Abstract
Background:
Prenatal exposure to persistent organic pollutants, including polychlorinated dibenzo-p-dioxins (PCDDs), polychlorinated dibenzofurans (PCDFs), dioxin-like polychlorinated biphenyls (DL-PCBs), and nondioxin-like PCBs (NDL-PCBs), has been hypothesized to have a detrimental impact on neurodevelopment. However, the association of prenatal exposure to a dioxin and PCB mixture with neurodevelopment remains largely inconclusive partly because these chemical levels are correlated.
Objectives:
We aimed to elucidate the association of in utero exposure to a mixture of dioxins and PCBs with neurodevelopment measured at 6 months of age by applying multipollutant methods.
Methods:
A total of 514 pregnant women were recruited between July 2002 and October 2005 in the Sapporo cohort, Hokkaido Study on Environment and Children’s Health. The concentrations of individual dioxin and PCB isomers were assessed in maternal peripheral blood during pregnancy. The mental and psychomotor development of the study participants’ infants was evaluated using the Bayley Scales of Infant Development-2nd Edition (n = 259). To determine both the joint and individual associations of prenatal exposure to a dioxin and PCB mixture with infant neurodevelopment, Bayesian kernel machine regression (BKMR) and quantile-based g-computation were employed.
Results:
Suggestive inverse associations were observed between in utero exposure to a dioxin and PCB mixture and infant psychomotor development in both the BKMR and quantile g-computation models. In contrast, we found no association of a dioxin and PCB mixture with mental development. When group-specific posterior inclusion probabilities were estimated, BKMR suggested prenatal exposure to mono-ortho PCBs as the more important contributing factors to early psychomotor development compared with the other dioxin or PCB groups. No evidence of nonlinear exposure-outcome relationships or interactions among the chemical mixtures was detected.
Conclusions:
Applying the two complementary statistical methods for chemical mixture analysis, we demonstrated limited evidence of inverse associations of prenatal exposure to dioxins and PCBs with infant psychomotor development.
Keywords: Child health, Prenatal exposure, Chemical mixtures, Persistent organic pollutants, Dioxins, Polychlorinated biphenyls, Polychlorinated dibenzo-p-dioxins, Polychlorinated dibenzofurans, Neurological development, Bayley scales of infant development, Bayesian kernel machine regression, Quantile g-computation, Birth cohort study
1. Introduction
Persistent organic pollutants (POPs) are a public health concern due to their resistance to environmental degradation, bioaccumulation, and long-range transport potential (Kodavanti, 2006). Types of POPs include dioxins, such as polychlorinated dibenzo-p-dioxins (PCDDs) and polychlorinated dibenzofurans (PCDFs), dioxin-like polychlorinated biphenyls (DL-PCBs), and nondioxin-like PCBs (NDL-PCBs) (Jones and de Voogt, 1999; Van den Berg et al., 2006). These organic chemicals are mostly of anthropogenic origins, such as industrial and agriculture sectors (Guo et al., 2019; Jones and de Voogt, 1999). Despite a consistent decline in concentrations since the 1970s after being banned (Dopico and Gomez, 2015; Hansen, 2002; van den Berg et al., 2017), dioxins and PCBs are still found in the environment (Bettinetti et al., 2016; Hung et al., 2016; Jamieson et al., 2017; Letcher et al., 2010).
One of the key potential health risks of dioxins and PCBs to humans includes detrimental impacts on neurological outcomes (Goodman et al., 2010; Ribas-Fito et al., 2001; Starling et al., 2015). Moreover, accumulative evidence indicates that many of these dioxins and PCBs may cross the placental barrier, suggesting prenatal exposure (Jeong et al., 2018; National Children’s Study Placenta et al., 2014; Porpora et al., 2013). Given that doses much lower than those affecting the adult brain could still cause perturbations of programmed neuronal development and network connectivity of the developing fetus, the impact of in utero exposure to dioxins or PCBs during this critical period of susceptibility could be even more substantial than later in life (Gore et al., 2014; Grandjean and Landrigan, 2006; Peterson et al., 1993).
Previous epidemiological studies have reported the associations of early-life exposure to dioxins and PCBs with neurodevelopmental outcomes mostly using traditional statistical methods such as multivariable regression approaches, typically adjusting for all chemicals in the same model (Ames et al., 2019; Caspersen et al., 2016a, 2016b; Daniels et al., 2003; Gladen et al., 1988; Ikeno et al., 2018; Koopman-Esseboom et al., 1996; Nakajima et al., 2006, 2017; Neugebauer et al., 2015; Nghiem et al., 2019; Nowack et al., 2015; Pham et al., 2015, 2019; Pham The et al., 2020; Tai et al., 2013; Tran et al., 2016; Walkowiak et al., 2001; Wilhelm et al., 2008; Winneke et al., 1998). For instance, Nakajima et al. examined the associations of in utero exposure to dioxins and PCBs with neurodevelopment at 6 months old in the Hokkaido Study Sapporo Cohort data, showing a stronger association in motor development compared with mental development(Nakajima et al., 2006). However, traditional approaches are limited due to several possible issues, including multicollinearity and model misspecification (Braun et al., 2016; Claus Henn et al., 2014; Gibson et al., 2019; Taylor et al., 2016). To address these issues, multiple advanced statistical methods, such as the Bayesian Kernel Machine Regression (BKMR) (Bobb et al., 2015) and quantile-based g-computation (Keil et al., 2020), have been introduced to the field. However, few previous studies have applied such newly developed methods to evaluate the effect of prenatal exposure to POPs on neurodevelopment (Lenters et al., 2019; Vuong et al., 2020).
Recognizing this research gap, the present study aimed to investigate overall and individual associations of prenatal exposure to multiple dioxins and PCBs with infant neurodevelopment using statistical mixture approaches—BKMR and quantile g-computation. BKMR is a semi-parametric statistical method that can be employed to estimate the overall mixture effect and individual chemical impact within a mixture on health outcomes. Without prior knowledge requirements, the BKMR is well suited to flexibly model a dose–response relationship between a chemical mixture and health outcomes, exploring potential nonlinearity and nonadditivity (Bobb et al., 2015, 2018). Quantile-based g-computation is a relatively new method that adapts the weighted quantile regression approach and enhances causal inferential aspects by g-computation. In comparison to BKMR, quantile g-computation can generate a single interpretable slope estimate for the overall effect (i.e., a single coefficient per unit increase in all chemical congeners within a mixture as a joint exposure for the change in the outcome) and is insensitive to outliers because of quantization. However, prior knowledge on nonlinearity and interactions must be known for accurate model specification, as in general for other traditional methods (Keil et al., 2020). These two methods take different statistical approaches to answer the similar research questions (i.e., joint and individual associations in a context of mixture), and we thus aimed to corroborate our findings by comparing the results from these two models. More specifically, our research objectives were to (i) estimate the joint associations of prenatal exposure to multiple dioxins and PCBs with infant neurodevelopment, which is accurately addressed by quantile-based g-computation, (ii) identify the most influential dioxin or PCB congener in relation to infant neurodevelopment, which is addressed by both methods, and (iii) explore nonlinear relationships or nonadditivity within a mixture in association with infant neurodevelopment, which is accurately addressed by BKMR. To our knowledge, our study is the first to assess the influence of in utero exposure to a dioxin and PCB mixture on neurodevelopment using both BKMR and quantile g-computation.
2. Materials and methods
2.1. Study population
The Sapporo Cohort, the Hokkaido Study on Environment and Children’s Health, was conducted between July 2002 and October 2005, with a total of 514 pregnant women who were recruited from the Sapporo Toho Hospital in Hokkaido, Japan. Of these, 10 pregnant women withdrew from the study before delivery, and among those who remained in the study, seven mothers delivered twins for a total of 511 children. All subjects were native Japanese residing in Sapporo and its surrounding areas. The study subjects completed a self-administered questionnaire after the second trimester during their last pregnancy, inquiring about their dietary habits, exposure to chemicals during daily life and at their work site, home environment, smoking habits, and medical histories of themselves and their partners. Additional information of the participating mothers and their children was obtained from medical records and face-to-face interviews. Details have been described elsewhere (Kishi et al., 2011, 2013, 2017, 2021). Of 511 infants, 271 (53.0%) completed the neurodevelopment test at 6 months of age. We further excluded any children who were not born singletons (n = 2) or any mothers who were missing the exposure (n = 10), leaving 259 mother-infant pairs for the final analytic sample (Figure S1). All study subjects’ written informed consent was obtained. The study was approved by the Institutional Ethical Board for Epidemiologic Studies of the Center for Environmental and Health Sciences, Hokkaido University Graduate School of Medicine.
2.2. Exposure measures
We collected a 40 ml venous blood sample from all participating mothers after the second trimester during their last pregnancy. Details regarding blood sampling and specimen storage have been previously described (Nakajima et al., 2006). All samples were stored at −80 °C until analysis. Dioxin and PCB concentrations in the maternal blood were measured using high-resolution gas chromatography/high-resolution mass spectrometry (HRGC/HRMS) equipped with a solvent-cut large--volume injection system (SGE Ltd, Victoria, Australia) at Fukuoka Institute of Health and Environmental Sciences. The gas chromatograph was an Agilent 6890 (Agilent Technologies Inc, Palo Alto, CA, USA) equipped with an AutoSpec-Ultima NT (Micromass Ltd, Manchester, UK). The dioxins and PCB levels were measured in each isomer (7 PCDDs, 10 PCDFs, 4 non-ortho DL-PCBs, 7 mono-ortho DL-PCBs, and 2 di-ortho NDL-PCBs). In cases where the values were below the detection limit, a value equal to half the detection limit was inputted for each isomer (Table S1) (Longnecker et al., 2003). Congeners with <50% of the detection rate were excluded from the analysis (1,2,3,7,8,9-HxCDF, 1,2,3,4,7,8,9-HpCDF, and 344′5-TCB(#81)) (Ikeno et al., 2018), leaving a total of 27 dioxin and PCB isomers for analysis.
