Abstract
Parkinson's disease (PD) is a common neurodegenerative disorder characterized by selective dopaminergic cell loss in the substantia nigra, but its pathogenesis remains unclear. The recessively inherited familial PD genes PARK2 and PARK6 have been attributed to mutations in the Parkin and PTEN-induced kinase 1 (PINK1) genes, respectively. Recent reports suggest that PINK1 works upstream of Parkin in the same pathway to regulate mitochondrial dynamics and/or conduct autophagic clearance of damaged mitochondria. This phenomenon is preserved from Drosophila to human cell lines but has not been demonstrated in a vertebrate animal model in vivo. Here, we developed a medaka fish (Oryzias latipes) model that is deficient in Pink1 and Parkin. We found that despite the lack of a conspicuous phenotype in single mutants for Pink1 or Parkin, medaka that are deficient in both genes developed phenotypes similar to that of human PD: late-onset locomotor dysfunction, a decrease in dopamine levels and a selective degeneration of dopaminergic neurons. Further analysis also revealed defects in mitochondrial enzymatic activity as well as cell death. Consistently, PINK1 and Parkin double-deficient MEF showed a further decrease in mitochondrial membrane potential and mitochondrial complex I activity as well as apoptosis compared with single-deficient MEF. Interestingly, these mitochondrial abnormalities in Parkin-deficient MEF were compensated by exogenous PINK1, but not by disease-related mutants. These results suggest that PINK1 and Parkin work in a complementary way to protect dopaminergic neurons by maintaining mitochondrial function in vertebrates.
INTRODUCTION
Parkinson's disease (PD) is a common neurodegenerative disorder that is characterized by late onset of motor symptoms associated with selective dopaminergic cell loss in the substantia nigra. Although the majority of cases are sporadic, several genes responsible for familial PD have been identified. Mutations in the genes encoding the E3 ubiqutin ligase Parkin (1) and PTEN-induced kinase 1 (PINK1) (2) are the leading causes of autosomal recessive-familial PD. To gain insights into the mechanisms of the disease, many PINK1 and Parkin-related animal models of PD have been created. Studies using Drosophila melanogaster show that a loss of PINK1 or Parkin similarly results in sperm and flight muscle defects with mitochondrial dysfunction and muscular degeneration associated with only a small decrease in dopaminergic neurons. Interestingly, the PINK1-mutant phenotype can be rescued by Parkin, while the Parkin-mutant phenotype cannot be rescued by PINK1 leading to the hypothesis that PINK1 and Parkin work in a single pathway in maintaining mitochondrial homeostasis and function with PINK1 upstream of Parkin (3–5). On the other hand, mice lacking PINK1 or Parkin exhibit less conspicuous changes; alterations in dopamine release without locomotor dysfunction or dopaminergic cell death (6–13) or even no phenotype (14). Only several conditional knockout or overexpression mice of PD-related genes, such as Parkin conditional knockout mice (15) and α-synuclein conditional transgenic mice (16), show dopaminergic cell loss.
Recently, teleost fish have gained wide interest in the modeling of PD. Indeed, several genetic models have been developed using knockdown or transgenic expression of genes that are related to PD including pink1 or parkin (17–20). We previously reported that administration of classical (21,22) and non-classical (22,23) neurotoxins in medaka induces the hallmark changes seen in PD: loss of dopaminergic neurons and locomotor dysfunction. To study the effects of familial PD genes on medaka, we also created a Pink1 homozygous mutant medaka (24) that only showed late-onset locomotor dysfunction and anomalies in the metabolism of dopamine.
In this study, we created two new medaka models: one with a mutation in Parkin and the other in both Pink1 and Parkin. Although Parkin single-deficient medaka did not show any prominent PD-related phenotypes, Pink1 and Parkin double-deficient medaka showed late-onset selective death of dopamine neurons in the middle diencephalon, a decrease in dopamine levels and a deterioration of locomotor behavior which recapitulate cardinal features of human PD. Concomitant to this, we found a decrease in mitochondrial complex activities as well as cell death in Pink1 and Parkin double-deficient medaka. Moreover, we also confirmed these findings using mouse embryonic fibroblasts (MEFs), in which deficiency in both PINK1 and Parkin function led to a further deterioration of mitochondrial dysfunction compared with PINK1 or Parkin single deficiency. Interestingly, this decrease in mitochondrial activity in a Parkin-deficient MEF was partially rescued by PINK1 but not by its mutants. Our results suggest that PINK1 and Parkin maintain mitochondrial respiratory function in a complementary way and that loss of both leads to PD-related phenotypes in vertebrates.
RESULTS
Parkin-deficient medaka did not develop a pronounced phenotype
We previously generated several Parkin-mutant medaka lines by ENU mutagenesis (25). A BLAST search using the Oryzias latipes EST database showed a single orthologue of the human Parkin gene that was highly homologous to that of human, mouse and fly (Supplementary Material, Fig. S1A). Using in situ hybridization we found that parkin mRNA was ubiquitously expressed throughout the medaka brain similarly to humans (Supplementary Material, Fig. S2A–D). We selected a strain that has a nonsense mutation resulting in a stop codon at the position Y320X of the medaka Parkin protein (Supplementary Material, Fig. S1B). This mutation also caused nonsense-mediated mRNA decay (Supplementary Material, Fig. S1C) and the mRNA expression was almost absent in the brain of the homozygous mutant (Supplementary Material, Fig. S2E–H). Hereafter, this homozygous-mutant medaka will be referred to as Parkin-deficient medaka. To assess whether this mutation in Parkin could lead to PD-related changes in medaka, we first analyzed their swimming behavior. Despite a slight decrease in body weight at 12 months (Fig. 1A), there were no changes in swimming distance, duration and velocity in 4- and 12-month-old Parkin-deficient medaka (Fig. 1B–D). Furthermore, there was no dopaminergic neuronal loss in this fish (Fig. 2C, D). These data indicate that Parkin deficiency does not lead to conspicuous PD-related phenotypic changes in medaka fish similar to previous results in zebrafish (19).
Figure 1.
Body weight and spontaneous swimming movement of medaka. (A) Body weight (g). (B) Total swimming distance (cm). (C) Duration of swimming movement (s). (D) Swimming velocity (cm/s). Pink1+: Pink1 wild-type medaka, Pink1−: Pink1-deficient medaka, Parkin+: Parkin wild-type medaka, Parkin−: Parkin-deficient medaka. *P < 0.05, **P < 0.01, ***P < 0.001. Error bars represent SEM. n = 12 (A), and n = 15 for each group (B–D).
Figure 2.
Number of TH+ neurons in the middle diencephalon and medulla oblongata. (A) Western blot analysis of TH and TPH proteins at 12 months. (B) Ratio of TH/β-actin proteins and TPH/β-actin proteins. (C) Representative photographs of middle diencephalic dopaminergic neurons in Pink1+/Parkin+, Pink1−/Parkin+, Pink1+/Parkin− and Pink1−/Parkin−. (D) Number of TH+ neurons in the middle diencephalon at 4 and 12 months (top). Number of TH+ neurons in the medulla oblongata at 12 months (bottom). *P < 0.05, **P < 0.01, ***: P < 0.001. Error bars represent SEM. n = 8 for each group (B and D).
