Abstract
Background and Objectives
Patients with acute ischemic stroke (AIS) treated with endovascular thrombectomy (EVT) in the late window (6–24 hours) can be evaluated with CT perfusion (CTP) or with noncontrast CT (NCCT) only. Whether outcomes differ depending on the type of imaging selection is unknown. We conducted a systematic review and meta-analysis comparing outcomes between CTP and NCCT for EVT selection in the late therapeutic window.
Methods
This study is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses 2020 guidelines. A systematic literature review of the English language literature was conducted using Web of Science, Embase, Scopus, and PubMed databases. Studies focusing on late-window AIS undergoing EVT imaged through CTP and NCCT were included. Data were pooled using a random-effects model. The primary outcome of interest was rate of functional independence, defined as modified Rankin scale 0–2. The secondary outcomes of interest included rates of successful reperfusion, defined as thrombolysis in cerebral infarction 2b–3, mortality, and symptomatic intracranial hemorrhage (sICH).
Results
Five studies with 3,384 patients were included in our analysis. There were comparable rates of functional independence (odds ratio [OR] 1.03, 95% CI 0.87–1.22; p = 0.71) and sICH (OR 1.09, 95% CI 0.58–2.04; p = 0.80) between the 2 groups. Patients imaged with CTP had higher rates of successful reperfusion (OR 1.31, 95% CI 1.05–1.64; p = 0.015) and lower rates of mortality (OR 0.79, 95% CI 0.65–0.96; p = 0.017).
Discussion
Although recovery of functional independence after late-window EVT was not more common in patients selected by CTP when compared with patients selected by NCCT only, patients selected by CTP had lower mortality.
Acute ischemic stroke (AIS) due to a large vessel occlusion (LVO) of the internal carotid artery or first part of the middle cerebral artery may result in significant morbidity and mortality, but treatment by endovascular thrombectomy (EVT) leads to substantially improved functional outcomes. Patients with AIS-LVO must undergo brain imaging to determine EVT eligibility. The randomized controlled trials that demonstrated the benefit of EVT in the late time window (6–24 hours from last known well) used CT perfusion (CTP) to determine EVT eligibility.1,2 Eligible patients should have evidence of a relatively small ischemic core on CTP1,2 and a salvageable penumbra on CTP2 or based on the severity of clinical symptoms.1
Yet, it is possible that CTP may be overly restrictive for patient selection, which may potentially exclude patients who could otherwise benefit from EVT.3 Furthermore, CTP is an advanced imaging modality that is resource intensive and not universally accessible. Due to the potential limitations of CTP in late-window AIS, the use of noncontrast CT (NCCT) ± CT angiography (CTA) has gained traction for late-window EVT patient selection. Several recent studies have attempted to compare CTP and NCCT for EVT selection in late-window AIS-LVO patients, but these studies have shown varying results.4-8 Therefore, we conducted a systematic review and meta-analysis to compare clinical outcomes between patients with AIS-LVO treated by EVT after CTP and NCCT imaging evaluation.
Methods
Search Strategy
On October 24, 2022, a systematic literature review was conducted within the Nested Knowledge AutoLit software (version 1.46; Nested Knowledge, Saint Paul, MN) from inception, using PubMed, Embase, Web of science, and Scopus.9 Combinations of keywords and/or Medical Subject Headings (MeSH) terms were used to conduct the search. Keywords and MeSH terms included the following: “imaging,” “advanced imaging,” “thrombectomy,” “late window,” “beyond 6 hours,” “extended,” “6–24 h,” and others (full search strategy can be found in eAppendix 1, links.lww.com/WNL/C725). In addition to the systematic literature review, we retrieved and evaluated references from articles included in the meta-analysis and from previous meta-analyses to ensure that all relevant articles were screened. This study is reported following the Preferred Reporting Items for Systematic Reviews and Meta-analyses 2020 guidelines.
