Abstract
A comprehensive structure-activity relationship study on antibody Fc-glycosylation has been performed using chimeric anti-SSEA4 antibody chMC813-70 as model. The α-2,6 sialylated biantennary complex type glycan was identified as the optimal Fc-glycan with significant enhancement on antibody effector functions, including binding to different Fc receptors, and ADCC.
GRAPHICAL ABSTRACT

Monoclonal antibodies (mAbs) represent a growing class of drugs for the treatment of life-threatening conditions such as cancers, inflammation, infectious and various autoimmune diseases.1–3 Therapeutic mAbs are generally target specific, highly potent, and stable in circulation, and in certain instances, are able to recruit immune cells to the target site to exhibit their effector functions, such as antibody-dependent cellular cytotoxicity (ADCC)4, 5 and complement-dependent cytotoxicity (CDC).6 ADCC is a mechanism of the adaptive immune system to ward off pathogenic and cancerous cells. In this cell-mediated immune defence mechanism, the antibody activates the effector cell, which is classically known to be a natural killer (NK) cell, by interacting with Fcγ receptors (such as FcγRIIIA, FcγRIIA, and FcγRIIB) on the effector cell through its crystallizable fragment (Fc) domain. Activation of effector cells results in the secretion of cytotoxic molecules followed by lysis and elimination of the target cell.7 CDC is triggered by the activation of complement cascade through interactions of IgG Fc domain and the complement component 1q, known as C1q.8 Antibody-dependent cellular phagocytosis (ADCP) provides alternate mechanisms by which antibody activates macrophages through the FcγRs on macrophages and triggers phagocytosis, leading to the internalization and clearance of target cell through phagosome acidification.9
The N-linked glycan at N297 in each of the two heavy chains of antibody plays a critical role in modulating the IgG Fc-FcγRs interactions that ultimately affect the effector functions.10 In addition, modification of the Fc-glycan affects the stability, conformation, immunogenicity, serum half-life, and pharmacokinetics of antibodies thereby impacting their therapeutic efficacy.11 The Fc-glycosylation of IgG antibodies manufactured in mammalian expression systems such as HEK293, mouse myeloma NS0, Sp2/0, and the most predominantly used Chinese hamster ovary (CHO) cells, is usually highly heterogeneous with the majority of G0, along with limited galactosylated (G1/G2), and sialylated (G1S1/G2S1/G2S2) glycoforms.12, 13 Typically, the G0 glycoform is composed of core fucosylated heptasaccharide (GlcNAc2Man3GlcNAc2), in which two N-acetyl glucosamine (GlcNAc) residues are linked via β-1,2 linkage to the terminal mannose (Man) residues of the Man3GlcNAc2 core. In addition to core fucose, the Fc-glycan can be modified by various glycosyl transferases; for example, the addition of β-1,4 galactose (by B4GALT1) to terminal GlcNAc, β-1,4 GlcNAc (by GnTIII) to core Man, β-1,4/β-1,6 GlcNAcs (by GnTIV and GnTV) to core Man to form tri- and tetra antennary structures, and α-2,6/2,3 sialic acids (by ST6GAL1) to terminal Gal residues. GlcNAc transferases (GnTIV and GnTV) also play a major role in determining N-glycan complexity and the resulting level of terminal galactosylation and sialylation. Therefore, the IgG-Fc N-glycans exhibit tremendous diversity and heterogeneity, with >400 different glyco-variants when pairing the two CH2 domains, and each of the glycoforms potentially modulates the effector functions in a slightly different manner.14
Type I and type II FcγRs are categorized by their capacity to bind the IgG Fc-domain with specific glycoform.15 Type I FcγRs such as FcγRI, FcγRIIa, FcγRIIb, FcγRIIc, FcγRIIIa, and FcγRIIIb are members of the immunoglobulin superfamily,16 while type II FcγRs are represented by C-type lectin receptors such as the human dendritic cell–specific ICAM3-grabbing non-integrin (DC-SIGN/CD209) and CD23, which specifically binds to the sialylated Fc domains within immune complexes.15 The presence of α-1,6 fucose attached to the innermost GlcNAc on IgG-Fc N-glycan has the most significant impact on antibody functions, including FcγRIIIa binding to affect the ADCC of NK cells in-vitro and in-vivo, and this finding has led to the development of therapeutic antibodies with low core fucose content to treat cancer and inflammatory diseases.17, 18 Moreover, some specific glycosylation patterns, such as bisecting GlcNAc enhances ADCC, while terminal galactose increases the CDC and terminal sialic acid increases anti-inflammatory activities.7, 19 Since the sugar residue on Fc-glycan has a profound effect on biological activity, synthesis of homogeneous antibody glycoforms with all possible Fc-glycan structures is necessary for the study of therapeutic outcomes and improvement of treatment quality.
