Summary
Background
Effective adjuvant therapy for patients with resected localised renal cell carcinoma represents an unmet need, with surveillance a current standard of care. We report results from a phase 3 randomised trial (part A) assessing the efficacy and safety of adjuvant nivolumab plus ipilimumab versus placebo.
Methods
The double-blind, randomised, multinational, phase 3 CheckMate 914 trial (NCT03138512) enrolled patients with localised clear cell renal cell carcinoma at high risk of relapse after radical or partial nephrectomy between 4–12 weeks before randomisation. Patients were randomised (1:1) to nivolumab (240 mg) intravenously every 2 weeks for 12 doses plus ipilimumab (1 mg/kg) every 6 weeks for four doses, or matching placebo. Expected treatment period was 24 weeks, and could be continued until week 36, allowing for treatment delays. Randomisation was stratified by TNM stage and partial/radical nephrectomy. The primary endpoint was disease-free survival by blinded independent central review; safety was a secondary endpoint.
Findings
Between August 28, 2017, and March 16, 2021, 405 patients were randomised to nivolumab plus ipilimumab and 411 to placebo. With median follow-up of 37·0 months, median disease-free survival was not reached with nivolumab plus ipilimumab versus 50·7 months (95% CI 48·1-not estimable) with placebo (HR 0·92; 95% CI 0·71–1·19; p=0·53). A total of 33 deaths occurred in the nivolumab plus ipilimumab arm and 28 in the placebo arm. All-cause grade 3–5 adverse events occurred in 38% of patients treated with nivolumab plus ipilimumab and in 10% of patients receiving placebo; any-grade adverse events led to treatment discontinuation in 32% and 2% of patients, respectively. Four deaths were attributed to treatment with nivolumab plus ipilimumab.
Interpretation
Adjuvant therapy with nivolumab plus ipilimumab did not improve disease-free survival versus placebo in patients with localised renal cell carcinoma at high risk of recurrence after nephrectomy.
Funding
Bristol Myers Squibb and Ono Pharmaceutical.
Keywords: Renal cell carcinoma, adjuvant, nivolumab
Introduction
Current standard treatment for localised, nonmetastatic (stage I–III) renal cell carcinoma is partial or radical nephrectomy.1,2 Although radical surgical resection of the kidney can be curative for a proportion of patients with localised disease, up to 40% of surgically resected patients with stage II-III disease will eventually relapse, and most will die of metastatic disease.1–4
Safe and effective adjuvant treatment options that provide durable disease control and long-term survival benefits are limited for patients with renal cell carcinoma.1,2,5 Studies of adjuvant therapy with cytokines, radiotherapy, and vaccine-based regimens failed to show benefit, and inconsistent results have been reported with VEGFR-targeted therapies in this setting.1,5 Despite notable drug-related toxicity and conflicting results across trials, adjuvant sunitinib is approved in the United States for high-risk patients with renal cell carcinoma based on improved disease-free survival versus placebo in the S-TRAC trial.6–8
Immune checkpoint blockade has revolutionised the first-line treatment landscape for advanced renal cell carcinoma. Due to the success in advanced disease, significant interest in exploring immunotherapy regimens in localised renal cell carcinoma has emerged with the goal of eliminating any residual, undetectable microscopic disease after curative resection.2,5,8,9 Immune checkpoint inhibitors maintain efficacy after treatment discontinuation, and may eradicate micrometastatic disease.7,10 Therefore, the demonstrated efficacy of immune checkpoint inhibitors in patients with advanced disease, together with the ability to provide enduring responses in patients, provided much of the rationale for evaluating adjuvant immune checkpoint blockade for the treatment of patients with localised disease.
Adjuvant pembrolizumab, an anti-programmed death 1 (PD-1) antibody, demonstrated disease-free survival benefits versus placebo in patients with high-risk clear cell renal cell carcinoma in the prespecified first interim analysis of the KEYNOTE-564 trial, leading to regulatory approval in Europe and the United States.11–14 However, recent reports from the IMmotion010 (adjuvant atezolizumab, a programmed death ligand 1 [PD-L1] inhibitor) and PROSPER RCC (perioperative nivolumab, a PD-1 inhibitor) trials evaluating the use of immunotherapy in renal cell carcinoma have shown no improvement in the primary endpoints of disease-free or recurrence-free survival, respectively.15,16
Nivolumab monotherapy has previously demonstrated efficacy as adjuvant treatment in multiple malignancies, including high-risk muscle-invasive urothelial carcinoma (CheckMate 274),17 resected oesophageal or gastro-oesophageal junction cancer (CheckMate 577),18 and stage 3–4 melanoma (CheckMate 238).19 Dual immune checkpoint blockade with nivolumab plus ipilimumab (an inhibitor of cytotoxic T-lymphocyte-associated protein 4 [CTLA-4]), has demonstrated significant long-term survival and durable response benefits versus sunitinib in previously untreated patients with advanced renal cell carcinoma, supporting evaluation of this combination regimen as adjuvant treatment for localised disease.20
We conducted the phase 3 CheckMate 914 trial to assess disease-free survival per blinded independent central review (BICR) of adjuvant treatment with nivolumab plus ipilimumab versus placebo (primary endpoint in part A) and adjuvant treatment with nivolumab monotherapy versus placebo (primary endpoint in part B) in mutually exclusive patients with localised renal cell carcinoma at high risk of recurrence after radical or partial nephrectomy. Study results for parts A and B will be analysed and reported separately. Here, we report the results from part A of CheckMate 914.
Methods
Study design and participants
CheckMate 914 is a double-blind, randomised, phase 3 trial of adjuvant nivolumab plus ipilimumab versus placebo (part A) and adjuvant nivolumab monotherapy versus placebo (part B). Part A, reported here, was conducted in 145 hospitals and cancer centres across 20 countries.
We recruited adult patients (≥18 years old) with localised renal cell carcinoma with a predominantly clear cell histology at high risk of relapse after partial or radical nephrectomy. Patients had negative surgical margins with no clinical or radiological evidence of macroscopic residual disease or distant metastases (M0) after nephrectomy per local review and confirmed by BICR, and pathological TNM staging pT2a (grade 3–4) N0M0, pT2b (any grade) N0M0, pT3 (any grade) N0M0, pT4 (any grade) N0M0, or pT any (any grade) N1M0.21 Additional enrolment criteria included an Eastern Cooperative Oncology Group performance status of ≤1; and available tumour tissue for analysis obtained within 3 months before enrolment.
Patients were excluded if they had active, known, or suspected autoimmune disease or a condition that required systemic treatment with either corticosteroids (>10 mg of prednisone equivalent per day) or other immunosuppressive medications within 14 days before first dose of study treatment; prior active malignancies within the previous 3 years (except for locally curable cancers that have been apparently cured); receipt of live or attenuated vaccine within 30 days of first dose of study treatment; or previous systemic therapy for renal cell carcinoma in the neoadjuvant, adjuvant, or metastatic setting. Full eligibility criteria are listed in the protocol (appendix).
CheckMate 914 was approved by an institutional review board or independent ethics committee and regulatory authorities at each site and conducted in accordance with Good Clinical Practice guidelines defined by the International Council for Harmonisation, ethical principles underlying European Union Directive 2001/20/EC, and US code of Federal Regulations Title 21, part 50 (21CFR50). Enrolled patients provided written informed consent according to the principles of the Declaration of Helsinki. Between March 22, 2017, and February 13, 2022, five protocol amendments were made which included changes that affected study design and recruitment (appendix p 10). Full details of the revisions are available in the protocol (appendix).
Randomisation and masking
In part A of this trial, patients were randomised (1:1) to the nivolumab plus ipilimumab arm or the placebo arm via an interactive response technology system. Randomisation occurred at greater than 4 weeks and no more than 12 weeks from the date of nephrectomy. The Bristol Myers Squibb (Princeton, NJ, USA) interactive response technology group created the computer-generated randomisation schedule; the screening of patients was done by study investigators at each site and the randomisation to trial groups was performed using the interactive voice response system. Patients were stratified according to pathological TNM staging (American Joint Committee on Cancer staging, 7th edition, 2010) and Fuhrman nuclear grading categories (pT2a, grade ≥3, N0 M0 and pT2b, any grade, N0M0; vs pT3, any grade, N0M0; vs pT4, any grade, N0M0 and pT any, any grade, N1M0)21; and type of nephrectomy (partial vs radical). Randomisation was carried out via permuted blocks within each stratum using a block size of two in each treatment group. The study was double-blinded; the patients, physicians, physicians’ staff, and the study sponsor were blinded to treatment assignment, and nivolumab and ipilimumab each had its own matching placebo. The site pharmacist was unblinded to allow preparation of study drug or placebo. Designated staff at Bristol Myers Squibb Research and Development were allowed to be unblinded to treatment before database lock to facilitate the bioanalytical analysis of pharmacokinetic samples and immunogenicity. A patient’s study treatment could have been unblinded to the investigator in the event of disease recurrence in order to determine subsequent treatment, or in the event of a medical emergency or pregnancy, using interactive response technology.