Screening Process
Studies satisfying our predetermined PECO were included. The population was patients with late-window AIS undergoing EVT, the Exposure was CTP, the Control group was NCCT ± CTA, and the Outcomes of interest were as follows:
Primary outcome: functional independence defined as modified Rankin scale (mRS) 0–2
Secondary outcomes: successful reperfusion defined as thrombolysis in cerebral infarction (TICI) score 2b–3, symptomatic intracranial hemorrhage (sICH), and mortality
We excluded studies that did not compare patient outcomes based on imaging modality, studies that did not report late-window AIS, abstracts, case reports, or case series with less than 5 patients, studies that did not use EVT, letters to the editor and technical notes, duplicate records, and secondary analyses. Only studies that reported both CTP and NCCT ± CTA were included, and single-arm studies were excluded. “Late-window” AIS was defined as AIS presenting 6–24 hours from stroke onset or after last known well. Wake-up strokes were not included.
The initial title and abstract screening was followed by a full-text screening, both of which were performed by 2 authors. In both the initial and full-text screening stages, a third senior author was consulted to adjudicate any discrepancies.
Data Extraction
Data extraction was conducted within the Nested Knowledge AutoLit software by 2 authors to ensure accuracy. The extracted data included study characteristics, baseline characteristics of included patients, and outcomes of interest. After the initial extraction, an additional author assessed data for any inconsistencies or discrepancies.
Risk of Bias
The Newcastle Ottawa Scale was used to assess the risk of bias.10 Risk of bias assessment was conducted by 2 authors independently, and a third senior author adjudicated any conflicting assessment when necessary. The following rating system was used: “good” quality: 3 or 4 stars in selection domain AND 1 or 2 stars in comparability domain AND 2 or 3 stars in outcome/exposure domain; “fair” quality: 2 stars in selection domain AND 1 or 2 stars in comparability domain AND 2 or 3 stars in outcome/exposure domain; “poor” quality: 0 or 1 star in selection domain OR 0 stars in comparability domain OR 0 or 1 stars in outcome/exposure domain.11
Standard Protocol Approvals, Registrations, and Patient Consents
Ethics approval was not required for this study. This systematic review and meta-analysis is registered in PROSPERO (registration number: CRD42022377408).
Statistical Analysis
All data were analyzed using R software version 4.2.1. Using the “meta” package, we computed odds ratios (ORs), mean differences (MDs) and their corresponding 95% CIs using a random-effects model. To calculate the CI of the random-effects estimate, we used a restricted maximum likelihood estimator with Jackson modification of the Hartung-Knapp/Sidik-Jonkman variance correction.12-15 In case of significant effect sizes (p < 0.05), we conducted leave-one-out analyses as sensitivity analyses to explore the influence of individual studies on the main results.
Heterogeneity was assessed with Q statistic and I2 test. Heterogeneity was considered significant when I2 value >50% or p < 0.05.16,17 In case of significant heterogeneity, a sensitivity analysis was performed with removal of outlier studies to bring the heterogeneity to an insignificant level. Outlier studies were identified using the method previously described in the literature.18 Because less than 10 studies were included in the analysis, neither Egger test for assessing publication bias nor meta-regression was possible.19
Data Availability
Data that support this study are available from the corresponding author on reasonable request.
Results
Search and Screening Results
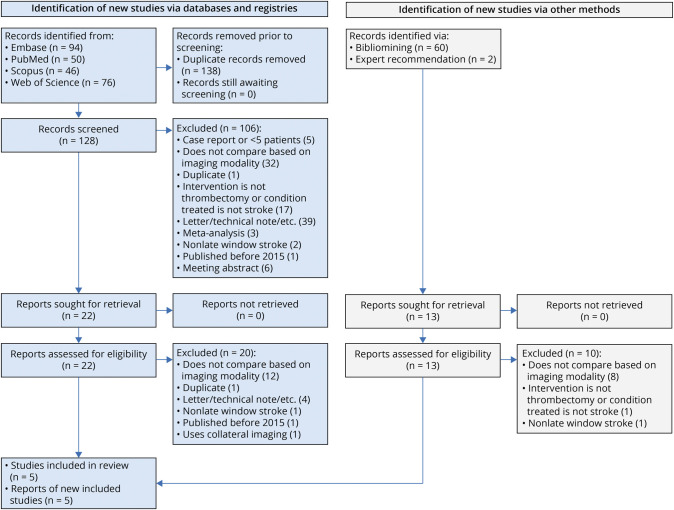
After the removal of 138 duplicate records, we retrieved 128 records for further screening. An additional 60 records were sought for retrieval through bibliomining, and 2 records were identified through expert recommendation. Ultimately, 5 studies were determined to satisfy our PECO and inclusion and exclusion criteria (Figure 1).
