Background:
Ceftolozane/tazobactam, a cephalosporin–β-lactamase inhibitor combination, is approved for the treatment of complicated urinary tract infections and complicated intra-abdominal infections (cIAI). The safety and efficacy of ceftolozane/tazobactam in pediatric participants with cIAI were assessed.
Methods:
This phase 2 study (NCT03217136) randomized participants to either ceftolozane/tazobactam+metronidazole or meropenem for treatment of cIAI in pediatric participants (<18 years). The primary objective was to assess the safety and tolerability of intravenous ceftolozane/tazobactam+metronidazole. Clinical cure at end of treatment (EOT) and test of cure (TOC) visits were secondary end points.
Results:
The modified intent-to-treat (MITT) population included 91 participants (ceftolozane/tazobactam+metronidazole, n = 70; meropenem, n = 21). Complicated appendicitis was the most common diagnosis (93.4%); Escherichia coli was the most common pathogen (65.9%). Adverse events (AEs) occurred in 80.0% and 61.9% of participants receiving ceftolozane/tazobactam+metronidazole and meropenem, drug-related AEs occurred in 18.6% and 14.3% and serious AEs occurred in 11.4% and 0% of participants receiving ceftolozane/tazobactam+metronidazole and meropenem, respectively. No drug-related serious AEs or discontinuations due to drug-related AEs occurred. Rates of the clinical cure for ceftolozane/tazobactam+metronidazole and meropenem at EOT were 80.0% and 95.2% (difference: −14.3; 95% confidence interval: −26.67 to 4.93) and at TOC were 80.0% and 100.0% (difference: −19.1; 95% confidence interval: −30.18 to −2.89), respectively; 6 of the 14 clinical failures for ceftolozane/tazobactam+metronidazole at TOC were indeterminate responses imputed as failures per protocol.
Conclusion:
Ceftolozane/tazobactam+metronidazole was well tolerated in pediatric participants with cIAI and had a safety profile similar to the established safety profile in adults. In this descriptive efficacy analysis, ceftolozane/tazobactam+metronidazole appeared efficacious.
Keywords: adolescent, cIAI, children, Gram-negative, Enterobacterales
Complicated intra-abdominal infections (cIAI) encompass a wide spectrum of pathologic conditions that arise from an infection originating in an abdominal organ and extending into the peritoneal space to form an abscess or peritonitis.1 In the majority of cIAI cases, the infection is polymicrobial and caused by aerobic Gram-negative bacteria, mainly of the Enterobacterales family, with anaerobic bacteria also commonly isolated.2–4 Treatment of cIAI generally involves a combination approach that includes a procedure to achieve control of the source of infection and appropriate antimicrobial therapy.5,6
The proliferation of resistant pathogens, such as extended-spectrum β-lactamase (ESBL)–producing Escherichia coli, Klebsiella pneumoniae and Pseudomonas aeruginosa is a concerning trend in cIAI.2,3,7,8 Antibacterial resistance poses a major global threat, having been shown to be associated with poor outcomes, including an increased mortality rate.8–10 Thus, new safe and effective treatments for cIAI are needed to treat these emerging drug-resistant pathogens.
Ceftolozane/tazobactam is a combination of the antipseudomonal cephalosporin ceftolozane with the established β-lactamase inhibitor tazobactam and is approved for the treatment of cIAI and complicated urinary tract infections (cUTIs) in adults and children birth to 18 years of age, and nosocomial pneumonia in adults.11 Results from the randomized, double-blind, phase 3 study (ASPECT-cIAI) of ceftolozane/tazobactam plus metronidazole in hospitalized adults with cIAI demonstrated that the regimen was noninferior to meropenem with respect to clinical cure rates, with a similar rate of adverse events (AEs).12
Data describing the use of ceftolozane/tazobactam in children and adolescents are limited; however, a pharmacokinetic study indicated drug exposure levels in pediatric participants are comparable with those observed in adults.13,14 Additionally, ceftolozane/tazobactam had potent activity against Enterobacterales and P. aeruginosa, with susceptibility rates of 98% and 95%, respectively, among 1336 isolates collected from pediatric participants in the United States between 2017 and 2019.15 This study evaluated the safety, tolerability, and efficacy of ceftolozane/tazobactam plus metronidazole compared with meropenem for the treatment of cIAI in pediatric participants.
MATERIALS AND METHODS
Study Design
This was a phase 2, randomized, double-blind study (protocol MK-7625A-035; NCT03217136) in pediatric participants with cIAI, conducted in 27 centers in 11 countries across Europe, North America, Africa, Asia/Pacific, and South America between April 2018 and December 2020. The study was conducted in accordance with the principles of Good Clinical Practice and the protocol was approved by the appropriate institutional review boards and regulatory agencies. All participants had a legally acceptable representative and documented informed consent/assent was provided for the study. Blinding, randomization, and masking procedures are included in Supplemental Digital Content 1 http://links.lww.com/INF/E990.
