Abstract
Although sleep loss is theorized to increase aggression risk, knowledge regarding the sleep-aggression relationship, or explanatory psychological processes, is limited. This study examined whether recent sleep duration predicted subsequent laboratory aggression, and whether neurocognitive indices of attentional and motor inhibition and negative emotional processing explained the sleep-aggression relationship. Participants (n=141) wore Fitbit Flex devices and kept a sleep diary for three days. Event-related potentials were measured during an Emotional-Linguistic Go/No-Go task, followed by a laboratory aggression paradigm. Results of mixed-model repeated measures ANOVAs linked shorter sleep duration with reduced motor inhibition processing during negative and neutral word blocks, and greater aggression. However, neurocognitive indices did not explain the sleep-aggression link. This is the first evidence that naturally occurring sleep loss predicts increases in laboratory aggression across the task and suggests that shorter sleepers are more vulnerable to rash action in negative and neutral contexts. Implications of these findings for understanding aggression will be discussed.
Keywords: Sleep, aggression, ERP
Insufficient sleep (i.e., sleeping less than 7 hours a night) is a growing public health problem, affecting 30% of American adults (Hafner, Stepanek, Taylor, Troxel, & Van Stolk, 2017). Although numerous health concerns are linked with shorter sleep duration (Van Cauter, Spiegel, Tasali, & Leproult, 2008), fewer studies have examined whether sleep duration impacts engagement in aggression, and studies yield diverging results. In general, the psychological processes that link sleep and aggression are poorly understood (Verona & Bozzay, 2017), which limits ability to explore the viability of sleep duration as an upstream target for aggression prevention. This study addressed these critical gaps in the literature by examining the prediction of aggression from recent sleep duration, and emotional and cognitive processing patterns that potentially link sleep and aggression.
Sleep and Aggression
Despite descriptive evidence connecting sleep duration to irritability and aggression, the research literature thus far has yielded mixed findings, partly due to distinctions in how sleep loss is measured across studies, specifically naturalistic sleep duration vs. sleep deprivation studies. The vast majority of studies in this vein have examined associations between measures of naturally occurring sleep duration (measured subjectively via sleep diaries and/or objectively via actigraph watches) and self-reported aggression. These cross-sectional (Randler & Vollmer, 2013; Vogler, Perkinson-Gloor, Brand, Grob, & Lemola, 2014) and longitudinal (Langsrud et al., 2018; Sheridan et al., 2013) studies show small-to-moderate between-subjects relationships between shorter sleep duration (i.e., several hours of sleep less than what is recommended) and aggression. In contrast, results from studies experimentally depriving subjects of sleep show more inconsistent results in regard to aggression. In one study, military personnel deprived of sleep for 55 hours were more likely than rested controls to blame others for problems and less willing to accept blame on the Rosenzweig Picture-Frustration projective test (Kahn-Greene, Lipizzi, Conrad, Kamimori, & Killgore, 2006). In another such study using a point subtraction aggression task, provoked men deprived of 33 hours of sleep were less aggressive than rested controls (Cote, McCormick, Geniole, Renn, & MacAulay, 2013). Finally, a third study found no effects of sleep deprivation on aggression, such that individuals who completed a cognitive depletion procedure were more aggressive, regardless of whether they had been sleep deprived (Vohs, Glass, Maddox, & Markman, 2011). Part of the problem is that studies using total sleep deprivation (e.g., 24 or more hours of sleep loss) produce extreme levels of fatigue, amotivation, and disengagement in participants (Alhola & Polo-Kantola, 2007; Cote et al., 2008), and these effects could override effects of degrees of sleep loss that more naturally occur in the real world (i.e., a few hours a night) on cognition and behavior (Van Dongen, Maislin, Mullington, & Dinges, 2003). Further exacerbating the confounding effects of fatigue, many of the studies failing to find the expected sleep-aggression relationship relied on aggression paradigms that are particularly susceptible to fatigue effects (e.g., reaction-time tasks). And, to our knowledge, no studies have examined sleep-aggression effects in the laboratory when measuring sleep duration as it naturally occurs. One strength of this study is that we examine aggression outcomes under conditions of naturally occurring sleep duration, uniquely combining daily assessments of naturalistic sleep and experimental aggression procedures.
Inhibition Processing, Sleep Loss, and Aggression
The role of reduced inhibitory control in aggression has been documented across various literatures. In personality research, trait disconstraint (or disinhibition) is a key personality construct associated with aggression proneness (Derefinko, DeWall, Metze, Walsh, & Lynam, 2011; Krueger, McGue, & Iacono, 2001; Verona & Patrick, 2002). Correlational studies link deficits in cognitive control and prefrontal cortex functioning to aggression risk (Giancola, 2004; J. Sprague & Verona, 2010; Jenessa Sprague, Verona, Kalkhoff, & Kilmer, 2011). And, research has shown that experimentally-primed cognitive control decreases aggressive impulses and increases forgiveness (Wilkowski, Robinson, & Troop-Gordon, 2010).
Disruption of inhibitory processes, thus, is one proposed mechanism that links shorter sleep with higher aggression (Krizan & Herlache, 2016). Decrements in cognition emerge following less than seven hours of sleep (Belenky et al., 2003), increase with greater sleep loss (Belenky et al., 2003; Cote et al., 2008), and compound over multiple nights of shortened sleep (Dinges & Kribbs, 1991; Doran, Van Dongen, & Dinges, 2001). Since restoring cognitive functioning requires “catch-up sleep”(Banks, Van Dongen, Maislin, & Dinges, 2010), a few nights of shorter sleep may impair cognition over the short-term, including response inhibition, the stopping of an automatic or impulsive response not conducive to meeting goals (Lezak, Howieson, Loring, & Fischer, 2004). Response inhibition is regulated by the prefrontal cortex (PFC) (Botvinick, Braver, Barch, Carter, & Cohen, 2001), a brain region particularly vulnerable to sleep loss (Harrison & Horne, 2000; Horne, 1993). Indeed, the PFC is theorized to rejuvenate during sleep through Non-Rapid-Eye-Movement (NREM) and Rapid-Eye-Movement (REM) episodes (Vyazovskiy & Delogu, 2014). The slow-wave oscillations during NREM are thought to enable brain network recovery (Vyazovskiy & Harris, 2013), with the REM stage following NREM thought integral in identifying brain networks still requiring recovery during the next NREM cycle (Vyazovskiy & Delogu, 2014). Since these stages repeat iteratively throughout the night, several hours of sleep loss may not allow enough time for the PFC to recover. Indeed, the PFC is impaired following a night of sleep deprivation, evidenced by reduced blood flow to prefrontal areas that correspond to deteriorations in performance on executive control tasks including inhibition (Harrison & Horne, 2000; Horne, 1993).
Notably, sleep deprivation has been linked to other, more temporally-precise brain indices of response inhibition, including event-related potentials like the no-go N2 and no-go P3a (hereafter termed N2 and P3). According to these literatures, the amplitude of frontal N2 or P3 to No-Go versus Go trials in a go/no-go tasks indexes the deployment of cognitive resources to response inhibition, with the N2 capturing attention inhibition (i.e., encompassing processes such as detection of a conflict between initiated and required responses, action monitoring, and/or effortful attention to conflicting pieces of information) and P3 assessing motor inhibition (i.e., inhibition of motor responses) (Qi et al., 2010). The behavioral indices of inhibition include commission errors (Demos et al., 2016; Qi et al., 2010). Findings showing links between sleep loss and no-go N2/P3 and commission errors are notable, given that the N2 and P3 are often the components of choice in studies of inhibition-related aggression in the laboratory (e.g., No-Go P3: (Verona & Bresin, 2015); behavioral indices: (Vigil-Colet & Codorniu-Raga, 2004). Thus, we expect that sleep loss over a short time period (a few days) will be partially linked with subsequently-assessed aggression via reduced response inhibition.
Emotional Processing, Sleep Loss, and Aggression
Experimental research has confirmed links between negative affective processes and aggression. Based on extensive evidence from laboratory and other studies (Berkowitz, 1990), researchers have theorized that stress exposure and the concomitant negative affect that arises from it can promote aggression via neural associative links between emotion centers (e.g., amygdala) that govern defense and cortical areas involved in inhibition and motor control (Verona & Kilmer, 2007; Verona & Patrick, 2002). In the animal literature, behavioral neuroscientists have identified mutual feedback links between the adrenocortical stress response and aggression attack centers in rat brains (Kruk, Halasz, Meelis, & Haller, 2004). Further, previous research has found that individuals high in trait anger, a construct highly related to aggression proneness (Arnold H. Buss & Perry, 1992; Martin, Watson, & Wan, 2000), have a bias to attend to words related to anger, angry faces, or perceptions of threat (Cohen, Eckhardt, & Schagat, 1998; Putman, Hermans, & van Honk, 2004; Wilkowski, Robinson, Gordon, & Troop-Gordon, 2007).
Sleep loss has also been linked to reduced thresholds for negative reactivity or biased attention to threat (Barclay & Ellis, 2013). Neuroimaging research links sleep deprivation with reduced inhibitory connections between the prefrontal cortex and amygdala, potentially disrupting emotional regulation and increasing reactivity to negative emotional stimuli (Yoo, Gujar, Hu, Jolesz, & Walker, 2007). These changes could promote the types of negative affective states (e.g., anger and hostility (Kahn-Greene, Killgore, Kamimori, Balkin, & Killgore, 2007)) that are implicated in aggression. Alternatively, recent sleep loss may bias attention to emotional/arousing information more broadly, rather than negative affect-specific stimuli. Indeed, sleep deprivation has also been associated with increased brain activation to positively valent stimuli (Gujar, Yoo, Hu, & Walker, 2011; Volkow et al., 2009). In event-related potentials research, the P3 to emotionally laden stimuli indexes attentional processing of salient information, and P3 contrasts can be used to understand whether attention is more biased to emotional arousal (negative/positive vs neutral; Arousal P3) or negatively valenced information (negative vs positive; Valenced P3). This information can aid in understanding the contexts that promote sleep-related inhibition problems. Unfortunately, existing research has not used ERP measurement that allows for more temporally-precise assessments of emotional processing or its interplay with response inhibition as a function of naturalistic sleep duration.
