Abstract
Background and Objectives
Cluster headache and migraine have circadian features at multiple levels (cellular, systems, and behavioral). A thorough understanding of their circadian features informs their pathophysiologies.
Methods
A librarian created search criteria in MEDLINE Ovid, Embase, PsycINFO, Web of Science, and Cochrane Library. Two physicians independently performed the remainder of the systematic review/meta-analysis using Preferred Reporting Items for Systematic Review and Meta-Analyses guidelines. Separate from the systematic review/meta-analysis, we performed a genetic analysis for genes with a circadian pattern of expression (clock-controlled genes or CCGs) by cross-referencing genome-wide association studies (GWASs) of headache, a nonhuman primate study of CCGs in a variety of tissues, and recent reviews of brain areas relevant in headache disorders. Altogether, this allowed us to catalog circadian features at the behavioral level (circadian timing, time of day, time of year, and chronotype), systems level (relevant brain areas where CCGs are active, melatonin and corticosteroid levels), and cellular level (core circadian genes and CCGs).
Results
For the systematic review and meta-analysis, 1,513 studies were found, and 72 met the inclusion criteria; for the genetic analysis, we found 16 GWASs, 1 nonhuman primate study, and 16 imaging reviews. For cluster headache behavior, meta-analyses showed a circadian pattern of attacks in 70.5% (3,490/4,953) of participants across 16 studies, with a clear circadian peak between 21:00 and 03:00 and circannual peaks in spring and autumn. Chronotype was highly variable across studies. At the systems level, lower melatonin and higher cortisol levels were reported in cluster headache participants. At the cellular level, cluster headache was associated with core circadian genes CLOCK and REV-ERBα, and 5 of the 9 cluster headache susceptibility genes were CCGs. For migraine behavior, meta-analyses showed a circadian pattern of attacks in 50.1% (2,698/5,385) of participants across 8 studies, with a clear circadian trough between 23:00 and 07:00 and a broad circannual peak between April and October. Chronotype was highly variable across studies. At the systems level, urinary melatonin levels were lower in participants with migraine and even lower during an attack. At the cellular level, migraine was associated with core circadian genes CK1δ and RORα, and 110 of the 168 migraine susceptibility genes were CCGs.
Discussion
Cluster headache and migraine are highly circadian at multiple levels, reinforcing the importance of the hypothalamus. This review provides a pathophysiologic foundation for circadian-targeted research into these disorders.
Trial Registration Information
The study was registered with PROSPERO (registration number CRD42021234238).
Two primary headache disorders—cluster headache and migraine—share pathophysiology (hypothalamic and trigeminovascular activation)1,2 and treatments (corticosteroids, galcanezumab, melatonin, noninvasive vagus nerve stimulation, sumatriptan, topiramate, and zolmitriptan).3,4 They are also intermittent pain disorders, and the timing of attacks appears to follow a 24-hour pattern: both cluster headache and migraine attacks have been proposed to occur in the late evening or early morning.5,6 Many of these features—hypothalamic activation, treatment with corticosteroids and melatonin, and a 24-hour pattern of attacks—suggest a role for the circadian clock.
The circadian clock, or our internal 24-hour biological timer, at its core consists of a handful of proteins that regulate each other's expression in a process that takes approximately 24 hours7 (Figure 1A). This transcriptional-translational feedback loop, which involves the transcription and translation of core clock genes, is present in virtually every cell type. Cell types often synchronize together such that specific organs and specific brain nuclei have their own circadian rhythms. These organs and brain nuclei are ordered hierarchically, and the master biological clock is the suprachiasmatic nucleus (SCN) in the hypothalamus (Figure 1B). The SCN receives specific inputs, such as light and exercise, that align the internal clock to the external world; these specific inputs are called zeitgebers. The SCN then synchronizes the body's circadian systems via hormonal communications and neuronal connections. This synchronization ultimately leads to circadian regulation of human physiology such as the daily rhythm of blood pressure and the timing of neurologic and other diseases (Figure 1C).
Figure 1. Circadian Rhythms.
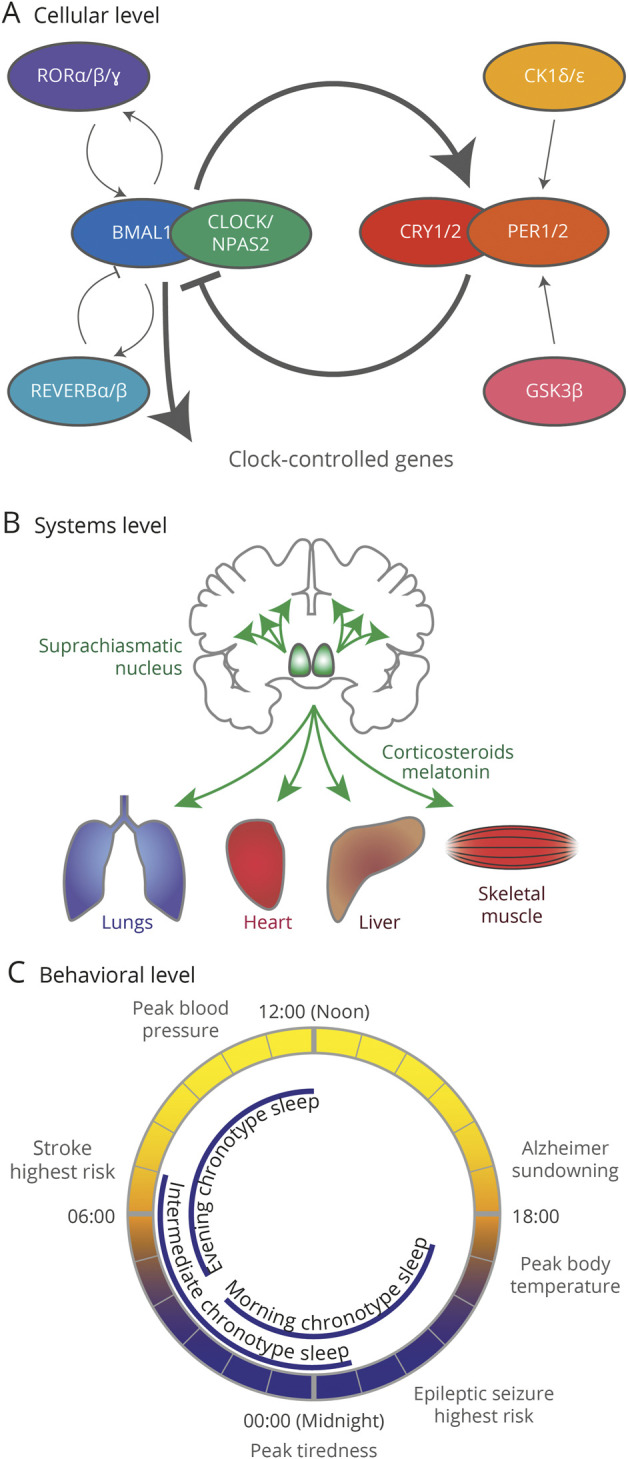
(A) The 24-hour circadian transcriptional-translational feedback loop. Full gene names: CLOCK = circadian locomotor output cycles kaput; NPAS2 = neuronal PAS domain protein 2; BMAL1 = brain and muscle ARNT like 1; PER = period; CRY = cryptochrome; REV-ERB = reverse strand of erb; ROR = retinoid acid–related orphan receptor; CK1 = casein kinase 1; GSK3 = glycogen synthase kinase 3. (B) The suprachiasmatic nucleus (green) acts as a master clock, coordinating the clocks of other brain areas and other organs through neuronal connections and hormones (especially corticosteroids and melatonin). (C) Peak timing of physiologic processes and neurologic diseases and chronotype sleep patterns.