Pink1 and Parkin double deficiency led to a deterioration of motor function
Several studies present evidence that PINK1 and Parkin hold various functions in maintaining mitochondrial homeostasis including mitochondrial fission/fusion, mitochondrial transport, mitochondrial respiration and quality control (26). These studies altogether indicate that PINK1 and Parkin have a multitude of functions that are either dependent on or independent of each other, some of which compensate for the other (27–29). Because of the lack of striking phenotypic changes in medaka that were deficient in either Pink1 or Parkin and suspecting that functional loss of both genes would lead to a loss of compensation, we crossed the Parkin-mutant medaka with Pink1-mutant medaka (22). From here on, we shall refer to the Pink1 and Parkin double homozygous-mutant medaka as double-deficient medaka. Though there were no apparent differences between wild-type, Pink1-deficient, Parkin-deficient and double-deficient medaka at 4 months (Fig. 1A–D), 12-month-old Pink1-deficient medaka showed a decrease in swimming distance, duration, velocity and body weight (Fig. 1A–D) consistent with our previous results (22). Moreover, we found that at the same age, double-deficient medaka showed a worsening of swimming behavior compared with single mutants (Fig. 1B–D). Interestingly, though Pink1 or Parkin single-deficient medaka had a reduction in body weight at 12 months, this weight loss was further aggravated in the double-deficient medaka (Fig. 1A). These data suggest that loss of both Pink1 and Parkin function in medaka causes a deterioration of locomotive behavior compared with single loss in either Pink1 or Parkin.
Pink1 and Parkin double-deficiency in medaka led to a selective loss of the middle diencephalic dopaminergic neurons
To understand the mechanisms underlying the locomotor dysfunction seen in 12-month-old Pink1 and Parkin double-deficient medaka, we examined whether, like in human PD, the locomotor phenotype is associated with dopaminergic cell loss. First, we measured the amount of tyrosine hydroxylase (TH), an enzyme specific to catecholaminergic neurons and used as a marker for dopaminergic and noradrenergic neurons. In medaka whole brains, we found a reduction in the amount of TH protein levels in the double-deficient medaka compared with wild-type or single-deficient medaka (Fig. 2A, B). To confirm whether the loss of TH protein levels was associated with dopaminergic neuronal loss, we performed immunohistochemical analysis of the medaka diencephalon. Similar to our previous neurotoxin-treated medaka PD models (22,23), 12-month-old double-deficient medaka showed a statistically significant decrease in TH-positive (TH+) cells in the middle diencephalon compared with other genotypes, with no differences present at 4 months (Fig. 2C and D). No changes were observed among TH+ neurons in the caudal diencephalon and rostro-ventral diencephalon (Supplementary Material, Fig. S3A).
In zebrafish, the ventral telencephalon has been shown to be the equivalent of the human striatum, receiving inputs from dopaminergic neurons from the diencephalon (18). To examine whether the loss of middle diencephalic dopaminergic neurons is associated with a loss of dopaminergic fibers in the ventral telencephalon, we examined TH immunoreactivity in this region. Indeed, at 12 months, we observed a decrease in TH staining in the ventral telencephalon of double-deficient medaka (Supplementary Material, Fig. S4), suggesting a loss of dopaminergic fibers in this region. Altogether, these results suggest that mutations in both Pink1 and Parkin lead to an age-related, selective decrease in dopaminergic neurons in the middle diencephalon associated with a loss of dopaminergic fibers in the telencephalon. We further noted that double-deficient medaka also showed a decrease in TH+ noradrenergic cells in the medulla oblongata, while no differences were found in Pink1- or Parkin-deficient medaka (Fig. 2D).
To address the effect of Pink1 and Parkin deficiency on non-catecholaminergic neurons, we measured the amount of tryptophan hydroxylase (TPH), a marker of serotonergic neurons, and found no difference among the four genotypes (Fig. 2A and B). In addition, there were no changes in the number of TPH-positive (TPH+) neurons in the raphe (Supplementary Material, Fig. S3B). These results suggest that Pink1 and Parkin deficiency selectively affects catecholaminergic neurons but not serotonergic neurons.
Collectively, these results indicate that though deficiency in either Pink1 or Parkin did not induce changes in the number of dopaminergic neurons in the brain, deficiency in both Pink1 and Parkin led to an age-dependent selective reduction of dopaminergic neurons.
The amount of dopamine was decreased in Pink1 and Parkin double-deficient medaka
To further examine the effects of Pink1 and Parkin double deficiency, we measured the amounts of several neurotransmitters by high-performance liquid chromatography (HPLC). Again though there were no changes seen at 4 months among the four groups, we found a significant decrease in dopamine (Fig. 3A) and noradrenaline (Fig. 3B) levels in 12-month-old double-deficient medaka reflecting the loss of TH+ neurons in the middle diencephalon and the medulla oblongata, respectively. We also measured serotonin and found no differences among all the groups at 4 and 12 months (Fig. 3C), compatible with the absence of reduction in TPH+ neurons. This offers further evidence of a selective degeneration of cathecolaminergic neurons in Pink1 and Parkin double-deficient medaka. These results strongly indicate that in medaka there is an age-dependent loss of dopaminergic and noradrenergic neurons associated with a decrease in dopamine and noradrenaline levels when Pink1 and Parkin are both deficient.
Figure 3.

Dopamine, noradrenaline and serotonin levels in medaka whole brain. (A) Amount of dopamine. (B) Amount of noradrenaline. (C) Amount of serotonin. All values are expressed as a percentage of the amount (ng) per protein weight (mg) for Pink1+/Parkin+. *: P < 0.05, **: P < 0.01, ***: P < 0.001. Error bars represent SEM. n = 12 for each group (A–C).
Pink1 and Parkin double-deficient medaka showed mitochondrial damage
Mitochondrial dysfunction has been classically linked to sporadic and familial forms of PD (26). Given the age-dependent appearance of locomotor dysfunction and dopaminergic cell loss in double-deficient medaka, we also examined whether mitochondrial dysfunction is present in these fish. Using mitochondria from medaka brains, we found that at 12 months of age, though Pink1 and Parkin single depletion did not cause any defects in mitochondrial complex activities, double-deficient medaka showed a significant decrease in both complex I and II activities (Fig. 4A). These results are consistent with previous studies where mitochondrial dysfunction was found in leukocytes (30) and fibroblasts (31,32) of PARK2 and PARK6 patients.
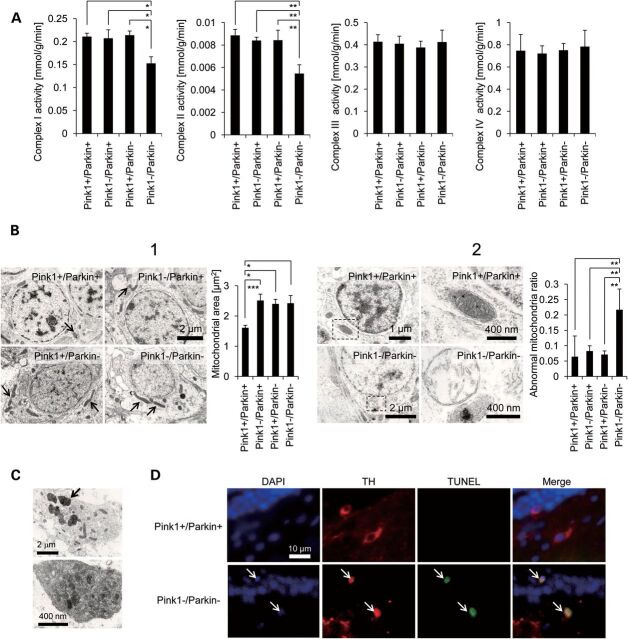
Figure 4.
