ABSTRACT
Background and Aims:
Vitiligo, an acquired sometimes familial depigmentary disorder of the skin and hair that results from selective destruction of melanocytes or pigment cells. It is the single most important non-neo plastic disease that involves both the immune system and melanocytes which are subsequently destroyed and the affected area turns pale and becomes white. The prevalence of the disease is between 1% and 2% in general population.
Methods:
It is a prospective, randomized, and controlled study. Over 90 vitiligo patients attending Dermatology OPD and vitiligo clinic are enrolled in the study. About 35 apparently healthy, age and sex matched individuals are selected to serve as control. A prescribed proforma containing all the demographic data, relevant questionnaire were recorded for each and every case with brief clinical history suggestive of any thyroid disease as well as those referred by the clinicians. P value <0.05 is considered significant. The Quantitative Determination of Thyroglobulin (Tg) Autoantibodies in Human Serum or Plasma by a Microplate Enzyme Immunoassay.
Results:
Vitiligo group, thirty four (37.78%) patients have clinical hypothyroidism while 9 (10%) patients have clinical hyperthyroidism. This difference in the distribution is statistically significant (P < 0.05) with a Chi-square value of 10.08. The data are entered, analyzed and computed with SPSS version 15 software and well-known statistical test like Chi-square, students’ ‘t’ test have been advocated wherever found applicable. P value <0.05 is considered significant.
Conclusion:
There is increased incidence of autoimmune thyroid diseases among vitiligo patients. The reason being vitiligo usually precedes the onset of thyroid dysfunction.
Keywords: Anti-thyroid peroxidase, antithyroglobulin antibody, thyroid disorders, vitiligo
Introduction
Vitiligo is an acquired skin disorder caused due to destruction of melanocytes which clinically presents with well-defined depigmented macules and patches with or without hairs.[1] Vitiligo, a depigmenting skin disorder, is characterized by the selective loss of melanocytes, which in turn leads to pigment dilution in the affected areas of the skin. The characteristic lesion is a totally amelanotic, nonscaly, chalky-white macule with distinct margins. Considerable recent progress has been made in our understanding of the pathogenesis of vitiligo, and now it is clearly classified as autoimmune disease, associated with genetic and environmental factors together with metabolic, oxidative stress and cell detachment abnormalities.[2] The association of vitiligo with autoimmune disorders of other organs like pernicious anemia, thyroid disease, insulin dependent diabetes mellitus (IDDM) and Addison’s disease are well evident. Vitiligo is an acquired depigmenting disorder due to destruction of melanocyte. Over half of the people with vitiligo have acquired some loss of pigment cells before the age of 20 years.[3] The findings of[4] also suggested that patients with vitiligo have an immunological disturbance and that the disappearance of melanocytes may be the result from the pathogenesis of the same autoantibody reaction. In recent years, increasing attention has been paid to exploring the genetics of vitiligo, more than 4 loci are thought to be associated with vitiligo.[5]
Materials and Methods
The study was conducted in the Department of Biochemistry in collaboration with the Department of Dermatology, Regional Institute of Medical Sciences (RIMS) Hospital, Imphal, Manipur.
Study duration
The study duration is of 19 months Jan’2014 to July’2015.
Study design
Prospective, randomized and controlled study.
Inclusion criteria
About 90 vitiligo patients attending the Dermatology OPD and vitiligo clinic are enrolled in the study. Thirty five apparently healthy, age and sex matched individuals are selected to serve as control.
Exclusion criteria
Patients who undergone thyroid surgery and under medication for thyroid diseases, individual with pregnancy, were excluded for the study.
A prescribed proforma containing all the demographic data, relevant questionnaire were recorded for each and every case with brief clinical history suggestive of any thyroid disease as well as those referred by the clinicians.
Ethics issues
Approval was sought from Research Ethics Board RIMS, Imphal.
Consent taken before taking blood samples.
Confidentiality maintained.