Participants
Male and female participants from birth (defined as >32 weeks gestational age and ≥7 days postnatal) to <18 years of age were eligible. The following were required for study entry: intravenous (IV) antibacterial therapy for presumed or documented cIAI, an operative procedure for the management of cIAI planned or completed within 24 hours of the first dose of an antibacterial drug (participants with necrotizing enterocolitis were exempt from this criteria) and a baseline intra-abdominal specimen collected in compliance with the protocol. Participants were excluded from the study if they had a history of cIAI within the previous year caused by a pathogen known to be resistant to either IV study treatment, had a concomitant infection requiring nonstudy systemic antibacterial therapy, had received potentially therapeutic antibacterial therapy for >24 hours during the 48 hours preceding the first dose of study treatment (except in cases of participants receiving >48 hours of prior antibacterial therapy that were deemed treatment failures) or had moderate or severe renal function impairment (estimated creatinine clearance <50 mL/min/1.73 m2). A full list of inclusion and exclusion criteria is provided in Supplemental Digital Content 1 http://links.lww.com/INF/E990.
The modified intent-to-treat (MITT) population comprised all randomized participants who received any amount of study treatment. The microbiologic-modified intent-to-treat (mMITT) population was the subset of the MITT population with ≥1 pathogen identified from the baseline intra-abdominal culture, regardless of susceptibility to study treatment. The clinically evaluable (CE) population was the subset of participants in the MITT population who adhered to trial procedures and had a clinical outcome at the visit of interest; an interpretable culture was not required. The safety population consisted of all randomized participants who received any amount of study treatment.
Treatment
Participants were stratified and dosed by age group as summarized in Table 1. The selected doses were based on population pharmacokinetic modeling and simulations.14 Treatment duration was 5–14 days. Optional open-label oral step-down therapy with a protocol-defined standard-of-care regimen was permitted after 3 days (9 doses) of IV therapy. Recommended oral step-down therapy options included β-lactam/β-lactamase inhibitor combinations, second- or third-generation cephalosporins in combination with metronidazole, or quinolones (if ciprofloxacin or levofloxacin were chosen, it was to be used in combination with metronidazole), with choice of therapy guided by culture results and based on local antibacterial susceptibility patterns.
TABLE 1.
Summary of Dosing and Pharmacokinetic Sampling Schedule by Age Cohort
| Age group | C/T + MTZ* | MEM + pbo* | ||
|---|---|---|---|---|
| n | Dose | n | Dose | |
| 12 to <18 years | 16 | IV ceftolozane 1 g/ tazobactam 0.5 g† + IV MTZ‡10 mg/kg | 5 | IV 20 mg/kg every 8 hours |
| 6 to <12 years | 30 | IV ceftolozane 20 mg/kg/ tazobactam 10 mg/kg§ + IV MTZ‡ 10 mg/kg | 9 | |
| 2 to <6 years | 22 | 7 | ||
| 3 months to <2 years | 1 | 0 | ||
| Birth¶ to <3 months | 1 | IV Ceftolozane 20 mg/kg/ tazobactam 10 mg/kg§ + IV MTZ‖,** 10-15 mg/kg | 0 | IV 20 mg/kg every 8 hours |
*Each dose of C/T or MTZ or MEM or pbo was administered as a 60-minute (±10 minutes) infusion. C/T + MTZ or MEM + pbo was to be dosed every 8 hours (±1 hour) after the previous infusion. The second IV dose had a ±4-hour window for dosing to facilitate adjustment of the dosing schedule (once every 8 hours) to be carried out throughout the dosing period.
†Children 12 to <18 years received the dosage indicated for adult patients with cIAI.11
‡Maximum dose was 1.5 g/day.
§Maximum dose was ceftolozane 1 g/tazobactam 0.5 g.
¶Birth was defined as >32 weeks gestational age and ≥7 days postnatal.
‖Participants >28 days of age: MTZ 10 mg/kg every 8 hours (maximum dose 1.5 g/day). For participants ≤28 days of age, the suggested dosing regimen was as follows: participants ≤28 days of age and ≤2 kg: MTZ 15 mg/kg loading dose, then 7.5 mg/kg/dose every 12 hours; participants ≤28 days of age and >2 kg: MTZ 15 mg/kg loading dose, then 10 mg/kg dose every 8 hours. However, other site-specific standard of care MTZ dosing was permitted at the investigator’s discretion.
**Participants 7–28 days of age who received MTZ with a frequency other than every 8 hours were required to receive placebo at the same frequency to maintain blinding.
C/T indicates ceftolozane/tazobactam; MEM, meropenem; MTZ, metronidazole; pbo, placebo; IV, intravenous.
Specimen Collection
Details regarding specimen collection and pathogen characterization are provided in Supplemental Digital Content 1 http://links.lww.com/INF/E990. Briefly, specimens were collected at the beginning of the interventional procedure before debridement, removal or disinfection of the primary infection site. Culture of the intra-abdominal specimen, isolation of pathogen(s), initial identification of pathogen(s) and susceptibility testing were conducted by local laboratories. Isolates were submitted to a central laboratory for identification and evaluation of antibacterial susceptibility profiles using the Clinical and Laboratory Standards Institute (CLSI) reference testing methodology and quality control recommendations.16 Isolates that displayed predefined minimum inhibitory concentration criteria were screened for the presence of ESBL-encoding genes.
Assessments and End Points
Clinical and microbiologic assessments were performed at the end of the IV treatment visit, which was scheduled <24 hours after the last IV dose of therapy, the end of treatment (EOT) visit, which was scheduled <48 hours after the last dose of oral step-down therapy (if applicable), and the test of cure (TOC) visit, which occurred 7–14 days after the last dose of study treatment. The EOT visit only applied to those participants who were switched to oral step-down therapy.
