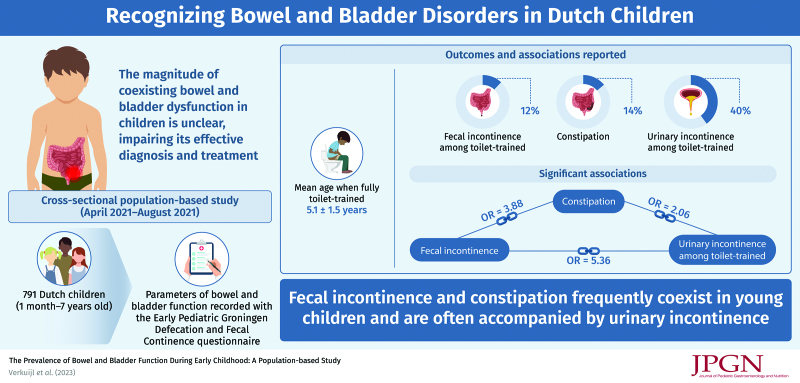
Objectives:
Our primary aim was to determine bowel and bladder function in children aged 1 month to 7 years in the general Dutch population. Second, we aimed to identify demographic factors associated with the presence of bowel and bladder dysfunction, and their coexistence.
Methods:
For this cross-sectional, population-based study, parents/caregivers of children aged from 1 month to 7 years were asked to complete the Early Pediatric Groningen Defecation and Fecal Continence questionnaire. Different parameters of bowel and bladder function were assessed using validated scoring systems such as the Rome IV criteria.
Results:
The mean age of the study population (N = 791) was 3.9 ± 2.2 years. The mean age at which parents/caregivers considered their child fully toilet-trained was 5.1 ± 1.5 years. Prevalence of fecal incontinence among toilet-trained children was 12%. Overall prevalence of constipation was 14%, with a constant probability and severity at all ages. We found significant associations between fecal incontinence and constipation [odds ratio (OR) = 3.88, 95% CI: 2.06–7.30], fecal incontinence and urinary incontinence (OR = 5.26, 95% CI: 2.78–9.98), and constipation and urinary incontinence (OR = 2.06, 95% CI: 1.24–3.42).
Conclusions:
Even though most children are fully toilet-trained at 5 years, fecal incontinence is common. Constipation appears to be common in infants, toddlers, and older children. Fecal incontinence and constipation frequently coexist and are often accompanied by urinary incontinence. Increased awareness of bowel and bladder dysfunction in infants, toddlers, and young children is required to prevent these problems from continuing at older ages.
Keywords: constipation, gastrointestinal, incontinence, infant, toddler
What Is Known
Bowel dysfunction is already prevalent in infants, toddlers, and preschool children.
However, recognizing bowel dysfunction in young children is difficult, especially because existing studies neither demonstrate the continuity of bowel function from one age to the next nor the magnitude of coexisting bowel and bladder dysfunction.
What Is New
At the age of 5 years most children are toilet-trained.
Nevertheless, bowel and bladder dysfunction are common and treatment is lacking. Awareness for these conditions in young children is needed to prevent these problems from persisting at older ages.
Bowel and bladder dysfunction are common among children. A recent meta-analysis of population-based studies showed that constipation is prevalent in 10% of children (1). Fecal incontinence is estimated to occur in 1%–4% of all toilet-trained children (2). These bowel complaints impair children’s quality of life and lead to considerable and increasing health care costs (3–5).
Contrary to popular belief, recent studies showed that bowel dysfunction is already prevalent in infants, toddlers, and preschool children (6–11). Existing studies, however, do not demonstrate the continuity of bowel function from one age to the next, but divide their samples into age groups (6–11). The result is lack of insight into the physiological bowel function during the first years of life and this in turn impairs recognizing bowel dysfunction in infants, toddlers, and young children.
According to current literature 3%–47% of constipated children also suffer from urinary incontinence (12). The magnitude of coexisting bowel and bladder dysfunction in infants, toddlers, and young children is unclear. This impairs effective diagnosis and treatment of young children presenting with symptoms of either bowel or bladder dysfunction.
Our primary aim was to determine bowel and bladder function according to age in children aged 1 month to 7 years in the general Dutch population. Second, we aimed to identify demographic factors that were associated with the presence of bowel and bladder dysfunction, and their coexistence.
METHODS
Study design
We performed this cross-sectional study between April 2021 and August 2021. Parents/caregivers (hereafter referred to as parents) of children aged 1 month to 7 years were invited to complete the validated Early Pediatric Groningen Defecation and Fecal Continence (EP-DeFeC) questionnaire (13). The EP-DeFeC contains validated scoring systems and criteria regarding bowel and urinary functioning, but also questions regarding demographic characteristics, therapies, and the parents’ classification of their child’s bowel health (13).