Manipulation of host biosynthetic pathway to enrich the desired antibody glycoform, including, for example, overexpression of different enzymes, knock-out/knock-in of certain genes, and use of carbohydrate analogues as inhibitors of the biosynthetic pathway, etc., offered promising ways to edit the glycosylation pattern but these approaches still result in a heterogenous mixture of glycoforms.13, 20, 21 Despite tremendous progress in the genetic engineering of glycosylation in mammalian and non-mammalian cells to produce glycoengineered antibodies, these methods are still limited by complicated protocols, low production yield, and difficult access to homogenous glycoforms.22, 23 An alternative powerful method to edit the glycosylation pattern is to use endoglycosidase mediated in-vitro glycoremodeling approach, where the intact heterogeneously glycosylated antibody is first deglycosylated to generate GlcNAc at Asn297, then a new glycan is added enzymatically in a stepwise manner or en-bloc in the form of oxazoline through transglycosylation to form a homogeneous glycan with well-defined structure.24
In this study, as a part of our ongoing efforts to optimize the IgG Fc-glycan for desired effector functions, we sought to investigate how the N-glycans, such as high mannose, hybrid- and complex-type glycans with various degrees of antennae, sialic acid linkage, terminal fucose linkage, bi-secting GlcNAc, and modifications of terminal sialic acid etc., modulate the binding of an antibody to FcγRIIIa for ADCC and CDC activity and to FcγRIIa for vaccinal effect. Based on our previous25, 26 and this studies, we demonstrated that the α-2,6 sialylated bi-antennary complex type glycan is the optimal glycan composition with significant improvement in effector functions.