The primary end points were rates of AEs and changes in laboratory values and vital signs from the first dose of study treatment through the last follow-up visit (21–28 days after the last dose) in all participants of the treated population. Key secondary end points were clinical success rate at the EOT and TOC visits, defined as the proportion of participants who had a clinical response of cure, and per-participant microbiologic success rate at the EOT and TOC visits, defined as the proportion of participants who had microbiologic eradication or presumed eradication (defined in Supplemental Digital Content 1, http://links.lww.com/INF/E990) of all baseline pathogens.
Definitions of End Points
Clinical cure was considered complete resolution or marked improvement in signs and symptoms of infection or return to preinfection status, such that no further antibacterial therapy or surgical or drainage procedure was required for treatment of the cIAI. Participants were considered to have experienced a partial improvement if they had partial resolution of the signs and symptoms of infection, such that no further intravenous antibacterial therapy was required for treatment of the cIAI, but additional oral step-down therapy was required. The clinical outcome was considered a failure for those who required antibacterial therapy beyond the protocol-defined treatment duration of 14 days or for those with persisting or recurrent infection within the abdomen requiring additional intervention (either nonstudy antibacterials or surgery).
Eradication was defined as an absence of the baseline pathogen(s) in a postbaseline specimen obtained from the original site of infection. Presumed eradication was defined as an absence of material to culture in a participant who is assessed as having partial improvement or clinical cure. Persistence was defined as the presence of the baseline pathogen(s) in a postbaseline specimen from the site of infection, and presumed persistence was an absence of material to culture in a participant assessed as a clinical failure. Participants were classified as having persistence-acquired resistance if the baseline pathogen(s) could be obtained from a postbaseline specimen and was found to be susceptible to study treatment pretreatment but resistant post-treatment.
Statistical Analysis
The study was designed as a descriptive study and was not powered for formal hypothesis testing to compare safety or efficacy between treatment groups. For the primary safety analysis, 95% CIs were derived for the between-treatment differences in the percentage of participants with events, and these analyses were performed using the unstratified Miettinen & Nurminen method, an unconditional, asymptotic method.17 Changes in the laboratory and vital sign values from baseline were summarized using descriptive statistics. For the secondary efficacy analyses, 2-sided 95% CI based on the Miettinen & Nurminen method17 and stratified by age group was used to evaluate the treatment differences for clinical success and per-participant microbiologic eradication at the EOT and TOC visits.
Sample Size Calculation
This study was not powered for inferential statistical comparisons of safety or efficacy end points in this study alone. This study was designed to contribute data to an integrated safety analysis in combination with data from a separate study of ceftolozane/tazobactam in pediatric participants with cUTI. The combined sample size for these studies was based on the enrollment of approximately 180 participants receiving ceftolozane/tazobactam in the combined safety analysis population of this study (MK-7625A-035; NCT03217136) and the study in pediatric cUTI (MK-7625A-034; NCT03230838), which would allow for a 97.3% probability of detecting AEs with an underlying true incidence of ≥2% within the ceftolozane/tazobactam treatment group. Ultimately, 170 participants were enrolled in the ceftolozane/tazobactam treatment group of the combined studies, which allowed for a 96.7% probability of detecting an AE with an underlying true incidence of ≥2%. Decreasing enrollment did not negatively impact the key goals or scientific validity of the studies and allowed for sufficient data to evaluate safety, efficacy and pharmacokinetics in both study populations.
RESULTS
Study Participants
A total of 94 participants were randomized and 91 were included in the MITT population, with 70 treated with ceftolozane/tazobactam plus metronidazole and 21 treated with meropenem. The clinically evaluable (CE) population included 78 participants at EOT (ceftolozane/tazobactam plus metronidazole, n = 59; meropenem, n = 19) and 77 participants at TOC (ceftolozane/tazobactam plus metronidazole, n = 58; meropenem, n = 19) (Supplemental Digital Content 2, http://links.lww.com/INF/E991). Overall, 94.4% of participants treated with ceftolozane/tazobactam plus metronidazole and 87.0% of participants treated with meropenem completed the study.
In the MITT population, demographics and baseline characteristics were generally comparable between those treated with ceftolozane/tazobactam plus metronidazole and those treated with meropenem, with the exception of sex and the percentage of participants who had an open vs. laparoscopic abdominal procedure (Table 2). A higher proportion of males were in the ceftolozane/tazobactam plus metronidazole group (67.1%) compared with the meropenem group (28.6%) and a greater proportion of participants in the meropenem group had an open procedure (42.9%) compared with the ceftolozane/tazobactam plus metronidazole group (28.6%). The most common diagnosis was complicated appendicitis (91.4% for ceftolozane/tazobactam plus metronidazole and 100.0% for meropenem). Polymicrobial cIAI infections were present in 54.3% and 66.7% of participants in the ceftolozane/tazobactam plus metronidazole and meropenem groups, respectively, and bacteremia was present at baseline in 2.9% and 0% of participants, respectively. Few (<2.0%) participants had failed prior antibacterial therapy for their cIAI before study entry. Most participants in both groups had baseline creatinine clearance ≥80 mL/min/1.73 m2.
TABLE 2.