In addition, the toxic equivalent (TEQ) was calculated by the sum of the concentration of each dioxin or PCB congener multiplied by its toxic equivalency factor (TEF) value (Van den Berg et al., 2006). The concept of TEF was developed as a risk assessment tool, recognizing that most of the biological effects of dioxins and PCBs are mediated through the aryl hydrocarbon receptor (AhR), a cytosolic receptor protein found in most vertebrate tissues. The relative effect potency for individual PCDD, PCDF, DL-PCBs, and NDL-PCB compounds was determined for producing toxic effects relative to 2,3,7,8-TCDD as a reference congener (Van den Berg et al., 1998).
2.3. Developmental measures
The Bayley Scales of Infant Development second edition (BSID-II) was used to measure the infants’ mental and psychomotor (motor) development at 6 months of age (Bayley, 1993). Because the BSID-II items are different by the assessment age, the evaluation period was strictly limited to 5 months 16 days to 6 months 15 days. The BSID-II consists of the Mental Developmental Index (MDI), which assesses cognitive, receptive, and expressive language development, and the Psychomotor Developmental Index (PDI), which evaluates fine and gross motor development (Johnson and Marlow, 2006). The standardized mean score for the MDI and PDI was 100 (standard deviation 15). While BSID-II is limited in that the test is time-consuming, representing mostly the U.S. population and requires appropriate training for administration (Balasundaram and Avulakunta, 2021; Gollenberg et al., 2010), it has been one of the most widely used development evaluation tools and is often regarded as “the gold standard in the assessment of early child development” (Walder D. J, 2012).
Each participating child was brought to the community center in Sapporo and tested in a quiet, private room in the presence of the parent (s). The developmental assessment was performed by the three occupational therapists who had clinical experience in developmental disabilities and were blinded to the children’s dioxin and PCB exposure levels. For all study participants, one examiner implemented the evaluation and scored each child’s performance, which was double-checked by two other examiners based on video recordings of each session. Because the BSID-II had not been standardized in Japan, the tool was translated in consultation with a manual for BSID (Kato T et al., 1987; Kato T et al., 1988).
2.4. Data analysis
We calculated and compared summary statistics of the baseline covariates for the final analytic sample versus those who were excluded from the analysis. Comparisons of continuous or categorical variables were performed using the Mann–Whitney U test or chi-square test, respectively. Geometric means and standard deviations were computed to show the distributions of the exposure concentrations and also compared between those included versus excluded from the analysis. Dioxin and PCB levels were natural-log transformed to approximate a normal distribution. To estimate the degree of correlation among dioxins and PCBs, Spearman rank correlation coefficients were calculated.
As a preliminary analysis, multiple linear regression models were used as a traditional approach to examine the association of prenatal exposure to dioxins and PCBs with infant neurodevelopment. First, each dioxin or PCB congener was evaluated separately in association with the BSID-II MDI and PDI for 6 months (single-congener model), followed by a single model with all dioxin and PCB congeners included together (multicongener model). All models were adjusted for maternal age at baseline, maternal education level (high school graduate or less, some college graduate or more), annual household income (<3 million yen, 3–5 million yen, or >5 million yen), working during pregnancy (yes or no), smoking during pregnancy (yes or no), and fish intake during pregnancy (inshore and deep-sea fish; 2 times per month or less, 1–2 times per week, or more than 2 times per week or more). Potential confounding factors were selected a priori based on subject knowledge (Nakajima et al., 2006), with possible mediators, such as gestational age, not included. The possibility of multicollinearity was checked using the variation inflation factor (VIF). To minimize the amount of multicollinearity in our models, we excluded exposures with high (>10) VIF, such as 1,2,3,6,7,8-HxCDF, 33′44′55′-HxCB(#169), 23′44′5-PeCB(#118), 233′44′-PeCB(#105), 23′44′55′-HxCB(#167), 233′44′5-HxCB(#156), 233′44′5′-HxCB(#157), 22′33′44′5-HpCB(#170), and 22′344′55′-HpCB(#180), from the multicongener model.
In the main analysis, we used BKMR to assess overall and individual associations of dioxins and PCBs as a mixture with neurodevelopment (Bobb et al., 2015). To determine the joint association, we subtracted the mean value of the outcome when the dioxin and PCB mixture concentrations were at the 25th percentile from the mean value of the outcome when the mixture concentrations were at the 75th percentile while holding the covariates constant. Posterior inclusion probabilities (PIPs) were estimated to identify the relative importance levels of each dioxin and PCB congener within a mixture in association with the neurodevelopmental outcomes (Bobb et al., 2018). We also performed the hierarchical variable selection function, which is recommended in the presence of high within-group correlation but relatively lower across-group correlation. Given the moderate-to-high correlation within each cluster observed in our data, and distinguished neurodevelopmental toxicity according to different chemical structures (Fischer et al., 1998; Lee and Yang, 2012; Niemi et al., 1998; Shain et al., 1991), we categorized the dioxin and PCB congeners into five groups (PCDDs, PCDFs, non-ortho DL-PCBs, mono-ortho DL-PCBs, and di-ortho NDL-PCBs). To identify which dioxin or PCB group is more important in association with neurodevelopment, group importance scores, or group hierarchical PIPs were estimated. Possible nonlinearity and interactions were also examined among the mixture members. The model convergence was checked by visually inspecting trace plots. Models were run for 10,000 iterations using the Markov chain Monte Carlo sampler.
To investigate whether we observed consistent findings across different multipollutant approaches, we employed quantile g-computation as a complementary method (Keil et al., 2020). First, the combined effect estimate was computed for prenatal exposure to dioxins and PCBs in relation to neurodevelopment using a one-quartile change in mixture, assuming Gaussian distribution. The mixture slope and overall model confidence bounds were iterated by 500 bootstraps. Each dioxin or PCB was assigned a positive or negative weight. Prior knowledge from the BKMR, including possible nonlinearity or nonadditivity, was fed to the quantile g-computation if necessary. Additional details on these two methods are provided in the Supplemental Digital Content 2.
To test the robustness of our main findings, the following sensitivity analyses were conducted. First, given that almost half of the study population was excluded from our analysis, the issue of selection bias was raised. The potential structure of selection bias is described in more detail in the Supplemental Digital Content 2. To account for potential selection bias, we combined the linear regression model with inverse probability of censoring weights (IPCW) (Hernan MA, 2020). IPCW is a statistical technique to assign study participants the inverse of the probability of being included in the analysis as a weight according to each individual’s exposure and covariate values (Oppenheimer et al., 2021). To construct a stabilized IPCW, we included the dioxin and PCB mixture components and the following covariates that were associated with censoring in this study: maternal age, education level, annual household income, smoking during pregnancy, and fish intake during pregnancy (deep-sea fish) (Table 1). The IPCW was estimated based on the information from a total of 425 out of the original 511 mother-infant pairs, who were not missing the covariates selected. To address under-estimation of standard error calculated by IPCW due to clustering, a robust standard error was estimated (Hernan et al., 2000; van der Wal and Geskus, 2011). Second, we assessed how much other endocrine disrupting chemicals could affect our findings by adding mono (2-ethylhexyl) phthalate (MEHP), perfluorooctanoic acid (PFOA), and perfluorooctane sulfonate (PFOS) in the mixture of interest as these toxicants could share the same sources as our exposures of interest (Koch et al., 2006; Xiao et al., 2015). Third, given the nonnegative integer outcomes in the current study, we applied the Poisson distribution instead of the Gaussian distribution in quantile g-computation. Fourth, we investigated whether different numbers of quantiles could impact the results from quantile g-computation. Fifth, this study included 27 dioxin and PCB congeners and covariates in the final analytic sample size of 259 participants. To examine whether similar results are found after reducing the number of variables in the model, we repeated our analysis using TEQ values. As a secondary analysis, BKMR and quantile g-computation were repeat-stratified by sex, given the sex-specific relationships of endocrine disruptors on neurodevelopment (Dong et al., 2019; Lenters et al., 2019; Nakajima et al., 2017; Rebuli and Patisaul, 2016). To facilitate comparability across the different statistical approaches, we included the same set of covariates in all models. All analyses were conducted using R (version 4.0.4; R Development Core Team) with the packages “bkmr,” “qgcomp,” and “ipw” for BKMR, quantile g-computation, and IPCW, respectively.
Table 1.