Participants were approached by email by an external survey company (Dynata, Rotterdam, the Netherlands). They recruited a randomly selected sample of Dutch-speaking inhabitants from all regions of the Netherlands, whereby the sex and age ratios according to the current population pyramid of the Netherlands, as published by Statistics Netherlands, were taken into account (14). We excluded respondents who reported a physically impossible combination of age, weight, and height according to the Dutch child growth charts (15), and/or invalid answers to open questions. We did not exclude children with congenital diseases or previous surgery. All the questionnaires were completed digitally and anonymously. To avoid missing data, all applicable questions of the digital EP-DeFeC questionnaire had to be completed to submit the questionnaire. Based on the known prevalences of bowel and bladder dysfunction from previous population-based samples, we calculated that a sample size of 800 children would be efficient (see Text, Supplemental Digital Content 1, http://links.lww.com/MPG/D143) (16,17). The study was conducted in compliance with the ethical standards of the Medical Ethical Review Board of University Medical Center Groningen.
Outcome Measures
We assessed stool consistency, stool frequency, and urinary frequency. We determined stool consistency according to the Bristol Stool Scale, ranging between 1 and 7 with a higher number indicating softer stool (18). Additionally, we examined the prevalence and severity of constipation, fecal incontinence, and urinary incontinence. To define these conditions, we used the validated scoring systems and criteria that are incorporated in the EP-DeFeC questionnaire (13).
We defined constipation according to the Rome IV criteria for neonates/toddlers (0–3 years) and children (≥4 years), depending on the age of the child (2,19). The status of toilet training of the child was defined as “fully toilet-trained,” “is currently being toilet-trained,” or “did not yet start toilet training.” We defined fecal incontinence in fully toilet-trained children as any involuntary loss of stool within the last month. We diagnosed nonretentive fecal incontinence as fecal incontinence without evidence of constipation in children of at least 4 years, according to the Rome IV criteria (2). We defined urinary incontinence according to the definition recommended by the International Children’s Continence Society (20). Additionally, we defined the following 3 subtypes: daytime incontinence, enuresis, or combined daytime incontinence and enuresis (20).
We assessed severity of constipation with the age-adapted Agachan score, ranging from 0 to 30 (21). We assessed severity of fecal incontinence with the Wexner score, ranging from 0 to 20 (22). For both scores a higher score indicates worse functioning. We also used the Pediatric Incontinence and Constipation scores (PICS) for constipation and fecal incontinence, ranging from 0 to 29 and from 0 to 32, respectively (23). Worse functioning is indicated by lower PICS scores. Finally, we asked parents how they would qualify their child’s bowel health and whether they had sought advice regarding bowel dysfunction of their child.
Definitions of Demographic Characteristics
We gathered the children’s demographic characteristics on sex, weight, and preterm birth from the EP-DeFeC questionnaire (13). We classified the children’s weight as underweight, normal weight, overweight, or obese according to the guidelines of the Dutch association of Pediatrics (24,25). In this way, a weight normal for children younger than 2 years was defined as between −2 SD and +1 SD from the Dutch child growth charts (15,24). A weight lower than −2 SD was defined as underweight (15,25), between +1 SD and +2 SD as overweight, and above +2 SD as obese (15,24). For children aged at least 2 years, we used the standardized body mass index cut-off points (24,25). Preterm birth was defined as birth at fewer than 37 weeks’ gestation, according to the definition of the World Health Organization.
Statistical Analysis
We expressed continuous variables as mean ± standard deviation (SD) or median and interquartile range (IQR). Categorical variables were shown as number (percentage) and compared using Pearson chi-square tests. We calculated Spearman correlations between age and stool consistency, stool frequency, and urinary frequency. We used cubic spline regression to calculate the probability of constipation, fecal incontinence, and urinary incontinence according to age. We also used cubic spline regression to express the observed and expected constipation and fecal incontinence scores according to age. We used univariable logistic regression analysis to investigate the association between demographic characteristics and constipation, fecal incontinence, and urinary incontinence, and the coexistence of these 3 conditions. We performed all analyses with SPSS software, Version 23.0 (IBM Corp, Armonk, NY) and R Version 3.6.3 (R Foundation of Statistical Computing, Vienna, Austria). We considered P values below 0.05 as statistically significant.
RESULTS
A total of 1021 parents of children aged 1 month to 7 years completed the EP-DeFeC questionnaire. We excluded 215 respondents on account of an invalid combination of the child’s age, weight, and height. Another 15 respondents were excluded on account of illogical answers to open questions. The study population finally comprised 791 respondents. The mean age of the children was 3.9 ± 2.2 years and 55% were boys (see Table, Supplemental Digital Content 2, http://links.lww.com/MPG/D144).
Stool Consistency, Stool Frequency, and Urinary Frequency
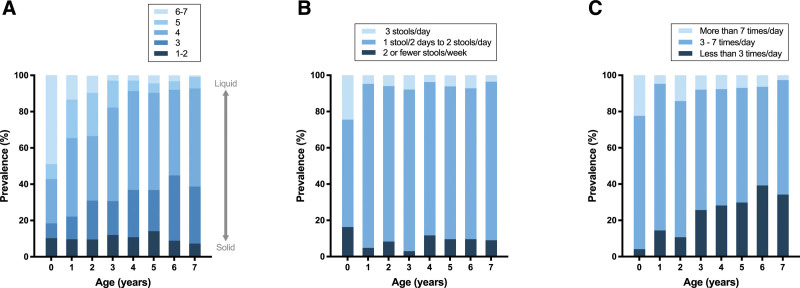
Mean stool consistency according to the Bristol Stool Scale was 3.8 ± 1.1. Stool consistency was harder in older children (Fig. 1A). Additionally, we found a correlation between children’s age and their stool consistency (rho, −0.249; P < 0.001).
