be targeted for screening, a widely embraced screening effort would lead to substantial economic costs, from screening endoscopy and the need for subsequent surveillance, as well as costs associated with subsequent care of neoplasia and any complications of the screening program. Economic modeling studies have found BE screening followed by surveillance in hypothetical populations (50-year-old male subjects with GERD symptoms) to be cost-effective, with acceptable incremental cost-effectiveness ratios ranging from $10 to 50,000/quality-adjusted life year (QALY) gained (48–50). However, all these studies assumed participation rates of 100% and endoscopy accuracy rates of 100%. This clearly overestimates the potential of such programs, given that lower participation rates have been described in prospective studies of BE screening (51) and lower accuracy rates for endoscopy are reported in previous studies (52).
Recent reports have described the creation of risk prediction scores for BE and EAC using a combination of clinical risk factors (53,54). These risk scores synthesize multiple risk factors (GERD, age, obesity measures, and smoking) into a single numerical score and may make BE screening more efficient by targeting a higher yield population. However, accuracy for BE prediction with these expanded models incorporating non-GERD risk factors, though improved when compared with using only GERD symptoms to stratify risk, remains modest (area under the receiver operating curve 0.66–69 for all risk factors vs area under the receiver operating curve 0.58 for GERD alone) (54). Previously, it was reported that the addition of circulating cytokines and adipokines in combination with clinical factors improved the accuracy for Barrett’s prediction (54); however, improvement in discrimination by such biomarkers was not validated in a recent comparative study (55). Further clinical implementation of these scores will require determination of thresholds at which screening should be triggered, which are not yet determined. These thresholds will depend on the invasiveness, cost, and performance characteristics of the tool used for screening and will likely require additional prospective and modeling studies before clinical implementation.
Unsedated transnasal endoscopy (uTNE) as a minimally invasive alternate modality for BE screening has been found to have comparable performance characteristics to endoscopy for the diagnosis of BE, with a sensitivity of 91% and specificity of 96% (56). The comparative effectiveness of uTNE to sedated endoscopy in BE screening in the community has also been demonstrated in randomized trials (51,57). Esophagoscopes with disposable sheaths, eliminating the need for standard disinfection, can be alternatives for BE screening, but are not currently commercially available (58). BE screening with uTNE seems to be cost-effective (59). Non-physician providers have been trained to perform this procedure reducing costs further. Hence, BE screening with uTNE is an alternative acceptable option, associated with excellent tolerance and good accuracy of diagnosis compared with sedated oral endoscopy (60). Unfortunately, the utilization of uTNE for BE screening in clinical practice has been suboptimal, likely due to both physician- and patient-related barriers.
Finally, the panel discussed the issue of restricting recommendation for BE screening to only those with chronic symptoms of gastroesophageal reflux. A substantial proportion of EACs (34% in a SEER-based modeling study (44)) are diagnosed in those without chronic reflux symptoms. Other estimates place this proportion at 40% (61). Population-based studies have reported that as many as 40%–50% of patients with EAC do not endorse chronic reflux. Multiple studies have reported substantial rates of BE in those without chronic reflux (62–64). Hence, limiting screening to those only with chronic reflux symptoms reduces the targeting of those at risk for BE and EAC by 50% and likely substantially reduces the effectiveness of a reflux symptom based strategy. A recent Veterans Affairs–based prospective study demonstrated the inadequate sensitivity (39%–43%) and modest specificity (67%–76%) of current guidelines requiring the presence of reflux symptoms for screening (65). In addition, as noted above, there are other independent risk factors for BE and EAC, which can be used to stratify risk. However, a challenge of screening those without chronic reflux symptoms is the larger population (120 million adults aged >40 years in the United States vs 30 million adults aged >40 years in the United States with chronic reflux), which will have to be targeted, if reflux symptoms as an essential criterion were to be removed. Hence, a population with a lower incidence of EAC compared with those with chronic reflux symptoms would require screening in this expanded approach. The cost-effectiveness and practical implications (such as costs, personnel issues) of expanding screening to this larger population with an invasive technique such as esophagogastroduodenoscopy are largely unknown. It is, however, conceivable that the availability of a safe, less expensive, minimally invasive screening option may alter this equation. Given the lack of relevant data at this time, the panel did not make specific recommendations on expanding BE screening to those without chronic reflux symptoms.
Recommendation.
-
6
We suggest that a swallowable, nonendoscopic capsule sponge device combined with a biomarker is an acceptable alternative to endoscopy for screening for BE in those with chronic reflux symptoms and other risk factors (strength of recommendation: conditional; quality of evidence: very low).
Summary of evidence.
Over the last decade, substantial progress has been made in developing a minimally invasive, nonphysician and office administered BE detection test. Most data are available on tests which use swallowable esophageal cell collection devices, consisting of dissolvable gelatin or vegetable capsules containing a compressible spherical polyurethane sponge attached to a string/suture which expands to a sphere when the capsule is dissolved [Cytosponge, EsophaCap], or an inflatable silicone balloon [EsoCheck]. These devices are swallowed, then withdrawn orally, obtaining esophageal cytology samples (Figure 3). These samples are then used for the assessment of biomarkers associated with BE: either a protein marker expressed in IM (trefoil factor 3 [TFF3]) or methylated DNA markers (MDMs) associated with BE mucosa to predict the presence of BE. TFF3 staining is performed by immunohistochemistry (IHC) with subsequent interpretation by a pathologist, whereas MDMs are quantitatively analyzed by a polymerase chain reaction–based test.
Figure 3.
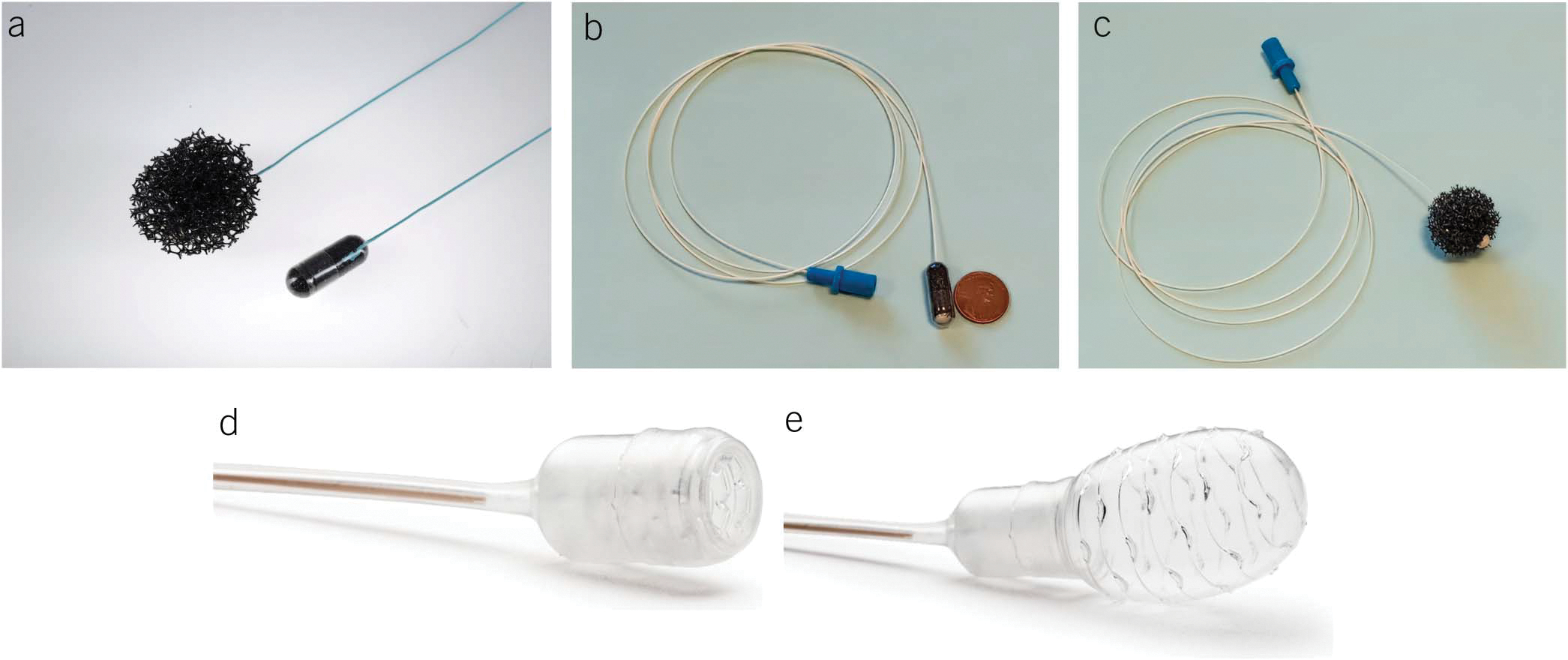
Nonendoscopic Barrett’s esophagus detection devices. (a) Encapsulated and expanded Cytosponge device. (b and c) Encapsulated and expanded EsophaCap device. (d and e) Retracted and inflated Esocheck device.
In addition, these tests can be performed in an office setting, by non–physician-trained providers and do not require the use of sedation. A local anesthetic spray to the oropharynx may be used to reduce discomfort during administration and withdrawal. The safety of this minimally invasive approach has been reported in multiple studies. More than 90% of enrolled participants were able to swallow these esophageal cell collection devices. Adverse events reported with these devices have included mild gagging and throat discomfort. Detachment of the string from the sponge has been reported in an extremely small proportion of patients. If detachment occurs, endoscopic removal of the detached sponge has been performed. In a pooled analysis of 2,672 patients from 5 clinical trials using the Cytosponge, detachment requiring endoscopy was reported in 1 case (66).
Several prospective studies performed in the United States and United Kingdom (summarized in Table 4) have demonstrated the feasibility and accuracy of this approach, with variable performance characteristics. The greatest experience to date and largest available evidence base has been with the Cytosponge device. Of note, all the studies reporting the operating characteristics of these devices are case-control studies performed in populations that have been enriched for BE.
Table 4.
Summary of performance characteristics of minimally invasive nonendoscopic swallowable cell collection devices combined with biomarkers for the nonendoscopic detection of BE
| Device Biomarker used Country of origin | Design Sample size | Sensitivity (%) | Specificity (%) |
|---|---|---|---|
|
| |||
| 30-mm capsule sponge (Cytosponge) (226) | Case-control | 80a | 92 |
| TFF3 | Cases: 647 | ||
| United Kingdom | Controls: 463 | ||
| 30-mm capsule sponge (Medtronic) (227) | Case-control | 76 | 77 |
| TFF3 | Cases: 129 | ||
| United States | Controls: 62 | ||
| 25-mm capsule sponge (EsophaCap) (228) | Case-control | 92 | 94 |
| MDMs | Cases: 112 | ||
| United States | Controls: 89 | ||
| 25-mm capsule sponge (EsophaCap) (229) | Case-control | 93 | 93 |
| MDMs | Training set: cases 110, controls 89 | ||
| United States | Test set: cases 60, controls 29 | ||
| 18-mm swallowable and inflatable balloon | Case-control | 92 | 88 |
| (EsoChek) (230) | Cases: 50 | ||
| MDMs | Controls: 36 | ||
| United States | |||
| 20-mm capsule sponge (EsophaCap) (231) | Case-control | 94 | 62 |
| MDMs | Training set: cases 18, controls 34 | ||
| United States | Test set: cases 14, controls 14 | ||
BE, Barrett’s esophagus; MDM, methylated DNA marker; TFF3, trefoil factor3.
BE defined as >2 cm segment length.
In a landmark pragmatic trial set in primary care clinics and performed in the United Kingdom (67), patients with chronic reflux (defined as those using antireflux medications for at least 6 months), who were randomized to the Cytosponge-TFF3 test, had a 10-fold higher likelihood of being diagnosed with BE by confirmatory endoscopy (2% BE prevalence) compared with those randomized to a usual care arm, where endoscopy was performed only if the provider thought it was indicated (<1% BE prevalence). Of those invited to participate, 39% of patients expressed interest in undergoing the Cytosponge test. The positive predictive value of this test in this screening population was 59%. In addition, more patients in the Cytosponge-TFF3 arm were also diagnosed with dysplastic BE and early-stage EAC (9) compared with the usual care arm (0), suggesting the potential utility of this strategy in identifying those who could benefit from therapeutic intervention. Importantly, this technique was also safe and well tolerated. A previous study has shown this test to be cost-effective (when compared with no screening) (68) when used in a hypothetical sample of 50-year-old White men with chronic reflux. Trials to assess the performance of MDM-based minimally invasive BE detection tests in screening populations are ongoing to determine their performance characteristics in this setting.
Another noninvasive BE screening technology that is being developed is the analysis of exhaled volatile organic compounds by a handheld device (Aeonose; eNose Company, Zutphen, the Netherlands) containing a metal oxide sensor array. Sensor measurements generate a digital signal, which can be analyzed by artificial neural networks for BE detection. In a preliminary study reported from the Netherlands, a sensitivity and specificity of 91% and 74% for BE detection using endoscopy as a gold standard were reported (69).
Recommendation.
-
7
We suggest against repeat endoscopic screening in patients who have undergone an initial negative screening examination by endoscopy (strength of recommendation: conditional; quality of evidence: low).
Summary of evidence.
The yield of a repeat endoscopy for diagnosing BE following an initial negative BE screening endoscopy is low. In a study of the Clinical Outcomes Research Initiative database, which included over 24,000 patients undergoing repeat endoscopy, only 561 (2.3%) patients had suspected BE on repeat endoscopy after an initial negative examination. Esophagitis on the index endoscopy, reflux as an indication for endoscopy (compared with other indications), and male sex were predictors of BE being diagnosed at subsequent endoscopy (70). In patients with esophagitis described at initial endoscopy, 9.9% were found to have suspected BE on repeat examination. However, of note, more than 85% of the repeat examinations were performed within 2 years of the initial examination. Hence, additional data on the detection of BE at endoscopic evaluation performed at longer intervals after an initial negative screening endoscopy would better clarify long-term risks. In another smaller retrospective study from Turkey, only 0.66% of 2,701 patients undergoing repeat endoscopy within 6 years of an initial negative examination had BE on the second endoscopy (71). In addition, the ProGERD study was a prospective cohort study of reflux patients under treatment with proton pump inhibitor (PPI) who underwent endoscopy at enrollment and again 5 years later. Of the 1,224 patients with nonerosive reflux disease at baseline undergoing a year 5 endoscopy, only 51 (4.2%) demonstrated BE, 79% of which was 2 cm or less in length (72).
One important caveat to the issue about repeating endoscopy is that erosive esophagitis, if Los Angeles grade B or worse, may mask the presence of BE. Studies have also assessed the rate of detection of BE after endoscopic confirmation of healing of esophagitis and found that a significant minority of patients with severe erosive esophagitis will show BE after healing. Ina prospective study of 172 patients with erosive esophagitis undergoing repeat endoscopy at a mean of 11 weeks after treatment with PPIs, BE was confirmed in 21 (12%) of patients (73). Nineteen of these patients had short-segment BE. Patients with more severe degrees of esophagitis (Los Angeles Grades C and D) were numerically more likely to have BE diagnosed at repeat endoscopy (17.4% vs 9.4% with Los Angeles Grades A or B). Similar results were also reported in a retrospective study, which evaluated 102 patients undergoing repeat endoscopy after finding esophagitis. BE was detected in 9% of patients, all of whom had severe (grade 4) esophagitis (74). Hence, patients with esophagitis on initial endoscopic evaluation should undergo repeat endoscopy 8–12 weeks after treatment with PPIs to ensure healing of esophagitis and to determine the presence of BE.
SURVEILLANCE OF BE
Recommendation.
-
8
We recommend both white light endoscopy and chromoendoscopy in patients undergoing endoscopic surveillance of BE (quality of evidence: moderate; strength of recommendation: strong).
Summary of evidence.
The goal of endoscopic surveillance of BE is the detection of dysplasia or carcinoma at an early and treatable stage. Initial evaluation of the Barrett’s segment should commence with high-definition white light endoscopy including a retroflexed view of the cardia. Adequate inspection of the columnar-lined segment is necessary, as longer inspection times are associated with increased ability to detect HGD or EAC (75). However, even with careful white light inspection, subtle lesions may be missed. Routine use of chromoendoscopy may enhance the detection of dysplasia and carcinoma. This may be accomplished by either vital dyes such as acetic acid or by electronic chromoendoscopy. Furthermore, the simple use of chromoendoscopy after careful white light inspection increases the time spent examining the Barrett’s mucosa.
Acetic acid chromoendoscopy involves applying dilute acetic acid to the Barrett’s mucosa, which leads to an initial whitening of the Barrett’s segment. However, areas of neoplasia lose this whitening more rapidly than nondysplastic Barrett’s epithelium. A meta-analysis of 9 acetic acid chromoendoscopy studies including 1,379 patients found a pooled sensitivity and specificity of 0.92 (95% CI 0.83–0.97) and 0.96 (95% CI 0.85–0.99) for the detection of HGD and EAC with no significant heterogeneity (76).
Electronic chromoendoscopy systems, now a part of all endoscope platforms, allow for a better view of the mucosal surface and vascular patterns. A randomized crossover trial has compared high-definition white light endoscopy using the Seattle protocol to narrow band imaging with targeted biopsies of abnormal areas for the detection of neoplasia in 123 patients with BE (77). Detection of dysplasia was higher in the narrow band imaging examination than in the high-definition white light examination (30% vs 21%, P = 0.01). Furthermore, all areas of dysplasia or carcinoma were characterized by an irregular mucosal or vascular pattern with narrow band imaging.
An international working group has developed and validated a simple classification system of mucosal and vascular pattern of the Barrett’s mucosa, characterizing both as either regular or irregular, to identify HGD and EAC (78). Using this simple system, they found the sensitivity to be 80%, with a specificity of 88%. A meta-analysis of 9 electronic chromoendoscopy studies examining 625 patients found that narrow band imaging targeted biopsies compared with standard biopsy protocols had a pooled sensitivity of 94.2% (95% CI 83%–98%) and specificity of 94.4% (95% CI 81%–99%) for the detection of dysplasia or EAC, both with high heterogeneity (79). In addition, 2 recent studies have demonstrated that electronic chromoendoscopy enhances the visualization and delineation of early Barrett’s-associated neoplasia in expert endoscopists, nonexpert endoscopists, and trainees when compared with high-definition white light endoscopy alone (80,81). However, chromoendoscopy-directed biopsies should not yet be used as a substitute for the standardized biopsy protocol. Taken together, the evidence supports routine use of either acetic acid or electronic chromoendoscopy in all BE surveillance examinations.
Advanced imaging.
A variety of additional advanced imaging techniques have been developed in an effort to improve the detection of dysplasia and EAC and thereby improve on the Seattle protocol in combination with high-definition white light endoscopy. Confocal laser endomicroscopy uses blue laser light to illuminate the esophageal tissue after intravenous injection of fluorescein. This then allows for real-time in vivo imaging at high magnification to obtain optical biopsies in a targeted fashion. To date, 2 systems have been developed; endoscope and probe based, with only the latter still being commercially available. The most recent systematic review and meta-analysis of 7 studies of 473 patients who combined both probe-based and endoscope-based systems found a pooled sensitivity for per patient analysis when compared with histopathology of 89% (95% CI 0.82–0.94; I2 = 31.6%) and specificity of 83% (95% CI 0.78–0.86; I2 = 90.1%) (82). Although these numbers are encouraging, there are multiple caveats, including the considerable capital cost, the need for intravenous fluorescein, training in image interpretation, and time to complete the examination. Given that many of these studies were performed in centers with a high prevalence of dysplasia/neoplasia, the applicability of these data to a general surveillance population is unknown. That being said, in centers with a high prevalence of neoplasia or dysplasia, confocal endomicroscopy may be helpful in targeting biopsies and guiding therapy, although the value above that of high-definition white light and electronic chromoendoscopy is unclear (83).