Finally, emotional and inhibitory processes that result from recent sleep loss may interact to increase aggression risk. Initial research has shown that aggression proneness is related to reduced processing of inhibitory cues (decreased no-go P3) specifically in negative (i.e., threatening) emotional vs. neutral conditions (Verona & Bresin, 2015). Indeed, the experience of negative affect can decrease the amount and influence of “cool” information processing (Metcalfe & Mischel, 1999), potentially disrupting adequate problem solving and inhibition in the face of intense affect (J. Sprague & Verona, 2010). Further, negative affect activates moodcongruent information in working memory (Dolcos, LaBar, & Cabeza, 2004) and increases expectations that punishing and aversive events will occur (Handley, Lassiter, Nickell, & Herchenroeder, 2004). In turn, negative attentional biases can impair the ability to reappraise negative situations by limiting the information being considered (De Houwer & Tibboel, 2010). Shorter sleep is associated with limited energy resources so that the processing emotional information (considered of survival value) is prioritized (Wyble, Sharma, & Bowman, 2008) over higher-order cognitive functions, such as working memory and cognitive control. That is, less sleep can promote reliance on habitual responses (e.g., aggression), decreased goal-directed responding (e.g., behavior inhibition;(Dias-Ferreira et al., 2009), and the externalizing of blame (Kahn-Greene et al., 2006). Taken together, and given dual process theories implicating trade-offs between higher-order cognitive control (e.g., inhibitory control) and reflexive emotion processing (Strack & Deutsch, 2004), we expected reduced attentional and motor inhibitory control processing in the face of emotional information to be exacerbated under less sleep (e.g., sleep loss-related reductions in No-Go N2 and P3 in negative emotional conditions), which may represent the interactive processes that explain the sleep-aggression link.
Aims and Hypotheses
Prior research links sleep with aggression (Randler & Vollmer, 2013) and with emotional and cognitive processing disruptions that are associated with aggression risk (Verona & Bresin, 2015). However, existing studies have failed to examine relationships between naturally-occurring sleep duration and indices of rapid processing of emotional and inhibitory cues. While theories implicate emotional processing and response inhibition in the sleep-aggression relationship (Qi et al., 2010; Yoo et al., 2007), empirical support is required to directly test this assumption. This study examined whether recent sleep duration was associated with subsequently-measured decrements in attentional and motor inhibition, biases in emotional processing, and increased laboratory aggression. We assessed naturalistic sleep duration across three nights and recorded subsequent provoked aggression in the laboratory, given our interests in emotional processes in relation to aggression. Aim 1 tested the hypothesis that shorter naturalistic sleep duration would be associated with self-reported aggressive tendencies and predict subsequent laboratory-provoked aggressive behavior. Aim 2 tested the hypothesis that less sleep duration would relate to prioritization of emotional processing (enhanced emotion P3 and N2) and reduced response inhibition (relatively blunted no-go P3 and N2, more commission errors) during negative emotional conditions. Finally, Aim 3 explored whether inhibitory and emotional processing indices separately or together (i.e., inhibitory processing within negative conditions specifically) explained the sleep-aggression relationship.
Method
Participants
A total of 141 participants aged 18 to 40 were recruited for a study examining cognitive and affective mechanisms involved in aggression proneness. Unselected individuals between the ages of 18 and 40 were recruited from the community through flyers, newspaper and electronic advertisements, and word of mouth (See Table 1). Prospective participants completed a phone screen to assess for inclusion and exclusion criteria. Individuals reporting medical (Parkinson’s disease, epilepsy, or traumatic brain injury) or mental health diagnoses (bipolar disorder, schizophrenia, or pervasive developmental disorder), which are low base rate and could contribute to disinhibition, were excluded, as were persons with auditory or visual impairments (e.g., colorblind). Other diagnoses that are commonly present among persons with aggression were allowed, so as to avoid limiting generalizability. Approximately 38% of the sample met criteria for a lifetime substance use disorder (10% had a current diagnosis), 40% had a lifetime alcohol use disorder (10% had a current alcohol use disorder), and 43% met for a lifetime diagnosis of major depressive disorder (3% endorsed current depression). Participants were evenly divided by gender, were predominantly Caucasian or African American, and most were employed (see Table 1).
Table 1.
Sample Characteristics
| Full Sample (n=141) | |
|---|---|
|
| |
| Age (M (SD)) | 29.32(6.34) |
| Gender (n(%)) | |
| Male | 65(46.1) |
| Female | 67(47.5) |
| Transgender (M to F) | 2(1.4) |
| Transgender (F to M) | 1(0.7) |
| Other | 1(0.7) |
| Race (n(%)) | |
| Caucasian | 79(55.2) |
| African American | 42(29.4) |
| Asian | 9(6.3) |
| American Indian or Alaskan Native | 4(2.8) |
| Other | 8(5.6) |
| Missing | 1(.7) |
| Ethnicity (n(%Hispanic)) | 23(16.1) |
| Employment Status (n(%)) | |
| Employed | 93(65.0) |
| Unemployed | 21(14.7) |
| Homemaker | 7(4.9) |
| Other (e.g., Retired) | 21(14.7) |
| Income (n(%)) | |
| <$15,000 | 27(18.9) |
| $15–30,000 | 40(28.0) |
| $30–45,000 | 27(18.9) |
| $45–60,000 | 23(16.1) |
| $60–75,000 | 8(5.6) |
| >$75,000 | 15(10.5) |
| Recruitment Source (n(%)) | |
| Friend/Relative | 14(9.8) |
| Electronic Ads/Flyers | 127(89.5) |
| Other | 1(0.7) |
The sample had considerable variability in measures of sleep duration and aggression proneness as well. During the three days of naturalistic sleep assessment, participants slept 6 hours and 59 minutes on average (SD=79 minutes, Range: 191–510 minutes). Approximately 48% of the sample slept less than recommended 7 hours, above the 34% reported in epidemiological research within the general population (Hafner et al., 2017). Further, 48% of the sample endorsed moderate (36%) to severe (12%) problems with sleep quality over the prior month on the Pittsburgh Sleep Quality Index (Buysse, Reynolds III, Monk, Berman, & Kupfer, 1989). Finally, approximately 35% of men and 57% of women who participated in our study scored above published averaged normed scores on the Aggression Questionnaire (Arnold H Buss & Warren, 2000). These descriptive statistics suggest sufficient endorsement of clinically severe problems with sleep and aggression to test study aims.
In this manuscript, we have reported all measures, conditions, and data exclusions used in this project. This study was not pre-registered. However, this project was conducted as part of a dissertation, and all study procedures (including hypotheses, data processing decisions, and analytic strategies) were planned and approved by the lead author’s dissertation committee prior to data collection. There were no deviations from planned procedures in this study.
Overview of Procedures
Participants in the study completed two sessions. During Session 1, participants provided informed consent, completed measures and interviews to assess lifetime substance use and psychiatric disorders using the Mini International Neuropsychiatric Interview (Sheehan et al., 1998), administered by graduate students supervised by a licensed clinical psychologist. Participants also underwent a shock threat procedure (Moberg & Curtin, 2009) during another cognitive task (Attention Network Task; (Fan, McCandliss, Sommer, Raz, & Posner, 2002). Participants in Session 1 were offered the opportunity to participate in a second session which made up the current study. All participants agreed to come back for the second session and were trained to track sleep using a daily sleep diary and Fitbit Flex. For three days before Session 2, participants wore the Fitbit Flex to track sleep and completed a sleep diary for those 3 days.
In Session 2, participants returned the Fitbit Flex. First, each participant and a study confederate (matched on gender and ethnic minority status) drew slips of paper from a cup to learn their purported roles in the interpersonal judgment task (i.e., aggression paradigm). Next, participants completed an emotional go/no-go task, followed by the laboratory aggression paradigm. Finally, participants were debriefed about the true study goals and compensated up to $80, depending on how many days of sleep tracking were completed. Study procedures were approved by the university IRB and completed in accordance with the provisions of the World Medical Association Declaration of Helsinki.
Sleep Tracking and Measurement
We assessed sleep duration over three nights to produce a measure of recent cumulative sleep loss. Objective sleep duration was measured using the Fitbit Flex (Fitbit, Inc., Boston, USA). Fitbit devices demonstrate high inter-device sleep monitoring reliability (Evenson, Goto, & Furberg, 2015), and the Fitbit Flex identifies differences in sleep quantity between primary insomniacs and good sleepers (Nie et al., 2015). However, since wearable sleep measurement devices can misidentify wakefulness as sleep, we cross-validated Fitbit information using the National Sleep Foundation Sleep Diary (the “gold standard” in subjective sleep measurement (Buysse, Ancoli-Israel, Edinger, Lichstein, & Morin, 2006)), as recommended by experts (Montgomery-Downs, Insana, & Bond, 2012), Consistent with procedures used in clinical trials research by sleep experts (van der Zweerde et al., 2016), we harmonized Fitbit data with sleep diary data when these measures differed by more than 30 minutes of time asleep/awake (e.g., participant did not cue Fitbit into ‘wake’ mode).1 Most participants had Fitbit data for all three nights (n=108, 76%). Most participants had Fitbit data for at least one night (n=123, 86%). Our rates of usable Fitbit data fall in the higher range of studies that use manually activated devices to track sleep (Lillehei et al., 2015). For nights in which no Fitbit data was available (e.g., device or operational failure), we substituted sleep diary data (18.5–21.2% of cases across nights; M =0.62, SD =1.03 nights). Analyses conducted using only sleep diary data yielded similar results to those reported using the diary-adjusted Fitbit data reported in this manuscript (see Supplemental Information). Diary and Fitbit measures of sleep duration were highly correlated (rs of .89–.93 across nights).2
Emotional Go/No-Go Task
The Emotional Go/No-Go task (EGNG), based on Goldstein et al. (2007) and modified for ERP research (Verona, Sprague, & Sadeh, 2012), was used to assess emotional and inhibitory control processing. The EGNG task requires inhibitory control to respond to word features (normal vs. italicized font) rather than content. Participants press a button to words appearing in a normal font (Go trial) and inhibit responding to italicized words (No-Go trial) within three emotional word blocks (neutral, negative, and positive words). Blocks contained 32 emotionally neutral (e.g., umbrella, lamp), negative (e.g., violent, hate), or positive (e.g., mighty, terrific) words from the Affective Norms for English Words (Bradley & Lang, 1999). Words were matched across conditions on length, and frequency of use in the English language. The negative words used in our task were previously selected to be particularly salient for participants with affective and behavioral regulation problems (J. Sprague & Verona, 2010). We further modified this task for the current study to include a positive word condition, with words matched to negative words on valence and arousal (based on ratings from ANEW). There were 20 practice trials, and 6 blocks for each emotion word category (18 blocks). Words were randomized within each emotional category block, and the sequence of blocks was counterbalanced across participants. Words selected for No-Go trials differed across blocks, with a rest period between blocks. Each block was comprised of fewer No-Go trials (9) than Go trials (23). Words were presented for 1400 ms, with a 750–1000ms intertrial interval.