Figure 1. PRISMA Flowchart Detailing the Literature Review Process.
PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-analyses.
Study Characteristics and Risk of Bias
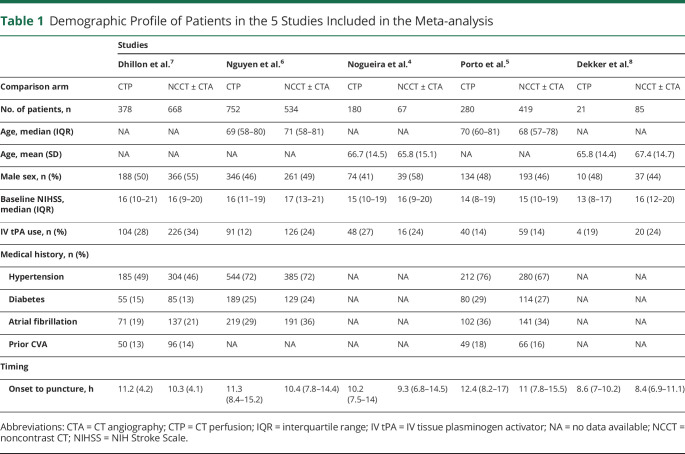
Of the 5 included studies, 3 used a retrospective design and all 5 were multicenter studies, with a total sample size of 3,384 patients. The size of the included studies ranged from 106 patients to 1,286 patients. All 5 of the studies included in our analysis were determined to have “good” quality (eTable 1, links.lww.com/WNL/C725). Patient characteristics are reported in Table 1. There were no significant differences between CTP-selected and NCCT-selected patients in the frequency of male sex (OR 0.88, 95% CI 0.76–1.01; p = 0.07), IV thrombolysis (OR 0.75, 95% CI 0.51–1.10; p = 0.14), and baseline NIHSS score (MD −0.86, 95% CI −1.97 to 0.25; p = 0.13). Compared with NCCT-selected patients, CTP-selected patients had a 45.6-minute greater onset to puncture time (MD 0.76 hours, 95% CI 0.43–1.09; p < 0.001) (eFigures 1 and 2).
Table 1.