Volumetric laser endomicroscopy is a probe-based technique using optical coherence tomography technology to obtain a 6-cm circumferential scan of the esophagus that allows 2-dimensional visualization of the mucosa and submucosa of the esophagus to a depth of 3 mm (84). The technology has evolved over time to include laser markings to delineate areas of interest and recently, a computer-assisted detection algorithm to facilitate interpretation of the large data sets generated by the probe. As such, summary estimates of the utility of this technology to detect dysplasia and early carcinoma using the entire 1,200 image scan are not available. One recent study of 29 such full scan videos from 15 patients with neoplasia and 14 patients with NDBE found that experts correctly labeled 73% of neoplastic cases and 52% of nondysplastic cases with fair interobserver agreement (85). Currently, volumetric laser endomicroscopy is not commercially available.
Considerable efforts are now underway to harness the power of artificial intelligence for enhanced dysplasia and early carcinoma detection in BE. Work in the Netherlands and the United States has already validated a deep learning computer-aided detection system that has the ability to delineate areas within the Barrett’s mucosa that contain early neoplasia while simultaneously demarcating the most abnormal aspect of the region (86). Furthermore, the computer-aided detection algorithm had superior performance characteristics when compared with 53 nonexpert endoscopists. This system has subsequently been assessed in a pilot study during live endoscopy with promising results (87). This field is rapidly advancing and has considerable potential to impact our approach to BE in the coming years.
Recommendation.
-
9
We recommend a structured biopsy protocol be applied to minimize detection bias in patients undergoing endoscopic surveillance of BE (quality of evidence: low; strength of recommendation: strong).
Summary of evidence.
The Seattle protocol, first described in 1993, consists of careful visual inspection of the Barrett’s segment with biopsies of any endoscopically visible lesions, followed by 4 quadrant biopsies at intervals ≤2 cm from the level of the lower esophageal sphincter to the squamocolumnar junction (88). This protocol was initially developed to distinguish HGD from early EAC in an era before high-definition endoscopy and EET. The rationale for this structured biopsy protocol is that more dysplasia may be detected by reducing sampling error, given that areas of dysplasia may not be visible, lesions are often focal, and the distribution is highly variable in the Barrett’s segment.
Support for a structured biopsy protocol comes from several lines of evidence. In a cohort study from the United Kingdom, the institution of a structured biopsy protocol led to an increase in the detection of HGD and early EAC when compared with the time period before the start of the rigorous protocol (89). Another UK cohort study compared a systematic 4 quadrant biopsy protocol performed by a surgical service to a nonsystematic biopsy protocol performed by the medical service and found a 13-fold increase in both prevalent low-grade and HGD in the systematic biopsy group (90). Finally, a single-center case series from Nottingham examined the yield of dysplasia with high-definition white light endoscopy comparing a systematic 4 quadrant biopsy technique to only targeted biopsies of mucosal abnormalities and found the yield of dysplasia or EAC to be higher in the former (73%) than the latter (27%) (91).
However, although we continue to advocate for the Seattle protocol, its limitations must be acknowledged. Even with a systematic biopsy protocol, only a small subset of the Barrett’s segment is sampled, considerable time and expense are involved, and adherence is highly variable. A community-based database study of 2,245 surveillance cases found adherence to the Seattle protocol in only 51.2% of the cases (92). Furthermore, adherence was inversely associated with increasing length of the Barrett’s segment. When stratified by length, nonadherence was associated with significantly decreased dysplasia detection (OR 0.53; 95% CI 0.35–0.82). As outlined below, this is especially problematic given that segment length is a risk factor for progression to HGD and EAC. Others have confirmed that increasing BE length is a predictor of nonadherence (93). A systematic review and meta-analysis of 45 studies found that worldwide, adherence to the Seattle protocol is low at 49% (95% CI 36%–62%) albeit with considerable heterogeneity (I2 = 98.8%) (94). Taken together, the body of evidence continues to support the use of a systematic biopsy protocol with the Seattle protocol.
Recommendation.
-
10
We suggest endoscopic surveillance be performed in patients with BE at intervals dictated by the degree of dysplasia noted on previous biopsies (quality of evidence: very low; strength of recommendation: conditional).
Summary of evidence.
There are no randomized controlled trials to support endoscopic surveillance in BE. However, 1 such study underway in the United Kingdom is examining surveillance at 2-year intervals compared with no surveillance (95). A community-based case-control study of BE in the Kaiser Permanente system compared the surveillance histories of 38 patients who died of esophageal EAC with 101 matched patients with BE who were alive (96). They found that surveillance within 3 years was not associated with a decreased risk of death from EAC (OR 0.99; 95% CI 0.36–2.75). Cases were found to have had surveillance comparably to controls in the preceding 3 years (55.3% vs 60.4%). A subsequent systematic review and meta-analysis examined cohort study evidence for the effect of endoscopic surveillance (40). This identified 4 cohort studies that found lower EAC mortality in the surveillance groups compared with the no or incomplete surveillance groups (relative risk [RR] 0.60; 95% CI 0.50–0.71) with no heterogeneity (I2 = 0%). Similarly, 3 studies compared surveillance with either incomplete or no surveillance and found a reduction in all-cause mortality (HR 0.75; 95% CI 0.50–0.94) with low heterogeneity (I2 = 22%). Finally, when looking at early-stage EAC, patients undergoing surveillance were more likely to be diagnosed with early-stage disease than those with either absent or inadequate surveillance (RR 2.11; 95% CI 1.08–4.11) albeit with considerable heterogeneity. However, when these results were adjusted for lead and length time biases, the above outcomes were either eliminated or attenuated.
Taken as a whole, studies suggesting a mortality benefit for surveillance are all retrospective studies (low quality of evidence at best), with the better designed case-control demonstrating no difference. Thus, the quality of evidence supporting endoscopic surveillance is very low. Lead and/or length bias likely further attenuate any reported survival benefits, meaning that the evidence supporting a survival benefit in endoscopically surveyed patients is weak.
Management of BE with IND
IND is a common finding encountered in an estimated 4.3%–8.4% of BE biopsies (97). This diagnosis is made when the pathologist is unable to determine whether the histology truly represents dysplasia or may be due to inflammatory changes (98). A recent systematic review and meta-analysis of 8 studies reporting outcomes in patients with BE IND found a pooled annual incidence of HGD and/or EAC to be 1.5/100 person-years (95% CI 1.0–2.0) with modest heterogeneity (I2 = 56.5%). This rate is comparable to the progression rate seen in LGD as outlined below. In that same analysis, the pooled annual incidence rate of progression to EAC alone from 5 studies was 0.6/100 person-years (95% CI 0.1–1.1) with considerable heterogeneity (I2 = 89%). The pooled annual incidence of LGD in patients IND was 11.4/100 patient-years (95% CI 0.06–0.2), derived from 4 studies with considerable heterogeneity (I2 = 83.6%). Subsequently, a single-center cohort study identified persistent IND as a risk factor for progression to LGD (OR 3.23; 95% CI 1.04–9.98) (99).
There is uniform agreement across international guidelines that a diagnosis of IND should first be confirmed by an expert GI pathologist (100–102). For confirmed cases, antireflux therapy should be intensified, followed by a repeat endoscopy within 6 months. For those downgraded to NDBE, surveillance should then follow the intervals for NDBE. However, for patients with confirmed and persistent indefinite dysplasia, some international guidelines suggest following the approach used for NDBE, whereas others suggest surveillance at 6-month intervals (100–102). The work cited above suggests that surveillance should continue annually until the findings normalize similar to recommendations for LGD as outlined below. Figure 2 demonstrates the recommended endoscopic surveillance for such patients.
MANAGEMENT OF BE WITH LGD OR HGD
Management of BE with LGD involves either endoscopic surveillance or EET. Management of BE with HGD generally is by EET. A full discussion of the management of BE with LGD or HGD is presented in the section on endoscopic management of BE.
Recommendation.
-
11
We recommend that length of the NDBE segment be considered when assigning surveillance intervals such that longer segments of BE (≥3 cm) are surveyed on a 3-year interval and shorter segments of BE (<3 cm) are surveyed on a 5-year interval (quality of evidence: moderate; strength of recommendation: strong).
Summary of evidence.
For many years, there has been uniform agreement among American guidelines that all patients with NDBE undergo surveillance at intervals of 3 to 5 years, based largely on expert opinion. However, a number of international guidelines including those from Europe, the United Kingdom, and Australia now recommend stratifying surveillance intervals based on the length of the Barrett’s segment (100–102). Support for the concept of using Barrett’s segment length as a risk stratification tool comes from a number of studies. A systematic review and meta-analysis of 20 studies that examined risk factors for progression to HGD or EAC found that increasing segment length per centimeter was associated with an increased risk of progression (OR 1.25; 95% CI 1.16–1.36; I2 = 45) (43). A multicenter cohort study of 1,883 patients with NDBE found that patients with segments <3 cm had a lower annual progression rate to EAC or the combined end point of HGD and EAC than those with segments ≥3 cm: 0.07% vs 0.25%, P = 0.001, and 0.29% vs 0.91%, P < 0.001 (103). Notably, this effect persisted in a multivariable analysis that corrected for other risk factors, including BMI, and use of aspirin (ASA), statins, and H2 receptor antagonists. Perhaps the strongest evidence addressing the importance of length as a predictor of progression comes from a meta-analysis of 10 studies with a minimum of 12 months of follow-up examining 1,979 Patients with a segment length of <3 cm and 2,118 patients with a segment length of ≥3 cm (104). Again, the annual rate of progression was lower for short-segment than for long-segment BE: 0.06% vs 0.31% (OR 0.25; 95% CI 0.11–0.56; P < 0.001). For the combined end point of HGD and EAC, progression rates were also lower for short-segment compared with long-segment BE: 0.24% vs 0.76% (OR 0.35; 95% CI 0.21–0.58; P < 0.001). Notably, little heterogeneity was found in this analysis as well (I2 = 8%). Finally, a model developed from a multicenter cohort study incorporating segment length, male sex, cigarette smoking, and LGD was found to predict progression to HGD or EAC by categorizing patients as low, intermediate, and high risk for progression to HGD or EAC (105). This model has subsequently been validated in a population-based cohort from Northern Ireland (106).
Taken together, there is a large amount of evidence that BE length can risk stratify patients for development of HGD and EAC. As such, it is our recommendation that surveillance for patients with <3 cm be extended to 5 years. Patients with a segment length of ≥3 cm undergo surveillance at 3-year intervals. The above recommendations assume a high-quality baseline endoscopic examination with adequate tissue sampling per the Seattle protocol. Table 5 outlines endoscopic surveillance recommendations for patients with short-segment NDBE, long-segment NDBE, BE IND, and BE with LGD opting for endoscopic surveillance.
Table 5.
Recommended endoscopic surveillance intervals based on degree of dysplasia and segment length
| Baseline endoscopic finding | Suggested endoscopic surveillance |
|---|---|
|
| |
| Nondysplastic BE of <3 cm length | EGD every 5 yr |
| Nondysplastic BE of ≥3 cm length | EGD every 3 yr |
| BE indefinite for dysplasia, any length (confirmed by a second pathologist) | Repeat EGD within 6 mo after increasing PPI
to twice-daily dosing, if not already on high-dose PPI If repeat EGD yields diagnosis of NDBE or LGD, treat using that algorithm If repeat EGD demonstrates BE indefinite for dysplasia, EGD annually |
| BE with LGD (confirmed by a second pathologist and opting for endoscopic surveillance) | EGD at 6 mo from diagnosis EGD 12 mo from diagnosis EGD annually thereafter |
BE, Barrett’s esophagus; EGD, esophagogastroduodenoscopy; LGD, low-grade dysplasia; NDBE, nondysplastic BE; PPI, proton pump inhibitor.
Recommendation.
-
12
We could not make a recommendation on the use of wide-area transepithelial sampling with computer-assisted 3-dimensional (WATS-3D) analysis in patients undergoing endoscopic surveillance of BE.
Summary of evidence.
When using WATS-3D, an abrasive cytology brush is passed through the channel of the endoscope to sample deeper layers of the glandular Barrett’s epithelium across an extensive area. The brush sample is smeared on a slide, yielding a tissue specimen that is up to 150 μm in thickness, unlike a typical forceps biopsy slide in which tissue sectioning produces samples that are only 3 to 5 μm thick. A neural network computer system that captures up to 50 optical slices (each 3 μm in thickness) of the specimen reconstructs it into 3-dimensional images of the sampled Barrett’s glands. The computer then scans these images and flags areas with high-risk features to bring to the attention of the pathologist for final interpretation.
We identified 2 meta-analyses of studies in which WATS-3D was used as an adjunct to forceps biopsies to detect dysplasia in patients undergoing screening or surveillance for BE using white light endoscopy (107,108). In those studies, the yield of dysplasia detection by WATS-3D was compared with that of forceps biopsies alone. One meta-analysis (107) included 6 studies, and the more recent second meta-analysis (108) included 9 (including 6 of the same studies from the earlier meta-analysis). In the latter meta-analysis of 19,950 screening and surveillance endoscopies performed in dysplasia-naive patients with BE, the addition of WATS-3D to forceps biopsies led to an absolute increase in the detection of dysplasia of 2% (95% CI 0.01–0.03) and a relative increase of 2.05-fold (95% CI 1.42–2.98) (108). There was considerable heterogeneity in both meta-analyses, but no evidence of publication bias. A major issue in most studies of this technology is that the incremental benefit in dysplasia detection is not confirmed in subsequent forceps biopsy sampling. Thus, it is difficult to know how much of the incremental benefit is truly due to more complete sampling of the mucosa by WATS-3D or better detection of dysplasia by the analysis algorithm and how much might be due to over diagnosis of dysplasia and false-positive examinations by WATS-3D. Also, no study yet has evaluated the addition of WATS-3D to forceps biopsies for detection of dysplasia during Barrett’s surveillance when forceps biopsies are guided both by white light and chromoendoscopy. In addition, no studies have been performed reproducing these results using pathologists not employed by CDx. Finally, most of the studies do not separate dysplasia diagnosed by WATS-3D into LGD and HGD. Those studies that do stratify by degree of dysplasia demonstrate that most of the additional dysplasia diagnosed by WATS-3D is LGD. All these factors complicate the interpretation of data supporting this technology in surveillance of BE.
Although we found no studies assessing cost-effectiveness of WATS-3D as an adjunct to forceps biopsies for surveillance of BE, there was 1 recent cost-effectiveness analysis using a decision-analytic model comparing forceps biopsies with WATS-3D vs forceps biopsies alone in screening for BE (109). In this model, a cohort of 60-year-old White men with GERD were screened for BE, and those with BE detected by either forceps biopsy or WATS-3D were entered into surveillance protocols with radiofrequency ablation (RFA) performed for those found to have LGD. Two-way sensitivity analysis of the additional yield of WATS-3D to forceps biopsies for a diagnosis of BE over a range (5%, 15%, and 25%) of false-positive WATS-3D results (i.e., forceps biopsies do not reveal BE) demonstrated that cost-effectiveness at $100,000/QALY was 98.7% of the stimulated trials; at a cost of $150,000/QALY, the sensitivity increased to 100%.
Given our recommendation that patients with BE undergoing routine endoscopic surveillance should have both chromoendoscopy and white light endoscopy for dysplasia detection, and with the additional factors noted above, the panel could not make a recommendation on the use of WATS-3D in BE surveillance at this time. We include recommendation 12 to document that this recommendation went through the formal GRADE review process with consideration by the authoring panel and to provide the data underpinning this decision.
Recommendation.
-
13
We could not make a recommendation on the use of predictive tools (p53 staining and TissueCypher) in addition to standard histopathology in patients undergoing endoscopic surveillance of BE.
Summary of evidence.
The annual incidence of cancer progression in BE is estimated at 0.2%–0.05% per year for NDBE and approximately 0.7% per year for LGD (110). Such low annual risks of progression highlight the need for a risk stratification biomarker to make surveillance of BE more effective. The detection of p53 abnormalities has the largest body of evidence as a biomarker for risk stratification. p53 is an important tumor suppressor gene whose alteration in function seems to be a key event, occurring early and often during Barrett’s carcinogenesis. Immunostaining of esophageal biopsy specimens revealing aberrant expression of p53 protein (either overexpression or absent expression) is evidence of alterations in p53 function. We identified 3 meta-analyses assessing p53 IHC (111–113) for risk-stratifying patients with BE enrolled in surveillance programs. Although there are a number of techniques to identify p53 alterations, we selected IHC because it is a relatively easy technique that is most commonly used in clinical practice and thus would have widespread applicability. ORs for aberrant p53 expression in cases (progression to HGD or EAC) compared with controls (no progression to HGD or EAC) ranged from approximately 4–17 in the 3 meta-analyses. One meta-analysis determined the OR and RR for both case-control and cohort studies that exclusively enrolled LGD (111). In the LGD cohort studies, the RR of progression in patients with abnormal p53 staining was 14.25 (95% CI 6.76–30.02), and in the case-control studies, the OR was 5.95 (95% CI 2.68–13.22) (111). One meta-analysis calculated the sensitivity and specificity of p53 overexpression at 62% (95% CI 59%–64%) and 80% (95% CI 79%–81%), respectively, and loss of p53 expression at 31% (95% CI 25%–28%) and 98% (95% CI 97%–98%), respectively (112). However, the meta-analyses and the studies included in those meta-analyses are methodologically problematic. All 3 meta-analyses included predominantly retrospective case-control or cohort studies. In 2 of the meta-analyses, case-control and cohort studies were incorporated together to calculate the OR (112,113), whereas the other meta-analysis determined an OR for case-control studies and an RR for cohort studies (111). There was heterogeneity among studies in the definition of BE (columnar-lined esophagus vs intestinal-lined esophagus), in the proportion of patients with no, indefinite, and LGD, and in the definition of aberrant p53 expression (overexpression alone, loss of expression alone, or the combination of both).
The TissueCypher tissue systems pathology assay integrates the 15 best-performing quantitative image analysis features derived from fluorescence images of 9 protein-based biomarkers, nuclear morphology, and tissue architecture to provide a risk score (0–10) that classifies patients as low, intermediate, or high risk for progression to HGD/EAC within 5 years (114,115). There have been 4 validation studies to predict incident progression in patients with BE and no, indefinite, or LGD and 1 study demonstrating its ability to detected prevalent HGD/EAC missed by the Seattle biopsy protocol (115–119). For patients with BE and a diagnosis of no, indefinite, or LGD, the prevalence-adjusted sensitivity and specificity of TissueCypher at 5 years for the 3-tiered classification system were 29% and 86%, respectively (117). Further assay validation in patients with NDBE found the prevalence-adjusted sensitivity and specificity at 5 years to be 30.4% and 95%, respectively (118). The sensitivity of this assay was further increased to 49.8% when biopsies obtained at multiple spatial levels were evaluated, without a change in its specificity (118). Further validation in patients with LGD demonstrated that the risk of progression was similar in the intermediate- and high-risk groups, allowing for a combined classification. Sensitivity and specificity for this 2-tiered classification system (high and low risk) for patients with low-grade dysplasia were 67.7% and 78.6%, respectively (119). Finally, 1 study evaluated the performance of TissueCypher for detection of prevalent HGD/EAC that was missed by random biopsies following the Seattle protocol and found an OR of 46 (95% CI 14.86–169) for patients BE and no, indefinite, and LGD that scored high risk vs those that scored low risk (116). A cost-effectiveness analysis using a hybrid decision tree/Markov model comparing TissueCypher with standard of care surveillance and treatment protocols based on those used at Geisinger Health System over a 5-year time period demonstrated that the required sensitivity of the assay for cost-effectiveness at $100,000/quality-adjusted life years was 51% over a range of specificities (80%–100%) in patients with no, indefinite, or LGD (120).
The aforementioned studies suggest that biomarkers may be better than routine histology alone in predicting cancer progression, but their grading as a diagnostic test is hindered by their low sensitivity and specificity. To enhance performance characteristics of the biomarkers for predicting disease progression, a Barrett’s progression score that incorporates clinical and biomarker variables has been proposed (121), but the value of such a prediction tool in NDBE is unclear because to date, no study has evaluated the combination of clinical and biomarker variables. It is also important to recognize that not even a perfect biomarker (1 that is 100% sensitive and 100% specific) will completely eliminate cancer development and cancer deaths as demonstrated in a Markov modeling study (122). One of the reasons for this is that the characteristics of the test used to detect the biomarker are not perfect. For example, IHC results for p53 are affected by the antibodies used, the staining method, and the subjectivity in the definition and interpretation of aberrant staining (123). Although TissueCypher uses automated image analysis to eliminate subjectivity in interpretation, various external factors such as cell stress, DNA damage, and ongoing GERD might alter some, if not all, of the 15 features detected on the panel producing erroneous estimates; the same holds true for these factors in altering expression levels of p53.