Mitochondrial complex I–IV activity, transmission electron microscopic images and TUNEL assay of medaka brains. (A) Mitochondrial complex I–activities. Two micrograms of medaka brain lysate was used for complex I and II assays, and 10 µg for complex III and IV assays. n = 8 for each group. (B) B1: Elongated mitochondria in single- and double-deficient medaka. B2: Damaged mitochondria in Pink1−/Parkin− brains with an abnormal cristae structure. In the left panel, images on the right are magnified from areas indicated on the left. n = 4 (brains) for each group, 10 different regions per brain were used for the statistics. (C) Inclusion bodies containing filamentous materials in Pink1−/Parkin− brains. (D) TUNEL assay in Pink1−/Parkin− brains. White arrows indicate TH and TUNEL double-positive neurons in the middle diencephalon. *P < 0.05, **P < 0.01, ***P < 0.001. Error bars represent SEM.
Electron microscopy of the middle diencephalon of Pink1 and Parkin double-deficient brains further revealed grossly enlarged mitochondria with fragmented cristae when compared with normal mitochondria from wild-type medaka (Fig. 4B). These morphological changes are similar to those observed in PINK1- or Parkin-mutant Drosophila (3,4). Interestingly, though the degree of mitochondrial enlargement was similar between single-deficient medaka and double-deficient medaka, the number of abnormal mitochondria with disrupted cristae structure was much higher in double-deficient medaka than in single-deficient medaka (Fig. 4B). These data suggest that depletion of both Pink1 and Parkin leads to mitochondrial respiratory and morphological alterations. Interestingly, we also found inclusion bodies containing filamentous materials in the double-deficient medaka (Fig. 4C).
Because of the key role that the mitochondria plays in the activation of cell death pathway and the roles of PINK1 and Parkin in the release of the initiator of apoptosis, cytochrome c (27,33,34), we checked whether cell death occurred resulting in dopaminergic cell loss. TH and TUNEL double-positive neurons were detected in the middle diencephalon of double-deficient medaka (Fig. 4D), suggesting that the loss of TH+ neurons could be attributed to cell death. Cell death of TH+ neurons was not detected in the diencephalon of wild-type, Pink1- or Parkin single-deficient medaka (data not shown). These results are consistent with previous studies where TUNEL-positive dopaminergic cells as well as activated caspases have been found in the substantia nigra of sporadic PD patients (35–37).
Altogether these data suggest that a loss of normal functions of both PINK1 and Parkin induces mitochondrial respiratory chain dysfunction, mitochondrial morphological alterations as well as cell death.
Deficiency in both PINK1- and Parkin-induced mitochondrial respiratory dysfunction in mouse embryonic fibroblasts
To further assess the roles of PINK1 and Parkin in mitochondrial homeostasis, we performed cell culture studies using MEF. First, we confirmed the expression of Parkin protein in MEF immediately after immortalization, and also confirmed the absence of Parkin protein in Parkin-deficient MEF through immunoblotting (Supplementary Material, Fig. S5). To illustrate that Parkin can work in cooperation with PINK1 in the maintenance of mitochondrial integrity, we used MEF treated with the protonophore, carbonyl cyanide m-chlorophenyl hydrazone that dissipates the mitochondrial membrane potential (ΔΨm) and confirmed the finding that Parkin is recruited to impaired mitochondria in a PINK1-dependent manner (29) (Supplementary Material, Fig. S6). First, to create an MEF that are deficient in both Parkin and PINK1, we have knocked down PINK1 in WT and Parkin-deficient MEF (Fig. 5A). As in human primary leukocytes and fibroblasts (30,31), we found that Parkin-deficient MEF have a decrease in mitochondrial complex I activity (Fig. 5A). Furthermore, a PINK1-deficient MEF derived from PINK1-deficient mouse or treated with siRNA against Pink1 also showed a decrease in complex I activity (Fig. 5A) consistent with previous studies in PINK1-deficient mice (7,38) and PARK6 patient fibroblasts (32). Surprisingly, knockdown of PINK1 in Parkin-deficient MEF led to a further reduction in complex I activity (Fig. 5B). These results show that though mitochondrial complex I activity is decreased in PINK1 or Parkin deficiency, loss of both PINK1 and Parkin leads to a further decrease in this activity.
Figure 5.

Mitochondrial function and apoptosis in MEFs. (A) RT–PCR showing PINK1 knockdown in WT and Parkin−/− MEF (left) and mitochondrial complex I activity (right). (B) Mitochondrial complex I activity in double-deficient MEFs. (C) TMRE fluorescence. (D) Annexin V staining. (E) Caspase 3 and cleaved caspase 3 immunoblotting. (F) Representative photographs of ssDNA staining in MEF (left). Number of ssDNA-positive cells over the total number of cells (right). More than 160 cells were analyzed per group. *P < 0.05, **P < 0.01, ***P < 0.005, ****P < 0.001. Error bars represent SD (A—C) and SEM (D). n = 3 for (A and B), n ≥ 5 for (C).
We also measured ΔΨm, often used as an indicator of mitochondrial condition, using tetramethylrhodamine ethyl ester (TMRE), a mitochondrial potentiometric fluorescent dye (39). In agreement with our results on mitochondrial complex I function and with previous findings (31,40,41), Parkin-deficient MEF and PINK1-deficient MEF displayed a decrease in ΔΨm (Fig. 5C). We also confirmed that PINK1 knockdown led to a decrease in ΔΨm. As would be expected, Parkin-deficient MEF treated with Pink1 siRNA showed a further deterioration of ΔΨm (Fig. 5C). These data suggest that PINK1 and Parkin function to maintain fundamental mitochondrial homeostasis, as measured here by complex I activity and ΔΨm, and concomitant absence of PINK1 and Parkin leads to an additive decline in mitochondrial functions.
To confirm whether apoptosis plays a role in cell death in our MEF and because mitochondrial dysfunction, including loss of ΔΨm, is thought to activate the apoptotic pathway (37,42), we checked increases in three different markers of apoptotic cell death: Annexin V staining, cleaved caspase 3 as well as ssDNA staining. Indeed, deficiency in both PINK1 and Parkin led to an increase in Annexin V staining (Fig. 5D). Furthermore, we found that though MEF that were deficient in PINK1 or Parkin showed only a few apoptotic cells, deficiency in both PINK1 and Parkin led to a prominent increase in apoptosis as revealed by the activation of caspase 3 (Fig. 5E) and the increased number of cells positive for ssDNA (Fig. 5F). These results indicate that in the deficiency of both PINK1 and Parkin, MEF shows typical signs of apoptosis.
Altogether, these results suggest that in the absence of PINK1 or Parkin, there exist some compensational mechanisms to maintain mitochondrial function that are lost or perturbed in the concomitant absence of both PINK1 and Parkin. Illustrating one of these compensational mechanisms, we found that in a Parkin-deficient MEF, there seemed to be a transcriptional upregulation of Pink1 mRNA, whereas in PINK1-deficient MEF Parkin mRNA was upregulated (Supplementary Material, Fig. S7). In medaka fish, however, pink1 mRNA upregulation was not observed in Parkin-deficient medaka, although parkin upregulation was conserved between MEF and medaka fish (Supplementary Material, Fig. S7).