Assessment of Aggression
Laboratory aggression.
The laboratory aggression paradigm was based on a task initially developed by Buss (Arnold H Buss, 1961) and modified in other studies (Verona, Sadeh, & Curtin, 2009). Participants were told that this study examined work-related processes, including employee-supervisor relationships. At the beginning of the session, study staff provided an overview of the session procedures to the participant and the confederate. The confederate followed a specific script, acting disinterested and impatient to increase believability of his/her behavior later in the session. Both then drew rigged slips of paper to determine their roles in two task phases. The first task was the interpersonal-judgment phase, used to induce provocation. The participant was given 5 minutes to write an essay describing his/her fit for a job that was then scored by the confederate. The confederate was told within earshot of the participant that detailed review of the essays normally took about 10 minutes. Within 3 to 4 minutes, the confederate handed in a feedback form which described the essay by the participant as “defensive” and “uninteresting,” with low attractiveness and likeability. A member of the research staff then appeared to accidentally leave behind the feedback form for the participant to view, to induce anger and hostility. Approximately 96.4% of participants looked at and read the feedback form; participants who did not read the form, and thus were presumably not provoked, were excluded from analyses of this task (n = 6).
Immediately afterwards, the participant switched roles within the employee-supervisor phase. The participant provided “supervisory” feedback on the correctness of the confederate’s (i.e., employee’s) responses on a digit recall task. Participants observed the confederate’s supposed responses on a computer monitor and provided shock feedback using a multi-button box. Participants pressed the “correct” button for correct responses and provided feedback on “incorrect” responses by pressing a no shock button (0) or any of 7 levels of increasing shock intensities (1–7). That participants experienced shocks during Session 1 increased the believability of this part of the task; however, no actual shocks were administered to the confederate in the laboratory aggression procedure. The task was comprised of four blocks, each with 10 trials, and 40% of trials involved incorrect responses. Aggression was the average shock intensity administered during incorrect responses per block. Across blocks, participants shocked at an average level of 2.33 (SD=2.06, Range: 0–7). When participants chose the no-shock feedback option for all confederate incorrect responses across the task (n = 30; 24.2%), their average shock intensity was recorded as “0.” Performance on this task relates to hostile and aggressive tendencies (Verona & Patrick, 2002).
Self-report aggression.
We supplemented the laboratory aggression index by assessing self-report aggression across two measures, administered in Session 1. The 34-item Aggression Questionnaire (α=.91) was used to assess aggression proneness (AQ;(Arnold H. Buss & Perry, 1992). It is comprised of subscales measuring physical, verbal, indirect aggression, anger, and hostility, with items rated using a 5-point Likert scale (1= “extremely uncharacteristic of me” to 5= “extremely characteristic of me”). We used the total score in analyses. The AQ demonstrates strong psychometric properties (Arnold H Buss & Warren, 2000). Lifetime aggressive acts were measured by the Life History of Aggression (LHA) (Brown et al., 1982) interview conducted in Session 1. The LHA is comprised of subscales measuring the frequency of self and other-directed aggression and antisocial behavior since the age of 13, rated on a 5-point scale (ranging from 0=no events to 5= so many events they can’t be counted). We used the Aggression subscale (e.g., physical fights and assaults) to index lifetime history of aggressive behavior (α =.72). The LHA demonstrates strong psychometric properties in community samples (Coccaro, Berman, & Kavoussi, 1997).
Manipulation Check and Debriefing
To validate the manipulation, changes in affect across the experiment were measured via the Positive and Negative Affect Scales (PANAS(D. Watson & Clark, 1994)) at (1) the beginning of the laboratory session, (2) following the EGNG Task, (3) following provocation, and (4) after the employee-supervisor phase. Participants rated how they were feeling “right now” on the 10-item Positive Affect (e.g., “excited,” “strong”) and 10-item Negative Affect (e.g., “hostile,” “scared”) subscales, using a 5-point Likert scale (ranging from 1=very slightly or none at all to 5= extremely). The PANAS is sensitive to short-term fluctuations in mood (David Watson, Clark, & Tellegen, 1988).
At the end of the session, participants completed a Post-Study Questionnaire developed in our laboratory (Verona et al., 2009), reporting perceptions of the confederate (e.g., ranging from “Immature” to “Mature”), and reasons for administering shocks (e.g., to encourage better performance, upset at employee or about employee’s performance) using several Likert Scales. They also completed a debriefing interview with 7 open-ended questions regarding the purpose of the study, experiences working with the confederate, and whether anything seemed “off or unusual” during the task. Participants who guessed the purpose of the study or identified the confederate as study staff (n=13, 9%) were excluded from laboratory aggression analyses.
Covariates
Since men are generally more aggressive than women (Staniloiu & Markowitsch, 2012), and substance use is implicated in both aggressive behavior (Boles & Miotto, 2003) and poor sleep (Brower, 2003), we covaried for these factors in secondary analyses of Aims 1 and 2, to rule out confounding effects. Past-year alcohol (11 items; e.g., tolerance) and drug use disorder (11 items; e.g., withdrawal) symptoms were assessed using the MINI for the DSM-5 (MINI 7.0) (Sheehan et al., 1998). Symptoms were rated by doctoral students trained by a licensed clinical psychologist. The MINI demonstrates good validity and reliability (Sheehan et al., 1998).
Physiological Data Acquisition and Processing
ERPs were recorded using Electrical Geodesics hydrocel 64-channel sensor nets and amplifiers (EGI, Eugene, OR). Nets were placed using known anatomical landmarks. Consistent with similar studies of the EGNG task (Verona et al., 2012), electrodes were selected from the frontocentral sites (3 electrodes), as the central focus of this study was inhibitory control. Consistent with research examining relationships between inhibitory control and aggression (Verona & Bresin, 2015), the greatest Go/No-Go differentiation in our study was apparent frontocentrally (F(1,110)=32.85, p<.001, ηp2=.23). Analog signals were digitized online at 250 Hz and bandpass-filtered (.15–200 Hz) and amplified using Net Amps amplifiers. Eye movements were recorded using electrodes underneath the eyes. Impedances were kept below 50 kΩ. Stimuli were presented using E-Prime (PST Inc., Pittsburgh, PA), and behavioral responses on EGNG task were collected with a 4-button keypad.
Offline data processing was completed in Netstation. Data were re-referenced to average head and epoched 200 ms before and 800 ms after stimulus onset. A 0.10 to 30 Hz filter was applied with a baseline correction. Trials with artifact deflections greater than 140 mV or with eye movements greater than 55 mV in absolute value were discarded, with a moving average of 80 ms. Channel replacement using spline interpolation from nearby electrodes was performed for channels where more than 20% of trials were discarded. We excluded participants with fewer than 20 total possible trials per each of the 6 conditions (n=24) to ensure the minimum number of trials needed for statistically stable (e.g., internally consistent) measurement of the N2 and P3 (Rietdijk, Franken, & Thurik, 2014). An additional 3 subjects were excluded due to missing (n=1) and corrupted (n=2) data files, for a final n of 111 in ERP analyses. Participants excluded from ERP analyses did not differ on demographic or study variables (e.g., sleep duration, self-reported aggressive tendencies and lifetime acts, laboratory aggression) from those not excluded.
An average of 83% of trials were retained for Go trials (M = 361.45, SD = 44.20, Range: 251–427 trials), and 83% were retained for No-Go trials (M = 119.53, SD = 16.83, Range: 76–144 trials). To characterize internal reliability of the ERP components within our sample, we computed dependability estimates using the ERP Reliability Analysis Toolbox v 0.4.8 (Clayson & Miller, 2017), which uses formulas based on generalizability theory. Results from the toolbox suggested that our ERP components had good internal reliability (N2 and P3 reliabilities: >.85).
The P3 was identified via visual inspection of the waveform in concert with established guidelines (Duncan-Johnson & Donchin, 1982). The P3 was defined as the adaptive mean peak amplitude (+/−50ms) across the three frontal-central electrodes within the 400 to 600ms post-stimuli time window. As visual inspection indicated no apparent frontocentral N2, we defined the N2 as the adaptive mean peak amplitude (+/− 10ms) within 200 to 350 ms post-stimuli at parietal sites (average across three parietal electrodes). Research suggests that the parietal N2 indexes visual attention or the processing of stimulus context and features (Folstein & Van Petten, 2008).3
Power and Data Analysis
An a priori power analysis using G*Power (Faul, Erdfelder, Lang, & Buchner, 2007) indicated that 100 participants would be necessary for .8 power to detect a medium effect (f=.25) for a 3-way interaction in a mixed-model repeated measures analysis of variance. According to guidelines for mediation analyses, assuming .8 power and an alpha of .05, 78 participants were required to detect a medium effect for bootstrap tests of indirect effects (Fritz & MacKinnon, 2007). However, all participants from the parent study beyond the 100 proposed who consented to participate in this sub-study were included in analyses to increase our power and maximize measurement of our constructs of interest.
