Abstract
Purpose:
In adults with long-term HIV infection, low bone density and increased fracture risk have emerged as significant comorbidities. Our aim was to assess the association of exercise, nutrition, and medications with bone quality in adults with long-term HIV infection.
Methods:
Forty-three adults with HIV infection were enrolled (median BMI 25.7, range 18.2–35.6 kg/m2; median age 57, range 50–69 years). Participants underwent ultradistal radius and tibia high-resolution peripheral quantitative CT (HR-pQCT). Questionnaires included the revised Community Healthy Activities Model Program for Seniors (CHAMPS), the Mini Nutritional Assessment (MNA) as well as medication assessments. Multivariable linear regression models were used to evaluate the association of exercise, nutritional status, tenofovir disoproxil fumarate (TDF) and protease inhibitor (PI) use with bone density and microstructure, adjusting for demographic risk factors.
Results:
In regression models, higher nutrition scores were associated with higher tibia cortical thickness (R2 = 0.23; β = 0.03; p = 0.044) and higher radius cortical BMD (R2 = 0.43; β = 8.4; p = 0.026). Higher weekly frequency of all physical activities was significantly associated with higher radius trabecular BMD (R2 = 0.38; p = 0.96; p = 0.050), higher radius trabecular number (R2 = 0.31; β = 0.01; p = 0.026), lower tibia and radius trabecular separation (tibia: R2 = 0.30; p = −0.003; p = 0.038; radius: R2 = 0.35; β = −0.003; p = 0.021), and higher radius bone stiffness (R2 = 0.45; β = 0.38; p = 0.047). Higher frequency of bone loading physical activities was significantly associated with higher tibia trabecular density (R2 = 0.44; β = 4.06; p = 0.036), higher tibia bone stiffness (R2 = 0.46; = 3.06; p = 0.050), and higher tibia estimated failure load (R2 = 0.46; β = 0.17; p = 0.049). TDF used in combination with a PI was associated with lower radius trabecular BMD (R2 = 0.39; β = −41.2; p = 0.042), lower radius trabecular number (R2 = 0.34; β = −0.44; p = 0.009) and greater radius trabecular separation (R2 = 0.42; 0 = 0.16; p = 0.002), while TDF use without a PI was not associated with reduced bone quality.
Conclusions:
In adults with HIV infection, malnutrition is associated with poor cortical bone quality, while reduced frequency of physical activities and specifically reduced frequency of mechanical loading activities are associated with deficient trabecular bone structure and reduced estimates of bone strength. TDF use in combination with a PI is associated with deleterious effects on trabecular bone structure.
Keywords: HIV, Bone, HR-pQCT, Physical activity, Nutrition
1. Introduction
In adults with long-term human immunodeficiency virus-1 (HIV) infection, low bone mineral density (BMD) and increased fracture risk have emerged as significant comorbidities representing a multifactorial challenge [1–3]. Increased risk of fracture and decreased rate of fracture healing has been shown to significantly affect life expectancy and quality of life [4]. Important contributing factors to the high prevalence of bone disease include overrepresentation of traditional risk factors such as low body mass index (BMI), inactivity, tobacco and alcohol use, poor dietary intake and vitamin D deficiency.
In PLWH low BMD has most commonly been linked to low body weight [5–10]. Despite major improvements in antiretroviral medications leading to significant reductions in the morbidity and mortality of HIV infection, weight loss and wasting remain important problems in PLWH [11]. The etiology of HIV-associated weight loss and wasting is multifactorial. Causes include inadequate dietary intake, altered metabolism, socioeconomic factors, access to care, psychological factors and complications of HIV or side-effects of antiretroviral medication [11]. A meta-analysis showed that on average PLWH were 5.1 kg lighter compared to controls [5], however the effect of malnutrition and low body weight on bone structure and microstructure in PLWH is not well understood.
Regular physical activity is recommended for optimal bone health in the general population [12]. Although physical activity likely has similar effects in PLWH, research on the impact of physical activity on bone loss in PLWH has been limited. Bonato et al. enrolled 27 PLWH and documented changes in BMD after a moderate-aerobic exercise [brisk walking) intervention of 12 weeks [13]. They found improved spinal BMD, as well as femoral BMD [13]. Santos et al. measured changes in BMD in 20 adults with HIV and lipodistrophy who participated in a 12-week strength training intervention (36 sessions), resulting in improved BMD at the lumbar spine and femoral neck and the radius [14].
Another factor predisposing people living with HIV (PLWH) to bone loss is the lifelong treatment with antiretroviral therapy. Standard antiretroviral therapy consists of the combination of at least three antiretroviral drugs to suppress the HIV virus and reduce disease related complications and the risk of HIV transmission [15], Antiretroviral therapy has been shown to trigger adverse osteogenic effects, either indirectly-through renal phosphorous wasting and hyperparathyroidism - or directly - through stimulation of osteoclastogenesis [16–18]. A previous study demonstrated that BMD decreases by 2%−6% within the first 2 years of antiretroviral therapy initiation, regardless of the choice of therapy [19].
Regarding the influence of disease severity on bone health, contradicting results were found by two studies measuring disease severity with CD4 count [20,21], Sawlani et al. found lower CD4 count to be associated with reduced BMD, while Biver et al. found no significant difference [20,21]. However, the mechanism and extent of how nutrition, physical activity, antiretroviral therapy and disease severity influence bone quality, and in particular bone microstructure, in long-term HIV seropositive individuals is not yet well understood.
While DXA (dual-energy X-ray absorptiometry) is the gold standard to measure areal BMD (aBMD) and to clinically assess bone fragility [22], it cannot provide information on cortical and trabecular compartments individually, nor quantitative microarchitecture information [23]. High-resolution peripheral quantitative computed tomography (HR-pQCT) provides measurements of both bone quality and mass of the cortical and trabecular compartments including bone microstructure [24]. A previous study comparing bone quality in PLWH to uninfected controls only detected impaired trabecular bone microstructure with HR-pQCT but no differences in aBMD by DXA, indicating that HR-pQCT may be more sensitive than DXA for the assessment of bone health in this population [25].
The aim of this study was therefore to analyze the association of nutrition, physical activity, antiretroviral medications, and HIV disease severity with bone density and microarchitecture measured using HR-pQCT in long-term HIV seropositive individuals. These data will inform efforts to identify treatment and prevention strategies to ultimately reduce the prevalence of bone loss and fracture risk in the aging HIV population.
2. Materials and methods
2.1. Study participants
Forty-three HIV seropositive adults were enrolled in our study, all with known HIV infection for ≥7 years. All participants were on a stable antiretroviral therapy regimen in the prior year. Informed consent was obtained from all participants; the study was compliant with the Health Insurance Portability and Accountability Act and approved by the local institutional review board.
Participants with chronic diseases associated with poor bone quality such as diabetes mellitus, rheumatologic diseases, chronic kidney disease, malabsorption syndromes and hepatitis C virus (HCV) infection were excluded. Participants treated with medications known to impact bone and mineral metabolism including current use of calcitonin, systemic glucocorticoids, thiazolidinedione, thyroid hormone replacement, and use of bisphosphonate or teriparatide in the last year or for > 12 months ever were also excluded. We further excluded participants with fractures at the imaging sites, an episode of immobilization lasting longer than 1 week in the previous six months, with conditions excluded by x-ray safety guidelines, illicit drug use and pre- or perimenopausal women (last menses ≤ 3 years). The target age range for enrollment was 50–70 years.
2.2. Medical history and clinical examination
The following information was obtained from patient records or recorded at study visit: Duration of HIV infection, disease complications, current medications including supplements containing vitamin D and/or calcium, alcohol or tobacco use, and history of fractures including history of low impact trauma fractures.
