Abstract
Purpose
The circle of Willis is a circulatory anastomosis that supplies blood to the brain. If any of the bridging segments are hypoplastic or absent, the capacity for collateral flow in the setting of large vessel occlusion may be decreased. Outside of the neonatal period, the prevalence of a complete circle of Willis (CoW) in the pediatric population has not been well described. Our objectives include determining the prevalence of a complete CoW in children and identifying if there is an age-related “loss” of arterial segments.
Methods
Following IRB approval, angiograms of the CoW performed on a 3-T MR platform from 2016 to 2020 on patients 21 years or younger were retrospectively reviewed. Any patient with underlying arterial pathology that may affect the CoW was excluded. Patient age and gender at the time of imaging were obtained.
Results
In total, 592 pediatric CoW were assessed. Frequencies of completeness were calculated in two different fashions: scenario 1 where a CoW was characterized as complete even if it contained hypoplastic vessels (88.8%), and scenario 2 where it was characterized as complete after excluding hypoplastic vessels (44.0%). In both scenarios, our data showed that older age was more associated with an incomplete CoW (p < 0.0001). In addition, we found a higher percentage of males with an incomplete CoW compared with females (p < 0.0001).
Conclusions
The presence of a complete CoW is greater in our pediatric population than what has been reported in adults. The prevalence of an incomplete circle of Willis also increases significantly with age.
Keywords: Circle of Willis, MRI, Radiology
Introduction
The circle of Willis (CoW) is a circulatory anastomosis that supplies blood to the brain and surrounding structures. Named after Thomas Willis (1621–1675), it is the primary source of blood flow to the brain through its seven arterial segments. If one (or more) of the bridging segments is hypoplastic or absent, the capacity for collateral flow in the setting of large vessel occlusion may be decreased (Fig. 1). Since more robust collateral blood supply is associated with a greater chance of functional outcome following an ischemic stroke [1], the presence of an incomplete CoW has clear implications in this setting.
Fig. 1.
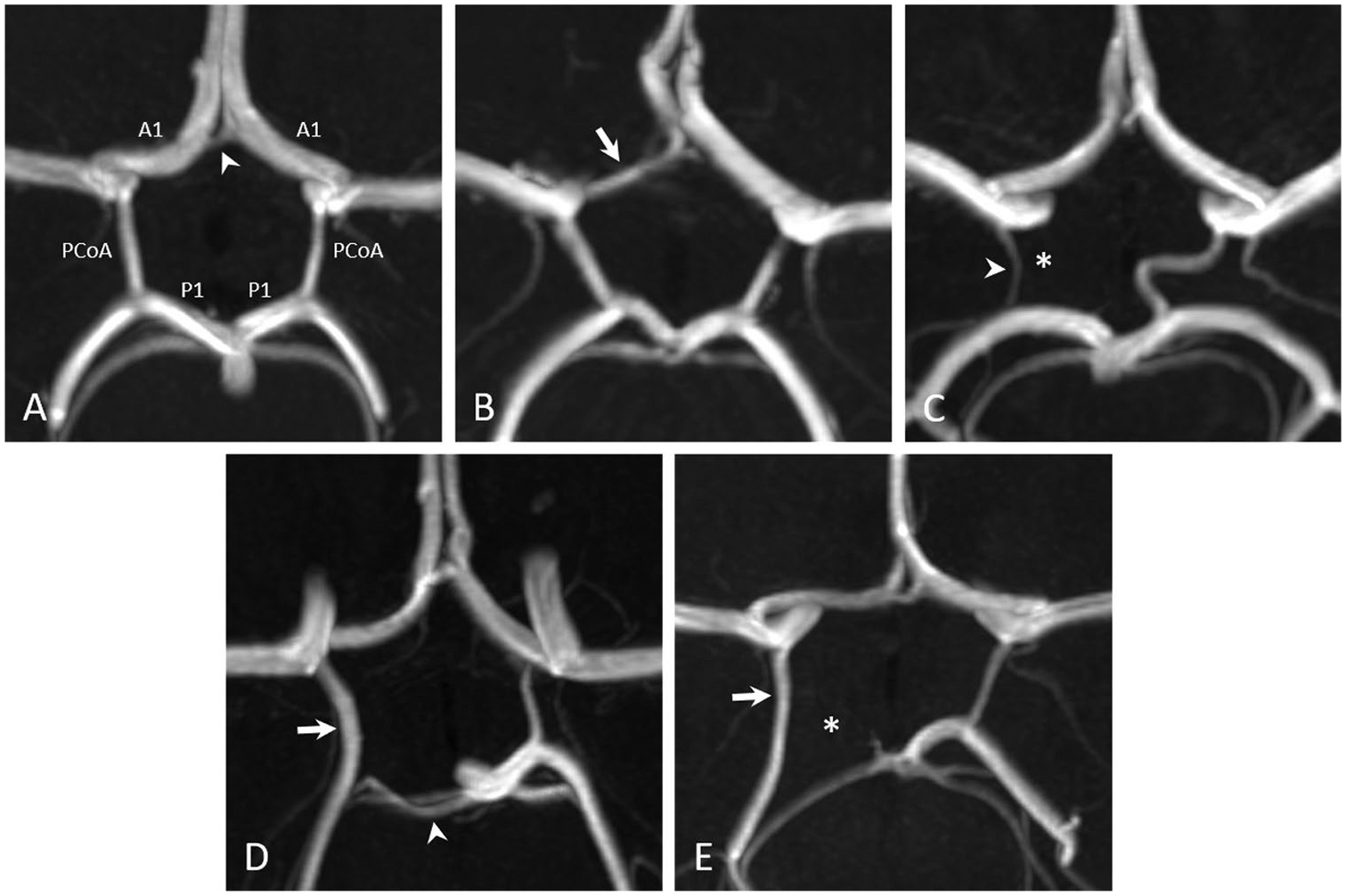
3D reconstructed time-of-flight magnetic resonance angiographic images of five different pediatric patients demonstrate (A) a complete circle of Willis (white arrowhead: anterior communicating artery), (B) a CoW with a hypoplastic right A1 segment (white arrow), (C) a CoW with an absent right posterior communicating artery (white asterisk; white arrowhead: right anterior choroidal artery), (D) a partial fetal-type right posterior cerebral artery (white arrow) with a hypoplastic right P1 segment (white arrowhead), and (E) a full fetal-type right posterior cerebral artery (white arrow) with an absent right P1 segment (white asterisk)