No variables departed from normality (assessed via outliers, skewness, kurtosis, and the Kolmogorov-Smirnov test). As previously noted, participants with invalid data were excluded; thus, sample sizes differed across analyses: n=124 for Aim 1 analyses, n=111 for Aim 2 analyses, and n=99 for Aim 3 analyses (due to excluding participants with invalid data in either Aim 1 or Aim 2). For Aim 1, we conducted a mixed model repeated measures ANOVA (RMANOVA) to examine the effect of continuous sleep duration (between subjects) and experimental block (1–4, within subjects) on average shock intensity delivered during the laboratory aggression paradigm, and correlated sleep duration with self-report measures of aggression proneness and lifetime acts. For Aim 2, we conducted mixed-model RMANOVAs to examine separate and interactive effects of sleep duration, trial type (Go, No-Go) and emotion word category (Negative, Neutral, Positive) on the amplitude of the P3 and behavioral indices of performance on the task (commission errors, reaction time). Inclusion of gender, substance use symptoms, and type of sleep measurement as covariates did not alter the size or direction of effects reported for Aims 1 and 2; therefore, we report results without these covariates for ease of analytic interpretation. For Aim 3, we used bootstrapped path analyses with maximum likelihood estimation to explore whether computed indices of emotional processing (Emotional – Neutral P3) and attentional or motor inhibition (No-Go – Go N2 or P3), or their interaction (No-Go P3 in the negative word condition), explained variance in the sleep-aggression relationship. Bootstrapped path analyses resample the collected data 10,000 times to provide a percentile-based and bias-corrected confidence interval for the indirect effect (Preacher & Hayes, 2004). A significant indirect effect is considered present when zero is not contained in its confidence interval (MacKinnon, Lockwood, & Williams, 2004).
Results
Aggression Paradigm Validity
Mood changes as a function of provocation.
A one-way RMANOVA with polynomial orthogonal contrasts (linear, quadratic, cubic) of time (1–4: beginning of experiment, after EGNG task, after provocation, after aggression paradigm) was conducted to examine change in affect (measured via PANAS) across the experimental session. There was a significant cubic effect of time on PANAS negative affect, F(1,123) = 13.05, p<001, ηp2=.10, such that negative affect decreased slightly from baseline (M=12.20, SD=3.72) to following the EGNG task (M=12.13, SD=3.35; Time 1 to Time 2 p>.05, ηp2=.001), increased following the provocation (M=13.29, SD=4.59; Time 2 to Time 3 p<.01, ηp2=.09), and declined at the end of the aggression paradigm (M=12.39, SD=3.87; Time 3 to Time 4 p<.01, ηp2=.09). There were larger, moderate-sized increases in hostility (t(123)=4.51, p<.001, d=0.40) and irritability (t(123)=3.22, p<.01, d=0.29) after provocation.
Construct validity of aggression procedure.
Average shock intensity was associated with post-study questionnaire reports of selecting higher shock levels due to being upset at the confederate (r=0.67, p<.001), which we considered a measure of reactive motives for aggression, and to encourage the confederate to improve his/her performance (r=0.70, p<.001), potentially instrumental motive for aggression. Based on these associations, our laboratory index of aggression may have indexed multiple motives. Nonetheless, other data indicated primarily emotion-based aggression. Higher shock was associated with negative perceptions of the confederate (e.g., competence, r=−0.24, p<.01; maturity, r=−0.19, p<.05; likeability, r=−0.19, p<.05) and with self-reported angry and aggressive tendencies (AQ r=.25, p<.01), albeit not lifetime acts of aggression (LHA r=.06, p>.05).
Main Analyses
Aim 1: Sleep Duration and Aggression.
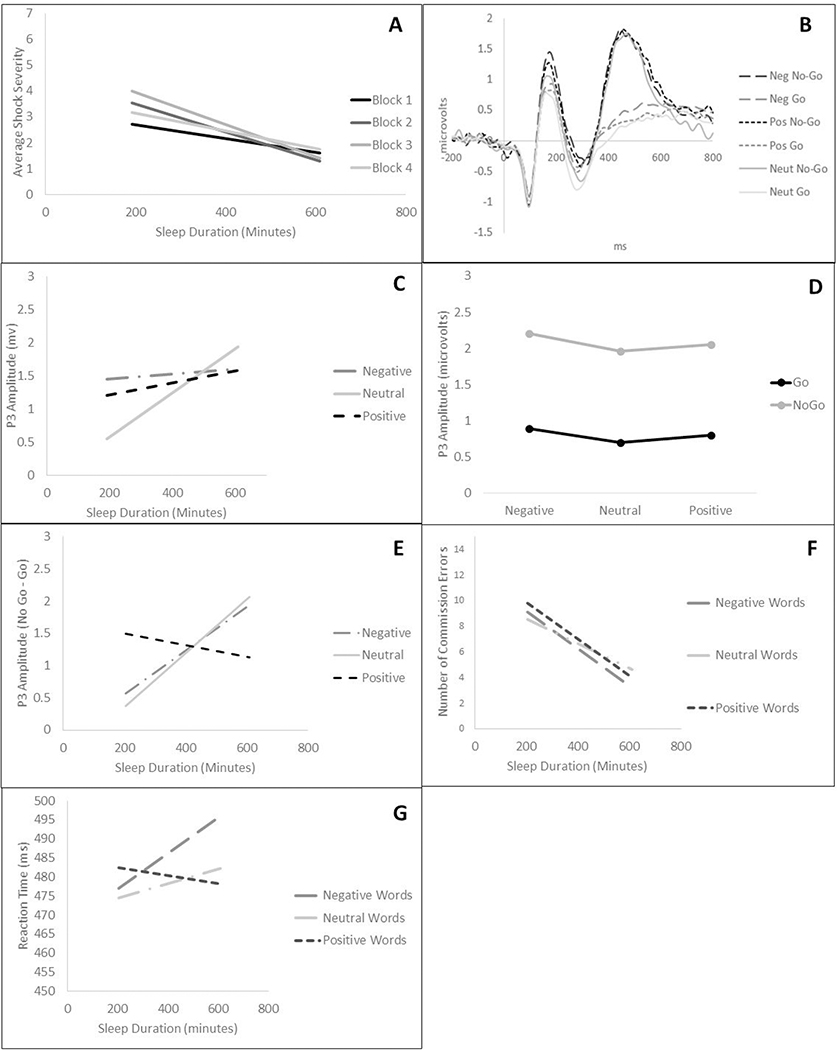
A priori planned contrasts (linear, quadratic, and cubic) were used to examine effects of block on laboratory aggression over time as a function of sleep duration, based on prior findings of quadratic effects of aggression across the experiment among aggressive participants (Verona et al., 2009). A Sleep Duration × Block (1–4; using linear, quadratic, and cubic polynomial contrasts) RMANOVA on average shock intensity scores revealed a quadratic effect of Block (shock intensity increased across the course of the experiment, peaking during middle blocks, and declining towards the end of the experiment). There was also a Sleep Duration × quadratic Block interaction (see Table 2), such that aggression was more pronounced in middle blocks as sleep duration decreased (Block 1 r: −.10, p=.27; Block 2 r: −.20, p<.05; Block 3 r: −.22, p<.05; Block 4 r: −.11, p=.06) (see Figure 1A).4,5 These results suggest that the pattern of aggression across blocks varies as a function of average sleep duration.
Table 2.
RMANOVA Results for five models examining interrelationships of sleep duration with average shock intensity and event-related potential and behavioral variables.
| Predictor | F | df | p | ηp2 | 95% CI |
|---|---|---|---|---|---|
|
| |||||
| Model DV: Average Shock Intensity | |||||
|
| |||||
| Block (Linear) | 0.80 | (1,121) | .37 | .01 | [0.0, 0.06] |
| Block (Quadratic) | 6.45 | (1,121) | <.05 | .05 | [0.002, 0.14] |
| Block (Cubic) | 0.32 | (1,121) | .57 | .00 | [0.0, 0.05] |
| Sleep Duration × Linear Block | 0.23 | (1,121) | .63 | .00 | [0.0, 0.04] |
| Sleep Duration × Quadratic Block | 4.89 | (1,121) | <.05 | .04 | [0.001, 0.12] |
| Sleep Duration × Cubic Block | 0.10 | (1,121) | .75 | .00 | [0.0, 0.04] |
| Sleep Duration (Between Subjects) | 3.61 | (1,121) | .06 | .03 | [0.0, 0.10] |
|
| |||||
| Model DV: Frontocentral P3 Amplitude | |||||
|
| |||||
| Emotion Valence | 0.25 | (1,108) | .62 | .00 | [0.0, 0.05] |
| Emotion Arousal | 14.37 | (1,108) | <.001 | .12 | [0.03, 0.23] |
| Trial Type | 0.02 | (1,108) | .90 | .00 | [0.0, 0.03] |
| Sleep Duration (Between Subjects) | 0.76 | (1,108) | .39 | .01 | [0.0,0.07] |
| Emotion Valence × Sleep Duration | 0.06 | (1,108) | .81 | .00 | [0.0, 0.04] |
| Emotion Arousal × Sleep Duration | 11.43 | (1,108) | <.01 | .10 | [0.02, 0.21] |
| Trial Type × Sleep Duration | 2.66 | (1,108) | .11 | .02 | [0.0, 0.11] |
| Emotion Valence × Trial Type | 2.85 | (1,108) | .09 | .03 | [0.0, 0.11] |
| Emotion Arousal × Trial Type | 4.47 | (1,108) | <.05 | .04 | [0.001, 0.11] |
| Emotion Valence × Trial Type × Sleep Duration | 3.20 | (1,108) | .08 | .03 | [0.0, 0.11] |
| Emotion Arousal × Trial Type × Sleep Duration | 4.43 | (1,108) | <.05 | .04 | [0.001, 0.11] |
|
| |||||
| Model DV: Parietal N2 Amplitude | |||||
|
| |||||
| Emotion Valence | 0.00 | (1,108) | .99 | .00 | [0.0,0.02] |
| Emotion Arousal | 0.11 | (1,108) | .75 | .00 | [0.0,0.04] |
| Trial Type | 1.74 | (1,108) | .19 | .02 | [0.0,0.09] |
| Sleep Duration (Between Subjects) | 0.16 | (1,108) | .69 | .00 | [0.0,0.05] |
| Emotion Valence × Sleep Duration | 0.19 | (1,108) | .67 | .00 | [0.0,0.02] |
| Emotion Arousal × Sleep Duration | 0.01 | (1,108) | .92 | .01 | [0.0,0.02] |
| Trial Type × Sleep Duration | 0.33 | (1,108) | .57 | .00 | [0.0,0.05] |
| Emotion Valence × Trial Type | 0.07 | (1,108) | .79 | .00 | [0.0,0.04] |
| Emotion Arousal × Trial Type | 1.32 | (1,108) | .25 | .00 | [0.0,0.08] |
| Emotion Valence × Trial Type × Sleep Duration | 0.07 | (1,108) | .79 | .00 | [0.0,0.04] |
| Emotion Arousal × Trial Type × Sleep Duration | 0.81 | (1,108) | .37 | .01 | [0.0,0.07] |
|
| |||||
| Model DV: Commission Errors | |||||
|
| |||||
| Sleep Duration (Between Subjects) | 3.53 | (1,108) | .06 | .03 | [0.0, 0.12] |
| Emotion Valence | 0.14 | (1,108) | .71 | .001 | [0.0, 0.04] |
| Emotion Arousal | 1.85 | (1,108) | .18 | .02 | [0.0, 0.09] |
| Sleep Duration × Emotion Valence | 0.01 | (1,108) | .92 | .00 | [0.0, 0.20] |
| Sleep Duration × Emotion Arousal | 2.20 | (1,108) | .14 | .02 | [0.0, 0.10] |
|
| |||||
| Model DV: Reaction Time | |||||
|
| |||||
| Sleep Duration (Between Subjects) | 0.03 | (1,105) | .87 | .00 | [0.0, 0.03] |
| Emotion Valence | 1.52 | (1,105) | .22 | .01 | [0.0, 0.09] |
| Emotion Arousal | 0.26 | (1,105) | .61 | .002 | [0.0, 0.05] |
| Sleep Duration × Emotion Valence | 3.18 | (1,105) | .08 | .03 | [0.0, 0.12] |
| Sleep Duration × Emotion Arousal | 0.00 | (1,105) | .96 | .00 | [0.0, 0.07] |
Figure 1.