Characteristics of mothers and infants who were included in the final analytic sample (n = 259) and those who were not included (n = 252).
| The final analytic sample (n = 259) | The participants who were not included (n = 252) | P a | |
|---|---|---|---|
| Characteristic | No. (%) | ||
| Maternal characteristics | |||
| Age (years)b | 31.3 ± 4.9 | 30.2 ± 4.7 | 0.01 |
| Education level (years) | 0.03 | ||
| ≤12 | 101 (39.0) | 124 (49.2) | |
| >12 | 158 (61.0) | 128 (50.8) | |
| Economic status: annual income (yen) | 0.04 | ||
| < 3,000,000 | 43 (16.6) | 51 (20.5) | |
| 3,000,000–5,000,000 | 120 (46.3) | 132 (53.0) | |
| >5,000,000 | 96 (37.1) | 66 (26.5) | |
| Missing | 0 (0) | 3 (1.2) | |
| Worked during pregnancy | 30 (11.6) | 25 (9.9) | 0.64 |
| Smoked during pregnancy | 32 (12.4) | 56 (22.2) | <0.01 |
| Alcohol intake during pregnancy | 78 (30.1) | 77 (30.6) | 0.99 |
| Fish intake during pregnancy | |||
| Inshore fish | 0.50 | ||
| ≤2 times/month; rarely/never | 146 (56.4) | 137 (54.4) | |
| 1–2 times/week | 100 (38.6) | 96 (38.1) | |
| 3+ times/week | 13 (5.0) | 19 (7.5) | |
| Deep-sea fish | 0.05 | ||
| ≤2 times/month; rarely/never | 125 (48.3) | 109 (43.3) | |
| 1–2 times/week | 124 (47.9) | 120 (47.6) | |
| 3+ times/week | 10 (3.9) | 23 (9.1) | |
| Pregnancy-related complications | 31 (12.1) | 23 (9.2) | 0.36 |
| Missing | 0 (0) | 1 (0.4) | |
| Pre-pregnancy body mass index (kg/m2)b | 21.3 ± 3.2 | 21.17 ± 3.30 | 0.41 |
| Missing | 0 (0) | 1 (0.4) | |
| Child characteristics | |||
| Sex | 1.00 | ||
| Boy | 125 (48.3) | 121 (48.0) | |
| Girl | 134 (51.7) | 131 (52.0) | |
| First-born | 126 (48.8) | 116 (46.0) | 0.84 |
| Gestational age (days)b | 275.6 ± 9.6 | 273.3 ± 12.0 | 0.14 |
| Birth weight (g)b | 3084.5 ± 374.8 | 2965.1 ± 456.9 | 0.01 |
| Length (cm)b | 48.1 ± 1.8 | 47.6 ± 2.5 | 0.05 |
| Head circumference (cm)b | 33.3 ± 1.3 | 33.1 ± 1.5 | 0.23 |
| BSID-II mental index score: | 90.7 ± 5.9 | 86.2 ± 9.0 | 0.03 |
| MDIb | |||
| Missing | 0 (0) | 240 (95.2) | |
| BSID-II psychomotor index score: PDIb | 90.8 ± 10.8 | 86.5 ± 12.3 | 0.09 |
| Missing | 0 (0) | 240 (95.2) |
BSID-II: Bayley Scales of Infant Development second edition.
Comparison of continuous data between those included and excluded was performed using Mann–Whitney U test. Categorical variables were compared using χ2 test.
Mean±SD.
3. Results
The characteristics of the participating mothers and infants are shown in Table 1. Overall, more than half of the participating mothers received more than 12 years of education and ate fish once or more per week, with an average childbirth age of 31 years old. The majority of the mothers did not work or smoke during pregnancy. Among those who were included in the final analytic sample, approximately half of the participating infants were first-born (48.8%), and the average scores of the BSID-II mental developmental index (MDI) and psychomotor developmental index (PDI) were 90.7 ± 5.9 and 90.8 ± 10.8, respectively, both of which were lower than the mean standardized scores. The main reason for not being included in the analysis was missing information on the Bayley Scales of Infant Development 2nd Edition (BSID-II). Compared with those who were not included in the analysis, the final analytic participants were more likely to have higher socioeconomic status, such as education and household income, and less likely to smoke, eat fish, and use alcohol during pregnancy. However, the values were similar between the two groups for most variables.
The dioxin and PCB concentrations are shown as geometric means and standard deviations in both final analytic sample and the participants who were not included in this study (Table S1). Overall, maternal exposure to dioxins and PCBs during pregnancy was higher among those included. However, the difference was not significant for most dioxin or PCB congeners. Additional explanations comparing the concentrations of dioxins in this study with those from a previous similar study are provided in the Supplemental Digital Content 2.
The Spearman rank correlation coefficients within a dioxin and PCB mixture varied by clustering. The correlation was the highest among the mono-ortho DL-PCBs (ranging from 0.64 to 0.98), followed by PCDDs (ranging from 0.42 to 0.82) and non-ortho DL-PCBs (ranging from 0.41 to 0.71). The correlation among the PCDFs showed a wide range, from −0.04 to 0.84. The correlation coefficient between the two di-ortho NDL-PCBs was 0.95. While dioxins and PCBs were generally highly correlated among the same clusters, a few exceptions were detected. For instance, 2,3,4,7,8-PeCDF was also highly correlated with mono-ortho DL-PCBs (ranging from 0.66 to 0.79). Figure S2 displays how dioxins and PCBs cluster with each other, showing predominant clustering among the PCDD and PCB congeners compared with the weak to mild correlation across clusters. The correlation between the main mixture components (i.e. the 27 dioxins or PCBs) and other endocrine disrupting chemicals, i.e., mono (2-ethylhexyl) phthalate (MEHP), perfluorooctanoic acid (PFOA), and perfluorooctane sulfonate (PFOS), was weak, ranging between 0.2 and 0.4.
Figure S3 presents the associations of prenatal exposure to dioxins and PCBs with infant neurodevelopment using multiple linear regression models. In the adjusted single-congener models including one dioxin or PCB congener at a time, most of the mixture chemicals were negatively associated with neurodevelopment for both the BSID-II subscales, but particularly for the PDI (1,2,3,4,6,7,8-HpCDD, 1,2,3,7,8-PeCDF, 33′44′5-PenCB(#126), 23′44′5-PeCB(#118), 23′44′55′-HxCB(#167), 233′44′5-HxCB(#156), 233′44′5′-HxCB(#157), and 22′33′44′5-HpCB(#170)). In contrast, the results from the adjusted multicongener models showed that only a few congeners reached statistical significance (P < 0.05) with the MDI (2,3,7,8-TCDD, 1,2,3,7,8,9-HxCDD, and 1,2,3,4,6,7,8-HpCDD) but not with the PDI after excluding all dioxins and PCBs with high VIF (>10).
Fig. 1 shows the overall association of prenatal exposure to the mixture of dioxins and PCBs at each quantile with the expected differences in the BSID-II MDI and PDI scores by BKMR. The MDI score increased by 0.15 (95% credible interval [CI]: 1.13, 1.43), but PDI score decreased by 1.65 (95% CI: 4.59, 1.29) when concentrations of the dioxins and PCBs increased from 25th to 75th percentiles. However, the model yielded wide credible intervals, with a suggestive inverse relationship detected only for psychomotor development, not mental development.
Fig. 1.
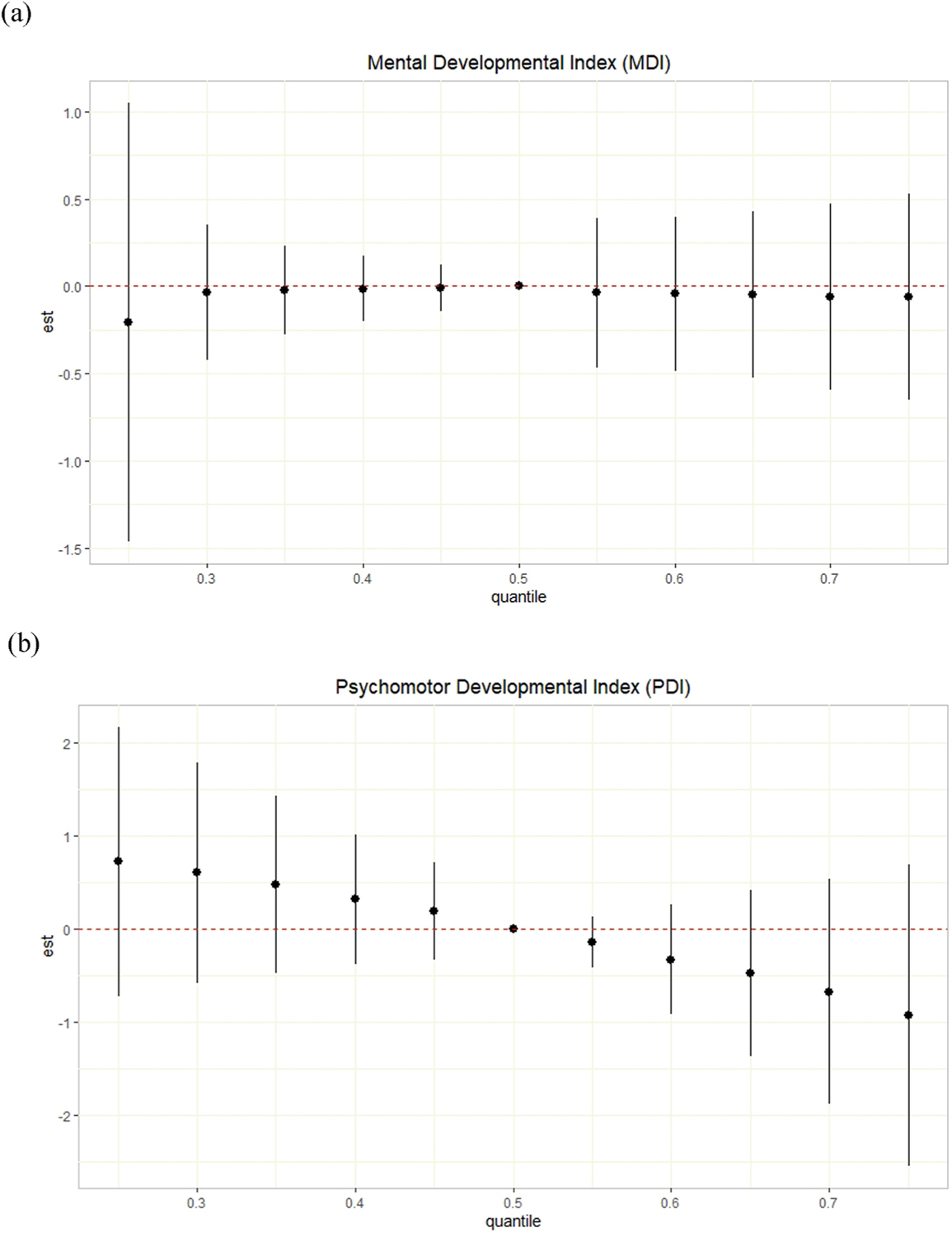
Joint effect (95% CI) of the log-transformed dioxin and PCB mixture on the Bayley Scales of Infant Development second edition (BSID-II) (a) Mental Developmental Index (MDI) and (b) Psychomotor Developmental Index (PDI) by Bayesian kernel machine regression (BKMR) model in the Hokkaido Study Small Cohort of the Hokkaido Study on Environment and Children’s Health (n = 259). The overall effect of prenatal exposure to dioxins and PCBs on infant neurodevelopment was plotted by comparing the impact when all dioxins and DL-PCBs are at a particular percentile with the effect when all of the congeners are at their medians. Models were adjusted for maternal age, education, household income, work status during pregnancy, smoking status during pregnancy, and fish intake during pregnancy (inshore and deep-sea fish).