Given the low sensitivity and specificity of the above biomarkers, the panel could not make a recommendation for routine use of p53 IHC or TissueCypher for risk stratification in patients with BE undergoing surveillance. Nevertheless, the panel does not recommend against the use of these biomarkers given that their predictive performance has been shown to be better in some cases than the histologic diagnosis, raising the possibility that these biomarkers may provide some benefit in a subset of patients with BE, particularly in those without dysplasia. The challenge for future research is to better define this subset and to demonstrate that the use of biomarkers in Barrett’s populations improves on risk stratification available by clinical prediction models. The use of biomarkers ultimately should impact harder end points such as cancer incidence or death. We include recommendation 13 to document that this recommendation went through the formal GRADE review process with consideration by the authoring panel and to provide the data underpinning this decision.
Key concept.
-
1
Consider cessation of endoscopic surveillance when a patient is no longer a candidate for EET.
Any discussion of cessation of endoscopic surveillance is by nature arbitrary given the lack of data to guide decision making. This is highlighted by a recent study of surveillance endoscopy for BE in Medicare enrollees that found that 79% of men aged 80–84 years, with a life expectancy less than 5 years, still underwent repeat endoscopy within 5 years (124). Only the ESGE makes an explicit recommendation to stop endoscopic surveillance in individuals at age 75 years in the absence of a prior history of dysplasia, whereas the British Society of Gastroenterology recommends considering fitness for repeated endoscopy should EET be merited and the patient’s ability to tolerate chemotherapy and/or radiation therapy should EAC be found. From a pragmatic perspective, it is reasonable to cease endoscopic surveillance in patients with an estimated survival of less than 5 years and those who are no longer fit for repeated endoscopy or cannot tolerate endoscopic, surgical, or oncological intervention for esophageal neoplasia. A recent modeling study suggested that the optimal age of last surveillance of a patient with NDBE was between 69 and 81 years and was dependent on the sex and comorbidities of the patient (125). Although it is difficult to be dogmatic given the wide variability in life-limiting comorbidities, given the current average American life expectancy, discussion of cessation of further endoscopic surveillance is merited when most patients reach 75 years of age, if it has not occurred prior.
Key concept.
-
2
Consider utilization of published quality indicators to benchmark your unit’s performance against published standards.
In this era of value-based and quality-based health care, quality indicators that benchmark performance and ensure the delivery of high-quality care in patients with BE have been proposed (126,127). The quality of care can be measured by comparing the performance of an individual or a group of individuals with an ideal or benchmark and nonadherence to a quality indicator reflects suboptimal care. Quality indicators for screening and surveillance focus on documentation of landmarks and extent of BE, not obtaining biopsies in the setting of a normal-appearing squamocolumnar junction, sampling using the Seattle biopsy protocol, and performing surveillance endoscopy in patients with NDBE no sooner than 3–5 years (126,127). Other quality indicators proposed, but not endorsed by societal statements, include Barrett’s inspection time and neoplasia detection rate (analogous to adenoma detection rate) (128). Available data using a national benchmarking quality registry (GI Quality Improvement Consortium Registry) suggest suboptimal adherence rates to these proposed quality indicators in BE (28,93,129,130). Implementation of specific quality indicators in BE will require an infrastructure for continuous monitoring of upper endoscopy quality by endoscopy practices performing BE screening and surveillance.
One likely future quality metric is the postendoscopy esophageal cancer rate or PEEC. Similar to the phenomenon of postcolonoscopy colorectal cancer, there is growing literature describing the diagnosis of BE-related HGD and EAC before the next recommended endoscopic evaluation after an upper endoscopy that was negative for HGD or EAC (131). Missed lesions during endoscopy may comprise most of these cases, with others being secondary to rapidly progressive cancer or incompletely resected or ablated lesions after EET. Two studies provide contemporary estimates of missed HGD and EAC. A recent retrospective cohort study using data from large commercial and Medicare Advantage health plans identified 50,817 individuals with incident BE, 366 of whom developed EAC. Of these EACs, 67.2%, 13.7%, and 19.1% were classified as prevalent EAC, postendoscopy EAC, and incident EAC, respectively (132). In an updated systematic review and meta-analysis that included 52 studies and 145,726 patients, the proportion of postendoscopy EAC was 21% (95% CI 13–31) and that of postendoscopy HGD/EAC was 26% (95% CI 19–34), outcomes defined by the diagnosis of HGD/EAC within the first year after an index endoscopy that demonstrated NDBE, LGD, or IND (133). Restricting this analysis to patients with NDBE only, postendoscopy EAC proportion was 17% (95% CI 11–23), and postendoscopy HGD/EAC proportion was 14% (95% CI 8–19). Interestingly, meta-regression analysis demonstrated a strong inverse association between postendoscopy EAC and incident EAC. These findings clearly question the effectiveness of current screening and surveillance practices and highlight the importance of a high-quality endoscopy to the success of BE screening and surveillance programs designed to reduce the incidence and mortality associated with EAC. Table 6 provides a simple 10 step approach to a high-quality endoscopic examination of Barrett’s esophagus.
Table 6.
Ten-step approach to endoscopic examination of Barrett’s esophagus
| Approach | Rationale |
|---|---|
|
| |
| Identify esophageal landmarks, including the location of the diaphragmatic hiatus, gastroesophageal junction, and squamocolumnar junction | Critical for future examinations |
| Consider use of a distal attachment cap (especially in patients with prior diagnosis of dysplasia) | Facilitate visualization |
| Clean mucosa well using water jet channel and carefully suction the fluid | Remove any distracting mucus or debris and minimize mucosal trauma |
| Use insufflation and desufflation | Fine adjustments to luminal insufflation can help with detection of subtle abnormalities |
| Spend adequate time inspecting the Barrett’s segment and gastric cardia in retroflexion | Careful examination increases dysplasia detection |
| Examine the Barrett’s segment using high-definition white light endoscopy | Standard of care |
| Examine the Barrett’s segment using chromoendoscopy (including virtual chromoendoscopy) | Enhances mucosa pattern and surface vasculature |
| Use the Prague classification to describe the circumferential and maximal Barrett’s segment length | Standardized reporting system |
| Use the Paris classification to describe superficial neoplasia | Standardized reporting system |
| Use the Seattle protocol (in conjunction with electronic chromoendoscopy) with a partially deflated esophagus to sample the Barrett’s segment | Increases dysplasia detection |
Adapted from Kolb and Wani (232).
NONENDOSCOPIC TREATMENT OF BE
This section addresses the role of chemoprevention (pharmacologic interventions) and antireflux interventions in reducing the risk of neoplastic progression in patients with BE (BE).
Recommendation.
-
14
We suggest at least once-a-day PPI therapy in patients with BE without allergy or other contraindication to PPI use (strength of recommendation: conditional; quality of evidence: very low).
Summary of evidence.
Several observational studies have demonstrated that GERD symptoms are a strong risk factor for EAC and the risk of EAC increases with increasing duration and severity of GERD symptoms (134,135). Similarly, GERD symptoms are also strongly associated with BE. PPIs are commonly prescribed in patients with BE, given the high proportion of patients with BE with symptomatic GERD and its impact on their quality of life (136). In addition, preclinical (biomarker based) and some observational studies have shown that PPIs may prevent neoplastic progression in patients with BE supporting its role as a chemopreventive agent (137–139).
Epidemiologic evidence supports a significant decrease in the risk of progression to HGD and EAC in patients with BE with PPI therapy, a critical outcome for this clinical question. A systematic review and meta-analysis of observational studies showed that PPI therapy was associated with a 71% reduction in the risk of HGD or EAC (adjusted OR 0.29; 95% CI 0.12–0.79) (139). In 4 cohort studies that reported the time to progression to HGD or EAC, PPI users were also significantly less likely to progress to HGD or EAC (aHR 0.32; 95% CI 0.15–0.67). There was insufficient information in these studies to allow estimation of the effect of PPIs on risk of progression to EAC alone or to HGD alone, and there was no information on whether taking PPI twice daily would provide any added benefit over once daily administration. This study also highlighted the lack of any significant effect with the use of histamine receptor antagonists. Another systematic review and meta-analysis reported no benefit with the use of PPIs in decreasing the risk of neoplastic progression in BE (140). These results were of questionable value given that this review combined different designs of observational studies, decreasing its impact in the drafting of this recommendation.
The panel considered several other important questions. The utility of increasing the dose of PPI therapy from once to twice daily, beyond what is required to control reflux symptoms, is unclear. Several studies have shown that pathologic acid reflux often persists despite PPI therapy in patients with BE and that control of GERD symptoms with PPI treatment does not guarantee that esophageal acid exposure is controlled (138,141–143). However, as reviewed below, the benefits of increasing the dose and frequency of PPIs are also unclear (144). The panel also considered the potential harms of long-term PPI therapy and the suggested associations between PPI therapy and the risk of pneumonia, dementia, cardiovascular events, cerebrovascular events, chronic renal failure, fractures, enteric infections, small bowel bacterial overgrowth, Clostridium difficile–associated diarrhea, anemia, and all-cause mortality (145,146). Evidence is inadequate to establish causal relationships between PPI and any of these proposed associations, with the exception of enteric infection. The largest randomized controlled trial that assessed the safety of PPIs in a 3-year trial among 17,598 receiving rivaroxaban or ASA reported no difference in the risk of all-cause mortality (HR 1.03; 95% CI 0.92–1.15), myocardial infarction (HR 0.94; 95% CI 0.79–1.12), fractures (OR 0.96; 95% CI 0.79–1.17), pneumonia (OR 1.02; 95% CI 0.87–1.19), chronic kidney disease (OR 1.17; 95% CI 0.94–1.45), and dementia (OR 1.2; 95% CI 0.81–1.78) between the PPI and placebo groups (146). There was a statistically significant difference between the 2 groups for the end point of enteric infections (OR 1.33; 95% CI 1.01–1.75) with a number needed to harm of >900 for each year of PPI therapy. Similar results highlighting the safety of PPIs have been reported among randomized controlled trials comparing PPIs with antireflux surgery (147). Given the unclear benefit of higher doses of PPI on oncogenesis, the panel recommended at least daily dosing, with higher doses considered for those requiring them for symptom control.
Recommendation.
-
15
We could not make a recommendation on combination therapy with ASA and PPI in patients with BE to reduce the risk of progression to HGD/EAC.
Summary of evidence.
ASA and nonsteroidal anti-inflammatory drugs (NSAIDs) inhibit several pathways important in oncogenesis including EAC, specifically the cyclooxygenase pathway, which is a key mediator of inflammation that upregulates a number of oncogenic factors (148,149). Patients taking ASA and NSAIDs seem less likely to develop EAC in epidemiologic studies (150–153). A phase II randomized controlled trial evaluated 114 patients with BE taking esomeprazole 40 mg twice daily and randomized participants to ASA 325 mg vs ASA 81 mg or placebo and demonstrated a statistically significant decrease in tissue prostaglandin E2 levels in the esophageal biopsies of patients allocated to the high-dose ASA arm (137). However, the unfavorable risk-benefit ratio makes NSAIDs unsuitable as a chemopreventive agent in reducing the risk of progression in patients with BE.
The AspECT chemoprevention study aimed to evaluate the efficacy of high-dose esomeprazole and ASA for improving outcomes in patients with BE, with a primary outcome of time to all-cause mortality, EAC, or HGD (144). This study was conducted across 84 centers in the United Kingdom and 1 in Canada using a 2 × 2 factorial design. Patients with BE (n = 2,557) were randomized to esomeprazole 20 mg once daily or 40 mg twice daily, with or without ASA (300 mg/d in the United Kingdom and 325 mg/d in Canada) and followed for a median follow-up of 8.9 years and >20,000 patient-years of follow-up. This study demonstrated that high-dose PPI was superior to low-dose PPI for lengthening the time to reach the combined end point of death from any cause, EAC, or HGD (time ratio [TR] 1.27; 95% CI 1.01–1.58, P = 0.038). Outcomes in the ASA group were not significantly better than no ASA group (TR 1.24; 95% CI 0.98–1.57). However, when censoring those with the use of concurrent NSAIDs, ASA was better than no ASA (TR 1.29; 95% CI 1.01–1.66; P = 0.04). Finally, combining high-dose PPI with ASA had the strongest effect compared with low-dose PPI without ASA (TR 1.59; 95% CI 1.14–2.23; P = 0.006). The safety data were reassuring, with only 1% of study participants reported serious adverse events; 69 reported bleeding, 38 of which occurred among individuals receiving ASA.
Despite these data, the panel was unable to make any recommendation with regard to the use of ASA along with PPI therapy due to several study limitations and caveats. This study did not demonstrate any significant differences for cancer-related outcomes. This trial was not double blinded, the event rate was low with wide 95% CIs, and a small effect size was noted, with the benefit largely driven from reductions in all-cause mortality, as opposed to the cancer-related outcomes most concerning in a BE population. Although it is still uncertain whether all patients with BE should be prescribed PPI and ASA for chemoprevention, the panel acknowledges that substantial proportion of patients with BE will be candidates for ASA for cardioprotection. Given that we recommend at least daily PPI for all patients with BE without contraindications, it therefore stands to reason that a large proportion of patients with BE will be prescribed combination therapy with PPI and ASA. We include recommendation 15 to document that this recommendation went through the formal GRADE review process with consideration by the authoring panel and to provide the data underpinning this decision.
Recommendation.
-
16
We suggest against the use of antireflux surgery as an antineoplastic measure in patients with BE (strength of recommendation: conditional; quality of evidence: low).
Summary of evidence.
Surgical antireflux procedures are highly effective at reducing gastroesophageal reflux episodes, healing esophagitis, and decreasing the symptoms associated with reflux. It is logical, therefore, to consider their application in the setting of BE to reduce the risk of progression to cancer.
Several issues argue against the routine application of surgical antireflux procedures as an antineoplastic measure in the setting of BE. First, the risk of progression to cancer in the setting of NDBE is so low that incurring the risks inherent in surgery, even a surgery with a low rate of life-threatening complications such as laparoscopic fundoplication, may not be merited in patients who do not require fundoplication for symptoms not controllable by medical therapy. Second, fundoplication comes with its own set of short- and long-term complications, which can occasionally be severe in nature and duration. Finally, and most importantly, data do not convincingly demonstrate that patients with BE treated with surgical antireflux procedures have a lower risk of progression to neoplasia than those treated medically.
Studies comparing the risk of progression to neoplasia in patients with BE treated medically and surgically have several shortcomings. Compliance with medical therapy is not routinely documented and is unclear among medically treated patients. Among surgically treated patients, the effectiveness of the wrap in averting reflux is not generally reported, and the use of concurrent medical therapy after surgical antireflux therapy, which is known to occur commonly, is not well described. Several attempts have been made to perform meta-analysis of studies documenting progression outcomes in patients with BE treated with medical and surgical management (154–156). These studies do not document consistent superiority of surgical management over medical management. The single randomized controlled trial of medical vs surgical management of BE for progression to neoplasia showed no statistically significant difference in outcomes between the groups but was inadequately powered (157).
ENDOSCOPIC TREATMENT OF BE
EET has revolutionized the management of patients with BE-related neoplasia and offers an effective, minimally invasive treatment approach, avoiding the morbidity and mortality associated with esophagectomy (158). The basic premise of EET is that patients with BE with HGD and intramucosal cancer (IMC) have a very low risk of lymph node metastasis (0% in HGD, up to 2% in IMC) (159). Contemporary practice includes endoscopic resection (ER) of any visible lesion within the BE segment, followed by ablative techniques such as RFA and cryotherapy to achieve complete eradication of dysplasia (CED) and IM (CEIM). Among the available ablative modalities, RFA has the widest breadth of demonstrated efficacy (from randomized controlled trials), effectiveness, and safety data (160).
Recommendation.
-
17
We recommend EET compared with esophagectomy in patients with BE with HGD or IMC (strength of recommendation: strong; quality of evidence: moderate).
Summary of evidence.
BE with HGD is an actionable diagnosis, and surveillance is not are commended management option for this patient population. To address this clinical question, the panel considered the following patient-centered outcomes: overall survival, EAC-related mortality, adverse events, CED/IMC, and recurrence rates. An updated systematic review and meta-analysis demonstrated no difference between EET and esophagectomy with regard to overall 1-, 3-, and 5-year survival and EAC mortality (158,161). For the critical outcome of 5-year survival, there was no difference between the 2 groups (RR 0.88, 95% CI 0.74–1.04). Lower rates of adverse events were noted among patients undergoing EET compared with esophagectomy (RR 0.38, 95% CI 0.20–0.73). Esophagectomy is associated with an operative mortality of 2% and a high morbidity rate (bleeding, anastomotic leakage, infection, stricture, and prolonged hospitalization) even at high-volume centers (158,162). The effectiveness and safety profile of EET in BE-related neoplasia is well established (160,163–168). A systematic review and meta-analysis that included 37 studies and 9,200 patients reported a pooled adverse event rate of 8.8%(95%CI 6.5–11.9) related to RFA with or without endoscopic mucosal resection (EMR), estimates that need to be discussed with patients before embarking on EET. Esophageal stricture was by far the most common adverse event (5.6%, 95% CI 4.2–7.4) followed by bleeding (1%, 95% CI 0.8%–1.3%) and perforation (0.6%, 95% CI 0.4%–0.9%) (160). Although higher rates of neoplastic recurrence were noted in patients undergoing EET (RR 9.5, 95% CI 3.26–27.75), there was no difference between EET and esophagectomy for the end point of complete eradication of HGD/IMC (RR 0.96, 95% CI 0.91–1.01). Available data suggest that most patients achieve CEIM, the primary end point of EET, within 3 endoscopy sessions (169–174). Figure 4 demonstrates the recommended management of patients with neoplastic BE.
Figure 4.