Sample collection and processing
About 4 cc of whole blood was drawn from antecubital vein from each patient as well as control, collected in sterile plain vials, sample was allowed to clot and centrifuged to separate the serum. Serum sample is stable in 2–8°c for a maximum period of 5 days and up to 30 days when stored in refrigerator at 20°c. In the study, all estimations were done within 3 days of collection and storage of sample was done in freezer compartment of refrigerator.
Estimation of serum anti-thyroglobulin (Anti-Tg)
Intended use: The Quantitative Determination of Thyroglobulin (Tg) Autoantibodies in Human Serum or Plasma by a Microplate Enzyme Immunoassay. Measurements of Tg autoantibodies may aid in the diagnosis of certain thyroid diseases such as Hashimoto’s and Grave’s as well as nontoxic goiter.
Upon mixing biotinylated antigen and a serum containing the autoantibody, reaction results between the antigen and the antibody to form an immune-complex.
The anti-h-IgG enzyme conjugate that binds to the immune complex in a second incubation is separated from unreacted material by a wash step. The enzyme activity in this fraction is directly proportional to the antibody concentration in the specimen. By utilizing several different serum references of known antibody activity, a reference curve can be generated from which the antibody activity of an unknown can be ascertained.
Specimen collection and preparation
The specimen shall be serum or plasma separated from a venipuncture collected blood sample. For accurate comparison to established normal values, a fasting morning serum sample is obtained, blood collected in a plain redtop venipuncture tube without additives or anti-coagulant containing EDTA or heparin. Centrifuge the specimen for separating serum or plasma from the cells.
Samples are refrigerated at 2–8°C for maximum period of 5 days. If the specimen cannot be assayed within this time, store at −20°C for up to 30 days. Avoid repetitive freezing and thawing. When assayed in duplicate, 0.100 ml of diluted sample is required.
Sensitivity
The anti-Tg Accubind™ ELISA has a sensitivity of 5 IU/ml.
Specificity
Interferences from ANA, DNA, thyroid peroxidase (TPO) and rheumatoid antibodies were found to be insignificant in the assay system (Vole R, 1994).
Estimation of anti-thyroid peroxidase (Anti-TPO)
Intended use
Anti-TPO is a solid phase enzyme immunoassay employing recombinant human thyroid peroxidase (TPO) from a eukaryotic expression system for the quantitative detection of antibodies against TPO in human serum. Only recombinant human antigen expressed in eukaryotic cells displays specific conformational epitopes that are accessible for human anti-TPO autoantibodies. The assay is a tool in the diagnosis of autoimmune thyroid diseases.
Principle of the assay and clinical application
It is the major enzyme involved in multiple steps of thyroid hormone synthesis.
TPO is one out of three major thyroid autoantigens besides thyroglobulin (Tg) and the TSH-receptor. The presence of auto antibodies to TPO and Tg today is an established tool for diagnosing chronic autoimmune thyroiditis as well as for the differential diagnosis of hypothyroidism including its subclinical and latent type.
Principle of the test
Serum samples diluted in the ratio 1:101, are incubated in the microplates coated with the specific antigen. Patient’s antibodies, if present in the specimen, bind to the antigen. The unbound fraction is washed off in the following step. Afterwards anti-human immunoglobulins conjugated to horseradish peroxidase (conjugate) are incubated and react with the antigen-antibody complex of the samples in microplates. Unbound conjugate is washed off in the following steps. Addition of TMB-substrate generates an enzymatic colorimetric (blue) reaction, which is stopped by diluted acid (color changes to yellow). The rate of color formation from the chromogen is a function of the amount of conjugate bound to the antigen-antibody complex and this is proportional to the initial concentration of the respective antibodies in the patient sample.
Storage and shelf life
All reagents and the microplate are stored at 2–8°C/35–46°F, in their original containers. Once prepared, reconstituted solutions are stable for 1 month at 2–8°C/35–46°F, at least.