Participant Baseline Characteristics (MITT Population)
| Characteristic | C/T + MTZ (N = 70) | MEM + placebo (N = 21) |
|---|---|---|
| Male sex, n (%) | 47 (67.1) | 6 (28.6) |
| Age, n (%) | ||
| Birth to <3 months | 1 (1.4) | 0 |
| 3 months to <2 years | 1 (1.4) | 0 |
| 2 to <6 years | 22 (31.4) | 7 (33.3) |
| 6 to <12 years | 30 (42.9) | 9 (42.9) |
| 12 to <18 years | 16 (22.9) | 5 (23.8) |
| White race, n (%) | 61 (87.1) | 19 (90.5) |
| Median (range) weight, kg | 27.7 (3.1–90.0) | 30.5 (11.0–61.1) |
| Complicated appendicitis baseline diagnosis, n (%) | 64 (91.4) | 21 (100.0) |
| Type of abdominal procedure, n (%) | ||
| Percutaneous | 2 (2.9) | 0 |
| Laparoscopic | 44 (62.9) | 11 (52.4) |
| Open | 20 (28.6) | 9 (42.9) |
| Other | 3 (4.3) | 1 (4.8) |
| Missing | 1 (1.4) | 0 |
| Bacteremia at baseline | 2 (2.9) | 0 |
| Baseline CrCl (mL/min/1.73 m2),* n (%) | ||
| CrCl ≥80 | 61 (87.1) | 21 (100.0) |
| CrCl ≥50 to <80 | 8 (11.4) | 0 |
| CrCl ≥30 to <50 | 1 (1.4) | 0 |
| Failure of prior antibacterial therapy, n (%) | 1 (1.4) | 0 |
| Number of baseline pathogens, n (%) | ||
| Polymicrobial | 38 (54.3) | 14 (66.7) |
| Monomicrobial | 26 (37.1) | 5 (23.8) |
| Missing | 6 (8.6) | 2 (9.5) |
*CrCl rates were calculated by the revised Schwartz equation at baseline.
C/T indicates ceftolozane/tazobactam; CrCl, creatinine clearance; MEM, meropenem; MITT, modified intent-to-treat; MTZ, metronidazole.
Pathogens at Baseline
The incidence and distribution of baseline intra-abdominal pathogens were generally comparable across age and treatment groups. The most common Gram-negative pathogens were E. coli, P. aeruginosa and Bacteroides fragilis (Table 3). All P. aeruginosa, Enterobacterales and Bacteroides fragilis isolates were susceptible to both ceftolozane/tazobactam and meropenem. Participants in each treatment group (12.5% ceftolozane/tazobactam plus metronidazole; 11.1% meropenem) had ESBL-producing Enterobacterales at baseline (the majority of which were E. coli). All of these participants had baseline isolates susceptible to ceftolozane/tazobactam and meropenem, based on CLSI breakpoints.16
TABLE 3.
Pathogen Identified at Baseline (MITT Population)*
| Pathogen category organism/group, n/N (%) | C/T + MTZ | MEM + placebo |
|---|---|---|
| Aerobic Gram-negative | 56/70 (80.0) | 18/21 (85.7) |
| Escherichia coli | 47/70 (67.1) | 13/21 (61.9) |
| ESBL producer† | 8/56 (14.3) | 3/18 (16.7) |
| Pseudomonas aeruginosa | 19/70 (27.1) | 6/21 (28.6) |
| Klebsiella pneumonia | 4/70 (5.7) | 0/21 (0.0) |
| ESBL producer† | 2/56 (3.6) | 0/18 (0.0) |
| Aerobic Gram-positive | 20/70 (28.6) | 10/21 (47.6) |
| Streptococcus anginosus | 9/70 (12.9) | 3/21 (14.3) |
| Streptococcus constellatus | 9/70 (12.9) | 3/21 (14.3) |
| Anerobic Gram-negative | 16/70 (22.9) | 5/21 (23.8) |
| Bacteroides fragilis | 13/70 (18.6) | 4/21 (19.0) |
| Bacteroides thetaiotaomicron | 4/70 (5.7) | 2/21 (9.5) |
| Anaerobic Gram-positive | 8/70 (11.4) | 2/21 (9.5) |
*Limited to pathogens with prevalence of ≥5% in ≥1 treatment arm.
†ESBL-producer status was determined for pathogens isolated from participants in the mMITT population with baseline aerobic Gram-negative pathogens.
C/T indicates ceftolozane/tazobactam; ESBL, extended-spectrum β-lactamase; MEM, meropenem; MITT, modified intent-to-treat; MTZ, metronidazole.
Overall, ceftolozane/tazobactam susceptibility rates were lower compared with meropenem for Gram-negative anaerobes [ceftolozane/tazobactam, 21/34 (61.8%); meropenem, 35/36 (97.2%)] and Gram-positive anaerobes [ceftolozane/tazobactam, 4/10 (40.0%); meropenem, 10/10 (100.0%)], based on CLSI breakpoints.16
Safety
The mean (SD) overall treatment duration (IV only or IV + oral) was 9.3 (3.6) days and 9.0 (3.2) days for ceftolozane/tazobactam plus metronidazole and meropenem, respectively. The mean (SD) duration of IV treatment in the ceftolozane/tazobactam plus metronidazole group was 6.4 (2.8) days and was 5.8 (1.8) days in the meropenem group. A total of 35 (50.0%) and 12 (57.1%) participants transitioned to optional oral step-down therapy in the ceftolozane/tazobactam plus metronidazole and meropenem groups, for a median of 6.3 and 5.2 days, respectively. The most common oral step-down antibacterial agents (>10% in either treatment group) were metronidazole, amoxicillin/clavulanate, and ciprofloxacin.
