ABSTRACT
Aim: The current study aimed at analyzing the effect of non-surgical periodontal treatment accompanied by systemic antibiotics on salivary enzyme activities, periodontal parameters, and glycemic control in type−2 diabetic (T2D) patients with chronic periodontitis.
Methods: The study included 125 type−2 diabetic patients with chronic periodontitis who had good glycemic control (T2Dc), 125 type−2 diabetics who had bad glycemic control (T2Dpc). The 125 T2Dpc were divided randomly into two groups. The first one enrolled 63 T2Dpc and received a non-surgical periodontal treatment (T2Dpc + NST). The second group enrolled 62 T2Dpc and received the non-surgical treatment accompanied by systemic antibiotics (T2Dpc+NST+A). HbA1c, periodontal indices, and salivary enzyme activities were assessed for all groups. The Glycated hemoglobin (HbA1c) was assessed. The Salivary alkaline phosphatase (ALP), aspartate aminotransferase (AST), alanine aminotransaminase (ALT), lactate dehydrogenase (LDH), and creatine kinase (CK) activities were measured.
Results: The T2Dpc were characterized by the highest probing depth (PPD) and clinical attachment loss (CAL) periodontal scores, as well as ALP, AST, and ALT enzymatic activities. However, BOP did not differ significantly between T2Dc and T2Dpc. Whereas the rest of clinical parameters PI, GI, and OHI-S did not significantly differ between groups. The Pearson’s analysis revealed three correlations between ALP-PPD, ALP-CAL, and ALP-BOP (bleeding on probing) in both T2Dc and T2Dpc (P < 0.05). Interestingly, a significant decrease in periodontal indices, salivary enzyme activities, and HbA1c was recorded in T2Dpc+NST+A group.
Conclusion: The increase in ALP, AST, and ALT activities reflects the impact of uncontrolled T2D on periodontal tissue alteration. The ALP activity increase was associated with the severity of periodontal status in diabetic patients. In comparison to non-surgical treatment alone, the adjunct use of systemic antibiotics improves periodontal state, enzyme activity, and glycemic control.
KEYWORDS: salivary enzyme activities, glycemic control, non-surgical treatment, antibiotics, periodontitis
1. Introduction
Type 2 diabetes (T2D) prevalence is still in perpetual worldwide increase. This burden is estimated at 15.1% in Tunisia [1]. T2D is a chronic metabolic disease caused by insulin resistance, which progressively leads to an insulin absolute deficit in case of beta cell destruction [2]. The persistence of hyperglycemia leads to the formation of advanced glycation end products generating several systemic as well as oral complications. The latter include salivary gland dysfunction through basement membrane alteration and autonomic neuropathy [3,4]. Saliva is the main fluid of the oral cavity. It contains electrolytes, proteins, enzymes, and molecules derived from blood. In T2D, alteration in saliva composition predispose to several diseases such as xerostomia, dental caries, and periodontal diseases.
Periodontal diseases are generated by dysbiosis in the oral microflora promoting the colonization of Gram-negative bacteria in periodontal pockets [5]. The injury of damaged tissues is often accompanied by a release of intracellular enzymes from host cells to the gingival crevicular fluid including alkaline phosphatase (ALP), aspartate aminotransferase (AST), alanine aminotransaminase (ALT), lactate dehydrogenase (LDH), and creatine kinase (CK) [6–8].
Periodontal therapies based on surgical and non-surgical come in a wide range of modalities. These protocols show varying efficacy depending on the administered dosage and combination of systemic antimicrobials. Subjects with metabolic syndrome may have a higher risk of acquiring cardiovascular disease if they have periodontal disease (PD) and concentric left ventricular remodeling, a marker of cardiovascular events, may be linked to periodontitis. The shape of the left ventricle may change in patients who have periodontitis. These results highlight the significance of periodontitis prevention, diagnosis, and management [9].
In literature, both periodontitis and TD2 are two chronic related diseases. Thus, it is of interest to assess the impact of glycemic imbalance on salivary enzyme activities and to find a reliable salivary enzyme biomarker for the periodontal disease monitoring. The current literature studied more sensitive inflammatory indicators to confirm tissue loss as well as the possibility for tissue regeneration and recovery. The geographic area where the study was carried out, which, in our opinion, the reader of this particular community demographic has to know. Furthermore, in order to identify more effective periodontal therapies for diabetics, in this context, the present study aimed to look first into the effects of glycemic imbalance on salivary enzyme activities and periodontal parameters. The second objective was to explore whether these parameters are linked and which one is the most relevant biomarker for disease monitoring. The third goal was to evaluate the effect of non-surgical treatment accompanied by systemic antibiotics on salivary enzyme activities, periodontal indices, and glycemic control (HbA1c) in T2D patients with chronic periodontitis (T2Dpc).
2. Materials and methods
2.1. Study design and setting
The present study is a single-center cross-sectional study involving 125 adequately glycemic controlled type 2 diabetic patients (HbA1c ≤ 7%) with chronic periodontitis (T2Dc), 125 poorly glycemic controlled type 2 diabetic patients (HbA1c > 7%) with chronic periodontitis (T2Dpc), and 125 periodontally healthy controls without T2D recruited from Sahloul Hospital of Sousse, Tunisia (Figure 1). The research was carried out at the Dental Medicine Department from March 2019 to February 2020 over the course of a year. The data was obtained in accordance with the STROBE principles using hospital medical records [10].
Figure 1.
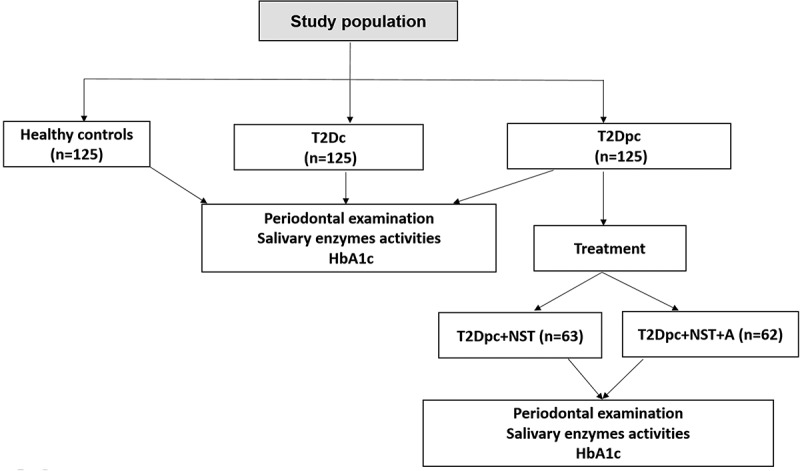
Flowchart showing distribution of the study population : T2Dc(controlled type 2 diabetes with chronic periodontitis), T2Dpc (poorly controlled type 2 diabetes with chronic periodontitis), HbA1c (glycated hemoglobin), NST (non-surgical treatment (scaling+ root planning)), A: antibiotics (amoxicillin and metronidazole).