FIGURE 1.
The prevalence of different stool consistencies (A), stool frequencies (B), and urinary frequencies (C) among children from 0 up to 7 years of age.
Overall, 85% of the children had a stool frequency ranging from 1 stool every 2 days and 2 stools a day. The prevalence of this stool frequency was 59% among children younger than 1 year and varied between 83% and 90% for older children (Fig. 1B). We found no significant correlation between children’s age and their stool frequency (rho, −0.066; P = 0.062).
Most children urinated 3–7 times a day (67%). The urinary frequency decreased in older children (Fig. 1C). We found a correlation between the children’s age and urinary frequency (rho, −0.232; P < 0.001).
Toilet Training and Fecal Incontinence
The mean age at which parents considered their child “fully toilet-trained for stool” was 5.1 ± 1.5 years. We found no significant difference between boys and girls (5.0 vs 5.2 years, respectively, P = 0.901). The mean age at which parents indicated that their child “was currently being toilet-trained for stool” was 2.7 ± 1.4 years.
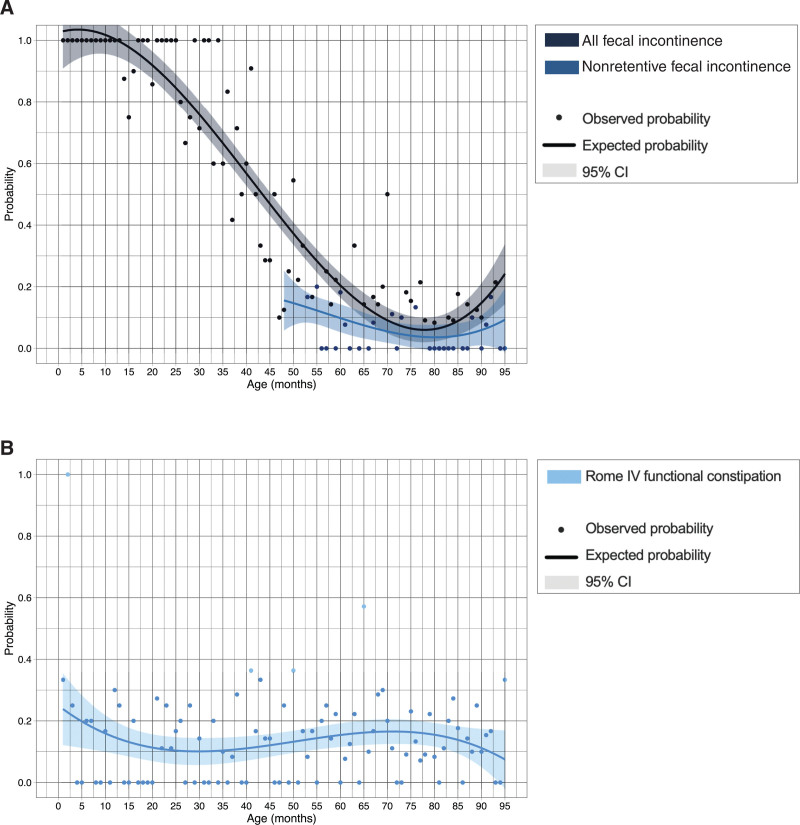
The prevalence of fecal incontinence among toilet-trained children was 12% and the prevalence of nonretentive fecal incontinence was 7%. Probability of fecal incontinence decreased in older children and ranged from 1.0 in children younger than 12 months to 0.07 in children aged 77 months (Fig. 2A). Univariable logistic regression analysis confirmed that older children were less likely to suffer from fecal incontinence (OR = 0.98, 95% CI: 0.97–1.00, P = 0.013, Table 1).
FIGURE 2.
The probability of fecal incontinence (A), nonretentive fecal incontinence (A), and constipation (B) according to age. CI = confidence interval.
TABLE 1.