The commercially available mouse mAb IgG3 MC813-70, which is specific for the glycan Stage-Specific Embryonic Antigen-4 (SSEA4), was humanized to a chimeric IgG called chMC813-70 and used as a model antibody for glycoengineering. The antibody starting material was produced by our previously reported methods27, 28 in WT Expi293TM cells to give a heterogenous Fc-glycan mixture with most of them core fucosylated, whereas the chMC813-70 produced in GnTI deficient Expi293TM cells gave the glycoforms mainly with high mannose type Man5 glycan without core fucose.27, 28 In general, deglycosylation of antibodies produced in Expi293TM required treatment with WT Endo-β-N-acetylglucosaminidase S2 (EndoS2) to trim the glycans of high mannose, hybrid- and complex-types, followed by α-fucosidase to remove core fucose and generate the antibody with core GlcNAc. However, treatment of antibodies produced from GnTI-Expi293TM with EndoH was sufficient to generate homogeneous glycoforms with core GlcNAc (Figure 1).28 Next, a new glycan was used in the form of oxazoline for transglycosylation on the IgG-GlcNAc acceptor in the presence of a glycosynthase derived from site-specific mutation of EndoS2 (such as D184M) with reduced hydrolytic activities and improved transglycosylation efficiency.25, 26, 29, 30
figure 1:
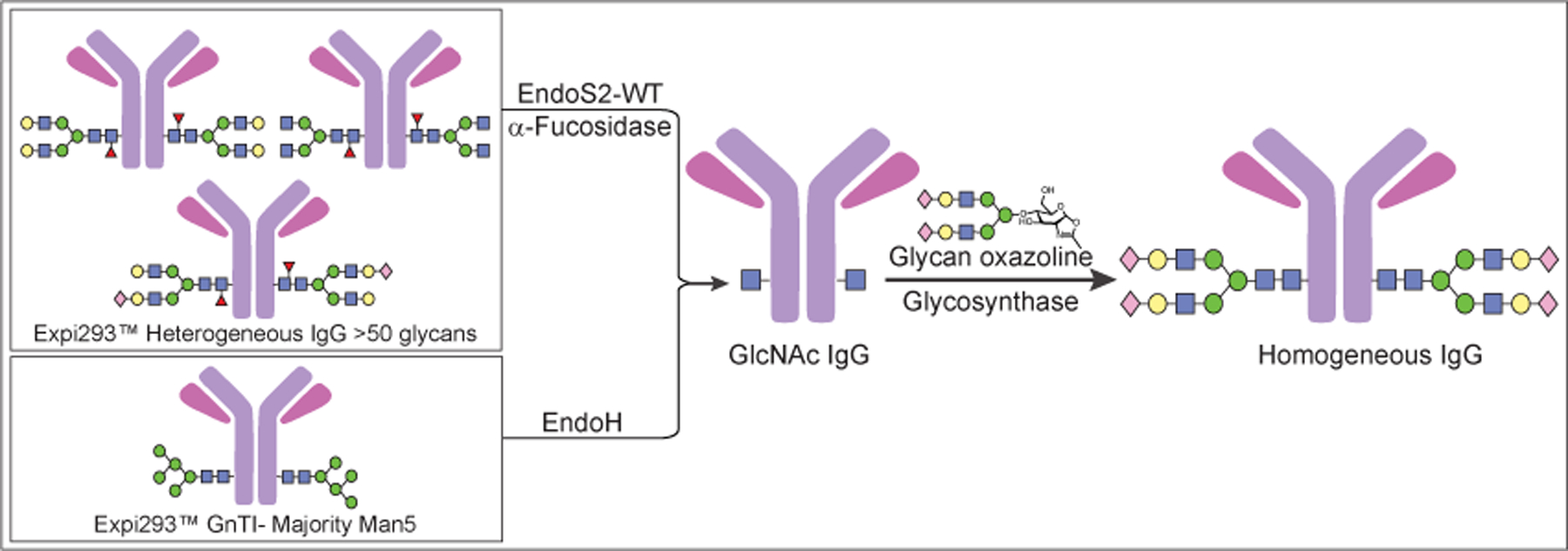
a general strategy for in-vitro glycan remodeling through the combined use of endoglycosidase and glycosynthase.