The individual, group, and conditional PIPs from the BKMR model are summarized in Table 2. Overall, the estimated individual PIPs were small, with 33′44′-TCB(#77) showing the largest value (0.11) on infant mental development and 23′44′55′-HxCB(#167) on infant psychomotor development (0.22). In the grouped analysis, the PCDD and mono-ortho DL-PCB groups showed the largest groupPIPs for mental and psychomotor development (0.23 and 0.50), respectively, indicating their relatively large influence within the mixture. Of the clusters with the highest groupPIPs, 1,2,3,7,8-PeCDD and 23′44′55′-HxCB (#167) were assigned the largest conditional PIPs for mental and psychomotor development, respectively, which are consistent with the individual PIPs. These findings suggest that different primary dioxins or PCBs may influence mental and psychomotor development at 6 months of age. The univariate exposure-response curves from BKMR are depicted in Figure S4. In both the mental and psychomotor developmental domains, individual dioxins and PCBs showed little evidence of associations with neurodevelopment, as shown in the estimated individual PIPs. Furthermore, no evidence of a nonlinear relationship was observed. The BKMR model also explored potential interactions among the 27 dioxin and PCB congeners (Figure S5). The associations of each dioxin or PCB isomer with neurodevelopmental outcomes were mainly unchanged while holding the other dioxin and PCB congeners within the mixture at fixed percentiles, indicating no synergistic or multiplicative interactions.
Table 2.
Posterior inclusion probabilities (PIPs) into infant neurodevelopment measured by the Bayley Scales of Infant Development second edition (BSID-II) by Bayesian kernel machine regression (BKMR) model in the Hokkaido Study Small Cohort (n = 259).
| Dioxin and PCB congeners | Individual PIP | Group PIP | Conditional PIP |
|---|---|---|---|
| (a) Mental Developmental Index (MDI) | |||
| PCDD | |||
| 2,3,7,8-TCDD | 0.03 | 0.23 | 0.16 |
| 1,2,3,7,8-PeCDD | 0.06 | 0.55 | |
| 1,2,3,4,7,8-HxCDD | <0.01 | 0.04 | |
| 1,2,3,6,7,8-HxCDD | <0.01 | 0.01 | |
| 1,2,3,7,8,9-HxCDD | <0.01 | 0.02 | |
| 1,2,3,4,6,7,8-HpCDD | 0.08 | 0.16 | |
| OCDD | <0.01 | 0.06 | |
| PCDF | |||
| 2,3,7,8-TCDF | 0.02 | 0.16 | 0.05 |
| 1,2,3,7,8-PeCDF | <0.01 | 0.07 | |
| 2,3,4,7,8-PeCDF | 0.01 | 0.05 | |
| 0.0141,2,3,4,7,8-HxCDF | 0.02 | 0.01 | |
| 1,2,3,6,7,8-HxCDF | 0.03 | 0.60 | |
| 2,3,4,6,7,8-HxCDF | 0.05 | 0.21 | |
| 1,2,3,4,6,7,8-HpCDF | <0.01 | <0.01 | |
| OCDF | <0.01 | <0.01 | |
| Non-ortho DL-PCB | |||
| 33′44′-TCB(#77) | 0.11 | 0.19 | 0.91 |
| 33′44′5-PenCB(#126) | <0.01 | 0.05 | |
| 33′44′55′-HxCB(#169) | <0.01 | 0.04 | |
| Mono-ortho DL-PCB | |||
| 2′344′5-PeCB(#123) | 0.02 | 0.03 | 0.22 |
| 23′44′5-PeCB(#118) | <0.01 | 0.02 | |
| 2344′5-PeCB(#114) | <0.01 | 0.09 | |
| 233′44′-PeCB(#105) | <0.01 | 0.04 | |
| 23′44′55′-HxCB(#167) | <0.01 | 0.08 | |
| 233′44′5-HxCB(#156) | <0.01 | 0.14 | |
| 233′44′5′-HxCB(#157) | <0.01 | 0.41 | |
| Di-ortho DL-PCB | |||
| 22′33′44′5-HpCB(#170) | <0.01 | <0.01 | NA |
| 22′344′55′-HpCB(#180) | <0.01 | NA | |
| (b) Psychomotor Developmental Index (PDI) | |||
| PCDD | |||
| 2,3,7,8-TCDD | <0.01 | 0.20 | 0.06 |
| 1,2,3,7,8-PeCDD | <0.01 | 0.08 | |
| 1,2,3,4,7,8-HxCDD | 0.02 | 0.18 | |
| 1,2,3,6,7,8-HxCDD | <0.01 | 0.05 | |
| 1,2,3,7,8,9-HxCDD | 0.03 | 0.08 | |
| 1,2,3,4,6,7,8-HpCDD | 0.15 | 0.47 | |
| OCDD | 0.10 | 0.09 | |
| PCDF | |||
| 2,3,7,8-TCDF | <0.01 | 0.22 | 0.11 |
| 1,2,3,7,8-PeCDF | 0.08 | 0.53 | |
| 2,3,4,7,8-PeCDF | 0.01 | 0.07 | |
| 1,2,3,4,7,8-HxCDF | <0.01 | 0.06 | |
| 1,2,3,6,7,8-HxCDF | 0.02 | 0.14 | |
| 2,3,4,6,7,8-HxCDF | 0.05 | 0.09 | |
| 1,2,3,4,6,7,8-HpCDF | <0.01 | <0.01 | |
| OCDF | <0.01 | <0.01 | |
| Non-ortho DL-PCB | |||
| 33′44′-TCB(#77) | 0.01 | 0.18 | 0.25 |
| 33′44′5-PenCB(#126) | 0.02 | 0.57 | |
| 33′44′55′-HxCB(#169) | 0.01 | 0.18 | |
| Mono-ortho DL-PCB | |||
| 2′344′5-PeCB(#123) | 0.03 | 0.50 | 0.07 |
| 23′44′5-PeCB(#118) | 0.06 | 0.10 | |
| 2344′5-PeCB(#114) | 0.04 | 0.08 | |
| 233′44′-PeCB(#105) | 0.06 | 0.08 | |
| 23′44′55′-HxCB(#167) | 0.22 | 0.37 | |
| 233′44′5-HxCB(#156) | 0.01 | 0.12 | |
| 233′44′5′-HxCB(#157) | 0.07 | 0.18 | |
| Di-ortho DL-PCB | |||
| 22′33′44′5-HpCB(#170) | 0.02 | 0.03 | 0.70 |
| 22′344′55′-HpCB(#180) | <0.01 | 0.30 | |
Models were adjusted for maternal age, education, household income, work status during pregnancy, smoking status during pregnancy, and fish intake during pregnancy (inshore and deep-sea fish).
Quantile g-computation revealed a suggestive negative association of in utero exposure to dioxins and PCBs in relation to neurodevelopment, indicating its association with lower or delayed neurodevelopment at 6 months of age (Fig. 2). Given no evidence of nonlinearity or nonadditivity shown from BKMR, we did not include any polynomial or interaction terms of exposures in the model. Similar to the BKMR model, quantile g-computation also yielded wide confidence bands. Overall, as suggested in the BKMR models, the joint influence of dioxin and PCB exposure on neurodevelopment at 6 months of age was not statistically significant. Increasing one quartile in the dioxin and PCB mixture was associated with −2.61 (95% CI: −5.70, 0.49) and −4.71 (95% CI: −10.35, 0.94) changes in the BSID-II MDI and PDI scores, respectively (Table 3).
Fig. 2.