Demographic Profile of Patients in the 5 Studies Included in the Meta-analysis
Dhillon et al. reported data from a national stroke registry. Alberta Stroke Program Early CT Scores (ASPECTS), clot location, and parenchymal imaging findings were not reported.7
Nguyen et al. reported data from a multinational cohort. ASPECTS were comparable between CTP-selected patients (median 8, interquartile range [IQR] 7–9) and NCCT-selected patients (median 8, IQR 7–9). Patients with NIH Stroke Scale (NIHSS) scores of 6 or more, occlusion of the internal carotid artery or proximal middle cerebral artery (M1/M2 segments), prestroke mRS scores of 0–2, and time last seen well (TLSW) to treatment of 6–24 hours were included.6
Nogueira et al. reported data from the Trevo Retriever Registry, a prospective multicenter registry. ASPECTS were comparable between CTP-selected patients (median 8, IQR 7–9) and NCCT-selected patients (median 8, IQR 7–9). Patients with occlusions involving the intracranial internal carotid artery or the M1/M2-segments of the middle cerebral artery, premorbid mRS score of 0–2, and TLSW to arterial puncture 6–24 hours were included.4
Porto et al. reported data from the Stroke Thrombectomy and Aneurysm registry. ASPECTS were comparable between CTP-selected patients (median 8, IQR 7–9) and NCCT-selected patients (median 8, IQR 7–9). Patients with NIHSS score of 6 or higher, pre-stroke mRS of 0–2, anterior circulation large vessel (internal carotid artery, middle cerebral artery M1/M2 segment) occlusion, and TLSW to arterial puncture of 6–24 hours were included.5
Dekker et al. reported data from the Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands (MR CLEAN) database, a multicenter, prospectively maintained registry. ASPECTS were comparable between CTP-selected patients (median 7, IQR 5–9) and NCCT-selected patients (median 9, IQR 7–10). Patients with anterior circulation large vessel (intracranial carotid artery, middle cerebral artery M1/M2 segment, or anterior cerebral artery A1/A2 segment) occlusion and TLSW or symptom onset to arterial puncture of beyond 6.5 hours were included.8
Functional Independence (mRS 0–2)
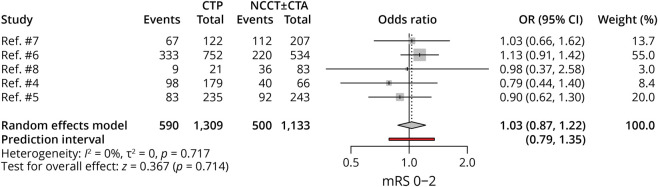
Five studies, with 2,442 patients, compared functional independence rates between CTP-selected and NCCT-selected patients. There were comparable rates of mRS 0–2 between the 2 groups (OR 1.03, 95% CI 0.87–1.22; p = 0.71), and no heterogeneity was noted among these studies (Figure 2).
Figure 2. Forest Plot of Rates of mRS 0–2.
CTA = CT angiography; CTP = CT perfusion; mRS = modified Rankin scale; NCCT = noncontrast CT; OR = odds ratio.
Successful Reperfusion (TICI 2b–3)
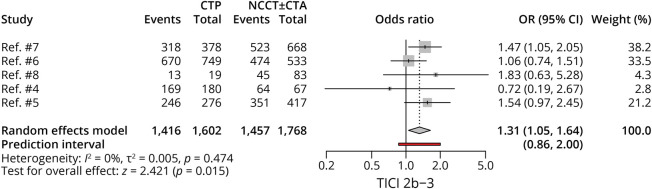
Five studies, with 3,370 patients, compared successful reperfusion rates between CTP-selected and NCCT-selected patients. Patients with CTP selection achieved higher rates of successful reperfusion (OR 1.31, 95% CI 1.05–1.64; p = 0.015), with no heterogeneity noted among studies (Figure 3).
Figure 3. Forest Plot of Rates of TICI 2b–3.
CTA = CT angiography; CTP = CT perfusion; NCCT = noncontrast CT; OR = odds ratio; TICI = thrombolysis in cerebral infarction.
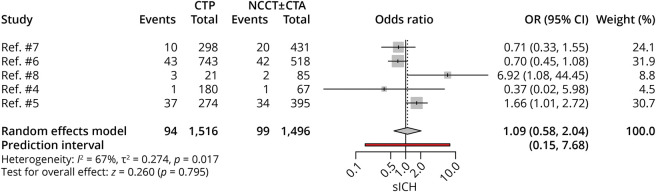
sICH
Five studies, with 3,012 patients, compared sICH rates between CTP-selected and NCCT-selected patients. There were comparable rates of sICH between the 2 groups (OR 1.09, 95% CI 0.58–2.04; p = 0.80); however, there was significant heterogeneity noted among studies (I2 = 67%, p = 0.017) (Figure 4). On removal of the outlier study,5 the results still yielded comparable rates of sICH between the 2 groups (OR 0.76, 95% CI 0.52–1.10; p = 0.15) (eFigures 3 and 4, links.lww.com/WNL/C725).
Figure 4. Forest Plot of Rates of sICH.