In total, ≥1 AE occurred in 80.0% and 61.9% of participants receiving ceftolozane/tazobactam plus metronidazole and meropenem; 18.6% and 14.3% were considered treatment-related, respectively (Table 4). The most frequently reported AEs and the most commonly reported drug-related AEs are summarized in Table 4. Serious AEs (SAEs) occurred in 11.4% (8/70) and 0% (0/21) of participants receiving ceftolozane/tazobactam plus metronidazole and meropenem, respectively. Three of the SAEs in the ceftolozane/tazobactam plus metronidazole group occurred during the IV treatment period (abdominal sepsis and pneumonia in 1 participant; intra-abdominal fluid collection in 1 participant) or during oral step-down therapy (fecaloma in 1 participant); all other SAEs were reported after study therapy was completed. All SAEs were resolved and none were considered drug-related. Two participants in the ceftolozane/tazobactam plus metronidazole group were discontinued from study therapy because of nondrug-related SAEs: 1 participant discontinued from study intervention owing to SAEs of abdominal sepsis and pneumonia on day 3, and 1 participant owing to an SAE of pneumonia on day 10. No study drug discontinuations occurred because of a drug-related AE.
TABLE 4.
Adverse Events (Safety Population)
| AE category, n (%) | C/T + MTZ (N=70) | MEM + placebo (N=21) | Difference* (95% CI) |
|---|---|---|---|
| Overall AE summary | |||
| ≥1 AE | 56 (80.0) | 13 (61.9) | 18.1 (−2.6 to 41.1) |
| No AE | 14 (20.0) | 8 (38.1) | −18.1 (−41.1 to 2.6) |
| Drug-related† AE | 13 (18.6) | 3 (14.3) | 4.3 (−17.6 to 19.1) |
| Serious AE | 8 (11.4) | 0 | 11.4 (−4.6 to 21.0) |
| Serious drug-related† AE | 0 | 0 | – |
| Death | 0 | 0 | – |
| Discontinued due to AE | 2 (2.9) | 0 | 2.9 (−12.9 to 9.9) |
| Discontinued due to drug-related† AE | 0 | 0 | – |
| Discontinued due to serious AE | 2 (2.9) | 0 | 2.9 (–12.9 to 9.9) |
| Discontinued due to serious drug-related† AE | 0 | 0 | – |
| Most commonly reported AEs‡ | |||
| Diarrhea | 12 (17.1) | 5 (23.8) | −6.7 (−29.5 to 10.6) |
| Pyrexia | 9 (12.9) | 3 (14.3) | −1.4 (−23.0 to 12.6) |
| Vomiting | 7 (10.0) | 1 (4.8) | 5.2 (−13.5 to 15.8) |
| Abdominal pain | 7 (10.0) | 0 | 10.0 (−6.0 to 19.3) |
| Nasopharyngitis | 3 (4.3) | 3 (14.3) | −10.0 (−30.9 to 1.8) |
| Most commonly reported drug-related AEs†,§ | |||
| Diarrhea | 4 (5.7) | 1 (4.8) | – |
| Increased alanine aminotransferase | 3 (4.3) | 1 (4.8) | – |
| Increased aspartate aminotransferase | 4 (5.7) | 1 (4.8) | – |
| Blood alkaline phosphatase increased | 2 (2.9) | 0 | – |
| Vulvovaginal mycotic infection | 0 | 1 (4.8) | – |
| Dysgeusia | 2 (2.9) | 0 | – |
*The percent difference (C/T + MTZ minus MEM) was based on the unstratified Miettinen & Nurminen method.
†Determined by the investigator to be related to the drug.
‡Incidence ≥10% in ≥1 treatment arm.
§Incidence ≥4% or ≥2 participants in ≥1 treatment arm.
AE, adverse event; C/T, ceftolozane/tazobactam; CI, confidence interval; MEM, meropenem; MTZ, metronidazole.
Efficacy
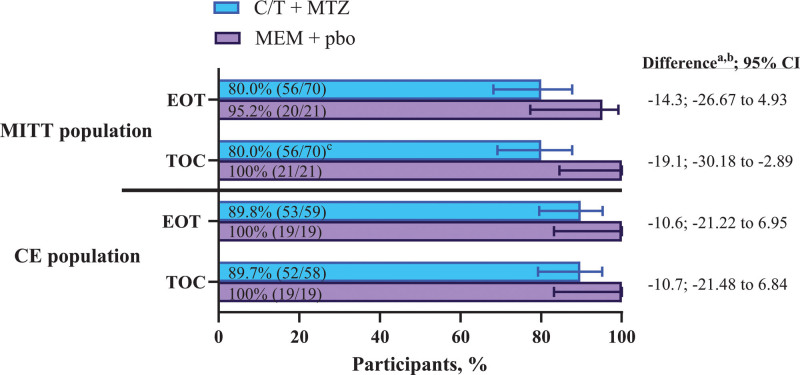
In the MITT population, rates of clinical cure for ceftolozane/tazobactam plus metronidazole and meropenem at EOT were 80.0% and 95.2%, and at TOC were 80.0% and 100.0%, respectively (Fig. 1). In the CE population, rates of clinical cure for ceftolozane/tazobactam plus metronidazole and meropenem were 89.8% and 100.0% at EOT, and 89.7% and 100.0% at TOC, respectively (Fig. 1). When clinical success rates were stratified by prespecified subgroups, they were generally comparable between treatment groups and consistent with the overall clinical success rate in the MITT population at the TOC visit (Supplemental Digital Content 3, http://links.lww.com/INF/E992). These subgroups included sex, geographic region, duration of IV study treatment, number of intra-abdominal abscesses, peritonitis type, procedure type, prior antibacterial agent use, site of infection, presence of bacteremia at baseline, and number of baseline pathogens. Among participants with ESBL-producing pathogens isolated at baseline (mMITT population), rates of clinical cure at the TOC visit were 80.0% (8/10) in the ceftolozane/tazobactam plus metronidazole group vs. 100.0% (3/3) in the meropenem group.