Sample collection, handling and storage
Freshly collected serum samples are used. Blood withdrawal must follow national requirements.
Not to use icteric, lipemic, hemolyzed or bacterially contaminated samples. Sera with particles should be cleared by low-speed centrifugation (<1000 m/s). Blood samples should be collected in clean, dry and empty tubes. After separation, the serum samples should be used immediately, respectively stored, tightly closed at 2–8°C/35–46°F up to three days or frozen at −20°C/−4°F for longer periods
Sensitivity
The anti-Tg Accubind™ ELISA has a sensitivity of 5 IU/ml.
Specificity
Interferences from ANA, DNA, thyroid peroxidase (TPO), and rheumatoid antibodies were found to be insignificant in the assay system.
Estimation of anti-thyroid peroxidase (Anti- TPO)
Anti-TPO is a solid phase enzyme immunoassay employing recombinant human thyroid peroxidase (TPO) from a eukaryotic expression system for the quantitative detection of antibodies against TPO in human serum. Only recombinant human antigen expressed in eukaryotic cells displays specific conformational epitopes that are accessible for human anti-TPO autoantibodies. The assay is a tool in the diagnosis of autoimmune thyroid diseases.
Principle of the assay and clinical application
Thyroid peroxidase (TPO) is a large (105 kDa). It is the major enzyme involved in multiple steps of thyroid hormone synthesis.
TPO is one out of three major thyroid autoantigens besides thyroglobulin (Tg) and the TSH-receptor. The presence of auto antibodies to TPO and Tg today is an established tool for diagnosing chronic autoimmune thyroiditis as well as for the differential diagnosis of hypothyroidism including its subclinical and latent type.
Statistical method used
The data are entered, analyzed, and computed with SPSS version 15 software and well-known statistical test like Chi-square, students’ ‘t’ test have been advocated wherever found applicable. The necessary interpretations are made. P value < 0.05 is considered significant.
Results
Demographic data such as age, sex, regional distribution, etc., and biochemical and immunological parameters such as TSH, T3, T4, anti-TG, and anti-TPO are recorded. The data are entered and computed with SPSS version 15 software and well-known statistical test like Chi-square, students ‘t’ test, etc., are used wherever found applicable and necessary.
Discussion
The study was carried out to look for any association of vitiligo and thyroid dysfunction with autoimmune thyroid disease and to find out clinical characteristics of vitiligo, which may predict such an association. It is a prospective, randomized, and controlled study in which 90 vitiligo patients attending the Dermatology OPD and vitiligo clinic of RIMS Hospital, in period of January 2006–July 2007 were enrolled for this study. Besides recording the age, sex, regional distribution, clinical features of vitiligo and thyroid disease, antithyroid autoantibody assays (anti-thyroglobulin, anti-TPO), and thyroid hormone profiles were done in these cases and 35 appropriately age and sex matched controls.
Previous studies were inconsistent as to whether male and female patients were affected by vitiligo with equal frequency. In this study, there were 63 (70%) female vitiligo patients as compared to 27 (30%) male patients. This is in agreement with the findings of Lacovelli P. et al.,[6] but in contrast with the findings of Shyria D. et al.,[7] and Zhang XJ. et al.,[5] where equal distribution among the sexes were reported as shown in Figure 1. However, prevalence distributions might be different in different ethnic groups. In my opinion, the differences probably result from: variable factors underlying vitiligo in different ethnic groups, fewer participants in the study, or preponderance of female respondents in this region where the study is taken up.
Figure 1.