Imaging and autopsy studies in adults have reported wide ranges of a complete CoW [2–10]. An incomplete CoW has generally been thought to be congenital in etiology although more recent studies have shown that the rate of complete CoW decreases with increasing age in the adult population [7, 10]. Outside of the neonatal time period, the prevalence of a complete CoW in the pediatric population has not been well described [11]. Data on the rate of a complete CoW in children versus adults could aid in understanding the etiology of hypoplastic variants (congenital versus acquired) and inform data on differences in stroke outcome between these age populations. Our primary objective in this study is to determine the prevalence of a complete CoW in children utilizing modern magnetic resonance imaging techniques. A secondary objective is to identify if there is an age-related “loss” of CoW arterial segments.
Methods
Participants
Following IRB approval, MR angiograms (MRA) of the CoW performed on the Philips Ingenia 3-T MR platform from 2016 to 2020 were retrospectively reviewed. All MRAs were performed with 3D TOF technique. The cohort included patients 21 years or younger. Any patient with acute or chronic underlying arterial pathology that could affect the CoW was excluded. Patient age at the time of MRA and gender were obtained.
Image analysis
Images were reviewed by pediatric neuroradiologists to assess the integrity (presence or absence) and the caliber (normal or hypoplastic) of the CoW segments. Bilateral A1 anterior cerebral artery (ACA) segments, bilateral posterior communicating arteries (PCoAs), and bilateral proximal or horizontal (P1) posterior cerebral artery (PCA) segments were categorized as normal, hypoplastic, or absent (Fig. 1). The anterior communicating artery (ACoA) was designated present or absent, as its small size makes determination of hypoplasia difficult.
A vessel was categorized as absent if flow-related enhancement along the expected course of the artery was not identified. The A1 segments of the ACAs and P1 segments of the PCAs were labelled hypoplastic if the vessel was 50% smaller than the contralateral artery in the setting of a normal contralateral vessel or if it was visually attenuated when the contralateral vessel was also attenuated, resulting in bilateral hypoplasia. Classifying the PCoA as hypoplastic was more subjective, as it is typically small in caliber. Most commonly we reserved that classification for when the vessel was significantly attenuated, however still visible, or if smaller than the contralateral side, when the contralateral vessel was categorized as normal. In cases that were borderline, a consensus was reached after consultation with additional neuroradiologists.
CoW completeness was defined differently based on two different scenarios. In the first scenario, the CoW was considered incomplete if any segment was absent and complete if all segments were either normal or hypoplastic. In scenario 2, the CoW was classified as incomplete if any segment was absent or hypoplastic and complete only if all segments were normal.