Mitochondrial function was independently regulated by PINK1 and Parkin
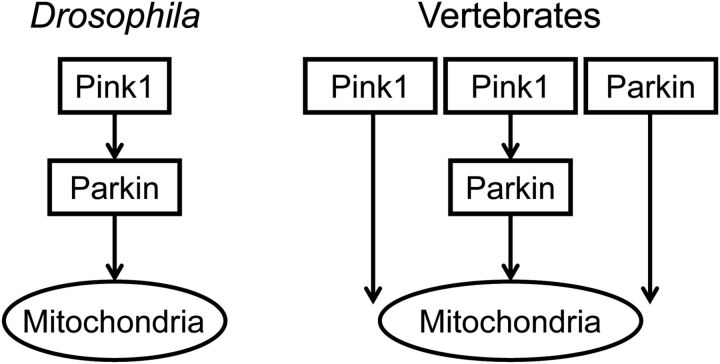
To further show that PINK1 and Parkin may work in parallel pathways to protect mitochondria, we overexpressed PINK1 in Parkin-deficient MEF and assessed mitochondrial activity. First, we confirmed the expression, in Parkin-deficient MEF, of exogenous Parkin, PINK1 and two PINK1 mutants; L347P PINK1, a disease-related kinase-inactive, and D362A; D384A PINK1, a kinase-dead mutant containing mutations in catalytically important residues that have been shown individually to inactivate PINK1 kinase function (33) (Fig. 6A). Overexpression of Parkin in Parkin-deficient MEF rescued mitochondrial complex I activity and ΔΨm (Fig. 6B, C). Interestingly, PINK1 overexpression rescued the Parkin-deficient MEF phenotype in both mitochondrial complex I activity (Fig. 6B) and ΔΨm (Fig. 6C). Overexpression of PINK1 mutants could rescue neither mitochondrial complex I activity nor ΔΨm, suggesting the importance of the kinase function of PINK1. Furthermore, Parkin overexpression also rescued ΔΨm of the PINK1-knockdowned MEF (Supplementary Material, Fig. S8). These results further indicate that PINK1 and Parkin operate in parallel pathways where they can both independently protect mitochondria including mitochondrial membrane potential and complex I activity in vertebrates (Fig. 7).
Figure 6.
PINK1 rescue of Parkin-deficient mitochondrial phenotypes. (A) Immunoblotting of overexpressed Parkin, PINK1, L347P PINK1 and D362A; D384A PINK1 in Parkin-deficient MEF. (B) Mitochondrial complex I activity. (C) TMRE fluorescence. *P < 0.05. Error bars represent SD. n = 3 for (B) and n ≥ 5 for (C).
Figure 7.
Proposed roles of Parkin and PINK1 in protecting mitochondrial function in Drosophila and vertebrates.
DISCUSSION
In this study, we have shown that PINK1- or Parkin-deficient medaka did not show any striking PD-related phenotypes, while double-deficient medaka developed the hallmark changes in PD; age-dependent loss of dopaminergic neurons, locomotor dysfunction, mitochondrial dysfunction and cell death. Consistent with the medaka data, we observed that double deficiency in both PINK1 and Parkin in MEF cells, compared with single deficiency, caused a further decrease in mitochondrial function as well as apoptosis. Moreover, the findings that PINK1 could rescue the mitochondrial phenotype of the Parkin-deficient MEF show that PINK1 can maintain mitochondrial function, in part, independently of Parkin.
The phenotype of Pink1- and Parkin-deficient medaka was quite different from that of Drosophila and mice. In Drosophila single deficiency in PINK1 or Parkin was enough to cause severe mitochondrial and muscular phenotypes (3–5), whereas in mice even double deficiency in PINK1 and Parkin did not cause drastic phenotypical changes (6–8,11–14). In medaka, only double deficiency, not single deficiency, causes PD-related phenotypes, suggesting that PINK1 and Parkin are complementary to each other in medaka and have redundant functions. We have also observed this functional redundancy in the maintenance of mitochondrial function in MEF. These results are consistent with the idea that compensational mechanisms may be responsible for the survival of dopaminergic neurons (15,43–45). Indeed, the lack of an obvious PD-related phenotype in mice in vivo suggests the existence of evolutionary, compensational mechanisms capable of overcoming the deficiency in PINK1 and Parkin. Consistent with this idea, a recent paper by Shin et al. (15) showed that only controlled conditional knockdown of Parkin in adult mice could cause dopaminergic neuronal loss. We believe that as the authors have speculated compensational mechanisms are responsible for the lack of apparent phenotypes in other germline deletion mouse models. On the other hand, in humans, a longer lifespan exposes dopaminergic neurons to environmental factors that may eventually overcome these compensational mechanisms leading to PD (46).
In what mechanisms can PINK1 and Parkin work complementarily and at least in some parts independently of each other to maintain cellular homeostasis? One possible mechanism is the anti-apoptotic effect of these proteins. Indeed, only double-deficient medaka showed the evidence of cell death. Similarly, in double-deficient MEF, not in Parkin or PINK1 single-deficient MEF, the number of Annexin V and ssDNA-positive cells was increased and, moreover, caspase 3 was also activated, indicating the presence of apoptosis in this model. It has recently been shown that Parkin regulates the release of cytochrome c, whose release from the mitochondria initiates apoptosis (27), and Parkin also inactivates Bax, a pro-apoptotic protein of the Bcl-2 family, through ubiquitination independently of PINK1 (47). In parallel, PINK1 also inhibits cytochrome c release (33,34) whereas mutations or loss of PINK1 leads to apoptosis (33,41). These lines of evidence are in line with our results that only double deficiency in PINK1 and Parkin caused apoptosis accompanied by mitochondrial abnormality, and suggest the possible redundant roles of PINK1 and Parkin against cell death including mitochondrial apoptotic cell death.
Recently, PINK1 and Parkin have also been shown to operate in the degradation of damaged mitochondria through autophagy (29,48,49). In addition to this, PINK1 and Parkin also play important roles in the dynamic remodeling of the mitochondria through fission and fusion (50). This process allows cells to either fuse dysfunctional mitochondria to healthier ones to ‘dilute’ the effects of the damaged mitochondria or separate the unhealthy mitochondria through fission to be later degraded by autophagy. In our study, mitochondria was enlarged in the absence of either PINK1 or Parkin, consistent with previous studies (50–52). On the other hand, mitochondrial function was maintained in the absence of either PINK1 or Parkin probably due to their compensatory activity, while loss of both led to mitochondrial functional and morphological abnormalities.
Finally, we have succeeded in creating a genetic vertebrate model of PD that recapitulates the main phenotypes of human PD in an age-dependent manner. The decreased complex I activity observed in this model is a common phenomenon observed in idiopathic as well as PINK1- and Parkin-related PD (30–32), and could be the key to the selective loss of catecholaminergic neurons. Indeed, mitochondrial quality control is critical to catecholaminergic neurons which are constantly exposed to high levels of oxidative stress (43). From this point of view, our PD medaka model is a reasonable and relevant animal model of human PD, and would be useful in understanding the pathological mechanisms of PD, as well as in developing therapies that would target specific processes in the development of this disease. Though the loss of dopaminergic neurons could not account for the behavioral phenotype in our study, we believe that the deterioration in swimming behavior was dependent on PINK1. Indeed, PINK1 deficiency resulted in a decrease in motor function and deficiency in both PINK1 and Parkin led to a further decrease, at least in swimming distance. Further studies involving pharmacological treatment with l-dopa or dopamine agonists should be performed to see whether these motor defects could be dependent on the dopaminergic loss or not.