Prevalence and univariable logistic regression analysis of fecal incontinence, constipation, and urinary incontinence
| Fecal incontinence* | Constipation† | Urinary incontinence‡ | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Prevalence | Univariable logistic regression | Prevalence | Univariable logistic regression | Prevalence | Univariable logistic regression | ||||
| Variables | n (%) | OR (95% CI) | P value | n (%) | OR (95% CI) | P value | n (%) | OR (95% CI) | P value |
| Demographic characteristics | |||||||||
| Age, mo | – | 0.98 (0.97–1.00) | 0.013* | – | 1.00 (0.99–1.01) | 0.909 | – | 0.97 (0.96–0.98) | < 0.001** |
| Sex | |||||||||
| Boys | 27 (10.3) | Reference | 58 (13.4) | Reference | 103 (39.8) | Reference | |||
| Girls | 34 (13.5) | 1.35 (0.79–2.32) | 0.272 | 50 (13.9) | 1.04 (0.69–1.57) | 0.838 | 103 (41.0) | 1.05 (0.74–1.50) | 0.771 |
| Classification of weight | |||||||||
| Normal weight | 42 (12.2) | Reference | 67 (12.7) | Reference | 131 (38.6) | Reference | |||
| Overweight | 9 (17.3) | 1.51 (0.69–3.31) | 0.309 | 13 (15.3) | 1.24 (0.65–2.37) | 0.509 | 24 (47.1) | 1.41 (0.78–2.55) | 0.254 |
| Obese | 7 (10.9) | 0.88 (0.38–2.06) | 0.774 | 14 (14.7) | 1.19 (0.64–2.22) | 0.585 | 27 (45.0) | 1.30 (0.75–2.26) | 0.354 |
| Underweight | 3 (5.7) | 0.43 (0.13–1.45) | 0.173 | 14 (16.9) | 1.40 (0.74–2.62) | 0.299 | 24 (40.0) | 1.06 (0.60–1.86) | 0.842 |
| Preterm birth | |||||||||
| No | 55 (11.9) | Reference | 97 (13.6) | Reference | 180 (39.3) | Reference | |||
| Yes | 6 (11.8) | 0.99 (0.40–2.42) | 0.977 | 11 (14.5) | 1.08 (0.55–2.12) | 0.827 | 26 (50.0) | 1.54 (0.87–2.75) | 0.138 |
| Bowel and urinary function | |||||||||
| Fecal incontinence* | |||||||||
| No | 44 (9.7) | Reference | 152 (35.2) | Reference | |||||
| Yes | N/A | N/A | N/A | 18 (29.5) | 3.88 (2.06–7.30) | <0.001** | 40 (74.1) | 5.26 (2.78–9.98) | <0.001** |
| Constipation | |||||||||
| No | 43 (9.5) | Reference | 167 (38.0) | Reference | |||||
| Yes | 18 (29.0) | 3.88 (2.06–7.30) | <0.001** | N/A | N/A | N/A | 39 (55.7) | 2.06 (1.24–3.42) | 0.006* |
| Urinary incontinence‡ | |||||||||
| No | 14 (4.8) | Reference | 31 (10.2) | Reference | |||||
| Yes | 40 (20.8) | 5.26 (2.78–9.98) | <0.001** | 39 (18.9) | 2.06 (1.24–3.42) | 0.006* | N/A | N/A | N/A |
CI = confidence interval; OR = odds ratio.
Only the 513 children who were toilet-trained for stool were included in the analyses.
All 791 children were included in the analyses.
Only the 510 children who were toilet-trained for urine were included in the analyses.
§Significance of P < 0.05,
∥Significance of P < 0.005.
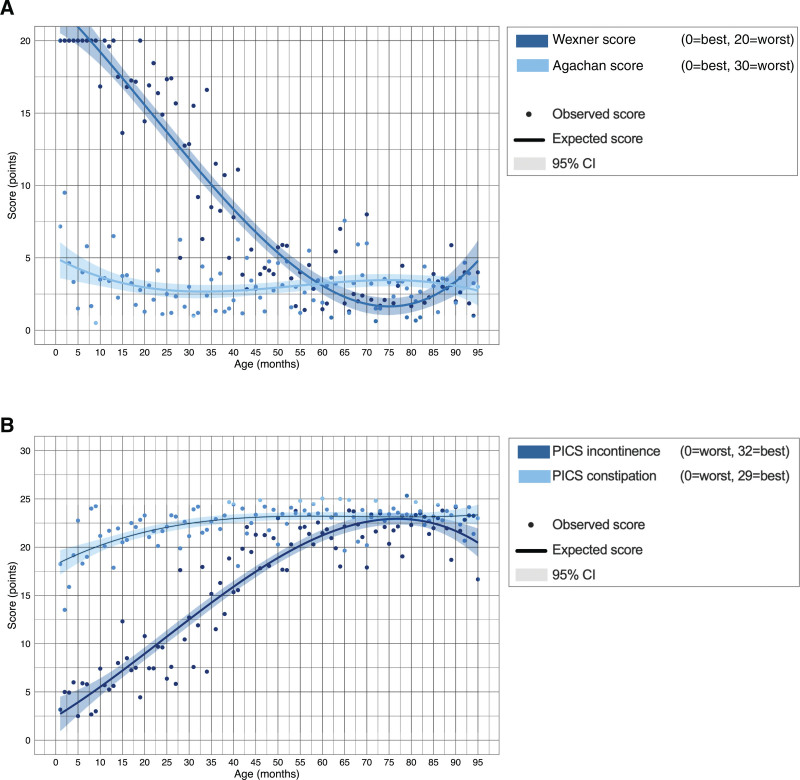
The median Wexner score was 0 (IQR 0–5) and the median PICS incontinence score was 23 (IQR 19–26). The Wexner score steeply decreased from 20 in children younger than 12 months to 2 in children of 75 months (Fig. 3A). Likewise, the PICS incontinence score increased from 3 in children aged 1 month to 23 in children aged 75 months (Fig. 3B).
FIGURE 3.