A) Shock severity across block as a function of sleep duration; B) Grand average frontocentral waveform; Amplitude of frontocentral P3 as a function of C) sleep duration × emotion word category; D) trial type × emotion word category; and E) sleep duration × inhibition processing (no-go P3); F) Commission errors and G) Reaction time as a function of emotion word category and sleep duration.
Besides its association with the time course of laboratory aggression, sleep duration was negatively associated with self-reported angry and aggressive tendencies in real life (AQ; r= −.26, p<.01; see Table 3), although not with a lifetime history of aggressive acts (LHA), suggesting that decreases in sleep duration relate to increases in more general aggression proneness, rather than lifetime acts of aggression.
Table 3.
Correlations between sleep duration and average shock intensity with P3, behavior performance, and self-report measures.
| 1 | 2 | 3 | M(SD) | |
|
| ||||
| 1. Sleep Duration (Minutes) | -- | -- | -- | 419.3(79.7) |
|
| ||||
| 2. Average Shock Intensity | −.17 | -- | -- | 2.3(2.1) |
| 3. Aggressive Tendencies (AQ) | −0.26** | .25** | -- | 72.4(20.5) |
| 4. Lifetime Aggression (LHA) | −0.12 | .06 | 0.45*** | 9.2(4.9) |
|
| ||||
| Sleep Duration | Avg. Shock Intensity | M(SD) | ||
|
| ||||
| Inhibitory Conditions | -- | -- | -- | |
| Go P3 | .01 | −.23* | 0.76(1.24) | |
| No-Go P3 | .11 | −.23* | 2.04(2.05) | |
| Negative P3 | .01 | −.22* | 1.55(1.75) | |
| Neutral P3 | .13 | −.22* | 1.33(1.66) | |
| Positive P3 | .03 | −.21* | 1.42(1.52) | |
| Go N2 | .02 | .04 | −0.41(1.84) | |
| No-Go N2 | .05 | −.01 | −0.83(2.10) | |
| Negative N2 | .03 | −.08 | −0.82(1.13) | |
| Neutral N2 | .04 | −.08 | −1.06(1.13) | |
| Positive N2 | −.01 | −.05 | −0.92(1.13) | |
| Inhibitory Conditions by Emotion Category | -- | -- | -- | |
| No-go vs. Go P3 | .12 | −.09 | 1.32(1.44) | |
| No-go vs. Go P3 (Negative) | .14 | −.16 | 1.32(1.58) | |
| No-go vs. Go P3 (Neutral) | .18 | −.07 | 1.30(1.76) | |
| No-go vs. Go P3 (Positive) | −.04 | .03 | 1.30(1.75) | |
| No-Go vs. Go N2 | .05 | .01 | −0.43(1.22) | |
| No-go vs. Go N2 (Negative) | .12 | .03 | −0.29(0.96) | |
| No-go vs. Go N2 (Neutral) | .17 | −.11 | −0.39(0.96) | |
| No-go vs. Go N2 (Positive) | .05 | −.07 | −0.30(0.92) | |
| Behavioral Indices by Valence | -- | -- | -- | |
| Commission Errors Total | −.18 | .06 | 18.97(16.51) | |
| Commission Errors (Negative) | −.20* | .06 | 5.90(5.60) | |
| Commission Errors (Neutral) | −.13 | .07 | 6.42(5.81) | |
| Commission Errors (Positive) | −.19 | .06 | 6.64(5.85) | |
| RT (ms) | .01 | .06 | 481.29(91.41) | |
| RT (Negative) | .04 | .02 | 486.62(94.81) | |
| RT (Neutral) | .01 | .10 | 477.78(91.34) | |
| RT (Positive) | −.01 | .05 | 479.46(91.27) | |
Note. AQ = Aggression Questionnaire. LHA = Lifetime History of Aggression Interview.
p<.05
p<.01
p<.001.
RT = Reaction Time.
Aim 2: Sleep Duration, Attentional and Motor Inhibition, and Emotional Processing.
The grand-average frontocentral waveform is illustrated in Figure 1B (n=111). First, RMANOVAs were conducted examining Sleep Duration × Emotion Word Category (3) × Trial Type (2) on the amplitude of the P3 and N2. For emotion word category, a priori planned contrasts were used to separately characterize the effects in terms of Emotion Arousal contrast (positive/negative versus neutral) and Valence contrast (negative versus positive).
For analyses involving the frontocentral P3, there was an effect of Emotion Arousal contrast (positive/negative vs. neutral) on P3 amplitude, with greater P3 amplitude to emotional versus neutral words, indicative of greater processing of salient cues under these conditions. Significant interactions included Sleep Duration × Emotion Arousal, Emotional Arousal × Trial Type, and Sleep Duration × Emotion Arousal × Trial Type interactions. We decomposed the three-way interaction, which superseded each of the two-way interactions (displayed in Figure 1C and Figure 1D, respectively), by conducting analyses within each emotion arousal condition. There was a Sleep Duration × Trial Type interaction within neutral (F(1,108)=5.90, p<.05, ηp2=.05), but not emotional (F(1,108)=0.94, p=.34, ηp2=.01) words. As sleep duration decreased, there was less go/no-go differentiation during neutral blocks (r=.18, p=.10), relative to emotional blocks (r=.09, p=.33), although these simple effects were not significant. As displayed in Figure 1E, the emotional arousal effect was driven primarily by No-Go P3 during the positive word condition. Specifically, as sleep duration decreased, there was a non-significant decrease in motor inhibitory processing (go/no-go P3) in neutral (r=.18, p=.10) and negative (r=.14, p=.15) blocks, but go/no-go differentiation within positive blocks was similar (r= −.04, p=.60) across the range of sleep duration. These results indicate that as sleep duration decreases on average, there is reduced motor inhibition processing within negative and neutral but not positive contexts.6 Additional decomposition of the Sleep × Quadratic Emotion Word interaction indicated that shorter sleep duration was linked with greater P3 differentiation to emotional versus neutral word blocks (emotion-neutral P3; r=−.29, p<.01; Figure 1C).
For analyses of the parietal N2, a Sleep Duration × Emotion Word (3) × Trial Type (2) RMANOVA did not yield any significant results (all ps .19–.99; ηp2s=.00–.02).
In terms of behavioral indices, RMANOVA revealed that sleep duration was not significantly associated with commission errors or reaction time overall or as a function of either contrast for Emotion Word Category (see Figure 1F and Figure 1G).7
Aim 3: Explaining the Sleep-Aggression Relationship.
Zero-order correlations between all variables of interest were first examined to explore the pattern of correlations. As shown in Table 2, sleep duration and aggression did not show the same patterns of correlations with the P3, N2, or behavioral indices. First, shorter sleep duration was associated with larger emotion arousal P3 (positive or negative – neutral) but not emotion valence P3 (negative – positive) amplitudes: Positive-Neutral (r =−.25, p<.01), Negative-Neutral (r =−.25, p<.05), Negative-Positive words (r =−.04, p =.51). Second, shorter sleep duration was related to smaller motor inhibition P3 (no-go – go) during negative and neutral conditions (Negative: r=.16, p=.09; Neutral: r=.18, p=.06), but not the positive condition (r = −.04, p=.68); and with more commission errors across conditions (Negative: r=−.20, p<.05; Neutral: r=−.13, p=.19; Positive: r=−.19, p=.051). Third, lab aggression (average shock intensity) was associated with overall reduced P3 magnitude across conditions (see Table 2), consistent with prior work. Average shock intensity also showed a modest, nonsignificant correlation with reduced motor inhibition in negative blocks (No-Go P3: r=−.16, p=.12; commission errors: r=.06, p=.51), but not in positive (No-Go – Go P3: r=.03, p=.74; commission errors: r=.06, p.55) or neutral conditions (No-Go – Go P3: r=−.07, p.45; commission errors: r=.07, p.55). Neither sleep duration nor laboratory aggression was significantly associated with N2.
Contrary to our hypotheses, bootstrapped path analyses revealed that none of the indices of attentional inhibition, motor inhibition, or emotional processing explained variance in the sleep-aggression relationship (see Supplementary Table 2).
Discussion
Although sleep loss is theorized to increase aggression risk (Kamphuis, Meerlo, Koolhaas, & Lancel, 2012), the sleep-aggression relationship and associated psychological processes are poorly understood. This study examined relationships between recent naturally-occurring sleep loss on provocation-induced aggression in the laboratory, and assessed brain potentials and behavioral performance that could help uncover putative mechanisms linking sleep loss and aggression. Findings indicated that, consistent with hypothesis, the lower the sleep duration, the higher the laboratory aggressive behavior, especially in the middle blocks of the experiment. Sleep duration was also related to emotional processing and motor inhibition indices in interesting ways. First, less sleep was associated with inhibited processing of neutral stimuli but allowed for intact processing of emotional stimuli, which may have been prioritized under limited resources. Second, less sleep was associated with smaller motor inhibition P3 (no-go – go) in negative and neutral conditions, but not positive conditions. However, decrements in performance were not specific to condition, as decreases in sleep duration were associated with generally more commission errors. Further, sleep duration was not related to attentional inhibition (i.e., No-Go N2), regardless of emotion condition. Finally, unlike our expectations, ERP/behavioral indices of inhibition and emotion processing failed to explain the sleep-aggression relationship. These results can help elucidate the emotional, cognitive and behavioral sequalae of recent cumulative sleep loss representing risk for aggression.