3. Ethical statement
The Ethics Committee approved the study’s methods in accordance with the Helsinki Declaration (IRB 00008931). Before being included in the study, each participant completed a written informed consent. The following inclusion criteria had to be met: adults aged from 35 to 70 years able to provide informed consent, and a willingness to cooperate with the study protocol.
4. Study population
The exclusion criteria were complete edentulism, pregnancy or breastfeeding, smoking, alcoholism; antimicrobial, anti-inflammatory, and periodontal therapies 3 months prior to the study; allergy to metronidazole and amoxicillin, and evidence for systemic illnesses deemed a risk factor for periodontitis such as rheumatoid arthritis, osteoporosis, and metabolic syndrome.
5. Sample size calculation
Based on the prevalence of type 2 diabetes in Tunisia (15.1%) [1], the sample size was estimated using the conventional formula [10]. n = (z)2 p(1-p)/d2, where n= sample size, z = 1.96 for a 95% confidence level, p= disease prevalence in a specific demographic, and d = tolerable margin of error (d = 0.05).
6. Grouping
The 125 T2Dpc were randomly divided into two subgroups using a simple randomization method with 2 arms study trial. The sequence of random numbers is generated by a computer using a 1:1 allocation (63 receiving the first protocols vs. 62 receiving the second one; Figure 1).
7. Protocol of the intervention
The first therapeutic protocol involved a non-surgical periodontal treatment (NST) during two consecutive days. The study group’s non-surgical management included instructions for good dental hygiene, full mouth subgingival and supragingival scaling, and root planning carried out in two quadrants while under local anesthetic with manual and ultrasonic equipment. Patients subjected to this treatment were designated as T2Dpc + NST.
The second therapeutic protocol comprised of NST accompanied by a combination of systemic antibiotics consisting of 500 mg amoxicillin and 500 mg metronidazole (NST+A) taken twice daily for 7 days consecutively. Patients were instructed to take the first dose of the antibiotics in the morning of the first day of NST. Patients subjected to this second treatment were designated as T2Dpc+NST+A.
8. Oral periodontal examination
The periodontal examination was carried out in accordance with the guidelines published by the American Academy of Periodontology and the European Federation of Periodontology at the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions [10,11]. Six sites per tooth (mesiobuccal, mediobuccal, disto-buccal, disto-lingual, medio-lingual, and mesio-lingual), excluding the third molars, were subjected to the complete mouth clinical evaluations. The same periodontist measured periodontal indices such as periodontal probing depth (PPD), clinical attachment loss (CAL), percentage of bleeding on probing (BOP%), O’Leary plaque index (PI), Löe and Silness gingival index (GI), and the simplified oral hygiene index (OHI-S) of Greene and Vermillion using a William’s graduated periodontal probe. All T2Dpc were subjected to a second oral examination after 1 month since receiving the treatments.
9. Study investigations
9.1. Saliva collection
All participants were instructed to refrain from eating or drinking as well as from tooth brushing at least 3 hours before the sampling. Subjects were asked to rinse their mouth with water for 30 seconds to remove any debris. After that, the unstimulated saliva was allowed to accumulate on the floor of the mouth and the subjects spit it out into the test tube every 60 seconds for 5 min [12]. The collected salivary specimens were subjected to centrifugation at 5000 rpm for 10 min at 4°C to reduce salivary debris and viscosity. The type of saliva selection was unstimulated. For T2Dpc, a second saliva collection was performed after 1 month since receiving the treatments. Samples were immediately put on ice and then transferred to the laboratory. After sampling, aliquots were prepared, and the samples were frozen at−80 C until an analysis was carried out [13].
10. Assessment of salivary enzyme activities
Enzyme activities were estimated in triplicate using the Beckman Coulter AU680 (Beckman-Coulter, USA) autoanalyzer in the Biochemistry Laboratory. The ALP activity was determined by a kinetic color test. Whereas AST, ALT, CK, and LDH were measured by kinetic UV tests where the centrifugation has no effect on salivary enzymes. For T2Dpc, a second assessment of salivary enzyme activities was performed after 1 month since receiving the treatments.
11. Glycated hemoglobin (HbA1c) measure
Blood samples were collected in ethylenediaminetetraacetic acid-containing tubes (EDTA) on the same day just before the oral examination and the saliva analysis. The fully automated high-performance liquid chromatography (HPLC) variant II analyzer (BioRad, USA) was used for the HbA1c measure. The HbA1c criterion smaller than or equal to 7% was used to determine the T2D control.
For T2Dpc, a second HbA1c measurement was performed after 1 month since receiving the treatments. Furthermore, follow-up visits for continuous evaluation of the patients were scheduled
12. Statistical analysis
SPSS 20.0 was used to conduct the statistical tests. The distribution of the collected data was normal and so the two-way analysis of variance (ANOVA) and Student’s t-test were used to compare quantitative parameters. They were given as mean standard deviation. To assess which group comparisons differed significantly, the Tamhane’s T2 Post-Hoc Test was used because the groups were unequal. The Pearson’s correlation coefficient was used to evaluate the relationships between salivary enzyme activity and periodontal markers. The p-values below p < 0.05 have been statistically significant.
The AST blood test is used to measure the level of the enzyme AST in the blood, which is found in high levels in the liver, heart, and muscles. The kinetic UV test for AST, ALT, CK, and LDH is performed by using an enzymatic kinetic UV rate method. For example, the aspartate aminotransferase activity is measured by an enzymatic kinetic UV rate method at 340 nm. In this reaction, aspartate aminotransferase catalyzes the reversible transamination of L-aspartate and α-ketoglutarate to oxaloacetate and L-glutamate.