FIGURE 1.
Rates of clinical cure in the MITT and clinical evaluable populations at EOT and TOC. CE indicates clinically evaluable; C/T, ceftolozane/tazobactam; EOT, end of treatment; MEM, meropenem; MITT, modified intent-to-treat; MTZ, metronidazole; pbo, placebo; TOC, test of cure. aDifference in C/T + MTZ minus MEM. bThe percent difference was based on the Miettinen & Nurminen method stratified by age group with Cochran-Mantel-Haenszel weights. If there was a zero count in any class of the stratum, the groups with the lower count were pooled with the near age group stratum in the model. cSix of the 14 failures at the TOC visit in the C/T plus MTZ group were based on indeterminate or missing clinical responses and not on observed clinical failures.
The overall per-participant microbiologic success rates at the TOC visit in the MITT population were high (>84%) and comparable between treatment groups (Supplemental Digital Content 4, http://links.lww.com/INF/E993). Most microbiologic outcomes were presumed based on clinical outcomes in participants who did not have follow-up intra-abdominal cultures and, therefore, reflect clinical outcomes. The per-participant microbiologic success rates at the EOT visit were consistent with those reported at the TOC visit (Supplemental Digital Content 4, http://links.lww.com/INF/E993).
DISCUSSION
This study and the companion pediatric study of cUTI (NCT03230838; reported separately18) are the first randomized clinical studies to evaluate ceftolozane/tazobactam treatment in pediatric populations. No clinically meaningful safety concerns were identified for ceftolozane/tazobactam plus metronidazole. Although the rates of both AEs and SAEs were higher in the ceftolozane/tazobactam plus metronidazole group than in the meropenem group, the significance of this is unclear, as the study was not powered for formal hypothesis testing of between-treatment group comparisons. Of note, the most common AEs seen in this study (diarrhea, pyrexia, and vomiting) are frequently observed signs of cIAI in children.19 In addition, the rates of drug-related AEs were similar between treatment groups. None of the 10 SAEs that occurred in 8 participants treated with ceftolozane/tazobactam plus metronidazole were considered drug-related, and only 3 (occurring in 2 participants) were reported during IV therapy; the remaining events manifested either during oral step-down therapy or during the post-treatment follow-up periods, suggesting that these events were unlikely to be associated with ceftolozane/tazobactam. In addition, there were no individual AEs or SAEs that predominated and would indicate a safety signal.
Clinical success rates at the TOC visit were numerically lower in the ceftolozane/tazobactam plus metronidazole group compared with the meropenem group. However, efficacy was a secondary end point and the study was not powered for formal hypothesis testing of between-treatment group comparisons. It is notable that the difference in clinical cure rates was not driven by lower-than-expected efficacy in the ceftolozane/tazobactam plus metronidazole group, as efficacy in this pediatric study population was comparable to that seen in the pivotal phase 3 study of adults with cIAI, in which treatment with ceftolozane/tazobactam plus metronidazole resulted in an 83.0% clinical cure rate.12 Although the study was randomized to facilitate balance in baseline characteristics between the 2 treatment groups, there were some numerical imbalances, notably in the type of surgery and prevalence of polymicrobial infection, which could have differentially influenced clinical outcomes between treatment groups.
There was no clear pattern in participant or disease characteristics (eg, cIAI diagnosis, medical history, and baseline pathogens) between those in the ceftolozane/tazobactam plus metronidazole group who experienced clinical failure versus the rest of the study population. It also should be noted that a substantial proportion [6/14 (43%)] of those classified as clinical failures in the ceftolozane/tazobactam plus metronidazole group were reported as having an indeterminate response, meaning that the participant was lost to follow-up or a clinical assessment could not be made owing to another reason. In the CE population, in which only observed failures were included, the response rates for ceftolozane/tazobactam plus metronidazole were higher than in the MITT population.
More than 10% of the study population had infections caused by ESBL-producing Enterobacterales, which pose a particular challenge in the treatment of pediatric infections; among this subgroup, 80% of those treated with ceftolozane/tazobactam plus metronidazole achieved a clinical cure. Similarly, the clinical cure rate for participants with ESBL-producing Enterobacterales from the phase 3 study in adults with intra-abdominal infections was 95.8% in the ceftolozane/tazobactam plus metronidazole treatment group.12 A recent study of isolates from pediatric participants found that apart from cephalosporins, ESBL-producing strains of E. coli and K. pneumoniae were less likely to be susceptible to other antibacterial agents, such as amoxicillin/clavulanic acid, quinolones, gentamicin, netilmicin, and cotrimoxazole.20 Thus, having options that are effective against these multidrug-resistant organisms, such as ceftolozane/tazobactam, is important.