CTA = CT angiography; CTP = CT perfusion; NCCT = noncontrast CT; OR = odds ratio; sICH = symptomatic intracranial hemorrhage.
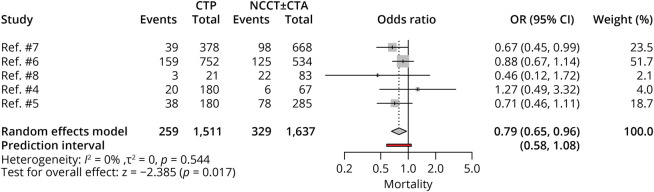
Mortality
Five studies, with 3,148 patients, compared mortality rates between CTP-selected and NCCT-selected patients. Patients with CTP selection had lower rates of mortality (OR 0.79, 95% CI 0.65–0.96; p = 0.017), with no heterogeneity noted among studies (Figure 5).
Figure 5. Forest Plot of Rates of Mortality.
CTA = CT angiography; CTP = CT perfusion; NCCT = noncontrast CT; OR = odds ratio.
Discussion
In this systematic review and meta-analysis, we found that patients with AIS-LVO selected for EVT in the late therapeutic window by CTP achieved similar rates of functional independence and had similar rates of sICH when compared with patients selected by NCCT only, despite having higher rates of successful reperfusion. Our results are important because they suggest that NCCT might be sufficient to select patients with AIS for EVT during the late therapeutic window.
The main finding of our meta-analysis is the comparable rates of functional independence among patients selected for EVT in the late window using CTP or NCCT. This finding was consistent across all 5 studies included. Our meta-analysis showed higher rates of successful reperfusion and lower rates of mortality among patients selected by CTP. The higher rate of reperfusion seen in the CTP group is noteworthy because successful reperfusion is strongly associated with better functional outcomes in patients undergoing EVT.20,21 Thus, better functional outcomes would have been expected in the CTP group based on the greater rate of successful reperfusion in this group; yet, such difference was not seen. The difference in mortality in favor of the CTP group remains unexplained, though one could speculate that centers with access to CTP may also have greater intensive care resources. Without individual patient data, it is difficult to ascertain why lower rates of mortality were observed in CTP-imaged patients while functional independence was comparable between NCCT-imaged and CTP-imaged patients. It is possible that the analysis for mortality was underpowered, given that deaths are less frequent than functional independence.
Of note, a recent meta-analysis found that there was no difference between CTP and NCCT not only for functional independence and sICH but also for successful reperfusion and mortality.22 However, this previous meta-analysis experienced a high degree of potential bias because single-arm studies were included. A meta-analysis from 2018 analyzing randomized trials found that patients undergoing EVT within 8 hours of symptom onset with advanced imaging achieved higher rates of successful reperfusion, which is consistent with our present findings.23
Utilization of NCCT would provide multiple clinical and logistic benefits. NCCT is more cost-effective and readily available compared with more advanced imaging, such as CTP.24,25 In addition, CTP is associated with potential treatment delays, complications due to contrast administration, and increased radiation exposure.26-28 Noninferiority of NCCT for late-window AIS would therefore increase access to EVT for patients who are at facilities where CTP is not available. However, it remains to be determined whether a substantial number of patients selected for late-window EVT based on NCCT are undergoing futile treatment. In early time windows (0–6 hours), the FRAME study found that approximately 20% of patients who underwent EVT lacked evidence of a salvageable penumbra, and the frequency of favorable outcomes in these patients was no better than that in patients historically treated with medical therapy alone.29 Further study is needed to determine whether a similar issue may occur in the late window. In addition, evaluating ASPECTS using NCCT requires expertise, and centers contemplating the use of NCCT may consider the need for adequate training to ensure that scoring is accurate.