13. Results
The present study enrolled 125 healthy controls, 125 T2Dc, and 125 T2Dpc. For T2Dpc, periodontal indices, salivary enzyme activities, and HbA1c were assessed twice before and after 1 month of treatment. The comparison of periodontal status revealed that T2Dpc exhibited significantly the highest scores of PPD and CAL (P < 0.05, Table 1). However, BOP did not differ significantly between T2Dc and T2Dpc. Whereas the rest of clinical parameters PI, GI, and OHI-S did not significantly differ between groups. Concerning the comparison of salivary enzyme activities, T2Dpc presented the highest ALP, AST, and ALT activities (P < 0.05). However, CK and LDH activities did not significantly differ between the groups.
Table 1.
Glycated hemoglobin, periodontal scores, and salivary enzyme activities of the study population.
| Parameters (mean ± SD) |
Controls (n = 125) |
T2Dc (n = 125) |
T2Dpc | p-value |
|---|---|---|---|---|
| HbA1c (%) | 4.83 ± 0.43 | 7.21 ± 0.61 | 8.10 ± 0.88 | |
| Periodontal scores PPD (mm) CAL (mm) BOP (%) PI (%) GI OHI-S Salivary enzymes ALP (U/L) AST (U/L) ALT (U/L) LDH (U/L) CK (U/L) |
2.24 ± 0.46 0.82 ± 0.68 9.16 ± 3.14 31.26 ± 15.03 0.78 ± 0.41 1.26 ± 0.24 10.34 ± 2.23 9.41 ± 1.69 8.93 ± 2.46 91.23 ± 12.19 7.84 ± 1.41 |
4.61 ± 0.59 b 4.57 ± 0.77b 46.75 ± 23.61b 37.79 ± 15.65 1.32 ± 0.52 1.81 ± 0.47 29.04 ± 8.31b 20.36 ± 6.79b 19.27 ± 3.21b 93.56 ± 12.67 9.74 ± 1.56 |
6.22 ± 0.67a,b 5.69 ± 0.86a,b 50.39 ± 24.78b 41.45 ± 15.99 1.72 ± 0.62 2.22 ± 0.66 40.31 ± 9.31a,b 31.42 ± 7.09a,b 30.27 ± 3.51a,b 95.49 ± 12.93 11.84 ± 2.75 |
<0.001 <0.001 0.014 0.060 0.072 0.101 <0.001 <0.001 <0.001 0.106 0.069 |
Note: SD: standard deviation; T2c: controlled type 2 diabetes with chronic periodontitis; T2pc: poorly controlled type 2 diabetes with chronic periodontitis; HbA1c: glycated hemoglobin; PPD: periodontal probing depth; CAL: clinical attachment loss; BOP: bleeding index; PI: plaque index; GI: gingival index; OHI-S: simplified oral hygiene index; ALP: alkaline phosphatase; AST: aspartate transaminase; ALT: alanine transaminase; LDH: lactate dehydrogenase; CK: creatine kinase.
a: p < 0,05 compared to T2Dc (The Tamhane’s T2 Post-Hoc analysis); b: p < 0,05 compared to controls (The Tamhane’s T2 Post-Hoc analysis).
Correlations relating periodontal indices to salivary enzyme activities in the study population are summarized in Table 2. The Spearman’s analysis demonstrated the presence of three significant correlations between ALP activity and three periodontal indices: PPD (P = 0.002, r = 0.587; Figure 2a), CAL (P = 0.019, r = 0.640; Figure 3a) and BOP (P = 0.030, r = 0.354; Figure 4a) in T2Dc (Table 2). The same three correlations were more pronounced in T2Dpc as described, ALP-PPD (P < 0.001, r = 0.726; Figure 2b), ALP-CAL (P < 0.001, r = 0.830; Figure 3b), and ALP-BOP (P = 0.020, r = 0.492; Figure 4b).
Table 2.
Correlations between periodontal parameters and salivary enzyme activities of the study population.
| controls |
T2Dc |
T2Dpc |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ALP | AST | ALT | LDH | CK | ALP | AST | ALT | LDH | CK | ALP | AST | ALT | LDH | CK | |
| PPD | p = 0.101 r = 0.216 |
p = 0.128 r=−0.269 |
p = 0.436 r=−0.694 |
p = 0.671 r = 0.017 |
p = 0.142 r=−0.349 |
p = 0.002 r = 0.587** |
p = 0.133 r=−0.299 |
p = 0.236 r=−0.394 |
p = 0.681 r = 0.027 |
p = 0.132 r=−0.339 |