Participants completed the Community Healthy Activities Model Program for Seniors (CHAMPS) Physical Activity Questionnaire [26,27], to assess the associations of bone microarchitecture and weekly frequency of participation in physical activities. Study participants were asked to self-report whether they had engaged in 27 light, moderate, and vigorous physical activities to which participants reported their weekly frequency of participation in a typical week over the last 4 weeks. Frequency measures were calculated as a sum of frequency per week for all activities (light, moderate, and vigorous). Given the importance of mechanical loading for bone adaptation [28–31], we also assessed the association of frequency of all bone-loading physical activities and frequency of upper body loading activities.
The Mini Nutritional Assessment (MNA) was used to determine nutritional status. The MNA is a validated nutritional assessment tool for older people [32–36], consisting of self-reported questions derived from four parameters of assessment: anthropometric assessment, general assessment, dietary assessment and self-assessment. The questionnaire permits detection of a decline in ingestion (loss of appetite, decline of food intake, digestive problems, chewing or swallowing difficulties), weight loss, current mobility impairment, acute illness or major stress in the past three months, neuropsychological problems (dementia or depression) and a decrease in BMI. The following scoring system is used: normal nutritional status [12–14], at risk of malnutrition [8–11] and malnourished (0–7) [37].
HIV-related laboratory parameters (current CD4 cell count and HIV-RNA within 3 months of the study visit) were obtained from clinical records.
2.3. HR-pQCT bone imaging and quantification
All participants were imaged using a HR-pQCT system (XtremeCT, Scanco Medical AG, Bruttisellen, Switzerland), according to the manufacturer’s standard in vivo protocols (60 kVp, 900 μA, 100 ms integration time, 750 projections over 180°). HR-pQCT images were obtained at 82 μm nominal voxel size in 9.02 mm stack lengths, at theultra-distal radius (starting 9.5 mm proximal to the distal endplate) and ultra-distal tibia [starting 22.5 mm proximal to the distal endplate), as described in detail elsewhere [38]. The non-dominant extremity was scanned unless there was a history of injury or surgery at that location. Trabecular and cortical density and structure parameters were quantified from these images using the manufacturer’s standard in vivo analysis protocol [38–40]. Linear micro-finite element (μFF.) analysis was performed to calculate apparent biomechanical properties (Scanco FE Software Version 1.12, Scanco Medical AG) using previously described techniques [41–43]. Homogeneous properties were assumed for all bone elements, with each element assigned an elastic modulus of 6 GPa and a Poisson ratio of 0.3 [41]. A uniaxial compression test in the superoinferor direction was performed with an applied strain of 1%. Failure load was estimated using methods validated by Mueller and colleagues [41]; failure was reached when a 7.5% critical bone volume reached a 0.7% strain threshold. From these analyses, the following quantitative trabecular and cortical parameters were obtained for tibia and radius, respectively: trabecular density, trabecular number, trabecular separation, cortical density, and cortical thickness. To provide an estimate of bone strength we also included the parameters bone stiffness and estimated failure load for the tibia and radius, respectively.
Reproducibility measurements previously obtained in elderly subjects in our facility indicated the least significant change and root mean square coefficient of variation LSC (RMSCV) for cortical density of the tibia to be 14 mg HA/cm3 (0.6%), for cortical thickness of the tibia 50 μm (1.5%), for cortical density of the radius 17 mg HA/cm3 (0.8%), and for cortical thickness of the radius 80 μm (3.9%) [39], LSC and RMSCV for trabecular parameters are lower than for cortical parameters [44].
2.4. DXA evaluation
DXA exams of the femur and lumbar spine were obtained as part of standard clinical care in 37 participants (13 participants: Prodigy, GE/Lunar, Milwaukee, WI, USA; 24 participants: Horizon A, Hologic, Marlborough, MA, USA). Areal BMD was automatically calculated at the proximal femur (femur neck and total femur) and the lumbar spine (Ll-4). Fractured vertebrae and degenerated segments were excluded from the analysis. Online available conversion tables from the manufacturers were used to convert aBMD values between the different scanners.
2.5. Statistical analysis
Statistical analysis was performed with SPSS software (version 23; IBM, Armonk, NY, USA), using a 2-sided 0.05 level of significance. Multivariable linear regression models were used to evaluate the associations of nutritional status, physical activity (frequency of all activities, frequency of all bone loading activities, and frequency of upper body loading activities), disease severity (duration of HIV infection, CD4 count, and HIV viral load), and antiretroviral therapy on bone density and microarchitecture, adjusting all models for age, sex, and race. For the regression analysis nutritional status, physical activity, and CD4 count were Included as continuous variables; HIV viral load, duration of HIV infection, and use of antiretroviral medications were included as categorical variables. HIV viral load was included as a categorical variable (not detectable i.e. HIV RNA < 40 copies/ml vs. detectable). Duration of HIV infection was included as a categorical variable (under 20 years vs. over 20 years). We analyzed the associations of two specific antiretroviral medications (tenofovir disoproxil fumarate and protease inhibitors), that were previously shown to particularly affect bone structure [45–49]. We analyzed [1] the use of regimens including tenofovir disoproxil fumarate vs. the use of regimens not including tenofovir disoproxil fumarate, [2] the use of regimens including a protease inhibitor vs. the use of regimens not including a protease inhibitor, and [3] the use of regimens including both tenofovir disoproxil fumarate and a protease inhibitor vs. regimens not including the combination of tenofovir disoproxil fumarate and a protease inhibitor. Due to the explorative nature of this study, no adjustments were made for multiple testing. Physical activity and nutritional status were considered primary predictors. Disease severity parameters and medication on bone microarchitecture were considered secondary predictors. This analysis strategy was also applied to DXA data to evaluate the associations of nutritional status, physical activity, disease severity, and antiretroviral medication on DXA-derived aBMD.
3. Results
3.1. Study participants
The median age of participants in this study was 57.0 years (range 50–69), with a median BMI of 25.7 kg/m2 (range 18.2–35.6) and more males (86%, 37/43) than females. The racial distribution consisted of 79% (34/43) Caucasian participants, 19% (8/43) African-American participants, and one Asian participant. None of the participants reported current HIV associated disease complications. Previous reported HIV associated complications included one case of extensive leukoencephalopathy (Balint’s syndrome), six cases of anal low grade squamous intraepithelial lesions, one case of anal squamous cell carcinoma and one case of limited cutaneous Kaposi sarcoma. None of the participants were coinfected with either hepatitis B virus (HBV) or HCV. This was defined as a negative blood test for anti-HCV/ anti-HBV antibodies and HBV antigenes, or HCV ribonucleic acid (RNA)/HBV desoxyribonucleic acid (DNA). Seven participants reported a previous fracture (5 wrist fractures, 1 mandibular fracture, 1 humerus fracture). The humerus fracture was caused by a fall from standing height (low impact trauma). The other fractures were reported to be caused by high-impact trauma: the wrist fractures were caused by bicycle accidents (n = 2), in-line skating (n = 1), a motor vehicle accident (n = 1) and a skiing accident (n = 1); the mandibular fracture was caused by direct trauma (hit from the side). Participant characteristics are demonstrated in Table 1.
Table 1.