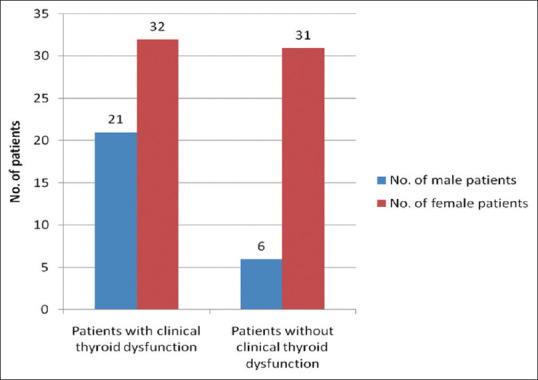
The sex-wise distribution with relation to the associated clinical thyroid dysfunction in the vitiligo (cases) group
As to the racial/religion wise distribution, depicted in Table 1, Hindu contributed maximum number of patients in both groups with 51 (56.67%) and 30 (85.71%) patients in vitiligo and control groups respectively as compared to other religions. Muslim shared 8 (8.89%) cases and 3 (8.57%) controls, whereas Christian contributed 31 (34.44%) cases and 2 (5.72%) control. The difference between Hindus and other religions is statistically P =0.00 with a Chi-square value of 12.11, which may be explained by the fact that majority of patients attending the RIMS Hospital are from the Hindu dominated valley districts. There are similar studies conducted in the dark races especially from the Indian subcontinent. Shyria D. et al.,[7] have attempted to demonstrate the association of vitiligo with thyroid disease in South Indian population where the frequency of thyroid dysfunction is high.
Table 1.
The religion wise (racial) distribution of patients in the two groups
| Religion (racial) | Cases (No) | Control (No) | Chi- square | Degree of Freedom | ‘P’ and Inference |
|---|---|---|---|---|---|
| Hindu | 51 (56.67%) | 30 (85.71%) | |||
| Muslim | 8 (8.89%) | 3 (8.57) | 12.11 | 2 | P=0.00* |
| Christian | 31 (34.44%) | 2 (5.72%) | |||
| Total | 90 (100%) | 35 (100%) |
*Significant (figure within the parenthesis indicates percentage)
As an important feature of complex diseases, the age of disease onset has routinely been analyzed in association studies. The mean ages of onset of vitiligo that we observed at about 8 years were almost identical to those found by Norlund JJ. et al.,[8] and Majumder PP. et al.,[9] Our findings were also in conformity with the findings of by Roth C. et al.,[10] as depicted in Table 2. Seven subtypes of vitiligo are recorded along with age wise distribution with maximum number of patients of 23 (25.56%) each in vespula vulgaris and vitiligo acrofacialis subtype while segmental has 22 (24.44%) patients. Universalis subtype has got the least with 1 (1.11%) patient which is same as findings of Zhang XJ et al.,[5] who also reported vitiligo vulgaris as maximum among their study group. It can be inferred that ages of vitiligo onset were closely associated with patients’ clinical phenotypes. Age of onset for universalis vitiligo is the least, which may be an indicative of an important environmental factor underlying its etiopathogenesis. However, it needs verification in future study.
Table 2.