To understand the contribution of each monosaccharide of IgG-Fc N-glycan to effector functions, we included the glycoforms prepared in this study and those from our previous work (Figure 2) for comparison. These include the glycoforms of high mannose type (Man3, Man5, Man9)(G1–3) and hybrid type (G4–5),26 the glycoforms of G0 analogues (G6–9)25 with terminal GlcNAc residue on the α-1,3 arm, the α-1,6 arm, and both arms w/o bi-secting GlcNAc, G2 analogues (G10–15) including G1, G2, G2 with bisecting GlcNAc, G2 with varying degrees of α-1,2/1,3 fucose on terminal galactose or GlcNAc respectively,26 G16–19 with terminal sialic acid, G20–21 with terminal 3-F sialic acid (G20) and 9-azido sialic acid (G21), and G22–26 of tri-antennary complex type N-glycans, of which G25–26 were reported before.26 The synthesis of homogenous chMC813-70 antibody glycoforms G8, G11, G17, G18, and G21–24 is described in this work while all other glycoforms using Rituximab and Herceptin as models were reported by our group previously.25, 26, 31
figure 2:
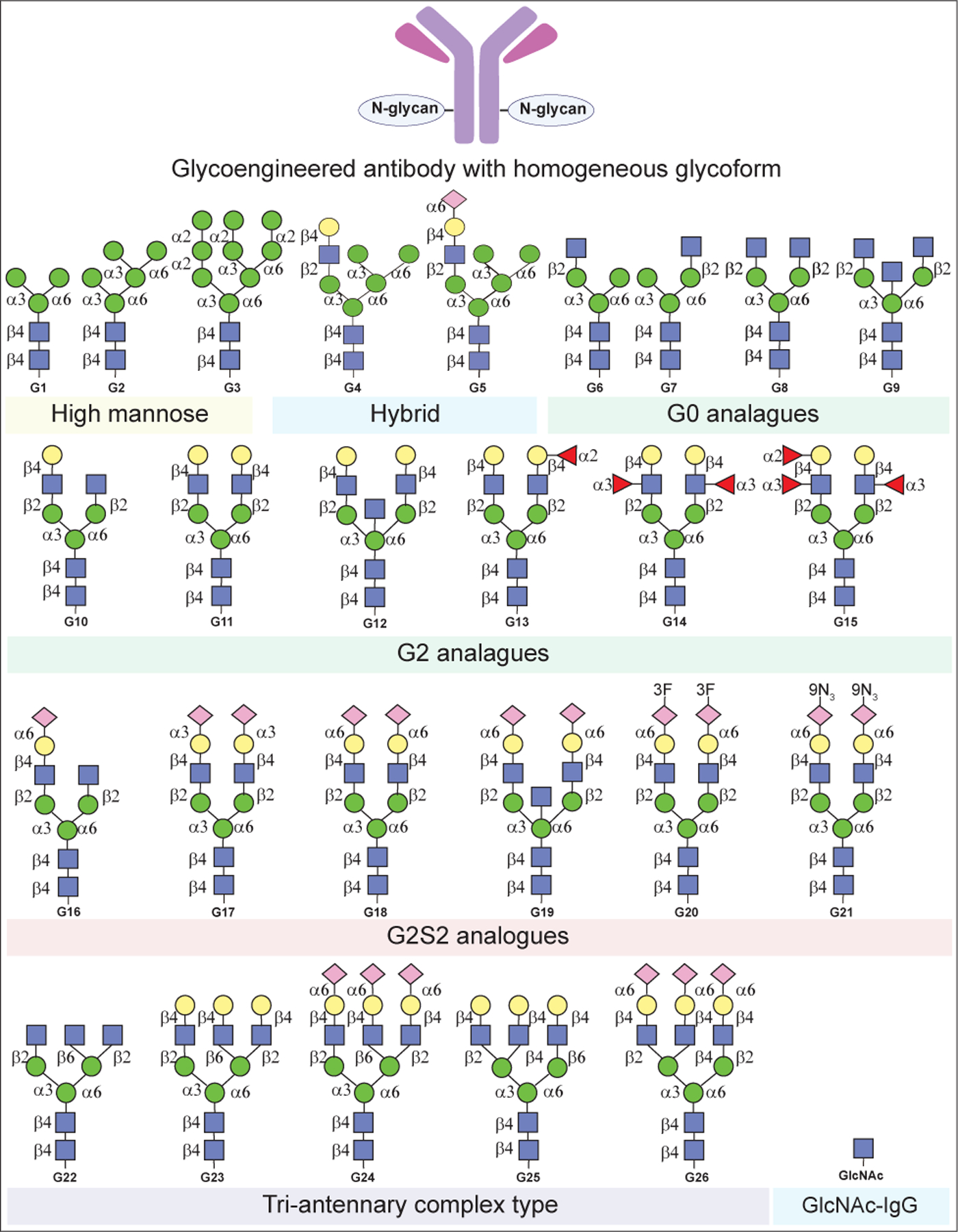
glycoengineered antibodies with homogeneous glycoforms of high mannose, hybrid, and complex types. homogeneous antibodies g8, g11, g17, g18, and g20–24 are prepared using chmc813-70, while all other glycoforms are reported using rituximab.
The synthesis and mass spectrometric analysis of bi- and tri-antennary glycoforms are described in supporting information, (Scheme S1, and Scheme S2 ESI†). The glycan oxazolines were prepared in the presence of 2-chloro-1,3-dimethylimidazolinium chloride (DMC) and transglycosylated to IgG-GlcNAc, catalyzed by a mutant of EndoS2 (D184M). The transglycosylation protocol was optimized to enhance reaction yield and minimize non-specific antibody modification. Final purified antibodies G8, G11, G17, G18 and G20–24 were characterized by SDS-PAGE for purity and mass spectrometric analysis to confirm glycan homogeneity (Figure S4 ESI†).