Cluster headache has strong links to the circadian system, ranging from the behavioral (a very predictable timing of headaches5) to the genetic (variants in the core clock genes circadian locomotor output cycles kaput [CLOCK] and CRY1 have been associated with cluster headache8,9) to the pharmacologic (corticosteroids, melatonin, and lithium are all effective treatments and are all strong modulators of the circadian transcriptional-translational feedback loop10). Thus, a systematic review of the circadian features of cluster headache, while useful, is perhaps unsurprising. However, migraine has separate lines of research that suggest it too is strongly associated with the circadian system. Many of the most common migraine triggers (bright light, exercise, skipping meals, and sleep-wake changes like undersleeping and oversleeping)11 are also well-known zeitgebers.12 In addition, 2 small studies provide convincing evidence of a circadian influence on migraine. First, variations in a core clock gene (casein kinase delta, CK1δ, also known as CSNK1d) are associated with migraine and a circadian rhythm disorder (familial advanced sleep phase syndrome).13 Second, in a pilot study of chronic migraine, circadian misalignment (via dim light melatonin onset) is associated with an increased frequency of attacks while sleep parameters (via actigraphy) were not.14 This suggests that the circadian cycle, not sleep, is associated with migraine attack frequency.
There are several aspects of the circadian clock worth noting when studying headache disorders. First, there are multiple circadian behaviors and physiologic changes than can be investigated, ranging from the timing of attacks to circadian blood pressure changes. We chose to focus on 2—the timing of headache attacks and chronotype—as these are more directly linked to the master clock and other neural clocks. Animal studies of SCN ablation and SCN transplantation have shown dramatic changes in the timing of activities and sleep.15-17 In contrast, circadian blood pressure changes could implicate the SCN, the autonomic system (which is known to be involved in cluster headache and migraine1,2), and/or changes in non-neurologic clocks such as the cardiac circadian clock. Second, a specific analysis of corticosteroids, melatonin, and core clock genes is important in headache disorders. Corticosteroids (a daytime signal) and melatonin (a nighttime signal) are primary circadian-related hormones10 and are also effective treatments in cluster headache and migraine.3,4,18 As above, variations in the core clock gene CK1δ are associated with both advanced sleep-wake phase disorder and migraine.13 Finally, core clock genes have downstream effects on gene expression. At least 43% of all protein-coding genes in mice19 and 82% of all protein-coding genes in nonhuman primates20 follow a circadian pattern of expression in 1 or more tissues and are called clock-controlled genes (CCGs). CCGs may be different in each organ and brain area, and one of the most comprehensive investigations of CCGs is a study of gene expression in a variety of baboon tissues collected every 2 hours for 24 hours.20 The baboon is the most closely related species to humans for which comprehensive tissue data are available.
Understanding the role of the circadian system in cluster headache and migraine would expand our understanding of the hypothalamus and the initiation of attacks. However, few studies have systematically examined multiple aspects of the circadian system. In this study, we synthesized the current circadian features of cluster headache and migraine, focusing on the timing of attacks, chronotype, corticosteroids, melatonin, core circadian genes, and CCGs.
Methods
Systematic Review
Protocol and Eligibility Criteria
The study was registered with PROSPERO (registration number CRD42021234238), and we followed Guidelines for the Preferred Reporting Items for Systematic Review and Meta-Analyses.21 For details of inclusion and exclusion criteria, see eMethods in eAppendix 1 (links.lww.com/WNL/C703).
Information Sources and Search
An experienced librarian (author E.S.) developed searches in 5 databases: the main search was conducted in MEDLINE Ovid on April 16, 2021 (search terms available in eTable 1, links.lww.com/WNL/C709) and then translated into Cochrane Library, Embase, PsycINFO, and Web of Science on April 20, 2021. Search terms focused on cluster headache, migraine, and circadian terms (for details, see eMethods in eAppendix 1, links.lww.com/WNL/C703). The librarian-developed search was not the only source of studies; a small number of additional studies were found when assessing bias (see the Risk of Bias Across Studies section).
Study Selection, Data Collection Process, and Data Items
Screening was performed independently by a senior neurology resident (author B.B.) and a headache specialist (author M.J.B.), first by title and abstract in Rayyan22 and then by full-length articles. The same 2 physicians then extracted data elements from each study (for details, see eMethods in eAppendix 1, links.lww.com/WNL/C703).
Risk of Bias in Individual Studies
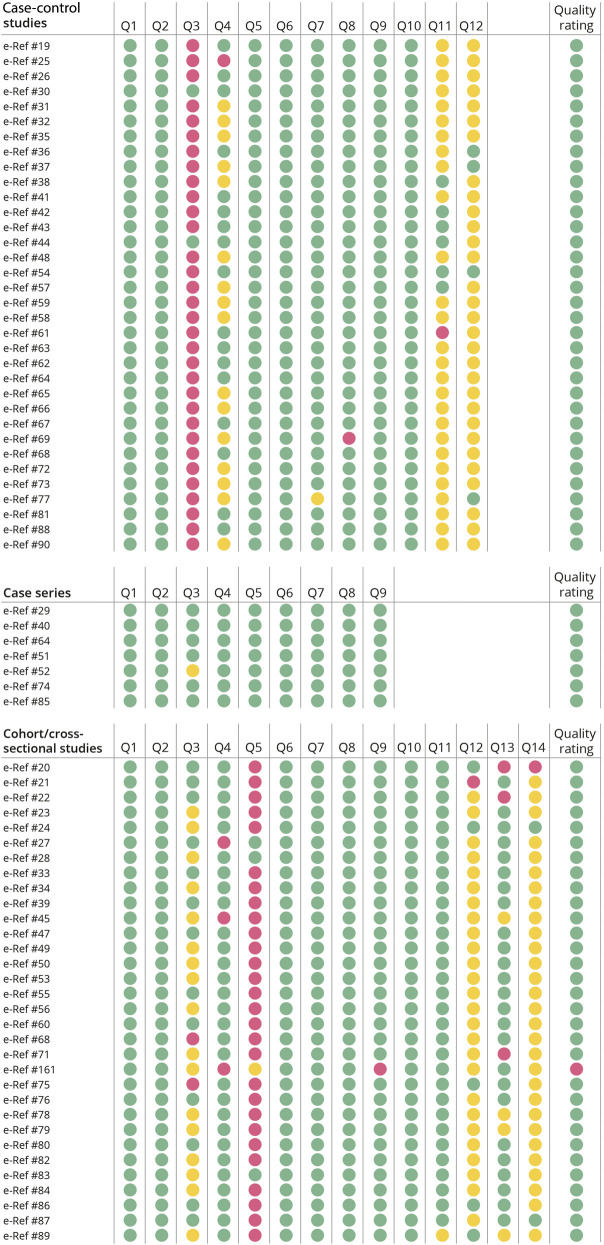
Two authors (B.B. and M.J.B.) independently used the NIH quality assessment tools for case series studies, case-control studies, and observational cohort and cross-sectional studies23 (Figure 2 and eTable 2, links.lww.com/WNL/C709). To reduce the risk of bias, we excluded 1 study,e161 which was given a poor quality rating.
Figure 2. Quality Assessment.
NIH quality assessment tools for case series studies (top), case-control studies (middle), and observational cohort and cross-sectional studies (bottom). Color codes: green indicates yes, not applicable, good quality rating, and fair quality rating; yellow indicates unclear or cannot determine; red indicates no or poor quality rating. One study (Romo-Nava 2010) had headache data that received a poor quality rating (although this rating is not a reflection of its other data); this study was excluded from the systematic review and meta-analysis. Details are available in eTable 2 (links.lww.com/WNL/C709).
Risk of Bias Across Studies
Several items such as core circadian genes and hormonal changes could not easily be assessed with funnel plots. Instead, we reviewed the literature to find systematic reviews, narrative reviews, and other articles that had previously attempted to catalog circadian data in cluster headache and migraine (see eResults in eAppendix 1, links.lww.com/WNL/C703). This analysis led to several studies being added to the systematic review/meta-analysis outside of the librarian's established search. The studies that were added this way were still assessed for bias within studies by a single author (M.J.B.) using the NIH quality assessment tools.
