Abstract
The DNA photosensitisers m-iodo Hoechst and m-iodo, p-methoxy Hoechst have been co-crystallised with the oligonucleotide d(CGCGAATTCGCG)2 and their crystal structures determined. The crystals were then subjected to slow dehydration, which reduced their solvent contents from 40 (normal) to 30 (partially dehydrated) and then 20% (fully dehydrated) and caused a reduction in cell volume from 68 000 to 60 000 then 51 000 Å3. The dehydration resulted in a dramatic enhancement of diffraction resolution from ~2.6 to beyond 1.5 Å. Crystal structures have also been determined for the partially and fully dehydrated states. The fully dehydrated crystals consist of an infinite polymeric network, in which neighbouring dodecamer duplexes are crosslinked through phosphate oxygens via direct bonding to bridging magnesium cations. This unique three-dimensional structure for DNA is described in detail in the following companion paper. The present paper details evidence from the sequence of crystal structures that the DNA is able to breathe locally, allowing the ligand to leave the minor groove, re-orient in the surrounding solvent medium and then re-enter the groove in a different orientation and location. The rearrangement of the minor groove binding ligands during the dehydration process mimics the binding behaviour of these ligands in solution and in vivo. We also present details of the DNA–ligand interactions that are consistent with a hydrogen atom abstraction mechanism for photocleavage of DNA.
INTRODUCTION
Radiosensitisers have the potential for a range of applications in cancer radiotherapy. There has been much interest in halogenated DNA precursors since the discovery in 1980 that incorporation of iodo- or bromodeoxyuridine into DNA sensitises cells to both UV and ionising radiation (1). The extent of sensitisation is much less for ionising radiation than for UV irradiation, but nevertheless sufficient to prompt clinical trials of iodo- and bromodeoxyuridine as radiosensitisers in cancer radiotherapy (2). The sensitisation is mediated by the uracilyl radical, which abstracts a hydrogen atom from a nearby deoxyribosyl carbon atom, resulting in lesions and subsequent DNA strand breakage. This mechanism was extended to a new concept in radiosensitiser design in which a halogen atom was incorporated into a minor groove-binding ligand. Thus UVA irradiation of m-iodo Hoechst 33258 bound to DNA results in strand cleavage at the ligand-binding sites, mediated by the ligand radical species formed by photodehalogenation (3). Subsequent studies with m-iodo Hoechst 33258 and three new analogues with the iodine atom substituted in the three possible positions in the phenyl ring showed significant differences in the sites and extent of UVA-induced strand cleavage (4). The ligands o-, m- and p-iodo Hoechst and m-iodo Hoechst 33258 are illustrated in Figure 1. The extent of single-strand cleavage varies over a >35-fold range in the order o-iodo Hoechst >> m- and p-iodo Hoechst >> m-iodo Hoechst 33258. The ortho isomer induces single-strand cleavage of either strand of the DNA, but the para and meta isomers produce cleavage of only one strand in a process which is believed to occur by hydrogen atom abstraction at conserved C5′ sites. The crystal structures of two m-iodo Hoechst ligands (IA and IB, Fig. 1) bound to DNA were determined to help explain the cleavage data.
Figure 1.
Molecular structures of Hoechst 33258, the iodo Hoechst ligands and p-dimethylamino Hoechst.
After these structural studies were completed, the capillary mounted crystals were subjected to dehydrating conditions as described in Materials and Methods. As each crystal dehydrated, the resolution of its X-ray diffraction data increased from 2.6 (‘normal’ crystals, with 40% solvent content) to 2.2 (‘partially dehydrated’ crystals, with 30% solvent content) to 1.5 Å (‘fully dehydrated’ crystals, with 20% solvent content). No further dehydration occurred and the fully dehydrated crystals appear to be indefinitely stable. The solvent loss is accompanied by a reduction of 25% in the volume of the unit cells. Intensity data sets were also collected for partially and fully dehydrated crystals. All seven structures listed in Table 1 were solved and independently refined. Collectively, they offer multiple representations of the three states of crystal hydration and allow visualisation, at near atomic resolution, of ligand movement in response to conformational changes in the DNA.