Next, we asseessed the binding affinity of diverse homogeneous antibodies (G8, G11, G17, G18, and G20–24) towards FcγRIa, FcγRIIa, and FcγRIIIa using ELISA. We previously reported that, homogeneous Rituximab having high mannose type Man3 (G1) and Man5 (G2) glycans exhibited 3- and 6-fold enhancements in FcγRIIIa binding, respectively, while Man9 (G3) glycan did not show any improvement.26 In the same study, we also showed that there was only 2–3-fold enhancement in FcγRIIIa binding for hybrid-type glycoforms (G4-G5).26
Our group systematically studied the G0 analogues (G6-G9) with terminal GlcNAc, G2 analogues (G10-G15) with terminal galactose and G2S2 analogues (G16-G21) with terminal sialic acid residues, and their impact on FcγR/Ia/IIa/IIIa bindings using Rituximab as a model.25, 26 The presence of GlcNAc on the α-1,3 arm (G6), the α-1,6 arm (G7) or both arms (G8) resulted in 1.4-, 1.9-, and 4.3-fold enhancements respectively.25 Addition of bi-secting GlcNAc in G9, G12, and G19 did not affect the binding significantly.25, 26 We then sought to investigate the impact of adding α-1,3 or α-1,2 fucose to G17 on binding; however, the presence of α-1,2 fucose on G2 (G13), α-1,3 fucose on G2 (G14) and both α-1,3/1,2 fucose on G2 (G15) showed reduction in FcγRIIIa binding affinity compared to G2 (G13).26
Among various monosaccharide modifications on IgG-Fc glycans, the terminal sialic acid capping is particularly interesting, as sialylation of Fc-glycan was known to dramatically reduce the antibody affinity to FcγRIIIa and ultimately the ADCC activity.5, 19 Sialylation was shown to increase serum half-life of a number of glycoproteins.32 Sialic acid capping hides the galactose residue to be recognized by hepatic asialoglycoprotein receptors (ASGPR) and avoid hepatic clearance.32 Sialic acid residues can be linked to the galactose either by α-2,3 or 2,6 linkages. To study the influence of sialic acid on effector functions, we compared the homogeneous antibodies (G8, G11, G17, G18, and G20–24) with bi- and tri-antennary structures without sialic acid cap (terminal GlcNAc and Gal), with α-2,6 sialylation, with α-2,3 sialylation, and with unnatural sialic acid residues for their bindings towards FcγRIa, FcγRIIa, and FcγRIIIa. Binding affinity screening performed using ELISA and EC50 is reported in Table 1. The bi-antennary complex glycan with terminal GlcNAc (G8) showed 17-fold enhancement, while additional galactose residues (G11) resulted in 33-fold enhancements in binding towards FcγRIIIa. Further sialylation with α-2,6 linkage (G18) did not affect much on binding compared to G11 (EC50 = 15.11 nM for G18 vs EC50= 23.15 nM for G11); however, α-2,3 sialylated glycoform (G17) showed reduction in FcγRIIIa binding affinity. Chen et. al.33 reported that sialic acid residues affect conformation but did not interact with FcγRIIIa. However, the 3-OH of galactose interacted with the protein through E258 and α-2,3 sialylation (G17) disrupted those interactions and adopted another conformation. This could be an explanation behind the reduction in binding of G17 compared to G11 and G18. The same trend was observed for tri-antennary glycoforms (G22–24) that galactose exhibited more significant impact on FcγRIIIa binding than sialic acid, but increase in branching to tri-antennary structures did not affect much on effector functions.
Table 1:
Binding of glycoengineered chMC813-70 to FcγR (EC50 µg/ml).
| FcR/ mAb | WT | Glc NAc |
G8 | G11 | G17 | G18 | G20 | G21 | G22 | G23 | G24 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| FcγIIIa | 8.552 | 1250 | 0.516 | 0.256 | 1.233 | 0.224 | 0.224 | 1.107 | 0.456 | 0.216 | 0.24 |
| FcγIIa | 17.53 | N. M | N. D | 17.28 | 47.8 | 5.95 | 6.45 | N. D | N. D | N. D | N. D |
| FcγIa | 0.031 | 0.24 | 0.030 | 0.032 | 0.044 | 0.031 | 0.026 | N. D | 0.032 | 0.029 | 0.029 |
Regarding the binding affinity toward FcγRIIa, sialylation of the bi-antennary glycans increased binding as shown in Table 1, from G11 to G18 (EC50 = 17.28 vs 5.75 µg/ml), but the impact was insignificant for the tri-antennary glycans G23 and G24. We were not able to achieve plateau at the higher concentration that was tested (150 μM). Interestingly, the glycoform with 3-F sialic acid (G20) showed a similar binding affinity as that of sialic acid (G18); however, 9-azido sialic acid (G21) reduces the binding affinity. At last, none of the glycoforms showed significant improvement in binding towards FcγRIa (Table 1).