Observed and expected constipation and fecal incontinence severity scores according to age. The Wexner score for severity of fecal continence and the Agachan score for severity of constipation (A), and the PICS incontinence and constipation scores (B). CI = confidence interval; PICS = Pediatric Incontinence and Constipation Score.
Antidiarrheals were given to 7% of all fecally incontinent children and to 1% of the fecally continent children. Parents of fecally incontinent children did not qualify their child’s bowel health as significantly worse compared to parents of children without bowel disorders (poor to very poor bowel health in 2% vs 1%, P = 0.474, see Figure, Supplemental Digital Content 3, http://links.lww.com/MPG/D145). We found that 56% of the parents of fecally incontinent children did not discuss these problems with anyone (see Figure, Supplemental Digital Content 4, http://links.lww.com/MPG/D146).
Constipation
Overall prevalence of constipation was 14%, with excessive stool retention (75.9%) and painful/hard stools (78.7%) as the 2 most common symptoms. We found that the probability of constipation remained around 0.15 for children at all ages (Fig. 2B). Univariable logistic regression analysis confirmed that constipation was not associated with age (OR = 1.00, 95% CI: 0.99–1.01, P = 0.909, Table 1).
Regarding severity of constipation among all the children, the median Agachan score was 2 (IQR 1–4) and the median PICS constipation score was 24 (IQR 21–25). The Agachan score remained around 2.5 at all ages (Fig. 3A). The PICS constipation score increased slightly in older children, ranging from 18 in children aged 1 month to 23 in children of 40 months (Fig. 3B).
Laxatives and enemas were administered to 41% and 9% of the constipated children, respectively, and to 4% and 1% of the non-constipated children. We found that 12% of the parents of constipated children qualified their child’s bowel health as poor to very poor, compared to 1% of the parents of children without bowel disorders (P < 0.001, see Figure, Supplemental Digital Content 3, http://links.lww.com/MPG/D145). We found that 28% of the parents of constipated children did not discuss these problems with anyone (see Figure, Supplemental Digital Content 4, http://links.lww.com/MPG/D146).
Toilet Training and Urinary Incontinence
The mean age at which parents considered their child “fully toilet-trained for urine” was 5.0 ± 1.5 years. The mean age at which parents indicated that their child “was currently being toilet-trained for urine” was 3.0 ± 1.8 years. The prevalence of urinary incontinence among all toilet-trained children was 40%. The majority of these urinary incontinent children suffered from daytime incontinence (56%). Enuresis occurred in 22% and the other 22% suffered from combined daytime incontinence and enuresis.
The probability of urinary incontinence decreased with increasing age from 1.0 in children younger than 15 months to 0.32 in children aged 80 months (see Figure A, Supplemental Digital Content 5, http://links.lww.com/MPG/D147). Univariable logistic regression analysis confirmed that older children were less likely to suffer from urinary incontinence (OR = 0.97, 95% CI: 0.96–0.98, P < 0.001, Table 1). Analysis of the subtypes of urinary incontinence showed that while the probability of combined daytime incontinence and enuresis decreased steeply with age, the probability of daytime incontinence and enuresis increased (see Figure B, Supplemental Digital Content 5, http://links.lww.com/MPG/D147).
Associated Demographic Factors
Neither children’s sex nor preterm birth were associated with fecal incontinence, constipation, or urinary incontinence (Table 1). Likewise, there was no significant association between children’s weight and fecal incontinence, constipation, or urinary incontinence (Table 1).
Coexistence of Bowel and Bladder Dysfunction
In 29% of children fecal incontinence and constipation coexisted. Univariable regression analysis demonstrated a significant association between fecal incontinence and constipation (OR = 3.88, 95% CI: 2.06–7.30, P < 0.001, Table 1). Furthermore, 21% of the urinary incontinent children suffered from coexisting fecal incontinence and 19% from coexisting constipation. We found a significant association between fecal incontinence and urinary incontinence (OR = 5.26, 95% CI: 2.78–9.98, P < 0.001) and between constipation and urinary incontinence (OR = 2.06, 95% CI: 1.24–3.42, P = 0.006, Table 1).
DISCUSSION
This population-based study investigated the bowel and bladder function of Dutch infants, toddlers, and children up to 7 years of age. We showed that constipation, fecal incontinence, and urinary incontinence were present in considerable numbers of children and that these conditions frequently coexisted.
Our results also showed that Dutch children usually started toilet training around the age of 2½ years and were fully toilet-trained at the age of 5 years. Compared to studies performed 15 years ago, we found toilet training to be completed at an older age (23,26–28). This may indicate a trend and may be attributed to different potty training routines nowadays (27–29).
No fewer than 12% of toilet-trained children suffered from fecal incontinence, with a steeply decreasing probability and severity at older ages. This curve illustrated the physiological decline in fecal incontinence during the first years of life while acquiring toileting skills. A plateau in probability and severity of fecal incontinence was reached at the age of 5 years, which corresponded with the age at which parents indicated toilet training as completed.
Constipation was prevalent in 14% of the children from 1 month to 7 years, which was comparable with previously reported rates in children of the same age using the Rome III or IV criteria for functional constipation (6–11). Probability and severity of constipation remained constant in children from 1 month to 7 years of age. Furthermore, the overall prevalence of 14% constipation that we found in children up to 7 years old corresponded with previously described prevalence rates among older children using the same criteria (9,10,30). Given the equally high prevalence of constipation from infancy until puberty, it seems that children do not automatically outgrow constipation.