In conclusion, we have shown through our medaka model that Pink1 and Parkin play compensatory functions where functional deficiency in both leads to an age-dependent loss of dopaminergic neurons and deterioration of the swimming behavior. These phenotypes are accompanied by mitochondrial dysfunction and cell death in both medaka and MEF models. Our results suggest that PINK1 and Parkin work not only in a single pathway but also in independent parallel pathways, both of which protect dopaminergic neurons by maintaining mitochondrial function.
MATERIALS AND METHODS
Generation of Pink1 and Parkin double-deficient medaka
We previously generated Parkin loss-of-function mutants (25) and Pink1 loss-of-function mutants (22). Heterozygous fish generated by in vitro fertilization were back-crossed seven times with Kyoto-Cab, a substrain of Cab. Then Pink1 heterozygous mutants and Parkin heterozygous mutants were crossed and generated Pink1/Parkin double heterozygous mutants. After three additional back-crossings with Kyoto-Cab, Pink1/Parkin double heterozygous mutants were incrossed to generate a double mutant. Behavioral analysis, in situ hybridization, immunoblotting, TUNEL staining, transmission electron microscopy, immunohistochemistry and HPLC were conducted as previously described (21,22). RT–PCR of medaka parkin was conducted as previously described (25). Mitochondrial complex I, II (53), III and IV (18) activity were measured as previously reported. All the medaka experiments were conducted also with less-backcrossed lines, and only the consistent data among the different lines were used for publication.
Generation of PINK1- and Parkin-deficient MEF
Generation of PINK1-deficient MEF was described elsewhere (54). MEFs were also generated from wild-type and Parkin-deficient mice (55). Briefly, E13 embryos were cultured at 37°C (5% CO2) in DMEM (Gibco) containing 10% fetal bovine serum (Thermo Scientific) supplemented with 1 mg/ml of Gentamycin and 50 mm of 2-mercaptoethanol (Gibco). The cells underwent passages every 3 days, and were placed at 3 × 104 cells/10 cm culture dish at each passage. The cells were used for experiments after more than 30 passages. Established immortalized MEFs were cultured in DMEM supplemented with 10% fetal calf serum plus 100 IU/ml penicillin G, and 100 μg/ml streptomycin (Gibco).
Annexin V staining
Annexin V staining was performed by using the ApoAlert Annexin V Apoptosis kit (Clontech). Cells were washed and incubated with Annexin V-FITC for 15 min at room temperature. Cells were then washed and mounted with ProLong Gold Antifade Reagent with DAPI (Invitrogen). Cells were observed using a fluorescence microscope (BZ-9000, Keyence).
Immunostaining of ssDNA
MEFs were fixed using 4% PFA then permeabilized with 0.1% Triton X-100. Cells were then pre-treated with formamide at 75°C and were incubated with anti-ssDNA antibody (Dako), followed with Alexa-488 rabbit IgG (Invitrogen). Cells were mounted with ProLong Gold Antifade Reagent with DAPI (Invitrogen) and observed using a fluorescence microscope (BZ-9000, Keyence).
Plasmid transfection
Human flag-tagged Parkin (56) and flag-tagged PINK1 and its mutants (57) were transfected using Xfect (Clontech) according to the manufacturer's protocol.
siRNA transfection
Stealth siRNAs for mouse PINK1 (mPINK1) were purchased from Invitrogen. The targeted sequences are as follows: anti-sense, 5′-CCG CGU GGC UUU GGC UGG AGA GUA A-3′; sense, 5′-AUA CUC UCC AGC CAA AGC CAC GCG G-3′. Stealth RNAi negative control GC high (Invitrogen) was used as a control. siRNA transfection was performed with RNAiMax (Invitrogen) according to the manufacturer's instructions.
Real-time PCR
Total RNA was isolated according to the manufacturer's instructions using RNeasy Plus Mini (Qiagen) and first-strand cDNA was generated using Primescript RT (Takara Bio). For mRNA quantification, LightCycler 480 SYBR Green I Master (Roche Applied Bioscience) was used and quantified with LightCycler 480 System (Roche). The following oligonucleotides were used: mPink1 forward: GCTTTGGCTGGAGAGTATGG, mPink1 reverse: TAGTAGTGTGGGGGCAGCAT, mParkin forward: GAGCTTCCGAATCACCTGAC, mParkin reverse: ACTCCCATATGGAGCCCTCT, mGapdh forward: CGTCCCGTAGACAAAATGGT, mGapdh reverse: GAATTTGCCGTGAGTGGAGT.
Measurement of complex I activity in MEF
Mitochondrial fractions were first obtained as follows. MEFs were suspended with suspension buffer (250 mm sucrose, 5 mm Tris–HCl pH7.4 and 2 mm EGTA) and homogenized on ice using a Dounce homogenizer with 40 strokes. The homogenates were centrifuged at 1000g for 10 min at 4°C, then supernatants were transferred to new tubes. The supernatants were further centrifuged at 10 000g for 10 min at 4°C and the pellets were resuspended in suspension buffer (10 mm Tris–HCl pH 7.6) and sonicated, resulting in the mitochondrial fraction. The complex I assay was determined by the reduction of 2,6-dichloroindophenol (DCIP) (58). Briefly, 2 µg of the mitochondrial fraction was suspended in 200 µl reaction buffer consisting of 25 mm potassium phosphate, 3.5 g/l BSA (Nacalai Tesque), 60 µm DCIP, µm decylubiquinone (Santa Cruz), 0.2 mm NADH, pH 7.8. DCIP reduction was measured at 590 nm for 10 min using a microplate reader Multiscan JX (Thermo Fisher Scientific). Statistical analysis was performed using three independent samples in duplicate.
Measurement of mitochondrial membrane potential
Mitochondrial membrane potential was measured using the Cellomics VTI HCS Reader (Cellomics/Thermo Scientific). MEFs were seeded at 1 × 104 cells/well in a 96-well plate 1 day prior to analysis and at the day of analysis were incubated with a non-quenching concentration (10 nM) of TMRE (Sigma) and 5 µg/ml Hoechst 33342 (Invitrogen) for 30 min at 37°C. The values of TMRE fluorescence were normalized to that measured in WT MEF. Statistical analysis was performed using data from at least five separate wells.
Immunoblot analysis
Cells were lysed in lysis buffer containing 50 mm Tris–HCl (pH 8.0), 150 mm NaCl, 1% Triton-X 100 (Nacalai Tesque). Nuclei and membrane fractions were removed by centrifugation. Lysates were separated by SDS–PAGE and proteins were then transferred to a PVDF membrane (Millipore). The membrane was incubated with the appropriate primary antibody (anti-PINK1 antibody, Novus Biologicals; anti-Parkin antibody, Abcam; anti β-actin antibody, Abcam; anti-caspase 3 and cleaved caspase 3 antibody, Cell Signaling Technology and anti-flag antibody, Sigma Aldrich), followed by incubation with a horseradish peroxidise (HRP)-conjugated anti-rabbit or anti-mouse IgG (Santa Cruz). Immunoreactive proteins were visualized using the SuperSignal Immunoblotting detection system (Pierce) and an LAS3000 scanning system (Fuji Film).
Statistical analysis
Results were statistically evaluated for significance using the ANOVA test with post-hoc analysis using Bonferroni correction, if not mentioned. Differences were considered significant when P < 0.05.