Table 1. Experimental and statistical summaries for the m-iodo Hoechst and p-dimethylamino Hoechst (p-DH) structures in space group P212121.
| Normal | Partially dehydrated | Fully dehydrated | |||||
|---|---|---|---|---|---|---|---|
| IA | IB | IA | IB | p-DH | IA | IB | |
| Cell constants (Å) | a = 25.38 | a = 25.37 | a = 24.58 | a = 24.49 | a = 24.16 | a = 24.91 | a = 24.56 |
| b = 40.71 | b = 40.48 | b = 38.79 | b = 38.84 | b = 38.62 | b = 34.15 | b = 33.85 | |
| c = 66.24 | c = 66.08 | c = 63.10 | c = 63.52 | c = 63.86 | c = 61.30 | c = 61.15 | |
| Volume (Å3) | 68 440 | 67 860 | 60 160 | 60 420 | 59 590 | 52 150 | 50 840 |
| Crystallisation conditionsa | |||||||
| DNA (mM) | 0.52 | 0.57 | 0.63 | 0.63 | 0.44 | 0.63 | 0.57 |
| Ligand (mM) | 0.65 | 0.71 | 0.79 | 0.79 | 0.55 | 0.79 | 0.71 |
| MgCl2 (mM) | 5.2 | 8.6 | 3.2 | 3.2 | 2.2 | 3.2 | 8.6 |
| Spermine (mM) | 0.87 | 0.95 | 1.1 | 0.53 | 0.74 | 1.1 | 0.95 |
| MPD (%) | 3.5 | 3.8 | 8.4 | 4.2 | 11.9 | 8.4 | 3.8 |
| Drop volume (µl) | 11.5 | 10.5 | 9.5 | 9.5 | 13.5 | 9.5 | 10.5 |
| Reservoir MPD (%) | 40 | 40 | 40 | 40 | 30 | 40 | 40 |
| Resolution (Å) | 2.6 | 2.4 | 2.1 | 2.2 | 2.2 | 1.6 | 1.6 |
| Reflections | |||||||
| Observed data (>2σFobs) | 1901 | 2513 | 3281 | 2788 | 2628 | 8269 | 9015 |
| Completeness (%) | 97.1 | 98.8 | 97.9 | 95.9 | 96.0 | 99.3 | 97.5 |
| Rsymb (%) | 11.1 | 11.8 | 9.5 | 10.1 | 14.1 | 9.2 | 7.6 |
| R (Rfreec) (%) | 22.7 (24.6) | 20.5 (24.9) | 20.3 (25.4) | 19.8 (25.0) | 22.3 (31.3) | 15.8 (18.7) | 14.9 (16.7) |
| Refinement program | X-PLOR | XPLOR | X-PLOR | XPLOR | X-PLOR | SHELXL-97 | SHELXL-97 |
| RMS deviation from ideal | |||||||
| Bond length (Å)d | 0.012 | 0.010 | 0.007 | 0.007 | 0.011 | 0.009 | 0.007 |
| Bond angle (Å)d | 1.609 | 1.066 | 1.077 | 1.088 | 1.068 | 0.021 | 0.017 |
| NDB code | DD0006 | DD0005 | DD0009 | DD0008 | DD0007 | DD0004 | DD0003 |
aLigands dissolved in methanol, all other solutions in 30 mM sodium cacodylate, pH 7.0. Crystals were grown at 20°C. Reservoir volume 1 ml.
bRsym = ∑|[Fo2 – 〈Fo2〉]|/∑[Fo2]
cfree R factor is based on 10% randomly chosen data.
dSHELXL-97 values are for DFIX and DANG restraints.
MATERIALS AND METHODS
The structures described in this paper contain one of three minor groove-binding ligands: IA, 2′-(3-iodophenyl)-5-(4-methyl- 1-piperazinyl)-2,5′-bi-benzimidazole (m-iodo Hoechst); IB, 2′-(3-iodo-4-methoxyphenyl)-5-(4-methyl-1-piperazinyl)-2,5′-bi-benzimidazole (m-iodo, p-methoxy Hoechst); p-DH, 2′-(4-dimethylaminophenyl)-5-(4-methyl-1-piperazinyl)-2,5′-bi-benzimidazole (p-dimethylamino Hoechst). Molecular structures of these ligands are depicted in Figure 1.
Crystallography
The DNA was purchased from Oligos Etc. Inc. and was annealed before use. Synthesis of the iodo Hoechst and dimethylamino Hoechst ligands has been reported elsewhere (5). All ligands were in the free base form and were dissolved initially in 100% methanol for the crystallisation experiments. Crystals of the complexes were grown from hanging drops at ambient temperature under the conditions given in Table 1. Addition of the drug solutions to the crystallisation droplets resulted in significant initial precipitation but crystals suitable for X-ray studies formed in 1 week and grew to their full dimensions within 1 month. All iodo Hoechst experiments, including crystallisation, mounting and data collection, were conducted in a blackened room with filtered lighting to prevent UVA-induced DNA cleavage by the ligands.
Crystal mounting and dehydration
Crystals containing iodo Hoechst ligands IA and IB were sealed in X-ray capillaries using epoxy resin. A plug of mother liquor was placed close to each crystal. Intensity data sets were collected and the crystal structures solved in the normal manner as described below. It was later discovered that the epoxy resin had not formed a complete seal and that the crystals were slowly dehydrating within the capillaries. There was no sign of deterioration of crystal quality, but the crystals were noticeably smaller in volume. When the crystals were returned to the diffractometer, it was found that the X-ray diffraction patterns had markedly improved and the crystal unit cells were much smaller than before. After ~2 months, the crystals reached a point at which dehydration stopped. The unit cells had, by then, shrunk to ~75% of their initial volumes. Data sets were collected for the fully dehydrated crystal state and a partially dehydrated intermediate state, for each of the IA and IB ligands.
The partially dehydrated p-DH-containing crystal was produced in a controlled experiment in which the mother liquor plug was replaced by a saturated sodium chloride solution (6). Equilibrium was attained within 1 or 2 days.
Crystallographic data collection
Data were collected at 20°C on a Siemens SMART diffractometer equipped with CCD detector, graphite monochromator and sealed tube molybdenum X-ray source. Hemispheres of data were collected and processed using SAINT (7), SADABS (8) and XPREP (9). Details of the data collection and processing are given in Table 1.
Structure solution, refinement and treatment of disorder
The normal crystals have unit cells which are very similar in size to that of the native DNA and it was possible to commence refinement with rigid body minimisation of these native coordinates (10) using X-PLOR v.3.851 (11). The partially dehydrated crystals could similarly be solved commencing with the native coordinates. The fully dehydrated structures could not be solved in this manner as the molecular rearrangements were too great. The structure of the fully dehydrated IB complex was eventually solved by molecular replacement using the coordinates of just the eight central base pairs of the partially dehydrated IB structure and the program AmoRe (12). The fully dehydrated complex with IA was refined directly from the IB structure. Individual positional and temperature factor calculations completed the initial refinement of the DNA coordinates. The DNA refinement strategies have been outlined many times previously (see for example 13).
Electron density maps were produced and displayed using O v.5.10 (14). The DNA in all structures fitted the electron density well in all regions. Continuous lobes of residual electron density within the minor grooves showed sufficient detail to allow model coordinates for the IA and IB ligands to be assigned. The ligands in the normal crystals were distributed over two sites and two orientations, but they occupied single locations and orientations in the partially and fully dehydrated crystals. The maps for the normal, partially dehydrated and fully dehydrated crystals are shown in Figures 4 and 5.
Figure 4.
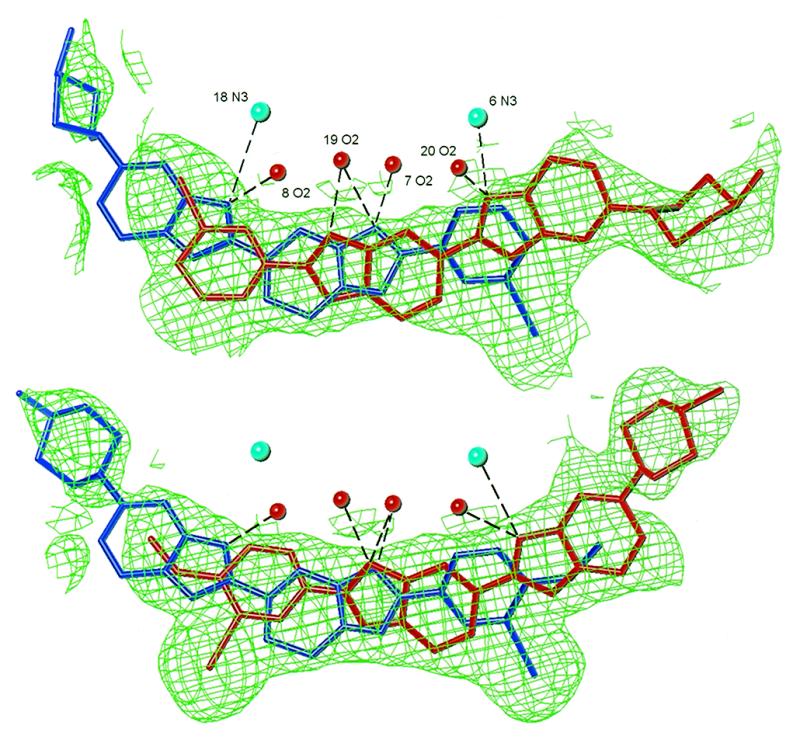
Ligand binding site and disorder in IA normal structure (top) and IB normal structure (bottom). Major ligand components are shaded in red, minor components in blue. Experimental electron density (2Fo – Fc map) before ligand refinement is drawn at the 0.7σ level using TURBO (29). Hydrogen bond distances are in the range 2.7–3.4 Å. In relation to the strand 1 sequence, the 3′-end of the binding site is to the left of the figure and the 5′-end to the right.
Figure 5.
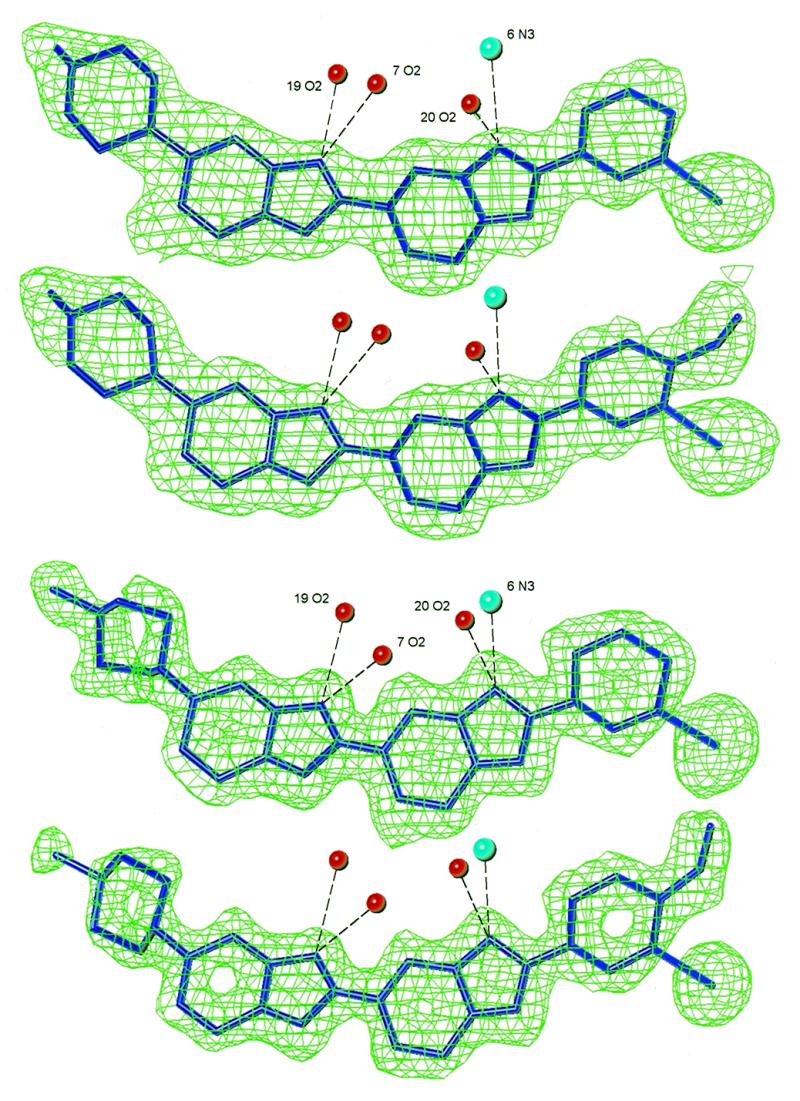
Ligand binding sites and 2Fo – Fc density maps for the partially dehydrated IA and IB structures (top) and for the fully dehydrated IA and IB structures (bottom). The experimental density before ligand refinement is drawn at the 1.0σ and 1.5σ levels for partially dehydrated and fully dehydrated structures, respectively. Hydrogen bond distances are in the range 2.8–3.3 Å.
The iodine atoms were fitted to obvious high density regions in the maps and the remainder of the ligand atom positions were found by manual rotation of a trial model to best fit the displayed electron density. In the major orientation of IA the iodine pointed inwards towards the groove floor and the piperazine pointed out; in the minor orientation the iodine pointed out and the piperazine pointed in. For both orientations of IB the iodine pointed out and the piperazine pointed in.
Electrostatic charges were calculated for ligand atoms using the cvff forcefield of InsightII (15). Ligand topology and parameter files were produced using the program XPLO2D [XPLO2D is freely available to academic users from http://alpha2.bmc.uu.se/usf/xutil.html ; commercial users without a license for the X-UTIL package may contact Gerard Kleywegt (gerard@xray.bmc.uu.se ) for licensing information] and edited manually to incorporate electrostatic charges. The ligands were included in the X-PLOR refinement of the DNA starting with positional refinement of atomic coordinates. Within the normal crystals the ligand atoms were assigned B factors taken from previous Hoechst–DNA structures and the final refined occupancies were determined using the grouped B factor protocol of X-PLOR. The two ligands of each normal structure were constrained from interacting with each other and only the X-ray terms of the X-PLOR calculations were used in their refinement. Ligand refinement for all structures was completed with individual B factors being calculated for each atom.
Water molecules were also located in density maps and individually refined. The criteria for acceptance were as outlined previously (16). In the fully dehydrated structures, two magnesium cations appeared as well-defined octahedral density (see figure 4 of the accompanying paper). The density about the magnesium cations was so well resolved that the magnesium and coordinated oxygen atoms could be refined free of any restraints. Because of the resolution and high number of observed reflections, the final refinements for the fully dehydrated structures were carried out using SHELXL-97 (17). This gave significantly improved structures compared to those obtained by the X-PLOR refinement. The location of water molecules was >90% consistent between X-PLOR and SHELXL-97 refinements.
Final refinement statistics and NDB deposition codes for all structures are listed in Table 1.
RESULTS AND DISCUSSION
Structural types
Representative complexes from normal, partially dehydrated and fully dehydrated crystals are illustrated in Figure 2. In all three structural types the DNA maintains B-form character, as found in previous X-ray structure determinations of dodecamers with ligands bound in the narrow central region of the minor groove (18–21). In the normal structures the DNA displays an ~8° bend in the helical axis around the junction of the G4–A5 sequence. In the partially dehydrated crystals, this bend is absent as a result of concerted changes in roll and propeller twist that are propagated along the helix and through the central AATT region (21). The fully dehydrated crystals represent a unique state of condensation, and their DNA conformations are described in detail in the following companion paper. The present paper focuses on the ligand interactions with the DNA and the changes in the minor groove widths of the three structural types as the crystals dehydrate.
Figure 2.
Structures of the three m-iodo, p-methoxy Hoechst IB complexes, drawn with XP (27). From left to right: normal hydration; partial dehydration; full dehydration. The normal structure shows just one of two possible ligand sites.
In all cases, the ligands bind in the narrow central region of the minor groove by a combination of van der Waals interactions and hydrogen bonding between benzimidazole NH groups and acceptor groups in the floor of the groove. Isohelicity and phasing are maintained by twists between aromatic groups. Hydrogen bonding distances have normal values.
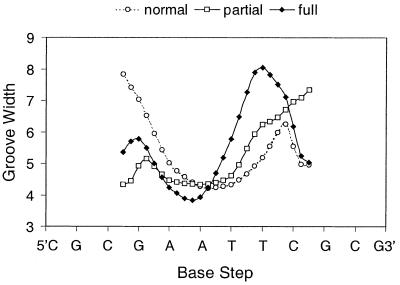
The van der Waals interactions, manifested by differences in minor groove width (Fig. 3), are the most important factor in determining site selectivity and binding strength. In the DNA of the partially dehydrated crystals, the C1 end of the AATT tract is significantly narrower than in the normal crystals as a result of the straightening of the helical backbone (21). The fully dehydrated DNA also has narrowing at the C1 end of the AATT tract, but in this case the groove width is determined by unusual crystal packing interactions involving covalent bridging of adjacent duplexes by magnesium cations. Crystal packing interactions in both partially and fully dehydrated structures also cause widening at the opposite end of the tract. In particular, the minor groove width in the T8 region has increased by ~3 Å during the transition from the normal to fully dehydrated structures. These observed variations in minor groove width symmetry provide a structural model for ligand binding changes in biological systems.
Figure 3.
Plot of minor groove width for the three m-iodo, p-methoxy Hoechst IB complexes. Distances (Å) are P–P and were calculated using CURVES (28).
Binding site of IA and IB ligands in the normal crystals
The IA and IB ligands both bind over two sites and in two orientations in the normal crystals (Fig. 4). The relative observed electron densities, and subsequent refinement, indicate that the two orientations are present in a 60:40 ratio. In relation to the strand 1 sequence, the major binding site is over the 5′-GAAT sequence with the iodine at the 3′-end of the site. The minor binding site is over the 5′-ATTC sequence with the iodine located at the 5′-end. Such binding disorder is not normally observed in dodecamer crystals containing non-iodinated Hoechst molecules and the minor groove width is usually asymmetrical over the binding site (18–21). In the present normal crystals, the presence of two ligand orientations results in a highly symmetrical minor groove over the binding sites, presumably as an artefact of structure averaging.
Major orientation. In the IB structure, in which the p-methoxy group influences the positioning of the phenyl ring, the iodine in the major orientation points out of the minor groove. In contrast, the iodine in the major orientation of IA faces into the floor of the groove. It might be expected that the bulkiness of the iodine atom would make its binding into the groove unfavourable. However, we note that the minor groove of the native uncomplexed dodecamer is comparatively wide at what would be the iodine end of the binding site in this orientation, and presumably there is room to accommodate the iodine (10). The bulky piperazine of IA points out of the groove instead of binding deeply within it.
Minor orientation. The ligands in the minor orientations of both the IA and IB normal structures are found in the same orientation as other Hoechst 33258 structures (18–21).
Binding site of IA and IB ligands in the partially dehydrated crystals
A key finding in the partially (and fully) dehydrated structures is that the IA and IB ligands occupy a single, identical binding site, 5′-AATT, with the iodines at the 5′-end of the sequence (Fig. 5). As this transformation has been observed to occur within a single crystal as it dehydrates from the normal to partial state, the ligand in the minor orientation must have slid along the groove to the new site, whereas the ligand in the major orientation must have exited the groove, re-oriented in the surrounding solvent medium and then re-entered the groove at the new site. This has not been found experimentally before. This movement of the ligands during the dehydration process can be thought of as mimicking the binding behaviour of minor groove binders in solution and in vivo.
In both the IA and IB partially dehydrated complexes, the iodines project out from, and the piperazines project into, the groove. This conformation is the same as the minor components of the normal crystals.
Figure 6 compares the sole binding site of the IB ligand in the partially dehydrated structure with that of the minor component of the IB ligand in the normal structure. The ligand of the partially dehydrated structure has moved along the groove by ~3 Å towards the 5′-end of the site. The binding sites are now similar to those of Hoechst 33258 in its 20 and 0°C structures (22,23).
Figure 6.
Comparison of the ligand binding site for the IB partially dehydrated structure (shaded bonds) with that of the minor component of the IB normal structure (open bonds).
During the partial dehydration process, ligands in the major orientation have exited the minor groove, re-oriented and then re-entered the groove in a single preferred orientation and binding site. The minor groove of the partially dehydrated structures narrows significantly at the 5′-AA sequence compared to the normal crystals (Fig. 3). This asymmetry favours binding of the iodinated phenyl ring in the narrowed groove region and it is comparatively disadvantageous for the bulky piperazine to bind there. Because of this, the ligand molecules have shifted along the groove to take full advantage of the narrowing groove at the 5′-end of the sequence. In order to check whether narrowing of the minor groove on dehydration was an inherent property of the DNA itself, and not a consequence of the particular iodo-substituted ligands, we determined the structure of partially dehydrated crystals containing the ligand p-dimethylamino Hoechst, p-DH (Fig. 1). This shows a similar 3 Å shift of the ligand along the minor groove compared to its normal crystal structure (C.J.Squire, in preparation). The normal and partially dehydrated p-DH structures are fundamentally similar to those of the normal and partially dehydrated IA and IB structures and hence we will not discuss them further in the present paper. Their coordinates have been deposited with the Nucleic Acids Database (24) with codes DD0011 and DD0007, respectively.
Binding site of IA and IB ligands in the fully dehydrated crystals
In the fully dehydrated IA and IB crystals, the single ligand binding sites and orientations are identical to those in their partially dehydrated structures (Fig. 5). This again highlights the requirement of the piperazine ring for a widened minor groove region and of the phenyl ring for a narrow one. The groove width asymmetry is even more marked in the fully dehydrated crystals (Fig. 3) where the minor groove is extremely wide (8 Å) in the region around T8 because of substantial DNA backbone distortions. The piperazine is located here, but makes few contacts with the DNA. During the dehydration process the number of close contacts between ligands and DNA decreases from 37, to 27, to 19. In the fully dehydrated structure, most of the lost contacts are at this piperazine-binding site. The minimal nature of the piperazine–groove contact is reflected in the higher temperature factors of the atoms of the piperazine ring compared to those of the other ring systems (Table 2).
Table 2. Average B factors (Å2) for ring systems in the IA and IB molecules in the fully dehydrated crystals.
| Ring system | Temperature factor IA | Temperature factor IB |
|---|---|---|
| Phenyl | 7.0 | 13.5 |
| Benzimidazole | 7.6 | 11.8 |
| Benzimidazole | 11.7 | 18.2 |
| Piperazine | 30.7 | 31.5 |
Photosensitisation by iodo Hoechst ligands
In our X-ray structures of the m-iodo Hoechst–DNA complexes, the ligand C3 atoms are close to C5′ atoms on the DNA, reinforcing a C5′ H abstraction mechanism (4). If the hydrogen atom abstraction is faster than the rate of rotation of the phenyl ring in the minor groove, then abstraction will occur statistically more often at the C5′ atom closest to the carbon centred radical. The estimated hydrogen atom abstraction rate of an aryl radical system is >105 M–1 s–1 (25), whereas the phenyl rotation rate of Hoechst 33258 has been estimated at >103 s–1 (26). The comparative rates support the hypothesis that the radical reaction will occur at the closest C5′ atom.
The hydrogen abstraction site predicted from the current X-ray study of the normal m-iodo crystal structures is at C21, which is one nucleotide away from the observed cleavage site G22 (4). However, in the partially and fully dehydrated structures, the repositioning of the ligand brings the predicted cleavage site up to G22. The use of three different structural types for the analysis of the photosensitisation mechanism has thus proven invaluable.
CONCLUSIONS
The present crystal structure determinations have established the detailed DNA conformations and provided an accurate description of the binding of the m-iodo Hoechst ligands in the minor groove. The structures of the DNA–ligand complexes are consistent with the proposed C5′ hydrogen abstraction mechanism and UVA-induced strand cleavage in the iodo Hoechst–DNA systems is a successful model for X-ray sensitisation.
The discovery of the three structural types (normal, partially dehydrated and fully dehydrated) has allowed examination of groove binding responses to changes in DNA conformation. The IA, IB and p-DH ligands migrate along the groove ~3 Å as the minor groove width changes on transforming from normal to dehydrated structures. A single ligand orientation (as opposed to the two disordered ligand orientations in the normal structure) results from the increased asymmetry in minor groove width. The total transformation from normal through partial to full dehydration occurred in single crystals, providing the first direct evidence that the central region of the DNA in the normal dodecamer crystals can breathe locally to allow the ligand to leave the minor groove, re-orient in the surrounding solvent medium and re-enter the groove in a single preferred orientation and binding site. This provides a good model for the behaviour of minor groove-binding ligands in solution and in vivo.
The process of controlled dehydration of B-form DNA crystals is capable of giving greatly enhanced X-ray diffraction resolution and significantly more accurate crystal structures. In addition to the IA, IB and p-DH structures described herein, we have successfully and reproducibly carried out partial dehydration experiments on other ligand systems employing d(CGCA-AATTTGCG)2 as well as d(CGCGAATTCGCG)2 (G.R.Clark, unpublished results). In many cases, even a very small water loss, by deliberate and controlled removal of ~4% of the crystal solvent, has shown a diffraction resolution increase of 0.5 Å. The universality of this effect may encourage other crystallographers, particularly those without synchrotron access, to employ such techniques to enhance resolution as standard practice in their drug design protocols.
Although the dehydrated structures described in these studies were obtained under conditions of low water activity, similar DNA conformations could exist in vivo in the complex spectrum of biological environments, including those that might be generated, for example, by protein–DNA interactions. We have used dehydration as a tool to probe alternative B-DNA conformations. In the context of structure-based design of DNA-binding ligands it could be advantageous to consider the full spectrum of structural detail available, including our dehydrated structures.
Acknowledgments
ACKNOWLEDGEMENTS
We thank Drs D. Kelly and R. Hooke for supplying samples of the ligands and the University of Auckland Research Committee for financial support (to C.J.S.).
NDB accession nos DD0003–DD0009
REFERENCES
- 1.Hutchinson F. and Kohnlein,W. (1980) Prog. Mol. Subcell. Biol., 7, 1–42. [Google Scholar]
- 2.McGinn C.J. and Kinsella,T.J. (1993) Curr. Probl. Cancer Res., 17, 273–320. [DOI] [PubMed] [Google Scholar]
- 3.Martin R.F., Murray,V., D’Cunha,G., Pardee,M., Kampouris,E., Haigh,A., Kelly,D.P. and Hodgson,G.S. (1990) Int. J. Radiat. Biol., 57, 939–946. [DOI] [PubMed] [Google Scholar]
- 4.Martin R.F., Kelly,D.P., Roberts,M., Green,A., Denison,L., Rose,M., Reum,M. and Pardee,M. (1994) Int. J. Radiat. Oncol. Biol. Phys., 29, 549–553. [DOI] [PubMed] [Google Scholar]
- 5.Kelly D.P., Bateman,S.A., Hook,R.J., Martin,R.F., Reum,M.E., Rose,M. and Whittaker,A.R.D. (1994) Aust. J. Chem., 47, 1751–1769. [Google Scholar]
- 6.Salunke D.M., Veerapandian,B., Kodandapani,R. and Vijayan,M. (1985) Acta Crystallogr., B41, 431–436. [Google Scholar]
- 7. Siemens (1994) SMART & SAINT. Siemens Analytical Instruments Inc., Madison, WI.
- 8.Blessing R.H. (1995) Acta Crystallogr., A51, 33–38. [DOI] [PubMed] [Google Scholar]
- 9. Siemens (1994) SHELXTL (XPREP). Siemens Analytical Instruments Inc., Madison, WI.
- 10.Westhof E. (1987) J. Biomol. Struct. Dyn., 5, 581–600. [DOI] [PubMed] [Google Scholar]
- 11.Brunger A.T. (1997) Nature Struct. Biol., 4(suppl.), 862–865. [PubMed] [Google Scholar]
- 12.Navaza J. (1994) Acta Crystallogr., A50, 157–163. [Google Scholar]
- 13.Clark G.R., Squire,C.J., Gray,E.J., Leupin,W. and Neidle,S. (1996) Nucleic Acids Res., 24, 4882–4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones T.A. and Kjeldgaard,M. (1995) O version 5.10. Uppsala University, Uppsala, Sweden.
- 15. Biosym Technologies (1993) Insight II version 2.3.0. Molecular Simulations Inc., San Diego, CA.
- 16.Squire C.J., Clark,G.R. and Denny,W.A. (1997) Nucleic Acids Res., 25, 4072–4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sheldrick G.M. (1997) SHELXL97. Program for the Solution of Crystal Structures. Universität Göttingen, Göttingen, Germany.
- 18.Vega M.C., Garcia-Saez,I., Aymami,J., Eritja,R., van der Marel,G.A., van Boom,J.H., Rich,A. and Coll,M. (1994) Eur. J. Biochem., 222, 721–726. [DOI] [PubMed] [Google Scholar]
- 19.Carrondo M.A., Coll,M., Aymami,J., Wang,A.H., van der Marel,G.A., van Boom,J.H. and Rich,A. (1989) Biochemistry, 28, 7849–7859. [DOI] [PubMed] [Google Scholar]
- 20.Sriram M., Van Der Marel,G.A., Roelen,H.L.P.F., Van Boom,J.H. and Wang,A.H.-J. (1992) EMBO J., 11, 225–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fratini A.V., Kopka,M.L., Drew,H.R. and Dickerson,R.E. (1982) J. Biol. Chem., 257, 14686–14707. [PubMed] [Google Scholar]
- 22.Teng M.-K., Usman,N., Frederick,C.A. and Wang,A.H.-J. (1988) Nucleic Acids Res., 16, 2671–2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quintana J.R., Lipanov,A.A. and Dickerson,R.E. (1991) Biochemistry, 30, 10294–10306. [DOI] [PubMed] [Google Scholar]
- 24.Berman H.M., Olson,W.K., Beveridge,D.L., Westbrook,J., Gelbin,A., Demeny,T., Hsieh,S.-H., Srinivasan,A.R. and Schneider,B. (1992) Biophys. J., 63, 751–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Packer J.E., House,D.B. and Rasburn,E.J. (1971) J. Chem. Soc., B, 1574–1578. [Google Scholar]
- 26.Embrey K.J., Searle,M.S. and Craik,D.J. (1993) Eur. J. Biochem., 211, 437. [DOI] [PubMed] [Google Scholar]
- 27. Siemens (1994) SHELXTL (XP). Siemens Analytical Instruments Inc., Madison, WI.
- 28.Lavery R. and Sklenar,H. (1996) CURVES version 5.0. Institut de Biologie Physico-Chimique, Paris, France.
- 29. BioGraphics (1997) TURBO-FRODO. AFMB-CNRS, Marseille, France.