p= <0.001 r = 0.726** |
p = 0.123 r= −0.199 |
p = 0.099 r = 0.113 |
p = 0.589 r = 0.123 |
p = 0.242 r= −0.193 |
| CAL | p = 0.241 r = 0.323 |
p = 0.126 r=−0.139 |
p = 0.131 r=−0.493 |
p = 0.445 r=−0.044 |
p = 0.216 r=−0.374 |
p = 0.019 r = 0.640** |
p = 0.226 r=−0.039 |
p = 0.111 r=−0.273 |
p = 0.435 r=−0.094 |
p = 0.346 r=−0.274 |
p= <0.001 r = 0.830** |
p = 0.126 r= −0.399 |
p = 0.181 r= −0.363 |
p = 0.419 r= −0.194 |
p = 0.166 r= −0.174 |
| BOP | p = 0.326 r =−0.123 |
p = 0.369 r=−0.496 |
p = 0.301 r=−0.608 |
p = 0.205 r=−0.073 |
p = 0.169 r=−0.049 |
p = 0.030 r = 0.354** |
p = 0.431 r=−0.486 |
p = 0.314 r=−0.018 |
p = 0.305 r=−0.093 |
p = 0.469 r=−0.094 |
p = 0.020 r = 0.492** |
p = 0.231 r= −0.086 |
p = 0.119 r= −0.218 |
p = 0.321 r= −0.183 |
p = 0.396 r= −0.190 |
| PI | p = 0.269 r=− 0.036 |
p = 0.601 r=−0.031 |
p = 0.221 r=−0.706 |
p = 0.439 r=−0.274 |
p = 0.147 r = 1.039 |
p = 0.260 r=− 0.035 |
p = 0.501 r=−0.021 |
p = 0.201 r=−0.306 |
p = 0.224 r=−0.284 |
p = 0.137 r=−0.248 |
p = 0.163 r=− 0.085 |
p = 0.301 r= −0.091 |
p = 0.291 r= −0.006 |
p = 0.242 r= −0.183 |
p = 0.197 r= −0.148 |
| GI | p = 0.419 r=−0.498 |
p = 0.693 r=−0.086 |
p = 0.616 r=−0.146 |
p = 0.116 r=−0.483 |
p = 0.210 r=−0.187 |
p = 0.414 r=−0.349 |
p = 0.623 r=−0.046 |
p = 0.416 r=−0.346 |
p = 0.106 r=−0.473 |
p = 0.329 r=−0.187 |
p = 0.214 r=−0.149 |
p = 0.423 r= −0.096 |
p = 0.426 r= −0.366 |
p = 0.129 r= −0.384 |
p = 0.429 r= −0.287 |
| OHI-s | p = 0.215 r=−0.369 |
p = 0.401 r = 0.121 |
p = 0.196 r=−0.167 |
p = 0.219 r=−0.286 |
p = 0.416 r=−0.329 |
p = 0.213 r=−0.339 |
p = 0.431 r = 0.101 |
p = 0.183 r=−0.196 |
p = 0.299 r = 0.046 |
p = 0.406 r=−0.239 |
p = 0.111 r= −0.139 |
p = 0.131 r = 0.201 |
p = 0.161 r= −0.134 |
p = 0.109 r = 0.126 |
p = 0.306 r= −0.181 |
Note: T2Dc: controlled type 2 diabetes with chronic periodontitis; T2Dpc: poorly controlled type 2 diabetes with chronic periodontitis; PPD: periodontal probing depth; CAL: clinical attachment loss; BOP: bleeding index; PI: plaque index; GI: gingival index; OHI-S: simplified oral hygiene index; ALP: alkaline phosphatase; AST: aspartate transaminase; ALT: alanine transaminase; LDH: lactate dehydrogenase; CK: creatine kinase; r: correlation coefficient; **: correlation is significant at the 0.01 level.
Figure 2.
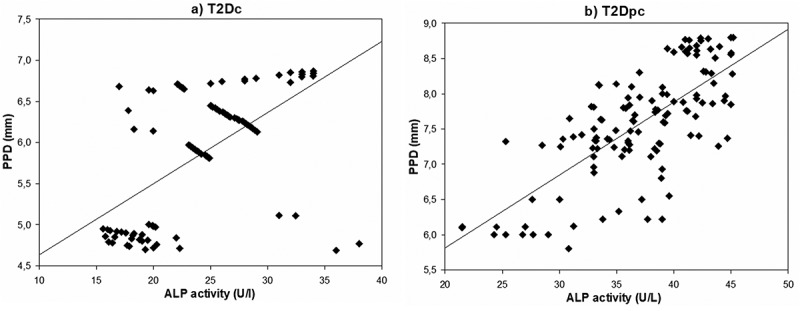
Correlation between ALP (alkaline phosphatase) activity and PPD (periodontal probing depth) in T2Dc (a) and in T2Dpc (b).
Figure 3.
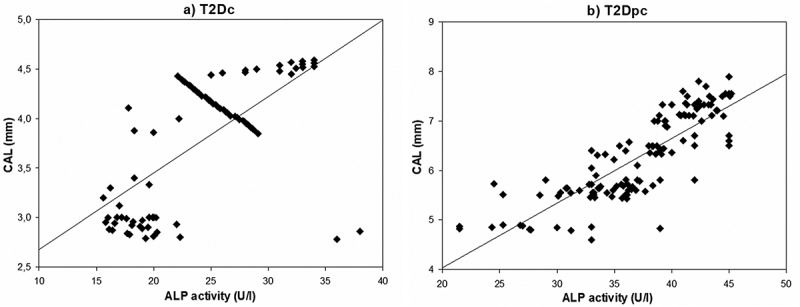
Correlation between ALP (alkaline phosphatase) activity and CAL (clinical attachment loss) in (a) T2Dc and in (b) T2Dpc.
Figure 4.
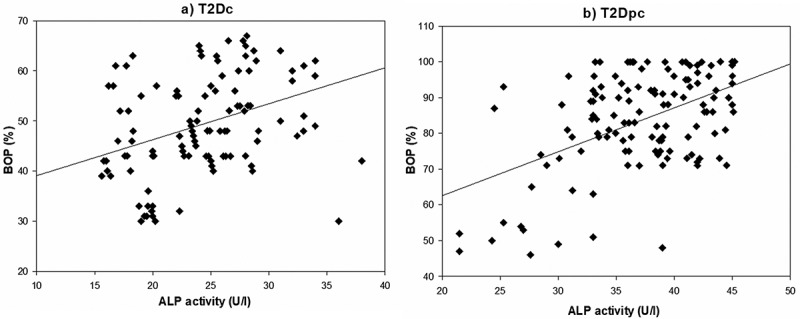
Correlation between ALP (alkaline phosphatase) activity and BOP (bleeding index) in (a) T2Dc and in (b) T2Dpc.
Compared to baseline, the first therapeutic protocol consisting in the non-surgical treatment, induced a non-significant decrease in periodontal indices, salivary enzyme activities, and HbA1c in T2Dpc+NST (Table 3). Whereas, the non-surgical treatment accompanied by antibiotics reduced significantly the periodontal indices, salivary enzyme activities, and HbA1c in T2Dpc+NST+A. Furthermore, the pairwise comparison between T2Dpc+NST and T2Dpc+NST+A showed a significant decrease in periodontal indices, salivary enzyme activities, and HbA1c following the antibiotic therapy (P < 0.05).
Table 3.