SUPPLEMENTARY MATERIAL
FUNDING
This work was supported by Grants-in-Aid for Scientific Research (B) (Grant number 23390232) from the Ministry of Education, Science, Sports and Culture of Japan and by Core Research for Evolutional Science and Technology (CREST) of the Japan Science and Technology Agency (JST).
Supplementary Material
ACKNOWLEDGEMENTS
We wish to thank the Kondoh Differentiation Signaling Project, JST, for the permission to use the Kyoto-cab strain. We are grateful to Dr Chang-Liang Zhang, Tomoyo Sawada, Ai Tanigaki, Rie Hikawa and Masae Kimura, who supported our experiments. We are also grateful to Satoshi Fukui for assistance with electron microscopy studies.
Conflicts of Interest statement : None declared.
Contributor Information
Hideaki Matsui, Department of Neurology, Kyoto University Graduate School of Medicine, Kyoto 606-8507, Japan, Core Research for Evolutional Science and Technology, Japan Science and Technology Agency, Kawaguchi, Saitama 332-0012, Japan
Roberto Gavinio, Department of Neurology, Kyoto University Graduate School of Medicine, Kyoto 606-8507, Japan, Core Research for Evolutional Science and Technology, Japan Science and Technology Agency, Kawaguchi, Saitama 332-0012, Japan
Takeshi Asano, Department of Neurology, Kyoto University Graduate School of Medicine, Kyoto 606-8507, Japan,
Norihito Uemura, Department of Neurology, Kyoto University Graduate School of Medicine, Kyoto 606-8507, Japan,; Core Research for Evolutional Science and Technology, Japan Science and Technology Agency, Kawaguchi, Saitama 332-0012, Japan
Hidefumi Ito, Department of Neurology, Kyoto University Graduate School of Medicine, Kyoto 606-8507, Japan,
Yoshihito Taniguchi, Department of Radiation Genetics, Kyoto University Graduate School of Medicine, Kyoto 606-8501, Japan, Core Research for Evolutional Science and Technology, Japan Science and Technology Agency, Kawaguchi, Saitama 332-0012, Japan
Yoshito Kobayashi, Department of Neurology, Kyoto University Graduate School of Medicine, Kyoto 606-8507, Japan,; Core Research for Evolutional Science and Technology, Japan Science and Technology Agency, Kawaguchi, Saitama 332-0012, Japan
Takakuni Maki, Department of Neurology, Kyoto University Graduate School of Medicine, Kyoto 606-8507, Japan,.
Jie Shen, Center for Neurologic Diseases, Brigham and Women's Hospital, Program in Neuroscience, Harvard Medical School, Boston, MA 02115, USA and,.
Shunichi Takeda, Department of Radiation Genetics, Kyoto University Graduate School of Medicine, Kyoto 606-8501, Japan,; Core Research for Evolutional Science and Technology, Japan Science and Technology Agency, Kawaguchi, Saitama 332-0012, Japan
Kengo Uemura, Department of Neurology, Kyoto University Graduate School of Medicine, Kyoto 606-8507, Japan,; Core Research for Evolutional Science and Technology, Japan Science and Technology Agency, Kawaguchi, Saitama 332-0012, Japan
Hodaka Yamakado, Department of Neurology, Kyoto University Graduate School of Medicine, Kyoto 606-8507, Japan,; Core Research for Evolutional Science and Technology, Japan Science and Technology Agency, Kawaguchi, Saitama 332-0012, Japan
Ryosuke Takahashi, Department of Neurology, Kyoto University Graduate School of Medicine, Kyoto 606-8507, Japan,; Core Research for Evolutional Science and Technology, Japan Science and Technology Agency, Kawaguchi, Saitama 332-0012, Japan
REFERENCES
- 1. Kitada T. Asakawa S. Hattori N. Matsumine H. Yamamura Y. Minoshima S. Yokochi M. Mizuno Y. Shimizu N. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392:605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- 2. Valente E.M. Abou-Sleiman P.M. Caputo V. Muqit M.M. Harvey K. Gispert S. Ali Z. Del Turco D. Bentivoglio A.R. Healy D.G. et al. Hereditary early-onset Parkinson's disease caused by mutations in PINK1. Science. 2004;304:1158–1160. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- 3. Clark I.E. Dodson M.W. Jiang C. Cao J.H. Huh J.R. Seol J.H. Yoo S.J. Hay B.A. Guo M. Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature. 2006;441:1162–1166. doi: 10.1038/nature04779. [DOI] [PubMed] [Google Scholar]
- 4. Park J. Lee S.B. Lee S. Kim Y. Song S. Kim S. Bae E. Kim J. Shong M. Kim J.M. et al. Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature. 2006;441:1157–1161. doi: 10.1038/nature04788. [DOI] [PubMed] [Google Scholar]
- 5. Yang Y. Gehrke S. Imai Y. Huang Z. Ouyang Y. Wang J.W. Yang L. Beal M.F. Vogel H. Lu B. Mitochondrial pathology and muscle and dopaminergic neuron degeneration caused by inactivation of Drosophila Pink1 is rescued by Parkin. Proc. Natl Acad. Sci. USA. 2006;103:10793–10798. doi: 10.1073/pnas.0602493103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kitada T. Pisani A. Porter D.R. Yamaguchi H. Tscherter A. Martella G. Bonsi P. Zhang C. Pothos E.N. Shen J. Impaired dopamine release and synaptic plasticity in the striatum of PINK1-deficient mice. Proc. Natl Acad. Sci. USA. 2007;104:11441–11446. doi: 10.1073/pnas.0702717104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gautier C.A. Kitada T. Shen J. Loss of PINK1 causes mitochondrial functional defects and increased sensitivity to oxidative stress. Proc. Natl Acad. Sci. USA. 2008;105:11364–11369. doi: 10.1073/pnas.0802076105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gispert S. Ricciardi F. Kurz A. Azizov M. Hoepken H.H. Becker D. Voos W. Leuner K. Muller W.E. Kudin A.P. et al. Parkinson phenotype in aged PINK1-deficient mice is accompanied by progressive mitochondrial dysfunction in absence of neurodegeneration. PloS One. 2009;4:e5777. doi: 10.1371/journal.pone.0005777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kitada T. Pisani A. Karouani M. Haburcak M. Martella G. Tscherter A. Platania P. Wu B. Pothos E.N. Shen J. Impaired dopamine release and synaptic plasticity in the striatum of parkin−/− mice. J. Neurochem. 2009;110:613–621. doi: 10.1111/j.1471-4159.2009.06152.x. [DOI] [PubMed] [Google Scholar]
- 10. Goldberg M.S. Fleming S.M. Palacino J.J. Cepeda C. Lam H.A. Bhatnagar A. Meloni E.G. Wu N. Ackerson L.C. Klapstein G.J. et al. Parkin-deficient mice exhibit nigrostriatal deficits but not loss of dopaminergic neurons. J. Biol. Chem. 2003;278:43628–43635. doi: 10.1074/jbc.M308947200. [DOI] [PubMed] [Google Scholar]
- 11. Itier J.M. Ibanez P. Mena M.A. Abbas N. Cohen-Salmon C. Bohme G.A. Laville M. Pratt J. Corti O. Pradier L. et al. Parkin gene inactivation alters behaviour and dopamine neurotransmission in the mouse. Hum. Mol. Genet. 2003;12:2277–2291. doi: 10.1093/hmg/ddg239. [DOI] [PubMed] [Google Scholar]