Distribution of vitiligo types with relation to the age in the cases group
| Segmental | Vitiligo vulgaris | Acrofa-Cialis | Focal | Mucosal | Mixed | Univer-salis | Statistical test | P | |
|---|---|---|---|---|---|---|---|---|---|
| Age range (in years) | |||||||||
| 6-10 | 2 (2.22%) | 3 (3.33%) | 2 (2.22%) | 2 (2.22%) | 1 (1.11%) | 0 (0%) | 0 (0%) | ||
| 11-15 | 1 (1.11%) | 2 (2.22%) | 0 (0%) | 0 (0%) | 1 (1.11%) | 1 (1.11%) | 0 (0%) | Chi- square | P>0.0 |
| 16-20 | 3 (3.33%) | 0 (0%) | 3 (3.33%) | 2 (2.22%) | 1 (1.11%) | 0 (0%) | 0 (0%) | value of 2.99 | 5 |
| 21-25 | 2 (2.22%) | 2 (2.22%) | 0 (0%) | 0 (0%) | 1 (1.11%) | 0 (0%) | 0 (0%) | ||
| 26-30 | 2 (2.22%) | 1 (1.11%) | 3 (3.33%) | 0 (0%) | 1 (1.11%) | 0 (0%) | 0 (0%) | ||
| 31-35 | 3 (3.33%) | 2 (2.22%) | 2 (2.22%) | 2 (2.22%) | 1 (1.11%) | 0 (0%) | 0 (0%) | ||
| 36-40 | 3 (3.33%) | 2 (2.22%) | 5 (5.55%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | ||
| 41-45 | 2 (2.22%) | 1 (1.11%) | 2 (2.22%) | 2 (2.22%) | 0 (0%) | 0 (0%) | 0 (0%) | ||
| 46-50 | 1 (1.11%) | 4 (4.44%) | 4 (4.44%) | 0 (0%) | 2 (2.22%) | 1 (1.11%) | 0 (0%) | ||
| 51-55 | 2 (2.22%) | 2 (2.22%) | 1 (1.11%) | 1 (1.11%) | 0 (0%) | 0 (0%) | 1 (1.11%) | ||
| 56 and above | 1 (1.11%) | 4 (4.44%) | 1 (1.11%) | 1 (1.11%) | 0 (0%) | 1 (1.11%) | 0 (0%) | ||
| Total | 22 | 23 | 23 | 10 | 8 | 3 | 1 |
(figure within the parenthesis indicates percentage)
The thyroid hormonal assay as shown in Tables 3-6 recorded an average (mean ± SD) serum TSH, T3 and T4 levels in both groups. The distribution of TSH levels in the two groups is statistically significant (P = 0.01) with ‘t’ test value of 2.51. Twenty five (27.78%) patients got increased serum TSH level in vitiligo as compared to 3 (8.57%) patients in control group. There exists a significant difference in the two groups with a Chi-square value of 6.41 (P < 0.05). Twelve (13.33%) patients in the vitiligo group have got raised serum T3 levels and 18 (20%) and 4 (11.43%) patients have low level below normal values in the study and control groups respectively. This distribution is statistically significant (P < 0.05) with a Chi-square value of 5.05. Likewise, 6 (6.67%) patients in vitiligo group have serum T4 levels above normal while there is none in control group. Low serum T4 levels are recorded in 31 (34.44%) and 6 (17.14%) patients of vitiligo and control group, respectively, which has statistical significance with Chi-square value of 6.42 (P < 0.05). When we study the vitiligo group, 34 (37.78%) patients have clinical hypothyroidism while 9 (10%) patients have clinical hyperthyroidism, which is statistically significant (P < 0.05) with Chi-square value of 10.08. This finding was in agreement with that of Parker F et al.,[11] and Czarnoka B et al.,[12] who also reported clinical hyper-thyroidism in some individuals. Also, findings of Kumar V et al.,[13] showed maximum individuals with clinical hypothyroid state, euthyroid vitiligo with tendency of developing hypothyroidism.
Table 3.
The distribution of Thyroid hormonal assay in the two groups
| Thyroid hormones | Observed assay | No of patients in the Cases | No of patients in the control | Chi-square | ‘P’ and Inference |
|---|---|---|---|---|---|
| TSH | Increase TSH | 25 (27.78%) | 3 (8.57%) | ||
| Decrease TSH | 4 (4.44%) | 0 (0%) | 6.41 | P<0.05* | |
| Normal | 61 (67.78%) | 32 (91.43%) | |||
| Total | 90 (100%) | 35 (100%) | |||
| T3 | Increase T3 | 12 (13.33%) | 0 (0%) | ||
| Decrease T3 | 18 (20%) | 4 (11.43%) | 5.05 | P<0.05* | |
| Normal | 60 (66.67%) | 31 (88.57%) | |||
| Total | 90 (100%) | 35 (100%) | |||
| T4 | Increase T4 | 6 (6.67%) | 0 (0%) | ||
| Decrease T4 | 31 (34.44%) | 6 (17.14%) | 6.42 | P<0.05* | |
| Normal | 53 (58.89%) | 29 (82.86%) | |||
| Total | 90 (100%) | 35 (100%) |
*Significant (figure within the parenthesis indicates percentage)
Table 6.