Aim 1: Linking Sleep Duration with Aggression
This study found that, as expected, naturally-occurring sleep loss in recent nights predicts greater provocation-induced laboratory aggression, even when controlling for potential confounds (e.g., substance use). Our results showed a quadratic block effect of aggression, consistent with previous studies using this experiment that have shown a similar pattern of aggression across time (Verona et al., 2009). Specifically, average degree of sleep loss predicted the time course of aggressive increases, such that shorter sleep duration was associated with a more pronounced quadratic block effect. In contrast, higher sleep duration was related to fairly low and stable levels of shock intensity across the experiment. Shorter sleep duration was associated with somewhat prolonged “burst” of aggression in the middle of the task (across blocks 2 and 3), which can reflect lapses in engagement of cognitive functions that are integral to regulating behavior (i.e., emotional regulation; (Altena et al., 2016). Our results indicate that these bursts of higher aggression do not appear to be driven by the consumption of cognitive resources by emotional processes, given that our indices of emotional and inhibitory processing did not explain relationships between sleep duration and laboratory aggression. However, the inconsistent recruitment of executive functions for other reasons (i.e., fatigue; (Hursh et al., 2004; Peng & Bouak, 2015; Van der Linden, Frese, & Meijman, 2003) may explain this “aggressive burst.”
Our finding of greater aggression across time as a function of decreased sleep diverges from experimental sleep deprivation studies that have found less aggressive behavior in the sleep deprived group or alternatively have found no sleep-aggression relationship (Cote et al., 2013; Vohs et al., 2011). Extreme sleep deprivation heightens fatigue and amotivation, and reductions in cortical arousal (Alhola & Polo-Kantola, 2007; Cote et al., 2008), which could reduce aggression. One interpretation of our findings, in light of the broader literature, is that the sleep-aggression relationship varies as a function of the degree of recent sleep loss, potentially following a U-shaped relationship. That is, greater aggression risk is observed with several hours of sleep loss across a few days, whereas extreme sleep deprivation (Alhola & Polo-Kantola, 2007; Cote et al., 2008) and temporary minor sleep loss (i.e., due to daylight savings time) (Umbach, Raine, & Ridgeway, 2017) can decrease aggression risk. This highlights the importance of conducting research that directly compares the effects of varying manipulations of sleep deprivation on aggressive behavior, to experimentally replicate current findings and confirm our hypotheses.
Aim 2: Linking Sleep Duration with Inhibitory and Emotional Processing
This study also examined relationships between recent sleep loss and cognitive processes using ERPs. Recent sleep loss was not associated with overall attention (smaller overall P3), diverging again from sleep deprivation research finding smaller P3 amplitude among sleep-deprived persons (Choudhary, Kishanrao, Dhanvijay, & Alam, 2016; Gosselin, De Koninck, & Campbell, 2005). This suggests that overall decrements in attention emerge at more extreme levels of sleep loss rather than more naturalistic sleep loss.
In contrast, the P3 index of attention to emotion and inhibitory cues varied as a function of sleep duration, which was as we predicted although not in the same pattern expected. First, decreased sleep did seem to reduce processing of neutral stimuli, but left the processing of emotional stimuli intact. Second, as sleep duration decreased, motor inhibition processing (decreased No-Go P3) decreased in both neutral and negative word blocks, but was similar across the whole range of sleep duration during the positive word blocks. This finding differed from our expectation that motor inhibition processing would be diminished specifically under negative emotional conditions as sleep duration decreased. Research has shown that sleep deprivation is associated with tendencies to evaluate neutral stimuli more negatively (Daniela et al., 2010), which may explain why processing both neutral and negative stimuli potentially interfered with attending to inhibitory cues at shorter durations of sleep. Although speculative, it is possible that shorter sleep induced cognitive adjustments and less focused attention on the goals of the task within contexts that are ambiguous (e.g., neutral) or are critical to survival (e.g., negative), although direct tests of these hypotheses are required before confirmation. Moreover, since our results also indicated that sleep duration was not associated with attentional inhibition (No-Go N2), our findings suggest that shorter sleep duration has more pronounced effects on comparatively later stages of cognitive processing. However, additional research is needed examining our index of attentional inhibition (N2) more frontally to be consistent with published research in this area (Verona et al., 2012), an important limitation of this study since our task did not seem to produce a visible frontal N2.
Notably, these alterations in motor inhibition processing as a function of emotion category and sleep did not map on to behavioral performance patterns on the task. Instead, shorter sleepers made more commission errors across conditions, which was contrary to our hypothesis that the effects would be specific to negative condition. However, the results support the idea that reduction in motor inhibition associated with less sleep is generalized, at least on a relatively simple laboratory paradigm. Under more complex, real-world conditions, the reduced inhibition processing we observed in neutral and negative conditions may translate to inhibitory behavioral impairments under specific salient contexts (Loeber et al., 2012) among those with less sleep. Alternatively, other factors influenced by sleep duration (e.g., sleepiness) may play a role in less effective behavioral performance on the overall task.
Aim 3: Explaining the Sleep-Aggression Relationship
This study also examined the potential mechanisms linking sleep and aggression. The zero-order correlations indicated that sleep duration and aggression were associated with some of the same processes, albeit in different ways, and many of the correlations did not reach conventional levels of significance (see Table 2). Specifically, sleep loss was associated with modest and non-significant reductions in motor inhibitory processing (reduced no-go P3) during negative and neutral word conditions. In contrast, laboratory aggression was associated with reduced motor inhibitory processing (No-Go P3) specifically under negative emotional conditions, consistent with previous work using the same experimental procedures (Verona & Bresin, 2015). Given the differences in patterns of relationships and small size of correlations among these key variables, it is not surprising, although inconsistent with our hypotheses, that the indirect effects of inhibitory or emotional processing were insignificant.
These results have several potential interpretations. First, since relationships in the path models were largely unchanged from their zero-order effects, it appears that sleep duration and indices of inhibition and emotional processing do not overlap in their effects on aggression. That is, the pathway to aggression involving disruption of inhibitory processing within negative contexts (Verona & Bresin, 2015) may be unrelated to the mechanisms that link sleep loss to aggression, an interpretation which diverges from theory implicating inhibitory deficits as a primary mechanism that drives the sleep-aggression relationship (Krizan & Herlache, 2016). Alternatively, the cognitive decrements associated with sleep loss that we examined in this study more distally contribute to aggression (e.g., by impacting emotion regulation or increasing negative affect), and we could not capture that distal impact in our experiment. Research is needed to examine whether intermediate processes (e.g., emotion dysregulation) that are more proximally related to aggression could explain this link. Such work should use controlled experimental designs that manipulate varying levels of sleep deprivation and incorporate measurement refinements. For example, measures of mechanisms at the neural level can be combined with real-time assessments of sleep and aggression (e.g., ecological momentary assessment, actigraphy, ambulatory psychophysiology) to examine how changes in naturalistic sleep duration map on to variations in emotional and cognitive processes and predict changes in aggressive tendencies and behavior in the real world. Alternatively, it is possible that indirect effects linking sleep and aggression are very small in nature, and that these effects would be detected with a larger sample size.
Limitations, Strengths and Conclusions
This study has several limitations. One limitation involves the operationalizations of sleep duration or sleep loss in this study. Because we did not manipulate sleep loss, we cannot make inferences about the causal effects of sleep duration on aggression, and we did not measure stages of sleep (e.g., REM sleep) that could contribute to sleep-aggression relationships (Fantini, Corona, Clerici, & Ferini-Strambi, 2005). It is possible that sleep loss several days prior to measurement could have had cumulative effects on the cognitive processes observed in our study. Although we addressed gaps in the literature by measuring three days of sleep duration data with both Fitbit and self-report data to improve the stability and generalizability of our sleep estimates, the use of the Fitbit Flex resulted in a small proportion of missing data, which we needed to substitute with sleep diary. Future work should strive to replicate the findings of this study using research-grade actigraphs. Finally, we examined sleep duration as a between-subjects variable, and thus are unable to draw conclusions about ways in which intra-individual variability in sleep (i.e., changes in sleep duration compared to an individual’s usual sleep patterns) may contribute to aggression.
A second weakness involves the ecological validity of our aggression measure. Despite methodological strengths of our aggression paradigm (e.g., internal validity; option to “not aggress”), this task may not fully represent real-world contexts of aggression. Indeed, the provocation produced only a small effect on negative affect. At the same time, the purpose of our induction was to induce hostility and irritability specifically, which the induction did produce at medium effect sizes. It is also the case that our laboratory paradigm cannot definitively distinguish different forms and functions of aggression (e.g., instrumental aggression). A final notable limitation is the sample. Although we recruited from a non-targeted community sample, our participants showed higher rates of some disorders (i.e., substance/alcohol use disorder) and lower rates of others (i.e., depression) relative to epidemiological rates (Kessler et al., 2014; Merikangas & McClair, 2012), which may limit generalizability of our findings.
This study also has several strengths. We examined mechanisms that have been theorized to account for relationships between sleep and aggression (Krizan & Herlache, 2016), and we found that we could not yet support these theoretical formulations. We used well-tested laboratory paradigms to measure inhibitory and emotional processing in real-time and mapped our sleep and psychophysiological data to aggressive behavior observed in the laboratory. The study advances our understanding of relationships between sleep, cognitive processes, and aggression. We found that greater sleep loss predicts increases in aggression across time in the laboratory, and that decreased sleep duration relates to reductions in inhibitory processing under non-positive contexts. However, we could not support the role of emotional and inhibitory indices as intermediary processes explaining the sleep-aggression relationship, at least not with our assessments. Nevertheless, our study provides the critically-needed initial evidence that sleep loss predicts more severe trajectories of aggression. If additional research finds mechanisms linking sleep and aggression, this could support sleep as an upstream intervention point for reducing aggression risk.