Procedures
Patients received nivolumab (240 mg) intravenously every 2 weeks for 12 doses and ipilimumab (1 mg/kg) intravenously every 6 weeks (or every third nivolumab dose if dosing is delayed) for four doses, or a placebo intravenously at the same frequency as nivolumab and ipilimumab administration. Treatment continued until completion of 12 cycles (12 nivolumab doses and four ipilimumab doses), week 36 of nivolumab treatment, unacceptable toxicity, recurrence, or withdrawal of consent, whichever occurred first. Dose delays for the management of adverse events or SARS-CoV-2 infection, and infusion interruptions or rate changes were allowed for nivolumab, ipilimumab, and placebo; if one drug was to be delayed or discontinued, both study drugs were to be delayed or discontinued. Dose escalations and dose reductions were not allowed for any study drug. All discontinuation criteria applied to nivolumab, ipilimumab and placebo are detailed in the trial protocol (appendix).
Tumour assessments were performed by CT and/or MRI of the chest, abdomen, and pelvis, and other known/suspected sites of disease. Assessment of disease-free status was performed at screening/baseline (greater than 4 weeks post-nephrectomy) and submitted with pre-nephrectomy scans (if available) for confirmation by BICR before randomisation. Subsequent tumour assessments were done at week 23 (±1 week) post-treatment initiation, weeks 36 and 52 (±1 week), then every 6 months (±2 weeks) for years 2–6, then annually to year 10. Tumour assessments were discontinued once recurrence was confirmed by BICR.
Adverse events were collected continuously during treatment and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0). Ongoing treatment-related adverse events were followed until resolution, return to baseline, or deemed irreversible. Immune-mediated adverse events were reported and defined as events occurring within 100 days of the last dose, regardless of causality, treated with immune-modulating medication (except endocrine events which were considered immune-mediated adverse events regardless of immune-modulating medication administration), with no clear alternate aetiology based on investigator assessment, or with an immune-mediated component. The use of glucocorticoids (≥40 mg prednisone daily or equivalent) to manage these events was also reported.
Outcomes
The primary endpoint of CheckMate 914 was disease-free survival per BICR. Disease-free survival was defined as the time from randomisation to development of local disease recurrence (ie, recurrence of primary tumour in situ or occurrence of a secondary renal cell carcinoma primary cancer), distant metastasis, or death, whichever occurred first. Disease-free survival was determined programmatically based on the disease recurrence date provided by BICR; of patients who received subsequent systemic anticancer therapy, tumour-directed radiotherapy, or tumour-directed surgery, those who received the new therapy before or without a documented recurrence were censored at the date of the last tumour assessment conducted on or before the initiation of the new therapy. The secondary definition of disease-free survival was defined similar to the primary definition excluding censoring for subsequent therapy. The full censoring scheme is provided in the appendix (p 11).
The secondary endpoints were overall survival, safety, and tolerability. Overall survival was defined as the time between the date of randomisation and the date of death. For patients without documentation of death, overall survival was censored on the last date the patient was known to be alive. Safety and tolerability included incidence, severity (graded by the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0), timing, seriousness, relatedness, and laboratory abnormalities up to 30 and 100 days of last dose of study therapy in all treated patients.
Exploratory endpoints of CheckMate 914 that are not reported here, and a full listing of exploratory study endpoints, are provided in the protocol (appendix). Assessment of efficacy by PD-L1 expression was part of the exploratory analysis but ongoing at the time of submission.
Statistical analysis
It was estimated that approximately 800 patients would undergo randomisation. A hierarchical testing procedure was used (disease-free survival, followed by overall survival) with an overall α level of 0·05. For the analysis of disease-free survival per BICR (primary endpoint), approximately 227 events were expected to provide 90% power to detect a disease-free survival HR of 0·65 at an α level of 0·05 (two-sided). If the between-group difference in disease-free survival was significant, it was specified that overall survival (secondary endpoint) would be tested hierarchically.
Disease-free survival was compared between treatment arms using a two-sided log-rank test stratified by the randomisation stratification factors (ie, pathological TNM staging and type of nephrectomy). The hazard ratio (HR) and confidence interval (CI) were calculated using a Cox proportional-hazards model with treatment arm as the sole covariate, stratified using the same stratification factors. Disease-free survival medians with 95% CIs and rates at fixed timepoints were estimated using Kaplan-Meier methods. The two-sided log-rank p value is reported.
Prespecified exploratory analyses of efficacy endpoints were done in subgroups of demographic and clinical characteristics at baseline, with stratification factors displayed per case report form. Adverse events and events leading to discontinuation of trial treatment or death were summarised descriptively.
Disease-free survival was analysed in all randomised patients (intention-to-treat population); exposure, safety, and tolerability were analysed in all patients who received at least one dose of study drug (all treated population). A data monitoring committee provided oversight of patient safety and evaluated available efficacy data. All statistical analyses were done with SAS (version 9.4). This study is registered with ClinicalTrials.gov, NCT03138512.
Role of the funding source
The funders contributed to the study design, data analysis, and data interpretation in collaboration with the authors. The funders did not have a role in data collection. Financial support for editorial and writing assistance was provided by the funders.
Results
Between August 28, 2017, and March 16, 2021, a total of 816 patients were randomly assigned to receive either adjuvant nivolumab plus ipilimumab (405 patients) or placebo (411 patients) in the intention-to-treat population (figure 1). A total of 404 patients received at least one dose of nivolumab plus ipilimumab, and 407 patients received at least one dose of placebo and were included in the safety analysis (all treated patients). Baseline demographic and clinical characteristics are shown in table 1. Patient characteristics at baseline were well balanced between the two arms. Most of the enrolled trial population had pathological TNM staging T3 N0M0 (nivolumab plus ipilimumab, 78%; placebo, 77%).
Figure 1: Trial profile for CheckMate 914 part A.
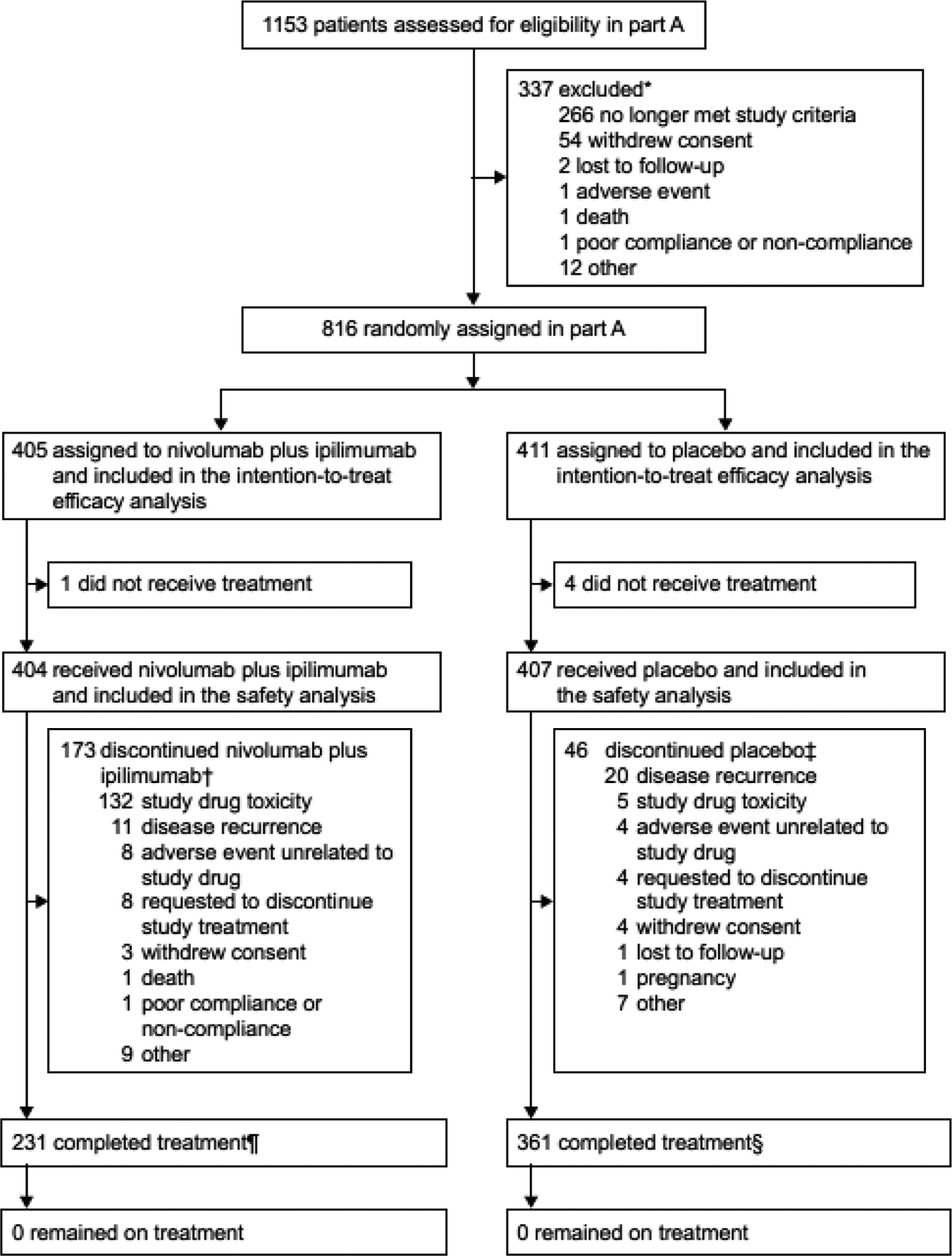
*Ten patients were not randomised due to COVID-19.