The distribution of patients with relation to the Immunoglobulin positivity and clinical thyroid diseases in the Vitiligo (cases)
| Immunoglobulin assay | No. of Vitiligo patients with clinical thyroid disease with positive Immunoglobulin assay | No. of Vitiligo patients without clinical thyroid disease with positive Immunoglobulin assay | Total | Chi- square | ‘P’ and Inference |
|---|---|---|---|---|---|
| Anti TG positive assay | 7 (7.78%) | 10 (11.11%) | 17 (18.89%) | ||
| Anti TG & anti TPO positive assay | 17 (18.89%) | 0 (0%) | 17 (18.89%) | 8.27 | P<0.05* |
| Anti TPO positive assay | 16 (17.78%) | 26 (28.88%) | 42 (46.67%) | ||
| Total | 40 (44.44%) | 36 (40%) | 76 (84.44%) |
*Significant (figure within the parenthesis indicates percentage)
Table 4.
The distribution of thyroid hormonal and anti-immunoglobulin assay in the two groups
| Hormones/Immunoglobulin | Cases (Mean±S.D) | Controls (Mean±S.D) | Student ‘t’ test | ‘P’ | Inference |
|---|---|---|---|---|---|
| TSH (µIU/ml) | 5.70±3.12 | 4.26±2.17 | 2.51 | 0.01 | P<0.05* |
| T4 (µg/dl) | 6.31±2.86 | 6.81±2.48 | 0.92 | 0.36 | P>0.05 |
| T3 (ng/ml) | 1.33±1.32 | 1.12±0.42 | 0.92 | 0.36 | P>0.05 |
| AntiTPO (IU/ml) | 170.18±99.49 | 96.42±38.45 | 4.2 | 0.00 | P<0.05* |
| Anti TG (IU/ml) | 136.94±98.34 | 95.64±22.33 | 2.46 | 0.02 | P<0.05* |
*Significant
Table 5.
The distribution of patients with relation to the associated clinical thyroid dysfunction in the vitiligo (cases) group
| Thyroid dysfunction | No of patients with clinical Thyroid dysfunction | No of patients without clinical Thyroid dysfunction | Chi-square | ‘P’ and Inference |
|---|---|---|---|---|
| Hypothyroid | 34 (37.78%) | |||
| Hyperthyroid | 9 (10%) | 47 (52.22%) | 10.08 | P<0.05* |
| Total | 43 (47.78%) | 47 (52.22%) |
*Significant (figure within the parenthesis indicates percentage)
As to the distribution of patients with relation to the immunoglobulin positivity and clinical thyroid diseases in vitiligo, the average (mean ± SD) concentration of anti-TG and anti-TPO are 136.94 ± 98.34 IU/ml and 170 ± 99.49 IU/ml while it is 95.64 ± 22.33 and 96.42 ± 38.45 IU/ml respectively in control group. There exists a significant difference (P = 0.02 and P = 0.02) in the distribution of these immunoglobulin in two groups with student ‘t’test value of 2.46 and 74.2, respectively. These findings were in conformity with the similar studies conducted by Kemp EH. et al.,[14] and Alkhateeb A. et al.[15]
Seventeen (18.89%) patients have only positive anti-TG assay while 42 (46.67%) patients were positive for anti-TPO assay in vitiligo group, 17 (18.89%) patients have both positive anti-TG and anti-TPO assay. Clinical thyroid diseases are noticed in 7 (7.78%), 17 (18.89%) and 16 (17.78%) patients with positive anti-TG, both anti-TG and anti-TPO positive and anti-TPO positive assay respectively in vitiligo group. This difference in the distribution is statistically significant (P < 0.05) with Chi-square value of 8.27. However, none of the patients in control group are positive for any of the studied immunoglobulin. Similar findings were observed by Roth C. et al.,[10] and Jameson JL. et al.,[16] who reported that autoimmune thyroid disorders were most frequently associated with 