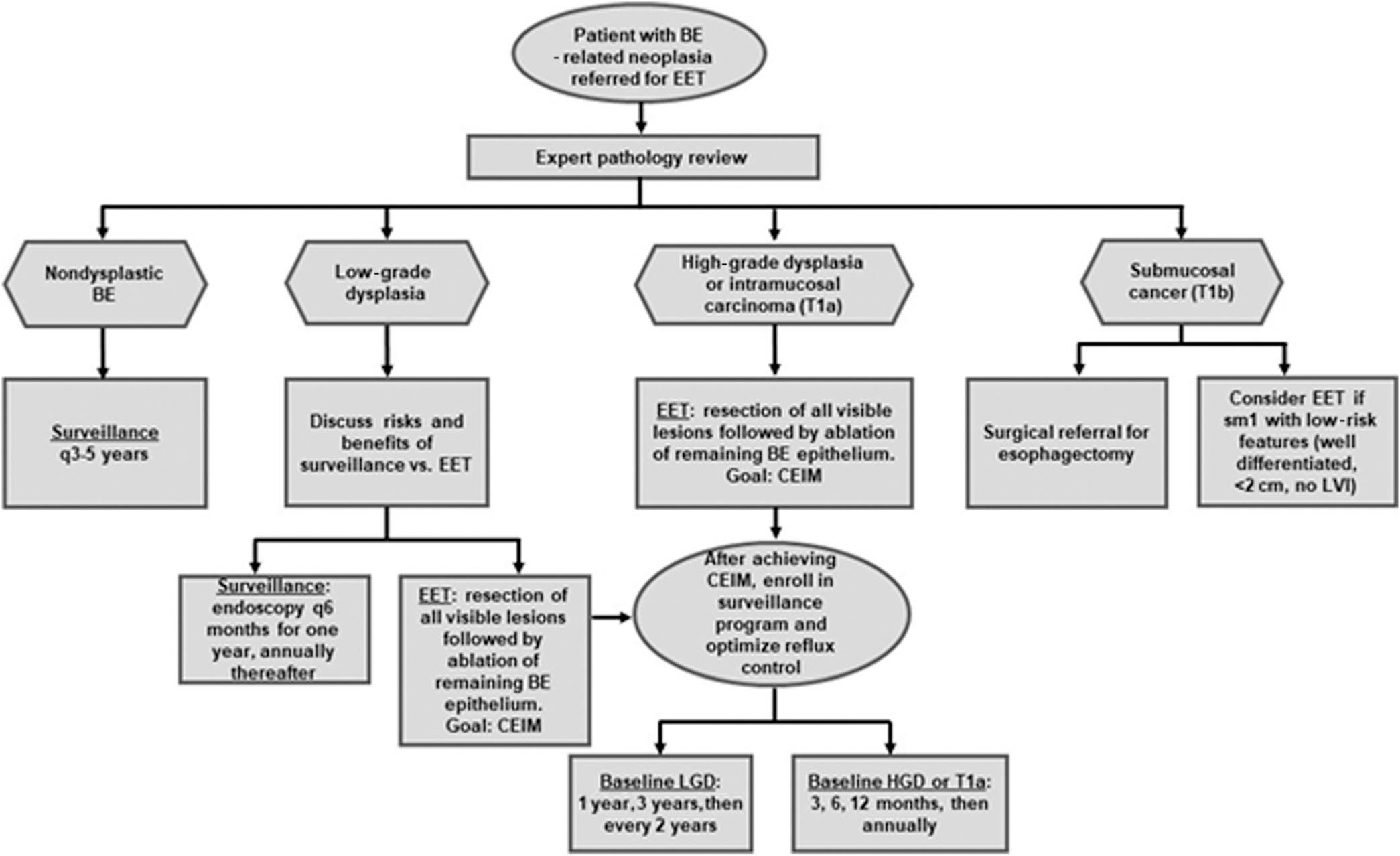
Algorithm for patients referred for consideration of EET. Please note that these procedures are to be performed using high-definition white light endoscopy and virtual chromoendoscopy and are generally performed after initiation of maximal acid suppressive therapy (twice-daily PPI). Resection of visible lesions should always precede ablative therapy, and this mucosal resection may upstage the BE, in which case the algorithm for the most severe histology should be followed. BE, Barrett’s esophagus; CEIM, complete eradication of intestinal metaplasia; EET, endoscopic eradication therapy; HGD, high-grade dysplasia; LGD, low-grade dysplasia; LVI, lymphovascular invasion; PPI, proton pump inhibitor.
The panel considered the limited comparative data between EET and esophagectomy and that the evidence supporting this recommendation was provided by observational studies (retrospective studies or population-based studies using the Surveillance, Epidemiology and End Results database).A randomized controlled trial comparing these 2 management strategies is highly unlikely. The panel primarily considered the comparative effectiveness and safety of EET to esophagectomy, in drafting this recommendation in favor of EET for superficial neoplasia.
Esophagectomy has traditionally been recommended in patients with EAC with submucosal invasion (T1b EAC) given the high risk of lymph node metastases (175). The panel noted the expanding role of EET in patients with superficial submucosal invasion. Observational data suggest that EET may be a viable alternative to esophagectomy for patients with T1b EAC with superficial submucosal invasion (sm1—invasion into the upper third of the submucosa to a depth <500 μm) and low-risk features such as deep margin negative, well-moderate differentiation and no lymphovascular invasion (176–178). The risk of lymph node metastases after EET in T1b sm1 EAC patients seems to be lower than the mortality rates associated with esophagectomy (176,179). Patients with high-risk histology are best treated with esophagectomy, unless the patient is a poor surgical candidate, for whom discussion at a multidisciplinary conference may be appropriate to consider alternative options such as adjuvant chemoradiation.
Recommendation.
-
18
We suggest endoscopic therapy in patients with BE with confirmed LGD to reduce the risk of progression to HGD/EAC, with endoscopic surveillance of confirmed LGD as an acceptable alternative (strength of recommendation: conditional; quality of evidence: moderate).
Summary of evidence.
LGD has long presented a management conundrum. This is due in part to considerable interobserver variability among pathologists in making the diagnosis as well as a variable natural history of progression to HGD/EAC. There is clear evidence that LGD increases the risk for neoplastic progression, but the magnitude of that risk is highly variable (43). Although a meta-analysis of 24 studies found the annual incidence of HGD/EAC to be 1.73%/patient-year, the Surveillance vs Radiofrequency Ablation study, a randomized controlled trial of RFAvs surveillance, found the risk of progression to be dramatically higher, at 11.8%/patient-year of follow-up (39,180). Thus, for patients diagnosed with LGD, several initial concerns must be addressed, including whether the LGD diagnosis is correct, whether undetected prevalent HGD or EAC is present, and what the most appropriate follow-up or additional therapy should be.
Any diagnosis of LGD merits a review by expert GI pathologists. Multiple studies demonstrate that a substantial proportion of patients thought to have LGD do not have the diagnosis confirmed on expert pathology review, reaffirming the importance of this step (33,34). Furthermore, several studies suggest that the risk of progression of LGD to HGD/EAC is highest in the first year after diagnosis, raising the specter of undetected HGD or EAC in patients with LGD. A systematic review and meta-analysis of 8 studies found that while the summary overall weighted annual incidence for progression to HGD/EAC was 4.6/100 patient-years (95% CI 2.0–7.2), the rate was higher,at 8.8/100 patients, in the first year after diagnosis (181). Others have found a similar increase in risk in the first year after diagnosis, thereby emphasizing the importance of a careful repeat examination (182). Finally, there is considerable evidence that a diagnosis of confirmed LGD that persists on a second examination increases the risk for progression still further (34,183).
Thus, for a patient with a diagnosis of LGD, the first step is to have expert pathologic review. If the diagnosis is downgraded to no dysplasia, surveillance should revert to the nondysplastic pathway. If the diagnosis of LGD is confirmed, the patient may choose to either consider endoscopic eradication therapy or to repeat an endoscopy within 6 months (181). Discussion based on shared decision making with the patient should review the pros and cons of proceeding to EET or continued surveillance. If surveillance is continued, there is no consensus among international guidelines on the frequency of surveillance intervals. Given the increase in progression risk in the first year, it makes sense to repeat surveillance every 6 months for 1 year followed by annual surveillance as long as LGD is present. If no dysplasia is then seen, the surveillance interval should revert to the nondysplastic pathway.
The panel considered several outcomes for this clinical question: progression rates to HGD and EAC, cancer-specific mortality, and adverse events between EET and surveillance. Two randomized controlled trials have compared RFA with surveillance in patients with BE with LGD. In a multicenter, randomized, sham-controlled trial, subjects with dysplastic BE were assigned 2:1 to either RFA or a sham procedure. A total of 127 patients were randomized (84 RFA, 43 sham), including 64 patients with LGD (42 RFA, 22 sham). At 12 months, complete eradication of LGD (90% vs 23%, P < 0.001) and CEIM (81% vs 4%, P < 0.001) rates was higher in the RFA group. Two patients in the RFA group progressed to HGD, with none progressing to EAC in either group (38). This study was not powered to study outcomes in patients with LGD alone. The study was a crossover design, and with longer follow-up, 3 patients progressed from LGD to HGD and 1 from LGD to EAC (184). The European multicenter randomized controlled trial—the Surveillance vs Radiofrequency Ablation study—randomized 136 patients with confirmed BE with LGD to either RFA or surveillance for the primary outcome of neoplastic progression (39). Ablation markedly reduced the risk of progression to a combined end point of HGD/EAC (1.5% vs 26.5%, P < 0.001) and EAC (1.5% vs 8.8%, P = 0.03). Ablation reduced the risk of neoplastic progression by 25% (95% CI 14.1–35.9) with a number needed to treat of 4. Similarly, ablation reduced the risk of progression to EAC by 7.4% (95% CI 0%–14.7%) with a number needed to treat of 13.6. A systematic review and meta-analysis compared the risk of neoplastic progression among BE patients with LGD treated with RFA compared with outcomes of LGD under endoscopic surveillance, using data from 22 studies, including the 2 randomized controlled trials (n = 2,746) (158). Lower rates of progression were reported in patients treated with RFA (RR 0.14). The cumulative rate of disease progression for a follow-up duration of up to 84 months was 12.6% (95% CI 9.8–15.9) in the surveillance group and 1.7% (95% CI 1.1–2.6) in the RFA group.
The panel recognized the several controversies surrounding the diagnosis and management of LGD and the several arguments that have been put forth supporting the rationale for continued surveillance in patients with LGD (36,158). These include (i) the phenomenon of regression of LGD, wherein the diagnosis of LGD cannot be confirmed on subsequent endoscopy, (ii) the limited generalizability of available data, as most effectiveness data are reported from expert centers, (iii) significant interobserver variability in the diagnosis of LGD among pathologists, (iv) the likelihood that surveillance of LGD (at least at expert centers) would detect progression to HGD/EAC at a stage amenable to EET and rarely requiring esophagectomy, and (v) EET is associated with adverse events (160). In addition, none of the published studies comparing EET with surveillance have incorporated patient-centered outcomes, and data on risk stratification in LGD remain limited. The panel considered requiring persistence of LGD before ablation, as some data suggest that ablation of persistent LGD is more cost-effective than ablation after a single confirmed reading. In contrast, other studies suggest that progression rates to HGD/EAC after a single confirmed reading of LGD may be in excess of those necessary for cost-effectiveness (185,186). Therefore, although persistence of LGD is a risk factor for progression, it is not mandatory to demonstrate persistence before considering EET in this setting. Of note, mucosal inflammation may lead to an errant diagnosis of LGD (187). Therefore, a repeat endoscopy after the institution of vigorous (twice daily) acid suppression may be advisable after an initial reading of LGD if accompanied by endoscopic and/or histological evidence of inflammation.
Consistent with previous documents (36,158,188), the panel suggests the concept of shared decision making in determining the optimal management strategy for patients with LGD. Among patients undergoing EET, RFA is the preferred ablative technique. In patients with LGD undergoing surveillance, we suggest that surveillance should be performed at 6-month intervals for 1 year and then annually unless there is reversion to NDBE in which case surveillance intervals can be extended to every 3 years. Sampling should be performed using the Seattle biopsy protocol (4 quadrants every 1 cm) (36).
Recommendation.
-
19
We suggest initial ER of any visible lesions before the application of ablative therapy in patients with BE undergoing EET (strength of recommendation: conditional; quality of evidence: very low).
Summary of evidence.
ER of visible lesions detected on careful screening or surveillance examination of the BE mucosa serves both a diagnostic and therapeutic purpose. Histologic interpretation of dysplasia grade (LGD, HGD, and IMC) on forceps biopsy specimens even by expert GI pathologists is limited by significant interobserver variation (189–191). However, larger histology specimens provided by EMR or endoscopic submucosal dissection (ESD) have been shown to reduce the interobserver variability associated with BE neoplasia assessment by pathologists (192–194).
In addition, likely due to the larger specimen size and deeper extent of the sample (inclusion of the muscularis mucosa and submucosa in most ER specimens), ER has also been shown to upstage/downstage the histologic grade of dysplasia and lead to a change in the management of 30%–40% of patients with BE undergoing endoscopic evaluation (195,196). In a retrospective study of 150 ERs performed for focal lesions, ER histology led to a change in diagnosis in 49% and a relevant change in treatment approach in 30% of patients (196). In another multicenter study of 138 patients with LGD, HGD, and EAC, 83% had visible lesions endoscopically resected. ER led to a change in histological diagnosis of 31% of patients with early neoplasia (195). This is especially relevant in the staging of early-stage EAC because conventional endoscopic ultrasound (EUS) has only modest accuracy in the staging of early-stage EAC (197,198). Thus, EUS is not routinely recommended in the evaluation of patients with BE with dysplasia (HGD or LGD) or early EAC referred for EET for the purpose of differentiating between mucosal vs submucosally invasive disease. However, EUS plays a role in appropriate staging of patients with T1b and more advanced EAC and in select cases with T1a EAC based on prognostic features described below (107).
Unlike EUS, ER of early-stage EAC also provides accurate tumor (T) staging and prognostic information. Tumor invasion into layers of the mucosa (lamina propria and muscularis mucosa) and submucosa can be precisely determined, in addition to lateral and deep margins of resection (199). In addition, prognostic features such as grade of differentiation and lymphovascular and perineural invasion can be accurately assessed and help in predicting prognosis and selecting appropriate management (200). Precise ascertainment of the depth of invasion is important, given the low prevalence of metastatic lymphadenopathy in T1a disease, compared with the substantial prevalence of metastatic lymphadenopathy in T1b disease invading the mid- to deep submucosa (20%–30%). Indeed, EET of early-stage EAC is associated with excellent long-term outcomes when compared with surgery (201,202). Hence, ER allows the selection of the most appropriate management strategy as dictated by histopathology of the resected lesion. Although some studies have attempted to correlate endoscopic appearance (based on the Paris classification of superficial neoplastic lesions) (203,204) and the likelihood of submucosal invasion, these correlations, while prognostic, are not perfect, and are inadequate to base clinical decision making on, in the absence of tissue sampling. Paris 0-I and 0-IIc lesions were found more likely to invade the submucosa in 1 study. The presence of deep ulceration (Paris III lesion) likely reflects deep submucosal invasion, making these lesions less optimal for ER (196).
Techniques of ER include cap-assisted EMR, multiband EMR, and ESD. Both techniques for EMR (cap-assisted EMR and multiband EMR) have equal efficacy and safety (Figure 5). Although cap-assisted EMR may allow for slightly larger samples of tissue because of ease of use, multiband EMR is the preferred resection technique in most cases. The safety of EMR is well described, whereas ESD (which allows en bloc resection of larger specimens with interpretation of lateral margins) has a steeper learning curve, requires more time to complete, and is associated with a higher rate of complications. Additional challenges include the lack of formal training pathways for ESD training in the West and the lack of dedicated billing codes, making reimbursement challenging. Despite these limitations, ESD may have a role in the resection of larger lesions (which are unsuitable for en bloc resection by EMR), lesions with potential submucosal invasion or lesions arising postablation, rendering EMR challenging due to scarring. Comparative data on the effectiveness of these 2 resection techniques followed by ablation are relatively limited (205). Available data suggest that these 2 techniques are likely comparable in terms of rates of CEIM when combined with ablation and complications when performed by expert endoscopists (206). Long-term recurrence data need to be assessed.
Figure 5.
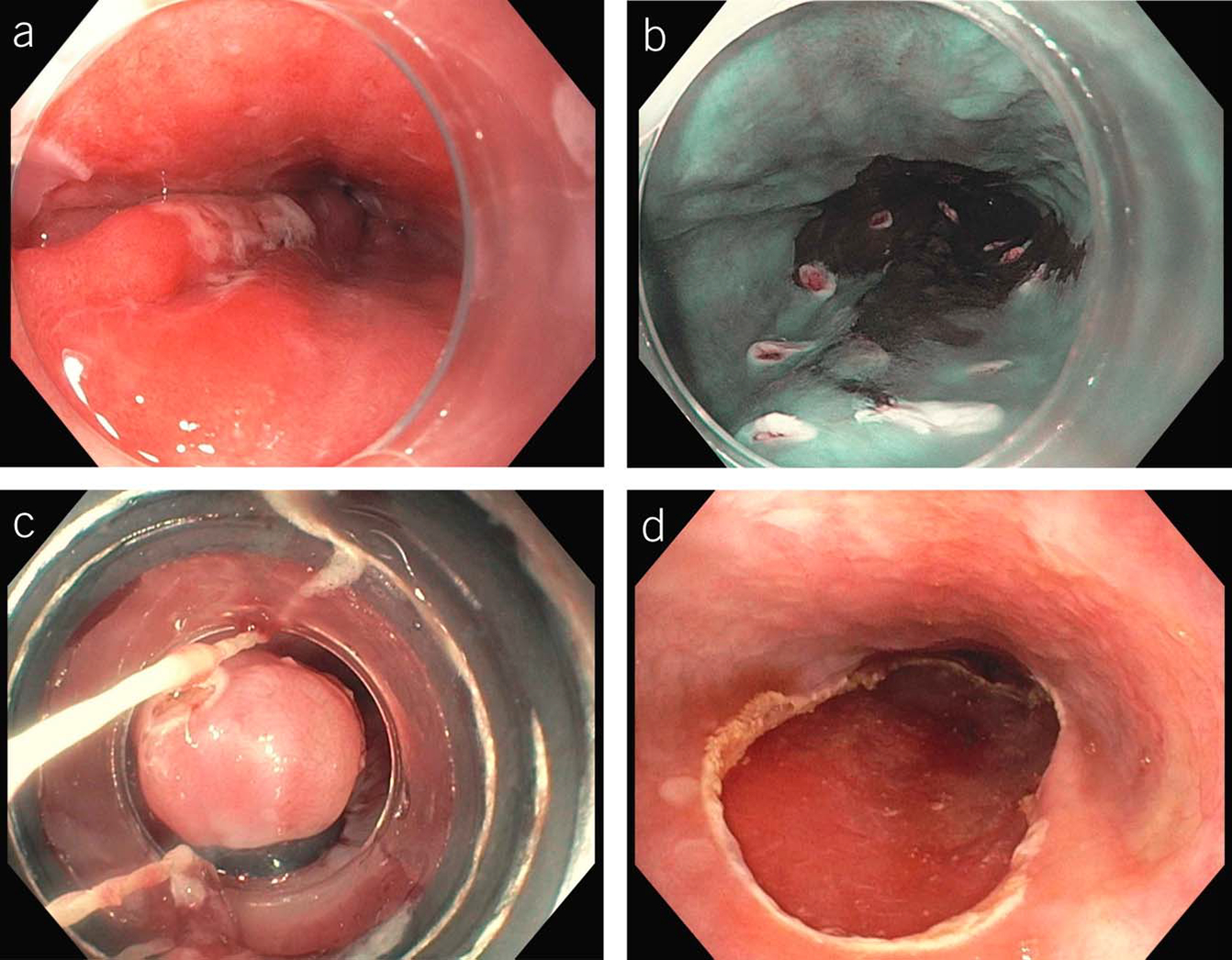
Images of band ligation endoscopic mucosal resection. (a) A lesion at the 9 o’clock position, (b) the same lesion, with borders marked with electrocautery, (c) the proximal portion of the lesion banded, and (d) complete resection of the lesion, with the absence of residual cautery markings.
Following successful ER of all visible abnormalities, ablation of the residual BE segment regardless of histology is recommended to reduce the risk of recurrent dysplasia/EAC. This recommendation is based on studies showing that residual BE predicts metachronous neoplasia following resection of HGD/EAC, as well as a randomized trial comparing ablation (with APC) of residual BE mucosa to continued surveillance, conclusively showing substantially higher rates of metachronous dysplasia in the surveillance arm (207). Ablation with RFA vs stepwise radical EMR of the residual BE mucosa were compared in a randomized trial and conclusively showed that the RFA arm reached CEIM faster, with fewer procedures and fewer complications than the EMR group (208).
Cryotherapy (spray or balloon) has also been used to ablate dysplastic BE mucosa. Unlike RFA, which is a thermal modality causing direct contact tissue necrosis, cryotherapy acts via multiple mechanisms initiated by rapid freezing and slow thawing including direct cell injury, apoptosis, and tissue ischemia. Two cryotherapy modalities are currently commercially available. Liquid nitrogen (LN) spray cryotherapy (truFreeze; STERIS, Mentor, OH) delivers LN at −1,960 °C using a flexible spray catheter via the working channel of the endoscope. A decompression tube is placed adjacent to the catheter given expansion of LN to gas while spraying. A newer cryotherapy technology is the C2CryoBalloon Ablation system (Pentax Medical, Redwood City, CA), which uses a rotatable diffuser within a compliant balloon to spray liquid nitrous oxide on the mucosa. There are currently no randomized trials assessing the efficacy of cryotherapy for the treatment of dysplastic BE. Retrospective and prospective cohort studies have reported CED and CEIM rates of 81%–88% and 57%–61%, respectively, with spray cryotherapy (209,210). A systematic review reported CED and CEIM rates of 76% and 46% in patients with dysplastic BE refractory to initial RFA who were treated with spray cryotherapy (211). Data on cryoballoon ablation are more limited, with a recent multicenter prospective cohort study reporting 76% and 72% of patients reaching CED and CEIM in an intention to treat analysis (212). Cryotherapy is associated with a 9%–12% stricture rate. There are currently no randomized trial data comparing cryotherapy and RFA, but a recent nonrandomized multicenter study reported comparable rates of CED and CEIM in patients with dysplastic BE treated with RFA and cryoballoon ablation with higher stricture rates in the cryoballoon cohort (213).
Recommendation.
-
20
We suggest that patients with BE undergoing EET be treated at high-volume centers (strength of recommendation: conditional; quality of evidence: very low).
Summary of evidence.
Several core competencies are necessary before embarking on EET. Acquisition of technical, cognitive, and integrative competencies is critical for quality of care and patient safety. These include but are not limited to (i) adequate training and expertise in the detection of mucosal lesions that harbor neoplasia with the use of high-definition white light endoscopy and virtual chromoendoscopy, (ii) appropriate selection of patients who merit EET, (iii) technical skills in performance of EMR and RFA, and (iv) recognition and management of potential adverse events related to EET (bleeding, perforation, stricture, and recurrence). There are limited data to determine the exact thresholds for training and education for the performance of EET.
However, as with most technical procedures in endoscopy, available data do suggest a volume-outcome effect in the management of patients with BE-related dysplasia and EAC undergoing endoscopic intervention. The outcomes considered for this clinical question included number of sessions required to achieve CEIM and CED rates, adverse events, recurrence rates, and mortality rates. Data from the US RFA Patient Registry that included patients who underwent RFA for BE at 148 centers demonstrated that with increasing number of cases performed by endoscopists and centers, the number of treatment sessions required to achieve CEIM decreased, a relationship that persisted after adjusting for patient variables such as age, sex, race, BE length, and pretreatment dysplasia status. This relationship between volume and treatment session necessary to achieve CEIM seemed to attenuate after treatment of at least 30 patients by the center or individual endoscopist (214). Center experience was not associated with overall rates of CEIM or CED, only the number of sessions necessary to achieve success. A retrospective study that predominantly included patients with HGD or IMC demonstrated a significant correlation between endoscopist RFA volume and CEIM rates (215). A recent retrospective cohort study that used the national Veterans Affairs health care system showed that treatment at high-volume RFA facilities was associated with a reduced risk of recurrence (comparing quartile 4 with quartile 1, HR 0.19, 95% CI 0.05–0.68) (216). Another observational study showed that endoscopist procedure volume was an independent predictor of adverse outcomes (30- and 90-day mortality and requirement for emergency intervention) among patients undergoing upper GI EMR (including patients with BE and esophageal cancer) (217). The results of these studies support a robust volume-outcome effect and suggest that outcomes might be improved by centralization of care for BE-related neoplasia at high annual case volume facilities. Centralized care for patients with BE-related neoplasia can include the following (101,218):
Treatment at centers with a high volume of patients with BE-related neoplasia by trained endoscopists. Although the annual case volume of new patients needs to be defined in future studies, the European Society of Gastrointestinal Endoscopy recommends an annual case volume of at least 10 new patients with HGD or early EAC per endoscopist.
Treatment by endoscopists trained in advanced imaging, EMR, and ablation who adhere to a standardized protocol and monitor outcomes with a commitment toward quality improvement.
Access to expert GI pathologists and to a multidisciplinary team that includes surgeons and medical and radiation oncologists.
Recommendation.
-
21
We recommend an endoscopic surveillance program in patients with BE who have completed successful EET (strength of recommendation: strong; quality of evidence: moderate).
Summary of evidence.
Recurrence of IM is well documented after successful EET, which is defined by the end point of CEIM. CEIM is variably described in the literature as 1 or 2 surveillance endoscopies negative for visible BE and IM in biopsies taken from the GEJ and the tubular esophagus. Recurrence is currently defined as the detection of IM (with or without dysplasia) from the tubular esophagus or the GEJ after achieving CEIM (219).
The annual incidence of recurrent IM after CEIM as described in a systematic review and meta-analysis including over 3,000 patients ranges from 8.6% to 10.5%, whereas the incidence of dysplastic IM recurrence and HGD/EAC recurrence was lower at 2.0% and 1.2%, respectively (172). The timing of detection of recurrences is variably reported in the literature as being mostly in the first year after CEIM (220) (in studies using 1 negative biopsy defining CEIM, but not in those with 2 negative biopsies defining CEIM), peaking at 18 months (173,174) after CEIM or continuing to increase with duration of follow-up (up to 5 years), both for nondysplastic and dysplastic BE recurrences (174). Hence, discontinuation of surveillance after EET is not recommended at this time, given the continued risk of recurrence after CEIM.
The location of recurrence after EET has been studied by multiple investigators. Recurrences may be detected either in the tubular esophagus or in the GEJ, and most recurrences appear distally (in the esophagus and at the GEJ). In a large cohort study (174), 75% of recurrences were diagnosed at the GEJ and the remainder in the tubular esophagus. In addition, most (60%) recurrences at the GEJ were described as not visible (to the treating endoscopist), whereas most recurrences in the tubular esophagus (80%) were visible. Most (87%) tubular esophagus recurrences were detected within 5 cm of the distal esophagus. This is consistent with other reports that also describe most recurrences in the distal 2 to 4 cm of the esophagus (221,222). In addition, the yield of random biopsies from a normal-appearing neosquamous epithelium was low (1% for any recurrence and 0.2% for any dysplastic recurrence) in these studies. This is in keeping with the low rate of subsquamous BE after successful EET with RFA. Hence, the utility of random biopsies from normal neosquamous epithelium is likely limited and may have the most utility if confined to the distal 2–4 cm of the tubular esophagus.
Predictors of recurrence after CEIM have been described in multiple studies and include HGD/EAC before ablation (vs LGD or NDBE), long-segment BE, and older age (172). Currently, surveillance recommendations are based on the preablation histology, with less frequent intervals for surveillance after EET for LGD, given the consistently lower rates of recurrence after EET for LGD. In a modeling study (223), which used recurrence data from the US RFA Registry and validated in the UK HALO Registry, using a 0.1% risk of recurrence of invasive EAC as a threshold (equivalent to the complication rate of sedated endoscopy in the elderly), surveillance at 1 year after CEIM and every 2 years thereafter in those with LGD preablation and at 3 months, 6 months, 12 months and annually thereafter was recommended for those with HGD preablation. These intervals are less frequent than those recommended previously. It is likely that intervals less frequent than those recommended for BE in the absence of ablation will be adequate to minimize the risk of missing recurrent dysplasia/EAC. Table 7 demonstrates the suggested surveillance intervals after successful EET of dysplastic BE.
Table 7.
Recommended endoscopic surveillance intervals following CEIM based on worst pretreatment histology
| Worst pretreatment histology | Suggested endoscopic surveillance |
|---|---|
|
| |
| Low-grade dysplasia | 1 yr following CEIM 3 yr following CEIM Every 2 yr thereafter |
| High-grade dysplasia | 3 mo following CEIM 6 mo following CEIM 12 mo following CEIM Annually thereafter |
| Intramucosal carcinoma | 3 mo following CEIM 6 mo following CEIM 12 mo following CEIM Annually thereafter |
CEIM, complete eradication of intestinal metaplasia.
Treatment of recurrent BE should follow similar principles of preablation EET, including ER for visible lesions and ablation for flat residual BE. Fortunately, results reported by multiple investigators indicate that the most (>90%) of all recurrences can be successfully treated endoscopically. Invasive EAC needing surgery or leading to death from metastatic disease is rare. The significance of nondysplastic IM recurrence at the GEJ/cardia is unclear, with some investigators suggesting that this is of limited clinical significance, comparing this with IM of the cardia in those without BE. A recent observational study described a lower rate of subsequent development of dysplasia in patients with nondysplastic GEJ recurrence who were followed without treatment compared with those who were treated, supporting this hypothesis (224). However, additional studies are needed to confirm this observation.
Given the data above, patients who achieve CEIM with EET should be placed into an endoscopic surveillance program, which includes careful inspection of the GEJ and neosquamous epithelium for any visible lesions with high-resolution white light endoscopy and virtual chromoendoscopy, followed by surveillance biopsies from the GEJ (in a separate bottle) and from the distal 2–5 cm of the esophagus (in a separate bottle). Figure 6 demonstrates an appropriate biopsy protocol for patients achieving CEIM. Post-CEIM surveillance intervals should be tailored to the preablation histology: those with LGD undergoing surveillance at 1 year after CEIM, 3 years after CEIM, and then every 2 years thereafter, and those with HGD undergoing surveillance at 3 months, 6 months, 12 months, and annually thereafter. Cessation of surveillance after CEIM is not recommended at this time, unless dictated by the overall medical status of the patient.
Figure 6.
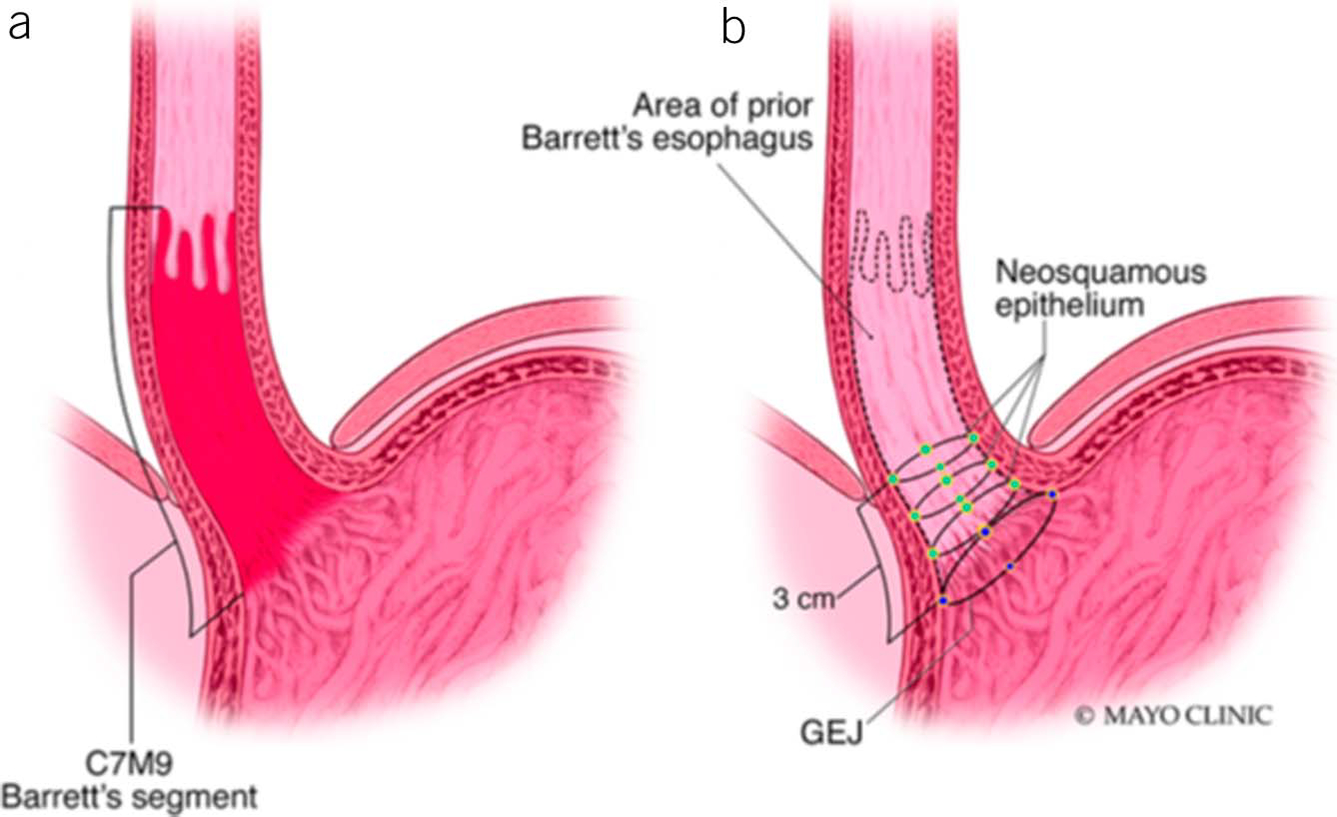
Suggested algorithm for post-CEIM surveillance in patients treated endoscopically for dysplastic BE. Panel a demonstrates the patient’s pretreatment long-segment BE, with a maximal extent of 9 cm and a circumferential extent of 7 cm. Panel b demonstrates the posttreatment esophagus, with previous areas of BE demonstrating neosquamous epithelium. Four quadrant biopsies are taken in the high cardia just below the Z line from the top of the gastric folds (blue dots). Four quadrant biopsies are additionally taken from each of the distal 2–3 cm of neosquamous epithelium (green dots). Biopsies taken of normal-appearing tissue above this range, even in previously long-segment disease, have not been demonstrated to have substantial additional yield for dysplasia or buried intestinal metaplasia. BE, Barrett’s esophagus; CEIM, complete eradication of intestinal metaplasia; GEJ, gastroesophageal junction. Adapted with permission from Kahn et al. (219).
Key concepts
-
3
Endoscopic cryotherapy may be considered as an alternative modality in patients unresponsive to RFA.
-
4
Patients with BE-related neoplasia embarking on EET should have a clear understanding of the risks and benefits associated with these therapies before initiation of therapy.
-
5
Endoscopists and centers performing EET should monitor their rates of CEIM, CED, and adverse events.
Given the increasing use of EET in patients with BE-related neoplasia, quality indicators for EET have recently been established and endorsed by the ACG and the American Society for Gastrointestinal Endoscopy (163,225). Appropriate quality indicators established as a part of this physician-led initiative using a formal methodologically rigorous process are highlighted in Table 8. Priority quality indicators established include monitoring (i) the rate at which CEIM is achieved by 18 months in patients with BE-related dysplasia and IMC referred for EET (outcome measure, threshold 70%), (ii) the rate at which CED is achieved by 18 months in patients with BE-related dysplasia and IMC referred for EET (outcome measure, threshold 80%), and (iii) the rate at which adverse events are being tracked and documented in individuals after EET (163,225). Compliance with this group of quality indicators has the potential to improve quality of care, identify performance gaps, reduce variability in health care, and ultimately improve patient outcomes. These quality indicators and benchmark targets may also be incorporated into the training curriculum of new endoscopists. Monitoring performance against these established quality indicators is critical in this era of value-based and quality-based health care.
Table 8.
Quality indicators for EET in dysplastic BE
| Metric | Numerator | Denominator | Type | Threshold |
|---|---|---|---|---|
|
| ||||
| For patients in whom a diagnosis of dysplasia has been made, the rate at which the reading is made by a GI pathologist or confirmed by a second pathologist before embarking on EET | Number of patients whose dysplasia diagnosis is made by a GI pathologist or a second pathologist before receiving EET | All patients who receive EET for treatment of dysplasia | Process | 90 (75–100) |
| The rate at which CEN is achieved by 18 mo in patients with Barrett’s-related dysplasia or intramucosal cancer referred for EET | Patients who are referred for EET for treatment of Barrett’s-related dysplasia or intramucosal cancer who achieve CED within 18 mo | All patients who are referred for EET for treatment of Barrett’s-related dysplasia or intramucosal cancer | Outcome | 80 (70–95) |
| The rate at which CEIM is achieved by 18 mo in patients with Barrett’s-related dysplasia and intramucosal cancer referred for EET | Patients who are referred for EET for treatment of Barrett’s-related dysplasia or intramucosal cancer who achieve CEIM within 18 mo | All patients who are referred for EET for treatment of Barrett’s-related dysplasia or intramucosal cancer | Outcome | 70 (50–80) |
| The rate at which adverse events are being tracked and documented in individuals post-EET | Adverse events that are tracked and documented | All endoscopic procedures involving EET | Process | 90 (50–100) |
BE, Barrett’s esophagus; CED, complete eradication of dysplasia; CEIM, complete eradication of intestinal metaplasia; CEN, complete eradication of neoplasia; EET, endoscopic eradication therapy; GI, gastrointestinal.
Adapted from Wani et al. (225).
CONCLUSION
BE is a common clinical condition that remains important, as it is the only known precursor lesion of EAC, a cancer that continues to increase in incidence in the Western world. This revised guideline synthesizes current best practices in the management of BE, with several key changes since the last iteration that reflect our evolving knowledge base. These include the broadening of acceptable screening modalities for BE to include new nonendoscopic methods, increasing surveillance intervals for segments <3 cm to 5 years, providing clear criteria and rationale for a quality endoscopic evaluation, volume thresholds for endoscopic therapy centers, and updated guidance on intervals and techniques for surveillance of patients after successful EET.
Multiple areas of uncertainty remain in the field of BE and are highlighted throughout this document. The reliance of GERD symptoms as a prerequisite for screening with either endoscopic or nonendoscopic techniques remains problematic, and further refinement of risk prediction algorithms can be anticipated. In the coming years, we expect to see increased use of nonendoscopic screening tools, which may have implications for general population screening in the future. Artificial intelligence has considerable potential for refining risk prediction algorithms used for screening and in the domains of endoscopic detection of neoplasia and pathologic interpretation of dysplasia. The predictive performance of biomarkers has been shown to be better in some cases than the histologic diagnosis of dysplasia, and our challenge now is defining this subset as we continue to assess biomarkers of increased risk to personalize our approach to these patients. The issue of postendoscopy esophageal cancer remains an area of concern. Is this due to suboptimal endoscopic surveillance or does it represent rapidly developing EAC? The maturing data in the field of EET have already allowed us to extend surveillance intervals and simplify biopsy protocols after complete eradication. This field will evolve further with accruing data on optimal surveillance intervals, tissue acquisition, and approaches to failure of eradication. Finally, we can anticipate continued refinement of quality metrics to ensure optimal strategies for diagnosis, surveillance, and therapy of BE.
Table 2.
Barrett’s esophagus recommendations
| Statement | Quality of evidence | Strength of recommendation |
|---|---|---|
|
| ||
| Diagnosis | ||
| 1. We suggest that a diagnosis of BE require the finding of intestinal metaplasia in the tubular esophagus | Very low | Conditional |
| 2. We suggest that columnar mucosa of
at least 1 cm in length be necessary for a diagnosis of
BE a. Patients with a normal-appearing Z line should not undergo routine endoscopic biopsies b. In the absence of any visible lesions, patients with a Z line demonstrating <1 cm of proximal displacement from the top of the gastric folds should not undergo routine endoscopic biopsies |
Low | Conditional |
| 3. We suggest at least 8 endoscopic biopsies be obtained in screening examinations with endoscopic findings consistent with possible BE, with the Seattle protocol followed for segments of longer than 4 cm | Low | Conditional |
| 4. We recommend that dysplasia of any grade detected on biopsies of BE be confirmed by a second pathologist with expertise in GI pathology | Low | Strong |
| Screening | ||
| 5. We suggest a single screening endoscopy in patients with chronic GERD symptoms and 3 or more additional risk factors for BE, including male sex, age >50 yr, White race, tobacco smoking, obesity, and family history of BE or EAC in a first-degree relative | Very low | Conditional |
| 6. We suggest that a swallowable, nonendoscopic capsule device combined with a biomarker is an acceptable alternative to endoscopy for screening for BE | Very low | Conditional |
| 7. We suggest against repeat screening in patients who have undergone an initial negative screening examination by endoscopy | Low | Conditional |
| Surveillance | ||
| 8. We recommend both white light endoscopy and chromoendoscopy in patients undergoing endoscopic surveillance of BE | Moderate | Strong |
| 9. We recommend a structured biopsy protocol be applied to minimize detection bias in patients undergoing endoscopic surveillance of BE | Low | Strong |
| 10. We suggest endoscopic surveillance be performed in patients with BE at intervals dictated by the degree of dysplasia noted on previous biopsies | Very low | Conditional |
| 11. We recommend that length of BE segment be considered when assigning surveillance intervals with longer intervals reserved for those with BE segments of <3 cm | Moderate | Strong |
| 12. We could not make a recommendation on the use of wide-area transepithelial sampling with computer-assisted 3-dimensional analysis in patients undergoing endoscopic surveillance of BE | ||
| 13. We could not make a recommendation on the use of predictive tools (p53 staining and TissueCypher) in addition to standard histopathology in patients undergoing endoscopic surveillance of BE | ||
| Treatment (Medical) | ||
| 14. We suggest at least once-a-day PPI therapy in patients with BE without allergy or other contraindication to PPI use | Very low | Conditional |
| 15. We could not make a recommendation on combination therapy with aspirin and proton pump inhibitor in patients with BE | ||
| 16. We suggest against the use of antireflux surgery as an antineoplastic measure in patients with BE | Low | Conditional |
| Treatment (Endoscopic) | ||
| 17. We recommend endoscopic eradication therapy in patients with BE with HGD or IMC | Moderate | Strong |
| 18. We suggest endoscopic eradication therapy in patients with BE with LGD to reduce the risk of progression to HGD or EAC vs close endoscopic surveillance | Moderate | Conditional |
| 19. We suggest initial endoscopic resection of any visible lesions before the application of ablative therapy in patients with BE undergoing endoscopic eradication therapy. | Very low | Conditional |
| 20. We suggest that patients with BE undergoing endoscopic eradication therapy be treated in high-volume centers | Very low | Conditional |
| 21. We recommend an endoscopic surveillance program in patients with BE who have completed successful endoscopic eradication therapy | Moderate | Strong |
BE, Barrett’s esophagus; EAC, esophageal adenocarcinoma; GEJ, gastroesophageal junction; GERD, gastroesophageal reflux disease; GI, gastrointestinal; HGD, high-grade dysplasia; IMC, intramucosal cancer; LGD, low-grade dysplasia; PPI, proton pump inhibitor.
ACKNOWLEDGEMENTS
This guideline was produced in collaboration with the Practice Parameters Committee of the American College of Gastroenterology. The Committee gives special thanks to Amir Soumekh, MD who served as the guideline monitor for this document.
Footnotes
Potential competing interests: N.J.S. receives research funding from Medtronic, Steris, Pentax, CDx Diagnostics, Interpace Diagnostics, and Lucid Medical; he is a consultant for Cernostics, Phathom Pharmaceuticals, Exact Sciences, Aqua Medical, and Cook Medical. G.W.F. receives research funding from Lucid and Interpace Diagnostics; he is a consultant for Lucid, CDx, Cernostics, Interpace, Exact Sciences, and Phathom Pharmaceuticals. P.G.I. receives research funding from Exact Sciences, Pentax Medical, and Cernostics; he is a consultant to Medtronic, Ambu, Pentax, and Symple Surgical. R.F.S. receives research funding from Sanofi and Phathom Pharmaceuticals; she is a consultant for Cernostics, Phathom Pharmaceuticals, Interpace Diagnostics, Ironwood Pharmaceuticals, ISOThrive, CDx Diagnostics, and AstraZeneca. R.H.Y. receives research funding from Ironwood Pharmaceuticals; she is a consultant for Medtronic, Phathom Pharmaceuticals, Eli Lilly, Ironwood Pharmaceuticals, Diversatek, StatLinkMD, and RJS Mediagnostix. B.G.S. is a consultant for Takeda Pharmaceuticals and Watermark Research Partners. S.W. receives research funding from Lucid Medical, Ambu, and CDx Medical; he is a consultant for Medtronic, Boston Scientific, Interpace Diagnostics, Exact Sciences, and Cernostics.
Guarantor of the article: Nicholas J. Shaheen, MD, MPH.
REFERENCES
- 1.Barrett NR. Chronic peptic ulcer of the oesophagus and “oesophagitis.” Br J Surg 1950;38:175–82. [DOI] [PubMed] [Google Scholar]
- 2.Winters C Jr, Spurling TJ, Chobanian SJ, et al. Barrett’s esophagus. A prevalent, occult complication of gastroesophageal reflux disease. Gastroenterology 1987;92:118–24. [PubMed] [Google Scholar]
- 3.Qumseya BJ, Bukannan A, Gendy S, et al. Systematic review and meta-analysis of prevalence and risk factors for Barrett’s esophagus. Gastrointest Endosc 2019;90:707–17. [DOI] [PubMed] [Google Scholar]
- 4.Thrift AP, El-Serag HB, Kanwal F. Global burden and epidemiology of Barrett oesophagus and oesophageal cancer. Nat Rev Gastroenterol Hepatol 2021;14(2):122–32. [DOI] [PubMed] [Google Scholar]
- 5.Katz PO, Dunbar KB, Schnoll-Sussman FH, et al. ACG clinical guideline: Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol 2022;117(1):27–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011;64:383–94. [DOI] [PubMed] [Google Scholar]
- 7.Balshem H, Helfand M, Schunemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 2011;64:401–6. [DOI] [PubMed] [Google Scholar]
- 8.Andrews JC, Schünemann HJ, Oxman AD, et al. GRADE guidelines: 15. Going from evidence to recommendation-determinants of a recommendation’s direction and strength. J Clin Epidemiol 2013;66:726–35. [DOI] [PubMed] [Google Scholar]
- 9.Que J, Garman KS, Souza RF, et al. Pathogenesis and cells of origin of Barrett’s esophagus. Gastroenterology 2019;157:349–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paull A, Trier JS, Dalton MD, et al. The histologic spectrum of Barrett’s esophagus. N Engl J Med 1976;295:476–80. [DOI] [PubMed] [Google Scholar]
- 11.Clermont M, Falk GW. Clinical guidelines update on the diagnosis and management of Barrett’s esophagus. Dig Dis Sci 2018;63:2122–8. [DOI] [PubMed] [Google Scholar]
- 12.Bhat S, Coleman HG, Yousef F, et al. Risk of malignant progression in Barrett’s esophagus patients: Results from a large population-based study. J Natl Cancer Inst 2011;103:1049–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bandla S, Peters JH, Ruff D, et al. Comparison of cancer-associated genetic abnormalities in columnar-lined esophagus tissues with and without goblet cells. Ann Surg 2014;260:72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kelty CJ, Gough MD, Van Wyk Q, et al. Barrett’s oesophagus: Intestinal metaplasia is not essential for cancer risk. Scand J Gastroenterol 2007;42: 1271–4. [DOI] [PubMed] [Google Scholar]
- 15.Gatenby PAC, Ramus JR, Caygill CPJ, et al. Relevance of the detection of intestinal metaplasia in non-dysplastic columnar-lined oesophagus. Scand J Gastroenterol 2008;43:524–30. [DOI] [PubMed] [Google Scholar]
- 16.Liu W, Hahn H, Odze RD, et al. Metaplastic esophageal columnar epithelium without goblet cells shows DNA content abnormalities similar to goblet cell-containing epithelium. Am J Gastroenterol 2009; 104:816–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shaheen NJ, Dulai GS, Ascher B, et al. Effect of a new diagnosis of Barrett’s esophagus on insurance status. Am J Gastroenterol 2005;100: 577–80. [DOI] [PubMed] [Google Scholar]
- 18.Crockett SD, Lippmann QK, Dellon ES, et al. Health-related quality of life in patients with Barrett’s esophagus: A systematic review. Clin Gastroenterol Hepatol 2009;7:613–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Westerhoff M, Hovan L, Lee C, et al. Effects of dropping the requirement for goblet cells from the diagnosis of Barrett’s esophagus. Clin Gastroenterol Hepatol 2012;10:1232–6. [DOI] [PubMed] [Google Scholar]
- 20.Harrison R, Perry I, Haddadin W, et al. Detection of intestinal metaplasia in Barrett’s esophagus: An observational comparator study suggests the need for a minimum of eight biopsies. Am J Gastroenterol 2007;102:1154–61. [DOI] [PubMed] [Google Scholar]
- 21.Sawas T, Killcoyne S, Iyer PG, et al. Identification of prognostic phenotypes of esophageal adenocarcinoma in 2 independent cohorts. Gastroenterology 2018;155:1720–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kerkhof M, Steyerberg EW, Kusters JG, et al. Predicting presence of intestinal metaplasia and dysplasia in columnar-lined esophagus: A multivariate analysis. Endoscopy 2007;39:772–8. [DOI] [PubMed] [Google Scholar]
- 23.Sharma P, Dent J, Armstrong D, et al. The development and validation of an endoscopic grading system for Barrett’s esophagus: The Prague C & M criteria. Gastroenterology 2006;131:1392–9. [DOI] [PubMed] [Google Scholar]
- 24.Vahabzadeh B, Seetharam AB, Cook MB, et al. Validation of the Prague C & M criteria for the endoscopic grading of Barrett’s esophagus by gastroenterology trainees: A multicenter study. Gastrointest Endosc 2012;75:236–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alvarez Herrero L, Curvers WL, van Vilsteren FG, et al. Validation of the Prague C&M classification of Barrett’s esophagus in clinical practice. Endoscopy 2013;45:876–82. [DOI] [PubMed] [Google Scholar]
- 26.Jung KW, Talley NJ, Romero Y, et al. Epidemiology and natural history of intestinal metaplasia of the gastroesophageal junction and Barrett’s esophagus: A population-based study. Am J Gastroenterol 2011;106: 1447–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thota PN, Vennalaganti P, Vennelaganti S, et al. Low risk of high-grade dysplasia or esophageal adenocarcinoma among patients with Barrett’s esophagus less than 1 cm (irregular Z line) within 5 years of index endoscopy. Gastroenterology 2017;152:987–92. [DOI] [PubMed] [Google Scholar]
- 28.Wani S, Williams JL, Falk GW, et al. An analysis of the GIQuIC Nationwide Quality Registry reveals unnecessary surveillance endoscopies in patients with normal and irregular Z-lines. Am J Gastroenterol 2020;115:1869–78. [DOI] [PubMed] [Google Scholar]
- 29.Khandwalla HE, Graham DY, Kramer JR, et al. Corrigendum: Barrett’s esophagus suspected at endoscopy but no specialized intestinal metaplasia on biopsy, what’s next? Am J Gastroenterol 2014;109:1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones TF, Sharma P, Daaboul B, et al. Yield of intestinal metaplasia in patients with suspected short-segment Barrett’s esophagus (SSBE) on repeat endoscopy. Dig Dis Sci 2002;47:2108–11. [DOI] [PubMed] [Google Scholar]
- 31.Montgomery E, Bronner MP, Goldblum JR, et al. Reproducibility of the diagnosis of dysplasia in barrett esophagus: A reaffirmation. Hum Pathol 2001;32:368–78. [DOI] [PubMed] [Google Scholar]
- 32.van der Wel MJ, Coleman HG, Bergman J, et al. Histopathologist features predictive of diagnostic concordance at expert level among a large international sample of pathologists diagnosing Barrett’s dysplasia using digital pathology. Gut 2020;69:811–22. [DOI] [PubMed] [Google Scholar]
- 33.Duits LC, Phoa KN, Curvers WL, et al. Barrett’s oesophagus patients with low-grade dysplasia can be accurately risk-stratified after histological review by an expert pathology panel. Gut 2015;64:700–6. [DOI] [PubMed] [Google Scholar]
- 34.Duits LC, van der Wel MJ, Cotton CC, et al. Patients with Barrett’s esophagus and confirmed persistent low-grade dysplasia are at increased risk for progression to neoplasia. Gastroenterology 2017;152:993–1001. [DOI] [PubMed] [Google Scholar]
- 35.Krishnamoorthi R, Lewis JT, Krishna M, et al. Predictors of progression in Barrett’s esophagus with low-grade dysplasia: Results from a multicenter prospective BE registry. Am J Gastroenterol 2017;112: 867–73. [DOI] [PubMed] [Google Scholar]
- 36.Wani S, Rubenstein JH, Vieth M, et al. Diagnosis and management of low-grade dysplasia in Barrett’s esophagus: Expert review from the Clinical Practice Updates Committee of the American Gastroenterological Association. Gastroenterology 2016;151:822–35. [DOI] [PubMed] [Google Scholar]
- 37.Codipilly DC, Sawas T, Dhaliwal L, et al. Epidemiology and outcomes of young-onset esophageal adenocarcinoma: An analysis from a population-based database.Cancer Epidemiol Biomarkers Prev 2021;30: 142–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablation in Barrett’s esophagus with dysplasia. N Engl J Med 2009;360:2277–88. [DOI] [PubMed] [Google Scholar]
- 39.Phoa KN, van Vilsteren FG, Weusten BL, et al. Radiofrequency ablation vs endoscopic surveillance for patients with Barrett esophagus and low-grade dysplasia: A randomized clinical trial. JAMA 2014;311:1209–17. [DOI] [PubMed] [Google Scholar]
- 40.Codipilly DC, Chandar AK, Singh S, et al. The effect of endoscopic surveillance in patients with Barrett’s esophagus: A systematic review and meta-analysis. Gastroenterology 2018;154:2068–86.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tan MC, Mansour N, White DL, et al. Systematic review with meta-analysis: Prevalence of prior and concurrent Barrett’s oesophagus in oesophageal adenocarcinoma patients. Aliment Pharmacol Ther 2020; 52:20–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ferrer-Torres D, Nancarrow DJ, Steinberg H, et al. Constitutively higher level of GSTT2 in esophageal tissues from African Americans protects cells against DNA damage. Gastroenterology 2019;156:1404–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krishnamoorthi R, Singh S, Ragunathan K, et al. Factors associated with progression of Barrett’s esophagus: A systematic review and meta-analysis. Clin Gastroenterol Hepatol 2018;16:1046–55. [DOI] [PubMed] [Google Scholar]
- 44.Rubenstein JH, Scheiman JM, Sadeghi S, et al. Esophageal adenocarcinoma incidence in individuals with gastroesophageal reflux: Synthesis and estimates from population studies. Am J Gastroenterol 2011;106:254–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moriarty JP, Shah ND, Rubenstein JH, et al. Costs associated with Barrett’s esophagus screening in the community: An economic analysis of a prospective randomized controlled trial of sedated versus hospital unsedated versus mobile community unsedated endoscopy. Gastrointest Endosc 2018;87:88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kamboj AK, Katzka DA, Iyer PG. Endoscopic screening for Barrett’s esophagus and esophageal adenocarcinoma: Rationale, candidates, and challenges. Gastrointest Endosc Clin N Am 2021;31:27–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kramer JR, Shakhatreh MH, Naik AD, et al. Use and yield of endoscopy in patients with uncomplicated gastroesophageal reflux disorder. JAMA Intern Med 2014;174:462–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barbiere JM, Lyratzopoulos G. Cost-effectiveness of endoscopic screening followed by surveillance for Barrett’s esophagus: A review. Gastroenterology 2009;137:1869–76. [DOI] [PubMed] [Google Scholar]
- 49.Gerson LB, Groeneveld PW, Triadafilopoulos G. Cost-effectiveness model of endoscopic screening and surveillance in patients with gastroesophageal reflux disease. Clin Gastroenterol Hepatol 2004;2: 868–79. [DOI] [PubMed] [Google Scholar]
- 50.Inadomi JM, Sampliner R, Lagergren J, et al. Screening and surveillance for Barrett esophagus in high-risk groups: A cost-utility analysis. Ann Intern Med 2003;138:176–86. [DOI] [PubMed] [Google Scholar]
- 51.Sami SS, Dunagan KT, Johnson ML, et al. A randomized comparative effectiveness trial of novel endoscopic techniques and approaches for Barrett’s esophagus screening in the community. Am J Gastroenterol 2015;110:148–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eloubeidi MA, Provenzale D. Does this patient have Barrett’s esophagus? The utility of predicting Barrett’s esophagus at the index endoscopy. Am J Gastroenterol 1999;94:937–43. [DOI] [PubMed] [Google Scholar]
- 53.Rubenstein JH, Morgenstern H, Appelman H, et al. Prediction of Barrett’s esophagus among men. Am J Gastroenterol 2013;108:353–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thrift AP, Garcia JM, El-Serag HB. A multibiomarker risk score helps predict risk for Barrett’s esophagus. Clin Gastroenterol Hepatol 2014;12: 1267–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rubenstein JH, McConnell D, Waljee AK, et al. Validation and comparison of tools for selecting individuals to screen for Barrett’s esophagus and early neoplasia. Gastroenterology 2020;158:2082–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shariff MK, Bird-Lieberman EL, O’Donovan M, et al. Randomized crossover study comparing efficacy of transnasal endoscopy with that of standard endoscopy to detect Barrett’s esophagus. Gastrointest Endosc 2012;75:954–61. [DOI] [PubMed] [Google Scholar]
- 57.Chang JY, Talley NJ, Locke GR III, et al. Population screening for barrett esophagus: A prospective randomized pilot study. Mayo Clin Proc 2011; 86:1174–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peery AF, Hoppo T, Garman KS, et al. Feasibility, safety, acceptability, and yield of office-based, screening transnasal esophagoscopy. Gastrointest Endosc 2012;75:945–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Honing J, Kievit W, Bookelaar J, et al. Endosheath ultrathin transnasal endoscopy is a cost-effective method for screening for Barrett’s esophagus in patients with GERD symptoms. Gastrointest Endosc 2019; 89:712–22. [DOI] [PubMed] [Google Scholar]
- 60.Alashkar B, Faulx AL, Hepner A, et al. Development of a program to train physician extenders to perform transnasal esophagoscopy and screen for Barrett’s esophagus. Clin Gastroenterol Hepatol 2014;12: 785–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vaughan TL, Fitzgerald RC. Precision prevention of oesophageal adenocarcinoma. Nat Rev Gastroenterol Hepatol 2015;12:243–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rex DK, Cummings OW, Shaw M, et al. Screening for Barrett’s esophagus in colonoscopy patients with and without heartburn. Gastroenterology 2003;125:1670–7. [DOI] [PubMed] [Google Scholar]
- 63.Ward EM, Wolfsen HC, Achem SR, et al. Barrett’s esophagus is common in older men and women undergoing screening colonoscopy regardless of reflux symptoms. Am J Gastroenterol 2006;101:12–7. [DOI] [PubMed] [Google Scholar]
- 64.Gerson LB, Shetler K, Triadafilopoulos G. Prevalence of Barrett’s esophagus in asymptomatic individuals. Gastroenterology 2002;123: 461–7. [DOI] [PubMed] [Google Scholar]
- 65.Nguyen TH, Thrift AP, Rugge M, et al. Prevalence of Barrett’s esophagus and performance of societal screening guidelines in an unreferred primary care population of U.S. veterans. Gastrointest Endosc 2021;93: 409–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Januszewicz W, Tan WK, Lehovsky K, et al. Safety and acceptability of esophageal cytosponge cell collection device in a pooled analysis of data from individual patients. Clin Gastroenterol Hepatol 2019;17:647–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fitzgerald RC, di Pietro M, O’Donovan M, et al. Cytosponge-trefoil factor 3 versus usual care to identify Barrett’s oesophagus in a primary care setting: A multicentre, pragmatic, randomised controlled trial. Lancet 2020;396:333–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Benaglia T, Sharples LD, Fitzgerald RC, et al. Health benefits and cost effectiveness of endoscopic and nonendoscopic cytosponge screening for Barrett’s esophagus. Gastroenterology 2013;144:62–73. [DOI] [PubMed] [Google Scholar]
- 69.Peters Y, Schrauwen RWM, Tan AC, et al. Detection of Barrett’s oesophagus through exhaled breath using an electronic nose device. Gut 2020;69:1169–72. [DOI] [PubMed] [Google Scholar]
- 70.Rodriguez S, Mattek N, Lieberman D, et al. Barrett’s esophagus on repeat endoscopy: Should we look more than once? Am J Gastroenterol 2008; 103:1892–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Suna N, Parlak E, Kuzu UB, et al. The prevalence of Barrett esophagus diagnosed in the second endoscopy: A retrospective, observational study at a tertiary center. Medicine (Baltimore) 2016;95:e3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Malfertheiner P, Nocon M, Vieth M, et al. Evolution of gastroesophageal reflux disease over 5 years under routine medical care—The ProGERD study. Aliment Pharmacol Ther 2012;35:154–64. [DOI] [PubMed] [Google Scholar]
- 73.Hanna S, Rastogi A, Weston AP, et al. Detection of Barrett’s esophagus after endoscopic healing of erosive esophagitis. Am J Gastroenterol 2006;101:1416–20. [DOI] [PubMed] [Google Scholar]
- 74.Modiano N, Gerson LB. Risk factors for the detection of Barrett’s esophagus in patients with erosive esophagitis. Gastrointest Endosc 2009;69:1014–20. [DOI] [PubMed] [Google Scholar]
- 75.Gupta N, Gaddam S, Wani SB, et al. Longer inspection time is associated with increased detection of high-grade dysplasia and esophageal adenocarcinoma in Barrett’s esophagus. Gastrointest Endosc 2012;76:531–8. [DOI] [PubMed] [Google Scholar]
- 76.Coletta M, Sami SS, Nachiappan A, et al. Acetic acid chromoendoscopy for the diagnosis of early neoplasia and specialized intestinal metaplasia in Barrett’s esophagus: A meta-analysis. Gastrointest Endosc 2016;83:57–67. [DOI] [PubMed] [Google Scholar]
- 77.Sharma P, Hawes RH, Bansal A, et al. Standard endoscopy with random biopsies versus narrow band imaging targeted biopsies in Barrett’s oesophagus: A prospective, international, randomised controlled trial. Gut 2013;62:15–21. [DOI] [PubMed] [Google Scholar]
- 78.Sharma P, Bergman JJ, Goda K, et al. Development and validation of a classification system to identify high-grade dysplasia and esophageal adenocarcinoma in Barrett’s esophagus using narrow-band imaging. Gastroenterology 2016;150:591–8. [DOI] [PubMed] [Google Scholar]
- 79.Committee AT, Thosani N, Abu Dayyeh BK, et al. ASGE Technology Committee systematic review and meta-analysis assessing the ASGE Preservation and Incorporation of Valuable Endoscopic Innovations thresholds for adopting real-time imaging-assisted endoscopic targeted biopsy during endoscopic surveillance of Barrett’s esophagus. Gastrointest Endosc 2016;83:684–98. [DOI] [PubMed] [Google Scholar]
- 80.de Groof AJ, Fockens KN, Struyvenberg MR, et al. Blue-light imaging and linked-color imaging improve visualization of Barrett’s neoplasia by nonexpert endoscopists. Gastrointest Endosc 2020;91:1050–7. [DOI] [PubMed] [Google Scholar]
- 81.Everson MA, Lovat LB, Graham DG, et al. Virtual chromoendoscopy by using optical enhancement improves the detection of Barrett’s esophagus-associated neoplasia. Gastrointest Endosc 2019;89:247–56. [DOI] [PubMed] [Google Scholar]
- 82.Xiong YQ, Ma SJ, Zhou JH, et al. A meta-analysis of confocal laser endomicroscopy for the detection of neoplasia in patients with Barrett’s esophagus. J Gastroenterol Hepatol 2016;31:1102–10. [DOI] [PubMed] [Google Scholar]
- 83.Sharma P, Meining AR, Coron E, et al. Real-time increased detection of neoplastic tissue in Barrett’s esophagus with probe-based confocal laser endomicroscopy: Final results of an international multicenter, prospective, randomized, controlled trial. Gastrointest Endosc 2011;74: 465–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Houston T, Sharma P. Volumetric laser endomicroscopy in Barrett’s esophagus: Ready for primetime. Transl Gastroenterol Hepatol 2020;5: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Struyvenberg M, Kahn A, Fleischer D, et al. Expert assessment on volumetric laser endomicroscopy full scans in Barrett’s esophagus patients with or without high grade dysplasia or early cancer. Endoscopy 2021;53:218–25. [DOI] [PubMed] [Google Scholar]
- 86.de Groof AJ, Struyvenberg MR, van der Putten J, et al. Deep-learning system detects neoplasia in patients with Barrett’s esophagus with higher accuracy than endoscopists in a multistep training and validation study with benchmarking. Gastroenterology 2020;158:915–29. [DOI] [PubMed] [Google Scholar]
- 87.de Groof AJ, Struyvenberg MR, Fockens KN, et al. Deep learning algorithm detection of Barrett’s neoplasia with high accuracy during live endoscopic procedures: A pilot study (with video). Gastrointest Endosc 2020;91:1242–50. [DOI] [PubMed] [Google Scholar]
- 88.Levine DS, Haggitt RC, Blount PL, et al. An endoscopic biopsy protocol can differentiate high-grade dysplasia from early adenocarcinoma in Barrett’s esophagus [see comments]. Gastroenterology 1993;105:40–50. [DOI] [PubMed] [Google Scholar]
- 89.Fitzgerald RC, Saeed IT, Khoo D, et al. Rigorous surveillance protocol increases detection of curable cancers associated with Barrett’s esophagus. Dig Dis Sci 2001;46:1892–8. [DOI] [PubMed] [Google Scholar]
- 90.Abela JE, Going JJ, Mackenzie JF, et al. Systematic four-quadrant biopsy detects Barrett’s dysplasia in more patients than nonsystematic biopsy. Am J Gastroenterol 2008;103:850–5. [DOI] [PubMed] [Google Scholar]
- 91.Nachiappan A, Ragunath K, Card T, et al. Diagnosing dysplasia in Barrett’s oesophagus still requires Seattle protocol biopsy in the era of modern video endoscopy: Results from a tertiary centre Barrett’s dysplasia database. Scand J Gastroenterol 2020;55:9–13. [DOI] [PubMed] [Google Scholar]
- 92.Abrams JA, Kapel RC, Lindberg GM, et al. Adherence to biopsy guidelines for Barrett’s esophagus surveillance in the community setting in the United States. Clin Gastroenterol Hepatol 2009;7:736–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wani S, Williams JL, Komanduri S, et al. Endoscopists systematically undersample patients with long-segment Barrett’s esophagus: An analysis of biopsy sampling practices from a quality improvement registry. Gastrointest Endosc 2019;90:732–41. [DOI] [PubMed] [Google Scholar]
- 94.Roumans CAM, van der Bogt RD, Steyerberg EW, et al. Adherence to recommendations of Barrett’s esophagus surveillance guidelines: A systematic review and meta-analysis. Endoscopy 2020;52:17–28. [DOI] [PubMed] [Google Scholar]
- 95.Old O, Moayyedi P, Love S, et al. Barrett’s Oesophagus Surveillance versus endoscopy at need Study (BOSS): Protocol and analysis plan for a multicentre randomized controlled trial. J Med Screen 2015;22:158–64. [DOI] [PubMed] [Google Scholar]
- 96.Corley DA, Mehtani K, Quesenberry C, et al. Impact of endoscopic surveillance on mortality from Barrett’s esophagus-associated esophageal adenocarcinoma. Gastroenterology 2013;145:312–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Krishnamoorthi R, Mohan BP, Jayaraj M, et al. Risk of progression in Barrett’s esophagus indefinite for dysplasia: A systematic review and meta-analysis. Gastrointest Endosc 2020;91:3–10. [DOI] [PubMed] [Google Scholar]
- 98.Frei NF, Stachler MD, Bergman J. Risk stratification in Barrett’s esophagus patients with diagnoses of indefinite for dysplasia: The definite silver bullet has not (yet) been found. Gastrointest Endosc 2020;91:11–3. [DOI] [PubMed] [Google Scholar]
- 99.Henn AJ, Song KY, Gravely AA, et al. Persistent indefinite for dysplasia in Barrett’s esophagus is a risk factor for dysplastic progression to low-grade dysplasia. Dis Esophagus 2020;33:doaa015. [DOI] [PubMed] [Google Scholar]
- 100.Whiteman DC, Appleyard M, Bahin FF, et al. Australian clinical practice guidelines for the diagnosis and management of Barrett’s esophagus and early esophageal adenocarcinoma. J Gastroenterol Hepatol 2015;30:804–20. [DOI] [PubMed] [Google Scholar]
- 101.Weusten B, Bisschops R, Coron E, et al. Endoscopic management of Barrett’s esophagus: European Society of Gastrointestinal Endoscopy (ESGE) position statement. Endoscopy 2017;49:191–8. [DOI] [PubMed] [Google Scholar]
- 102.Fitzgerald RC, di Pietro M, Ragunath K, et al. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett’s oesophagus. Gut 2014;63:7–42. [DOI] [PubMed] [Google Scholar]
- 103.Hamade N, Vennelaganti S, Parasa S, et al. Lower annual rate of progression of short-segment vs long-segment Barrett’s esophagus to esophageal adenocarcinoma. Clin Gastroenterol Hepatol 2019;17:864–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chandrasekar VT, Hamade N, Desai M, et al. Significantly lower annual rates of neoplastic progression in short- compared to long-segment nondysplastic Barrett’s esophagus: A systematic review and meta-analysis. Endoscopy 2019;51:665–72. [DOI] [PubMed] [Google Scholar]
- 105.Parasa S, Vennalaganti S, Gaddam S, et al. Development and validation of a model to determine risk of progression of Barrett’s esophagus to neoplasia. Gastroenterology 2018;154:1282–9.e2. [DOI] [PubMed] [Google Scholar]
- 106.Kunzmann AT, Thrift AP, Johnston BT, et al. External validation of a model to determine risk of progression of Barrett’s oesophagus to neoplasia. Aliment Pharmacol Ther 2019;49:1274–81. [DOI] [PubMed] [Google Scholar]
- 107.Qumseya B, Sultan S, Bain P, et al. ASGE guideline on screening and surveillance of Barrett’s esophagus. Gastrointest Endosc 2019;90: 335–59. [DOI] [PubMed] [Google Scholar]
- 108.Suresh Kumar VC, Harne P, Patthipati VS, et al. Wide-area transepithelial sampling in adjunct to forceps biopsy increases the absolute detection rates of Barrett’s oesophagus and oesophageal dysplasia: A meta-analysis and systematic review. BMJ Open Gastroenterol 2020;7:e000494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Singer ME, Smith MS. Wide area transepithelial sampling with computer-assisted analysis (WATS(3D)) is cost-effective in Barrett’s esophagus screening. Dig Dis Sci 2021;66(5):1572–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shaheen NJ, Falk GW, Iyer PG, et al. ACG clinical guideline: Diagnosis and management of Barrett’s esophagus. Am J Gastroenterol 2016;111: 30–50; quiz 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Snyder P, Dunbar KB, Cipher DJ, et al. Aberrant p53 immostaining in Barrett’s esophagus predicts neoplastic progression: Systematic review and meta-analyses. Dig Dis Sci 2019;64(5):1089–97. [DOI] [PubMed] [Google Scholar]
- 112.Altaf K, Xiong JJ, la Iglesia D, et al. Meta-analysis of biomarkers predicting risk of malignant progression in Barrett’s oesophagus. Br J Surg 2017;104:493–502. [DOI] [PubMed] [Google Scholar]
- 113.Janmaat VT, van Olphen SH, Biermann KE, et al. Use of immunohistochemical biomarkers as independent predictor of neoplastic progression in Barrett’s oesophagus surveillance: A systematic review and meta-analysis. PLoS One 2017;12:e0186305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Prichard JW, Davison JM, Campbell BB, et al. TissueCypher: A systems biology approach to anatomic pathology. J Pathol Inform 2015;6:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Critchley-Thorne RJ, Duits LC, Prichard JW, et al. A tissue systems pathology assay for high-risk Barrett’s esophagus. Cancer Epidemiol Biomarkers Prev 2016;25:958–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Critchley-Thorne RJ, Davison JM, Prichard JW, et al. A tissue systems pathology test detects abnormalities associated with prevalent high-grade dysplasia and esophageal cancer in Barrett’s esophagus. Cancer Epidemiol Biomarkers Prev 2017;26:240–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Davison JM, Goldblum J, Grewal US, et al. Independent blinded validation of a tissue systems pathology test to predict progression in patients with Barrett’s esophagus. Am J Gastroenterol 2020;115:843–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Frei NF, Konte K, Bossart EA, et al. Independent validation of a tissue systems pathology assay to predict future progression in nondysplastic Barrett’s esophagus: A spatial-temporal analysis. Clin Transl Gastroenterol 2020;11:e00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Frei NF, Khoshiwal AM, Konte K, et al. Tissue systems pathology test objectively risk stratifies Barrett’s esophagus patients with low-grade dysplasia. Am J Gastroenterol 2021;116(4):675–82. [DOI] [PubMed] [Google Scholar]
- 120.Hao J, Critchley-Thorne R, Diehl DL, et al. A cost-effectiveness analysis of an adenocarcinoma risk prediction multi-biomarker assay for patients with Barrett’s esophagus. Clinicoecon Outcomes Res 2019;11:623–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Prasad GA, Bansal A, Sharma P, et al. Predictors of progression in Barrett’s esophagus: Current knowledge and future directions. Am J Gastroenterol 2010;105:1490–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rubenstein JH, Vakil N, Inadomi JM. The cost-effectiveness of biomarkers for predicting the development of oesophageal adenocarcinoma. Aliment Pharmacol Ther 2005;22:135–46. [DOI] [PubMed] [Google Scholar]
- 123.Srivastava A, Appelman H, Goldsmith JD, et al. The use of ancillary stains in the diagnosis of barrett esophagus and Barrett esophagus associated dysplasia: Recommendations from the Rodger C. Haggitt Gastrointestinal Pathology Society. Am J Surg Pathol 2017;41:e8–21. [DOI] [PubMed] [Google Scholar]
- 124.Rubenstein JH, Noureldin M, Tavakkoli A, et al. Utilization of surveillance endoscopy for Barrett’s esophagus in Medicare enrollees. Gastroenterology 2020;158:773–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Omidvari AH, Hazelton WD, Lauren BN, et al. The optimal age to stop endoscopic surveillance of Barrett’s esophagus patients based on sex and comorbidity: A comparative cost-effectiveness analysis. Gastroenterology 2021;161(2):487–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Park WG, Shaheen NJ, Cohen J, et al. Quality indicators for EGD. Am J Gastroenterol 2015;110:60–71. [DOI] [PubMed] [Google Scholar]
- 127.Sharma P, Katzka DA, Gupta N, et al. Quality indicators for the management of Barrett’s esophagus, dysplasia, and esophageal adenocarcinoma: International consensus recommendations from the American Gastroenterological Association Symposium. Gastroenterology 2015;149:1599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sharma P, Parasa S, Shaheen N. Developing quality metrics for upper endoscopy. Gastroenterology 2020;158:9–13. [DOI] [PubMed] [Google Scholar]
- 129.Wani S, Williams JL, Komanduri S, et al. Over-utilization of repeat upper endoscopy in patients with non-dysplastic Barrett’s esophagus: A quality registry study. Am J Gastroenterol 2019;114:1256–64. [DOI] [PubMed] [Google Scholar]
- 130.Wani S, Williams JL, Komanduri S, et al. Time trends in adherence to surveillance intervals and biopsy protocol among patients with Barrett’s esophagus. Gastroenterology 2020;158:770–2. [DOI] [PubMed] [Google Scholar]
- 131.Wani S, Gyawali CP, Katzka DA. AGA clinical practice update on reducing rates of post-endoscopy esophageal adenocarcinoma: Commentary. Gastroenterology 2020;159:1533–7. [DOI] [PubMed] [Google Scholar]
- 132.Vajravelu RK, Kolb JM, Thanawala SU, et al. Characterization of prevalent, post-endoscopy, and incident esophageal cancer in the United States: A large retrospective cohort study. Clin Gastroenterol Hepatol 2021. (doi: 10.1016/j.cgh.2021.02.005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sawas T, Majzoub AM, Haddad J, et al. Magnitude and time-trend analysis of post-endoscopy esophageal adenocarcinoma: A systematic review and meta-analysis. Clin Gastroenterol Hepatol 2022;20:e31–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lagergren J, Bergstrom R, Lindgren A, et al. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med 1999;340:825–31. [DOI] [PubMed] [Google Scholar]
- 135.Cook MB, Corley DA, Murray LJ, et al. Gastroesophageal reflux in relation to adenocarcinomas of the esophagus: A pooled analysis from the Barrett’s and Esophageal Adenocarcinoma Consortium (BEACON). PLoS One 2014;9:e103508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Moayyedi P, Armstrong D, Hunt RH, et al. The gain in quality-adjusted life months by switching to esomeprazole in those with continued reflux symptoms in primary care: EncomPASS—A cluster-randomized trial. Am J Gastroenterol 2010;105:2341–6. [DOI] [PubMed] [Google Scholar]
- 137.Falk GW, Buttar NS, Foster NR, et al. A combination of esomeprazole and aspirin reduces tissue concentrations of prostaglandin E(2) in patients with Barrett’s esophagus. Gastroenterology 2012;143:917–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ouatu-Lascar R, Fitzgerald RC, Triadafilopoulos G. Differentiation and proliferation in Barrett’s esophagus and the effects of acid suppression. Gastroenterology 1999;117:327–35. [DOI] [PubMed] [Google Scholar]
- 139.Singh S, Garg SK, Singh PP, et al. Acid-suppressive medications and risk of oesophageal adenocarcinoma in patients with Barrett’s oesophagus: A systematic review and meta-analysis. Gut 2014;63:1229–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hu Q, Sun TT, Hong J, et al. Proton pump inhibitors do not reduce the risk of esophageal adenocarcinoma in patients with Barrett’s esophagus: A systematic review and meta-analysis. PLoS One 2017;12:e0169691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Spechler SJ, Sharma P, Traxler B, et al. Gastric and esophageal pH in patients with Barrett’s esophagus treated with three esomeprazole dosages: A randomized, double-blind, crossover trial. Am J Gastroenterol 2006;101:1964–71. [DOI] [PubMed] [Google Scholar]
- 142.Katzka DA, Castell DO. Successful elimination of reflux symptoms does not insure adequate control of acid reflux in patients with Barrett’s esophagus. Am J Gastroenterol 1994;89:989–91. [PubMed] [Google Scholar]
- 143.Wani S, Sampliner RE, Weston AP, et al. Lack of predictors of normalization of oesophageal acid exposure in Barrett’s oesophagus. Aliment Pharmacol Ther 2005;22:627–33. [DOI] [PubMed] [Google Scholar]
- 144.Jankowski JAZ, de Caestecker J, Love SB, et al. Esomeprazole and aspirin in Barrett’s oesophagus (AspECT): A randomised factorial trial. Lancet 2018;392:400–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Vaezi MF, Yang YX, Howden CW. Complications of proton pump inhibitor therapy. Gastroenterology 2017;153:35–48. [DOI] [PubMed] [Google Scholar]
- 146.Moayyedi P, Eikelboom JW, Bosch J, et al. Safety of proton pump inhibitors based on a large, multi-year, randomized trial of patients receiving rivaroxaban or aspirin. Gastroenterology 2019;157: 682–91. [DOI] [PubMed] [Google Scholar]
- 147.Attwood SE, Ell C, Galmiche JP, et al. Long-term safety of proton pump inhibitor therapy assessed under controlled, randomised clinical trial conditions: Data from the SOPRAN and LOTUS studies. Aliment Pharmacol Ther 2015;41:1162–74. [DOI] [PubMed] [Google Scholar]
- 148.Buas MF, He Q, Johnson LG, et al. Germline variation in inflammation-related pathways and risk of Barrett’s oesophagus and oesophageal adenocarcinoma. Gut 2017;66:1739–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hashemi Goradel N, Najafi M, Salehi E, et al. Cyclooxygenase-2 in cancer: A review. J Cell Physiol 2019;234:5683–99. [DOI] [PubMed] [Google Scholar]
- 150.Corley DA, Kerlikowske K, Verma R, et al. Protective association of aspirin/NSAIDs and esophageal cancer: A systematic review and meta-analysis. Gastroenterology 2003;124:47–56. [DOI] [PubMed] [Google Scholar]
- 151.Gammon MD, Terry MB, Arber N, et al. Nonsteroidal anti-inflammatory drug use associated with reduced incidence of adenocarcinomas of the esophagus and gastric cardia that overexpress cyclin D1: A population-based study. Cancer Epidemiol Biomarkers Prev 2004;13:34–9. [DOI] [PubMed] [Google Scholar]
- 152.Rothwell PM, Fowkes FG, Belch JF, et al. Effect of daily aspirin on long-term risk of death due to cancer: Analysis of individual patient data from randomised trials. Lancet 2011;377:31–41. [DOI] [PubMed] [Google Scholar]
- 153.Liao LM, Vaughan TL, Corley DA, et al. Nonsteroidal anti-inflammatory drug use reduces risk of adenocarcinomas of the esophagus and esophagogastric junction in a pooled analysis. Gastroenterology 2012; 142:442–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Corey KE, Schmitz SM, Shaheen NJ. Does a surgical anti-reflux procedure decrease the incidence of esophageal adenocarcinoma in Barrett’s esophagus? A meta-analysis. Am J Gastroenterol 2003;98(11): 2390–4. [DOI] [PubMed] [Google Scholar]