†Nine patients discontinued nivolumab plus ipilimumab due to COVID-19 (all nine patients discontinued due to “other” reason).
‡Eight patients discontinued placebo due to COVID-19 (one patient withdrew consent; seven patients discontinued due to “other” reason).
¶Two patients in the nivolumab plus ipilimumab arm were reported as having completed treatment by the investigators even though one patient skipped nivolumab at cycle 3 and one patient skipped nivolumab at cycle 12.
§One patient in the placebo arm was reported as having completed treatment by the investigator even though the patient skipped ipilimumab-placebo at cycle 7.
Table 1:
Demographic and clinical characteristics at baseline in the intent-to-treat population
| Characteristic | Nivolumab plus ipilimumab (N=405) |
Placebo (N=411) |
|---|---|---|
| Median age (Q1, Q3) — years | 58 (51, 65) | 57 (50, 65) |
| Age — n (%) | ||
| <65 | 293 (72) | 301 (73) |
| ≥65 | 112 (28) | 110 (27) |
| ≥65 and <75 | 93 (23) | 91 (22) |
| ≥75 and <85 | 19 (5) | 19 (5) |
| Sex — n (%) | ||
| Male | 286 (71) | 294 (72) |
| Female | 119 (29) | 117 (28) |
| Race — n (%) | ||
| White | 302 (75) | 321 (78) |
| Black or African American | 3 (<1) | 6 (1) |
| American Indian or Alaska native | 0 | 3 (<1) |
| Asian | 93 (23) | 65 (16) |
| Native Hawaiian or other Pacific Islander | 0 | 1 (<1) |
| Other | 7 (2) | 13 (3) |
| Not reported | 0 | 2 (<1) |
| Ethnicity — n (%) | ||
| Hispanic or Latino | 41 (10) | 44 (11) |
| Not Hispanic or Latino | 189 (47) | 198 (48) |
| Not reported | 175 (43) | 169 (41) |
| Region — n (%) | ||
| US/Canada/W. Europe/N. Europe | 224 (55) | 240 (58) |
| Rest of the world | 181 (45) | 171 (42) |
| ECOG performance status — n (%) | ||
| 0 | 341 (84) | 361 (88) |
| 1 | 64 (16) | 50 (12) |
| Type of nephrectomy* — n (%) | ||
| Radical | 378 (93) | 381 (93) |
| Partial | 27 (7) | 30 (7) |
| Pathological TNM staging* — n (%) | ||
| pT2a G3 or G4, N0 M0 / pT2b, G any, N0 M0 | 60 (15) | 62 (15) |
| pT3, G any, N0 M0 | 315 (78) | 316 (77) |
| pT4, G any, N0 M0 / pT any, G any, N1 M0 | 30 (7) | 33 (8) |
| Disease risk category — n (%)† | ||
| High risk | 228 (56) | 233 (57) |
| Moderate risk | 176 (43) | 177 (43) |
| Other | 1 (<1) | 1 (<1) |
| Fuhrman grade — n (%) | ||
| Grade 1–2 | 136 (34) | 147 (36) |
| Grade 2 | 126 (31) | 136 (33) |
| Grade 3 | 189 (47) | 173 (42) |
| Grade 4 | 80 (20) | 91 (22) |
| Sarcomatoid features — n (%) | ||
| Yes | 19 (5) | 21 (5) |
| No | 386 (95) | 390 (95) |
| Time from initial disease diagnosis to randomisation — n (%) | ||
| <1 year | 405 (100) | 411 (100) |
| LDH level — n (%) | ||
| ≤1·5 × ULN | 400 (99) | 408 (99) |
| >1·5 × ULN | 0 | 1 (<1) |
| Not reported | 5 (1) | 2 (<1) |
| Haemoglobin — n (%) | ||
| <LLN | 95 (23) | 90 (22) |
| ≥LLN | 310 (77) | 321 (78) |
| Corrected calcium — n (%) | ||
| ≤10 mg/dL | 368 (91) | 377 (92) |
| >10 mg/dL | 27 (7) | 17 (4) |
| Not reported | 10 (2) | 17 (4) |
| Alkaline phosphatase – n (%) | ||
| <ULN | 375 (93) | 373 (91) |
| ≥ULN | 29 (7) | 38 (9) |
| Not reported | 1 (<1) | 0 |
Per interactive response technology.
Disease-free survival was assessed in the high and moderate risk subgroups by using the following risk staging system: high risk (pT3, G3 or G4, N0 M0; pT4, Gany, N0 M0; pTany, Gany, N1 M0) and moderate risk (pT2a, G3 or G4, N0 M0; pT2b, Gany, N0 M0; PT3, G1 and G2, N0 M0).
ECOG-Eastern Cooperative Oncology Group. LLN=lower limit of normal. Q=quartile. ULN=upper limit of normal.
As of the clinical data cutoff date of June 28, 2022, 173 (43%) of 404 treated patients in the nivolumab plus ipilimumab arm had discontinued study drug without completing treatment, with the most common reason for discontinuation being study drug toxicity in 132 (33%) of 404 treated patients (figure 1). In the placebo arm, 46 (11%) of 407 treated patients discontinued study treatment, with the most common reason being disease recurrence in 20 (5%) of 407 patients. In total, 17 (2%) of 811 treated patients discontinued treatment due to COVID-19 (nivolumab plus ipilimumab, n=9; placebo, n=8). No patients are continuing to receive nivolumab plus ipilimumab or placebo at this time. Fifty-seven (14%) patients in the nivolumab plus ipilimumab arm and 77 (19%) patients in the placebo arm received subsequent systemic therapy; most commonly, a VEGF-targeted agent was used among patients who received subsequent systemic therapy in the nivolumab plus ipilimumab arm (55 patients [96%]); table S1).
At a median follow-up (time from an individual patient’s randomisation date to the date of clinical cutoff [last patient last visit date]) of 37·0 months (IQR 31·3, 43·7), 218 events of disease recurrence or death had occurred as assessed by BICR (110 events in the nivolumab plus ipilimumab arm and 118 in the placebo arm; figure 2). The median disease-free survival was not reached in the nivolumab plus ipilimumab arm and was 50·7 months (95% CI 48·1-not estimable) in the placebo arm (figure 2). The risk of disease recurrence or death was not significantly different with adjuvant nivolumab plus ipilimumab than with placebo (HR for recurrence or death, 0·92; 95% CI 0·71–1·19; p=0·53). The estimated percentage of patients who remained alive and recurrence-free at 24 months was 76% (95% CI 72–81) in the nivolumab plus ipilimumab arm and 74% (95% CI 69–78) in the placebo arm. The corresponding median disease-free survival as assessed by investigator was not reached in either arm (HR for recurrence or death, 0·92; 95% CI 0·71–1·20; p=0·54) and the percentages of patients who remained alive and recurrence-free at 24 months were 77% (95% CI 73–81) and 74% (95% CI 69–78), with nivolumab plus ipilimumab and placebo, respectively (figure S1). The concordance between BICR and investigator assessments for events of recurrence or death and censoring was approximately 94% in the nivolumab plus ipilimumab arm and 99% in the placebo arm (figure 2 and figure S1). Median disease-free survival for the secondary definition of disease-free survival (without censoring for subsequent therapy) was not reached with nivolumab plus ipilimumab (95% CI not estimable) versus 50·7 months (95% CI 48·1-not estimable) with placebo; the HR was 0·93 (95% CI 0·72–1·20; p=0·5658) (figure S2).
Figure 2: Kaplan-Meier estimates of disease-free survival (primary definition*) per blinded independent central review.