Subject characteristics.
| Subject characteristics | n = 43 |
|---|---|
|
| |
| Agea | 57.0 (54;62) |
| Sex | |
| Females | 14% |
| Males | 86% |
| Body mass index in kg/m2a | 25.7 (21.6;26.7) |
| Race | |
| Caucasian | 79% |
| African American | 19% |
| Asian | 2% |
| Risk factors | |
| Current tobacco consumption | 19% |
| Alcohol consumption ≥ 7 units weekly | 12% |
| Supplements | |
| Calcium | 23% |
| Vitamin D | 42% |
| HIV characteristics | |
| Time since diagnosis in yearsa | 24.0 (20;27) |
| HIV-RNA < 40 copies | 93% |
| CD4 cell count in cells/μla | 644 (316;804) |
| Percentage of participants on antiretroviral therapy regimens (HIV therapies) including the following antiretroviral medicationsb: | |
| Nucleoside reverse-transcriptase inhibitors | 98% |
| Integrase inhibitors | 51% |
| Non-nucleoside reverse-transcriptase inhibitors | 47% |
| Tenofovir alafenamide | 44% |
| Tenofovir disoproxil fumarate | 35% |
| Protease inhibitor | 26% |
| Tenofovir disoproxil fumarate and protease inhibitor | |
Numbers are median (25th and 75th percentiles).
All participants were on more than one type of antiretroviral medication, therefore numbers do not add lip to 100%.
3.2. Nutritional status
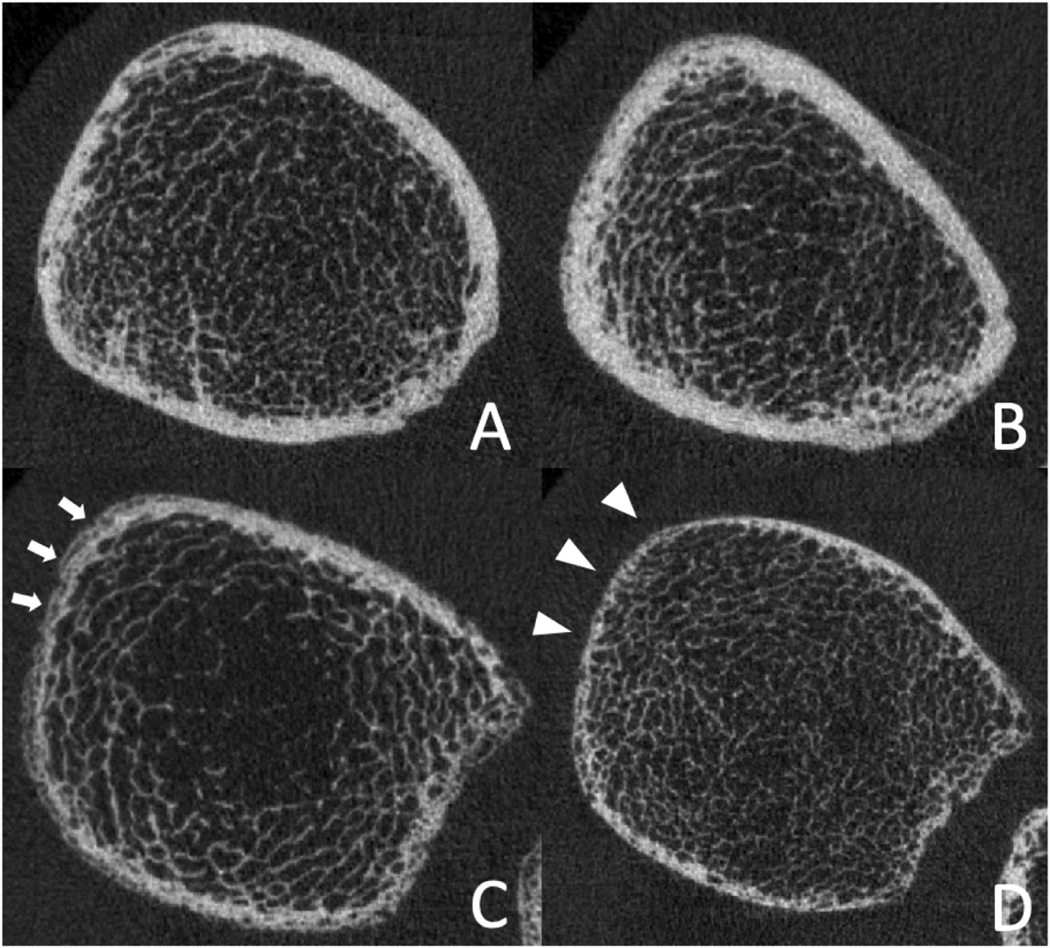
The mean MNA nutrition score was 12 ± 2.2; range 7–14. Two participants (2/43, 5%) met the definition of malnutrition (MNA score 0–7); 28% (12/43) were at risk of malnutrition (MNA score 8–11). The association of nutritional status and bone parameters is shown in Table 2 and representative images are presented in Fig. 1. In models adjusted for age, sex, and race, significant associations were found between higher nutritional scores and higher tibia cortical thickness (R2 = 0.23; coefficient: 0.03; 95% confidence interval (Cl): 0.01, 0.065; p = 0.044). Furthermore, radius cortical BMD was significantly higher in these participants (R2 = 0.43; coefficient: 8.4; 95% Cl: 1.0, 15.8; p = 0.026), Supplementary figures. Interestingly, no significant association was found between nutritional scores and trabecular HR-pQCT parameters (p > 0.05). Nutritional scores showed no significant associations with DXA aBMD measurements, Supplementary material Table 1.
Table 2.
Association between nutritional status and bone microarchitecture.
| Parameter (n = 43) | Nutritional status | |||
|---|---|---|---|---|
|
|
||||
| R2 base modela | R2 (p)b | β valuec | p-Value*d | |
|
| ||||
| Tibia | ||||
| Trabecular density | 0.36 | 0.36 | −0.12 | 0.955 |
| (0.005) | (−4.3;4.0) | |||
| Trabecular number | 0.18 | ns | ns | ns |
| Trabecular separation | 0.21 | ns | ns | ns |
| Cortical density | 0.28 | 0.33 (0.010) | 7.1 (−1.7;16.0) | 0.112 |
| Cortical thickness | 0.14 | 0.23 (0.071) | 0.03 (0.001;0.07) | 0.044 |
| Bone stiffness | 0.40 | 0.40 (0.001) | 1.0 (−2.3;4.3) | 0.539 |
| Failure load | 0.40 | 0.41 (0.001) | 0.04 (−0.15;0.22) | 0.686 |
| Radius | ||||
| Trabecular density | 0.31 | 0.32 (0.016) | −1.7 (−6.6;3.1) | 0.472 |
| Trabecular number | 0.20 | 0.23 (0.088) | −0.03 (−0.07;0.02) | 0.226 |
| Trabecular separation | 0.24 | 0.28 (0.037) | 0.01 (−0.004;0.02) | 0.162 |
| Cortical density | 0.34 | 0.43 (0.001) | 8.4 (1.0;15.8) | 0.026 |
| Cortical thickness | 0.24 | 0.24 (0.082) | 0.004 (−0.03;0.03) | 0.800 |
| Bone stiffness | 0.38 | 0.38 (0.004) | −0.05 (−2.1;2.0) | 0.961 |
| Failure load | 0.29 | 0.29 (0.029) | −0.01 (−0.16;0.14) | 0.917 |
ns: p > 0.1 for total model and individual predictor, respectively.
R2 for base model (age, sex, race).
R2 and p-value for total model.
Multivariable linear regression adjusting for age, sex and race.
p-Value for individual predicor (nutritional status).
Significant (p < 0.05) values are in bold.
Fig. 1.
Ultradistal tibia HR-pQCT images of two adults with HIV-infection with high nutritional scores (A: 53 years, male, white, 167 cm; B: 52 years, male, white, 170 cm) and two adults with HIV-infection with low nutritional scores (C: 55 years, male, white, 171 cm; D: 57 years, male, white, 168 cm). Those with low nutritional scores show reduced cortical BMD with visibly higher porosity (C, white arrows) and reduced cortical thickness (D, white arrowheads).