Differences in periodontal indices, salivary enzyme activity, and glycemic control between the systemic antibiotic non-surgical therapy group and the non-surgical group alone for T2Dpc.
| Parameters (mean ± SD) | At baseline | T2Dpc+NST (n = 63) | p-value* | T2Dpc+NST+A (n = 62) | P-value* | P-value# |
|---|---|---|---|---|---|---|
| HbA1c (%) | 8.10 ± 0.88 | 7.8 ± 0.63 | 0.051 | 7.5 ± 0.56 | 0.049 | 0.048 |
| Periodontal status | ||||||
| PPD (mm) | 6.22 ± 0.67 | 5.94 ± 0.46 | 0.059 | 5.41 ± 0.44 | 0.007 | 0.049 |
| CAL (mm) | 5.69 ± 0.86 | 5.09 ± 0.85 | 0.054 | 4.53 ± 0.82 | 0.009 | 0.044 |
| BOP (%) | 50.39 ± 24.78 | 48.39 ± 24.72 | 0.083 | 45.34 ± 21.69 | 0.011 | 0.035 |
| PI (%) | 41.45 ± 15.99 | 38.23 ± 14.71 | 0.091 | 35.74 ± 14.03 | 0.016 | 0.039 |
| GI | 1.72 ± 0.62 | 1.63 ± 0.52 | 0.129 | 1.02 ± 0.42 | 0.043 | 0.037 |
| OHI-S | 2.22 ± 0.66 | 2.01 ± 0.45 | 0.133 | 1.63 ± 0.41 | 0.032 | 0.031 |
| Salivary enzyme activities | ||||||
| ALP (U/L) | 40.31 ± 9.31 | 37.31 ± 9.29 | 0.061 | 28.03 ± 7.13 | <0.001 | <0.001 |
| AST (U/L) | 31.42 ± 7.09 | 28.53 ± 6.77 | 0.073 | 21.16 ± 5.29 | <0.001 | <0.001 |
| ALT (U/L) | 30.27 ± 3.51 | 25.21 ± 3.21 | 0.059 | 20.59 ± 3.41 | <0.001 | 0.001 |
| LDH (U/L) | 95.49 ± 12.93 | 90.49 ± 12.91 | 0.146 | 84.26 ± 10.72 | 0.003 | 0.009 |
| CK (U/L) | 11.84 ± 2.75 | 9.67 ± 0.70 | 0.122 | 6.36 ± 0.36 | 0.017 | 0.022 |
NOTE: SD: standard deviation; NST: non-surgical treatment (scaling+root planning); A: antibiotics (amoxicillin+metronidazole); PPD: periodontal probing depth; CAL: clinical attachment loss; BOP: bleeding index; PI: plaque index; GI: gingival index; OHI-S: simplified oral hygiene index; ALP: alkaline phosphatase; AST: aspartate transaminase; ALT: alanine transaminase; LDH: lactate dehydrogenase; CK: creatine kinase.
*: compared to baseline; #: the pair-wise comparisons between T2Dpc+NST and T2Dpc+NST+A.
14. Discussion
The present study was mainly conducted to show the impact of glycemic imbalance on periodontal indices, on salivary enzyme activities as well as to explore whether these parameters are linked. The investigation also intended to find the most sensitive salivary enzyme for the disease monitoring. Furthermore, the present work sought to compare the outcome of two curative protocols on salivary enzyme activities, periodontal indices, and HbA1c balance in T2Dpc with chronic periodontitis.
In this study, T2Dpc exhibited the most severe periodontal status depicted by high PPD, CAL, and BOP scores. This was in accordance with the reported negative impact of inappropriate glycemic control on diabetes promoting oral complications [11].
Both T2Dc and T2Dpc had higher activities of salivary ALP, AST, and ALT, whereas CK and LDH activities did not significantly differ compared to controls. These findings are in accordance with those of an early study [14]. Besides, previous studies reported high ALP activity in T2D patients with periodontitis [15,16], in T2D with dental caries in uncontrolled Type 1 diabetic children with periodontitis [17] and also with dental caries [18], in non-diabetic subjects with different periodontal conditions [19,20]. Moreover, AST and ALT high activities were reported in patients affected by periodontitis with [14,15] or without type 2 diabetes [20]. In contrast to our findings, AST activity difference did not reach a significant limit in T2D patients but was significant in Type 1diabetics versus controls.
In disagreement with our findings, LDH activity was increased in T2D patients with periodontitis [6,17]. CK activity was also increased in T2D patients with periodontitis [15], and in non-diabetic subjects with chronic periodontitis [21,22].
According to published research, diabetes neuropathy damages the parenchyma of the salivary glands, causing an aberrant basement membrane permeability. Therefore, an enhancement of salivary enzyme leakage into extracellular fluid arises progressively from locally destructed periodontal tissues by cytolysis or gingival bleeding [6,23]. Currently, the increase in AST and ALT activities particularly in T2Dpc could be considered as markers of salivary gland dysfunction and soft tissue inflammation.
The ALP enzyme is a calcium and phosphate membrane-bound glycoprotein also described as a phosphor-hydrolytic enzyme produced by several cells like polymorphonuclear leukocytes, osteoblasts, macrophages, and fibroblasts. ALP is also involved in the calcification process [24,25] and is mostly implicated in the normal turnover of the periodontal ligament, of the root cementum, and in adequate periodontal bone metabolism [17]. However, high ALP activity is considered as an indicator of its progressive increased leakage from damaged hard periodontal tissue after destructive process in the alveolar bone marking the advanced stages of periodontal disease [26]. Many oral enzymes are of bacterial origin; therefore, the destructive effect on periodontium might not be solely due to host enzymes. The increase in the mean level of ALP can be due to tissue alteration because of host-parasite reaction. During the progression of the disease, enzymes are released from dead and dying cells of the periodontium, polymorphonuclear leukocytes, inflammatory, epithelial, and connective tissue cells of the affected sites. Patel et al. (2016) revealed that study of salivary ALP levels were significantly raised in chronic periodontitis subjects as compared to gingivitis and healthy subjects [26].
In the present study, the Pearson’s analysis revealed significant positive correlations between ALP activity and periodontal parameters such as PPD, CAL, and BOP in T2D patients. Interestingly, the correlation was stronger in T2Dpc than in T2Dc. This result emphasizes prior findings showing the presence of a metabolic link between ALP activity and hard tissue destruction. According to the literature, two pathogenic processes have been elucidated in T2Dpc. First, prolonged hyperglycemia leads to an increase in glucose concentration in the gingival crevicular fluid shifting periodontal pockets to a competitive environment for virulent periodontal pathogens multiplication as Aggregatibacter Actinomycetemcomitans.29 Indeed, bacterial endotoxins boost the cascade of inflammatory responses leading to an apical migration of the junctional epithelium, to a collagen destruction, and to an alveolar bone loss. Hence, the intracellular ALP release from the damaged periodontal cells to the extracellular fluid is installed [27].