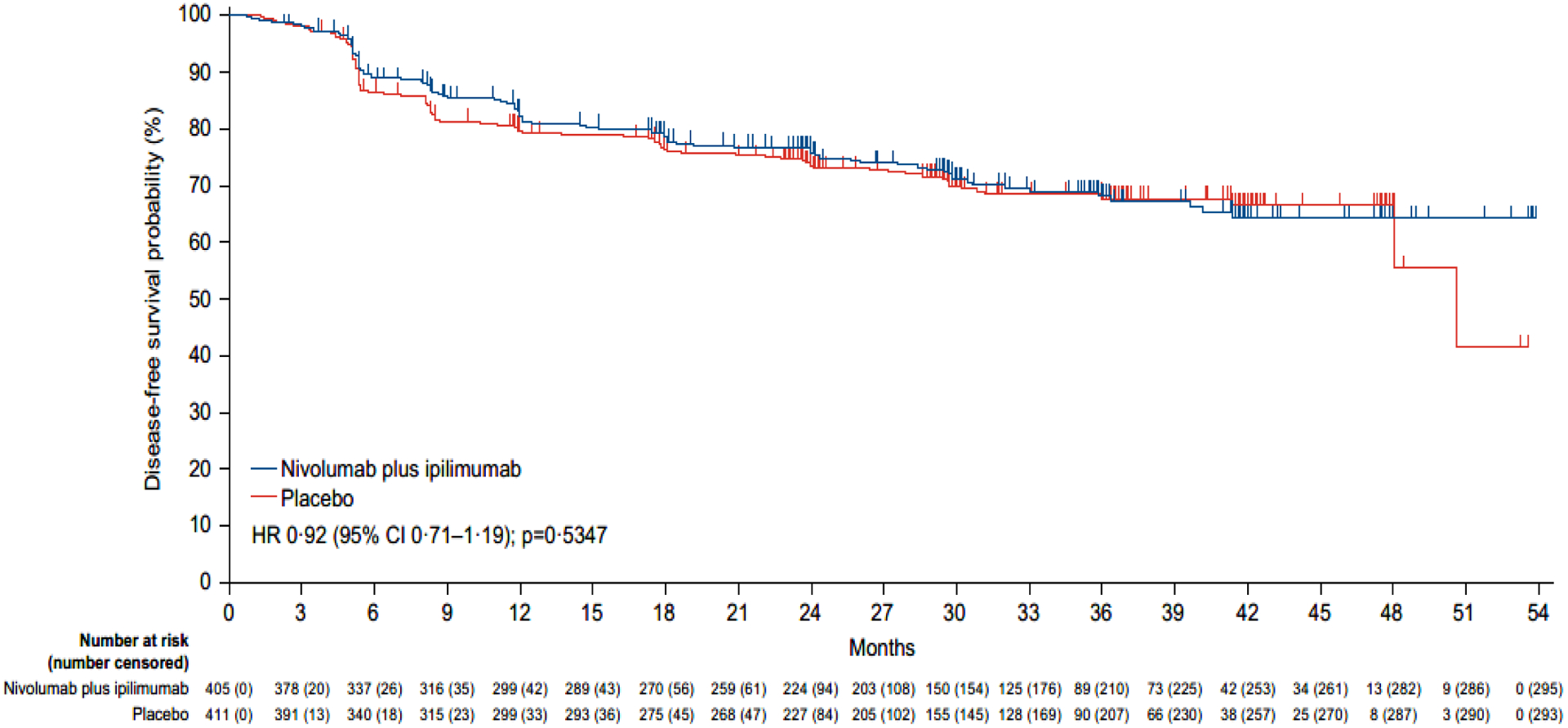
Disease-free survival according to blinded independent central review was assessed in all randomly assigned patients. The reported p value is two-sided. Tick marks represent data censored at the last time that the patient was known to be alive and free from disease recurrence.
*Subsequent therapy includes systemic anticancer therapy, tumour-directed radiotherapy, or tumour-directed surgery. Patients who died without a reported recurrence were considered to have recurred on the date of their death. The following censoring rules were applied for the primary definition of disease-free survival: 1. patients who did not recur or die were censored on the date of their last evaluable tumour assessment; 2. patients who did not have any on-study tumour assessments and did not die were censored on their date of randomisation; 3. patients who received subsequent systemic anticancer therapy, tumour-directed radiotherapy, or tumour-directed surgery before documented recurrence were censored at the date of the last tumour assessment conducted on or before the initiation of the new therapy; 4. patients who did not have a documented recurrence and received subsequent systemic anticancer therapy, tumour-directed radiotherapy, or tumour-directed surgery were censored at the date of the last tumour assessment conducted on or before the initiation of the new therapy (see supplemental methods for full censoring scheme).
Exploratory disease-free survival analyses by stratification factors and other subgroups of clinical interest were performed (figure 3). Across most subgroups, there was no difference between treatment arms. However, disease-free survival favoured nivolumab plus ipilimumab versus placebo in a small subgroup of 40 patients with sarcomatoid features; the HR for disease recurrence or death was 0·29 (95% CI 0·09–0·91) (figure 3).
Figure 3: Disease-free survival per blinded independent central review according to key subgroups.
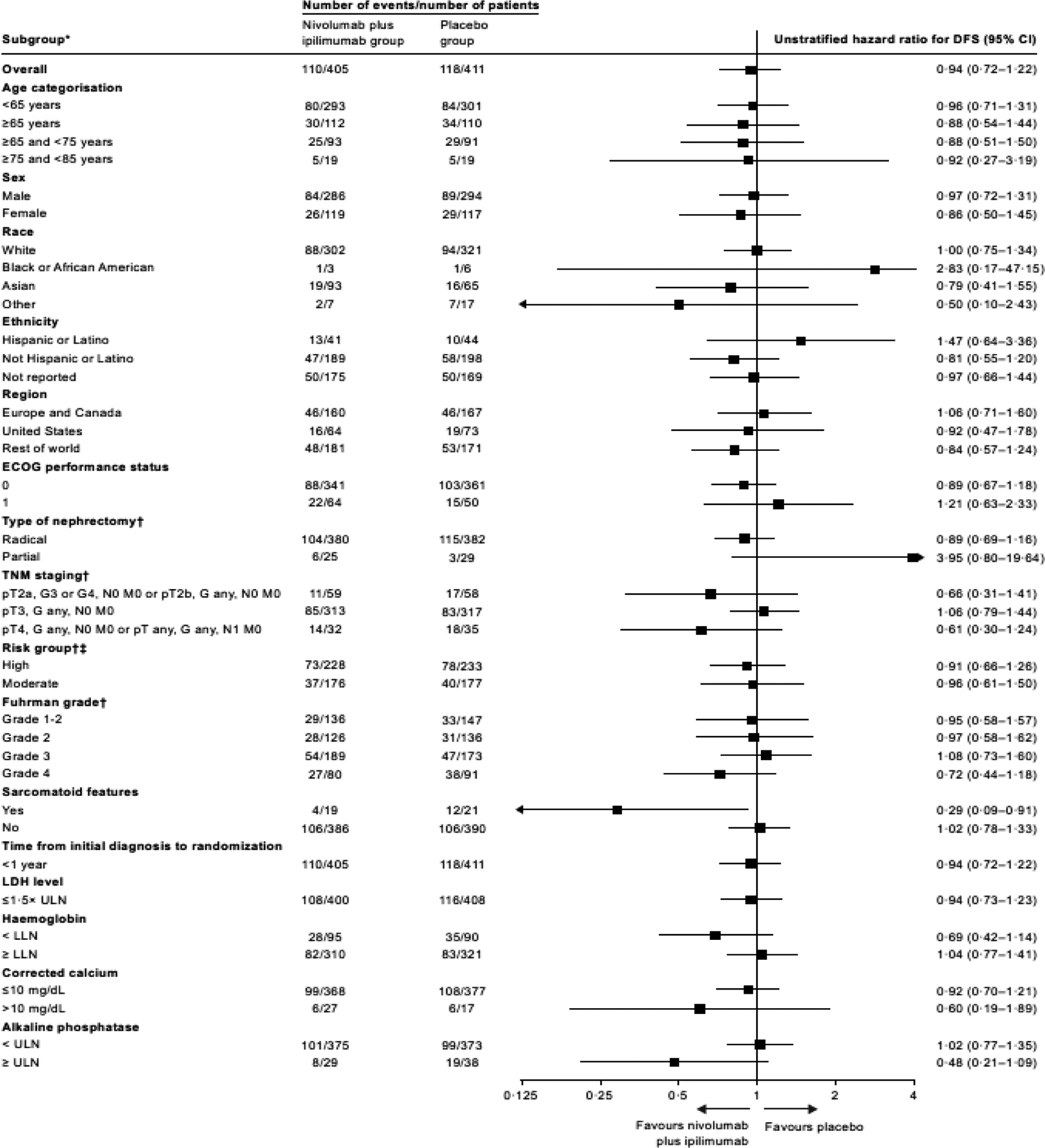
*The influence of demographic and baseline clinical characteristics on disease-free survival among randomised patients was assessed via exploratory subgroup analyses for age, sex, race, ethnicity, region, ECOG performance status, type of nephrectomy, TNM staging, risk group, Fuhrman grade, Sarcomatoid features, time from diagnosis to randomisation, lactate dehydrogenase level, haemoglobin, corrected calcium, and alkaline phosphatase. HR was not computed for subset (except age, race, region, and sex) category with fewer than 11 patients per treatment group.
†The statistical analysis plan prespecified that subgroup analyses for stratification factors (TNM staging and type of nephrectomy) would only be displayed using subgroups based on case report form data. Analysis of disease-free survival by high and moderate risk groups and by Fuhrman grade were also per case report form.
‡Disease-free survival was assessed in the high and moderate risk subgroups using the following risk staging system: high risk (pT3, G3 or G4, N0 M0; pT4, Gany, N0 M0; pTany, Gany, N1 M0) and moderate risk (pT2a, G3 or G4, N0 M0; pT2b, Gany, N0 M0; pT3, G1 and G2, N0 M0).
DFS=disease-free survival. ECOG=Eastern Cooperative Oncology Group. LDH=lactate dehydrogenase. LLN=lower limit of normal. ULN=upper limit of normal.