- 12. Von Coelln R. Thomas B. Savitt J.M. Lim K.L. Sasaki M. Hess E.J. Dawson V.L. Dawson T.M. Loss of locus coeruleus neurons and reduced startle in parkin null mice. Proc. Natl Acad. Sci. USA. 2004;101:10744–10749. doi: 10.1073/pnas.0401297101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kitada T. Tong Y. Gautier C.A. Shen J. Absence of nigral degeneration in aged parkin/DJ-1/PINK1 triple knockout mice. J. Neurochem. 2009;111:696–702. doi: 10.1111/j.1471-4159.2009.06350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Perez F.A. Palmiter R.D. Parkin-deficient mice are not a robust model of parkinsonism. Proc. Natl Acad. Sci. USA. 2005;102:2174–2179. doi: 10.1073/pnas.0409598102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shin J.H. Ko H.S. Kang H. Lee Y. Lee Y.I. Pletinkova O. Troconso J.C. Dawson V.L. Dawson T.M. PARIS (ZNF746) repression of PGC-1alpha contributes to neurodegeneration in Parkinson's disease. Cell. 2011;144:689–702. doi: 10.1016/j.cell.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lin X. Parisiadou L. Sgobio C. Liu G. Yu J. Sun L. Shim H. Gu X.L. Luo J. Long C.X. et al. Conditional expression of Parkinson's disease-related mutant alpha-synuclein in the midbrain dopaminergic neurons causes progressive neurodegeneration and degradation of transcription factor nuclear receptor related 1. J. Neurosci. 2012;32:9248–9264. doi: 10.1523/JNEUROSCI.1731-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Anichtchik O. Diekmann H. Fleming A. Roach A. Goldsmith P. Rubinsztein D.C. Loss of PINK1 function affects development and results in neurodegeneration in zebrafish. J. Neurosci. 2008;28:8199–8207. doi: 10.1523/JNEUROSCI.0979-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Flinn L. Mortiboys H. Volkmann K. Koster R.W. Ingham P.W. Bandmann O. Complex I deficiency and dopaminergic neuronal cell loss in parkin-deficient zebrafish (Danio rerio) Brain. 2009;132:1613–1623. doi: 10.1093/brain/awp108. [DOI] [PubMed] [Google Scholar]
- 19. Fett M.E. Pilsl A. Paquet D. van Bebber F. Haass C. Tatzelt J. Schmid B. Winklhofer K.F. Parkin is protective against proteotoxic stress in a transgenic zebrafish model. PloS one. 2010;5:e11783. doi: 10.1371/journal.pone.0011783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xi Y. Ryan J. Noble S. Yu M. Yilbas A.E. Ekker M. Impaired dopaminergic neuron development and locomotor function in zebrafish with loss of pink1 function. Eur. J. Neurosci. 2010;31:623–633. doi: 10.1111/j.1460-9568.2010.07091.x. [DOI] [PubMed] [Google Scholar]
- 21. Matsui H. Taniguchi Y. Inoue H. Uemura K. Takeda S. Takahashi R. A chemical neurotoxin, MPTP induces Parkinson's disease like phenotype, movement disorders and persistent loss of dopamine neurons in medaka fish. Neurosci. Res. 2009;65:263–271. doi: 10.1016/j.neures.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 22. Matsui H. Ito H. Taniguchi Y. Inoue H. Takeda S. Takahashi R. Proteasome inhibition in medaka brain induces the features of Parkinson's disease. J. Neurochem. 2010;115:178–187. doi: 10.1111/j.1471-4159.2010.06918.x. [DOI] [PubMed] [Google Scholar]
- 23. Matsui H. Ito H. Taniguchi Y. Takeda S. Takahashi R. Ammonium chloride and tunicamycin are novel toxins for dopaminergic neurons and induce Parkinson's disease-like phenotypes in medaka fish. J. Neurochem. 2010;115:1150–1160. doi: 10.1111/j.1471-4159.2010.07012.x. [DOI] [PubMed] [Google Scholar]
- 24. Matsui H. Taniguchi Y. Inoue H. Kobayashi Y. Sakaki Y. Toyoda A. Uemura K. Kobayashi D. Takeda S. Takahashi R. Loss of PINK1 in medaka fish (Oryzias latipes) causes late-onset decrease in spontaneous movement. Neurosci. Res. 2010;66:151–161. doi: 10.1016/j.neures.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 25. Taniguchi Y. Takeda S. Furutani-Seiki M. Kamei Y. Todo T. Sasado T. Deguchi T. Kondoh H. Mudde J. Yamazoe M. et al. Generation of medaka gene knockout models by target-selected mutagenesis. Genome Biol. 2006;7:R116. doi: 10.1186/gb-2006-7-12-r116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Narendra D.P. Youle R.J. Targeting Mitochondrial Dysfunction. Role for PINK1 and Parkin in Mitochondrial Quality Control. Antioxid. Redox Signal. 2011;14:1929–1938. doi: 10.1089/ars.2010.3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Berger A.K. Cortese G.P. Amodeo K.D. Weihofen A. Letai A. LaVoie M.J. Parkin selectively alters the intrinsic threshold for mitochondrial cytochrome c release. Hum. Mol. Genet. 2009;18:4317–4328. doi: 10.1093/hmg/ddp384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hao L.Y. Giasson B.I. Bonini N.M. DJ-1 is critical for mitochondrial function and rescues PINK1 loss of function. Proc. Natl Acad. Sci. USA. 2010;107:9747–9752. doi: 10.1073/pnas.0911175107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Narendra D.P. Jin S.M. Tanaka A. Suen D.F. Gautier C.A. Shen J. Cookson M.R. Youle R.J. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 2010;8:e1000298. doi: 10.1371/journal.pbio.1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Muftuoglu M. Elibol B. Dalmizrak O. Ercan A. Kulaksiz G. Ogus H. Dalkara T. Ozer N. Mitochondrial complex I and IV activities in leukocytes from patients with parkin mutations. Mov. Disord. 2004;19:544–548. doi: 10.1002/mds.10695. [DOI] [PubMed] [Google Scholar]
- 31. Mortiboys H. Thomas K.J. Koopman W.J. Klaffke S. Abou-Sleiman P. Olpin S. Wood N.W. Willems P.H. Smeitink J.A. Cookson M.R. et al. Mitochondrial function and morphology are impaired in parkin-mutant fibroblasts. Ann. Neurol. 2008;64:555–565. doi: 10.1002/ana.21492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hoepken H.H. Gispert S. Morales B. Wingerter O. Del Turco D. Mulsch A. Nussbaum R.L. Muller K. Drose S. Brandt U. et al. Mitochondrial dysfunction, peroxidation damage and changes in glutathione metabolism in PARK6. Neurobiol. Dis. 2007;25:401–411. doi: 10.1016/j.nbd.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 33. Pridgeon J.W. Olzmann J.A. Chin L.-S. Li L. PINK1 protects against oxidative stress by phosphorylating mitochondrial chaperone TRAP1. PLoS Biol. 2007;5:e172. doi: 10.1371/journal.pbio.0050172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang H.L. Chou A.H. Yeh T.H. Li A.H. Chen Y.L. Kuo Y.L. Tsai S.R. Yu S.T. PINK1 mutants associated with recessive Parkinson's disease are defective in inhibiting mitochondrial release of cytochrome c. Neurobiol. Dis. 2007;28:216–226. doi: 10.1016/j.nbd.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 35. Tatton N.A. Increased caspase 3 and Bax immunoreactivity accompany nuclear GAPDH translocation and neuronal apoptosis in Parkinson's disease. Exp. Neurol. 2000;166:29–43. doi: 10.1006/exnr.2000.7489. [DOI] [PubMed] [Google Scholar]