Regarding presence of a fetal-type PCA (FTP), we adopted van Raamt’s definition [12]. FTP was determined when the PCA received the majority of its arterial supply from the anterior circulation via a prominent ipsilateral PCoA. This was then further classified as a full FTP if the ipsilateral P1 segment was absent or as a partial FTP if the ipsilateral P1 segment was hypoplastic (Fig. 1). Additional anatomic variants of the CoW were noted, particularly of the ACoA, including complex or plexiform, duplication, and accessory A2 segments.
Statistical analysis
Statistical analyses were performed using custom scripts in the R language (v3.6.0) using the tidyverse library. Significance between age and sex distributions was calculated by applying the Student’s t-test to distributions generated using bootstrap resampling (n = 1000). Importance of right and left PCoA vessels was identified using a decision tree model. Dependency of CoW completeness and right and left PCoA were confirmed using logistic regression. Significance threshold was set to p < 0.0001.
Results
A total of 592 pediatric CoW were assessed. The breakdown of males and females per age is listed in Table 1. The frequencies of absent, hypoplastic, and normal vessels for the major branches of the CoW are presented in Table 2. Frequencies of CoW completeness were 88.8% for scenario 1 and 44.0% for scenario 2 (as defined above). In both scenarios, our data showed that there was a relationship between age and completeness of CoW; older age was more associated with an incomplete CoW (p < 0.0001). In addition, in both scenarios, we found a higher percentage of males with an incomplete CoW compared with females (p < 0.0001).
Table 1.
Frequency of patient sex stratified by patient age
| Age (years) | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | |
| Female | 20 | 12 | 10 | 17 | 9 | 9 | 11 | 9 | 15 | 13 | 19 | 14 | 19 | 19 | 27 | 19 | 13 | 18 | 17 | 6 | 2 | 0 |
| Male | 26 | 14 | 12 | 12 | 16 | 13 | 15 | 19 | 14 | 12 | 13 | 12 | 17 | 12 | 25 | 23 | 18 | 13 | 7 | 4 | 0 | 1 |
| Total | 46 | 26 | 22 | 29 | 25 | 22 | 26 | 28 | 29 | 25 | 32 | 26 | 36 | 31 | 52 | 42 | 31 | 31 | 24 | 10 | 2 | 1 |
Table 2.
Vessel status
| Absent | Hypoplastic | Normal | |
|---|---|---|---|
| ACoA | 5 | - | 591 |
| (0.84%) | (−) | (99.16%) | |
| Right A1 | 1 | 38 | 557 |
| (0.17%) | (6.37%) | (93.46%) | |
| Left A1 | 3 | 30 | 563 |
| (0.50%) | (5.04%) | (94.46%) | |
| Right PCoA | 29 | 127 | 440 |
| (4.87%) | (21.31%) | (73.82%) | |
| Left PCoA | 34 | 116 | 446 |
| (5.71%) | (19.46%) | (74.83%) | |
| Right P1 | 1 | 49 | 546 |
| (0.17%) | (8.22%) | (91.61%) | |
| Left P1 | 2 | 42 | 552 |
| (0.34%) | (7.04%) | (92.62%) |
Under scenario 1, 8.1% of females had an incomplete CoW versus 14.4% of males. In addition, the average age of a patient with a complete CoW was younger than an incomplete CoW: 9.37 versus 10.76 years. Under scenario 2, 57.0% of males had an incomplete CoW versus 55.0% of females. Similar to scenario 1, the average age for complete CoW is younger than that with an incomplete CoW: 9.32 versus 9.69 years. Finally, the left and right PCoAs are the most frequently affected vessels (i.e., hypoplastic or absent) and demonstrate significant dependence on age and sex (p < 0.0001), congruent with CoW status.
Regarding fetal-type posterior cerebral artery (FTP), there were 49 patients with partial FTP (8.2%) and 1 with full FTP (0.2%) on the right; 42 patients with partial FTP (7.0%) and 2 with full FTP (0.3%) on the left. Eight patients had bilateral partial FTP (1.3%). There were 52 patients with ACoA variants (8.7%), 2 patients with duplicated A1 segments (0.3%), and 2 patients with duplicated PCoA (0.3%).
Discussion
The circle of Willis (CoW) is the major source of collateral blood flow to the brain through its seven major branches. Hypoplastic or absent vessels have implications in patients with ischemic stroke [1, 13–15]. Previous studies in adults have reported wide ranging percentages for a complete CoW in the adult population: 21–85% [2–10]. This is in part due to differences in technique: autopsy versus imaging; conventional angiography versus CTA versus MRA. In addition, different studies utilized different definitions for what was considered a complete CoW (i.e., whether or not to characterize a CoW with a hypoplastic vessel as complete versus incomplete). In our MRI-based study of a large pediatric cohort (n = 596), the majority of patients had a complete CoW: 88.8% when including patients that had hypoplastic vessels. This number dropped to 44% if we removed any CoW with a hypoplastic vessel.