Second, advanced glycation end-products contribute to periodontal osteoclastogenesis through the activation of collagenases, hydrolases, and matrix metalloproteinases [28,29]. Taking together, the ALP activity could be considered as a reliable specific inflammatory biomarker of the hard tissue destruction.
Referring to the literature, correlations were also found between the AST activity and CAL in both T2Dc and T2Dpc, whereas the ALP activity was correlated to GI in T2Dpc, and ALT correlated to PPD in periodontitis subjects [14]. In a population with gingivitis and chronic periodontitis, Luke et al. found that ALP, AST, and ALT activities were well correlated to CAL, PPD, and OHI-S. Unlike our study, the AST activity was correlated to PPD and GI indices, whereas the CK activity was correlated to GI, PI, PPD, CAL, and BOP. The salivary enzyme activities increase seemed to be related to the type of the destructed tissue from which they were originated [20].
Currently, the adjunctive use of amoxicillin plus metronidazole provided a significant decrease of the periodontal indices and the salivary enzyme activities when compared to the mechanical treatment alone. Similar findings were reported in non-diabetic [30,31] and in diabetic subjects with periodontitis [32] treated with systemic antibiotics during the active phase of the disease. In fact, the adjunctive use of the systemic antibiotics diminishes the number of sites to be treated surgically [33]. The combination of systemic amoxicillin plus metronidazole has a wide spectrum of activity [34]. It can effectively reach and suppress multiple microorganisms in the deep pockets, in the root furcations, and in concavities that are inaccessible to the scaling instruments therapy, as well as unapparent non-diseased sites that could cause reinfection [35]. According to the documented literature, amoxicillin and metronidazole are the two antibiotics most frequently used in periodontal therapy [32]. Their combined effect is more important than each antibiotic used alone. Amoxicillin increases the uptake of metronidazole into the bacterial cells like strict anaerobic keystone pathogens [30].
The synergistic effect, mainly enhanced by amoxicillin, leads to the inhibition of the bacteria metabolic activities resulting in the proteolytic bacterial depletion [36]. In addition, the combined antibiotics are also implicated in the reduction of pro-inflammatory cytokines release from monocytes and fibroblasts that are stimulated by the lipopolysaccharides [37]. Furthermore, these types of antibiotics promote and maintain the recolonization of the beneficial compatible bacteria with a healthy periodontium [38,39]. All these benefits make amoxicillin and metronidazole combination the most effective antibiotic therapy for diabetics who present an increased risk of periodontitis progression.
In contrast to our findings, significant ameliorations of periodontal status and salivary enzyme activities following the NST without the use of antibiotics were reported [23,40]. The NST has been regarded as the standard non-surgical periodontal therapy. However, the use of mechanical instrumentation can only perturbate the subgingival biofilm making it more susceptible [41,42]. Besides, the mechanical instrumentation generates wound without reaching deeper bacteria that reside in the root concavities or in the dentin tubules. This leads to an uncompleted elimination of the subgingival biofilm [43,44], and increases the risk of eventual attachment loss [36,38].
There were certain limits in the present study. Re-evaluation of residual sites at a regular interval term follow-ups would be essential, which depend on patient availability to pursue enzyme activities and to achieve successful periodontal management. Further investigations dealing with other inflammatory markers are recommended
In conclusion, the current investigation revealed that T2Dpc exhibited severe periodontal status accompanied by the increased salivary ALP, AST, and ALT activities. The later could be regarded as inflammatory indicators of periodontal tissue injury.
Furthermore, ALP activity was praiseworthy correlated to periodontal indices that are related to glycemic balance. Thus, the ALP activity could be considered as a reliable marker for the diagnosis and prognosis of periodontitis. Interestingly, the administration of amoxicillin in combination with metronidazole adjunctively prescribed to subgingival instrumentation appropriately improved the periodontal status and reduced the activity of salivary enzymes.
Acknowledgments
The authors are grateful for the cooperation of the study participants. The authors especially acknowledge the head of the Biochemistry Department and LR12SP11, in Sahloul University Hospital, and the excellent technical team assistance for their valuable contribution.
Funding Statement
The author(s) reported there is no funding associated with the work featured in this article.
Abbreviations list
- A
Antibiotics
- ALP
Alkaline phosphatase
- ALT
Alanine aminotransaminase
- AST
Aspartate aminotransferase
- BOP
Bleeding on probing
- CAL
Clinical attachment loss
- CK
Creatine kinase
- GI
Gingival index
- HbA1c
Glycated hemoglobin
- LDH
Lactate dehydrogenase
- NST
Non-surgical treatment
- OHI-S
Oral hygiene index- simplified
- PI
Plaque index
- PPD
Periodontal probing depth
- T2Dc
Adequately glycemic controlled type 2 diabetic patients with chronic periodontitis
- T2Dpc
Poorly glycemic controlled type 2 diabetic patients with chronic periodontitis
Disclosure statement
No potential conflict of interest was reported by the authors.
Author contributions
Marwa Mrag and Yosra Ayed contributed to the study concepts. The study design was done by Dr Asma Kassab. Data acquisition was performed by Aishah Alhodhodi and Yassine Khalij. Marwa Mrag and Shadia A. Elsayed contributed to data analyses and interpretation. Asma Kassab contributed to the manuscript preparation and manuscript editing and reviewing. All authors read and approved the final manuscript.
References
- [1].Ben Romdhane H, Ali SB, Aissi W, et al. Prevalence of diabetes in northern African countries: the case of Tunisia. BMC Public Health. 2014;14:86. doi: 10.1186/1471-2458-14-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].International Diabetes Federation . IDF Diabetes Atlas Ninth. IDF. 2019.