The 2 most common constipation-associated symptoms were excessive stool retention and painful/hard stools, a finding that corroborated with the findings reported by other researchers (6,8,31). This indicates that young, constipated children present with different clinical symptoms than do older children and adults (30,32).
Our results demonstrated that fecal incontinence coexisted with constipation in one-third of the children up to 7 years of age. This was comparable to the 25% coexistence of fecal incontinence and constipation in children aged 8–17 years, but much more than the 15% coexistence in adolescents and 4% coexistence in adults (30,33). This emphasizes the importance of gaining information about obstructive complaints when young children present with fecal incontinence and vice versa. Nevertheless, the majority of the fecally incontinent children suffered from nonretentive fecal incontinence. The proportion of nonretentive fecal incontinence in the current study was higher than previously described (10,34,35), which most likely was a reflection of the younger age of our study population.
Urinary incontinence was present in no fewer than 40% of the children we studied, which was much higher than the prevalence reported in studies including older children (36,37). This corresponds with the steeply decreasing probability of urinary incontinence in older children. A further decline in urinary incontinence with ageing is found in older children (37), although others did not find this relation (36).
On account of our finding that urinary incontinence frequently coexisted with both constipation and fecal incontinence, even more than previously found in older children (12,36), it is important to inquire about lower urinary tract symptoms in young children who present with bowel dysfunction. Further problems may be prevented by timely and adequate diagnosis, as treatment of constipation and/or fecal incontinence often leads to the resolution of urinary incontinence (34).
Similar to previous reports in young children, we did not find a sex predominance for fecal incontinence or constipation (6–8,10). It has been suggested that the frequently reported higher prevalence of functional gastrointestinal disorders in women is related to sex hormones, which are not yet present in “adult concentrations” in young children (6). The fact that we did not find an association between preterm birth and bowel or bladder dysfunction corroborates with previous findings (8,38). Also in line with previous reports, we found no association between excessive bodyweight and constipation (6,8,39). Although a higher prevalence of obesity in constipated children of older ages had been described (40). Likewise, there was no association between underweight and constipation, which had previously been described (6). The number of children with underweight in the respective study sample was limited (6).
Remarkably, only 7% of all fecally incontinent children were treated with antidiarrheals, which are comparable with the treatment rate in older children, but less than that in adults (30). The low treatment rate corroborated with our findings that parents of fecally incontinent children did not qualify their child’s bowel health as poor and that more than half of the parents did not seek help. This may either indicate that parents considered involuntary loss of stool as normal, or it may show the taboo on talking about fecal incontinence. Conversely, almost half of the children who suffered from constipation were treated with laxatives. The majority of parents sought medical advice for their child’s problems regarding constipation, which was more than previously reported (6). Nevertheless, half of the children with constipation and almost all the children with fecal incontinence remained untreated. There is certainly room for improvement here because early treatment appears to be more successful (31,41). For the current treatment of constipation and fecal incontinence in young children, we refer to excellent recent reviews (32,34,42,43).
The strength of our study includes the use of a validated questionnaire and the fact that the study sample comprised a representative cohort of Dutch children in terms of sex and age ratios (14). The most important limitation of the current study is the lack of longitudinal data. Another limitation may be that the questionnaire was completed by the parents, which was inevitable because of the young age of the children we studied. However, this may have influenced the remarkably high prevalence of fecal and urinary incontinence. Furthermore, the parents may have given untruthful answers. We tried to minimize this bias by excluding respondents with physically impossible or illogical answers. Finally, we used the Rome IV criteria for constipation without considering the presence of a large fecal mass in the rectum. In this way, we may have underestimated the prevalence of constipation, although a recent study showed the influence of this criterion to be minimal (44).
CONCLUSIONS
At the age of 5 years most Dutch children are fully toilet-trained. Nevertheless, fecal incontinence is common even in fully toilet-trained children. Constipation appears to be common as well, with an equally high prevalence in infants, toddlers, and older children. Furthermore, fecal incontinence and constipation frequently coexist and are often accompanied by urinary incontinence, which requires attention. Remarkably, treatment of fecal incontinence and constipation is lacking in most of the affected children. Therefore, awareness is needed of both bowel and bladder dysfunction in infants, toddlers, and young children to prevent these problems from persisting at older ages. This awareness can, for instance, be created during regular developmental check-ups at the child health care center within the first years of life.
Acknowledgments
The authors thank T. van Wulfften Palthe, PhD for correcting the English manuscript and all respondents for participating.
Supplementary Material
Footnotes
The authors report no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.jpgn.org).