Similar to other authors, we found a statistically significant association between increasing patient age and increased rate of incomplete CoW. Recognition of this apparent “loss” of vessels with age can aid in interpreting imaging studies and also may have implications for neurological conditions.
Interestingly, we also found significant differences in the rate of incomplete CoW between sexes. Overall, males had a higher rate of incomplete CoW than females. It is unclear if this has any association with males being at higher risk for all stroke types than females.
Conclusion
The presence of a complete CoW is greater in our pediatric population than what has been reported previously in adults. The prevalence of an incomplete circle of Willis also increases significantly with age. Age-related changes should be considered when interpreting intracranial MRAs in children.
Footnotes
Conflict of interest On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
- 1.Felling RJ, Sun LR, Maxwell EC, Goldenberg N, Bernard T (2017) Pediatric arterial ischemic stroke: epidemiology, risk factors, and management. Blood Cells Mol Dis 67:23–33. 10.1016/j.bcmd.2017.03.003 [DOI] [PubMed] [Google Scholar]
- 2.Hashemi SM, Mahmoodi R, Amirjamshidi A (2013) Variations in the anatomy of the Willis’ circle: a 3-year cross-sectional study from Iran (2006–2009). Are the distributions of variations of circle of Willis different in different populations? Result of an anatomical study and review of literature. Surg Neurol Int 4:65. 10.4103/2152-7806.112185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hindenes LB et al. (2020) Variations in the circle of Willis in a large population sample using 3D TOF angiography: the Tromso Study. PLoS One 15:e0241373. 10.1371/journal.pone.0241373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jalali Kondori B, Azemati F, Dadseresht S (2017) Magnetic resonance angiographic study of anatomic variations of the circle of Willis in a population in Tehran. Arch Iran Med 20:235–239. [PubMed] [Google Scholar]
- 5.Karatas A, Coban G, Cinar C, Oran I, Uz A (2015) Assessment of the circle of Willis with cranial tomography angiography. Med Sci Monit 21:2647–2652. 10.12659/MSM.894322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yeniceri IO, Cullu N, Deveer M, Yeniceri EN (2017) Circle of Willis variations and artery diameter measurements in the Turkish population. Folia Morphol (Warsz) 76:420–425. 10.5603/FM.a2017.0004 [DOI] [PubMed] [Google Scholar]
- 7.Zaninovich OA, Ramey WL, Walter CM, Dumont TM (2017) Completion of the circle of Willis varies by gender, age, and indication for computed tomography angiography. World Neurosurg 106:953–963. 10.1016/j.wneu.2017.07.084 [DOI] [PubMed] [Google Scholar]
- 8.Kapoor K, Singh B, Dewan LI (2008) Variations in the configuration of the circle of Willis. Anat Sci Int 83:96–106. 10.1111/j.1447-073X.2007.00216.x [DOI] [PubMed] [Google Scholar]
- 9.Klimek-Piotrowska W et al. (2016) A multitude of variations in the configuration of the circle of Willis: an autopsy study. Anat Sci Int 91:325–333. 10.1007/s12565-015-0301-2 [DOI] [PubMed] [Google Scholar]
- 10.Eaton RG et al. (2020) Demographic age-related variation in circle of Willis completeness assessed by digital subtraction angiography. Brain Circ 6:31–37. 10.4103/bc.bc_43_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malamateniou C et al. (2009) The anatomic variations of the circle of Willis in preterm-at-term and term-born infants: an MR angiography study at 3T. AJNR Am J Neuroradiol 30:1955–1962. 10.3174/ajnr.A1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Raamt AF, Mali WP, van Laar PJ, van der Graaf Y (2006) The fetal variant of the circle of Willis and its influence on the cerebral collateral circulation. Cerebrovasc Dis 22:217–224. 10.1159/000094007 [DOI] [PubMed] [Google Scholar]
- 13.Hoksbergen AW et al. (2003) Absent collateral function of the circle of Willis as risk factor for ischemic stroke. Cerebrovasc Dis 16:191–198. 10.1159/000071115 [DOI] [PubMed] [Google Scholar]
- 14.Jin ZN et al. (2016) CTA characteristics of the circle of Willis and intracranial aneurysm in a Chinese crowd with family history of stroke. Biomed Res Int 2016:1743794. 10.1155/2016/1743794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chuang YM, Liu CY, Pan PJ, Lin CP (2008) Posterior communicating artery hypoplasia as a risk factor for acute ischemic stroke in the absence of carotid artery occlusion. J Clin Neurosci 15:1376–1381. 10.1016/j.jocn.2008.02.002 [DOI] [PubMed] [Google Scholar]