Summary Measures and Synthesis of Results
Summary measures were (1) proportion of participants with headache with a circadian pattern of attacks, (2) the specific time of day of attacks, (3) the specific time of year of attacks, (4) chronotype data, (5) core clock gene data, and (6) circadian-related hormones (corticosteroids and melatonin). Meta-analyses were performed for items 1–3. Meta-analyses were planned a priori for items 4–6 but were not performed because the data could not be combined. Specifically, item 4 used a variety of measures including the Morningness-Eveningness Questionnaire, reduced Morningness-Eveningness Questionnaire, Munich Chronotype Questionnaire, self-reported chronotype, and self-reported bedtime. Item 5 examined different genes that could not be directly compared. Item 6 examined different samples (such as urine or serum) at different time points.
Genetic Analysis
Detailed methodology is available in eMethods in eAppendix 1 (links.lww.com/WNL/C703). In brief, for susceptibility genes, we used the most recently available genome-wide association studies (GWASs) and additional GWASs from the GWAS catalog (ebi.ac.uk/gwas/) on April 19, 2022. For brain areas relevant to migraine and cluster headache, we reviewed recent imaging studies (between 2019 and 2022) in PubMed (pubmed.ncbi.nlm.nih.gov/) and extracted data from the large summary tables.
Statistical Measures
For the time of day and time of year, we calculated unimodal orientation using the Rayleigh test; we compared cluster headache and migraine using the Watson 2 test. These tests were calculated using the circular package (version 0.4-95) in R (version 4.1.3). Rose plots were drawn using ggplot2 (version 3.3.6) in R, and Photoshop (version 22.5.1; Adobe Inc., San Jose, CA) was used only to change the colors of the rose plots. For all other calculations, descriptive statistics were used.
Data Availability
All data were obtained, already anonymized, from published studies. Data not provided in this article may be shared at the request of any qualified investigator for the purposes of replicating results.
Results
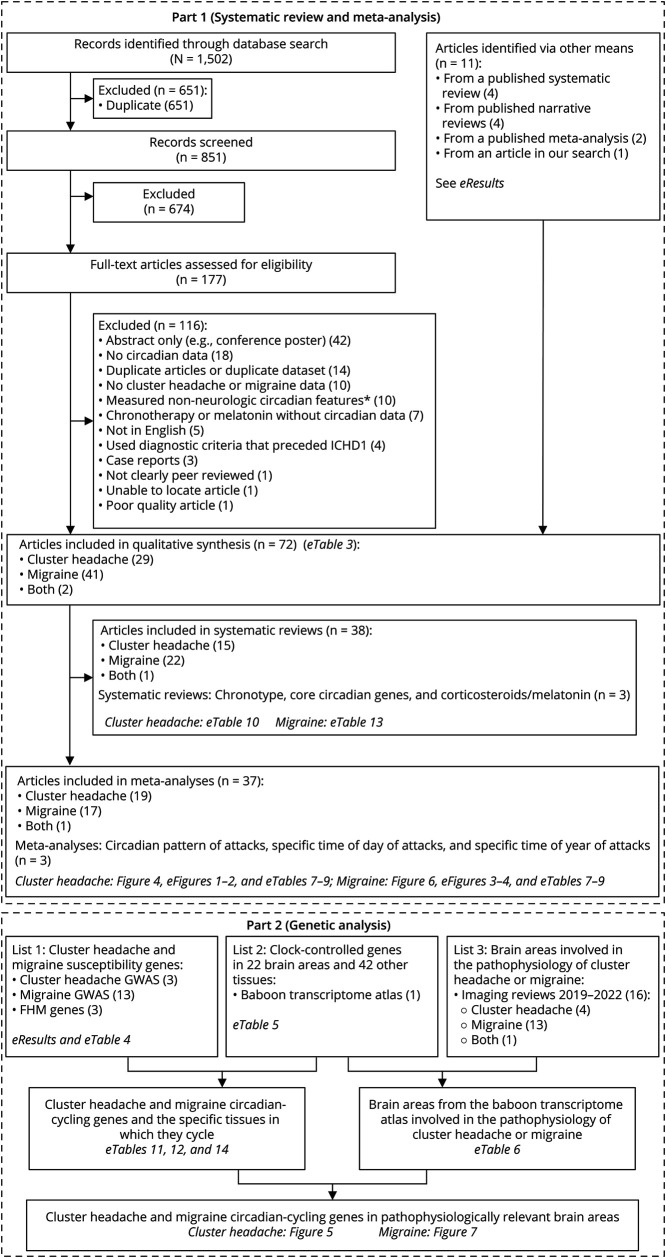
Circadian regulation of cluster headache and migraine was investigated with a systematic review/meta-analysis and a genetic analysis. The flow diagrams for the 2 disorders and 2 methodologies are shown in Figure 3. In our systematic review/meta-analysis, our search terms identified 1,502 records before deduplication, which ultimately yielded 61 studies (eTable 3, links.lww.com/WNL/C709). We also added 11 studies13,24-33 via our review of the literature (see eResults in eAppendix 1, links.lww.com/WNL/C703). Within these 72 studies, we extracted data for 6 circadian topics: a circadian pattern of attacks, a specific time of day of attacks, a specific time of year of attacks, chronotype, core clock genes, and circadian-related hormones (corticosteroids and melatonin).
Figure 3. Flow Diagrams for Each Phase of This Study.
GWAS = genome-wide association study; FHM = familial hemiplegic migraine; ICHD1 = International Classification of Headache Disorders, first edition (published in 1988). *Ten studies measured non-neurologic features in cluster headache or migraine, specifically the circadian features of the heart rate, blood pressure, reflexes, epinephrine, sodium, cyproheptadine, or tweets about headaches.
In our genetic analysis, we cross-referenced 3 sources: (1) GWASs of cluster headache and migraine genes, (2) a study of all CCGs in a variety of baboon tissues, and (3) recent review articles of relevant brain areas for cluster headache and migraine. Source 1 was derived from the GWAS catalog and included 3 cluster headache and 12 migraine studies (see eResults in eAppendix 1, links.lww.com/WNL/C703, and eTable 4, links.lww.com/WNL/C709). Source 2 was a single study20 and provided all CCGs in each of 64 different tissues including 14 nonhypothalamic brain areas and 8 hypothalamic areas (eTable 5). For source 3, we unfortunately could not find a definitive source, such as a recent systematic review. As an approximation of relevant brain areas, we performed a PubMed search for review articles of imaging studies published since 2019 (see eResults in eAppendix 1). We ultimately included 16 studies in our final review (4 cluster headache, 11 migraine, and 1 study with both disorders) (eTable 6). Our results for cluster headache and migraine are presented separately below.
Cluster Headache
Systematic Review and Meta-analysis
Behavioral Level (Circadian Timing, Time of Day, Time of Year, and Chronotype)
A circadian pattern of attacks was present in 70.5% (3,490/4,953) of participants with cluster headache across 16 studies (Figure 4A, eFigure 1, links.lww.com/WNL/C704, and eTable 7, links.lww.com/WNL/C709). The data were collected primarily (>99%) retrospectively (eTable 3). The definition of a circadian pattern of attacks, however, was not provided by most studies. Although most studies did not comment on longitudinal changes of the circadian pattern of attacks, 2 studies measured changes over at least 2 headache cycles (n = 139)34 or at least 5 years (n = 42).35 These studies noted that a majority (52.5% and 78.4%, respectively) of patients retained their same pattern but that the rest changed the time of day of, gained, or lost their circadian pattern of attacks.
Figure 4. Meta-analysis of Predictable Timing of Participants With Cluster Headache for (A) the Presence or Absence of a Circadian Pattern, (B) the Circadian Pattern of a Participant Hour by Hour, or (C) the Circannual Pattern of a Participant Month by Month.