The number of events required for the planned overall survival interim analysis was not reached at the time of the data cutoff, and only 61 events occurred (33 in the nivolumab plus ipilimumab arm and 28 in the placebo arm) at a median follow-up of 37·0 months. Due to immaturity of the overall survival data, Kaplan–Meier estimates of the median overall survival are not estimable for both arms. Kaplan–Meier curves are presented for each arm in figure S3.
Treatment exposure is summarised in table 2. The median duration of study therapy was 5·1 months (IQR 2·8–5·3) in the nivolumab plus ipilimumab arm and 5·1 months (IQR 5·1–5·3) in the placebo arm. Treated patients in the nivolumab plus ipilimumab arm received a median of 12 (range 1–12) nivolumab doses and four (range 1–4) ipilimumab doses. In the placebo arm, treated patients received a median of 12 (range 1–12) nivolumab-placebo doses and four (range 1–4) ipilimumab-placebo doses. The majority of patients completed all cycles of nivolumab (230 of 404, 57%) and ipilimumab (266 of 403, 66%; table 2). In the nivolumab plus ipilimumab arm, 141 (35%) of 404 patients had at least one dose delay of nivolumab, and 136 (34%) patients had at least one dose delay of ipilimumab, with each delay exceeding 3 days. In the placebo arm, 110 (27%) of 407 patients had at least one dose delay of nivolumab-placebo, and 104 (26%) patients had at least one dose delay of ipilimumab-placebo, each delay also exceeding 3 days. Dose delays due to adverse events were attributed to nivolumab in 123 (62%) of 197 total doses delayed, ipilimumab in 44 (26%) of 168 total doses delayed cases, nivolumab-placebo in 45 (31%) of 146 total doses delayed, and ipilimumab-placebo in 20 (16%) of 128 doses delayed.
Table 2:
Treatment exposure and dose delay in all treated patients
| Nivolumab plus ipilimumab (N=404) |
Placebo (N=407) |
|||
|---|---|---|---|---|
| Median duration of therapy (range), months | 5·1 (<0·1–8·3) | 5·1 (<0·1–8·1) | ||
| Q1, Q3 | 2·8, 5·3 | 5·1, 5·3 | ||
|
Nivolumab
n=404 |
Ipilimumab
n=403 † |
Nivolumab-placebo
n=407 |
Ipilimumab-placebo
n=406 † |
|
| Median number of doses received (range) * | 12 (1–12) | 4 (1–4) | 12 (1–12) | 4 (1–4) |
| Last cycle received before treatment period ends |
Nivolumab
n=404 |
Ipilimumab
n=403 |
Nivolumab-placebo
n=407 |
Ipilimumab-placebo
n=406 |
| Cycle 1 | 17 | 51 | 5 | 8 |
| Cycle 2 | 21 | – | 2 | – |
| Cycle 3 | 14 | – | 2 | – |
| Cycle 4 | 22 | 50 | 4 | 12 |
| Cycle 5 | 16 | – | 2 | – |
| Cycle 6 | 12 | – | 5 | – |
| Cycle 7 | 13 | 36 | 6 | 14 |
| Cycle 8 | 13 | – | 3 | – |
| Cycle 9 | 10 | – | 6 | – |
| Cycle 10 | 12 | 266 | 5 | 372 |
| Cycle 11 | 24 | – | 6 | – |
| Cycle 12‡ | 230 | – | 361 | – |
|
Nivolumab
n=404 |
Ipilimumab
n=403 |
Nivolumab-placebo
n=407 |
Ipilimumab-placebo
n=406 |
|
| Patients with at least one dose delay, n (%) ¶ | 141 (35) | 136 (34) | 110 (27) | 104 (26) |
| Relative dose intensity, n (%) § |
Nivolumab
n=404 |
Ipilimumab
n=403 |
Nivolumab-placebo
n=407 |
Ipilimumab-placebo
n=406 |
| ≥110 | 0 | 0 | – | – |
| 90 to <110 | 332 (82) | 346 (86) | – | – |
| 70 to <90 | 63 (16) | 52 (13) | – | – |
| 50 to <70 | 7 (2) | 4 (1) | – | – |
| <50 | 2 (<1) | 1 (<1) | – | – |
Dose units are mg for nivolumab and mg/kg for ipilimumab.
Treated patients are defined as having received at least one dose of study drug (nivolumab or ipilimumab). One patient in the nivolumab plus ipilimumab treatment arm and one patient in the placebo arm did not receive the scheduled dose of ipilimumab or ipilimumab-placebo, respectively, at the time that nivolumab or nivolumab-placebo was given.
One patient in the nivolumab plus ipilimumab arm skipped nivolumab at cycle 3 and one patient in the placebo arm skipped ipilimumab-placebo at cycle 7.
A dose was considered as delayed if the delay exceeded 3 days for nivolumab or ipilimumab. Reasons for dose delay of nivolumab only (based on total number of doses delayed): adverse event, 123 (62%); other, 73 (37%); not reported, one (<1%). Reasons for dose delay of ipilimumab only (based on total number of doses delayed): adverse event, 44 (26%); other, 30 (18%); not reported, 94 (56%). Reasons for dose delay of nivolumab-placebo (based on total number of doses delayed): adverse event, 45 (31%); other, 100 (68%); not reported, one (<1%). Reasons for dose delay of ipilimumab-placebo (based on total number of doses delayed): adverse event, 20 (16%); other, 41 (32%); not reported, 67 (52%).
Defined as the actual dose received relative to the planned dose.
Q=quartile.
In the all-treated population, 392 (97%) of 404 patients who received nivolumab plus ipilimumab and 361 (89%) of 407 patients who received placebo had at least one adverse event of any grade and of any cause (table 3). In total, 154 (38%) of 404 patients who received nivolumab plus ipilimumab and 42 (10%) of 407 patients who received placebo had an adverse event of grade 3–5. The most common adverse events of any cause in the two arms were pruritus (32% with nivolumab plus ipilimumab and 17% with placebo), fatigue (30% with nivolumab plus ipilimumab and 27% with placebo), and diarrhoea (27% with nivolumab plus ipilimumab and 21% with placebo). All-cause adverse events of any grade led to the discontinuation of nivolumab plus ipilimumab in 129 (32%) of 404 treated patients and of placebo in nine (2%) of 407 treated patients (table 3).
Table 3:
All-cause adverse events (≥10% cutoff) and immune-mediated adverse events in all treated patients in either treatment arm
| All-cause adverse events | ||||
|---|---|---|---|---|
| Nivolumab plus ipilimumab (N=404) |
Placebo (N=407) |
|||
| Event, n (%)* | Grade 1–2 | Grade 3–4† | Grade 1–2 | Grade 3–4 |
| Patients with any event | 237 (59) | 154 (38) | 319 (78) | 42 (10) |
| Pruritus | 126 (31) | 2 (<1) | 69 (17) | 0 |
| Fatigue | 120 (30) | 3 (<1) | 108 (27) | 1 (<1) |
| Diarrhoea | 95 (24) | 16 (4) | 83 (20) | 2 (<1) |
| Rash | 86 (21) | 5 (1) | 37 (9) | 1 (<1) |
| Headache | 69 (17) | 2 (<1) | 59 (14) | 0 |
| Nausea | 67 (17) | 2 (<1) | 50 (12) | 0 |
| Hyperthyroidism | 65 (16) | 1 (<1) | 5 (1) | 0 |
| Arthralgia | 64 (16) | 1 (<1) | 55 (14) | 0 |
| Hypothyroidism | 63 (16) | 2 (<1) | 20 (5) | 0 |
| Decreased appetite | 51 (13) | 1 (<1) | 8 (2) | 0 |
| Cough | 50 (12) | 0 | 52 (13) | 0 |
| Asthenia | 46 (11) | 2 (<1) | 31 (8) | 0 |
| Blood creatinine increased | 45 (11) | 1 (<1) | 37 (9) | 1 (<1) |
| Increased alanine aminotransferase | 35 (9) | 10 (2) | 12 (3) | 3 (<1) |
| Study treatment discontinuation due to an adverse event‡ | 47 (12) | 82 (20) | 1 (<1) | 8 (2) |
| Immune-mediated adverse events¶ | ||||
| Nivolumab plus ipilimumab (N=404) |
Placebo (N=407) |
|||
| Categories, n (%) | Grade 1–2 | Grade 3–4 | Grade 1–2 | Grade 3–4 |
| Hypothyroidism | 76 (19) | 2 (<1) | 13 (3) | 0 |
| Rash | 61 (15) | 10 (2) | 10 (2) | 2 (<1) |
| Hyperthyroidism | 62 (15) | 1 (<1) | 2 (<1) | 0 |
| Adrenal insufficiency | 24 (6) | 11 (3) | 2 (<1) | 0 |
| Hypophysitis | 18 (4) | 12 (3) | 0 | 0 |
| Diarrhoea/colitis | 15 (4) | 22 (5) | 3 (<1) | 0 |
| Hepatitis | 9 (2) | 14 (3) | 1 (<1) | 2 (<1) |
| Thyroiditis | 9 (2) | 2 (<1) | 0 | 0 |
| Pneumonitis | 7 (2) | 3 (<1) | 1 (<1) | 0 |
| Nephritis/renal dysfunction | 5 (1) | 5 (1) | 5 (1) | 1 (<1) |
| Diabetes mellitus | 1 (<1) | 8 (2) | 0 | 0 |
Shown are adverse events that occurred while patients were receiving the assigned treatment or within 30 days after the end of the trial treatment period of all treated patients. Events are listed in descending order of frequency in the nivolumab plus ipilimumab arm.