Anticancer antibodies rapidly initiate lysis of tumor cells by ADCC; however, long-term anti-cancer immune response can be generated by vaccinal effect. Recently, the Ravetch group demonstrated that, anti-cancer mAb must engage hFcγRIIIa on macrophages to mediate ADCC, but also interacts with hFcγRIIa, a receptor expressed by human dendritic cells to induce vaccinal effect in FcγR humanized mice model.34 Therefore, the ideal antibody must be optimized for both FcγRIIIa and FcγRIIa engagement for long-term anti-cancer immune response. Previously we showed that the ADCC of α-2,3 sialylated glycoforms (G17) was reduced compared to its α-2,6 counterpart (G18) In addition, no significant enhancement in ADCC was seen for G18 vs G11 in the context of Herceptin. Two independent studies by Kurogochi et al. 35 and Li et al. 36 reported similar findings using Herceptin and Rituximab, respectively. No significant difference in ADCC activity between G18 and G11 was observed (Figure S3 ESI†). Therefore, we aimed to compare the ADCC activity of newly prepared tri-antennary glycoforms (G23 and G24) with bi-antennary glycoform with natural sialic acid (G18) and sialidase resistant 3F-sialic acid (G20). Glycoforms G18, G20, and G23–24 were selected for evaluation of their ADCC effect against pancreatic adenocarcinoma epithelial cell line (HPAC). Glycoforms G18, G23, and G24 showed the same degree of ADCC enhancement as wild-type antibody, and deglycosylated antibody lost the ADCC activity completely. However, G20 with 3-F sialic acid showed 4–5 fold enhancement compared to WT and slightly better than G18, G23, and G24.(Figure 3).
figure 3:
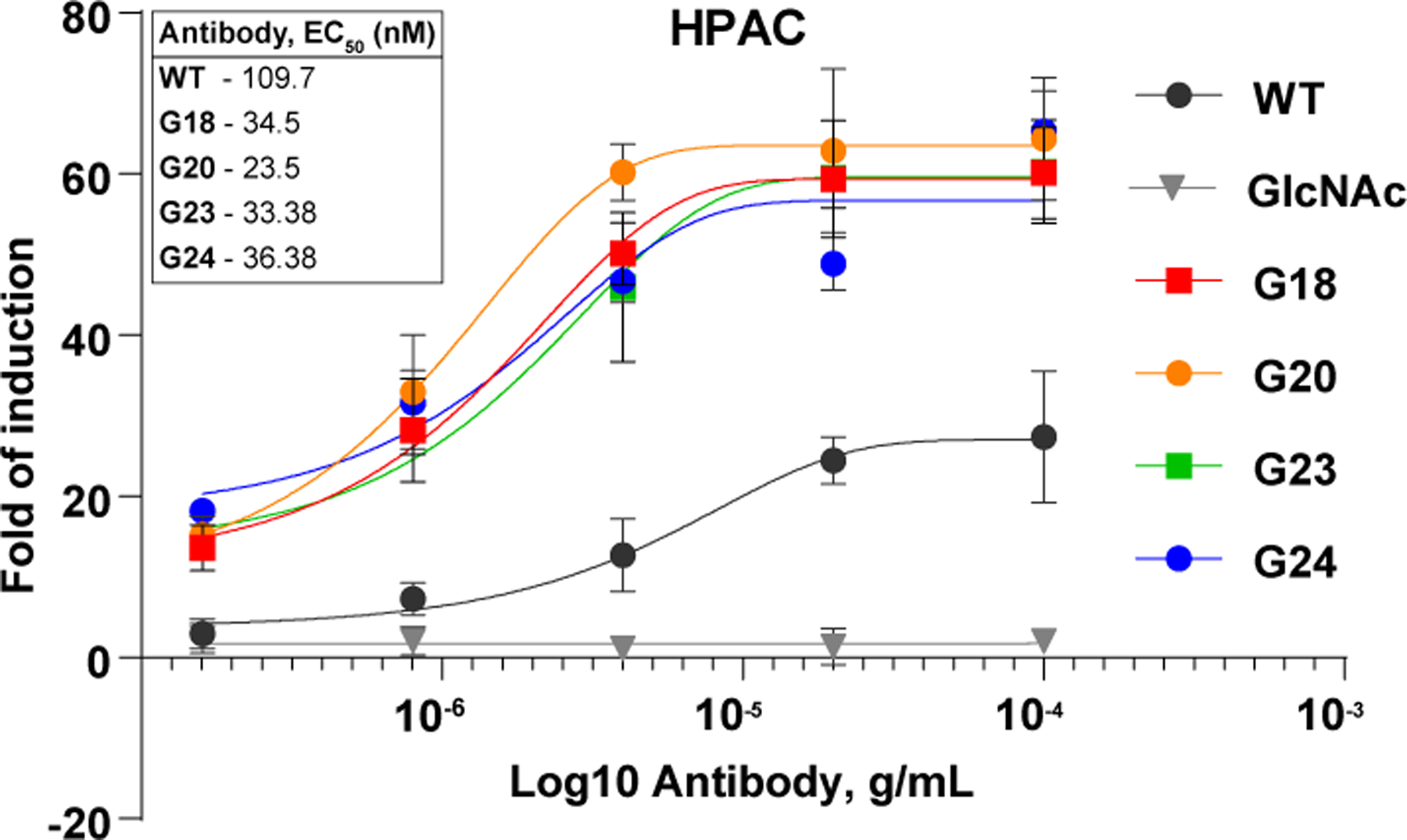
. adcc activity of selected glycoforms tested against pancreatic adenocarcinoma epithelial cell line (hpac).