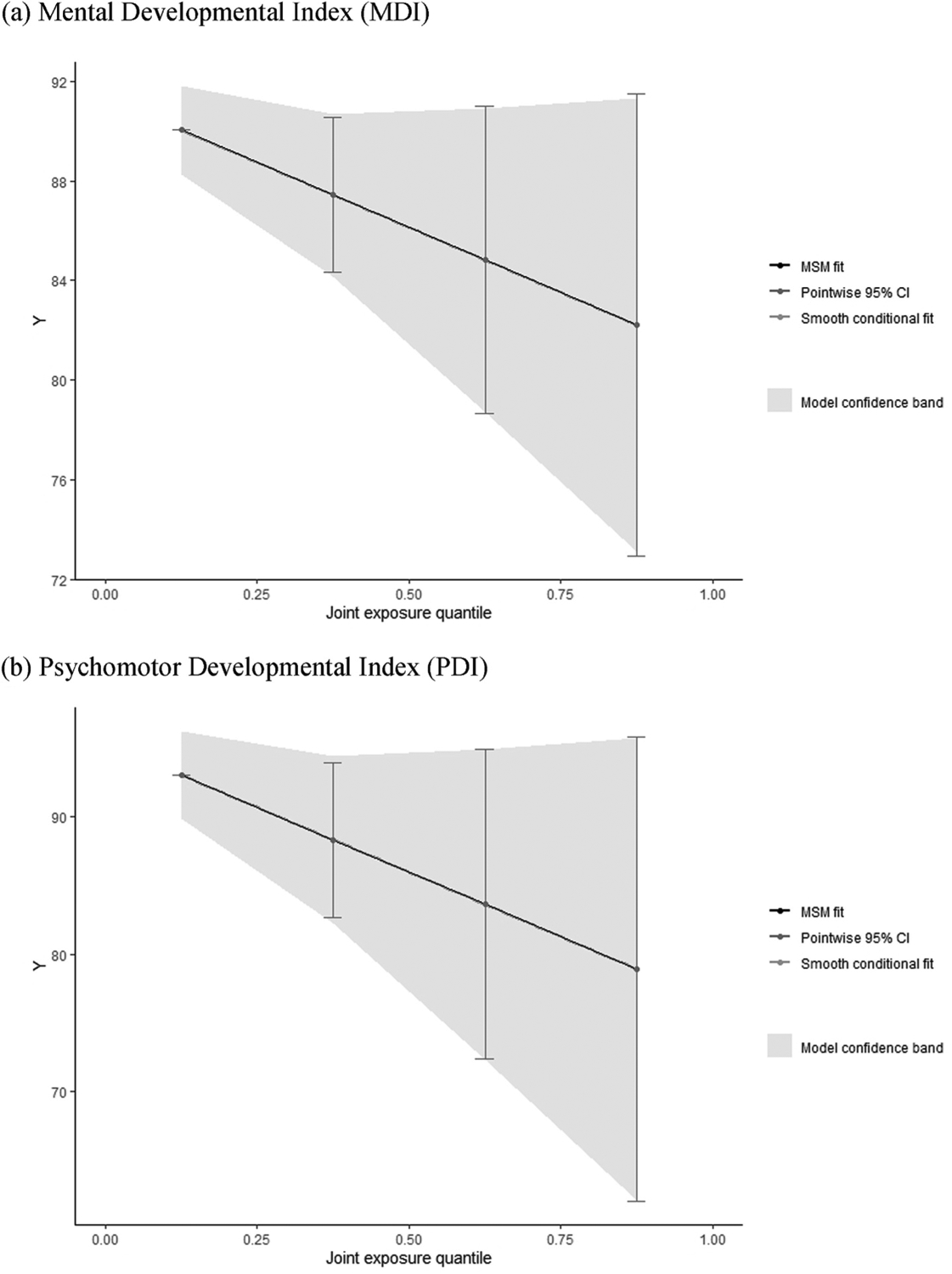
Smooth fit of the joint associations of the log-transformed dioxin and PCB mixture with infant neurodevelopment at 6 months using quantile g-computation for (a) MDI and (b) PDI in the Hokkaido Study Small Cohort of the Hokkaido Study on Environment and Children’s Health (n = 259). Models were adjusted for maternal age, education, household income, work status during pregnancy, smoking status during pregnancy, and fish intake during pregnancy (inshore and deep-sea fish).
Table 3.
Quantile-based g-computation estimates per quantile increase in the mixture of log-transformed dioxins and DL-PCBs with congener-specific weights in relation to infant neurodevelopment measured by the Bayley Scales of Infant Development second edition (BSID-II) (n = 259).
| (a) Mental Developmental Index (MDI) | |||
|---|---|---|---|
| Estimate (95% CI)a | P | Weights | |
| −2.61 (−5.70, 0.49) | 0.10 | ||
| Positive direction | Sum of positive coefficients = 7.45 | ||
| 1,2,3,7,8,9-HxCDD | 0.28 | ||
| 23′44′5-PeCB(#118) | 0.16 | ||
| 33′44′-TCB(#77) | 0.15 | ||
| 233′44′5-HxCB(#156) | 0.15 | ||
| 33′44′55′-HxCB(#169) | 0.11 | ||
| 2,3,4,7,8-PeCDF | 0.08 | ||
| 2′344′5-PeCB(#123) | 0.05 | ||
| 1,2,3,7,8-PeCDD | 0.01 | ||
| 1,2,3,6,7,8-HxCDF | 0.01 | ||
| Negative direction | Sum of negative coefficients = −10 | ||
| 2,3,7,8-TCDD | 0.30 | ||
| 1,2,3,4,7,8-HxCDD | 0.19 | ||
| 233′44′-PeCB(#105) | 0.12 | ||
| 33′44′5-PenCB(#126) | 0.06 | ||
| 1,2,3,4,7,8-HxCDF | 0.06 | ||
| 23′44′55′-HxCB(#167) | 0.05 | ||
| 233′44′5′-HxCB(#157) | 0.05 | ||
| OCDD | 0.04 | ||
| 22′344′55′-HpCB(#180) | 0.04 | ||
| 2344′5-PeCB(#114) | 0.03 | ||
| 2,3,7,8-TCDF | 0.03 | ||
| 1,2,3,6,7,8-HxCDD | 0.02 | ||
| 1,2,3,4,6,7,8-HpCDF | 0.01 | ||
| 1,2,3,4,6,7,8-HpCDD | 0.01 | ||
| 22′33′44′5-HpCB(#170) | <0.01 | ||
| 1,2,3,7,8-PeCDF | <0.01 | ||
| 2,3,4,6,7,8-HxCDF | <0.01 | ||
| OCDF | <0.01 | ||
| (b) Psychomotor Developmental Index (PDI) | |||
| Estimate (95% CI)a | P | Weights | |
| −4.71 (−10.35, 0.94) | 0.10 | ||
| Positive direction | Sum of positive coefficients = 7.94 | ||
| 2,3,4,7,8-PeCDF | 0.19 | ||
| 233′44′-PeCB(#105) | 0.18 | ||
| 1,2,3,7,8-PeCDD | 0.16 | ||
| 1,2,3,6,7,8-HxCDF | 0.16 | ||
| 2344′5-PeCB(#114) | 0.10 | ||
| 22′344′55′-HpCB(#180) | 0.09 | ||
| OCDD | 0.07 | ||
| 1,2,3,4,6,7,8-HpCDF | 0.04 | ||
| 33′44′-TCB(#77) | 0.02 | ||
| Negative direction | Sum of negative coefficients = −12.6 | ||
| 2,3,7,8-TCDD | 0.14 | ||
| 33′44′5-PenCB(#126) | 0.13 | ||
| 1,2,3,4,7,8-HxCDF | 0.12 | ||
| 233′44′5′-HxCB(#157) | 0.08 | ||
| 1,2,3,4,6,7,8-HpCDD | 0.08 | ||
| 22′33′44′5-HpCB(#170) | 0.07 | ||
| 233′44′5-HxCB(#156) | 0.07 | ||
| 1,2,3,4,7,8-HxCDD | 0.06 | ||
| 23′44′5-PeCB(#118) | 0.05 | ||
| 23′44′55′-HxCB(#167) | 0.05 | ||
| 33′44′55′-HxCB(#169) | 0.05 | ||
| 2,3,7,8-TCDF | 0.04 | ||
| 1,2,3,7,8,9-HxCDD | 0.02 | ||
| 2′344′5-PeCB(#123) | 0.02 | ||
| 1,2,3,6,7,8-HxCDD | 0.01 | ||
| 1,2,3,7,8-PeCDF | <0.01 | ||
| 2,3,4,6,7,8-HxCDF | <0.01 | ||
| OCDF | <0.01 | ||
Models were adjusted for maternal age, education, household income, work status during pregnancy, smoking status during pregnancy, and fish intake during pregnancy (inshore and deep-sea fish).
β can be interpreted as the change in the BSID-II score for one quartile increase in all dioxins and DL-PCBs based on 200 bootstrap iterations.
Using quantile g-computation, most dioxins and PCBs were assigned negative weights in association with neurodevelopment, which is consistent with the overall associations (Figure S6). For MDI, 1,2,3,7,8,9-HxCDD was assigned the largest positive weight (0.28), whereas 2,3,7,8-TCDD demonstrated the largest negative weight (0.30) (Table 3). For PDI, 2,3,4,7,8-PeCDF was the congener with the largest positive weight (0.19), whereas 2,3,7,8-TCDD had the greatest proportional negative contribution to the mixture effect (0.14).
In sensitivity analyses, results from traditional linear regression with IPCW (Figure S7) were similar to the models without IPCW (Figure S3). Further inclusion of MEHP, PFOA, and PFOS into the exposure mixture yielded significant, larger-in-magnitude effect estimates for psychomotor development (joint association estimate by quantile g-computation: −6.33 [95% CI: 12.32, −0.34]), but not for mental development (Figure S8 and Table S2). The analysis using Poisson distribution with quantile g-computation indicated similar results in both the mental and psychomotor domains (Figure S9 and Table S2). The association with mental development was attenuated when the number of quantiles was changed, whereas stronger, consistent evidence of suggestive inverse association was found in the psychomotor developmental domain (Figure S10 and Table S2). However, both associations were not significant. In the TEQ-based analysis, associations slightly shifted away from the null in the quantile g-computation, particularly for psychomotor development (joint association estimate: −1.40; 95% CI: −2.73, −0.08), while similar results were shown in BKMR models (Figure S11 and Table S2). In the sex-stratified analysis, the suggestive inverse association with mental development was detected among boys but not among girls (Figure S12). The association with psychomotor development was slightly attenuated in both infant sexes. However, as found in the main analysis, no association was significant in these sex-stratified analyses (Table S2).