One grade 5 event occurred in the nivolumab plus ipilimumab treatment arm.
Includes events leading to discontinuation of either nivolumab or ipilimumab at any time; all discontinuation criteria apply to nivolumab, ipilimumab, and placebo.
Immune-mediated adverse events are defined as adverse events consistent with an immune-mediated mechanism or immune-mediated component for which non-inflammatory aetiologies (eg, infection or tumour progression) have been ruled out. Includes all categories of immune-mediated adverse events reported in patients treated with nivolumab plus ipilimumab or placebo including diarrhoea/colitis, hepatitis, pneumonitis, nephritis/renal dysfunction, and rash, considered by investigators to be potentially immune-mediated, and met the following criteria: occurred within 100 days of the last dose, regardless of causality, treated with immune-modulating medication, had no clear alternate aetiology, or had an immune-mediated component. Adrenal insufficiency, hypophysitis, hypothyroidism/thyroiditis, hyperthyroidism, and diabetes mellitus were considered immune-mediated adverse events regardless of immune-modulating medication use, as these endocrine events were often managed without immune-modulating medication.
A total of 359 (89%) of 404 patients treated with nivolumab plus ipilimumab and 231 (57%) of 407 patients treated with placebo had at least one treatment-related adverse event of any grade, including an event of grade 3 or 4 in 28% of patients treated with nivolumab plus ipilimumab and 2% of patients treated with placebo (table S2). Treatment-related adverse events are listed in Table S2. Treatment-related adverse events of any grade led to the discontinuation of nivolumab plus ipilimumab in 117 (29%) of 404 treated patients and of placebo in four (1%) of 407 treated patients (table S2). The most common treatment-related adverse events leading to discontinuation were varied, with the most common being diarrhoea, hypophysitis, and increased alanine aminotransferase in 15 (4%), 10 (2%), and 10 (2%) of 404 treated patients, respectively, with nivolumab plus ipilimumab, and increased alanine aminotransferase, increased aspartate aminotransferase, increased blood creatinine, rash, and eczema in one each (<1%) of 407 patients treated with placebo. Four deaths (1% of treated patients in the nivolumab plus ipilimumab arm) were attributed to treatment with nivolumab plus ipilimumab, and were due to cardiac arrest, immunotherapy-induced diarrhoea/colitis, aortic dissection/ischaemic cerebral infarction/pulmonary embolism, and drug-induced myocarditis (in one patient each). There were no deaths attributed to treatment with placebo.
Immune-mediated adverse events in patients treated with nivolumab plus ipilimumab or placebo are reported in Table 3. The most frequently reported grade 3–4 immune-mediated adverse events in the nivolumab plus ipilimumab arm were diarrhoea/colitis (22 [5%] of 404), hepatitis (14 [3%] of 404), and hypophysitis (12 [3%] of 404). In the placebo arm, grade 3–4 immune-mediated adverse events were reported for rash (two [<1%] of 407), hepatitis (two [<1%] of 407), and nephritis/renal dysfunction (one [<1%] of 407). A total of 93 (23%) of 404 patients treated with nivolumab plus ipilimumab and 10 (2%) of 407 patients treated with placebo received corticosteroids (≥40 mg of prednisone daily or equivalent) for any duration of time to manage immune-mediated adverse events (occurring on therapy or ≤100 days after the end of the trial treatment period); 56 (14%) patients treated with nivolumab plus ipilimumab and four (1%) patients treated with placebo received corticosteroids (≥40 mg of prednisone daily or equivalent) continuously for at least 14 days, and 26 (6%) and one (<1 %) patients, respectively, continuously for at least 30 days.
Discussion
In this phase 3 trial assessing adjuvant nivolumab plus ipilimumab versus placebo for patients with localised renal cell carcinoma at high risk of post-nephrectomy recurrence, the primary efficacy endpoint of disease-free survival by BICR was not met (HR 0·92 [95% CI 0·71–1·19; p=0·53]). Disease-free survival was also not significantly different between the nivolumab plus ipilimumab and placebo arms, as assessed by the study investigators. Disease-free survival was similar between the treatment arms across most key subgroups. Of note, there was no disease-free survival benefit in the group of patients with pathologic tumour stage T3, encompassing the most heterogenous range of pathologic tumour features, including involvement of the renal vein and invasion of perirenal and/or renal sinus fat, which may be associated with a different prognosis. As expected, nivolumab plus ipilimumab was associated with higher rates of grade 3–5 adverse events of any cause, treatment-related adverse events, and adverse events leading to treatment discontinuation versus placebo. However, the overall safety of adjuvant nivolumab plus ipilimumab in patients with localised renal cell carcinoma in this trial was consistent with the known profile for the combination in advanced renal cell carcinoma.22 Together, these results do not indicate a role for the nivolumab plus ipilimumab combination as adjuvant therapy for patients with localised renal cell carcinoma at high risk by TNM criteria of post-nephrectomy recurrence.
Factors that may have contributed to the outcome reported in the CheckMate 914 trial include heterogeneity of the patient population studied, dosing schedule and duration of treatment chosen in this trial, decreased adverse event tolerability in the setting of adjuvant treatment for localised renal cell carcinoma, and other factors as discussed below.
Currently, pembrolizumab is the only immune checkpoint inhibitor approved as adjuvant therapy for localised renal cell carcinoma after nephrectomy, with specific approval in patients at increased risk of recurrence after nephrectomy or after nephrectomy and resection of metastatic lesions. This approval was based on data from the phase 3 KEYNOTE-564 trial comparing pembrolizumab monotherapy with placebo.11 With 24·1 months of median follow-up at a prespecified interim analysis, adjuvant pembrolizumab showed a significant disease-free survival benefit versus placebo.11 Overall survival reported in the primary analysis and with an extended median follow-up of 30·1 months has not shown a significant benefit, although results are still immature.11,12
While the results from CheckMate 914 and KEYNOTE-564 may appear conflicting, there are distinctions in the study designs that may have contributed to the divergent outcomes. For instance, differences in the planned and actual duration of therapy may have impacted results for each respective trial. In CheckMate 914, treatment with nivolumab plus ipilimumab was scheduled for 6 months, with an actual median duration of treatment of 5·1 months. In KEYNOTE-564, pembrolizumab treatment was scheduled for approximately 1 year, with an actual median duration of 11·1 months. Currently there is no consensus regarding the optimal treatment duration of adjuvant therapy for patients with localised renal cell carcinoma. The 6-month duration of treatment of nivolumab plus ipilimumab was designed to potentially minimise toxicity while maintaining expected efficacy, although this may have contributed to the lack of observed activity. Further distinctions between the trials included different screening methods for patient eligibility, stratification factors, primary endpoints, and documentation of disease progression, with KEYNOTE-564 using assessment by investigator versus by investigator with confirmation by BICR in CheckMate 914.11
Recently, results from two other phase 3 trials evaluating the use of adjuvant immunotherapy in renal cell carcinoma showed no improvements in disease-free survival. The IMmotion010 trial evaluated adjuvant checkpoint blockade with atezolizumab in patients with localised renal cell carcinoma at increased risk for recurrence after resection (including patients with both locally advanced intermediate- and high-risk M1 NED). With 45 months of median follow-up and a median treatment duration of 10 months, the primary analysis reported no improvement in median disease-free survival versus placebo (57·2 months vs 49·5 months; HR 0·93; p=0·50).15 Overall survival was reported with immature follow-up; however, there was no observed trend toward a survival advantage. As the trial was negative for disease-free survival, no formal analysis will be performed for overall survival. Treatment-related grade 3–4 adverse events and discontinuation rates were low compared with CheckMate 914.15 PROSPER is a phase 3 randomised open-label trial that evaluated priming the immune system with nivolumab before nephrectomy (1 dose) followed by adjuvant nivolumab (9 doses) versus surgery alone in patients with high-risk renal cell carcinoma.16 The primary endpoint of recurrence-free survival was similar in both arms (HR 0·97; 95% CI 0·74–1·28; p=0·43) with medians not reached. Overall survival was not mature at the time of analysis. Twenty percent of patients treated with nivolumab experienced at least one treatment-related grade 3–4 adverse event, compared with 6% in the control arm. The trial was stopped early by the data and safety monitoring committee due to futility.16
The primary outcomes of CheckMate 914, IMmotion010, and PROSPER contrast with that of KEYNOTE 564, likely reflecting differences in the patient populations and dosing schedules, as well as distinctions in the mechanism of action of the immunotherapy agents tested (anti-PD-1 vs anti-PD-L1 agents). IMmotion010, PROSPER, and KEYNOTE-564 permitted patients with disease stage M1 with no evidence of disease, while CheckMate 914 did not.11,15,23 IMmotion010 and PROSPER are the only trials that permitted patients with non-clear cell histology.15,23 Length of treatment assessed was predominantly 1 year, with the exception of CheckMate 914, which scheduled treatment for 6 months.11,15,23 IMmotion010 was the only trial evaluating a PD-L1 inhibitor (while anti-PD-1 inhibitors were studied in CheckMate 914, KEYNOTE-564, and PROSPER).11,15,23 Overall, PROSPER is difficult to interpret in the context of findings from other phase 3 trials (IMmotion010, CheckMate 914, and KEYNOTE-564) due to significant differences in trial design.11,15,23 Future subgroup and biomarker analyses may shed light on benefits in particular patient populations.