REFERENCES
- 1.Koppen IJN, Vriesman MH, Saps M, et al. Prevalence of functional defecation disorders in children: a systematic review and meta-analysis. J Pediatr. 2018;198:121–30. [DOI] [PubMed] [Google Scholar]
- 2.Hyams JS, Di Lorenzo C, Saps M, Shulman RJ, Staiano A, van Tilburg M. Childhood functional gastrointestinal disorders: child/adolescent. Gastroenterology. 2016;150:1456–68.e2. [Google Scholar]
- 3.Kovacic K, Sood MR, Mugie S, et al. A multicenter study on childhood constipation and fecal incontinence: effects on quality of life. J Pediatr. 2015;166:1482–7.e1. [DOI] [PubMed] [Google Scholar]
- 4.Vriesman MH, Rajindrajith S, Koppen IJN, et al. Quality of life in children with functional constipation: a systematic review and meta-analysis. J Pediatr. 2019;214:141–50. [DOI] [PubMed] [Google Scholar]
- 5.Park R, Mikami S, LeClair J, et al. Inpatient burden of childhood functional GI disorders in the USA: an analysis of national trends in the USA from 1997 to 2009. Neurogastroenterol Motil. 2015;27:684–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walter AW, Hovenkamp A, Devanarayana NM, Solanga R, Rajindrajith S, Benninga MA. Functional constipation in infancy and early childhood: epidemiology, risk factors, and healthcare consultation. BMC Pediatr. 2019;19:285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Tilburg MA, Hyman PE, Walker L, et al. Prevalence of functional gastrointestinal disorders in infants and toddlers. J Pediatr. 2015;166:684–9. [DOI] [PubMed] [Google Scholar]
- 8.Steutel NF, Zeevenhooven J, Scarpato E, et al. Prevalence of functional gastrointestinal disorders in European infants and toddlers. J Pediatr. 2020;221:107–14. [DOI] [PubMed] [Google Scholar]
- 9.Scarpato E, Kolacek S, Jojkic-Pavkov D, et al. Prevalence of functional gastrointestinal disorders in children and adolescents in the Mediterranean region of Europe. Clin Gastroenterol Hepatol. 2018;16:870–6. [DOI] [PubMed] [Google Scholar]
- 10.Robin SG, Keller C, Zwiener R, et al. Prevalence of pediatric functional gastrointestinal disorders utilizing the Rome IV criteria. J Pediatr. 2018;195:134–9. [DOI] [PubMed] [Google Scholar]
- 11.Saps M, Velasco-Benitez CA, Fernandez Valdes L, et al. The impact of incorporating toilet-training status in the pediatric Rome IV criteria for functional constipation in infant and toddlers. Neurogastroenterol Motil. 2020;32:e13912. [DOI] [PubMed] [Google Scholar]
- 12.van Summeren JJGT, Holtman GA, van Ommeren SC, Kollen BJ, Dekker JH, Berger MY. Bladder symptoms in children with functional constipation: a systematic review. J Pediatr Gastroenterol Nutr. 2018;67:552–60. [DOI] [PubMed] [Google Scholar]
- 13.Verkuijl SJ, Trzpis M, Broens PMA. Development and validation of the Early Pediatric Groningen Defecation and Fecal Continence questionnaire. Eur J Pediatr. 2023;182:615–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Statistics Netherlands (Centraal Bureau voor de Statistiek). Population pyramid. Age composition in the Netherlands 2021. Available at: https://www.cbs.nl/en-gb/visualisations/dashboard-population/population-pyramid. Accessed 17 November, 2021.
- 15.Van Buuren S. TNO growth charts, 2010. Available at: https://www.tno.nl/nl/gezond/werk-jeugd-gezondheid/jeugd/eerste-1000-dagen-kind/groeidiagrammen-groeicalculators/. Accessed 11 November, 2021.
- 16.Pourhoseingholi MA, Vahedi M, Rahimzadeh M. Sample size calculation in medical studies. Gastroenterol Hepatol Bed Bench. 2013;6:14–7. [PMC free article] [PubMed] [Google Scholar]
- 17.Daniel WW. Biostatistics: A Foundation for Analysis in the Health Sciences. New York: John Wiley & Sons; 1999. [Google Scholar]
- 18.Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32:920–4. [DOI] [PubMed] [Google Scholar]
- 19.Benninga MA, Nurko S, Faure C, et al. Childhood functional gastrointestinal disorders: neonate/toddler. Gastroenterology. 2016;150:1443–55. [DOI] [PubMed] [Google Scholar]
- 20.Austin PF, Bauer SB, Bower W, et al. The standardization of terminology of lower urinary tract function in children and adolescents: update report from the standardization committee of the International Children’s Continence Society. Neurourol Urodyn. 2016;35:471–81. [DOI] [PubMed] [Google Scholar]
- 21.de Abreu GE, Dias Souto Schmitz AP, Dourado ER, et al. Association between a constipation scoring system adapted for use in children and the dysfunctional voiding symptom score in children and adolescents with lower urinary tract symptoms. J Pediatr Urol. 2019;15:529. [DOI] [PubMed] [Google Scholar]
- 22.Jorge JM, Wexner SD. Etiology and management of fecal incontinence. Dis Colon Rectum. 1993;36:77–97. [DOI] [PubMed] [Google Scholar]
- 23.Fichtner-Feigl S, Sailer M, Höcht B, et al. Development of a new scoring system for the evaluation of incontinence and constipation in children. Coloproctology. 2003;25:10–5. [Google Scholar]
- 24.Van den Akker E, Vreugdenhil A, Hustinx SR, et al. Obesitas bij kinderen en adolescenten. Leidraad voor kinderartsen. January 8, 2018. Available at: https://docplayer.nl/106220771-08-01-2018-obesitas-bij-kinderen-en-adolescenten-leidraad-voor-kinderartsen.html. Accessed 11 November, 2021.