In summary, we evaluated a broad range of IgG-Fc N-glycan modifications, including high mannose, hybrid, and diverse complex type N-glycans. Based on the minimum glycan requirement, in-vivo stability, importance of terminal sialic acid, and the results from the systematic structure-activity studies led to the conclusion that the α-2,6 sialylated bi-antennary complex type structure (G18) and its sialidase resistant 3-F analogue (G20) is the optimal glycan that offered excellent biological outcomes in terms of FcγRIIIa binding for ADCC and FcγRIIa binding for vaccinal effect. Animal studies are ongoing to demonstrate the efficacy of glycoengineered anti-SSEA4 antibodies (G18 and G20) in pancreatic cancer.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (AI130227), and the National Science Foundation (CHE-1954031).
Footnotes
Electronic Supplementary Information (ESI). See DOI: 10.1039/x0xx00000x
Conflicts of interest
Authors declare no conflict of interest.
References
- 1.Carter PJ and Rajpal A, Cell, 2022, 185, 2789–2805. [DOI] [PubMed] [Google Scholar]
- 2.Kaplon H and Reichert JM, MAbs, 2021, 13, 1860476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu RM, Hwang YC, Liu IJ, Lee CC, Tsai HZ, Li HJ and Wu HC, J Biomed Sci, 2020, 27, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pereira NA, Chan KF, Lin PC and Song Z, MAbs, 2018, 10, 693–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomann M, Reckermann K, Reusch D, Prasser J and Tejada ML, Mol Immunol, 2016, 73, 69–75. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Q, Joubert MK, Polozova A, De Guzman R, Lakamsani K, Kinderman F, Xiang D, Shami A, Miscalichi N, Flynn GC and Kuhns S, Biotechnol Prog, 2020, 36, e3045. [DOI] [PubMed] [Google Scholar]
- 7.Natsume A, Niwa R and Satoh M, Drug Des Devel Ther, 2009, 3, 7–16. [PMC free article] [PubMed] [Google Scholar]
- 8.Ząbczyńska M, Polak K, Kozłowska K, Sokołowski G and Pocheć E, Biomolecules, 2020, 10, 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kamen L, Myneni S, Langsdorf C, Kho E, Ordonia B, Thakurta T, Zheng K, Song A and Chung S, J Immunol Methods, 2019, 468, 55–60. [DOI] [PubMed] [Google Scholar]
- 10.Dashivets T, Thomann M, Rueger P, Knaupp A, Buchner J and Schlothauer T, PLoS One, 2015, 10, e0143520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu L, J Pharm Sci, 2015, 104, 1866–1884. [DOI] [PubMed] [Google Scholar]
- 12.Goh JB and Ng SK, Crit Rev Biotechnol, 2018, 38, 851–867. [DOI] [PubMed] [Google Scholar]
- 13.Heffner KM, Wang Q, Hizal DB, Can Ö and Betenbaugh MJ, Adv Biochem Eng Biotechnol, 2021, 175, 37–69. [DOI] [PubMed] [Google Scholar]
- 14.Chen B, Liu W, Li Y, Ma B, Shang S and Tan Z, Molecules, 2022, 27, 8859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pincetic A, Bournazos S, DiLillo DJ, Maamary J, Wang TT, Dahan R, Fiebiger BM and Ravetch JV, Nat Immunol, 2014, 15, 707–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bournazos S, Gupta A and Ravetch JV, Nat Rev Immunol, 2020, 20, 633–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Satoh M, Iida S and Shitara K, Expert Opin Biol Ther, 2006, 6, 1161–1173. [DOI] [PubMed] [Google Scholar]