3.3. Physical activity
The mean weekly frequency for all activities in a typical week was 21 ± 10 events per week. The mean weekly frequency for bone-loading activities in a typical week was 1.9 ± 1.6 events per week. The mean weekly frequency upper-body loading activities in a typical week was 1.3 ± 1.8 events per week. The associations of weekly frequency of physical activities on bone parameters are summarized in Table 3. In models adjusted for age, sex, and race, greater frequency of physical activity was significantly associated with more robust trabecular bone at both the radius and tibia and higher estimates of bone strength. Higher frequency of all physical activities (light, moderate, and vigorous) was significantly associated with higher radius trabecular density (R2 = 0.38; coefficient: 0.96; 95% Cl: 0.001, 1.93; p = 0.050) and higher radius trabecular number (R2 = 0.31; coefficient: 0.01; 95% Cl: 0.001, 0.02; p = 0.026). Moreover, higher frequency of all physical activities was significantly associated with lower tibia and radius trabecular separation (R2 = 0.30; coefficient: −0.003; 95% Cl: −0.01, 0.00; p = 0.038 and R2 = 0.35; coefficient: −0.003; 95% Cl: −0.01, 0.00; p = 0.021, respectively) and higher radius bone stiffness (R2 = 0.45; coefficient: 0.38; 95% Cl: 0.01, 0.75; p = 0.047). Higher frequency of all bone loading physical activities was significantly associated with higher tibia trabecular density (R2 = 0.44; coefficient: 4.06; 95% Cl: 0.29, 7.84; p = 0.036), higher tibia bone stiffness (R2 = 0.46; coefficient; 3.06; 95% Cl; 0.00, 6.12; p = 0.050), and higher tibia estimated failure load (R2 = 0.46; coefficient: 0.17; 95% Cl: 0.00, 0.34; p = 0.049). Higher frequency of upper body loading activities had a trend towards higher radius trabecular number (R2 = 0.26; coefficient: 0.04; 95% Cl: 0.01, 0.09; p = 0.086) and lower radius trabecular separation (R2 = 0.30; coefficient: −0.01; 95% Cl: −0.03, 0.00; p = 0.074). No associations were found between physical activity and cortical HR-pQCT parameters (p > 0.05). Moreover, no significant associations were found between physical activity parameters and DXA aBMD measurements (Supplementary material Table 1).
Table 3.
Association between frequency of physical activities and bone microarchitecture.
| Parameter (n = 43) | Frequency of all physical activities | Frequency of bone-loading physical activities | Frequency of upper-body bone-loading physical activities | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||
| R2 base modela | R2 (p)b | β valuec | p-value*d | R2 (p)b | β valuec | p-value*d | R2 (p)b | β valuec | p-value*d | |
|
| ||||||||||
| Tibia | ||||||||||
| Trabecular density | 0.36 | 0.36 (0.005) | 0.07 (−0.81;0.95) | 0.871 | 0.44 (0.001) | 4.06 (0.29;7.84) | 0.036 | 0.37 (0.004) | 1.58 (−3.73;6.90) | 0.551 |
| Trabecular number | 0.18 | 0.24 (0.074) | 0.01 (−0.002;0.02) | 0.119 | 0.24 (0.063) | 0.04 (−0.01;0.08) | 0.093 | ns | ns | ns |
| Trabecular separation | 0.21 | 0.30 (0.023) | −0.003 (−0.01;0.00) | 0.038 | 0.27 (0.036) | −0.01 (−0.03;0.001) | 0.074 | 0.23 (0.080) | −0.01 (−0.03;0.01) | 0.270 |
| Cortical density | 0.28 | 0.28 (0.032) | −0.07 (−2.03;1.90) | 0.945 | 0.29 (0.027) | 3.0 (−5.9;11.9) | 0.503 | 0.29 (0.025) | −4.59 (−16.4;7.25) | 0.437 |
| Cortical thickness | 0.14 | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| Bone stiffness | 0.40 | 0.40 (0.002) | −0.10 (−0.81;0.60) | 0.769 | 0.46 (0.000) | 3.06 (0.00;6.12) | 0.050 | 0.40 (0.002) | 0.66 (−3.6;4.9) | 0.757 |
| Failure load | 0.40 | 0.40 (0.002) | −0.00 (−0.04;0.04) | 0.920 | 0.46 (0.000) | 0.17 (0.001;0.34) | 0.049 | 0.41 (0.002) | 0.05 (−0.19;0.29) | 0.666 |
| Radius | ||||||||||
| Trabecular density | 0.31 | 0.38 (0.004) | 0.96 (0.001;1.93) | 0.050 | 0.34 (0.011) | 2.76 (−1.77;7.29) | 0.225 | 0.34 (0.010) | 3.88 (−2.15;9.90) | 0.200 |
| Trabecular number | 0.20 | 0.31 (0.021) | 0.01 (0.001;0.02) | 0.026 | 0.23 (0.084) | 0.02 (−0.01;0.06) | 0.208 | 0.26 (0.049) | 0.04 (−0.01;0.09) | 0.086 |
| Trabecular separation | 0.24 | 0.35 (0.009) | −0.003 (−0.01;0.00) | 0.021 | 0.27 (0.039) | .−01 (−0.02;0.00) | 0.183 | 0.30 (0.022) | −0.01 (−0.03;0.00) | 0.074 |
| Cortical density | 0.34 | 0.36 (0.007) | 0.77 (−0.70;2.24) | 0.297 | 0.36 (0.008) | 2.88 (−4.59; 10.4) | 0.438 | 0.34 (0.011) | 0.96 (−8.12; 10.0) | 0.831 |
| Cortical thickness | 0.24 | 0.29 (0.030) | 0.004 (−0.001;0.01) | 0.114 | 0.25 (0.074) | 0.01 (−0.02;0.04) | 0.564 | 0.24 (0.084) | −0.001 (−0.04;0.03) | 0.957 |
| Bone stiffness | 0.38 | 0.45 (0.001) | 0.39 (0.01;0.75) | 0.047 | 0.40 (0.003) | 0.96 (−1.0; 2.96) | 0.326 | 0.39 (0.003) | 0.84 (−1.5;3.2) | 0.478 |
| Failure load | 0.29 | 0.46 (0.001) | 0.02 (0.00;0.04) | 0.054 | 0.41 | 0.05 (−0.06;0.16) | 0.359 | 0.40 (0.003) | 0.05 (−0.09;0.18) | 0.497 |
ns: p > 0.1 for total model and individual predictor, respectively.
R2 for base model (age, sex, race).
R2 and p-value for total model.
Multivariable linear regression adjusting for age, sex and race.
p-Value for individual predicor (frequency of all physical activities/bone-loading physical activities/upper-body loading physical activities).
Significant (p < 0.05) values are in bold.
3.4. Disease severity and antiretroviral medication
The mean duration of HIV infection (time since diagnosis) was 22 ± 8.4 years (range 7–39). Thirty-nine participants were suppressed on successful antiretroviral therapy (HIV RNA < 40 copies/ml; four participants had 44, 45, 58, and 214 detected copies/ml, respectively. The mean CD4 cell count was 657 ± 289 (range 119–1228). In models adjusted for age, sex, and race, participants with HIV infection > 20 years (n = 26) had significantly lower trabecular BMD of the radius (R2 = 0.43; coefficient: −25.7; 95% Cl: −47.0, −4.32; p = 0.020), reduced cortical thickness of the radius (R2 −0.33; coefficient: −0.13; 95% Cl; −0.25, −0.002; p = 0.046), reduced radius bone stiffness (R2 = 0.51; coefficient: −10.6; 95% Cl; −18.9, −2.3; p = 0.014), and lower radius estimated failure load (R2 = 0.40, coefficient: −0.68; 95% Cl: −1.3, −0.08; p = 0.028) compared to participants with HIV infection ≤ 20 years. In models adjusted for age, sex, and race, CD4 count and detectable viral load were not significantly associated with cortical or trabecular HR-pQCT parameters (p > 0.05).