30% of patients with vitiligo. Elevated levels of anti-TPO are seen in more than 90% cases of Hashimoto’s thyroiditis and 75% of Graves’ disease which were also observed by Ai J. et al.,[17] Hegedus L. et al.,[18] reported a close association of thyroid functional disorders and autoimmune diseases with vitiligo. In 1995, Vanderpump MP. et al.,[19] suggested there exist increased incidence of clinical and subclinical autoimmune thyroid diseases among vitiligo and the need for proper screening of such patients. Again, in 2004, Kemp EH, detected various thyroid antibodies in autoimmune thyroid disorders, suggesting these thyroid antibodies could act as a sensitive marker for detection of early and subclinical autoimmune disorders of thyroid gland. The studies of Maryam D. et al.,[20] also goes in favor of this study, suggesting that these can be used as a tool for detection of autoimmune thyroid diseases including Graves’ disease and Hashimoto’s thyroiditis. The reason being vitiligo usually precedes the onset of thyroid dysfunction.
Conclusion
There exist a correlation between the extent of depigmentation and level of vitiligo antibodies. There is increased incidence of autoimmune thyroid diseases among vitiligo patients. Various thyroid antibodies were detected in thyroid disorders, suggesting that these thyroid antibodies could act as sensitive markers for detection of early and subclinical autoimmune disorders of thyroid gland including Grave’s disease and Hashimoto’s thyroiditis. The reason being vitiligo usually precedes the onset of thyroid dysfunction. Therefore, these thyroid autoantibodies especially anti-TPO, anti-TG should be included along with thyroid parameters TSH, T3, T4 in routine investigation of vitiligo as association of vitiligo with autoimmune thyroid disease is very much common in the North-Eastern region of India.
Ethical statement
The study is in compliance with all Ethical Standards.
It is funded by Regional institute of Medical Sciences.
There is no conflict of interest.
Approved by Institutional Ethics Committee.
Informed consent was taken from all the patients involved which was signed by them.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
We would like to show our deep gratitude to Sir Gyaneshwor Singh and Madam Damayanti Devi, Department of Biochemistry for their support and help throughout. They will never be forgotten.
References
- 1.Kumar NA, N A, G S, Sangaiah S. Knowledge, attitude and behaviour study of vitiligo among general population. IP Indian J Clin Exp Dermatol. 2021;7:228–31. [Google Scholar]
- 2.Picardo M, Dell'Anna ML, Ezzedine K, Hamzavi I, Harris JE, Parsad D, et al. Vitiligo. Nat Rev Dis Primers. 2015;1:15011. doi: 10.1038/nrdp.2015.11. [DOI] [PubMed] [Google Scholar]
- 3.Aaron B. Lerner and Gisela Moeumam Vitiligo the immune connection in Dermatologic and Immunology and Allergy 1985. :641–3. [Google Scholar]
- 4.Brystyn JC, Naughton GK. The significance of vitiligo antibodies. J Dermatol. 1985;12:1–9. doi: 10.1111/j.1346-8138.1985.tb01532.x. [DOI] [PubMed] [Google Scholar]
- 5.Zhang XJ, Bo-Liu J, Gui JP, Li M, Xiong OG, Wu H, et al. Characteristics of genetic epidemiology and genetic models for vitiligo. J Am Acad Dermatol. 2004;51:383–90. doi: 10.1016/j.jaad.2003.12.044. [DOI] [PubMed] [Google Scholar]