In contrast to the results of CheckMate 914 adjuvant trial (part A), the combination of nivolumab plus ipilimumab has demonstrated substantial efficacy and tolerability compared with sunitinib in patients with untreated advanced renal cell carcinoma and intermediate/poor risk, including long-term survival benefits, durable responses, and a favourable safety profile.20 The differences in activity observed in the localised (CheckMate 914) and advanced (CheckMate 214) settings may have been brought about by the obvious differences in patient disease characteristics but also by adverse event tolerability and treatment discontinuation rates, as well as drug exposure to the nivolumab plus ipilimumab combination.24 Historically, trials evaluating adjuvant tyrosine kinase inhibitors have shown that a given therapy was not as tolerable in patients with localised disease post-nephrectomy versus when patients have advanced or metastatic disease, leading to increased discontinuation due to treatment-related adverse events in the latter setting.6,24 Additionally, the extended treatment period for adjuvant ipilimumab compared with the condensed induction regimen in advanced disease may have reduced effectiveness of the CTLA-4 inhibitor without improving tolerability.22
Finally, the CheckMate 914 trial included two parts, A and B, with each comprising mutually exclusive randomisation schemes and patients. The primary endpoint in part A assessed the efficacy of adjuvant treatment with nivolumab plus ipilimumab versus placebo whereas the primary endpoint in part B will assess adjuvant treatment with nivolumab monotherapy versus placebo. Part B enrolment largely followed that of part A, reported herein, and compares a 6-month course of nivolumab monotherapy with placebo. This trial completed enrolment and may provide further insight regarding tolerability and the impact of a 6-month treatment program of checkpoint inhibitor monotherapy as adjuvant therapy.
One of the limitations of our study was that the enrolled population was selected based on clinical features, without clear signals for relapse or efficacy based on underlying biology. Furthermore, part A of the CheckMate 914 trial was conducted in part during the COVID-19 pandemic, with patients randomised between August 2017 and March 2021 (clinical data cutoff date was June 28, 2022). Patient participation was likely impacted by the COVID-19 constraints and implications, such as the ability to travel for continuing treatment or adverse event management, which may have also increased the rate of treatment discontinuation.
In summary, adjuvant nivolumab plus ipilimumab did not demonstrate disease-free survival benefits over placebo in patients with localised renal cell carcinoma at high risk of post-nephrectomy recurrence. Patient disease characteristics, adverse events leading to treatment discontinuation, and length of drug exposure may have contributed to the lack of efficacy observed in the trial.
Research in context panel
Evidence before this study
Patients with localised renal cell carcinoma who undergo nephrectomy have limited adjuvant therapy options that can extend the time they live free of recurrence. We searched PubMed for published clinical trial reports, with no restrictions on language, from August 22, 2012, until August 22, 2022, using the terms “immunotherapy” OR “immune checkpoint inhibitor”, “renal cell carcinoma”, and “adjuvant”. The identified literature shows that none of the recently approved adjuvant treatments showed a significant benefit for both disease-free survival and overall survival in patients with localised renal cell carcinoma at high risk of recurrence after nephrectomy. The KEYNOTE-564 trial, which evaluated adjuvant pembrolizumab versus placebo in patients with clear cell renal cell carcinoma with intermediate- to high-risk and high-risk of relapse, including a group of patients after metastasectomy and no evidence of disease (M1 NED), was the first trial of an immune checkpoint inhibitor that reported significantly improved disease-free survival, although the overall survival data are not yet mature. The European Association of Urology renal cell carcinoma guideline panel issued a weak recommendation for adjuvant pembrolizumab for patients with high-risk clear cell renal cell carcinoma, until final overall survival data are available. Furthermore, European Society for Medical Oncology clinical practice guidelines similarly recommend that adjuvant pembrolizumab should be considered optional for patients with intermediate- or high-risk operable clear cell renal cell carcinoma until overall survival data are reported.
Added value of this study
In the CheckMate 914 trial (part A) assessing adjuvant nivolumab plus ipilimumab versus placebo in patients with localised renal cell carcinoma at high risk of recurrence after nephrectomy (median follow-up 37·0 months), we report that the primary endpoint of disease-free survival was not met. Exploratory analyses showed similar outcomes across most subgroups of patients analysed by baseline characteristics of clinical interest. Safety of nivolumab plus ipilimumab in this population was consistent with the known profile for this combination in advanced renal cell carcinoma, although the rate of discontinuation due to treatment-related adverse events was higher with adjuvant nivolumab plus ipilimumab versus placebo in this trial.
Implications of all the available evidence
Despite previously demonstrated long-term efficacy of dual immune checkpoint inhibition with nivolumab plus ipilimumab in patients with previously untreated advanced renal cell carcinoma, the data from the CheckMate 914 trial does not support a role for this combination as adjuvant therapy based on dosing and duration of treatment tested for unselected patients with localised renal cell carcinoma at high risk of post-nephrectomy recurrence. The results of our study contrast with those of the KEYNOTE-564 trial, which observed a disease-free survival benefit with adjuvant pembrolizumab. However, consistent with results reported in CheckMate 914, recent reports from two other phase 3 trials evaluating the use of adjuvant/perioperative immunotherapy in renal cell carcinoma showed no improvements in disease-free survival. In IMmotion010, treatment with adjuvant atezolizumab showed no improvement in disease-free survival versus placebo, nor was there any benefit with perioperative nivolumab versus observation in the PROSPER trial. These findings suggest an ongoing need for continued investigation of alternative therapy approaches to standard surgical management for this patient population with a high unmet medical need.
Supplementary Material
Acknowledgements
Supported by Bristol Myers Squibb and Ono Pharmaceutical. We thank the patients who participated in this study, the clinical study teams, and the representatives of the sponsor who were involved in data collection and analyses. We would like to acknowledge previous trial manager Tatiana Zelinksky and current trial manager Yasmin Candelo (Bristol Myers Squibb, Princeton, NJ, USA) for serving as global trial managers. Medical writing support was provided by Rachel Maddente, PhD, of Parexel, and was funded by Bristol Myers Squibb. Patients treated at Memorial Sloan Kettering Cancer Center were supported in part by Memorial Sloan Kettering Cancer Center Support Grant (Core Grant, number P30 CA008748).
Declaration of interests
RJM reports clinical trial support (institutional) from Bristol Myers Squibb (BMS) for this manuscript; advisory board fees from AstraZeneca, AVEO, Eisai, EMD Serono, Exelixis, Genentech/Roche, Incyte, Lilly Oncology, Merck, Novartis, and Pfizer; and fees (institutional) for coordinating PI from AVEO, BMS, Eisai, Exelixis, Genentech/Roche, Merck, and Pfizer.
PR has nothing to disclose.
VG reports medical writing and processing charges from BMS for this manuscript, research grants (institutional) from BMS, Ipsen, MSD, and Pfizer; speakers’ bureau fees from Astellas, AstraZeneca, BMS, Eisai, Ipsen, Janssen-Cilag, Merck Serono, MSD, Nanobiotix, Novartis, Ono Pharmaceutical, Pfizer, and Roche; advisory board fees from Apogepha, BMS, Debiopharm, Eisai, EUSA, MSD, Nanobiotix, Oncorena, PCI Biotech, Pfizer, Roche, and Merck Serono; leadership roles (unpaid) with AIO and Das Lebenshaus, patient advocacy; stock options from AstraZeneca, BMS, MSD, and Seattle Genetics; steering committee member for BMS, Eisai, Ipsen, and Novartis; and trial chair for PharmaMar.
YT reports research grant from Chugai and Ono Pharmaceutical; speakers’ bureau fees from Astellas, BMS, Merck, and Ono Pharmaceutical; and advisory board fees from Eisai, Ono Pharmaceutical, and MSD.
BZ has nothing to disclose.
OP has nothing to disclose.
SB reports research grants from Novartis; speakers’ bureau fees from BMS, Ipsen, MSD, and Novartis; payment for expert testimony from MSD, BMS, Ipsen, and Pfizer; advisory board fees from BMS, Ipsen, MSD, Novartis, and Pfizer; fees (institutional) for coordinating PI from BMS and Ipsen; and member of AIOM and Meet-URO group.