- 25.Joosten K, Hulst JM, Van den Berg A, et al. Richtlijn ondergewicht NVK. April 2, 2019. https://www.nvk.nl/themas/kwaliteit/richtlijnen/richtlijn?componentid=6881280. Accessed 11 November, 2021.
- 26.Wald ER, Di Lorenzo C, Cipriani L, Colborn DK, Burgers R, Wald A. Bowel habits and toilet training in a diverse population of children. J Pediatr Gastroenterol Nutr. 2009;48:294–8. [DOI] [PubMed] [Google Scholar]
- 27.Blum NJ, Taubman B, Nemeth N. Relationship between age at initiation of toilet training and duration of training: a prospective study. Pediatrics. 2003;111:810–4. [DOI] [PubMed] [Google Scholar]
- 28.Blum NJ, Taubman B, Nemeth N. Why is toilet training occurring at older ages? A study of factors associated with later training. J Pediatr. 2004;145:107–11. [DOI] [PubMed] [Google Scholar]
- 29.Li X, Wen JG, Xie H, et al. Delayed in toilet training association with pediatric lower urinary tract dysfunction: a systematic review and meta-analysis. J Pediatr Urol. 2020;16:352. [DOI] [PubMed] [Google Scholar]
- 30.Timmerman MEW, Trzpis M, Broens PMA. Prevalence of defecation disorders and their symptoms is comparable in children and young adults: cross-sectional study. Pediatr Gastroenterol Hepatol Nutr. 2021;24:45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loening-Baucke V. Constipation in early childhood: patient characteristics, treatment, and longterm follow up. Gut. 1993;34:1400–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vriesman MH, Koppen IJN, Camilleri M, Di Lorenzo C, Benninga MA. Management of functional constipation in children and adults. Nat Rev Gastroenterol Hepatol. 2020;17:21–39. [DOI] [PubMed] [Google Scholar]
- 33.Meinds RJ, van Meegdenburg MM, Trzpis M, Broens PMA. On the prevalence of constipation and fecal incontinence, and their co-occurrence, in the Netherlands. Int J Colorectal Dis. 2017;32:475–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koppen IJ, von Gontard A, Chase J, et al. Management of functional nonretentive fecal incontinence in children: recommendations from the International Children’s Continence Society. J Pediatr Urol. 2016;12:56–64. [DOI] [PubMed] [Google Scholar]
- 35.Rajindrajith S, Devanarayana NM, Benninga MA. Constipation-associated and nonretentive fecal incontinence in children and adolescents: an epidemiological survey in Sri Lanka. J Pediatr Gastroenterol Nutr. 2010;51:472–6. [DOI] [PubMed] [Google Scholar]
- 36.Linde JM, Nijman RJM, Trzpis M, et al. Prevalence of urinary incontinence and other lower urinary tract symptoms in children in the Netherlands. J Pediatr Urol. 2019;15:164. [DOI] [PubMed] [Google Scholar]
- 37.Abrams P, Cardozo L, Wagg A, et al. International Continence Society. Bristol, UK: Incontinence 6th Edition 2017.
- 38.Bekkali N, Moesker FM, Van Toledo L, et al. Bowel habits in the first 24 months of life: preterm- versus term-born infants. J Pediatr Gastroenterol Nutr. 2010;51:753–8. [DOI] [PubMed] [Google Scholar]
- 39.Koppen IJ, Velasco-Benítez CA, Benninga MA, Di Lorenzo C, Saps M. Is there an association between functional constipation and excessive bodyweight in children? J Pediatr. 2016;171:178–82.e1. [DOI] [PubMed] [Google Scholar]
- 40.Pashankar DS, Loening-Baucke V. Increased prevalence of obesity in children with functional constipation evaluated in an academic medical center. Pediatrics. 2005;116:e377377–e380. [DOI] [PubMed] [Google Scholar]
- 41.van den Berg MM, van Rossum CH, de Lorijn F, Reitsma JB, Di Lorenzo C, Benninga MA. Functional constipation in infants: a follow-up study. J Pediatr. 2005;147:700–4. [DOI] [PubMed] [Google Scholar]
- 42.Koppen IJ, Lammers LA, Benninga MA, Tabbers MM. Management of functional constipation in children: therapy in practice. Paediatr Drugs. 2015;17:349–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tabbers MM, DiLorenzo C, Berger MY, et al. Evaluation and treatment of functional constipation in infants and children: evidence-based recommendations from ESPGHAN and NASPGHAN. J Pediatr Gastroenterol Nutr. 2014;58:258–74. [DOI] [PubMed] [Google Scholar]
- 44.Pradhan S, Jagadisan B. Yield and examiner dependence of digital rectal examination in detecting impaction in pediatric functional constipation. J Pediatr Gastroenterol Nutr. 2018;67:570–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.