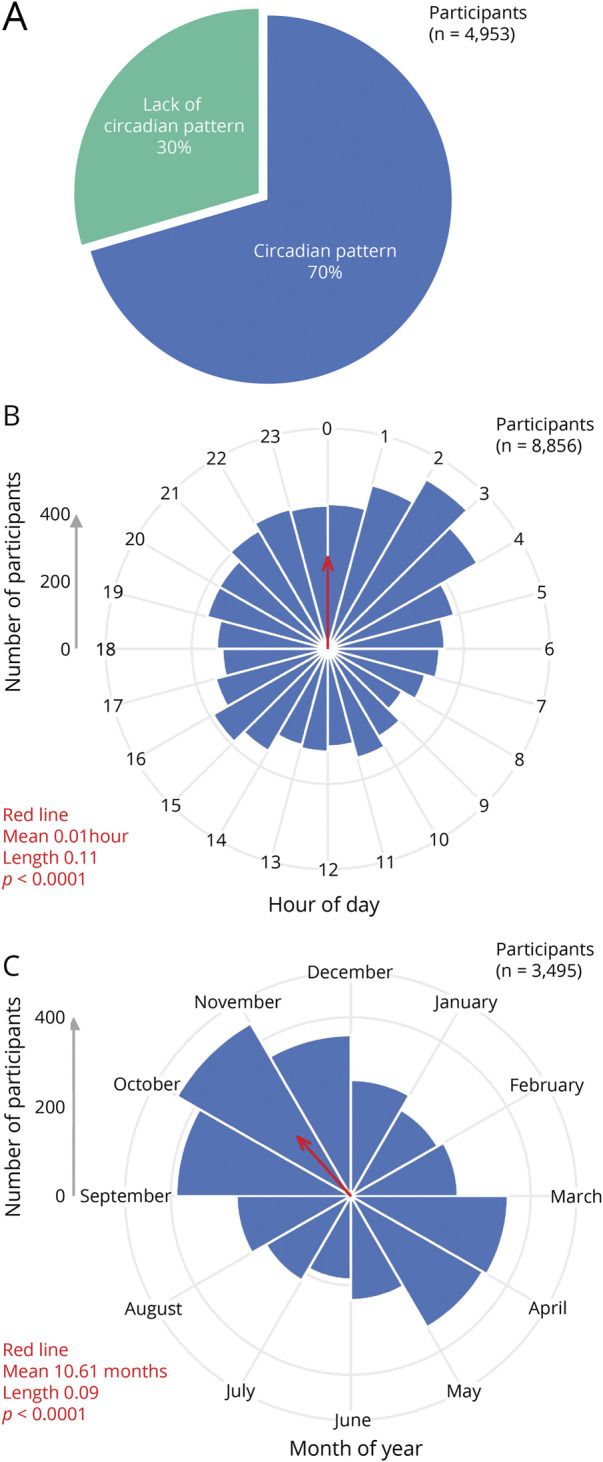
Rayleigh test data (in red) are shown for mean, length, and p value. The average time across all participants (red arrows) was 0.01 hours and 10.61 months or 00:01 on October 19. Both the hour-by-hour and month-by-month data were significantly different between cluster headache and migraine (Watson 2 test p < 0.01). Of note, a single participant could respond once for A but multiple times for B or C. Additional analysis, including bar graphs, circadian 4-hour blocks, circannual seasons, individual contributions from each study, and raw data, is available in eFigures 1 and 2 (links.lww.com/WNL/C704 and links.lww.com/WNL/C705) and eTables 7–9 (links.lww.com/WNL/C709).
Hour-by-hour timing of cluster headache attacks was available from 3 retrospective studies,e26,e55,e75 and the data were fairly consistent across studies (eFigure 2I, links.lww.com/WNL/C705). There was a clear peak between 21:00 and 03:00, as these 7 consecutive hours comprised all 7 highest values for the time of day of attacks (Figure 4B, eFigure 2, A and B, eTable 8, links.lww.com/WNL/C709). The single most likely hour was 02:00, present in 6.5% (508/8,856) of participants. Four-hour blocks were available for 6 studiese26,e39,e55,e68,e75,e80: the 4-hour data were similar to the 1-hour data with peaks between 20:00 and 04:00 (eFigure 2, C and D, eTable 8).
Month-by-month timing of cluster headache attacks was available from 5 retrospective studies,e26,e60,e74,e75,e82 and the data were fairly consistent across studies (eFigure 2J, links.lww.com/WNL/C705). Three studies reported the month in which the headache cycle began for participants with episodic cluster headache31,36,37; the fourth and fifth studies reported either the months in which the headaches were most likely to occur (for episodic cluster headache) or were most likely to have a worsening of attacks (for chronic cluster headache).38,39 There were clear peaks in spring (March and April) and autumn (September, October, and November) (Figure 4C, eFigure 2, E and F, eTable 9, links.lww.com/WNL/C709), and these 5 months accounted for 53.8% (1,882/3,495) of participants. Seasonal data were available for 7 studies,e26,e47,e60,e68,e74,e75,e82 and autumn was the most common season (30.7%, or 1,140/3,709 of participants) (eFigure 2, G and H).
Chronotype changes for cluster headache are shown in eTable 10 (links.lww.com/WNL/C709). Chronotype data were available from 5 studies and did not show a consistent pattern: in 2 studies, participants with cluster headache had Morningness-Eveningness scores in the intermediate range (means 53.2 and 51.4), whereas in 1 study, participants with episodic cluster headache were more likely to have late chronotypes than either chronic cluster headache or controls, and in 2 other studies, participants with cluster headache or chronic cluster headache self-reported being early risers more often than night owls.
Systems Level (Corticosteroids and Melatonin)
Data from 8 studies were available (eTable 10, links.lww.com/WNL/C709). Corticosteroid blood levels were higher (3 studies) or unchanged (1 study) compared with controls, whereas nocturnal urinary melatonin was lower (5 studies) and plasma melatonin was lower (1 study) compared with controls.
Cellular Level (Core Clock Genes)
Core clock genes include genes involved in the core clock transcriptional-translational feedback loop40 and PERIOD3. Our search for core clock genes showed a link between cluster headache and the CLOCK single nucleotide variation (SNV [formerly SNP]) rs12649507e44 as well as between cluster headache and lower levels of REV‐ERBα (also known as NR1D1) RNA in the lymphoblasts of participants with cluster headache compared with controlse38 (eTable 10, links.lww.com/WNL/C709). There was no association between cluster headache and the CLOCK SNVs rs1801260, rs11932595, or rs12649507.e42,e44 There was also no association between cluster headache and PERIOD3 variable number tandem repeat polymorphisms.e69
Circadian Genetic Analysis
We extracted gene names from 3 cluster headache GWASs,41-43 although all genes came from 2 GWASs41,42 as the third GWAS43 found no genes that met genome-wide significance. These genes were cross-referenced with a baboon study of CCGs in multiple tissues20 (eTable 11, links.lww.com/WNL/C709).
Systems Level (Relevant Brain Areas Where CCGs Are Active)
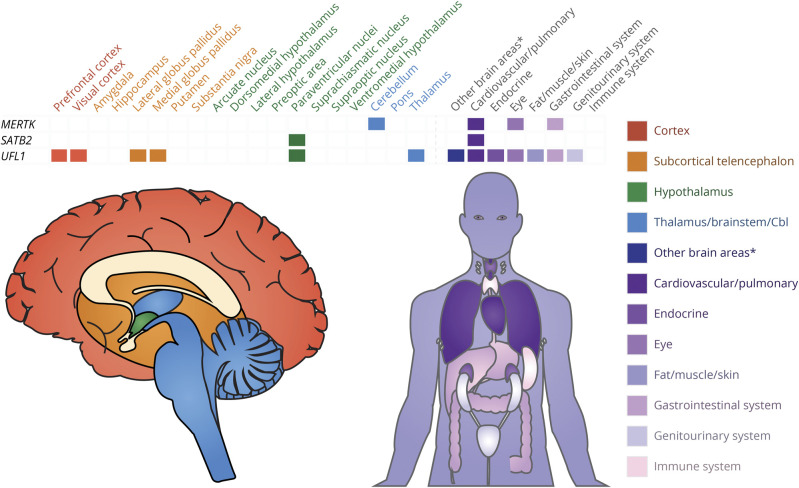
UFL1 showed 24-hour cycling in most tissues (15 of 64 tissues), followed by MERTK (6 of 64 tissues). Three of the 9 cluster headache genes showed 24-hour cycling in brain areas relevant for cluster headache, with UFL1 cycling over 24 hours in 6 relevant brain areas and MERTK and SATB2 each cycling in 1 relevant brain area (Figure 5 and eTable 12, links.lww.com/WNL/C709). The tissues with the most cluster headache CCGs were the paraventricular nucleus of the hypothalamus and the aorta, both with the same 2 genes (SATB2 and UFL1).
Figure 5. Clock-Controlled Genes for Cluster Headache Genes (First Column) and the Specific Tissues in Which They Cycle (First Row).
Tissues from red (prefrontal cortex) to blue (thalamus) were determined to be relevant in the pathophysiology of cluster headache (tissues colored shades of purple were not relevant). For the full uncondensed dataset, see eTable 12 (links.lww.com/WNL/C709). Cbl = cerebellum. *The other brain areas category consists of 3 tissues (habenula, olfactory bulb, and mammillary body) that were not mentioned as relevant in the pathophysiology of cluster headache.
Cellular Level (CCGs)
CCGs accounted for 55.6% (5/9) of cluster headache genes (eTable 12, links.lww.com/WNL/C709).
Migraine
Systematic Review and Meta-analysis
Behavioral Level (Circadian Timing, Time of Day, Time of Year, and Chronotype)
A circadian pattern of attacks was present in 50.1% (2,698/5,385) of participants with migraine across 8 studies (Figure 6A, eFigure 3, links.lww.com/WNL/C706, and eTable 7, links.lww.com/WNL/C709). Like cluster headache, however, the definition of a circadian pattern of attacks was not provided by most studies. Unlike cluster headache, some data (15%) were collected prospectively, whereas most (85%) were collected retrospectively (eTable 3). No longitudinal changes of the circadian pattern of attacks were noted in the migraine studies.
Figure 6. Meta-analysis of Predictable Timing of Participants With Migraine for (A) the Presence or Absence of a Circadian Pattern, (B) the Circadian Pattern of Attacks Hour by Hour, or (C) the Circannual Pattern of Attacks Month by Month.
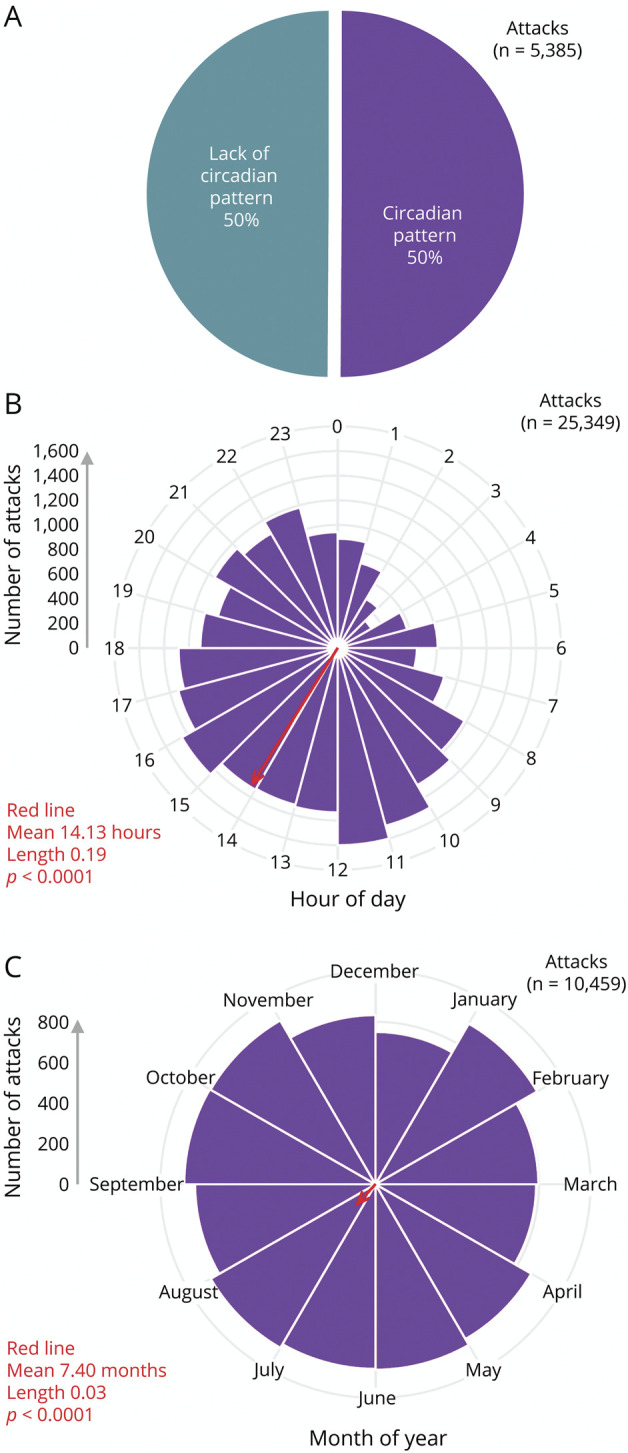
Rayleigh test data (in red) is shown for mean, length, and p value. The average time across all attacks (red arrows) was 14.13 hours and 7.4 months or 14:08 on July 12. Both the hour-by-hour and month-by-month data were significantly different between cluster headache and migraine (Watson 2 test p < 0.01). Of note, a single participant could respond once for A but multiple times for B or C. Additional analysis, including bar graphs, circadian 2-hour blocks, individual contributions from each study, and raw data, is available in eFigures 3 and 4 (links.lww.com/WNL/C706 and links.lww.com/WNL/C707) and eTables 7–9 (links.lww.com/WNL/C709).
Hour-by-hour timing of migraine attacks was available from 4 prospective studies,e20,40,e45,e79 although the peaks in hourly timing varied across studies (eFigure 4G, links.lww.com/WNL/C707). Unlike cluster headache, which reported the circadian timing of participants (i.e., the number of participants having attacks at each hour), the migraine studies reported the circadian timing of attacks (i.e., the number of attacks each hour, regardless of the participant). There was a clear trough between 23:00 and 07:00, as these 9 consecutive hours comprised all 9 lowest values for the time of day of attacks (Figure 6B, eFigure 4, A and B, eTable 8, links.lww.com/WNL/C709). There was a relatively broad peak starting in the late morning and lasting until the early evening. The single most likely hour was 11:00, present in 6.3% (1,601/23,349) of attacks. Two-hour blocks could be calculated for these 4e20,40,e45,e79 and 1 additional study.e78 However, the additional study added only 1% additional data; thus, the 2-hour data were nearly identical to the 1-hour data (eFigure 4, C and D, eTable 8).
Month-by-month timing of migraine attacks was available from 4 prospective studies,e21,e45,e49,e79 although the peaks in monthly timing varied across studies (eFigure 4H, links.lww.com/WNL/C707). Again in contrast to cluster headache, the migraine studies reported the circadian timing of attacks (i.e., the number of attacks each month, regardless of the participant). Across 4 studies, there was a mild broad trough between November and March and a mild broad peak between April and October (Figure 6C, eFigure 4, E and F, eTable 9, links.lww.com/WNL/C709). Of note, 1 study recorded data over 52 weeks but only reported weekly patterns (specifically less attacks on Sundayse22).
Chronotype data were available from 9 studies (eTable 13, links.lww.com/WNL/C709), and most showed no difference in chronotype between participants with migraine and controls (4 studies) or a score in the expected intermediate range (1 study). However, the remainder showed that participants with migraine were more likely to have a morning chronotype (2 studies), have a later bedtime (1 study), or have a bimodal pattern with more morning and evening types than controls (1 study).
Systems Level (Corticosteroids and Melatonin)
Data from 14 studies were available (eTable 13, links.lww.com/WNL/C709). CSF cortisol was lower in participants with migraine than controls (1 study), morning salivary cortisol was higher (1 study), and blood cortisol was either no different (1 study) or higher (1 study). Nocturnal urinary melatonin was lower in participants with migraine than controls in 2 studies but no different in 1 study. Nocturnal urinary 6-sulfatoxymelatonin was lower in participants with migraine than controls (1 study), and of interest, nocturnal urinary melatonin (1 study) and nocturnal urinary 6-sulfatoxymelatonin (1 study) were both lower in participants with migraine with an acute headache than in participants with migraine without an acute headache. Dim light melatonin onset was not different between participants with migraine and controls (1 study), although later dim light melatonin onset was associated with an increased frequency of monthly migraine days. Blood melatonin showed no consistent pattern, with 3 studies showing no difference in nocturnal serum melatonin (but one with a phase delay in the melatonin peak) and a fourth study showing more marked suppression in participants with migraine than controls.
Cellular Level (Core Clock Genes)
There was a positive association between migraine and 2 alleles of the CK1δ genee30 (eTable 13, links.lww.com/WNL/C709). There was a positive association between migraine and the retinoid acid–related orphan receptor α (RORα) SNV rs4774388 and no association with the RORα SNV rs11639084.e43 There was a positive association between migraine and the CLOCK SNV rs10462028 only when financial stress and migraine were combined.e24
Circadian Genetic Analysis
We extracted gene names from 13 GWASs and the 3 established familial hemiplegic migraine genes from the ICHD3 (CACNA1A, ATP1A2, and SCN1A).44 Similar to our cluster headache analysis, these migraine genes were cross-referenced with a study of CCGs in baboons.20
Systems Level (Relevant Brain Areas Where CCGs Are Active)
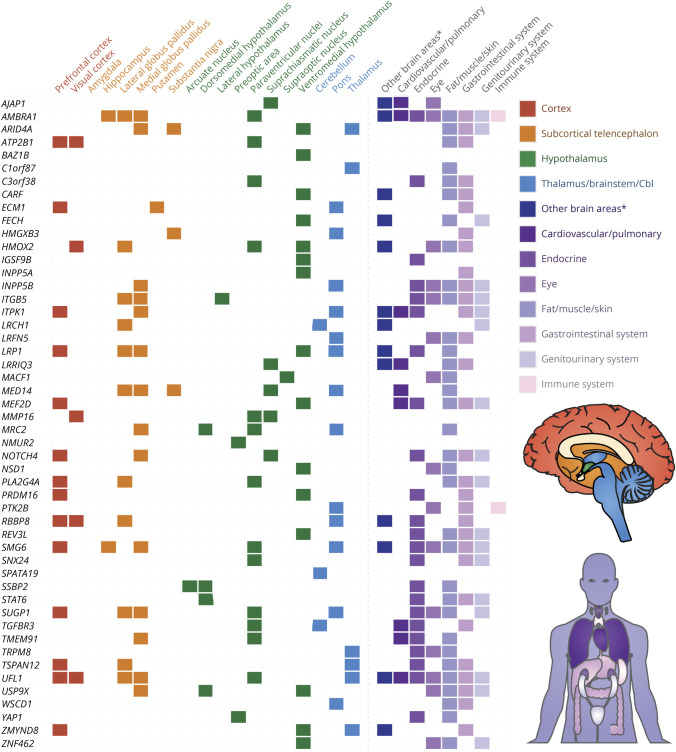
A total of 73 of the 110 migraine CCGs showed 24-hour cycling in brain areas relevant for migraine (Figure 7 and eTable 14, links.lww.com/WNL/C709), with UFL1 cycling in 6 relevant brain areas and 4 genes cycling over 24 hours in 5 relevant brain areas (LRP1, MED14, SMG6, and SUGP1). The tissues with the most migraine CCGs were the thyroid (31 of 110 genes), prefrontal cortex (27 of 110 genes), and stomach fundus (22 of 110 genes).
Figure 7. Clock-Controlled Genes for Migraine (First Column) and the Specific Tissues in Which They Cycle (First Row).
Given the numerous genes involved in migraine, here we show a subset, namely all migraine genes that cycle in the thalamus, hypothalamus, brainstem, or cerebellum (green and blue). For the full uncondensed dataset, including genes that cycle in only cortical and subcortical areas, see eTable 14 (links.lww.com/WNL/C709). Tissues from red (prefrontal cortex) to blue (thalamus) were determined to be relevant in the pathophysiology of migraine (tissues colored shades of purple were not relevant). Cbl = cerebellum. *The other brain areas category consists of 3 tissues (habenula, olfactory bulb, and mammillary body) that were not mentioned as relevant in the pathophysiology of migraine.
Cellular Level (CCGs)
CCGs accounted for 65.5% (110/168) of the migraine genes (eTable 14, links.lww.com/WNL/C709). AMBRA1 showed 24-hour cycling in most tissues (18 of 64 tissues), followed by SMG6 and UFL1 (at 16 and 15 tissues, respectively).
Discussion
We report on circadian features of cluster headache and migraine at the behavioral level (circadian timing, time of day, time of year, and chronotype), systems level (brain areas where CCGs are active, melatonin and corticosteroid levels), and cellular level (core circadian genes and CCGs).
At the behavioral level, 7 in 10 patients with cluster headache have a circadian pattern of attacks across nearly 5,000 participants, with 2 am as the most common time of day and October as the most common time of year. This suggests a robust circadian and circannual pattern but one that is highly variable between individuals: a minority of participants lacked a circadian pattern of attacks entirely, and many participants did not have attacks at 2 am in October. Chronotype data were inconsistent across studies. At the systems level, 2 cluster headache CCGs (SATB2 and UFL1) both cycle in the paraventricular nucleus of the hypothalamus, an area of particular relevance in cluster headache because of its role in the autonomic system.1,3 A third CCG, MERTK, cycles in the cerebellum, a brain region that has circadian cycles dependent on corticosteroids.45 The cerebellum is relevant in cluster headache not only because of the role of corticosteroids but also due to its role with the first-line preventive medication verapamil. Verapamil shifts the timing of cluster headache attacks by approximately 1 hour46; in addition, verapamil responders show changes in cerebellar gray matter concentration compared with nonresponders,47 and verapamil is known to cause core circadian gene changes in animal models including in the cerebellum.48 Hormonally, participants with cluster headache show lower melatonin levels and higher cortisol levels. At the cellular level in cluster headache, there were positive associations between cluster headache and the core circadian genes CLOCKe44 and REV‐ERBα.e38 In addition, 5 of the 9 cluster headache genes identified in GWASs were CCGs.
Although we did not perform a meta-analysis of hormone levels, our melatonin data are in line with a previous meta-analysis49 that found (1) lower serum melatonin during a cluster headache cycle but not outside of a cluster headache cycle and (2) lower urine 6-sulfatoxymelatonin levels both during and outside of a cluster headache cycle compared with controls.