- 36. Hartmann A. Troadec J.D. Hunot S. Kikly K. Faucheux B.A. Mouatt-Prigent A. Ruberg M. Agid Y. Hirsch E.C. Caspase-8 is an effector in apoptotic death of dopaminergic neurons in Parkinson's disease, but pathway inhibition results in neuronal necrosis. J. Neurosci. 2001;21:2247–2255. doi: 10.1523/JNEUROSCI.21-07-02247.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Viswanath V. Wu Y. Boonplueang R. Chen S. Stevenson F.F. Yantiri F. Yang L. Beal M.F. Andersen J.K. Caspase-9 activation results in downstream caspase-8 activation and bid cleavage in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinson's disease. J. Neurosci. 2001;21:9519–9528. doi: 10.1523/JNEUROSCI.21-24-09519.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Morais V.A. Verstreken P. Roethig A. Smet J. Snellinx A. Vanbrabant M. Haddad D. Frezza C. Mandemakers W. Vogt-Weisenhorn D. et al. Parkinson's disease mutations in PINK1 result in decreased complex I activity and deficient synaptic function. EMBO Mol. Med. 2009;1:99–111. doi: 10.1002/emmm.200900006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Green D.R. Narendra D.P. Jin S.M. Tanaka A. Suen D.-F. Gautier C.A. Shen J. Cookson M.R. Youle R.J. PINK1 Is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 2010;8:e1000298. doi: 10.1371/journal.pbio.1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Exner N. Treske B. Paquet D. Holmstrom K. Schiesling C. Gispert S. Carballo-Carbajal I. Berg D. Hoepken H.H. Gasser T. et al. Loss-of-function of human PINK1 results in, mitochondrial pathology and can be rescued by Parkin. J. Neurosci. 2007;27:12413–12418. doi: 10.1523/JNEUROSCI.0719-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gandhi S. Wood-Kaczmar A. Yao Z. Plun-Favreau H. Deas E. Klupsch K. Downward J. Latchman D.S. Tabrizi S.J. Wood N.W. et al. PINK1-associated Parkinson's disease is caused by neuronal vulnerability to calcium-induced cell death. Mol. Cell. 2009;33:627–638. doi: 10.1016/j.molcel.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Li P. Nijhawan D. Budihardjo I. Srinivasula S.M. Ahmad M. Alnemri E.S. Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 43. Dawson T.M. Ko H.S. Dawson V.L. Genetic animal models of Parkinson's disease. Neuron. 2010;66:646–661. doi: 10.1016/j.neuron.2010.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bezard E. Gross C.E. Brotchie J.M. Presymptomatic compensation in Parkinson's disease is not dopamine-mediated. Trends Neurosci. 2003;26:215–221. doi: 10.1016/S0166-2236(03)00038-9. [DOI] [PubMed] [Google Scholar]
- 45. Palop J.J. Chin J. Mucke L. A network dysfunction perspective on neurodegenerative diseases. Nature. 2006;443:768–773. doi: 10.1038/nature05289. [DOI] [PubMed] [Google Scholar]
- 46. Exner N. Lutz A.K. Haass C. Winklhofer K.F. Mitochondrial dysfunction in Parkinson's disease. Molecular mechanisms and pathophysiological consequences. EMBO J. 2012;31:3038–3062. doi: 10.1038/emboj.2012.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Johnson B.N. Berger A.K. Cortese G.P. Lavoie M.J. The ubiquitin E3 ligase parkin regulates the proapoptotic function of Bax. Proc. Natl Acad. Sci. USA. 2012;109:6283–6288. doi: 10.1073/pnas.1113248109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Geisler S. Holmström K.M. Skujat D. Fiesel F.C. Rothfuss O.C. Kahle P.J. Springer W. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat. Cell. Biol. 2010;12:119–131. doi: 10.1038/ncb2012. [DOI] [PubMed] [Google Scholar]
- 49. Vives-Bauza C. Zhou C. Huang Y. Cui M. de Vries R.L.A. Kim J. May J. Tocilescu M.A. Liu W. Ko H.S. et al. PINK1-dependent recruitment of Parkin to mitochondria in mitophagy. Proc. Natl Acad. Sci. USA. 2009;107:378–383. doi: 10.1073/pnas.0911187107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Deng H. Dodson M.W. Huang H. Guo M. The Parkinson's disease genes pink1 and parkin promote mitochondrial fission and/or inhibit fusion in Drosophila. Proc. Natl Acad. Sci. USA. 2008;105:14503–14508. doi: 10.1073/pnas.0803998105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yang Y. Ouyang Y. Yang L. Beal M.F. McQuibban A. Vogel H. Lu B. Pink1 regulates mitochondrial dynamics through interaction with the fission/fusion machinery. Proc. Natl Acad. Sci. USA. 2008;105:7070–7075. doi: 10.1073/pnas.0711845105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Poole A.C. Thomas R.E. Andrews L.A. McBride H.M. Whitworth A.J. Pallanck L.J. The PINK1/Parkin pathway regulates mitochondrial morphology. Proc. Natl Acad. Sci. USA. 2008;105:1638–1643. doi: 10.1073/pnas.0709336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Janssen A.J.M. Trijbels F.J.M. Sengers R.C.A. Smeitink J.A.M. van den Heuvel L.P. Wintjes L.T.M. Stoltenborg-Hogenkamp B.J.M. Rodenburg R.J.T. Spectrophotometric assay for complex I of the respiratory chain in tissue samples and cultured fibroblasts. Clin. Chem. 2007;53:729–734. doi: 10.1373/clinchem.2006.078873. [DOI] [PubMed] [Google Scholar]
- 54. Amo T. Sato S. Saiki S. Wolf A.M. Toyomizu M. Gautier C.A. Shen J. Ohta S. Hattori N. Mitochondrial membrane potential decrease caused by loss of PINK1 is not due to proton leak, but to respiratory chain defects. Neurobiol. Dis. 2011;41:111–118. doi: 10.1016/j.nbd.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 55. Kitao Y. Imai Y. Ozawa K. Kataoka A. Ikeda T. Soda M. Nakimawa K. Kiyama H. Stern D.M. Hori O. et al. Pael receptor induces death of dopaminergic neurons in the substantia nigra via endoplasmic reticulum stress and dopamine toxicity, which is enhanced under condition of parkin inactivation. Hum. Mol. Genet. 2007;16:50–60. doi: 10.1093/hmg/ddl439. [DOI] [PubMed] [Google Scholar]
- 56. Imai Y. Parkin suppresses unfolded protein stress-induced cell death through Its E3 ubiquitin-protein ligase Activity. J. Biol. Chem. 2000;275:35661–35664. doi: 10.1074/jbc.C000447200. [DOI] [PubMed] [Google Scholar]
- 57. Moriwaki Y. Kim Y.-J. Ido Y. Misawa H. Kawashima K. Endo S. Takahashi R. L347P PINK1 mutant that fails to bind to Hsp90/Cdc37 chaperones is rapidly degraded in a proteasome-dependent manner. Neurosci. Res. 2008;61:43–48. doi: 10.1016/j.neures.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 58. de Wit L.E.A. Scholte H.R. Sluiter W. Correct assay of complex I activity in human skin fibroblasts by timely addition of rotenone. Clin. Chem. 2008;54:1921–1922. doi: 10.1373/clinchem.2008.104802. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.