Supplementary Material
Acknowledgments
Efforts on this manuscript were supported by a grant from the National Institute of Mental Health awarded to Dr. Verona (R21-MH109853), and grants from Psi Chi, Sigma Xi, and the American Psychological Association to Dr. Bozzay. The funders had no role in the conduct of the study, manuscript preparation, or the decision to submit for publication. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the funders.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no conflicts of interest with respect to the authorship or the publication of this article.
Specifically, we compared each individual’s diary data with epoch-by-epoch data from the Fitbit to identify periods misidentified as sleep or wake. Harmonization procedures were employed when diary and Fitbit measures differed by more than 30 minutes of time asleep/awake. In general, these disagreements in Fitbit and diary reports occurred when 1) a participant forgot to tap the Fitbit into awake mode (i.e., Fitbit was tracking ‘sleep’ for the entire day), or 2) a participant forgot to tap the Fitbit into awake mode shortly after waking up (i.e., delayed tapping it into ‘awake’ mode by an hour or more). In these cases, we compared diary data with visible physical activity data across time from the actigraphs, used diary data to guide selection of the time awake or asleep, and used this consensus to adjust the Fitbit sleep start and end times. In cases where there were not clear bursts of physical activity data to demarcate sleep from wake, we adhered to the time provided by the sleep diary.
Average 3-night sleep duration measured in the study correlated moderately with participant reports on the Pittsburgh Sleep Quality Index of average number of hours slept per night over the month prior to study participation (r =.41, p<.001). The average 3-night sleep duration during the study was the following: M= 6 hours and 59 min, SD=79 minutes, Range: 191–510 minutes; whereas, the PSQI last-month average sleep duration, reported prior to monitoring their sleep for our study, was similar: M = 6 hours and 34 minutes, SD=96 minutes, Range: 120–600 minutes.
We also conducted analyses examining P3 effects parietally, to be consistent with other studies examining emotion P3 effects. These results are reported in supplemental materials.
We conducted post hoc RMANOVA analyses to explore whether participants who shocked during the experiment differed in sleep duration from those who did not shock at all. Analyses conducted on data from participants who had used some non-zero level of shock during the experiment (n=70), using an average shock severity score from shock responses only, yielded a Sleep Duration × Quadratic Block effect. Participants with shorter sleep durations showed more pronounced levels of aggression in middle blocks (F(1,68)=4.89, p<.05, ηp2=.07). We also conducted a separate analysis to examine whether sleep duration was associated with the number of no-shock choices administered across blocks in the full sample. The number of no-shock trials did not vary across blocks (n=123), and sleep duration was not associated with number of no-shock trials (all ηp2s=.00 -.03).
We examined potential curvilinear effects of sleep duration on aggression block by probing the interaction at varying levels of sleep duration using the Aiken & West (1991) simple slopes method (calculating +/− 80 minutes from the sample’s average sleep time of 418 minutes to index high and low sleep scores). Overall, shorter sleep durations were associated with greater changes in average shock intensity across blocks (See Figure 1A). Among participants with shorter (−1SD, or 5.65 hours: Linear Block Trend: F(1,121)=3.88, p=.051, ηp2=.03; Quadratic Block: F(1,121)=8.75, p<.01, ηp2=.07) and average (7 hours: Linear Block: F(1,121)=5.27, p<.05, ηp2=.04, Quadratic Block Trend: F(1,121)=3.77, p=.06, ηp2=.03) sleep durations, average shock intensity increased across the course of the experiment, peaking during middle blocks, and declining towards the end of the experiment. In contrast, there was not a significant Block effect at higher degrees (+1SD, or 8.27 hours) of sleep duration (F(2.62, 316.51)=.81, p=.49, ηp2=.01), indicating that individuals with high levels of sleep demonstrated similar shock intensities across blocks.
We also decomposed the three-way interaction using the Aiken & West (1991) simple slopes method (calculating +/− 80 minutes from the sample’s average sleep time of 418 minutes to index high and low sleep scores). We found that at low levels of sleep (−1SD), a non-significant Emotional Arousal × Trial Type interaction trended (F(1,108)=2.79, p=.10, ηp2=.02), showing reduced inhibitory motor processing (No-Go vs. Go) in neutral word blocks (F(1,108)=13.20, p<.001, ηp2=.11) and increased inhibitory processing in emotional word blocks (Positive: F(1,108)=28.34, p<.001, ηp2=.21; Negative: F(1,108)=20.83, p<.001, ηp2=.16) words. This interaction was not significant at average or high levels (+1SD) of sleep. These results provide some nuance to our main study findings, suggesting that the degree to which emotional context facilitates motor inhibitory processing is associated with the degree of sleep loss.
Notably, when analyses of the task were conducted without including sleep duration in the model (i.e., Trial Type (2) × Emotion Word (3) analysis), there was a significant and large main effect of trial type (F(1,110)=97.89, p<.001, ηp2=.47), with the pattern of results (i.e., more pronounced P3 in no-go vs go trials) supporting the no-go P3 as an index of inhibition.
References
- Alhola P, & Polo-Kantola P (2007). Sleep deprivation: Impact on cognitive performance. Journal of Neuropsychiatric disease and treatment. [PMC free article] [PubMed] [Google Scholar]
- Altena E, Micoulaud-Franchi J-A, Geoffroy P-A, Sanz-Arigita E, Bioulac S, & Philip P (2016). The bidirectional relation between emotional reactivity and sleep: From disruption to recovery. Behavioral neuroscience, 130(3), 336. [DOI] [PubMed] [Google Scholar]
- Banks S, Van Dongen HP, Maislin G, & Dinges DF (2010). Neurobehavioral dynamics following chronic sleep restriction: dose-response effects of one night for recovery. Sleep, 33(8), 1013–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay NL, & Ellis JG (2013). Sleep-related attentional bias in poor versus good sleepers is independent of affective valence. Journal of Sleep Research, 22(4), 414–421. [DOI] [PubMed] [Google Scholar]
- Belenky G, Wesensten NJ, Thorne DR, Thomas ML, Sing HC, Redmond DP, … Balkin TJ (2003). Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: A sleep dose-response study. Journal of Sleep Research, 12(1), 1–12. [DOI] [PubMed] [Google Scholar]
- Berkowitz L (1990). On the formation and regulation of anger and aggression: A cognitive-neoassociationistic analysis. American Psychologist, 45(4), 494. [DOI] [PubMed] [Google Scholar]
- Boles SM, & Miotto K (2003). Substance abuse and violence: A review of the literature. Journal of Aggression and Violent Behavior, 8(2), 155–174. [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, & Cohen JD (2001). Conflict monitoring and cognitive control. Psychological review, 108(3), 624. [DOI] [PubMed] [Google Scholar]
- Bradley MM, & Lang PJ (1999). Affective norms for English words (ANEW): Instruction manual and affective ratings. Retrieved from
- Brower KJ (2003). Insomnia, alcoholism and relapse. Journal of Sleep Medicine Reviews, 7(6), 523–539. [DOI] [PubMed] [Google Scholar]
- Brown GL, Ebert MH, Goyer PF, Jimerson DC, Klein WJ, Bunney WE, & Goodwin FK (1982). Aggression, suicide, and serotonin: relationships of CSF amine metabolites. The American Journal of Psychiatry. [DOI] [PubMed] [Google Scholar]
- Buss AH (1961). The psychology of aggression: Wiley. [Google Scholar]
- Buss AH, & Perry M (1992). The Aggression Questionnaire. Journal of Personality and Social Psychology, 63(3), 452–459. doi: 10.1037/0022-3514.63.3.452 [DOI] [PubMed] [Google Scholar]
- Buss AH, & Warren W (2000). Aggression questionnaire:(AQ): Western Psychological Services; Torrence, CA. [Google Scholar]
- Buysse DJ, Ancoli-Israel S, Edinger JD, Lichstein KL, & Morin CM (2006). Recommendations for a standard research assessment of insomnia. Sleep, 29(9), 1155–1173. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF III, Monk TH, Berman SR, & Kupfer DJ (1989). The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Journal of Psychiatry Research 28(2), 193–213. [DOI] [PubMed] [Google Scholar]
- Choudhary AK, Kishanrao SS, Dhanvijay AKD, & Alam T (2016). Sleep restriction may lead to disruption in physiological attention and reaction time. Sleep Science, 9(3), 207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayson PE, & Miller GA (2017). ERP Reliability Analysis (ERA) Toolbox: An open-source toolbox for analyzing the reliability of event-related brain potentials. International Journal of Psychophysiology, 111, 68–79. [DOI] [PubMed] [Google Scholar]
- Coccaro EF, Berman ME, & Kavoussi RJ (1997). Assessment of life history of aggression: development and psychometric characteristics. Psychiatry Research, 73(3), 147–157. [DOI] [PubMed] [Google Scholar]
- Cohen DJ, Eckhardt CI, & Schagat KD (1998). Attention allocation and habituation to anger-related stimuli during a visual search task. Aggressive Behavior, 24(6), 399–409. [Google Scholar]
- Cote KA, McCormick CM, Geniole SN, Renn RP, & MacAulay S. D. J. B. p. (2013). Sleep deprivation lowers reactive aggression and testosterone in men. 92(2), 249–256. [DOI] [PubMed] [Google Scholar]
- Cote KA, Milner CE, Osip SL, Baker ML, Cuthbert BPJP, & behavior. (2008). Physiological arousal and attention during a week of continuous sleep restriction. 95(3), 353–364. [DOI] [PubMed] [Google Scholar]
- Daniela T, Alessandro C, Giuseppe C, Fabio M, Cristina M, & Michele F (2010). Lack of sleep affects the evaluation of emotional stimuli. Brain Research Bulletin, 82(1–2), 104–108. [DOI] [PubMed] [Google Scholar]
- De Houwer J, & Tibboel H (2010). Stop what you are not doing! Emotional pictures interfere with the task not to respond. Psychonomic Bulletin Review, 17(5), 699–703. [DOI] [PubMed] [Google Scholar]