At the behavioral level in migraine, half (50.1%) of participants with migraine had a circadian pattern of attacks across more than 5,000 participants. Although this percentage is not as high as cluster headache, it is notable in a disorder for which timing is not considered a prominent feature. A particular time of day was not investigated as it was in cluster headache; instead, looking at the timing of attacks across participants, there is a clear nadir in the early morning and a clear peak in the late morning and early afternoon. A particular time of year was also not investigated as it was in cluster headache; again looking at the timing of attacks across participants, there was only a weak yearly pattern. Chronotype data were highly variable across studies. At the systems level, 27 CCGs cycled in the prefrontal cortex, whereas many also cycled in various basal ganglia and hypothalamic tissues. Of note, after our analysis was performed a fourth hemiplegic migraine gene, proline-rich transmembrane protein 2, was proposede160; this gene cycles in 4 brain areas (the habenula, mammillary body, paraventricular nucleus, and SCN). Unlike cluster headache, many of the migraine CCGs cycled in 2 or more brain tissues, suggesting a more distributed circadian network in migraine. This more distributed network is not surprising given the multiple brain areas activated in migraine ranging from the hypothalamus, thalamus, and brainstem to the visual cortex and pain neuromatrix.2 Hormonally, urinary melatonin levels appear to be lower in patients with migraine and even lower during an acute attack. At the cellular level, there were positive associations between migraine and the core circadian genes CK1δe30 and RORαe43 and a less clear association with CLOCKe24 as it was only positively associated with migraine when combined with financial stress. One hundred ten of the 168 migraine genes identified in GWASs were CCGs. Our time of day data and time of year data are in line with a recent meta-analysis finding the peak time for a migraine generally in the morning and no clear circannual peak.50
These data suggest that both headache disorders are highly circadian at multiple levels, especially cluster headache. The inherent circadian nature of these disorders reinforces the importance of the hypothalamus (where the SCN is located) and its role in both cluster headache1 and migraine.2 The inherent circadian nature also raises the question about the genetics of triggers. For example, sleep changes (undersleeping and oversleeping) are known triggers for migraine and are known zeitgebers. There is genetic overlap between several sleep traits and migraine.51 Moreover, insomnia is a risk factor for the development of migraine,52,53 raising the question whether the circadian features of insomnia genes54 are also relevant in migraine.
These data also raise the question of using circadian-based treatments as therapies for headache disorders. Circadian-based treatments include both chronotherapy (treatment based on the circadian rhythm such as taking medications at certain times of day) and treatments that cause circadian alterations. Of note, multiple cluster headache and migraine treatments are already known to alter core clock genes in animal models.40
Our study has several limitations. The circadian pattern of attack for both cluster headache and migraine may be inaccurate because a definition of circadian pattern was not provided by most studies. Separately, the time of day data for migraine is likely inaccurate in 3 respects. First, participants are presumably reporting the pain onset, and the pain phase is not the first phase of the attack.2 True migraine attack onset is likely the beginning of the premonitory phase, which is more difficult for participants to discern. Second, migraine attacks often have a gradual escalation of pain intensity, which may not awaken the participant. Thus, there is likely an artificially decreased reporting of nocturnal onset and an increased reporting of wake-up onset. Third, there was considerable variability in hourly (and monthly) peaks across a small number of studies, and future studies are needed to reconcile this variability. In contrast to migraine, the time of day for cluster headache would not be expected to have these limitations given the lack of a clear premonitory phase, the rapid onset of the attacks,55 the extreme severity of the pain,56 which would very likely waken participants from sleep, and the consistency of the hourly (and monthly) data across studies. As another limitation, we combined data from several study types (prospective headache diaries, retrospective questionnaires, and emergency department data); the data were primarily retrospective for cluster headache and primarily prospective for migraine. Also, in our meta-analyses, we lacked certain information that might influence the circadian cycle, for example, medications like melatonin, circadian rhythm issues like night shift workers, and comorbid disorders like bipolar disease. We also did not have sufficient information to examine subgroups, for example, chronic vs episodic, male vs female, or young vs old. There were also too few studies at different latitudes to evaluate the effect of latitude on circadian and especially circannual patterns; 1 group examined patients near the Arctic Circle,e20-e22 and it is unclear whether these data should be considered outliers. Finally, 11 studies were added via other means as our search term did not include them, although we should note that the addition of these studies: (1) did not substantially change our meta-analysis results, and (2) were incorporated from multiple previous reviews, so we suspect that the combination of our search term and prior reviews constitutes most of the published literature.
Glossary
- CCG
clock-controlled gene
- CLOCK
circadian locomotor output cycles kaput
- CK1δ
casein kinase delta
- GWAS
genome-wide association study
- ROR
retinoid acid–related orphan receptor
- SCN
suprachiasmatic nucleus
- SNV
single nucleotide variation
Appendix. Authors
Footnotes
Editorial, page 1035
CME Course: NPub.org/cmelist
Study Funding
This work is in part supported by grants in aid for scientific research from the Japan Society for the Promotion of Science (18H02600 and 19K22516) to K. Yagita, the NIH (RF1AG061901) to Z. Chen, The Welch Foundation (U-2127-20220331) and NIH (R35 GM145232-01) to S.-H. Yoo, and The Will Erwin Headache Research Foundation to M.J. Burish.
Disclosure
B. Benkli, S.Y. Kim, N. Koike, C. Han, C. Tran, E. Silva, Y. Yan, K. Yagita, Z. Chen, and S-H. Yoo report no disclosures relevant to the manuscript. M.J. Burish reports being an unpaid member of the medical advisory board of Clusterbusters and reports being a site investigator for a cluster headache clinical trial funded by Lundbeck. Go to Neurology.org/N for full disclosures.
References
- 1.May A, Schwedt TJ, Magis D, Pozo-rosich P, Evers S, Wang SJ. Cluster headache. Nat Rev Dis Prim. 2018;4:18006. [DOI] [PubMed] [Google Scholar]
- 2.Goadsby PJ, Holland PR, Martins-oliveira M, Hoffmann J, Schankin C, Akerman S. Pathophysiology of migraine: a disorder of sensory processing. Physiol Rev. 2017;97(2):553-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schindler EAD, Burish MJ. Recent advances in the diagnosis and management of cluster headache. BMJ. 2022;376:e059577. [DOI] [PubMed] [Google Scholar]
- 4.Ailani J, Burch RC, Robbins MS. The American Headache Society Consensus statement: update on integrating new migraine treatments into clinical practice. Headache. 2021;61(7):1021-1039. [DOI] [PubMed] [Google Scholar]
- 5.Barloese M. Current understanding of the chronobiology of cluster headache and the role of sleep in its management. Nat Sci Sleep. 2021;13:153-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baksa D, Gecse K, Kumar S, et al. Circadian variation of migraine attack onset: a review of clinical studies. Biomed Res Int. 2019;2019:4616417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takahashi JS. Transcriptional architecture of the mammalian circadian clock. Nat Rev Genet. 2017;18(3):164-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fourier C, Ran C, Sjöstrand C, Waldenlind E, Steinberg A, Belin AC. The molecular clock gene cryptochrome 1 (CRY1) and its role in cluster headache. Cephalalgia. 2021;41(13):1374-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fourier C, Ran C, Zinnegger M, et al. A genetic CLOCK variant associated with cluster headache causing increased mRNA levels. Cephalalgia. 2018;38(3):496-502. [DOI] [PubMed] [Google Scholar]