- [3].Prathibha MK. Evaluation of salivary profile among adult type 2 diabetes mellitus patients in South India. J Clin Diagnostic Res. 2013;7:1592–10. doi: 10.7860/JCDR/2013/5749.3232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Gowdar IM, Almuhaiza M. Diabetes and Oral Health – a Review. Ann Int Med Dent Res. 2016;2:2–8. [Google Scholar]
- [5].Kinane DF, Stathopoulou PG, Papapanou PN. Periodontal diseases. Nat Rev Dis Prim. 2017;3:17038. doi: 10.1038/nrdp.2017.38. Cited: in: PMID: 28805207. [DOI] [PubMed] [Google Scholar]
- [6].Malicka B, Skoskiewicz-Malinowska K, Kaczmarek U. Salivary lactate dehydrogenase and aminotransferases in diabetic patients. Med (United States). 2016;95:e5211. doi: 10.1097/MD.0000000000005211. Cited: in: PMID: 27893660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Shetty AS, Fuoad A, Hamed MS. Salivary alkaline phosphatase and oral health: a review. Ital J Dent Med. 2017;2(2):55–58. [Google Scholar]
- [8].Vinod K, Madathil L, Shetty P, et al. Salivary and serum aspartate aminotransferases and alanine aminotransferases in insulin-dependent diabetes mellitus and normal children: a comparative study. J Int Soc Prev Community Dent. 2018;8:229. doi: 10.4103/jispcd.JISPCD_60_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Nibali L, Donos N, Terranova V, et al. Left ventricular geometry and periodontitis in patients with the metabolic syndrome. Clin Oral Investig. 2019;23:2695–2703. Cited: in: PMID: 30350134. doi: 10.1007/s00784-018-2667-8 [DOI] [PubMed] [Google Scholar]
- [10].Tonetti MS, Greenwell H, Kornman KS. Staging and grading of periodontitis: framework and proposal of a new classification and case definition. J Periodontol. 2018;89:S159–S172. Cited: in: PMID: 29926952. doi: 10.1002/JPER.18-0006 [DOI] [PubMed] [Google Scholar]
- [11].Nascimento GG, Leite FRM, Vestergaard P, et al. Does diabetes increase the risk of periodontitis? A systematic review and meta-regression analysis of longitudinal prospective studies. Acta Diabetol 55. Springer Milan; 2018. pp. 653–667. 10.1007/s00592-018-1120-4 [DOI] [PubMed] [Google Scholar]
- [12].NAVAZESH M. Methods for collecting saliva. Ann N Y Acad Sci. 1993;694:72–77. doi: 10.1111/j.1749-6632.1993.tb18343.x. Cited: in: PMID: 8215087. [DOI] [PubMed] [Google Scholar]
- [13].Kassab A, Ayed Y, Elsayed SA, et al. Glycated hemoglobin influence on periodontal status, pathogens and salivary interleukins in type II diabetic Tunisian subjects with chronic periodontitis. J Dent Sci. 2021;16:614–620. doi: 10.1016/j.jds.2020.09.018. Internet. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Abdul-Wahab GA, Ahmed MA. Assessment of some salivary enzymes levels in type 2 Diabetic Patients with Chronic Periodontitis: clinical and Biochemical Study. J Baghdad Coll Dent. 2015;27(1):138–143. doi: 10.12816/0015278. [DOI] [Google Scholar]
- [15].Ikekpeazu EJ, Neboh EE, Maduka IC, et al. Periodontal disease and type 2 diabetes: effects on salivary enzyme activities. Int J Diabetes Dev Ctries. 2011;31:9–13. doi: 10.1007/s13410-010-0005-z [DOI] [Google Scholar]
- [16].Cutando A, Lopez-Valverde A, Gomez-de-Diego R, et al. Effect of gingival application of melatonin on alkaline and acid phosphatase, osteopontin and osteocalcin in patients with diabetes and periodontal disease. Med Oral Patol Oral y Cir Bucal. 2013;18:e657–e663. Cited: in: PMID: 23524437. doi: 10.4317/medoral.18832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Srisridharan R, Sravani P, Satyanarayan A, et al. Salivary alkaline phosphatase as a noninvasive marker for periodontal disease in children with uncontrolled type 1 diabetes mellitus. J Clin Pediatr Dent. 2017;41(1):70–74. doi: 10.17796/1053-4628-41.1.70. Cited: in: PMID: 28052205. [DOI] [PubMed] [Google Scholar]
- [18].Uppu K, Sahana S, Madu GP, et al. Estimation of salivary glucose, calcium, phosphorus, alkaline phosphatase, and immunoglobulin a among diabetic and nondiabetic children: a case–control study. Int J Clin Pediatr Dent. 2018;11:71–78. doi: 10.5005/jp-journals-10005-1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kumar R, Sharma G. Salivary Alkaline Phosphatase level as Diagnostic marker for periodontal disease. J Int Oral Heal. 2011;3:82–85. [Google Scholar]
- [20].Luke R, Khan SN, Iqbal PS, et al. Estimation of specific salivary enzymatic biomarkers in individuals with gingivitis and chronic periodontitis: a clinical and biochemical study. J Int Oral Health: JIOH. 2015;7(9):54–57. Cited: in: PMID: 26435618. [PMC free article] [PubMed] [Google Scholar]
- [21].Latti B, Kalburge J, Birajdar S, et al. Evaluation of relationship between dental caries, diabetes mellitus and oral microbiota in diabetics. J Oral Maxillofac Pathol. 2018;22(2):282. doi: 10.4103/jomfp.JOMFP_163_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Neaz S, Rahman SM, Jhura FT. Investigation of salivary enzyme levels in periodontitis patient of bangladeshi population: a preliminary study salivary enzyme. International Journal of Research and Scientific Innovation (IJRSI) | Volume VI, Issue VII. 2019;VI:111–113.2321–2705. July 2019. [Google Scholar]
- [23].Kudva P, Saini N, Kudva H, et al. To estimate salivary aspartate aminotransferase levels in chronic gingivitis and chronic periodontitis patients prior to and following non-surgical periodontal therapy: a clinico-biochemical study. J Indian Soc Periodontol. 2014;18:53. doi: 10.4103/0972-124X.128209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Alsaykhan K, Khan NS, Aljumah MI, et al. Comparative Evaluation of Salivary Enzyme in Patients with Gingivitis and Periodontitis: a Clinical-Biochemical Study. Cureus. 2022;14:e20991. Cited: in: PMID: 35004095. doi: 10.7759/cureus.20991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Totan A, Greabu M, Totan C, et al. Salivary aspartate aminotransferase, alanine aminotransferase and alkaline phosphatase: possible markers in periodontal diseases? Clin Chem Lab Med. 2006;44:612–615. doi: 10.1515/CCLM.2006.096. Cited: in: PMID: 16681433. [DOI] [PubMed] [Google Scholar]