- Demos K, Hart C, Sweet L, Mailloux K, Trautvetter J, Williams S, … McCaffery J (2016). Partial sleep deprivation impacts impulsive action but not impulsive decision-making. Physiology Behavior, 164, 214–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derefinko K, DeWall CN, Metze AV, Walsh EC, & Lynam DR (2011). Do different facets of impulsivity predict different types of aggression? Aggress Behav, 37(3), 223–233. doi: 10.1002/ab.20387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias-Ferreira E, Sousa JC, Melo I, Morgado P, Mesquita AR, Cerqueira JJ, … Sousa N (2009). Chronic stress causes frontostriatal reorganization and affects decision-making. Science, 325(5940), 621–625. [DOI] [PubMed] [Google Scholar]
- Dinges DF, & Kribbs NB (1991). Performing while sleepy: effects of experimentally-induced sleepiness: John Wiley & Sons. [Google Scholar]
- Dolcos F, LaBar KS, & Cabeza R (2004). Dissociable effects of arousal and valence on prefrontal activity indexing emotional evaluation and subsequent memory: an event-related fMRI study. Neuroimage, 23(1), 64–74. [DOI] [PubMed] [Google Scholar]
- Doran SM, Van Dongen HP, & Dinges D. F. J. A. i. d. b. (2001). Sustained attention performance during sleep deprivation: evidence of state instability. 139(3), 253–267. [PubMed] [Google Scholar]
- Duncan-Johnson CC, & Donchin E (1982). The P300 component of the event-related brain potential as an index of information processing. Biol Psychol, 14(1–2), 1–52. [DOI] [PubMed] [Google Scholar]
- Evenson KR, Goto MM, & Furberg RD (2015). Systematic review of the validity and reliability of consumer-wearable activity trackers. International Journal of Behavioral Nutrition and Physical Activity, 12(1), 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Sommer T, Raz A, & Posner MI (2002). Testing the efficiency and independence of attentional networks. Journal of cognitive neuroscience, 14(3), 340–347. [DOI] [PubMed] [Google Scholar]
- Fantini ML, Corona A, Clerici S, & Ferini-Strambi L (2005). Aggressive dream content without daytime aggressiveness in REM sleep behavior disorder. Neurology, 65(7), 1010–1015. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, & Buchner A (2007). G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39, 175–191. [DOI] [PubMed] [Google Scholar]
- Folstein JR, & Van Petten C (2008). Influence of cognitive control and mismatch on the N2 component of the ERP: a review. Psychophysiology, 45(1), 152–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz MS, & MacKinnon D (2007). Required sample size to detect the mediated effect. Psychological Science, 18(3), 233–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giancola PR (2004). Executive functioning and alcohol-related aggression. Journal of Abnormal Psychology, 113(4), 541. [DOI] [PubMed] [Google Scholar]
- Gosselin A, De Koninck J, & Campbell KB (2005). Total sleep deprivation and novelty processing: implications for frontal lobe functioning. Clinical neurophysiology, 116(1), 211–222. [DOI] [PubMed] [Google Scholar]
- Gujar N, Yoo S-S, Hu P, & Walker MP (2011). Sleep deprivation amplifies reactivity of brain reward networks, biasing the appraisal of positive emotional experiences. Journal of Neuroscience, 31(12), 4466–4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafner M, Stepanek M, Taylor J, Troxel WM, & Van Stolk C (2017). Why sleep matters—the economic costs of insufficient sleep: a cross-country comparative analysis. Rand health quarterly, 6(4). [PMC free article] [PubMed] [Google Scholar]
- Handley IM, Lassiter GD, Nickell EF, & Herchenroeder LM (2004). Affect and automatic mood maintenance. Journal of Experimental Social Psychology, 40(1), 106–112. [Google Scholar]
- Harrison Y, & Horne JA (2000). The impact of sleep deprivation on decision making: a review. Journal of experimental psychology: Applied, 6(3), 236. [DOI] [PubMed] [Google Scholar]
- Horne JA (1993). Human sleep, sleep loss and behaviour. The British Journal of Psychiatry, 162(3), 413–419. [DOI] [PubMed] [Google Scholar]
- Hursh SR, Redmond DP, Johnson ML, Thorne DR, Belenky G, Balkin TJ, … Eddy DR (2004). Fatigue models for applied research in warfighting. Aviation, space, and environmental medicine, 75(3), A44–A53. [PubMed] [Google Scholar]
- Kahn-Greene ET, Killgore DB, Kamimori GH, Balkin TJ, & Killgore WD (2007). The effects of sleep deprivation on symptoms of psychopathology in healthy adults. Sleep medicine, 8(3), 215–221. [DOI] [PubMed] [Google Scholar]
- Kahn-Greene ET, Lipizzi EL, Conrad AK, Kamimori GH, & Killgore WD (2006). Sleep deprivation adversely affects interpersonal responses to frustration. Journal of Personality and Individual Differences 41(8), 1433–1443. [Google Scholar]
- Kamphuis J, Meerlo P, Koolhaas JM, & Lancel M (2012). Poor sleep as a potential causal factor in aggression and violence. Sleep medicine, 13(4), 327–334. [DOI] [PubMed] [Google Scholar]
- Kessler RC, de Jonge P, Shahly V, van Loo HM, Wang PS-E, & Wilcox MA (2014). Epidemiology of depression.
- Krizan Z, & Herlache AD (2016). Sleep disruption and aggression: Implications for violence and its prevention. Psychology of Violence, 6(4), 542. [Google Scholar]
- Krueger RF, McGue M, & Iacono WG (2001). The higher-order structure of common DSM mental disorders: Internalization, externalization, and their connections to personality. Personality and Individual Differences, 30(7), 1245–1259. [Google Scholar]
- Kruk MR, Halasz J, Meelis W, & Haller J (2004). Fast positive feedback between the adrenocortical stress response and a brain mechanism involved in aggressive behavior. Behavioral neuroscience, 118(5), 1062. [DOI] [PubMed] [Google Scholar]
- Langsrud K, Kallestad H, Vaaler A, Almvik R, Palmstierna T, & Morken G (2018). Sleep at night and association to aggressive behaviour; patients in a psychiatric intensive care unit. Psychiatry Research, 263, 275–279. [DOI] [PubMed] [Google Scholar]
- Lezak MD, Howieson DB, Loring DW, & Fischer JS (2004). Neuropsychological assessment: Oxford University Press, USA. [Google Scholar]
- Loeber S, Grosshans M, Korucuoglu O, Vollmert C, Vollstädt-Klein S, Schneider S, … Kiefer F (2012). Impairment of inhibitory control in response to food-associated cues and attentional bias of obese participants and normal-weight controls. International journal of obesity, 36(10), 1334–1339. [DOI] [PubMed] [Google Scholar]
- MacKinnon DP, Lockwood CM, & Williams J (2004). Confidence limits for the indirect effect: Distribution of the product and resampling methods. Multivariate behavioral research, 39(1), 99–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R, Watson D, & Wan CK (2000). A three-factor model of trait anger: Dimensions of affect, behavior, and cognition. Journal of personality, 68(5), 869–897. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, & McClair VL (2012). Epidemiology of substance use disorders. Human genetics, 131(6), 779–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe J, & Mischel W (1999). A hot/cool-system analysis of delay of gratification: dynamics of willpower. Psychological review, 106(1), 3. [DOI] [PubMed] [Google Scholar]
- Moberg CA, & Curtin JJ (2009). Alcohol selectively reduces anxiety but not fear: startle response during unpredictable versus predictable threat. Journal of Abnormal Psychology, 118(2), 335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery-Downs HE, Insana SP, & Bond JA (2012). Movement toward a novel activity monitoring device. Sleep and Breathing, 16(3), 913–917. [DOI] [PubMed] [Google Scholar]
- Nie X, Shao Y, Liu S. y., Li H. j., Wan A. l., Nie S, … Dai X.-j. (2015). Functional connectivity of paired default mode network subregions in primary insomnia. Neuropsychiatric disease and treatment, 11, 3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H, & Bouak F (2015). Development of bio-mathematical models for human performance under fatigue. DRDC Scientific Report. [Google Scholar]
- Putman P, Hermans E, & van Honk J (2004). Emotional stroop performance for masked angry faces: it’s BAS, not BIS. Emotion, 4(3), 305–311. doi: 10.1037/1528-3542.4.3.305 [DOI] [PubMed] [Google Scholar]
- Qi J-L, Shao Y-C, Miao D, Fan M, Bi G-H, & Yang Z (2010). The effects of 43 hours of sleep deprivation on executive control functions: event-related potentials in a visual go/no go task. Social Behavior Personality: an international journal 38(1), 29–42. [Google Scholar]
- Randler C, & Vollmer C (2013). Aggression in young adults—a matter of short sleep and social jetlag? Psychological reports, 113(3), 754–765. [DOI] [PubMed] [Google Scholar]
- Rietdijk WJ, Franken IH, & Thurik AR (2014). Internal consistency of event-related potentials associated with cognitive control: N2/P3 and ERN/Pe. PloS one, 9(7), e102672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, … Dunbar GC (1998). The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. The Journal of clinical psychiatry. [PubMed] [Google Scholar]
- Sheridan A, Murray L, Cooper PJ, Evangeli M, Byram V, & Halligan SL (2013). A longitudinal study of child sleep in high and low risk families: Relationship to early maternal settling strategies and child psychological functioning. Sleep medicine, 14(3), 266–273. [DOI] [PubMed] [Google Scholar]
- Sprague J, & Verona E (2010). Emotional conditions disrupt behavioral control among individuals with dysregulated personality traits. J Abnorm Psychol, 119(2), 409–419. doi: 10.1037/a0019194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague J, Verona E, Kalkhoff W, & Kilmer A (2011). Moderators and mediators of the stress-aggression relationship: Executive function and state anger. Emotion, 11(1), 61. [DOI] [PubMed] [Google Scholar]
- Staniloiu A, & Markowitsch H (2012). Gender differences in violence and aggression–a neurobiological perspective. Procedia-social behavioral sciences, 33, 1032–1036. [Google Scholar]
- Strack F, & Deutsch R (2004). Reflective and impulsive determinants of social behavior. Personality and social psychology review, 8(3), 220–247. [DOI] [PubMed] [Google Scholar]
- Umbach R, Raine A, & Ridgeway G (2017). Aggression and sleep: a daylight saving time natural experiment on the effect of mild sleep loss and gain on assaults. Journal of Experimental Criminology, 13(4), 439–453. [Google Scholar]
- Van Cauter E, Spiegel K, Tasali E, & