This systematic review found evidence to suggest a possible association between kissing and oropharyngeal gonorrhea after controlling for other sexual practices, including fellatio and rimming.
Background
Tongue kissing is a poorly studied risk factor for sexually transmitted infections (STIs). We undertook the first systematic review to assess whether kissing is a risk factor for gonorrhea or chlamydia of the oropharynx.
Methods
Online databases (MEDLINE, EMBASE, CINAHL, Web of Science, Cochrane) and reference lists were searched until September 30, 2022. The eligibility criteria for studies included: any peer-reviewed study design in the English language; gonorrhea or chlamydia diagnosed by nucleic acid amplification test, or an infection self-reported by a patient; tongue kissing or its equivalent measured as an exposure. Studies were appraised using a quality scoring tool and qualitatively synthesized.
Results
Of 8248 studies screened, 6 were eligible for review. All were conducted among men who have sex with men in Australia, including 3 prospective cohort studies, 2 cross-sectional studies, and 1 age-matched case-control study. In summary, all 5 studies examining gonorrhea found an unadjusted association between kissing and oropharyngeal gonorrhea. Two cross-sectional studies found that tongue kissing was an independent risk factor for oropharyngeal gonorrhea after adjusting for other confounders, such as participant demographic characteristics and other sexual practices. In contrast, a single eligible prospective cohort study found no association between kissing and oropharyngeal chlamydia.
Conclusions
This systematic review summarized the existing evidence that suggests that tongue kissing may be a risk factor for oropharyngeal gonorrhea but not chlamydia. Reinforcing the message that oropharyngeal gonorrhea could be transmitted through kissing may inform the development of novel approaches to prevent and treat gonorrhea.
The World Health Organization estimates that at least 374 million curable sexually transmitted infections (STIs) were occurred globally in 2020.1 Of these, gonorrhea and chlamydia are of significant interest: chlamydia is the most commonly notifiable STI and gonorrhea is an urgent public health threat due to rising antimicrobial resistance.2 Since the 2010s, there has been a substantial increase in gonorrhea among gay, bisexual and other men who have sex with men (MSM).3 To better understand this concerning trend, investigators have started to reexamine its mode of transmission.4
Recent studies have demonstrated that Neisseria gonorrhoeae and Chlamydia trachomatis can be detected in the saliva of people diagnosed with oropharyngeal gonorrhea and chlamydia, respectively.5,6 These findings raise the possibility that tongue kissing (also referred to as simply kissing in this review), during which saliva is exchanged, may contribute to oropharyngeal transmission. The US Centers for Disease Control and Prevention has acknowledged that the role of kissing in STI transmission has not been well studied in their 2021 STI treatment guidelines.7 Although the morbidity of oropharyngeal gonorrhea and chlamydia is not considered to be high, there are implications for disease burden if transmission occurs from oropharyngeal infection to other body sites including pelvic inflammatory disease and epididymitis,8,9 and also the development of gonococcal antimicrobial resistance. These oropharyngeal infections are often asymptomatic, which means that individuals may not be aware of the infection, and this could result in onward transmission prior to detection. Hence, we conducted a systematic review to assess whether tongue kissing is a risk factor for oropharyngeal gonorrhea or chlamydia.
METHODS
This systematic review was undertaken following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for reporting systematic reviews.10 The protocol for this review was registered (CRD42018092368) with the International Prospective Register of Systematic Reviews (PROSPERO).
Search Strategy
We searched literature published in MEDLINE and EMBASE, through Ovid, CINAHL, Web of Science and the Cochrane Central Register of Controlled Trials until September 30, 2022. Date restrictions were not applied to our searches; however, studies were limited to human studies and those written in English.
The following search terms (with wild cards “*”) were included: (gonorrhoea OR gonorrhea OR N. gonorrhoeae OR chlamydia*) AND (kiss* OR oropharyn* OR pharyn* OR oral OR throat).
The initial title and abstract screenings were performed by 3 independent reviewers (F.C., A.K., J.T.). The full texts of screened articles were subsequently assessed against eligibility criteria (F.C., A.K.). All discrepancies were resolved in consultation with a fourth reviewer (E.P.F.C.).
Inclusion and Exclusion Criteria
We included all study designs (eg, cohort, case-control, cross-sectional) that provided primary data and were published in a peer-reviewed journal in the English language. We included studies if they provided data about kissing and its association with oropharyngeal gonorrhea or chlamydia diagnoses (either self-reported or a laboratory-confirmed test).
We excluded any studies that were case reports, editorials, reviews, mathematical modeling studies, or other articles, without primary data. Studies on mouth-to-mouth resuscitation were ineligible.
Data Extraction and Synthesis
Data were extracted by 2 independent reviewers (F.C. and A.K.). For each study, we extracted the following: first author, study period, study location, study design, sample size, age, gender, sexuality, the definition of kissing, the number of cases of incident or prevalent oropharyngeal gonorrhea or chlamydia diagnosed or reported and the diagnostic method used.
Outcome and Exposure Definition
The outcome of interest was prevalent or incident oropharyngeal gonorrhea or chlamydia of the oropharynx. The exposure of interest was tongue kissing (including open mouth kissing, deep kissing, and dry or wet kissing).
Analysis
Pooling of results using meta-analysis was not possible due to the differences in study design, measurements of kissing, and participant characteristics. Therefore, a narrative review was undertaken to describe the key findings for each study and these were stratified by STI and study design.
Quality Assessment
All studies were independently reviewed for quality by 2 reviewers (F.C. and R.C.) using a modified form of the Quality Index (QI),11 and any discrepancies were resolved through a consensus discussion. A higher QI score suggests greater methodological quality. Individual questions are either given a score of 0 (no/indeterminate) or 1 (yes). The QI scale includes 4 subcategories that are tallied for a final score: reporting (0–7), external validity (0–3), internal validity (0–5), and power (0–1). The assessment items are outlined in Supplementary Table 1 (http://links.lww.com/OLQ/A907).
RESULTS
We identified 12,239 records based on our search strategy. After removing duplicates, 8248 articles were identified from the databases, and a further article was identified through searching reference lists. After title and abstract screening, 41 articles underwent full-text review, and of these, 6 were eligible for inclusion (Fig. 1). The eligible studies included 3 prospective cohort studies,12–14 1 age-matched case-control study15 and 2 cross-sectional studies.16,17 All studies were conducted among gay, bisexual and other MSM in Australia. Participants were recruited from sexual health clinics (n = 4) and convenience samples (n = 2). The sample sizes ranged from 100 to 3677. Five studies reported on gonorrhea12,14–17 and 1 study reported on chlamydia.13 The characteristics and findings of all included studies are summarized in Table 1, and further detail is provided in Supplementary Table 2 (http://links.lww.com/OLQ/A908).
Figure 1.
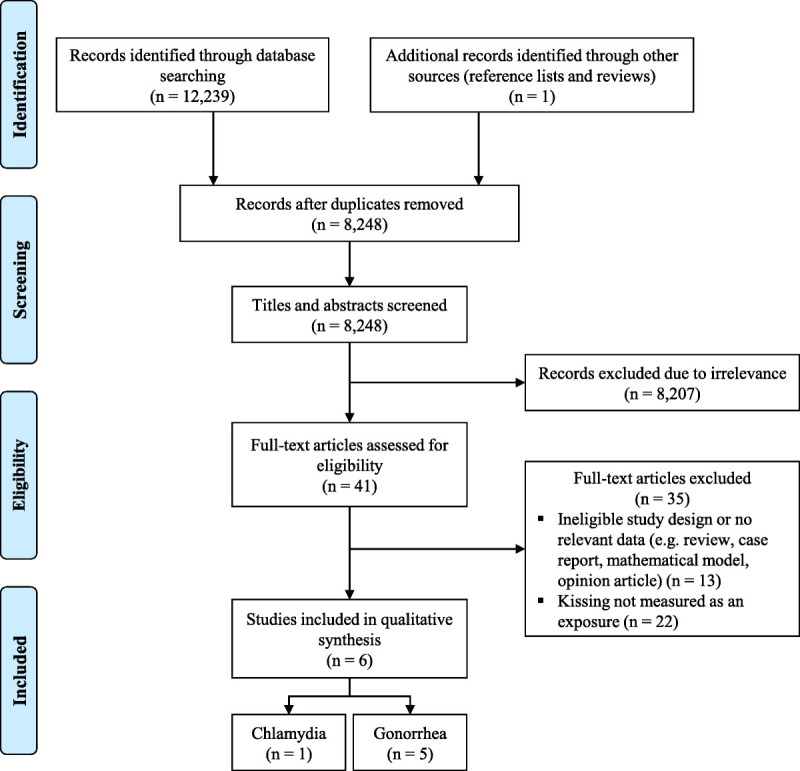
Study selection flowchart.
TABLE 1.
Summary of Included Study Findings
| Study (Year), Location, Type | Study Type | Study Population | n | Kissing Exposure | Measure of Association | Crude Result (95% CI) | Adjusted Result (95% CI) | Comments |
|---|---|---|---|---|---|---|---|---|
| Chlamydia | ||||||||
| Templeton (2008), Australia13 | Prospective cohort | HIV- MSM (non-random convenience sample) | 1427 | Dry kissing and wet kissing casual partners in last 6 mo (never, occasionally, or often) | HR | Dry kissing: Never: 1.0 (ref); Occasionally: 1.81 (0.52 to 6.29); Often: 0.92 (0.24 to 3.47) Wet kissing: Never: 1.0 (ref); Occasionally: 1.51 (0.34 to 6.77); Often: 0.85 (0.19 to 3.82) |
NR | Multivariate analysis/adjustment was not performed for kissing |
| Gonorrhea | ||||||||
| Chow (2021), Australia12 | Prospective cohort | HIV +/− MSM (full study population details Supplementary Table 2, http://links.lww.com/OLQ/A908) | 100 | No. tongue kissing partners over a 12-week study period | IRR | No. tongue kissing partners: 1.08 (1.03 to 1.12)* |
NR | Of the incident oropharyngeal gonorrhea cases, 2 men reported kissing only without penile-oral sex or insertive rimming |
| Chow (2019), Australia16 | Cross-sectional | HIV +/− MSM from a sexual health clinic | 3677 | Kissing-only or kissing-with-sex partners in the last 3 mo (kissing-only is kissing without other sexual contact in last 3 mo) | OR | No. kissing only partners in last 3 mo: 0–1 partners: 1 (ref), 2–3 partners: 1.34 (0.91 to 1.97), ≥4 partners: 2.02 (1.49 to 2.74)* | No. kissing only partners in last 3 mo: 0–1 partners: (ref), 2–3 partners: 1.30 (0.87 to 1.95), ≥4 partners: 1.46 (1.04 to 2.06)* Ptrend = 0.029* |
Also measured number of sex-only and kissing-with-sex partners to separate kissing from sex as an exposure Multivariable regression adjusting for kissing-only, sex-only and kissing-with-sex partners |
| Cornelisse (2018), Australia15 | Case-control | Age-matched MSM from a sexual health clinic | Cases (n = 177) Controls (n = 354) |
No. tongue kissing partners in last 3 mo | OR | Median number of casual sexual partners kissed (CSP): Cases: 4 vs controls: 2.5* CSP tongue kissed: No: (ref); Yes: OR, 2.17 (1.31 to 3.59)* Median duration of kiss (min): Cases: 10 vs controls: 10 Days since last kiss: Cases: 10 vs controls: 7 Proportion kissing > 75% of CSP: ≤75%: (ref); >75%: 1.38 (0.94 to 2.03) |
Proportion kissing > 75% of CSP: ≤75%: (ref); >75%: 1.55 (0.96 to 2.49) |
Multivariable regression adjusted for receptive fellatio with/without ejaculation and insertive rimming |
| Templeton (2010), Australia14 | Prospective cohort | HIV- MSM (non-random convenience sample) | 1427 | Dry kissing and wet kissing casual partners in last 6 mo (never, occasionally, or often) | HR | Dry kissing: Never: 1.0 (ref); Occasionally: 1.26 (0.74–2.14); Often: 1.61 (0.95–2.73)* Wet kissing: Never: 1.0 (ref); Occasionally: 2.55 (1.10–5.91); Often: 2.98 (1.32–6.75)* |
Dry kissing: Never: 1.0 (ref); Occasionally: 1.04 (0.61 to 1.77); Often: 1.18 (0.68 to 2.05) Wet kissing: Never: 1.0 (ref); Occasionally: 1.77 (0.77 to 4.06); Often: 1.83 (0.80 to 4.17) |
Adjusted for repeated measures in same individual and other sexual practices (number of male sexual partners, receptive penile-oral sex with and without ejaculation, insertive oro-anal sex) |
| Tran (2022), Australia17 | Cross-sectional | HIV +/− MSM from a sexual health clinic | 2322 | No. tongue kissing partners in last 3 mo | OR | No. kissing only partners in last 3 mo: 0 partners: 1 (ref), 1–2 partners: 1.87 (0.55 to 6.41), 3–4 partners: 3.27 (0.97 to 10.97), ≥4 partners: 6.28 (1.96 to 20.11)* Ptrend < 0.0001* |
No. kissing only partners in last 3 mo: 0 partners: 1 (ref), 1–2 partners: 1.81 (0.48 to 6.88), 3–4 partners: 2.58 (0.68 to 9.81), ≥4 partners: 3.59 (0.95 to 13.55)* Ptrend = 0.014* |
Adjusted for number of fellatio and rimming partners |
*P < 0.05.
QIS, Quality Index Scale; NR, not reported; OR, odds ratio; HR, hazards ratio; IRR, incidence rate ratio; +, positive; −, negative.
Kissing and Oropharyngeal Gonorrhea (n = 5)
All 5 studies that assessed gonorrhea found that kissing was associated with oropharyngeal gonorrhea in the univariable analyses.12,14–17 The 2 cross-sectional studies also found that kissing was associated with oropharyngeal gonorrhea in the multivariable analyses after adjusting for other confounding factors, such as participant demographic characteristics and other sexual practices.16,17
Case-Control Studies
Cornelisse et al15 conducted a 1:2 age-matched case-control study of 531 MSM attending a sexual health clinic in Melbourne in 2015, which included 177 cases of oropharyngeal gonorrhea and 354 controls. The univariable regression analyses demonstrated that men with oropharyngeal gonorrhea were 2.17 (95% confidence interval [CI], 1.31 to 3.59) times more likely to have tongue kissed their casual partners in the last 3 months compared with men who did not have oropharyngeal gonorrhea. However, kissing was highly correlated with receptive oral-penile sex and the total number of casual sexual partners (r = 0.91, P < 0.001) and in the multivariable analysis it was not significantly associated with oropharyngeal gonorrhea.
Prospective Cohort Studies
Templeton et al14 conducted a prospective cohort study of 1427 MSM between 2001 and 2007 in Sydney, Australia that investigated the association between “wet” and “dry” kissing and oropharyngeal gonorrhea. This analysis was part of the Australian Health in Men (HIM) study and included a total of 193 oropharyngeal gonorrhea cases with an incidence rate of 4.45 (95% CI, 3.86 to 5.12) per 100 person-year. In the univariate analysis, the authors found that the odds of acquiring oropharyngeal gonorrhea increased with an increasing frequency of dry kissing (Ptrend = 0.044 and wet kissing (Ptrend = 0.008) with their casual partners in the last 6 months. However, this association was not significant after adjusting for insertive oro-anal sex (i.e. rimming) and receptive penile-oral sex in the multivariable analysis.
Chow et al12 conducted a 12-week prospective cohort study between August and October 2019 of 100 MSM taking HIV preexposure prophylaxis attending a sexual health clinic in Melbourne in Australia, which included 14 incident oropharyngeal gonorrhea cases with an incidence of 62.43 (95% CI, 36.97 to 105.41) per 100 person-years. Weekly saliva specimens were self-collected and sent to the clinic by postal service and clinician-collected oropharyngeal swabs were obtained during clinic visits at weeks 0 and 12. Both saliva specimens and oropharyngeal swabs were tested for N. gonorrhoeae by nucleic acid amplification test. Incident oropharyngeal gonorrhea was found to be associated with the number of kissing partners in the past week (incidence rate ratio (IRR): 1.08; 95% CI, 1.03 to 1.12). However, the high collinearity of sexual practices made it difficult to establish the individual contribution of exposures to either mouth or urethra independently.
Cross-Sectional Studies
Chow et al16 recruited 3677 MSM in a cross-sectional study from a sexual health clinic in Melbourne between 2016 and 2017. The prevalence of oropharyngeal gonorrhea was 6.2% (229/3677). The study collected data on the number of kissing-only partners (where kissing occurred without sex), sex-only partners (where sex occurred without kissing) and kissing-with-sex partners (where both sex and kissing occurred) in the last 3 months. The authors defined “sex” as having oral or anal sexual encounters. The study found that, after adjusting for potential confounding factors, MSM who had ≥4 kissing-only partners had 1.46-fold (95% CI, 1.04 to 2.06) higher odds of having oropharyngeal gonorrhea compared with those who had 0–1 kissing-only partners in the last 3 months. There was also a significant trend seen with increasing numbers of kissing partners in the last 3 months (Ptrend = 0.029). However, one of the limitations of this study was that the study did not include direct measures of other sexual activities with potential exposure to the oropharynx, such as fellatio or rimming.
Tran et al17 recruited 2322 MSM in a cross-sectional study from a sexual health clinic in Melbourne between 2018 and 2020. The prevalence of oropharyngeal gonorrhea was 5.2% (120/2322). This study examined the association between kissing and oropharyngeal gonorrhea, and also adjusted for other oral sexual practices that exposed the oropharynx, including performing fellatio and performing rimming. In the multivariable regression analysis, oropharyngeal gonorrhea was significantly associated with increasing numbers of kissing partners (Ptrend = 0.014). It is worth noting that this study was conducted between November 2018 and December 2020, which included a period during the COVID-19 pandemic lockdown restriction, which might have influenced some changes in sexual practices during this period as suggested elsewhere.18
Kissing and Oropharyngeal Chlamydia (n = 1)
We only identified 1 prospective cohort study examining the association between kissing and oropharyngeal chlamydia. In the study by Templeton et al,13 also collected from the HIM cohort among 1427 MSM, which included 25 oropharyngeal chlamydia infections with an incidence of 0.58 (95% CI, 0.39–0.85) per 100 person-years. However, there was no association between kissing (either wet or dry kissing) and oropharyngeal chlamydia in the univariate analysis.
Assessment of Quality
Overall, the mean QIS for the eligible studies was 11.3 (standard deviation, 1.6; range, 9–14) (Table 2), which indicates imperfect methodological quality on average. Several items were reported clearly in all studies, such as how the outcomes were measured. However, the external validity of studies was low due to selection bias and the internal validity was compromised by potential confounding and recall bias in the analyses. Sample size and power calculations were rarely provided.
TABLE 2.
Quality Index Scale Scores With Means and Standard Deviations
| Study | Reporting | External Validity | Internal Validity | Power | Total |
|---|---|---|---|---|---|
| Chow (2019)16 | 7 | 1 | 3 | 0 | 11 |
| Chow (2021)12 | 7 | 1 | 3 | 0 | 11 |
| Cornelisse (2018)15 | 6 | 1 | 4 | 1 | 12 |
| Templeton (2008)13 | 5 | 1 | 3 | 0 | 9 |
| Templeton (2010)14 | 7 | 1 | 3 | 0 | 11 |
| Tran (2022)17 | 7 | 1 | 5 | 1 | 14 |
| Max score possible | 7 | 3 | 5 | 1 | 16 |
| Mean | 6.5 | 1.0 | 3.5 | 0.3 | 11.3 |
| SD | 0.8 | 0.0 | 0.8 | 0.5 | 1.6 |
SD, standard deviation.
DISCUSSION
To the best of our knowledge, this is the first systematic review assessing the association between kissing and gonorrhea or chlamydia of the oropharynx. We identified 6 eligible studies of which 5 examined gonorrhea and 1 examined chlamydia. Of the 5 studies examining oropharyngeal gonorrhea, all found that kissing was associated with oropharyngeal gonorrhea among MSM in their unadjusted analyses, whereas 2 studies found kissing was an independent risk factor for oropharyngeal gonorrhea after adjusting for other confounders such as participant demographic characteristics and other sexual practices.16,17 In particular, Tran et al17 found an association after specifically adjusting for other oral exposures (ie, performing fellatio and performing rimming). A single study examined chlamydia and did not find an association between kissing and oropharyngeal chlamydia.13 The consistency of the finding of an association between kissing and oropharyngeal gonorrhea suggests that it is likely to be a real finding although the studies were exclusively from Australia and should be repeated elsewhere.
The uncertainty created by multiple sexual activities with high collinearity occurring during a single sexual encounter is a key issue that impedes confirming the role of kissing as an independent risk factor.19,20 Chow et al16 showed that tongue kissing in the absence of sex was an independent factor for oropharyngeal gonorrhea after adjusting for multiple confounding factors, such as the number of sex-only partners (sex without kissing) and kissing-with-sex partners. However, sex-only partners could participate in a range of sexual activities, including fellatio and rimming, which are also known risk factors for oropharyngeal gonorrhea.14,15,17 The additional data collected by Tran et al attempted to address this limitation by specifically asking about the number of fellatio and insertive rimming partners, and reached the same conclusion with its findings suggesting tongue kissing is an independent risk factor for oropharyngeal gonorrhea after adjusting for performing fellation and performing rimming. Interestingly, Tran et al found that performing fellatio is not a significant risk factor for oropharyngeal gonorrhea. Supporting this conclusion, a prospective cohort study in the United States by Barbee et al,9 which did not fulfill the inclusion criteria for this review, found that oropharyngeal gonorrhea is likely to occur in the absence of fellatio—15% of the incident gonorrhea infections were identified in men who denied any recent oral-penile contact. A possible explanation is that urethral gonorrhea is usually symptomatic, which would make it less likely to be transmitted through performing fellatio.21,22 Taken together, accumulating evidence supports oropharyngeal gonorrhea being transmitted through kissing. The 2021 Centers for Disease Control and Prevention STI Treatment Guidelines stated kissing has not been well studied in gonorrhea and chlamydia transmission7; however, 3 important studies are published after this guideline9,12,17 and therefore these studies should be considered in the next update.
In this review, all included studies were conducted among MSM. We did not limit the study populations for this review, but we were unable to identify studies on non-MSM populations. Past studies have shown that oropharyngeal STIs are also found in other at-risk populations such as female sex workers and heterosexual men and women,23–25 and these populations also frequently report kissing.26,27 The lack of studies in non-MSM populations may also be due to the lack of universal screening of extragenital STIs in these populations. A Melbourne-based study of a sexual network, which included 4 females, 2 men and 1 nonbinary, non–gender-conforming participant who attended a music festival, found that 6 of 7 individuals within the sexual network had oropharyngeal gonorrhea but none had urogenital gonorrhea.28
There are several limitations in our systematic review. First, the specific measurements of kissing used and the study designs employed were not consistent, which prevented using a meta-analysis approach to calculate pooled summary estimates of association. Second, several studies did not conduct multivariable analyses, and as a result, confounding cannot be excluded from the results. Third, the 2 studies that identified kissing as an independent risk factor for oropharyngeal gonorrhea in the multivariable analyses were both cross-sectional studies; hence, a causal relationship between kissing and oropharyngeal gonorrhea cannot be established. Fourth, recall bias on sexual practices, particularly on the number of kissing partners might have occurred in the included studies. Fifth, all included studies were conducted in Australia. Sexual practices, including kissing practices, may vary across ethnicities and cultures29; therefore, further studies conducted outside Australia are required to support this evidence. Finally, some of the coauthors in this review have also served as coinvestigators in 4 of 6 of the studies included in this review. However, the full texts screening, data extraction, and quality assessments were performed by independent investigators who are not coauthors of the included studies.
Considerably more research is required to clarify the role of kissing as a route of transmission across populations other than MSM. This is important because recent work has also suggested that gonorrhea at the oropharynx appears to be an important source of gonorrhea transmission and hence future interventions may well focus on this site. If they do, it is important to establish transmission routes in heterosexuals as well. It is important to clarify the role of kissing in STI transmission in future research, as novel interventions may be required to target this mode of transmission. Concentrating on behavioral change alone might prove difficult. A previous study that surveyed 926 Australian MSM found that most (77.6%) MSM would be unlikely to stop tongue kissing to prevent oropharyngeal gonorrhea.30
CONCLUSION
This systematic review provided some evidence to suggest a possible association between kissing and oropharyngeal gonorrhea. In contrast, we found no evidence for an association between kissing and oropharyngeal chlamydia, but only a single relevant study on chlamydia was identified. Kissing has been considered a neglected risk factor for oropharyngeal STIs. Public health and safe sex messages around kissing and oropharyngeal STIs should be considered. Future studies will also be required to examine the role of kissing in oropharyngeal STI transmission in other settings and other at-risk populations. Furthermore, with the increase in condomless sex in the era of preexposure prophylaxis and the concern of antibiotic resistance, non–condom-based and non–antibiotic-based novel interventions are urgently required for gonorrhea prevention and control.
Footnotes
Conflicts of Interest: None declared.
Sources of Funding: E.P.F.C. is supported by the National Health and Medical Research Council (NHMRC) Emerging Leadership Investigator Grant (GNT1172873). C.K.F. is supported by an NHMRC Leadership Investigator Grant (GNT1172900). J.S.H. is supported by an NHMRC Senior Research Fellowship (GNT1136117). J.T. is supported by the Australian Government Research Training Program (RTP) Scholarship from Monash University.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (http://www.stdjournal.com).
Contributor Information
Julien Tran, Email: JTran@mshc.org.au.
Adam Kolobaric, Email: S3385831@student.rmit.edu.au.
Richard Case, Email: richard.case@wh.org.au.
Christopher K. Fairley, Email: Christopher.fairley@monash.edu.
Jane S. Hocking, Email: j.hocking@unimelb.edu.au.
Eric P.F. Chow, Email: echow@mshc.org.au;eric.chow@monash.edu.
REFERENCES
- 1.World Health Organization . Global health sector strategies on, respectively, HIV, viral hepatitis and sexually transmitted infections for the period 2022–2030. Geneva, Switzerland: World Health Organization; 2022. Available at: https://apps.who.int/iris/rest/bitstreams/1451670/retrieve. Accessed March 1, 2023.
- 2.U.S. Department of Health and Human Services . Sexually Transmitted Infections National Strategic Plan for the United States: 2021–2025. Washington, DC: U.S. Department of Health and Human Services; 2020. Available at: https://www.hhs.gov/sites/default/files/STI-National-Strategic-Plan-2021-2025.pdf. Accessed March 1, 2023.
- 3.Chow EPF, Grulich AE, Fairley CK. Epidemiology and prevention of sexually transmitted infections in men who have sex with men at risk of HIV. Lancet HIV 2019; 6:e396–e405. [DOI] [PubMed] [Google Scholar]
- 4.Fairley CK Cornelisse VJ Hocking JS, et al. Models of gonorrhoea transmission from the mouth and saliva. Lancet Infect Dis 2019; 19:e360–e366. [DOI] [PubMed] [Google Scholar]
- 5.Chow EP Tabrizi SN Phillips S, et al. Neisseria gonorrhoeae bacterial DNA load in the pharynges and saliva of men who have sex with men. J Clin Microbiol 2016; 54:2485–2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Phillips TR Fairley CK Maddaford K, et al. Bacterial load of Chlamydia trachomatis in the posterior oropharynx, tonsillar fossae, and saliva among men who have sex with men with untreated oropharyngeal chlamydia. J Clin Microbiol 2019; 58:e01375–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Workowski KA Bachmann LH Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep 2021; 70:1–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adamson PC, Klausner JD. The staying power of pharyngeal gonorrhea: Implications for public health and antimicrobial resistance. Clin Infect Dis 2021; 73:583–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barbee LA Soge OO Khosropour CM, et al. The duration of pharyngeal gonorrhea: A natural history study. Clin Infect Dis 2021; 73:575–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Page MJ McKenzie JE Bossuyt PM, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst Rev 2021; 10:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health 1998; 52:377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chow EPF Vodstrcil LA Williamson DA, et al. Incidence and duration of incident oropharyngeal gonorrhoea and chlamydia infections among men who have sex with men: Prospective cohort study. Sex Transm Infect 2021; 97:452–457. [DOI] [PubMed] [Google Scholar]
- 13.Templeton DJ Jin F Imrie J, et al. Prevalence, incidence and risk factors for pharyngeal chlamydia in the community based health in men (HIM) cohort of homosexual men in Sydney, Australia. Sex Transm Infect 2008; 84:361–363. [DOI] [PubMed] [Google Scholar]
- 14.Templeton DJ Jin F McNally LP, et al. Prevalence, incidence and risk factors for pharyngeal gonorrhoea in a community-based HIV-negative cohort of homosexual men in Sydney, Australia. Sex Transm Infect 2010; 86:90–96. [DOI] [PubMed] [Google Scholar]
- 15.Cornelisse VJ Walker S Phillips T, et al. Risk factors for oropharyngeal gonorrhoea in men who have sex with men: An age-matched case-control study. Sex Transm Infect 2018; 94:359–364. [DOI] [PubMed] [Google Scholar]
- 16.Chow EPF Cornelisse VJ Williamson DA, et al. Kissing may be an important and neglected risk factor for oropharyngeal gonorrhoea: A cross-sectional study in men who have sex with men. Sex Transm Infect 2019; 95:516–521. [DOI] [PubMed] [Google Scholar]
- 17.Tran J Ong JJ Bradshaw CS, et al. Kissing, fellatio, and analingus as risk factors for oropharyngeal gonorrhoea in men who have sex with men: A cross-sectional study. EClinicalMedicine 2022; 51:101557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chow EPF Hocking JS Ong JJ, et al. Changes in PrEP use, sexual practice, and use of face mask during sex among MSM during the second wave of COVID-19 in Melbourne, Australia. J Acquir Immune Defic Syndr 2021; 86:153–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kilner A Fairley CK Burrell S, et al. Age pattern of sexual activities with the most recent partner among men who have sex with men in Melbourne, Australia: A cross-sectional study. BMJ Sex Reprod Health 2021; 47:e4. [DOI] [PubMed] [Google Scholar]
- 20.Rosenberger JG Reece M Schick V, et al. Sexual behaviors and situational characteristics of most recent male-partnered sexual event among gay and bisexually identified men in the United States. J Sex Med 2011; 8:3040–3050. [DOI] [PubMed] [Google Scholar]
- 21.Martín-Sánchez M Ong JJ Fairley CK, et al. Clinical presentation of asymptomatic and symptomatic heterosexual men who tested positive for urethral gonorrhoea at a sexual health clinic in Melbourne, Australia. BMC Infect Dis 2020; 20:486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ong JJ Fethers K Howden BP, et al. Asymptomatic and symptomatic urethral gonorrhoea in men who have sex with men attending a sexual health service. Clin Microbiol Infect 2017; 23:555–559. [DOI] [PubMed] [Google Scholar]
- 23.Chow EPF Williamson DA Fortune R, et al. Prevalence of genital and oropharyngeal chlamydia and gonorrhoea among female sex workers in Melbourne, Australia, 2015–2017: Need for oropharyngeal testing. Sex Transm Infect 2019; 95:398–401. [DOI] [PubMed] [Google Scholar]
- 24.Chow EPF Chen MY Williamson DA, et al. Oropharyngeal and genital gonorrhea infections among women and heterosexual men reporting sexual contact with partners with gonorrhea: Implication for oropharyngeal testing of heterosexual gonorrhea contacts. Sex Transm Dis 2019; 46:743–747. [DOI] [PubMed] [Google Scholar]
- 25.Chan PA Robinette A Montgomery M, et al. Extragenital infections caused by Chlamydia trachomatis and Neisseria gonorrhoeae: A review of the literature. Infect Dis Obstet Gynecol 2016; 2016:5758387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phillips TR Constantinou H Fairley CK, et al. Oral, vaginal and anal sexual practices among heterosexual males and females attending a sexual health clinic: A cross-sectional survey in Melbourne, Australia. Int J Environ Res Public Health 2021; 18:12668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zappulla A Fairley CK Donovan B, et al. Sexual practices of female sex workers in Melbourne, Australia: An anonymous cross-sectional questionnaire study in 2017–18. Sex Health 2020; 17:53–60. [DOI] [PubMed] [Google Scholar]
- 28.Cornelisse VJ Bradshaw CS Chow EPF, et al. Oropharyngeal gonorrhea in absence of urogenital gonorrhea in sexual network of male and female participants, Australia, 2018. Emerg Infect Dis 2019; 25:1373–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Charleson FJ Fairley CK Hocking JS, et al. Age, ethnic and travel-related disparities in kissing and sexual practices among heterosexual men in Melbourne, Australia. Sex Health 2020; 17:279–287. [DOI] [PubMed] [Google Scholar]
- 30.Chow EPF Walker S Phillips T, et al. Willingness to change behaviours to reduce the risk of pharyngeal gonorrhoea transmission and acquisition in men who have sex with men: A cross-sectional survey. Sex Transm Infect 2017; 93:499–502. [DOI] [PubMed] [Google Scholar]