Not unexpectedly, there were few participants in the youngest age cohorts (3 months to <2 years and birth to <3 months of age) despite efforts to enroll participants <2 years of age, consistent with the epidemiology of cIAI.2 Nevertheless, the companion study in cUTI did include 34 ceftolozane/tazobactam-treated participants who ranged from birth to <2 years of age.18 In addition, a safety and pharmacokinetic study of ceftolozane/tazobactam in pediatric participants enrolled 6 participants who were 3 months to <2 years of age and 13 participants from birth to <3 months of age.13
A limitation of this study is that it was not powered for formal hypothesis testing of between-treatment group comparisons. Therefore, the results should be interpreted with caution.
In conclusion, these results show that ceftolozane/tazobactam plus metronidazole was well tolerated in pediatric participants with cIAI and had a safety profile similar to the established safety profile in adults. In this descriptive efficacy analysis, ceftolozane/tazobactam plus metronidazole appeared efficacious.
ACKNOWLEDGMENTS
The authors thank the study participants and the investigators who made this study possible, particularly, as they dealt with the challenges imparted by the COVID-19 pandemic. Investigators included Elisangela Mattos e Silva (Curitiba, Brazil), Rodrigo Melo Gallindo (Recife, Brazil), Simone Caldeira Silva (Bento Goncalves, Brazil), Laszlo Sasi Szabo (Debrecen, Hungary), Csaba Bereczki (Szeged, Hungary), Ferenc Dicso (Nyiregyhaza, Hungary), Tamas Decsi (Pecs, Hungary), Sandor Sarkozy/Zoltan Ringwald (Budapest, Hungary), Attila Szabo (Budapest, Hungary); Dalius Malcius (Klaipeda, Lithuania), Vidmantas Barauskas (Kaunas, Lithuania), Kestutis Trainavicius (Vilniaus, Lithuania); Yee Ian Yik (Kuala Lumpur, Malaysia), Marjmin Osman (Cheras, Malaysia), Tammy Han Qi Teoh (Georgetown, Malaysia), Mercedes Macias Parra (Mexico City, Mexico), Sarbelio Moreno Espinosa (Mexico City, Mexico), César Adrián Martínez Longoria (Monterrey, Mexico), Carlos Nicolas del Rio Almendarez (Emiliano Zapata, Mexico), Eugen-Sorin Boia (Timisoara, Romania), Horatiu Gocan (Cluj-Napoca, Romania), Antonina Petrovna Zouzova (Smolensk, Russia), Sergey Gennadievich Kovalenko (Chelyabinsk, Russia), Sergey Viktorovich Minaev (Stavropol, Russia), Natalia Grishina (Vologda, Russia), Jan Hendrik Reynor Becker (Pretoria, South Africa), Peter Nourse (Cape Town, South Africa), Antoni Noguera Julian (Barcelona, Spain), Federico Martinon Torres (Coruna, Spain), Emilio Monteagudo (Valencia, Spain), Maria Mendez Hernandez (Barcelona, Spain), Derya Alabaz (Adana, Turkey), Ates Kara (Ankara, Turkey), Ener Cagri Dinleyici (Eskisehir, Turkey), Nazan Dalgic (Istanbul, Turkey), Nataliia Dementieva (Dnipro, Ukraine), Ihor Ksonz (Poltava, Ukraine), Tetyana V. Lytvynova (Kryvyy Rig, Ukraine) Natalia Karpenko (Kyiv, Ukraine), Oleksandr Fofanov (Ivano-Frankivsk, Ukraine), Dmytro Dmytriiev (Vinnytsya, Ukraine), Kwabena Krow Ampofo (Salt Lake City, UT, USA), Delma Nieves (Orange, CA, USA), John S. Bradley (San Diego, CA, USA), Michael N. Neely (Los Angeles, CA, USA), Leonard B. Weiner (Syracuse, NY, USA), Danielle Zerr (Seattle, WA, USA), Jason G. Newland (St. Louis, MO, USA), Carl-Christian A. Jackson (Boston, MA, USA), and Mobeen H. Rathore (Jacksonville, FL, USA).
Medical writing and/or editorial assistance was provided by Meredith Rogers, MS, CMPP, of The Lockwood Group, Stamford, CT, USA. This assistance was funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
A portion of this study was conducted during the COVID-19 pandemic, and all standard operating procedures for study conduct, monitoring, and oversight during the pandemic were maintained, with a risk-based approach to assess and mitigate its impact on study conduct employed.
Supplementary Material
Footnotes
Affiliation at the time the study was conducted
Funding for this research was provided by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
J.L., F.-H.S, J.A.H., M.B., M.G.J., C.D.A., E.G.R., and C.J.B. are employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA (MSD), who may own stock and/or hold stock options in Merck & Co., Inc., Rahway, NJ, USA. M.W.P. was an employee of MSD at the time of the study conduct. C.-C.A.J. received consulting fees from MSD and holds stock in Merck & Co., Inc., Rahway, NJ, USA. J.N. reports funding to conduct the study from MSD to his institution. N.D. has no potential conflicts of interest to disclose.
All authors are responsible for the work described in this article and meet ICMJE authorship criteria. All authors were involved in at least one of the following: conception, design of work or acquisition, analysis, interpretation of data and drafting the article and/or revising/reviewing the article for important intellectual content. All authors provided final approval of the version to be published. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.pidj.com).
Contributor Information
Carl-Christian A. Jackson, Email: carl-christian.jackson@brownphysicians.org.
Jason Newland, Email: jgnewland@wustl.edu.
Nataliia Dementieva, Email: Nataliia60dementieva@gmail.com.
Julia Lonchar, Email: julia.lonchar@merck.com.
Feng-Hsiu Su, Email: feng-hsiu.su@merck.com.
Jennifer A. Huntington, Email: Jennifer.Huntington@merck.com.
Mekki Bensaci, Email: mekki.bensaci@merck.com.
Myra W. Popejoy, Email: myra.popejoy@gmail.com.
Matthew G. Johnson, Email: matthew.johnson1@merck.com.
Carisa De Anda, Email: carisa.de.anda@merck.com.
Elizabeth G. Rhee, Email: elizabeth.rhee@merck.com.
REFERENCES
- 1.Menichetti F, Sganga G. Definition and classification of intra-abdominal infections. J Chemother. 2009;21(suppl 1):3–4. [DOI] [PubMed] [Google Scholar]
- 2.Newman N, Wattad E, Greenberg D, et al. Community-acquired complicated intra-abdominal infections in children hospitalized during 1995-2004 at a paediatric surgery department. Scand J Infect Dis. 2009;41:720–726. [DOI] [PubMed] [Google Scholar]
- 3.Sartelli M, Catena F, Ansaloni L, et al. Complicated intra-abdominal infections in Europe: a comprehensive review of the CIAO study. World J Emerg Surg. 2012;7:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lob SH, Badal RE, Hackel MA, et al. Epidemiology and antimicrobial susceptibility of gram-negative pathogens causing intra-abdominal infections in pediatric patients in Europe-SMART 2011-2014. J Pediatric Infect Dis Soc. 2017;6:72–79. [DOI] [PubMed] [Google Scholar]
- 5.Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:133–164. [DOI] [PubMed] [Google Scholar]
- 6.Sartelli M, Chichom-Mefire A, Labricciosa FM, et al. The management of intra-abdominal infections from a global perspective: 2017 WSES guidelines for management of intra-abdominal infections. World J Emerg Surg. 2017;12:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Badal RE, Bouchillon SK, Lob SH, et al. Etiology, extended-spectrum β-lactamase rates and antimicrobial susceptibility of gram-negative bacilli causing intra-abdominal infections in patients in general pediatric and pediatric intensive care units--global data from the Study for Monitoring Antimicrobial Resistance Trends 2008 to 2010. Pediatr Infect Dis J. 2013;32:636–640. [DOI] [PubMed] [Google Scholar]
- 8.Blot S, Antonelli M, Arvaniti K, et al. ; Abdominal Sepsis Study (AbSeS) group on behalf of the Trials Group of the European Society of Intensive Care Medicine. Epidemiology of intra-abdominal infection and sepsis in critically ill patients: “AbSeS,” a multinational observational cohort study and ESICM Trials Group Project. Intensive Care Med. 2019;45:1703–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bassetti M, Poulakou G, Ruppe E, et al. Antimicrobial resistance in the next 30 years, humankind, bugs and drugs: a visionary approach. Intensive Care Med. 2017;43:1464–1475. [DOI] [PubMed] [Google Scholar]
- 10.Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis [Published online January 18, 2022]. Lancet. 2022;399:629–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zerbaxa® (ceftolozane and tazobactam). US Prescribing information. Merck Sharp & Dohme LLC;2022. [Google Scholar]
- 12.Solomkin J, Hershberger E, Miller B, et al. Ceftolozane/tazobactam plus metronidazole for complicated intra-abdominal infections in an era of multidrug resistance: results from a randomized, double-blind, phase 3 trial (ASPECT-cIAI). Clin Infect Dis. 2015;60:1462–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bradley JS, Ang JY, Arrieta AC, et al. Pharmacokinetics and safety of single intravenous doses of ceftolozane/tazobactam in children with proven or suspected gram-negative infection. Pediatr Infect Dis J. 2018;37:1130–1136. [DOI] [PubMed] [Google Scholar]
- 14.Larson KB, Patel YT, Willavize S, et al. Ceftolozane-tazobactam population pharmacokinetics and dose selection for further clinical evaluation in pediatric patients with complicated urinary tract or complicated intra-abdominal infections. Antimicrob Agents Chemother. 2019;63:e02578-–e025718.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lob S, Hackel M, Andrew DeRyke C, et al. 1273. Activity of ceftolozane/tazobactam and comparators against Enterobacterales and P. aeruginosa isolates from pediatric patients—SMART United States 2017-2019. Open Forum Infect Dis. 2021;8(Suppl 1):725–725. [Google Scholar]
- 16.Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing. 30th ed. Available at: https://www.nih.org.pk/wp-content/uploads/2021/02/CLSI-2020.pdf. Accessed November 30, 2021. [Google Scholar]
- 17.Miettinen O, Nurminen M. Comparative analysis of two rates. Stat Med. 1985;4:213–226. [DOI] [PubMed] [Google Scholar]
- 18.Roilides E, Ashouri N, Bradley JS, et al. Safety and efficacy of ceftolozane/tazobactam versus meropenem in neonatal and pediatric participants with complicated urinary tract infection, including pyelonephritis: a phase 2, randomized, clinical trial. Pediatr Infect Dis J. 2022;42:292–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thompson AE, Marshall JC, Opal SM. Intraabdominal infections in infants and children: descriptions and definitions. Pediatr Crit Care Med. 2005;6(3 Suppl):S30–S35. [DOI] [PubMed] [Google Scholar]
- 20.Sethaphanich N, Santanirand P, Rattanasiri S, et al. Pediatric extended spectrum β-lactamase infection: community-acquired infection and treatment options. Pediatr Int. 2016;58:338–346. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.