4. Discussion
In this prospective birth cohort study, we found limited evidence to indicate that prenatal exposure to a mixture of dioxins and PCBs had a negative trending association with psychomotor, not mental, neurodevelopment assessed by the BSID-II at 6 months of age, applying both the BKMR and quantile g-computation models. The overall mixture effect was spread across the 27 mixture components with weak individual effect estimates. With the feature of estimating group hierarchical importance in the BKMR models, PCDDs and mono-ortho DL-PCBs appeared to have a larger influence on mental and psychomotor development, respectively, compared with the other groups. However, neither of these groups was a strong driver of the associations. No evidence of nonlinear relationships or interactions among the individual dioxin or PCB isomers was found. While wide credible or confidence intervals from BKMR and quantile g-computation, respectively, might suggest possibly different underlying true relationships, the suggestive inverse associations of in utero exposure to dioxins and PCBs with infant psychomotor neurodevelopment remained robust in a series of sensitivity analyses. Particularly, statistically significant associations were found for psychomotor development when we included other endocrine disruptors in the models or repeated the analysis with fewer variables based on the TEQ values.
Animal studies suggest the inverse association of perinatal exposure to dioxins and PCBs with neurodevelopment (Endo et al., 2012; Haijima et al., 2010; Hojo et al., 2002; Kakeyama et al., 2014; Markowski et al., 2001; Mitsui et al., 2006; Schantz et al., 1996). Most of the existing studies in humans typically utilized multiple linear regression models to examine this association, showing inconsistent results (Boucher et al., 2014; Forns et al., 2012; Gladen and Rogan, 1991; Gladen et al., 1988; Goodman et al., 2011; Koopman-Esseboom et al., 1996; Lai et al., 2001; Lynch et al., 2012; Park et al., 2010; Ribas-Fito et al., 2001; Rogan and Gladen, 1991; Ruel et al., 2019; Walkowiak et al., 2001; Wilhelm et al., 2008; Winneke et al., 1998). For instance, a 2006 Japanese study based on the same birth cohort found that one PCDD isomer, total PCDD concentration, and total PCDF concentration were associated with lower or delayed mental development, whereas two PCDD isomers and three PCDF isomers were inversely associated with psychomotor development (Nakajima et al., 2006). Findings from a 2017 Japanese study from the same birth cohort also reported sex-specific impact at 18 months old, with 10 dioxin or PCB isomers negatively related to psychomotor development among boys, but only one isomer showed a significant inverse relationship with psychomotor development among girls (Nakajima et al., 2017). In contrast, our study observed the groups of PCDDs and mono-ortho DL-PCBs as the most influential dioxin or PCB groups in association with mental and psychomotor development, respectively. Moreover, we found that the association of maternal exposure to dioxins and PCBs during pregnancy with neurodevelopment in the offspring was more pronounced among boys in mental development domain. These discrepancies between the current study and previous studies from the same birth cohort could be attributable to the different statistical approaches and outcome measurement period.
However, some prior studies have similarly found a stronger inverse association between exposure to dioxins and PCBs with psychomotor development, rather than with mental development (Forns et al., 2012; Gladen et al., 1988; Koopman-Esseboom et al., 1996; Lynch et al., 2012; Park et al., 2010; Rogan and Gladen, 1991). For instance, a study of Vietnamese toddlers observed significant inverse associations between infant daily dioxin intake via breast feeding and motor development at 4 months (Tai et al., 2013). It should also be noted that these studies found inverse associations at younger age (<6 months) but null findings at older ages (>18 months). No information on nonlinearity in exposure-response relationships or additivity within a mixture has been reported in relation to neurodevelopment.
We applied two complementary multipollutant analytic methods, BKMR and quantile g-computation. Despite the different statistical approaches to mixtures, our findings from the BKMR models were generally in agreement with the results from the quantile g-computation models. For instance, the suggestive inverse association of prenatal exposure to multiple dioxins or PCBs with psychomotor development was consistently shown in both BKMR and quantile g-computation models. The discrepancy in the rank of the most influential dioxin or PCB component between BKMR and quantile g-computation could be attributable to the different way these techniques deal with the presence of highly correlated exposures, and small individual effects. First, in the presence of highly correlated chemicals within a mixture, BKMR is likely to exclude some covariates from the correlated clusters, while quantile g-computation is still subject to multicollinearity and might provide relevant weights in different directions to correlated exposures. In the current study, a moderate-to-high correlation was generally found within each cluster, among PCDDs, PCDFs, or PCBs in particular, but was also detected across clusters. Second, the deviation from the joint and individual trends of associations could be observed when the effect estimate is close to 0 (Lebeaux et al., 2020). In our study, the individual posterior inclusion probabilities from BKMR and weights from quantile g-computation were generally low, indicating the small, clinically negligible associations of individual dioxins and PCBs with infant neurodevelopment. However, even if individual chemicals might have small, clinically negligible effects, the joint effect could be significant and clinically relevant (Silva et al., 2002).
A significant inverse joint association was shown between the dioxin and PCB mixture and infant psychomotor development when the TEQ values were used. This finding could be due to the increased statistical power with the decreased number of exposures in the model. Another possibility suggests that the TEQ values are better indicators of the biological mechanisms of dioxins and PCBs. The common cellular mechanism of dioxin-like compounds is the action of the aryl hydrocarbon receptor (AhR) (Budinsky et al., 2014). Based on the potencies of dioxin-like compounds to activate various AhR-dependent endpoints, a toxic equivalence factor (TEF) approach for the risk assessment of mixtures was established, with the most toxic component (2,3,7, 8-TCDD, TEF = 1) as a reference (Van den Berg et al., 1998). The TEQ is then computed as the sum of the concentrations of individual dioxin or PCB isomers multiplied by their TEFs (Safe, 1997; Van den Berg et al., 2006). Given the link between TEQ values and the AhR mechanism, the significant inverse association detected for infant psychomotor development could be reassuring the biological and toxicological pathways of dioxins and PCBs to neurodevelopment through the AhR.
There are several limitations of this study. First, given that log-transformed dioxins and PCBs were used for a better model fit, we cannot provide reference dose or toxicity equivalent of the exposures in relation to the outcomes of interest. Second, other distribution assumptions were only available in quantile g-computation, not in BKMR. However, the results utilizing the Poisson distribution were consistent with the results estimated with the Gaussian distribution. Third, the present study was based on local Japanese pregnant mothers and infant pairs, of which the findings could not be generalizable to other populations. However, the underlying biological mechanisms linking prenatal exposure to the dioxin and PCB mixture with neurodevelopment are unlikely to differ in other populations. Fourth, nonlinearity or interactions might have been nondetectable due to the relatively small sample size. Fifth, selection bias was likely given that almost half of the study population was not included in the analysis. However, the results with IPCW to account for selection bias materially unchanged from the models without IPCW, suggesting selection bias could not be a major concern in this study. Nevertheless, the interpretation is limited as inverse probability weighting analysis does not guarantee protection from unknown or unmeasured confounding factors (Nohr and Liew, 2018).
Several strengths are worth being noted. To the best of our knowledge, the present study is the first to implement mixture methods to estimate both the overall and individual effects of correlated dioxin and PCB exposure on infant neurodevelopment. Despite their different modeling techniques, both BKMR and quantile g-computation were largely similar to each other, corroborating our findings. Second, the prospective nature of the current study allowed us to determine the temporality of prenatal exposure to the dioxin and PCB mixture in relation to neurodevelopment. Third, we used the direct assessment of prenatal exposure to dioxins and PCBs rather than a proxy such as breastmilk or fish consumption, with good quality control of laboratory evaluation. Fourth, both the exposures and outcomes were measured during a strict time window, which could inform future public health intervention or prevention programs and facilitate comparisons with other studies. Fifth, we performed extensive sensitivity analyses to ensure the robustness of the results.
5. Conclusion
Using two complementary mixture analysis methods, BKMR and quantile g-computation, we found suggestive inverse associations of prenatal exposure to multiple dioxins and PCBs with psychomotor neurodevelopment at 6 months of age in a prospective birth cohort study. Our study contributes to the growing literature on the associations between multiple chemicals and neurodevelopmental outcomes. Further research is warranted to replicate our findings in other study settings.
Supplementary Material
Acknowledgements:
We would like to thank the participants, staff, and collaborators of the Hokkaido Study on Environment and Children’s Health for their substantial contributions. Special thanks also to Dr. Jumboku Kajiwara and Dr. Takashi Todaka for their dioxin and PCB measurements.
Role of the funder/sponsor
This study was supported by a Grant-in-Aid for Health Scientific Research from the Japan Ministry of Health, Labour and Welfare (grant nos. 200301311A, 201624002Band 17932352); and a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (grant nos. 16,209,022, 25,253,050, 16H02645and 19H01071). Dr. Yim was supported by The Tao & Cheng Fund and the O’Friel Fund for graduate summer research grant from the Harvard University Asia Center for her visit to Hokkaido University, Japan. Dr. Kioumourtzoglou was supported by National Institute of Environmental Health Sciences (NIEHS) P30 ES009089, P30 ES000002, and R01 ES028805. The funders played no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; the preparation, review, and approval of the manuscript; or the decision to submit the manuscript for publication.
Footnotes
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envres.2022.112757.
References
- Ames J, et al. , 2019. Prenatal dioxin exposure and neuropsychological functioning in the seveso second generation health study. Int. J. Hyg Environ. Health 222, 425–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasundaram P, Avulakunta ID, 2021. Bayley Scales Of Infant and Toddler Development. StatPearls, Treasure Island (FL). [PubMed] [Google Scholar]
- Bayley N, 1993. Manual for the Bayley Scales of Infant Development, second ed. Psychological Corporation, New York. [Google Scholar]
- Bettinetti R, et al. , 2016. Recent DDT and PCB contamination in the sediment and biota of the como bay (lake como, Italy). Sci. Total Environ 542, 404–410. [DOI] [PubMed] [Google Scholar]
- Bobb JF, et al. , 2018. Statistical software for analyzing the health effects of multiple concurrent exposures via Bayesian kernel machine regression. Environ. Health 17, 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobb JF, et al. , 2015. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics 16, 493–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher O, et al. , 2014. Domain-specific effects of prenatal exposure to PCBs, mercury, and lead on infant cognition: results from the Environmental Contaminants and Child Development Study in Nunavik. Environ. Health Perspect 122, 310–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, et al. , 2016. What can epidemiological studies tell us about the impact of chemical mixtures on human health? Environ. Health Perspect 124, A6–A9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budinsky RA, et al. , 2014. Mode of action and dose-response framework analysis for receptor-mediated toxicity: the aryl hydrocarbon receptor as a case study. Crit. Rev. Toxicol 44, 83–119. [DOI] [PubMed] [Google Scholar]
- Caspersen IH, et al. , 2016a. The influence of maternal dietary exposure to dioxins and PCBs during pregnancy on ADHD symptoms and cognitive functions in Norwegian preschool children. Environ. Int 94, 649–660. [DOI] [PubMed] [Google Scholar]
- Caspersen IH, et al. , 2016b. Maternal dietary exposure to dioxins and polychlorinated biphenyls (PCBs) is associated with language delay in 3 year old Norwegian children. Environ. Int 91, 180–187. [DOI] [PubMed] [Google Scholar]
- Claus Henn B, et al. , 2014. Chemical mixtures and children’s health. Curr. Opin. Pediatr 26, 223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels JL, et al. , 2003. Prenatal exposure to low-level polychlorinated biphenyls in relation to mental and motor development at 8 months. Am. J. Epidemiol 157, 485–492. [DOI] [PubMed] [Google Scholar]
- Dong R, et al. , 2019. Lactational exposure to phthalates impaired the neurodevelopmental function of infants at 9 months in a pilot prospective study. Chemosphere 226, 351–359. [DOI] [PubMed] [Google Scholar]
- Dopico M, Gomez A, 2015. Review of the current state and main sources of dioxins around the world. J. Air Waste Manag. Assoc 65, 1033–1049. [DOI] [PubMed] [Google Scholar]
- Endo T, et al. , 2012. Executive function deficits and social-behavioral abnormality in mice exposed to a low dose of dioxin in utero and via lactation. PLoS One 7, e50741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer LJ, et al. , 1998. Symposium overview: toxicity of non-coplanar PCBs. Toxicol. Sci 41, 49–61. [DOI] [PubMed] [Google Scholar]
- Forns J, et al. , 2012. Prenatal exposure to organochlorine compounds and neuropsychological development up to two years of life. Environ. Int 45, 72–77. [DOI] [PubMed] [Google Scholar]
- Gibson EA, et al. , 2019. Complex mixtures, complex analyses: an emphasis on interpretable results. Curr Environ Health Rep 6, 53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladen BC, Rogan WJ, 1991. Effects of perinatal polychlorinated biphenyls and dichlorodiphenyl dichloroethene on later development. J. Pediatr 119, 58–63. [DOI] [PubMed] [Google Scholar]
- Gladen BC, et al. , 1988. Development after exposure to polychlorinated biphenyls and dichlorodiphenyl dichloroethene transplacentally and through human milk. J. Pediatr 113, 991–995. [DOI] [PubMed] [Google Scholar]
- Gollenberg AL, et al. , 2010. Concurrent validity of the parent-completed ages and stages questionnaires, 2nd ed. With the Bayley Scales of infant development II in a low-risk sample. Child Care Health Dev. 36, 485–490. [DOI] [PubMed] [Google Scholar]
- Goodman M, et al. , 2010. Using systematic reviews and meta-analyses to support regulatory decision making for neurotoxicants: lessons learned from a case study of PCBs. Environ. Health Perspect 118, 727–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman M, et al. , 2011. Using systematic reviews and meta-analyses to support regulatory decision making for neurotoxicants: lessons learned from a case study of PCBs. Cîencia Saúde Coletiva 16, 3207–3220. [DOI] [PubMed] [Google Scholar]
- Gore AC, et al. , 2014. Implications of prenatal steroid perturbations for neurodevelopment, behavior, and autism. Endocr. Rev 35, 961–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P, Landrigan PJ, 2006. Developmental neurotoxicity of industrial chemicals. Lancet 368, 2167–2178. [DOI] [PubMed] [Google Scholar]
- Guo W, et al. , 2019. Persistent organic pollutants in food: contamination sources, health effects and detection methods. Int. J. Environ. Res. Publ. Health 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haijima A, et al. , 2010. In utero and lactational exposure to low doses of chlorinated and brominated dioxins induces deficits in the fear memory of male mice. Neurotoxicology 31, 385–390. [DOI] [PubMed] [Google Scholar]
- Hansen LG, 2002. Persistent organic pollutants in food supplies. J. Epidemiol. Community Health 56, 820–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernan MA, et al. , 2000. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology 11, 561–570. [DOI] [PubMed] [Google Scholar]
- Hernan Ma RJ, 2020. Causal Inference: What If. Chapman & Hall/CRC, Boca Raton. [Google Scholar]
- Hojo R, et al. , 2002. Sexually dimorphic behavioral responses to prenatal dioxin exposure. Environ. Health Perspect 110, 247–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung H, et al. , 2016. Temporal trends of persistent organic pollutants (POPs) in arctic air: 20 years of monitoring under the arctic monitoring and assessment programme (AMAP). Environ. Pollut 217, 52–61. [DOI] [PubMed] [Google Scholar]
- Ikeno T, et al. , 2018. Effects of low-level prenatal exposure to dioxins on cognitive development in Japanese children at 42 months. Sci. Total Environ 618, 1423–1430. [DOI] [PubMed] [Google Scholar]
- Jamieson AJ, et al. , 2017. Bioaccumulation of persistent organic pollutants in the deepest ocean fauna. Nat Ecol Evol 1, 51. [DOI] [PubMed] [Google Scholar]
- Jeong Y, et al. , 2018. Placental transfer of persistent organic pollutants and feasibility using the placenta as a non-invasive biomonitoring matrix. Sci. Total Environ 612, 1498–1505. [DOI] [PubMed] [Google Scholar]
- Johnson S, Marlow N, 2006. Developmental screen or developmental testing? Early Hum. Dev 82, 173–183. [DOI] [PubMed] [Google Scholar]
- Jones KC, de Voogt P, 1999. Persistent organic pollutants (POPs): state of the science. Environ. Pollut 100, 209–221. [DOI] [PubMed] [Google Scholar]
- Kakeyama M, et al. , 2014. Disruption of paired-associate learning in rat offspring perinatally exposed to dioxins. Arch. Toxicol 88, 789–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato TAT, Maruno A, Yukawa R, Kato N, Kawasaki C, et al. , 1987. Computer analysis on the development of infants. Rep Stud Nippon Aiiku 22, 51–74. [Google Scholar]
- Kato TTE, Amino T, Maruno A, Hagiwara H, Yukawa R, et al. , 1988. Follow-up study of healthy infant from neonatal age. Rep Stud Nippon Aiiku 23, 25–46. [Google Scholar]
- Keil AP, et al. , 2020. A quantile-based g-computation approach to addressing the effects of exposure mixtures. Environ. Health Perspect 128, 47004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi R, et al. , 2017. The Hokkaido birth cohort study on environment and children’s health: cohort profile-updated 2017. Environ. Health Prev. Med 22, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi R, et al. , 2021. Hokkaido birth cohort study on environment and children’s health: cohort profile 2021. Environ. Health Prev. Med 26, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi R, et al. , 2013. Ten years of progress in the Hokkaido birth cohort study on environment and children’s health: cohort profile–updated 2013. Environ. Health Prev. Med 18, 429–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi R, et al. , 2011. Cohort profile: the Hokkaido study on environment and children’s health in Japan. Int. J. Epidemiol 40, 611–618. [DOI] [PubMed] [Google Scholar]
- Koch HM, et al. , 2006. Di(2-ethylhexyl)phthalate (DEHP): human metabolism and internal exposure- an update and latest results. Int. J. Androl 29, 155–165 discussion 181–5. [DOI] [PubMed] [Google Scholar]
- Kodavanti PR, 2006. Neurotoxicity of persistent organic pollutants: possible mode(s) of action and further considerations. Dose Response 3, 273–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopman-Esseboom C, et al. , 1996. Effects of polychlorinated biphenyl/dioxin exposure and feeding type on infants’ mental and psychomotor development. Pediatrics 97, 700–706. [PubMed] [Google Scholar]
- Lai TJ, et al. , 2001. Effect of prenatal exposure to polychlorinated biphenyls on cognitive development in children: a longitudinal study in Taiwan. Br. J. Psychiatr. Suppl 40, s49–52. [DOI] [PubMed] [Google Scholar]
- Lebeaux RM, et al. , 2020. Maternal serum perfluoroalkyl substance mixtures and thyroid hormone concentrations in maternal and cord sera: the HOME Study. Environ. Res 185, 109395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, Yang JH, 2012. Chlorination of ortho-position on polychlorinated biphenyls increases protein kinase C activity in neuronal cells. Toxicol Res 28, 107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenters V, et al. , 2019. Early-life exposure to persistent organic pollutants (OCPs, PBDEs, PCBs, PFASs) and attention-deficit/hyperactivity disorder: a multi-pollutant analysis of a Norwegian birth cohort. Environ. Int 125, 33–42. [DOI] [PubMed] [Google Scholar]
- Letcher RJ, et al. , 2010. Exposure and effects assessment of persistent organohalogen contaminants in arctic wildlife and fish. Sci. Total Environ 408, 2995–3043. [DOI] [PubMed] [Google Scholar]
- Longnecker MP, et al. , 2003. Comparison of polychlorinated biphenyl levels across studies of human neurodevelopment. Environ. Health Perspect 111, 65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch CD, et al. , 2012. The effect of prenatal and postnatal exposure to polychlorinated biphenyls and child neurodevelopment at age twenty four months. Reprod. Toxicol 34, 451–456. [DOI] [PubMed] [Google Scholar]
- Markowski VP, et al. , 2001. Altered operant responding for motor reinforcement and the determination of benchmark doses following perinatal exposure to low-level 2,3,7,8-tetrachlorodibenzo-p-dioxin. Environ. Health Perspect 109, 621–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsui T, et al. , 2006. Perinatal exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin suppresses contextual fear conditioning-accompanied activation of cyclic AMP response element-binding protein in the hippocampal CA1 region of male rats. Neurosci. Lett 398, 206–210. [DOI] [PubMed] [Google Scholar]
- Nakajima S, et al. , 2006. Effects of prenatal exposure to polychlorinated biphenyls and dioxins on mental and motor development in Japanese children at 6 months of age. Environ. Health Perspect 114, 773–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima S, et al. , 2017. Sex-specific differences in effect of prenatal exposure to dioxin-like compounds on neurodevelopment in Japanese children: Sapporo cohort study. Environ. Res 159, 222–231. [DOI] [PubMed] [Google Scholar]
- National Children’s Study Placenta C, et al. , 2014. Selected persistent organic pollutants in human placental tissue from the United States. Chemosphere 106, 20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer J, et al. , 2015. The influence of low level pre- and perinatal exposure to PCDD/Fs, PCBs, and lead on attention performance and attention-related behavior among German school-aged children: results from the Duisburg Birth Cohort Study. Int. J. Hyg Environ. Health 218, 153–162. [DOI] [PubMed] [Google Scholar]
- Nghiem GT, et al. , 2019. Adverse effects of maternal dioxin exposure on fetal brain development before birth assessed by neonatal electroencephalography (EEG) leading to poor neurodevelopment; a 2-year follow-up study. Sci. Total Environ 667, 718–729. [DOI] [PubMed] [Google Scholar]
- Niemi WD, et al. , 1998. PCBs reduce long-term potentiation in the CA1 region of rat hippocampus. Exp. Neurol 151, 26–34. [DOI] [PubMed] [Google Scholar]
- Nohr EA, Liew Z, 2018. How to investigate and adjust for selection bias in cohort studies. Acta Obstet. Gynecol. Scand 97, 407–416. [DOI] [PubMed] [Google Scholar]
- Nowack N, et al. , 2015. Influence of low-level prenatal exposure to PCDD/fs and PCBs on empathizing, systemizing and autistic traits: results from the duisburg birth cohort study. PLoS One 10, e0129906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer AV, et al. , 2021. Prenatal exposure to chemical mixtures and inhibition among adolescents. Toxics 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HY, et al. , 2010. Neurodevelopmental toxicity of prenatal polychlorinated biphenyls (PCBs) by chemical structure and activity: a birth cohort study. Environ. Health 9, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson RE, et al. , 1993. Developmental and reproductive toxicity of dioxins and related compounds: cross-species comparisons. Crit. Rev. Toxicol 23, 283–335. [DOI] [PubMed] [Google Scholar]
- Pham NT, et al. , 2019. Perinatal dioxin exposure and neurodevelopment of 2-year-old Vietnamese children in the most contaminated area from Agent Orange in Vietnam. Sci. Total Environ 678, 217–226. [DOI] [PubMed] [Google Scholar]
- Pham The T, et al. , 2020. Effects of perinatal dioxin exposure on learning abilities of 8-year-old children in Vietnam. Int. J. Hyg Environ. Health 223, 132–141. [DOI] [PubMed] [Google Scholar]
- Pham TT, et al. , 2015. Perinatal dioxin exposure and the neurodevelopment of Vietnamese toddlers at 1 year of age. Sci. Total Environ 536, 575–581. [DOI] [PubMed] [Google Scholar]
- Porpora MG, et al. , 2013. Placental transfer of persistent organic pollutants: a preliminary study on mother-newborn pairs. Int. J. Environ. Res. Publ. Health 10, 699–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebuli ME, Patisaul HB, 2016. Assessment of sex specific endocrine disrupting effects in the prenatal and pre-pubertal rodent brain. J. Steroid Biochem. Mol. Biol 160, 148–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas-Fito N, et al. , 2001. Polychlorinated biphenyls (PCBs) and neurological development in children: a systematic review. J. Epidemiol. Community Health 55, 537–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogan WJ, Gladen BC, 1991. PCBs, DDE, and child development at 18 and 24 months. Ann. Epidemiol 1, 407–413. [DOI] [PubMed] [Google Scholar]
- Ruel MVM, et al. , 2019. Prenatal exposure to organohalogen compounds and children’s mental and motor development at 18 and 30 months of age. Neurotoxicology 72, 6–14. [DOI] [PubMed] [Google Scholar]
- Safe S, 1997. Limitations of the toxic equivalency factor approach for risk assessment of TCDD and related compounds. Teratog. Carcinog. Mutagen 17, 285–304. [PubMed] [Google Scholar]
- Schantz SL, et al. , 1996. Effects of gestational and lactational exposure to TCDD or coplanar PCBs on spatial learning. Neurotoxicol. Teratol 18, 305–313. [DOI] [PubMed] [Google Scholar]
- Shain W, et al. , 1991. Neurotoxicity of polychlorinated biphenyls: structure-activity relationship of individual congeners. Toxicol. Appl. Pharmacol 111, 33–42. [DOI] [PubMed] [Google Scholar]
- Silva E, et al. , 2002. Something from “nothing”–eight weak estrogenic chemicals combined at concentrations below NOECs produce significant mixture effects. Environ. Sci. Technol 36, 1751–1756. [DOI] [PubMed] [Google Scholar]
- Starling P, et al. , 2015. Fish intake during pregnancy and foetal neurodevelopment–a systematic review of the evidence. Nutrients 7, 2001–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai PT, et al. , 2013. Dioxin exposure in breast milk and infant neurodevelopment in Vietnam. Occup. Environ. Med 70, 656–662. [DOI] [PubMed] [Google Scholar]
- Taylor KW, et al. , 2016. Statistical approaches for assessing health effects of environmental chemical mixtures in epidemiology: lessons from an innovative workshop. Environ. Health Perspect 124, A227–A229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran NN, et al. , 2016. Impacts of perinatal dioxin exposure on motor coordination and higher cognitive development in Vietnamese preschool children: a five-year follow-up. PLoS One 11, e0147655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Berg M, et al. , 1998. Toxic equivalency factors (TEFs) for PCBs, PCDDs, PCDFs for humans and wildlife. Environ. Health Perspect 106, 775–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Berg M, et al. , 2006. The 2005 World Health Organization reevaluation of human and Mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol. Sci 93, 223–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg M, et al. , 2017. WHO/UNEP global surveys of PCDDs, PCDFs, PCBs and DDTs in human milk and benefit-risk evaluation of breastfeeding. Arch. Toxicol 91, 83–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Wal WM, Geskus RB, 2011. Ipw: an R package for inverse probability weighting. J. Stat. Software 43, 1–23. [Google Scholar]
- Vuong AM, et al. , 2020. Prenatal exposure to a mixture of persistent organic pollutants (POPs) and child reading skills at school age. Int. J. Hyg Environ. Health 228, 113527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walder DJ, S JC, Pulsifer Margaret B., 2012. Neurodevelopmental assessment. In: Mowder FR Barbara A, Yasik Anastasia E. (Eds.), Evidence-Based Practice in Infant and Early Childhood Psychology. [Google Scholar]
- Walkowiak J, et al. , 2001. Environmental exposure to polychlorinated biphenyls and quality of the home environment: effects on psychodevelopment in early childhood. Lancet 358, 1602–1607. [DOI] [PubMed] [Google Scholar]
- Wilhelm M, et al. , 2008. The Duisburg birth cohort study: influence of the prenatal exposure to PCDD/Fs and dioxin-like PCBs on thyroid hormone status in newborns and neurodevelopment of infants until the age of 24 months. Mutat. Res 659, 83–92. [DOI] [PubMed] [Google Scholar]
- Winneke G, et al. , 1998. Developmental neurotoxicity of polychlorinated biphenyls (PCBS): cognitive and psychomotor functions in 7-month old children. Toxicol. Lett 102–103, 423–428. [DOI] [PubMed] [Google Scholar]
- Xiao F, et al. , 2015. Perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in soils and groundwater of a U.S. metropolitan area: migration and implications for human exposure. Water Res. 72, 64–74. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