Regarding medications, in models adjusted for age, sex, and race, the use of regimens including the combination of tenofovir disoproxil fumarate and a protease inhibitor was significantly associated with lower trabecular BMD of the radius (R2 = 0.39; coefficient: −41.2; 95% Cl: −80.8, −1.6; p = 0.042), lower radius trabecular number (R2 = 0.34; coefficient: −0.44; 95% Cl: −0.76, −0.11; p = 0.009), and greater radius trabecular separation (R2 = 0.42; coefficient: 0.16; 95% Cl: 0.06, 0.26; p = 0.002), compared to the use of regimens not including the combination of tenofovir disoproxil fumarate and a protease inhibitor. The use of tenofovir disoproxil fumarate or use of a protease inhibitor alone was not associated with lower bone quality. The associations of medication and bone microarchitecture are shown in Table 4. Disease severity parameters and antiretroviral therapy medication showed no significant associations with DXA aBMD measurements (Supplementary material Table 1).
Table 4.
Association between antiretroviral medications and bone microarchitecture.
| Parameter (n = 43) | TDF | PI | TDF and PI | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||
| R2 base modela | R2 (P)b | ß valuec | p-Value*d | R2 (P)b | ß valuec | p-Value*d | R2 (P)b | ß valuec | p-value*d | |
|
| ||||||||||
| Tibia | ||||||||||
| Trabecular density | 0.36 | 0.36 (0.005) | 3.5 (−16.3;23.3) | 0.723 | 0.36 (0.005) | 0.39 (−20.1;20.9) | 0.969 | 0.36 (0.005) | −1.10 (−37;35) | 0.951 |
| Trabecular number | 0.18 | 0.24 (0.064) | −0.17 (−0.39;0.04) | 0.107 | ns | ns | ns | ns | ns | ns |
| Trabecular separation | 0.21 | 0.26 (0.046) | 0.06 (−0.02;0.13) | 0.126 | 0.22 (0.089) | −0.03 (−0.11;0.05) | 0.417 | 0.22 (0.096) | 0.05 (−0.09;0.19) | 0.486 |
| Cortical density | 0.28 | 0.30 (0.019) | 21.7 (−21.8;65.2) | 0.318 | 0.35 (0.006) | 42.3 (−0.86;86) | 0.054 | 0.34 (0.007) | 72 (−4.6;148) | 0.065 |
| Cortical thickness | 0.14 | ns | ns | ns | 0.22 (0.092) | 0.15 (−0.01;0.21) | 0.064 | 0.22 (0.086) | 0.27 (−0.01;0.55) | 0.058 |
| Bone stiffness | 0.40 | 0.40 (0.001) | 3.9 (−11.9;19.7) | 0.620 | 0.44 (0.001) | 12.6 (−3.2;28.4) | 0.116 | 0.43 (0.001) | 19.1 (−8.9; 47.1) | 0.176 |
| Failure load | 0.40 | 0.40 (0.001) | 0.09 (−0.79;0.98) | 0.833 | 0.44 (0.000) | 0.69 (−0.19; 1.58) | 0.121 | 0.42 (0.001) | 0.91 (−0.67;2.48) | 0.251 |
| Radius | ||||||||||
| Trabecular density | 0.31 | 0.31 (0.018) | −5.56 (−29.1;18.0) | 0.635 | 0.34 (0.010) | −15.4 (−38.6;7.9) | 0.188 | 0.39 (0.003) | −41.2 (−80.8; −1.6) | 0.042 |
| Trabecular number | 0.20 | ns | ns | ns | ns | ns | ns | 0.34 (0.010) | −0.44 (−0.76;−0.11) | 0.009 |
| Trabecular separation | 0.24 | 0.27 (0.048) | 0.04 (−0.03;0.10) | 0.250 | 0.27 (0.042) | 0.04 (−0.02;0.10) | 0.201 | 0.42 (0.001) | 0.16 (0.06;0.26) | 0.002 |
| Cortical density | 0.34 | 0.35 (0.010) | −4.53 (−39.2;30.1) | 0.792 | 0.37 (0.006) | 19.7 (−15.6;55.1) | 0.264 | 0.37 (0.006) | 34.6 (−26;95) | 0.253 |
| Cortical thickness | 0.24 | 0.25 (0.070) | −0.04 (−0.17;0.9) | 0.502 | 0.26 (0.061) | 0.06 (−0.07;0.19) | 0.372 | 0.25 (0.069) | 0.08 (−0.15;0.31) | 0.482 |
| Bone stiffness | 0.38 | 0.39 (0.004) | −2.6 (−11.7;6.5) | 0.567 | 0.38 (0.004) | 0.70 (−8.8; 10.2) | 0.881 | 0.39 (0.004) | −1.8 (−18.1;14.4) | 0.820 |
| Failure load | 0.29 | 0.29 (0.029) | −0.01 (−0.65;0.63) | 0.975 | 0.29 (0.028) | 0.10 C−0.57;0.77) | 0.757 | 0.29 (0.029) | −0.14 (−1.3;1.0) | 0.806 |
ns: p > 0.1 for total model and individual predictor, respectively; abbreviations: tenofovir disoproxil fumarate (TDF), protease inhibitor (PI). Abbreviations: TDF (tenofovir disoproxil fumarate); PI (protease inhibitor).
R2 for base model (age, sex, race).
R2 and p-value for total model.
Multivariable linear regression adjusting for age, sex and race.
p-Value for individual predicor (TDF/PI/TDF and PI).
Significant (p < 0.05) values are in bold.
4. Discussion
This study examined factors associated with alterations of cortical and trabecular bone and estimates of bone strength in long term HIV seropositive individuals using HR-pQCT. We found an association of malnutrition with poor cortical bone quality in PLWH, while reduced frequency of physical activities and specifically reduced frequency of mechanical loading activities were associated with deficient trabecular bone structure and reduced estimates of bone strength. Moreover, longer duration of HIV infection and use of tenofovir disoproxil fumarate in combination with a protease inhibitor were associated with deficient cortical and trabecular bone structure and reduced estimates of bone strength.
Lower nutrition scores were associated with poor cortical bone quality in our cohort of adults with long-term HIV infection. Cortical bone is an important determinant of whole bone strength and fracture risk [50,51], substantially contributing to biomechanical load bearing in elderly subjects, in whom a large part of the trabecular compartment has been resorbed [52]. Therefore, adults with HIV at risk of malnutrition would likely benefit from nutritional support to abate deficiencies in cortical bone structure, improve biomechanical properties, and ultimately reduce the associated fracture risk. In postmenopausal women low body weight and weight-loss are also considered risk factors for osteoporosis, while weight gain appears to protect against bone loss [53]. Previous studies showed that specific dietary recommendations such as increased calcium and vitamin D intake and reduced salt and protein intake can reduce bone loss and decrease fracture risk in the general population [54]. These interventions could likely be translated into the population of PLWH. However, since the causes for malnutrition and weight loss in PLWH are multifactorial, other moderating factors such as access to care, socioeconomic factors or factors linked to médication side-effects would need to be taken into consideration.