The use of NCCT may be a reasonable choice for late-window AIS, but there are yet no randomized data to support this imaging triage pathway. The TWIST trial recently reported that in patients with wake-up stroke (including AIS due to LVO) triaged with NCCT, there was no benefit from IV tenecteplase over no thrombolysis.30 It is possible that patients selected with CTP have more favorable collateral blood flow, which may account for the higher rates of successful reperfusion after EVT in these patients. However, results for the DEFUSE-3 trial showed that good collaterals were associated with a reduced growth of the ischemic core but not with better functional outcome.31 Of note, most patients selected for EVT in the late window would be expected to have a CTA along with an NCCT. Yet, the value of collateral rating or core assessment on CTA was not evaluated in the studies included in our meta-analysis.
Our meta-analysis has limitations. Because comparing imaging modalities for late-window EVT is a topic that has gained interest recently, we were able to include only 5 studies in our analysis, most of which were retrospective analyses. Therefore, the impact of any individual study may have disproportionally influenced the results of our analyses. The low number of studies also meant we were unable to perform meta-regression to test for the effect of different individual confounders. We did not have access to patient-level data, further compromising our ability to test for confounders. Similar and relatively high ASPECTS in patients selected with both imaging modalities (median ASPECTS of 8) implies that patients in the included studies had small cores, which may have limited the difference in treatment effect.32 There was heterogeneity in the time frame in which outcomes were reported, with Dhillon et al. reporting functional independence at 6 months post-EVT and mortality in the hospital. Finally, we were unable to account for single-phase and multi-phase CTA due to inconsistent reporting among included studies.
In this meta-analysis, we found that patients with AIS treated with EVT in the late window (6–24 hours) had similar rates of functional independence and sICH but a lower rate of successful reperfusion and higher rate of mortality, when selected by NCCT when compared with CTP. While these findings suggest that that NCCT may be a valid alternative to CTP when evaluating EVT candidacy during the late therapeutic window, prospective and randomized studies comparing CTP and NCCT are needed to confirm this possibility.
Acknowledgment
The authors acknowledge the Nested Knowledge meta-analytical software.
Glossary
- AIS
acute ischemic stroke
- ASPECTS
Alberta Stroke Program Early CT Scores
- CTA
CT angiography
- CTP
CT perfusion
- EVT
endovascular thrombectomy
- IQR
interquartile range
- LVO
large vessel occlusion
- MD
mean difference
- MeSH
Medical Subject Headings
- mRS
modified Rankin scale
- NCCT
noncontrast CT
- NIHSS
NIH Stroke Scale
- OR
odds ratio
- sICH
symptomatic intracranial hemorrhage
- TICI
thrombolysis in cerebral infarction
- TLSW
time last seen well
Appendix. Authors
Footnotes
Editorial, page 1039
Study Funding
No targeted funding reported.
Disclosure
W. Brinjikji holds equity in Nested Knowledge, Superior Medical Editors, Piraeus Medical, Sonoris Medical, and MIVI Neurovascular; he receives royalties from Medtronic and Balloon Guide Catheter Technology; he receives consulting fees from Medtronic, Stryker, Imperative Care, Microvention, MIVI Neurovascular, Cerenovus, Asahi, and Balt; he serves in a leadership or fiduciary role for MIVI Neurovascular, Marblehead Medical LLC, Interventional Neuroradiology (Editor in Chief), Piraeus Medical, and WFITN. J.J. Heit is a consultant for Medtronic and MicroVention, a member of the medical and scientific advisory board for iSchemaView, and a member of the data safety monitoring board for Vesalio. A.A. Rabinstein serves in the CEC committee for trials sponsored by Boston Scientific and has participated in advisory board meetings for Astra Zeneca and Novo Nordisk. D.F. Kallmes holds equity in Nested Knowledge, Superior Medical Editors, and Conway Medical, Marblehead Medical, and Piraeus Medical. He receives grant support from MicroVention, Medtronic, Balt, and Insera Therapeutics; has served on the Data Safety Monitoring Board for Vesalio; and received royalties from Medtronic. The other authors report no relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378(1):11-21. doi: 10.1056/NEJMoa1706442 [DOI] [PubMed] [Google Scholar]