- [26].Patel RM, Varma S, Suragimath G, et al. Estimation and Comparison of Salivary Calcium, Phosphorous, Alkaline Phosphatase and pH Levels in Periodontal Health and Disease: a Cross-sectional Biochemical Study. J Clin Diagn Res. 2016;10:ZC58–61. doi: 10.7860/JCDR/2016/20973.8182. Cited: in: PMID: 27630955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Esteves-Lima R-P, Reis C, Santirocchi-Júnior F, et al. Association between periodontitis and serum c-reactive protein levels. J Clin Exp Dent. 2020;12:e838–e843. doi: 10.4317/jced.57041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ilea A, Băbţan AM, Boşca BA, et al. Advanced glycation end products (AGEs) in oral pathology. Arch Oral Biol. 2018;93:22–30. Cited: in: PMID: 29803117. doi: 10.1016/j.archoralbio.2018.05.013 [DOI] [PubMed] [Google Scholar]
- [29].Ramadan DE, Hariyani N, Indrawati R, et al. Cytokines and Chemokines in Periodontitis. Eur J Dent. 2020;14:483–495. doi: 10.1055/s-0040-1712718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Jentsch HFR, Dietrich M, Eick S. Non-surgical periodontal therapy with adjunctive amoxicillin/metronidazole or metronidazole when no aggregatibacter actinomycetemcomitans is detected—a randomized clinical trial. Antibiotics. 2020;9:686. doi: 10.3390/antibiotics9100686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].McGowan K, McGowan T, Ivanovski S. Optimal dose and duration of amoxicillin-plus-metronidazole as an adjunct to non-surgical periodontal therapy: a systematic review and meta-analysis of randomized, placebo-controlled trials. J Clin Periodontol. 2018;45:56–67. doi: 10.1111/jcpe.12830. Cited: in: PMID: 26362346. [DOI] [PubMed] [Google Scholar]
- [32].da CD, Duarte PM, Figueiredo LC, et al. Metronidazole and amoxicillin for patients with periodontitis and diabetes mellitus: 5-year secondary analysis of a randomized controlled trial. J Periodontol. 2021;92(4):479–487. doi: 10.1002/JPER.20-0196. Cited: in: PMID: 32905615. [DOI] [PubMed] [Google Scholar]
- [33].Teughels W, Feres M, Oud V, et al. Adjunctive effect of systemic antimicrobials in periodontitis therapy: a systematic review and meta-analysis. Cited: in: PMID: 31994207 J Clin Periodontol. 2020;47(2):257–281. doi: 10.1111/jcpe.13264 [DOI] [PubMed] [Google Scholar]
- [34].Gómez-Sandoval JR, Robles-Cervantes JA, Hernández-González SO, et al. Efficacy of clindamycin compared with amoxicillin-metronidazole after a 7-day regimen in the treatment of periodontitis in patients with diabetes: a randomized clinical trial. BMJ Open Diabetes Res Care. 2020;8(1):e000665. doi: 10.1136/bmjdrc-2019-000665. Cited: in: PMID: 31958293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Luchian I, Goriuc A, Martu MA, et al. Clindamycin as an Alternative Option in Optimizing Periodontal Therapy. Antibiot (Basel, Switzerland)2021. Vol. 10: doi: 10.3390/antibiotics10070814. Cited: in: PMID: 34356735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Tamashiro NS, Duarte PM, Miranda TS, et al. Amoxicillin Plus Metronidazole Therapy for Patients with Periodontitis and Type 2 Diabetes: a 2-year Randomized Controlled Trial. J Dent Res. 2016;95:829–836. doi: 10.1177/0022034516639274. Cited: in: PMID: 27013640. [DOI] [PubMed] [Google Scholar]
- [37].Rizzo A, Paolillo R, Guida L, et al. Effect of metronidazole and modulation of cytokine production on human periodontal ligament cells. Int Immunopharmacol. 2010;10:744–750. doi: 10.1016/j.intimp.2010.04.004. Cited: in: PMID: 20399284. [DOI] [PubMed] [Google Scholar]
- [38].Fritoli A, Gonçalves C, Faveri M, et al. The effect of systemic antibiotics administered during the active phase of non-surgical periodontal therapy or after the healing phase: a systematic review. J Appl Oral Sci. 2015;23:249–254. doi: 10.1590/1678-775720140453. Cited: in: PMID: 26221918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Abdallaoui-Maan L, Bouziane A. Effects of timing of adjunctive systemic antibiotics on the clinical outcome of periodontal therapy: a systematic review. J Clin Exp Dent. 2020;12:e300–e309. Cited: in: PMID: 32190202. doi: 10.4317/jced.56324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Jeyasree RM, Theyagarajan R, Sekhar V, et al. Evaluation of serum and salivary alkaline phosphatase levels in chronic periodontitis patients before and after nonsurgical periodontal therapy. 2018. doi: 10.4103/jisp.jisp_133_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Laugisch O, Auschill TM, Tumbrink A, et al.Influence of Anti-Infective Periodontal Therapy on Subgingival Microbiota Evaluated by Chair-Side Test Compared to Qpcr-A Clinical Follow-Up StudyAntibiot (Basel, Switzerland)2022. p. 11: doi; 10.3390/antibiotics11050577. Cited: in: PMID: 35625221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Cosgarea R, Juncar R, Heumann C, et al. Non-surgical periodontal treatment in conjunction with 3 or 7 days systemic administration of amoxicillin and metronidazole in severe chronic periodontitis patients. A placebo-controlled randomized clinical study. J Clin Periodontol. 2016;43:767–777. doi: 10.1111/jcpe.12559. Cited: in: PMID: 27027501. [DOI] [PubMed] [Google Scholar]
- [43].Karrabi M, Baghani Z, Venskutonis T. Amoxicillin/Metronidazole Dose Impact As An Adjunctive Therapy For Stage II - III grade C periodontitis (aggressive periodontitis) at 3- and 6-month follow-ups: a systematic review and meta-analysis. J Oral Maxillofac Res. 2022;13(1):e2. doi: 10.5037/jomr.2022.13102. Cited: in: PMID: 35574209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Moreno Villagrana AP, Gómez Clavel JF. Antimicrobial or subantimicrobial antibiotic therapy as an adjunct to the nonsurgical periodontal treatment: a meta-analysis. ISRN Dent. 2012;2012:1–11. doi: 10.5402/2012/581207 [DOI] [PMC free article] [PubMed] [Google Scholar]