- 10.Burish MJ, Chen Z, Yoo SH. Cluster headache is in part a disorder of the circadian system. JAMA Neurol. 2018;75(7):783-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gross EC, Lisicki M, Fischer D, Sándor PS, Schoenen J. The metabolic face of migraine: from pathophysiology to treatment. Nat Rev Neurol. 2019;15(11):627-643. [DOI] [PubMed] [Google Scholar]
- 12.Golombek DA, Rosenstein RE. Physiology of circadian entrainment. Physiol Rev. 2010;90(3):1063-1102. [DOI] [PubMed] [Google Scholar]
- 13.Brennan KC, Bates EA, Shapiro RE, et al. Casein kinase iδ mutations in familial migraine and advanced sleep phase. Sci Transl Med. 2013;5(183):183ra56, 1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ong JC, Taylor HL, Park M, et al. Can circadian dysregulation exacerbate migraines? Headache. 2018;58(7):1040-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990;247(4945):975-978. [DOI] [PubMed] [Google Scholar]
- 16.Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 1972;42(1):201-206. [DOI] [PubMed] [Google Scholar]
- 17.Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci USA. 1972;69(6):1583-1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Long R, Zhu Y, Zhou S. Therapeutic role of melatonin in migraine prophylaxis: a systematic review. Medicine (Baltimore). 2019;98(3):e14099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB. A circadian gene expression atlas in mammals: implications for biology and medicine. Proc Natl Acad Sci USA. 2014;111(45):16219-16224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mure LS, Le HD, Benegiamo G, et al. Diurnal transcriptome atlas of a primate across major neural and peripheral tissues. Science. 2018;359(6381):eaao0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264-269, W64. [DOI] [PubMed] [Google Scholar]
- 22.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan: a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Heart Lung and Blood Institute. Study Quality Assessment Tools [online]. Accessed December 23, 2021. nhlbi.nih.gov/health-topics/study-quality-assessment-tools. [Google Scholar]
- 24.Winner P, Rothner AD, Putnam DG, Asgharnejad M. Demographic and migraine characteristics of adolescents with migraine: Glaxo Wellcome clinical trials' database. Headache. 2003;43(5):451-457. [DOI] [PubMed] [Google Scholar]
- 25.Steinberg A, Fourier C, Ran C, Waldenlind E, Sjöstrand C, Belin AC. Cluster headache: clinical pattern and a new severity scale in a Swedish cohort. Cephalalgia. 2018;38(7):1286-1295. [DOI] [PubMed] [Google Scholar]
- 26.Bruni O, Russo PM, Violani C, Guidetti V. Sleep and migraine: an actigraphic study. Cephalalgia. 2004;24(2):134-139. [DOI] [PubMed] [Google Scholar]
- 27.Hoffmann J, Lo H, Neeb L, Martus P, Reuter U. Weather sensitivity in migraineurs. J Neurol. 2011;258(4):596-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vgontzas A, Li W, Mostofsky E, Rueschman M, Mittleman MA, Bertisch SM. Associations between migraine attacks and nightly sleep characteristics among adults with episodic migraine: a prospective cohort study. Sleep. 2020;43(7):zsaa001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kimoto K, Aiba S, Takashima R, et al. Influence of barometric pressure in patients with migraine headache. Intern Med. 2011;50(18):1923-1928. [DOI] [PubMed] [Google Scholar]
- 30.Bahra A, May A, Goadsby PJ. Cluster headache: a prospective clinical study with diagnostic implications. Neurology. 2002;58(3):354-361. [DOI] [PubMed] [Google Scholar]
- 31.Lin KH, Wang PJ, Fuh JL, et al. Cluster headache in the Taiwanese: a clinic-based study. Cephalalgia. 2004;24(8):631-638. [DOI] [PubMed] [Google Scholar]
- 32.Leone M, Frediani F, D'Amico D, et al. Dexamethasone suppression test, melatonin and TRH-test in cluster headache. Ital J Neurol Sci. 1992;13(3):227-232. [DOI] [PubMed] [Google Scholar]
- 33.Neeb L, Anders L, Euskirchen P, Hoffmann J, Israel H, Reuter U. Corticosteroids alter CGRP and melatonin release in cluster headache episodes. Cephalalgia. 2015;35(4):317-326. [DOI] [PubMed] [Google Scholar]
- 34.Lee MJ, Cho SJ, Park JW, et al. Temporal changes of circadian rhythmicity in cluster headache. Cephalalgia. 2020;40(3):278-287. [DOI] [PubMed] [Google Scholar]
- 35.Lee MJ, Choi HA, Shin JH, Park HR, Chung CSS. Natural course of untreated cluster headache: a retrospective cohort study. Cephalalgia. 2018;38(4):655-661. [DOI] [PubMed] [Google Scholar]
- 36.Rozen TD, Fishman RS. Cluster headache in the United States of America: demographics, clinical characteristics, triggers, suicidality, and personal burden. Headache. 2012;52(1):99-113. [DOI] [PubMed] [Google Scholar]
- 37.Urban GJ, Diamond S, Freitag FG, Diamond ML. Analysis of patients with cluster headache. Headache Q. 1994;5:236-240. [Google Scholar]
- 38.Barloese M, Lund N, Petersen A, Rasmussen M, Jennum P, Jensen R. Sleep and chronobiology in cluster headache. Cephalalgia. 2015;35(11):969-978. [DOI] [PubMed] [Google Scholar]
- 39.Popescu C. Familial periodicity in a multigenerational family of cluster headache: a case report. Cephalalgia Rep. 2019;2:1-4. [Google Scholar]
- 40.Burish MJ, Chen Z, Yoo SH. Emerging relevance of circadian rhythms in headaches and neuropathic pain. Acta Physiol. 2019;225(1):e13161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harder AVE, Winsvold BS, Noordam R, et al. Genetic susceptibility loci in genomewide association study of cluster headache. Ann Neurol. 2021;90(2):203-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O'Connor E, Fourier C, Ran C, et al. Genome-wide association study identifies risk loci for cluster headache. Ann Neurol. 2021;90(2):193-202. [DOI] [PubMed] [Google Scholar]
- 43.Bacchelli E, Cainazzo MM, Cameli C, et al. A genome-wide analysis in cluster headache points to neprilysin and PACAP receptor gene variants. J Headache Pain. 2016;17(1):114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38(1):1-211. [DOI] [PubMed] [Google Scholar]
- 45.Bering T, Hertz H, Rath MF. Rhythmic release of corticosterone induces circadian clock gene expression in the cerebellum. Neuroendocrinology. 2020;110(7-8):604-615. [DOI] [PubMed] [Google Scholar]
- 46.Barloese M, Haddock B, Lund NT, Petersen A, Jensen R. Chronorisk in cluster headache: a tool for individualised therapy? Cephalalgia. 2018;38(14):2058-2067. [DOI] [PubMed] [Google Scholar]
- 47.Tso AR, Brudfors M, Danno D, et al. Machine phenotyping of cluster headache and its response to verapamil. Brain. 2021;144(2):655-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Burish MJ, Han C, Mawatari K, et al. The first-line cluster headache medication verapamil alters the circadian period and elicits sex-specific sleep changes in mice. Chronobiol Int. 2021;38(6):839-850. [DOI] [PubMed] [Google Scholar]
- 49.Liampas I, Siokas V, Brotis A, et al. Meta-analysis of melatonin levels in cluster headache: review of clinical implications. Acta Neurol Scand. 2020;142(4):356-367. [DOI] [PubMed] [Google Scholar]
- 50.Poulsen AH, Younis S, Thuraiaiyah J, Ashina M. The chronobiology of migraine: a systematic review. J Headache Pain. 2021;22(1):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Daghlas I, Vgontzas A, Guo Y, Chasman DI, Saxena R. Habitual sleep disturbances and migraine: a Mendelian randomization study. Ann Clin Transl Neurol. 2020;7(12):2370-2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chu S, Wu Z, Wu Z, Wu J, Qian Y. Association between insomnia and migraine risk: a case-control and bidirectional Mendelian randomization study. Pharmgenomics Pers Med. 2021;14:971-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Odegard SS, Sand T, Engstrom M, Stovner LJ, Zwart JA, Hagen K. The long-term effect of insomnia on primary headaches: a prospective population-based cohort study (HUNT-2 and HUNT-3). Headache. 2011;51(4):570-580. [DOI] [PubMed] [Google Scholar]
- 54.Jansen PR, Watanabe K, Stringer S, et al. Genome-wide analysis of insomnia in 1,331,010 individuals identifies new risk loci and functional pathways. Nat Genet. 2019;51(3):394-403. [DOI] [PubMed] [Google Scholar]
- 55.Wei DY, Goadsby PJ. Comprehensive clinical phenotyping of nitroglycerin infusion induced cluster headache attacks. Cephalalgia. 2021;41(8):913-933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Burish MJ, Pearson SM, Shapiro RE, Zhang W, Schor LI. Cluster headache is one of the most intensely painful human conditions: results from the International Cluster Headache Questionnaire. Headache. 2020;61(1):117-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Access the eReferences here; : links.lww.com/WNL/C708.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data were obtained, already anonymized, from published studies. Data not provided in this article may be shared at the request of any qualified investigator for the purposes of replicating results.