- 2.Albers GW, Marks MP, Kemp S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378(8):708-718. doi: 10.1056/NEJMoa1713973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boned S, Padroni M, Rubiera M, et al. Admission CT perfusion may overestimate initial infarct core: the ghost infarct core concept. J Neurointerv Surg. 2017;9(1):66-69. doi: 10.1136/neurintsurg-2016-012494 [DOI] [PubMed] [Google Scholar]
- 4.Nogueira RG, Haussen DC, Liebeskind D, et al. Stroke imaging selection modality and endovascular therapy outcomes in the early and extended time windows. Stroke. 2021;52(2):491-497. doi: 10.1161/STROKEAHA.120.031685 [DOI] [PubMed] [Google Scholar]
- 5.Porto GBF, Chen C-J, Al Kasab S, et al. Association of noncontrast computed tomography and perfusion modalities with outcomes in patients undergoing late-window stroke thrombectomy. JAMA Netw Open. 2022;5(11):e2241291. doi: 10.1001/jamanetworkopen.2022.41291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nguyen TN, Abdalkader M, Nagel S, et al. Noncontrast computed tomography vs computed tomography perfusion or magnetic resonance imaging selection in late presentation of stroke with large-vessel occlusion. JAMA Neurol. 2022;79(1):22-31. doi: 10.1001/jamaneurol.2021.4082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dhillon PS, Butt W, Podlasek A, et al. Perfusion imaging for endovascular thrombectomy in acute ischemic stroke is associated with improved functional outcomes in the early and late time windows. Stroke. 2022;53(9):2770-2778. doi: 10.1161/STROKEAHA.121.038010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dekker L, Venema E, Pirson FAV, et al. Endovascular treatment in anterior circulation stroke beyond 6.5 hours after onset or time last seen well: results from the MR CLEAN Registry. Stroke Vasc Neurol. 2021;6(4):572-580. doi: 10.1136/svn-2020-000803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLos Med. 2009;6(7):e1000100. doi: 10.1371/journal.pmed.1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stang A Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603-605. doi: 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 11.Penson D, Krishnaswami S, Jules A, Seroogy J, McPheeters M. Newcastle-Ottawa Quality Assessment Form for Cohort Studies. Ottawa Hospital Research Institute; 2012. [Google Scholar]
- 12.Röver C, Knapp G, Friede T. Hartung-Knapp-Sidik-Jonkman approach and its modification for random-effects meta-analysis with few studies. BMC Med Res Methodol. 2015;15(1):99. doi: 10.1186/s12874-015-0091-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riley RD, Stewart LA, Tierney JF. Individual participant data meta-analysis for healthcare research. In: Riley RD, Stewart LA, Tierney JF, eds. Individual Participant Data Meta‐Analysis. Wiley; 2021:1-6. [Google Scholar]
- 14.Jackson D, Law M, Rücker G, Schwarzer G. The Hartung-Knapp modification for random-effects meta-analysis: a useful refinement but are there any residual concerns? Stat Med. 2017;36(25):3923-3934. doi: 10.1002/sim.7411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siemens W, Meerpohl JJ, Rohe MS, Buroh S, Schwarzer G, Becker G. Reevaluation of statistically significant meta-analyses in advanced cancer patients using the Hartung-Knapp method and prediction intervals-A methodological study. Res Synth Methods. 2022;13(3):330-341. doi: 10.1002/jrsm.1543 [DOI] [PubMed] [Google Scholar]
- 16.Higgins JP, Green S. Identifying and measuring heterogeneity. In: Higgins JP, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons; 2011. [Google Scholar]