PB reports research grants from BMS, Ipsen, MSD, Pfizer, Merck, AstraZeneca, and Janssen-Cilag; consulting fees from BMS, Ipsen, MSD, Merck, Pfizer, Janssen-Cilag, Astellas, Amgen, and Gilead; honoraria from BMS, Ipsen, MSD, Merck, Pfizer, Janssen-Cilag, Astellas, Seagen, Novartis, and AAA; travel expense support from Pfizer, Merck, BMS, and Ipsen; and advisory board fees from Merck and Pfizer.
JCG reports medical writing support from BMS for this manuscript; research grant (institutional) from BeiGene; honoraria from MSD and GlaxoSmithKline; travel expense support from AstraZeneca; advisory board fees from BMS, GlaxoSmithKline, MSD, Eisai, Janssen, and AstraZeneca; stock options from ICON Cancer Centres; meeting chair fees from Ipsen; and local PI fees from BMS.
DY has nothing to disclose.
AL has nothing to disclose.
J-BL reports consulting fees from BMS, Merck, Sanofi, Paladin, Pfizer, Novartis, Knights Pharmacy, Verity, and AbbVie; speakers’ bureau fees from Tersera and Tolmar; advisory board fees from BMS, Knights Pharmacy, and Verity; local PI for BMS; and member of AUA, Canadian Uro-Oncology Group, and Canadian Urological Association.
LA reports research grant (institutional) from BMS, consulting fees (institutional) from BMS, Ipsen, Roche, Novartis, Pfizer, Astellas Pharma, Merck, MSD, AstraZeneca, Janssen, and Eisai; and travel support from BMS and MSD.
SG reports advisory board fees from AVEO, Bayer, BMS, Corvus, Eisai, EMD Serono, Exelixis, Merck, Pfizer, QED Therapeutics, Sanofi/Genzyme, and Seattle Genetics; and non-financial interests from Agensys, Aravive, AVEO, Bayer, BMS, Calithera, Corvus, Eisai, Exelixis, Gilead, Merck, Novartis, Pfizer, Seattle Genetics, and Surface Oncology.
BSh reports research grants (institutional) from Allogene and Rebiotix; royalties from Up to Date; consulting fees from Veracyte and Merck; speakers’ bureau fees from Merck; advisory board fees from Genentech, Merck, and Johnson & Johnson; and board leadership fees from National Cancer Institute PDQ.
JSo reports consulting fees from Apexigen, Jazz Pharmaceuticals, and Iovance Biotherapeutics; and honoraria from Apexigen, Jazz Pharmaceuticals, and Iovance Biotherapeutics.
MS reports support from BMS for this manuscript; grants from Pfizer, GlaxoSmithKline, AVEO, BMS, Novartis, Bayer, Roche/Genentech, Immatics, Wilex, Ipsen, Exelixis, and Eisai; consulting fees from Pfizer, GlaxoSmithKline, Novartis, Bayer, Roche AVEO, EUSA Pharma, Astellas, Ipsen, Exelixis, Peloton Therapeutics, Eisai, BMS, MSD, and Apogepha; and travel support from Pfizer, EUSA Pharma, Ipsen, Eisai, BMS, MSD, and Apogepha.
SVE has nothing to disclose.
BSi is employed by and has stock ownership in BMS.
JSp is employed by and has stock ownership in BMS.
AC is employed by and has stock ownership in BMS.
AB reports research grants from Pfizer, advisory board fees from the International Kidney Cancer Coalition and the Kidney Cancer Association; and local PI and steering committee member from BMS and Roche/Genentech.
Footnotes
Data sharing
Bristol Myers Squibb’s policy on data sharing can be found online (https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html). De-identified and anonymised datasets of clinical trial information, including patient-level data, will be shared with external researchers for proposals that are complete and for which the scientific request is valid and the data are available, consistent with safeguarding patient privacy and informed consent. Upon execution of an agreement, the de-identified and anonymised datasets can be accessed via a secured portal that provides an environment for statistical programming with R as the programming language. The protocol and statistical analysis plan will also be available. Data will be available for 2 years from the study completion or termination of the programme (July 2024).
References
- 1.Motzer RJ, Jonasch E, Agarwal N, et al. Kidney cancer, version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2022; 20: 71–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martinez Chanza N, Tripathi A, Harshman LC. Adjuvant therapy options in renal cell carcinoma: Where do we stand? Curr Treat Options Oncol 2019; 20: 44. [DOI] [PubMed] [Google Scholar]
- 3.Motzer RJ, Ravaud A, Patard JJ, et al. Adjuvant sunitinib for high-risk renal cell carcinoma after nephrectomy: subgroup analyses and updated overall survival results. Eur Urol 2018; 73: 62–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harshman LC, Xie W, Moreira RB, et al. Evaluation of disease-free survival as an intermediate metric of overall survival in patients with localized renal cell carcinoma: a trial-level meta-analysis. Cancer 2018; 124: 925–33. [DOI] [PubMed] [Google Scholar]
- 5.Tacconi EMC, Tuthill M, Protheroe A. Review of adjuvant therapies in renal cell carcinoma: evidence to date. Onco Targets Ther 2020; 13: 12301–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ravaud A, Motzer RJ, Pandha HS, et al. Adjuvant sunitinib in high-risk renal-cell carcinoma after nephrectomy. N Engl J Med 2016; 375: 2246–54. [DOI] [PubMed] [Google Scholar]
- 7.Gul A, Rini BI. Adjuvant therapy in renal cell carcinoma. Cancer 2019; 125: 2935–44. [DOI] [PubMed] [Google Scholar]
- 8.Patel HD, Kates M, Allaf ME. Adjuvant therapy for urothelial and renal cell carcinoma. Eur Urol Focus 2020; 6: 3–6. [DOI] [PubMed] [Google Scholar]
- 9.Kuusk T, Abu-Ghanem Y, Mumtaz F, Powles T, Bex A. Perioperative therapy in renal cancer in the era of immune checkpoint inhibitor therapy. Curr Opin Urol 2021; 31: 262–9. [DOI] [PubMed] [Google Scholar]
- 10.Janjigian YY, Wolchok JD, Ariyan CE. Eradicating micrometastases with immune checkpoint blockade: Strike while the iron is hot. Cancer Cell 2021; 39: 738–42. [DOI] [PubMed] [Google Scholar]
- 11.Choueiri TK, Tomczak P, Park SH, et al. Adjuvant pembrolizumab after nephrectomy in renal-cell carcinoma. N Engl J Med 2021; 385: 683–94. [DOI] [PubMed] [Google Scholar]
- 12.Powles T, Tomczak P, Park SH, et al. Pembrolizumab versus placebo as post-nephrectomy adjuvant therapy for clear cell renal cell carcinoma (KEYNOTE-564): 30-month follow-up analysis of a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2022; 23: 1133–44. [DOI] [PubMed] [Google Scholar]
- 13.European Medicines Agency. Keytruda. 2022. https://www.ema.europa.eu/en/medicines/human/EPAR/keytruda (accessed October 12, 2022).
- 14.KEYTRUDA (pembrolizumab) [prescribing information]. Whitehouse Station, NJ: Merck & Co., Inc, 2022. [Google Scholar]
- 15.Pal SK, Uzzo R, Karam JA, et al. Adjuvant atezolizumab versus placebo for patients with renal cell carcinoma at increased risk of recurrence following resection (IMmotion010): a multicentre, randomised, double-blind, phase 3 trial. Lancet 2022; 400: 1103–16. [DOI] [PubMed] [Google Scholar]
- 16.Allaf ME, Kim SE, Harshman LC, et al. LBA67 Phase III randomized study comparing perioperative nivolumab (nivo) versus observation in patients (pts) with renal cell carcinoma (RCC) undergoing nephrectomy (PROSPER, ECOG-ACRIN EA8143), a National Clinical Trials Network trial. LBA67. Ann Oncol; 33(suppl 7): S808–S69. 10.1016/annonc/annonc1089. [DOI] [Google Scholar]
- 17.Bajorin DF, Witjes JA, Gschwend JE, et al. Adjuvant nivolumab versus placebo in muscle-invasive urothelial carcinoma. N Engl J Med 2021; 384: 2102–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelly RJ, Ajani JA, Kuzdzal J, et al. Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer. N Engl J Med 2021; 384: 1191–203. [DOI] [PubMed] [Google Scholar]
- 19.Weber J, Mandala M, Del Vecchio M, et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med 2017; 377: 1824–35. [DOI] [PubMed] [Google Scholar]
- 20.Motzer RJ, McDermott DF, Escudier B, et al. Conditional survival and long-term efficacy with nivolumab plus ipilimumab versus sunitinib in patients with advanced renal cell carcinoma. Cancer 2022; 128: 2085–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.AJCC Cancer Staging Manual. New York, NY: Springer; 2010. [Google Scholar]
- 22.Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med 2018; 378: 1277–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haas NB, Puligandla M, Allaf ME, et al. PROSPER: phase III randomized study comparing perioperative nivolumab versus observation in patients with renal cell carcinoma (RCC) undergoing nephrectomy (ECOG-ACRIN EA8143). J Clin Oncol 2020; 38(suppl 15): TPS5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Motzer RJ, Rini BI, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: extended follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial. Lancet Oncol 2019; 20: 1370–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