- 155.Maret-Ouda J, Konings P, Lagergren J, et al. Antireflux surgery and risk of esophageal adenocarcinoma: A systematic review and meta-analysis. Ann Surg 2016;263:251–7. [DOI] [PubMed] [Google Scholar]
- 156.Chang EY, Morris CD, Seltman AK, et al. The effect of antireflux surgery on esophageal carcinogenesis in patients with Barrett esophagus: A systematic review. Ann Surg 2007;246:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Parrilla P, Martinez de Haro LF, Ortiz A, et al. Long-term results of a randomized prospective study comparing medical and surgical treatment of Barrett’s esophagus. Ann Surg 2003;237:291–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Standards of Practice Committee, Wani S, Qumseya B, et al. Endoscopic eradication therapy for patients with Barrett’s esophagus-associated dysplasia and intramucosal cancer. Gastrointest Endosc 2018;87:907–31. [DOI] [PubMed] [Google Scholar]
- 159.Dunbar KB, Spechler SJ. The risk of lymph-node metastases in patients with high-grade dysplasia or intramucosal carcinoma in Barrett’s esophagus: A systematic review. Am J Gastroenterol 2012;107:850–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Qumseya BJ, Wani S, Desai M, et al. Adverse events after radiofrequency ablation in patients with Barrett’s esophagus: A systematic review and meta-analysis. Clin Gastroenterol Hepatol 2016;14:1086–95. [DOI] [PubMed] [Google Scholar]
- 161.Wu J, Pan YM, Wang TT, et al. Endotherapy versus surgery for early neoplasia in Barrett’s esophagus: A meta-analysis. Gastrointest Endosc 2014;79:233–41. [DOI] [PubMed] [Google Scholar]
- 162.Han S, Kolb JM, Hosokawa P, et al. The volume-outcome effect calls for centralization of care in esophageal adenocarcinoma: Results from a large national cancer registry. Am J Gastroenterol 2020;116(4):811–5. [DOI] [PubMed] [Google Scholar]
- 163.Wani S, Muthusamy VR, Shaheen NJ, et al. Development of quality indicators for endoscopic eradication therapies in Barrett’s esophagus: The TREAT-BE (Treatment with Resection and Endoscopic Ablation Techniques for Barrett’s Esophagus) Consortium. Gastrointest Endosc 2017;86(1):1–17. [DOI] [PubMed] [Google Scholar]
- 164.Wani S, Sharma P. Challenges with endoscopic therapy for Barrett’s esophagus. Gastroenterol Clin North Am 2015;44:355–72. [DOI] [PubMed] [Google Scholar]
- 165.Wolf WA, Pasricha S, Cotton C, et al. Incidence of esophageal adenocarcinoma and causes of mortality after radiofrequency ablation of Barrett’s esophagus. Gastroenterology 2015;149:1752–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Pech O, Behrens A, May A, et al. Long-term results and risk factor analysis for recurrence after curative endoscopic therapy in 349 patients with high-grade intraepithelial neoplasia and mucosal adenocarcinoma in Barrett’s oesophagus. Gut 2008;57:1200–6. [DOI] [PubMed] [Google Scholar]
- 167.Orman ES, Li N, Shaheen NJ. Efficacy and durability of radiofrequency ablation for Barrett’s esophagus: Systematic review and meta-analysis. Clin Gastroenterol Hepatol 2013;11:1245–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Phoa KN, Pouw RE, van Vilsteren FG, et al. Remission of Barrett’s esophagus with early neoplasia 5 years after radiofrequency ablation with endoscopic resection: A Netherlands cohort study. Gastroenterology 2013;145:96–104. [DOI] [PubMed] [Google Scholar]
- 169.Pasricha S, Bulsiewicz WJ, Hathorn KE, et al. Durability and predictors of successful radiofrequency ablation for Barrett’s esophagus. Clin Gastroenterol Hepatol 2014;12:1840–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Komanduri S, Kahrilas PJ, Krishnan K, et al. Recurrence of Barrett’s esophagus is rare following endoscopic eradication therapy coupled with effective reflux control. Am J Gastroenterol 2017;112:556–66. [DOI] [PubMed] [Google Scholar]
- 171.Fujii-Lau LL, Cinnor B, Shaheen N, et al. Recurrence of intestinal metaplasia and early neoplasia after endoscopic eradication therapy for Barrett’s esophagus: A systematic review and meta-analysis. Endosc Int Open 2017;5:E430–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Krishnamoorthi R, Singh S, Ragunathan K, et al. Risk of recurrence of Barrett’s esophagus after successful endoscopic therapy. Gastrointest Endosc 2016;83:1090–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wani S, Han S, Kushnir V, et al. Recurrence is rare following complete eradication of intestinal metaplasia in patients with Barrett’s esophagus and peaks at 18 months. Clin Gastroenterol Hepatol 2020;18:2609–17. [DOI] [PubMed] [Google Scholar]
- 174.Sami SS, Ravindran A, Kahn A, et al. Timeline and location of recurrence following successful ablation in Barrett’s oesophagus: An international multicentre study. Gut 2019;68:1379–85. [DOI] [PubMed] [Google Scholar]
- 175.Badreddine RJ, Prasad GA, Lewis JT, et al. Depth of submucosal invasion does not predict lymph node metastasis and survival of patients with esophageal carcinoma. Clin Gastroenterol Hepatol 2010;8:248–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Manner H, Pech O, Heldmann Y, et al. Efficacy, safety, and long-term results of endoscopic treatment for early stage adenocarcinoma of the esophagus with low-risk sm1 invasion. Clin Gastroenterol Hepatol2013; 11:630–5. [DOI] [PubMed] [Google Scholar]
- 177.Scholvinck D, Kunzli H, Meijer S, et al. Management of patients with T1b esophageal adenocarcinoma: A retrospective cohort study on patient management and risk of metastatic disease. Surg Endosc 2016;30: 4102–13. [DOI] [PubMed] [Google Scholar]
- 178.Otaki F, Ma GK, Krigel A, et al. Outcomes of patients with submucosal (T1b) esophageal adenocarcinoma: A multicenter cohort study. Gastrointest Endosc 2020;92:31–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Manner H, Pech O, Heldmann Y, et al. The frequency of lymph node metastasis in early-stage adenocarcinoma of the esophagus with incipient submucosal invasion (pT1b sm1) depending on histological risk patterns. Surg Endosc 2015;29:1888–96. [DOI] [PubMed] [Google Scholar]
- 180.Singh S, Manickam P, Amin AV, et al. Incidence of esophageal adenocarcinoma in Barrett’s esophagus with low-grade dysplasia: A systematic review and meta-analysis. Gastrointest Endosc 2014;79: 897–909.e4; quiz 983.e1, 983.e3. [DOI] [PubMed] [Google Scholar]
- 181.Rubenstein JH, Waljee AK, Dwamena B, et al. Yield of higher-grade neoplasia in Barrett’s esophagus with low-grade dysplasia is double in the first year following diagnosis. Clin Gastroenterol Hepatol 2018;16: 1529–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.O’Byrne LM, Witherspoon J, Verhage RJJ, et al. Barrett’s Registry Collaboration of academic centers in Ireland reveals high progression rate of low-grade dysplasia and low risk from nondysplastic Barrett’s esophagus: Report of the RIBBON network. Dis Esophagus 2020;33: doaa009. [DOI] [PubMed] [Google Scholar]
- 183.Kestens C, Offerhaus GJ, van Baal JW, et al. Patients with Barrett’s esophagus and persistent low-grade dysplasia have an increased risk for high-grade dysplasia and cancer. Clin Gastroenterol Hepatol 2016;14: 956–62. [DOI] [PubMed] [Google Scholar]
- 184.Shaheen NJ, Overholt BF, Sampliner RE, et al. Durability of radiofrequency ablation in Barrett’s esophagus with dysplasia. Gastroenterology 2011;141:460–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Omidvari AH, Ali A, Hazelton WD, et al. Optimizing management of patients with Barrett’s esophagus and low-grade or No dysplasia based on comparative modeling. Clin Gastroenterol Hepatol 2020;18:1961–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hur C, Choi SE, Rubenstein JH, et al. The cost effectiveness of radiofrequency ablation for Barrett’s esophagus. Gastroenterology 2012; 143:567–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Vennalaganti P, Kanakadandi V, Goldblum JR, et al. Discordance among pathologists in the United States and Europe in diagnosis of low-grade dysplasia for patients with Barrett’s esophagus. Gastroenterology 2017;152:564–70. [DOI] [PubMed] [Google Scholar]
- 188.American Gastroenterological A, Spechler SJ, Sharma P, et al. American Gastroenterological Association medical position statement on the management of Barrett’s esophagus. Gastroenterology 2011;140: 1084–91. [DOI] [PubMed] [Google Scholar]
- 189.Skacel M, Petras RE, Gramlich TL, et al. The diagnosis of low-grade dysplasia in Barrett’s esophagus and its implications for disease progression. Am J Gastroenterol 2000;95:3383–7. [DOI] [PubMed] [Google Scholar]
- 190.Downs-Kelly E, Mendelin JE, Bennett AE, et al. Poor interobserver agreement in the distinction of high-grade dysplasia and adenocarcinoma in pretreatment Barrett’s esophagus biopsies. Am J Gastroenterol 2008;103:2333–40. [DOI] [PubMed] [Google Scholar]
- 191.Wani S, Falk GW, Post J, et al. Risk factors for progression of low-grade dysplasia in patients with Barrett’s esophagus. Gastroenterology 2011; 141:1179–86, 1186. [DOI] [PubMed] [Google Scholar]
- 192.Mino-Kenudson M, Hull MJ, Brown I, et al. EMR for Barrett’s esophagus-related superficial neoplasms offers better diagnostic reproducibility than mucosal biopsy. Gastrointest Endosc 2007;66: 660–6. [DOI] [PubMed] [Google Scholar]
- 193.Podboy A, Kolahi KS, Friedland S, et al. Endoscopic submucosal dissection is associated with less pathologic uncertainty than endoscopic mucosal resection in diagnosing and staging Barrett’s-related neoplasia. Dig Endosc 2020;32:346–54. [DOI] [PubMed] [Google Scholar]
- 194.Wani S, Mathur SC, Curvers WL, et al. Greater interobserver agreement by endoscopic mucosal resection than biopsy samples in Barrett’s dysplasia. Clin Gastroenterol Hepatol 2010;8:783–8. [DOI] [PubMed] [Google Scholar]
- 195.Wani S, Abrams J, Edmundowicz SA, et al. Endoscopic mucosal resection results in change of histologic diagnosis in Barrett’s esophagus patients with visible and flat neoplasia: A multicenter cohort study. Dig Dis Sci 2013;58:1703–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Peters FP, Brakenhoff KP, Curvers WL, et al. Histologic evaluation of resection specimens obtained at 293 endoscopic resections in Barrett’s esophagus. Gastrointest Endosc 2008;67:604–9. [DOI] [PubMed] [Google Scholar]
- 197.Choi J, Chung H, Lee A, et al. Role of endoscopic ultrasound in selecting superficial esophageal cancers for endoscopic resection. Ann Thorac Surg 2021;111(5):1689–95. [DOI] [PubMed] [Google Scholar]
- 198.Bartel MJ, Wallace TM, Gomez-Esquivel RD, et al. Role of EUS in patients with suspected Barrett’s esophagus with high-grade dysplasia or early esophageal adenocarcinoma: Impact on endoscopic therapy. Gastrointest Endosc 2017;86:292–8. [DOI] [PubMed] [Google Scholar]
- 199.Prasad GA, Buttar NS, Wongkeesong LM, et al. Significance of neoplastic involvement of margins obtained by endoscopic mucosal resection in Barrett’s esophagus. Am J Gastroenterol 2007;102:2380–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Leggett CL, Lewis JT, Wu TT, et al. Clinical and histologic determinants of mortality for patients with Barrett’s esophagus-related T1 esophageal adenocarcinoma. Clin Gastroenterol Hepatol 2015;13:658–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Pech O, May A, Manner H, et al. Long-term efficacy and safety of endoscopic resection for patients with mucosal adenocarcinoma of the esophagus. Gastroenterology 2014;146:652–60. [DOI] [PubMed] [Google Scholar]
- 202.Prasad GA, Wu TT, Wigle DA, et al. Endoscopic and surgical treatment of mucosal (T1a) esophageal adenocarcinoma in Barrett’s esophagus. Gastroenterology 2009;137(3):815–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.The Paris endoscopic classification of superficial neoplastic lesions: Esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc 2003;58:S3–43. [DOI] [PubMed] [Google Scholar]
- 204.Endoscopic Classification Review Group. Update on the Paris classification of superficial neoplastic lesions in the digestive tract. Endoscopy 2005;37:570–8. [DOI] [PubMed] [Google Scholar]
- 205.Yang D, Zou F, Xiong S, et al. Endoscopic submucosal dissection for early Barrett’s neoplasia: A meta-analysis. Gastrointest Endosc 2018;87: 1383–93. [DOI] [PubMed] [Google Scholar]
- 206.Codipilly DC, Dhaliwal L, Oberoi M, et al. Comparative outcomes of cap assisted endoscopic resection and endoscopic submucosal dissection in dysplastic Barrett’s esophagus. Clin Gastroenterol Hepatol 2022;20: 65–73.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Manner H, Rabenstein T, Pech O, et al. Ablation of residual Barrett’s epithelium after endoscopic resection: A randomized long-term follow-up study of argon plasma coagulation vs. surveillance (APE study). Endoscopy 2014;46:6–12. [DOI] [PubMed] [Google Scholar]
- 208.van Vilsteren FG, Pouw RE, Seewald S, et al. Stepwise radical endoscopic resection versus radiofrequency ablation for Barrett’s oesophagus with high-grade dysplasia or early cancer: A multicentre randomised trial. Gut 2011;60:765–73. [DOI] [PubMed] [Google Scholar]
- 209.Ghorbani S, Tsai FC, Greenwald BD, et al. Safety and efficacy of endoscopic spray cryotherapy for Barrett’s dysplasia: Results of the National Cryospray Registry. Dis Esophagus 2016;29:241–7. [DOI] [PubMed] [Google Scholar]
- 210.Shaheen NJ, Greenwald BD, Peery AF, et al. Safety and efficacy of endoscopic spray cryotherapy for Barrett’s esophagus with high-grade dysplasia. Gastrointest Endosc 2010;71:680–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Visrodia K, Zakko L, Singh S, et al. Cryotherapy for persistent Barrett’s esophagus after radiofrequency ablation: A systematic review and meta-analysis. Gastrointest Endosc 2018;87:1396–404.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Canto MI, Trindade AJ, Abrams J, et al. Multifocal cryoballoon ablation for eradication of Barrett’s esophagus-related neoplasia: A prospective multicenter clinical trial. Am J Gastroenterol 2020;115:1879–90. [DOI] [PubMed] [Google Scholar]
- 213.Agarwal S, Alshelleh M, Scott J, et al. Comparative outcomes of radiofrequency ablation and cryoballoon ablation in dysplastic Barrett’s esophagus: A propensity score-matched cohort study. Gastrointest Endosc 2022;95(3):422–431. [DOI] [PubMed] [Google Scholar]
- 214.Pasricha S, Cotton C, Hathorn KE, et al. Effects of the learning curve on efficacy of radiofrequency ablation for Barrett’s esophagus. Gastroenterology 2015;149:890–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Fudman DI, Lightdale CJ, Poneros JM, et al. Positive correlation between endoscopist radiofrequency ablation volume and response rates in Barrett’s esophagus. Gastrointest Endosc 2014;80:71–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Tan MC, Kanthasamy KA, Yeh AG, et al. Factors associated with recurrence of Barrett’s esophagus after radiofrequency ablation. Clin Gastroenterol Hepatol 2019;17:65–72. [DOI] [PubMed] [Google Scholar]
- 217.Markar SR, Mackenzie H, Ni M, et al. The influence of procedural volume and proficiency gain on mortality from upper GI endoscopic mucosal resection. Gut 2018;67:79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Kolb JM, Davis C, Wani S. Durability of endoscopic eradication therapy for Barrett’s esophagus-related neoplasia: A call for centralized care. Gastroenterology 2022;162:343–5. [DOI] [PubMed] [Google Scholar]
- 219.Kahn A, Shaheen NJ, Iyer PG. Approach to the post-ablation Barrett’s esophagus patient. Am J Gastroenterol 2020;115:823–31. [DOI] [PubMed] [Google Scholar]
- 220.Sawas T, Iyer PG, Alsawas M, et al. Higher rate of Barrett’s detection in the first year after successful endoscopic therapy: Meta-analysis. Am J Gastroenterol 2018;113:959–71. [DOI] [PubMed] [Google Scholar]
- 221.Omar M, Thaker AM, Wani S, et al. Anatomic location of Barrett’s esophagus recurrence after endoscopic eradication therapy: Development of a simplified surveillance biopsy strategy. Gastrointest Endosc 2019;90:395–403. [DOI] [PubMed] [Google Scholar]
- 222.Cotton CC, Wolf WA, Pasricha S, et al. Recurrent intestinal metaplasia after radiofrequency ablation for Barrett’s esophagus: Endoscopic findings and anatomic location. Gastrointest Endosc 2015;81:1362–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Cotton CC, Haidry R, Thrift AP, et al. Development of evidence-based surveillance intervals after radiofrequency ablation of Barrett’s esophagus. Gastroenterology 2018;155:316–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Solfisburg QS, Sami SS, Gabre J, et al. Clinical significance of recurrent gastroesophageal junction intestinal metaplasia after endoscopic eradication of Barrett’s esophagus. Gastrointest Endosc 2021;93(6): 1250–7. [DOI] [PubMed] [Google Scholar]
- 225.Wani S, Muthusamy VR, Shaheen NJ, et al. Development of quality indicators for endoscopic eradication therapies in Barrett’s esophagus: The TREAT-BE (Treatment With Resection and Endoscopic Ablation Techniques for Barrett’s Esophagus) Consortium. Am J Gastroenterol 2017;112:1032–48. [DOI] [PubMed] [Google Scholar]
- 226.Ross-Innes CS, Debiram-Beecham I, O’Donovan M, et al. Evaluation of a minimally invasive cell sampling device coupled with assessment of trefoil factor 3 expression for diagnosing Barrett’s esophagus: A multicenter case-control study. PLoS Med 2015;12:e1001780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Shaheen NJ, Komanduri S, Muthusamy VR, et al. Acceptability and adequacy of a non-endoscopic cell collection device for diagnosis of Barrett’s esophagus: Lessons learned. Dig Dis Sci 2022;67:177–86. [DOI] [PubMed] [Google Scholar]
- 228.Iyer PG, Taylor WR, Johnson ML, et al. Accurate nonendoscopic detection of Barrett’s esophagus by methylated DNA markers: A multisite case control study. Am J Gastroenterol 2020;115:1201–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Iyer PG, Taylor WR, Slettedahl SW, et al. Validation of a methylated DNA marker panel for the nonendoscopic detection of Barrett’s esophagus in a multi-site case-control study. Gastrointest Endosc 2021; 94(3):498–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Moinova HR, LaFramboise T, Lutterbaugh JD, et al. Identifying DNA methylation biomarkers for non-endoscopic detection of Barrett’s esophagus. Sci Transl Med 2018;10:eaao5848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Wang Z, Kambhampati S, Cheng Y, et al. Methylation biomarker panel performance in EsophaCap cytology samples for diagnosing Barrett’s esophagus: A prospective validation study. Clin Cancer Res 2019;25: 2127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Kolb JM, Wani S. Endoscopic eradication therapy for Barrett’s oesophagus: State of the art. Curr Opin Gastroenterol 2020;36:351–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