- 17.Hashan MR, Ghozy S, El-Qushayri AE, Pial RH, Hossain MA, Al Kibria GM. Association of dengue disease severity and blood group: a systematic review and meta-analysis. Rev Med Virol. 2021;31:1-9. doi: 10.1002/rmv.2147 [DOI] [PubMed] [Google Scholar]
- 18.Viechtbauer W, Cheung MW. Outlier and influence diagnostics for meta-analysis. Res Synth Methods. 2010;1(2):112-125. doi: 10.1002/jrsm.11 [DOI] [PubMed] [Google Scholar]
- 19.Higgins JP, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons; 2019. [Google Scholar]
- 20.Manning NW, Warne CD, Meyers PM. Reperfusion and clinical outcomes in acute ischemic stroke: systematic review and meta-analysis of the stent-retriever-based, early window endovascular stroke trials. Front Neurol. 2018;9:301. doi: 10.3389/fneur.2018.00301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mohammaden MH, Haussen DC, Pisani L, et al. Lack of reperfusion rather than number of passes defines futility in stroke thrombectomy: a matched case-control study. Stroke. 2021;52(9):2757-2763. doi: 10.1161/STROKEAHA.120.033539 [DOI] [PubMed] [Google Scholar]
- 22.Sequeiros JM, Rodriguez-Calienes A, Chavez-Malpartida SS, et al. Stroke imaging modality for endovascular therapy in the extended window: systematic review and meta-analysis. J Neurointerv Surg. 2022. doi: 10.1136/neurintsurg-2022-018896 [DOI] [PubMed] [Google Scholar]
- 23.Tsivgoulis G, Katsanos AH, Schellinger PD, et al. Advanced neuroimaging in stroke patient selection for mechanical thrombectomy. Stroke. 2018;49(12):3067-3070. doi: 10.1161/strokeaha.118.022540 [DOI] [PubMed] [Google Scholar]
- 24.Boltyenkov AT, Martinez G, Pandya A, et al. Cost-consequence analysis of advanced imaging in acute ischemic stroke care. Front Neurol. 2021;12:774657. doi: 10.3389/fneur.2021.774657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martinez G, Katz JM, Pandya A, et al. Cost-effectiveness study of initial imaging selection in acute ischemic stroke care. J Am Coll Radiol. 2021;18(6):820-833. doi: 10.1016/j.jacr.2020.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheth KN, Terry JB, Nogueira RG, et al. Advanced modality imaging evaluation in acute ischemic stroke may lead to delayed endovascular reperfusion therapy without improvement in clinical outcomes. J Neurointerv Surg. 2013;5(suppl 1):i62-i65. doi: 10.1136/neurintsurg-2012-010512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meinel TR, Kaesmacher J, Mosimann PJ, et al. Association of initial imaging modality and futile recanalization after thrombectomy. Neurology. 2020;95(17):e2331-e2342. doi: 10.1212/wnl.0000000000010614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wichmann JL, Katzberg RW, Litwin SE, et al. Contrast-induced nephropathy. Circulation. 2015;132(20):1931-1936. doi: 10.1161/CIRCULATIONAHA.115.014672 [DOI] [PubMed] [Google Scholar]
- 29.Olivot JM, Albucher JF, Guenego A, et al. Mismatch profile influences outcome after mechanical thrombectomy. Stroke. 2021;52(1):232-240. doi: 10.1161/STROKEAHA.120.031929 [DOI] [PubMed] [Google Scholar]
- 30.Roaldsen MB, Eltoft A, Wilsgaard T, et al. Safety and efficacy of tenecteplase in patients with wake-up stroke assessed by non-contrast CT (TWIST): a multicentre, open-label, randomised controlled trial. Lancet Neurol. 2023;22(2):117-126. doi: 10.1016/S1474-4422(22)00484-7 [DOI] [PubMed] [Google Scholar]
- 31.de Havenon A, Mlynash M, Kim-Tenser MA, et al. Results from DEFUSE 3: good collaterals are associated with reduced ischemic core growth but not neurologic outcome. Stroke. 2019;50(3):632-638. doi: 10.1161/strokeaha.118.023407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim-Tenser M, Mlynash M, Lansberg MG, et al. CT perfusion core and ASPECT score prediction of outcomes in DEFUSE 3. Int J Stroke. 2021;16:288-294. doi: 10.1177/1747493020915141 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data that support this study are available from the corresponding author on reasonable request.