- 18.Golay J, Andrea AE and Cattaneo I, Front Immunol, 2022, 13, 929895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomann M, Schlothauer T, Dashivets T, Malik S, Avenal C, Bulau P, Rüger P and Reusch D, PLoS One, 2015, 10, e0134949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Narimatsu Y, Büll C, Chen YH, Wandall HH, Yang Z and Clausen H, J Biol Chem, 2021, 296, 100448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Q, Chung CY, Chough S and Betenbaugh MJ, Biotechnol Bioeng, 2018, 115, 1378–1393. [DOI] [PubMed] [Google Scholar]
- 22.Zhang P, Woen S, Wang T, Liau B, Zhao S, Chen C, Yang Y, Song Z, Wormald MR, Yu C and Rudd PM, Drug Discov Today, 2016, 21, 740–765. [DOI] [PubMed] [Google Scholar]
- 23.Edwards E, Livanos M, Krueger A, Dell A, Haslam SM, Mark Smales C and Bracewell DG, Biotechnol Bioeng, 2022, 119, 1343–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang LX, Tong X, Li C, Giddens JP and Li T, Annu Rev Biochem, 2019, 88, 433–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin CW, Tsai MH, Li ST, Tsai TI, Chu KC, Liu YC, Lai MY, Wu CY, Tseng YC, Shivatare SS, Wang CH, Chao P, Wang SY, Shih HW, Zeng YF, You TH, Liao JY, Tu YC, Lin YS, Chuang HY, Chen CL, Tsai CS, Huang CC, Lin NH, Ma C, Wu CY and Wong C-H, Proc Natl Acad Sci U S A, 2015, 112, 10611–10616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shivatare SS, Huang LY, Zeng YF, Liao JY, You TH, Wang SY, Cheng T, Chiu CW, Chao P, Chen LT, Tsai TI, Huang CC, Wu CY, Lin NH and Wong C-H, Chem Commun (Camb), 2018, 54, 6161–6164. [DOI] [PubMed] [Google Scholar]
- 27.Lin CW, Wang YJ, Lai TY, Hsu TL, Han SY, Wu HC, Shen CN, Dang V, Chen MW, Chen LB and Wong CH, Proc Natl Acad Sci U S A, 2021, 118, e2114774118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee RH, Wang YJ, Lai TY, Hsu TL, Chuang PK, Wu HC and Wong C-H, ACS Chem Biol, 2021, 16, 1526–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li T, Tong X, Yang Q, Giddens JP and Wang LX, J Biol Chem, 2016, 291, 16508–16518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang W, Giddens J, Fan SQ, Toonstra C and Wang LX, J Am Chem Soc, 2012, 134, 12308–12318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lo HJ, Krasnova L, Dey S, Cheng T, Liu H, Tsai TI, Wu KB, Wu CY and Wong C-H, J Am Chem Soc, 2019, 141, 6484–6488. [DOI] [PubMed] [Google Scholar]
- 32.Bork K, Horstkorte R and Weidemann W, J Pharm Sci, 2009, 98, 3499–3508. [DOI] [PubMed] [Google Scholar]
- 33.Chen CL, Hsu JC, Lin CW, Wang CH, Tsai MH, Wu CY, Wong CH and Ma C, ACS Chem Biol, 2017, 12, 1335–1345. [DOI] [PubMed] [Google Scholar]
- 34.DiLillo DJ and Ravetch JV, Cell, 2015, 161, 1035–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kurogochi M, Mori M, Osumi K, Tojino M, Sugawara S, Takashima S, Hirose Y, Tsukimura W, Mizuno M, Amano J, Matsuda A, Tomita M, Takayanagi A, Shoda S and Shirai T, PLoS One, 2015, 10, e0132848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li T, DiLillo DJ, Bournazos S, Giddens JP, Ravetch JV and Wang LX, Proc Natl Acad Sci U S A, 2017, 114, 3485–3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