Our findings that higher frequency of all physical activity (light, moderate, and vigorous) may attenuate decreases in BMD are in agreement with longitudinal studies of populations at high risk for bone loss (e.g., postmenopausal women) showing that consistent participation in physical activity is associated with reduced bone loss [55–58]. Furthermore, strength training (including any strength training in the last week) has been shown to reduce bone loss in PLWH [10], Bonato et al. enrolled 27 PLWH and documented changes in BMD after a moderate-aerobic exercise (brisk walking) intervention of 12 weeks [13], They found improved spinal BMD, as well as femoral BMD [13]. Santos et al. tested a 12-week strength training intervention in 20 adults with HIV and lipodystrophy [14] and found that participants had significant increases in BMD at the lumbar spine, femoral neck and radius at the end of the intervention [14]. The strength training was composed by the following exercises: warm-up (active stretching), bench press, lat pull-down, leg extension, leg flexion, elbow flexion, elbow extension, abdominal exercise, sole flexion, and cool-down (active stretching) [14]. While these studies only included small sample sizes, they produced promising foundational evidence for the efficacy of physical activity interventions to improve BMD and prevent bone loss in PLWH [13,14]. In addition exercise interventions for other at-risk populations could be used in PLWH including training programs that induce stress to the skeleton by means of either ground-reaction or muscle-induced forces [59].
Concerning the effects of exercise on bone microstructure, previous studies with cohorts of postmenopausal women found that increased amounts of total physical activity (light, moderate, and vigorous) mainly maintains cortical bone rather than trabecular bone components [53,56]. Another review concluded that increased amounts of total physical activity maintains both cortical and trabecular components [57]. To the best of our knowledge only one study evaluated the association of bone microarchitecture and exercise assessed by frequency questionnaires in PLWH (no specific information was provided on which questionnaire was used). In analyses including 28 HIV positive and 112 HIV negative participants, physical activity was significantly associated with higher trabecular and cortical parameters (adjusted for age and BMI), however not significantly associated with bone microarchitecture parameters in analyses adjusted for age, BMI and HIV status [21].
Previous studies by Robling et al. have shown that short periods of exercise with rest periods in between are more effective to prevent bone loss compared to a single sustained exercise session [28,29,31]. Bone cells have been shown to desensitize after a few minutes of loading with the cellular response subsequently reducing. Moreover, higher levels of cortisol caused by high volumes and intensities of exercise potentially increase catabolic actions on bone [60]. Physical activities specifically targeting mechanical loading were previously shown to be a particularly potent stimulus for bone cells [30]. These findings are in line with the results of our study showing that higher frequency of bone loading activities was significantly associated with higher tibia trabecular density, higher tibia bone stiffness, and higher tibia estimated failure load.
We found different relationships between the weight-bearing tibia and the non-weightbearing radius in our study population. As expected those more frequently engaging in upper body loading activities (e.g. tennis) showed a trend towards higher trabecular number and lower trabecular separation at the radius. However the reason for differing relationships for nutrition and antiretroviral medications are unclear. Moreover, those with HIV infection over 20 years had significantly worse trabecular and cortical parameters, and reduced estimates of bone strength, only at the radius but not at the tibia. As models were adjusted for age, the association does not seem to be influenced by the fact that those with HIV infection over 20 years might be older. These findings indicate that after long-term HlV-infection, bone loss is exacerbated at non-weightbearing sites but maintained at weightbearing sites. However, further studies conducting analyses of bone microarchitecture at multiple time points and anatomic sites are warranted to evaluate longitudinal changes.
In accordance with previously published studies [10,21] we detected no significant associations of CD4 count with parameters of bone quality. Sawlani et al. found a correlation of low CD4 count and low BMD levels [20]. However, it should be noted that the mean CD4 count observed by Sawlani et al. (281 ± 113) was notably lower compared to our mean CD4 count [657 ± 289). Therefore, it seems plausible that disease severity could detrimentally affect bone density in adults with particularly low CD4 count, while not in adults with a CD4 count above a certain level.
Antiretroviral therapy combinations including tenofovir disoproxil fumarate have previously been associated with increased bone loss and higher fracture risk in adults with HIV infection [45–47]. Moreover, a randomized, double-blind, placebo-controlled trial evaluating tenofovir disoproxil fumarate/Emtricitabine pre-exposure prophylaxis among HIV-negative men found that those randomized to tenofovir disoproxil fumarate/Emtricitabine demonstrated greater decreases in BMD than those randomized to the placebo arm [49]. Tenofovir disoproxil fumarate plasma concentrations have been shown to be increased when combined with protease inhibitors [47,48], therefore potentially increasing detrimental effects on bone composition. We identified the combined use of tenofovir disoproxil fumarate with a protease inhibitor to be associated with lower trabecular bone quality, while tenofovir disoproxil fumarate use or protease inhibitor use alone were not associated with bone quality deterioration. Therefore, as expected, avoiding this specific medication combination could reduce the fracture risk in PLWH.
In our previous publication we found that HR-pQCT is a powerful tool to assess bone health in HIV-infected men who show no differences to healthy males by DXA aBMD [25], With this new cohort and in the context of questions about nutrition, exercise, antiretroviral therapy medications, and disease severity, our results replicated these previous findings, supporting the conclusion that DXA may be less useful for the evaluation of bone structure in PLWH.
Some limitations are pertinent to this study. While information on current viral load and CD4 count were available, current testosterone levels were unknown; therefore the influence of testosterone level on bone quality could not be assessed. Moreover, we did not have sufficient information on previous disease control and this may also have an effect on bone parameters, given the mean disease duration of 22 years. Another limitation is that only three participants in our study were currently using regimens including tenofovir disoproxil fumarate and a protease inhibitor, because in the past few years physicians have typically changed regimens including this combination due to the known detrimental effects on bone structure. However, this medication combination was of specific interest for this exploratory study because tenofovir disoproxil fumarate plasma concentrations have been shown to increase when combined with protease inhibitors [47,48]. Studies including larger groups of participants on this specific regimen would be necessary to replicate this finding. Finally, because physical activity was assessed using questionnaires, we cannot fully exclude a certain extent of response bias. To minimize socially desirable responding and overreporting of physical activities we used the CHAMPS questionnaire. This questionnaire includes a number of questions on activities other than physical activities in the list, subsequently enabling individuals who are less physically active to report participation in other types of valued activities (e.g. volunteering, attending church, visiting friends, playing a musical instrument) and thus minimizing the likelihood of less active individuals having to report “no” to the majority of questions [26].
5. Conclusions
In conclusion, in the context of HIV infection, regular exercise could help maintain or improve trabecular bone structure and bone strength while nutritional support is specifically relevant for maintaining cortical bone structure. Patients on specific antiretroviral therapy combinations such as tenofovir disoproxil fumarate and protease inhibitors may require close monitoring to assess bone loss and extent of fracture risk.
Supplementary Material
Funding
This work was supported by the National Institutes of Health [NIH NIAID R01 All25080, NIH P30 AR066262, NIH P30 DK098722] and the UCSF RAP Core Exploratory Award.
Abbreviations:
- HIV
human immunodeficiency virus-1
- BMD
bone mineral density
- BMI
body mass index
- PLWH
people living with HIV
- HR-pQCT
high-resolution peripheral quantitative computed tomography
- DXA
dual-energy x-ray absorptiometry
- aBMD
areal BMD
- HCV
hepatitis C virus
- HBV
hepatitis B virus
- LSC
least significant change
- RMSCV
root mean square coefficient of variation
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bone.2019.115210.
References
- [1].Overton ET MK, Bush TJ, Conley U, Kojic EM, Henry K, Hammer J, Wood KC, Lichtenstein KA, Brooks JT, and SUN Study Investigators, Factors Associated With Low Bone Mineral Density (BMD) in a Large Cohort of HIV-Infected U.S. Adults - Baseline Results from the SUN Study. 14th CROI. 2007. Los Angeles, USA, Abstract 836. [Google Scholar]
- [2].Borderi M, Gibellini D, Vescini F, De Crignis E, Cimatti L, Biagetti C, et al. , Metabolic bone disease in HIV infection, AIDS 23 (11) (2009) 1297–1310. [DOI] [PubMed] [Google Scholar]
- [3].Mallon PW, HIV and bone mineral density, Curr. Opin. Infect. Dis 23 (1) (2010) 1–8. [DOI] [PubMed] [Google Scholar]
- [4].Barkhordarian R. Ajaj, Ramchandani MH, Demeijian G, Cayabyab R, Danaie S, et al. , Osteoimmunopathology in HIV/AIDS: a translational evidence-based perspective, Pathol. Res. Int 2011 (2011) 359242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bolland MJ, Grey AB, Gamble GD, Reid IR, CLINICAL review #\ low body weight mediates the relationship between HIV infection and low bone mineral density: a meta-analysis, J. CUn. Endocrinol. Metab 92 (12) (2007) 4522–4528. [DOI] [PubMed] [Google Scholar]
- [6].Knobel H, Guelar A, Vallecillo G, Nogues X, Diez A, Osteopenia in HIV-infected patients: is it the disease or is it the treatment? AIDS 15 (6) (2001) 807–808. [DOI] [PubMed] [Google Scholar]
- [7].Bruera D, Luna M, David DO, Bergoglio LM, Zamudio J, Decreased bone mineral density in HIV-infected patients is independent of antiretroviral therapy, AIDS 17 (13)(2003) 1917–1923. [DOI] [PubMed] [Google Scholar]
- [8].Amiel C, Osier tag A, Slama L, Baudoin C, N’Guyen T, Lajeunie E, et al. , BMD is reduced in HIV-infected men irrespective of treatment, J. Bone Miner. Res 19 (3) (2004) 402–409. [DOI] [PubMed] [Google Scholar]
- [9].Brown TT Ruppe MD, Kassner R, Kumar P, Kehoe T, Dobs AS, et al. , Reduced bone mineral density in human immunodeficiency virus-infected patients and its association with increased central adiposity and postload hyperglycemia, J. Clin. Endocrinol. Metab 89 (3) (2004) 1200–1206. [DOI] [PubMed] [Google Scholar]
- [10].Jacobson DL, Spiegelman D, Knox TK, Wilson IB, Evolution and predictors of change in total bone mineral density over time in HIV-infected men and women in the nutrition for healthy living study, J. Acquir. Immune Defic. Syndr 49 (3) (2008) 298–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mangili A, Murman DH, Zampini AM, Wanke CA, Nutrition and HIV infection: review of weight loss and wasting in the era of highly active antiretroviral therapy from the nutrition for healthy living cohort, Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America 42 (6) (2006) 836–842. [DOI] [PubMed] [Google Scholar]
- [12].National Osteoporosis Foundation, Are you at risk? Retrieved from, 2016. http://nof.org/artides/2.
- [13].Bonato M, Moderate aerobic exercise (brisk walking) increases bone density in cART-treated persons, J. Int. AIDS Soc 15 (6) (2012) 18318. [Google Scholar]
- [14].Santos WR, Santos WR, Paes PP, Ferreira-Silva IA, Santos AP, Vercese N, et al. , Impact of strength training on bone mineral density in patients infected with HIV exhibiting lipodystrophy, J Strength Cond Res 29 (12) (2015) 3466–3471. [DOI] [PubMed] [Google Scholar]
- [15].Currier JS, Havlir DV, Complications of HIV disease and antiretroviral therapy, Top HIV Med 13 (1) (2005) 16–23. [PubMed] [Google Scholar]
- [16].Bagnis CI, Karie S, Deray G, Essig M, Hypophosphataemia: an easy strategy for diagnosis and treatment in HIV patients, Antivir. Ther 14 (4) (2009) 481–488. [PubMed] [Google Scholar]
- [17].Waheed S, Atta MG, Predictors of HIV-associated nephropathy, Expert Rev. Antj-Infect. Ther 12 (5) (2014) 555–563. [DOI] [PubMed] [Google Scholar]
- [18].Waheed S, Attia D, Estrella MM, Zafar Y, ALta MG, M Lucas G, et al. , Proximal tubular dysfunction and kidney injury associated with tenofovir in HIV patients; a case series, Clin. Kidney J 8 (4) (2015) 420–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].McComsey GA, Kitch D, Daar ES Tierney C, Jahed NC, Tebas P, et al. , Bone mineral density and fractures in antiretroviral-naive persons randomized to receive abacavir-lamivudine or tenofovir disoproxil fumarate-emtricitabine along with efavirenz or atazanavir-ritonavir: Aids Clinical Trials Group A5224s, a substudy of ACTG A5202, J. InfecL Dis 203 (12) (2011) 1791–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kamal K, Sawlani SS, Chaudhary SC, Himanshu Reddy D, Usman K, Atam V, A study of bone mineral density among people living with HIV in India and its correlation with CD4 count, International Journal of Research in Medical Sciences 5 (2) (2017) 563–568. [Google Scholar]
- [21].Biver E, Calmy A, Delhumeau C, Durosier C, Zawadynski S, Rizzoli R, Microstmcturai alterations of trabecular and cortical bone in long-term HIV-infected elderly men on successful antiretroviral therapy, AIDS 28 (16) (2014) 2417–2427. [DOI] [PubMed] [Google Scholar]
- [22].Blake GM, Fogelman I, The role of DXA bone density scans in the diagnosis and treatment of osteoporosis. Postgrad. Med. J 83 (982) (2007) 509–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Nishiyama KK, Shane E, Clinical imaging of bone microarchitecture with HR pQCT, Curr Osteoporos Rep 11 (2) (2013) 147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Riggs BL, Melton LJ, A Robb R, Camp JJ, Atkinson EJ, McDaniel L, et al. , A population-based assessment of rates of bone loss at multiple skeletal sites: evidence for substantial trabecular bone loss in young adult women and men, J. Bone Miner. Res 23 (2) (2008) 205–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kazakia GJ, CarbaUido-Gamio J, Lai A, Nardo L, Facchetti L, Pasco C, et al. Trabecular bone microstructure is impaired in the proximal femur of human immunodeficiency virus-infected men with normal bone mineral density, Quant Imaging Med Surg 8 (1) (2018) 5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Stewart AL, Mills KM, King AC, L Haskell W, Gillis D, Ritter PL, CHAMPS physical activity questionnaire for older adults: outcomes for interventions, Med. Sri. Sports Exerc 33 (7) (2001) 1126–1141. [DOI] [PubMed] [Google Scholar]
- [27].Stewart AL, Verboncoeur CJ, McLellan BY, Gillis DE, Rush S, Mills KM, et al. , Physical activity outcomes of CHAMPS II: a physical activity promotion program for older adults, J. Gerontol. A Biol. Sci. Med. Sci, 56 (8) (2001) M465–M470, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Burr DB, Robling AG, Turner CH, Effects of biomechanical stress on bones in animals, Bone 30 (5) (2002) 781–786. [DOI] [PubMed] [Google Scholar]
- [29].Robling AG, Burr DB, Turner CH, Partitioning a daily mechanical stimulus into discrete loading bouts improves the osteogenic response to loading, J. Bone Miner. Res 15 (8) (2000) 1596–1602. [DOI] [PubMed] [Google Scholar]
- [30].Robling AG, Castillo AB, Turner CH, Biomechantcal and molecular regulation of bone remodeling, Anna. Rev. Biomed. Eng 8 (2006) 455–498. [DOI] [PubMed] [Google Scholar]
- [31].Robling AG, Hinant FM, Burr DB, Turner CH, Improved bone structure and strength after long-term mechanical loading is greatest if loading is separated into short bouts, J. Bone Miner. Res 17 (8) (2002) 1545–1554. [DOI] [PubMed] [Google Scholar]
- [32].Wikby K, Ek AC, Christensson L, The two-step mini nutritional assessment procedure in community resident homes, J. Clin. Nurs 17 (9) (2008) 1211–1218. [DOI] [PubMed] [Google Scholar]
- [33].Charlton KE, Kolbe-Alexander TL, Nel JH, The MNA. but not the DETERMINE, screening tool is a valid indicator of nutritional status in elderly Africans, Nutrition 23 (7–8) (2007) 533–542. [DOI] [PubMed] [Google Scholar]
- [34].Kuzuya M, Kanda S, Koike T, Suzuki Y, Satake S, A Iguchi, Evaluation of mini-nutritional assessment for Japanese frail elderly, Nutrition 21 (4) (2005) 498–503. [DOI] [PubMed] [Google Scholar]
- [35].Visvanathan R, Penhall R, Chapman I, Nutritional screening of older people in a sub-acute care facility in Australia and its relation to discharge outcomes, Age Ageing 33 (3) (2004) 260–265. [DOI] [PubMed] [Google Scholar]
- [36].Cohendy R, Rubenstein LZ, iiledjam JJ, The Mini Nutritional Assessment-Short Form for preoperative nutritional evaluation of elderly patients, Aging (Milano) 13 (4) (2001) 293–297. [DOI] [PubMed] [Google Scholar]
- [37].Vellas B, Guigoz Y, Garry PJ, Nourhashemi F, Beimahum D, Lauque S, et al. , The Mini Nutritional Assessment (MNA) and its use in grading the nutritional state of elderly patients, Nutrition 15 (2) (1999) 116–122. [DOI] [PubMed] [Google Scholar]
- [38].Kazakia GJ, Tjong W, Nirody JA, Burghardt AJ, Carballido-Gamio J, Patsch JM, et al. The influence of disuse on bone microstructure and mechanics assessed by HR-pQCT, Bone 63 (2014) 132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].BurghardL H AJ.Buie R, Laib A, Majumdar S, Boyd SK, Reproducibility of direct quantitative measures of cortical bone microarchitecture of the distal radius and tibia by HR-pQCT, Bone 47 (3) (2010) 519–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Laib A, Hauselmann HJ, Ruegsegger P, In vivo high resolution 3D-QCT of the human forearm, Technol. Health Care 6 (5–6) (1998) 329–337. [PubMed] [Google Scholar]
- [41].Mueller TL, Christen D, Sandercott S, Boyd SK, van Rietbergen B, Eckstein F, et al. , Computational finite element bone mechanics accurately predicts mechanical competence in the human radius of an elderly population, Bone 48 (6) (2011) 1232–1238. [DOI] [PubMed] [Google Scholar]
- [42].Macneil JA, Boyd SK, Bone strength at the distal radius can be estimated from high-resolution peripheral quantitative computed tomography and the finite element method, Bone 42 (6) (2008) 1203–1213. [DOI] [PubMed] [Google Scholar]
- [43].Muller R, Ruegsegger P, Three-dimensional finite element modelling of non-in vasively assessed trabecular bone structures, Med. Eng. Phys 17 (2) (1995) 126–133. [DOI] [PubMed] [Google Scholar]
- [44].BurghardL AJ, Pialat JB, Kazakia GJ, Boutroy S, Engelke K, M Patsch J, et al. , Multicenter precision of cortical and trabecular bone quality measures assessed by high-resolution peripheral quantitative computed tomography, J. Bone Miner. Res 28 (3) (2013) 524–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Gallant JE, Staszewski S. L Pozniak A, DeJesus E, Suleiman JM, Miller MD, et al. , Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial, JAMA 292 (2) (2004) 191–201. [DOI] [PubMed] [Google Scholar]
- [46].Komatsu A, Ikeda A, Kikuchi A, Minami C, Tan M, Matsushita S, Osteoporosis-related fractures in HIV-infected patients receiving long-term tenofovir disoproxil fumarate: an observational cohort study, Drug Saf. 41 (9) (2018) 843–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Woodward CL, Hall AM, Williams IG, Madge S, Copas A, Nair D, et al. , Tenofovir-associated renal and bone toxicity, HIV Med 10 (8) (2009) 482–487. [DOI] [PubMed] [Google Scholar]
- [48].Kearney BP, Mathias A, Mittan A, Sayre J, Ebrahimi R, Cheng AK, Pharmacokinetics and safety of tenofovir disoproxil fumarate on coadministration with lopinavir/ritonavir, J. Acquir. Immune Defic. Syndr 43 (3) (2006) 278–283. [DOI] [PubMed] [Google Scholar]
- [49].Liu AY, Vittinghoff E, Sellmeyer DE, Irvin R, Mulligan K, Mayer K, et al. , Bone mineral density in HIV-negative men participating in a tenofovir pre-exposure prophylaxis randomized clinical trial in San Francisco, PLoS One 6 (8) (2011) e23688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Silva MJ, Biomechanics of osteoporotic fractures, Injury 38 (Suppl. 3) (2007) S69–S76. [DOI] [PubMed] [Google Scholar]
- [51].Orwoll ES, Toward an expanded understanding of the role of the periosteum in skeletal health, J. Bone Miner. Res 18 (6) (2003) 949–954. [DOI] [PubMed] [Google Scholar]
- [52].Nilsson M, Sundh D, Mellstrom D, Lorentzon M, Current physical activity is independently associated with cortical bone size and bone strength in elderly Swedish women, J. Bone Miner. Res 32 (3) (2017) 473–485. [DOI] [PubMed] [Google Scholar]
- [53].Hannan MT, Felson DT, Dawson-Hughes B, Tucker KL, Cupples LA, Wilson PW, et al. , Risk factors for longitudinal bone loss in elderly men and women: the Framingham Osteoporosis Study, J. Bone Miner. Res 15 (4) (2000) 710–720. [DOI] [PubMed] [Google Scholar]
- [54].Nordin BE, Need AG, Steurer T, Morris HA, Chatterton BE, Horowitz M, Nutrition, osteoporosis, and aging, Ann. N. Y. Acad. Sci 854 (1998) 336–351. [DOI] [PubMed] [Google Scholar]
- [55].Muir JM, Ye C, Bhandari M, Adachi JD, Thabane L, The effect of regular physical activity on bone mineral density in post-menopausal women aged 75 and over: a retrospective analysis from the Canadian multicentre osteoporosis study, BMC Musculoskelet. Disord 14 (2013) 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Hamilton CJ, Swan VJ, Jamal SA, The effects of exercise and physical activity participation on bone mass and geometry in postmenopausal women; a systematic review of pQCT studies, Osteoporos. int 21 (1) (2010) 11–23. [DOI] [PubMed] [Google Scholar]
- [57].Polidoulis I, Beyene J, Cheung AM, The effect of exercise on pQCT parameters of bone structure and strength in postmenopausal women—a systematic review and meta-analysis of randomized controlled trials, Osteoporos. Int 23 (1) (2012) 39–51. [DOI] [PubMed] [Google Scholar]
- [58].Kemmler W, Engelke K, von Stengel S, Long-term exercise and bone mineral density changes in postmenopausal women—are there periods of reduced effectiveness? J. Bone Miner. Res 31 (1) (2016) 215–222. [DOI] [PubMed] [Google Scholar]
- [59].Kohrt WM, Ehsani AA, Birge SJ Jr, Effects of exercise involving predominantly either joint-reaction or ground-reaction forces on bone mineral density in older women, J. Bone Miner. Res 12 (8) (1997) 1253–1261. [DOI] [PubMed] [Google Scholar]
- [60].Jacks DE, Sowash J, Anning J, McGloughlin T, Andres F, Effect of exercise at three exercise intensities on salivary cortisol, J Strength Cond Res 16 (2) (2002) 286–289. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.