- 6.Lacovelli P, Sinagra JL, Vidolin AP, Marenda S, Capitanio B, Leone G, et al. Relevance of thyroiditis and other autoimmune diseases in children with vitiligo. Dermatology. 2005;210:26–30. doi: 10.1159/000081479. [DOI] [PubMed] [Google Scholar]
- 7.Shyria D, Mariette D'Souza, Devinder MT, Reddy KS, Zachariah B. High frequency of thyroid dysfunction in Indian patients with vitiligo. Indian J Dermatol. 2003;48:68–72. [Google Scholar]
- 8.Norlund JJ. The epidemiology and genetics of vitiligo. Clin Dermatol. 1997;15:875–8. doi: 10.1016/s0738-081x(97)00128-4. [DOI] [PubMed] [Google Scholar]
- 9.Majumder PP, Norlund JJ, Nath SK. Pattern of familial aggregation of vitiligo. Arch Dermatol. 1993;129:994–8. [PubMed] [Google Scholar]
- 10.Roth C, Scortea M, Stubbe P, Ruschenburg M, Zappel H, Becker W, et al. Autoimmune thyroiditis in childhood epidemiology and laboratory findings. Exp Clin Endo Diabetes. 1994;105:66–9. doi: 10.1055/s-0029-1211937. [DOI] [PubMed] [Google Scholar]
- 11.Parker F. Skin and Hormones Textbook of Endocrinology William RH WB Saunders Co Philadelphia. (6th Edition) 19811080:23. [Google Scholar]
- 12.Czarnoka B, Ruff J, Ferrand M, Carayon P, Lissitzky S. Purification of the human thyroid and its identification as the microsomal antigen involved in the human thyroid disease. FEBS Lett. 1985;190:147–52. doi: 10.1016/0014-5793(85)80446-4. [DOI] [PubMed] [Google Scholar]
- 13.Kumar V, Shankar V, Chaudhary S, Bhatia KK, Mehta LK, Arora N, et al. Radio-active iodine uptake in vitiligo. J Dermatol. 1990;17:41–3. doi: 10.1111/j.1346-8138.1990.tb01607.x. [DOI] [PubMed] [Google Scholar]
- 14.Kemp EH. Autoantibodies as diagnostic and predictive markers of vitiligo. Autoimmunity. 2004;37:287–90. doi: 10.1080/08916930410001710857. [DOI] [PubMed] [Google Scholar]
- 15.Alkhateeb A, Fain PR, Thody A, Bennett DC, Spritz RA. Epidemiology of vitiligo and associated autoimmune diseases in Causatian probands and their families. Pigment Cell Res. 2003;16:208–14. doi: 10.1034/j.1600-0749.2003.00032.x. [DOI] [PubMed] [Google Scholar]
- 16.Jameson JL, Anthony, Weetman P. Disorders of Thyroid Gland in Harrison's Principles of Internal Medicine Braunwald, Kasper, Fauci Mc Graw Hill. 15th Edition. New York: 2001. pp. 2060–82. [Google Scholar]
- 17.Ai J, Leonhardt MJ, Heymann RW. Autoimmune thyroid diseases Etiology pathogenesis and dermatological manifestations. Am Acad Dermatol. 2003;48:641–59. doi: 10.1067/mjd.2003.257. [DOI] [PubMed] [Google Scholar]
- 18.Hegedus L, Heidenheim M, Gervil M, Hjalgrim H, Hoier M. High frequency of thyroid dysfunction in patients with vitiligo. Acta Derm Venereol. 1994;74:120–3. doi: 10.2340/0001555574120123. [DOI] [PubMed] [Google Scholar]
- 19.Vanderpump MP, Tunbrige WM, French JM. The incidence of thyroid disorders in the community a twenty year follow-up of the Whickham survey. Clin Endocrinol (Oxf) 1995;43:55–68. doi: 10.1111/j.1365-2265.1995.tb01894.x. [DOI] [PubMed] [Google Scholar]
- 20.Maryam D, Mahtab MG, Javad B, Maryam A, Reza MR. Anti-thyroid peroxidase antibody and vitiligo. BMC Dermatol. 2007;6:3. doi: 10.1186/1471-5945-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


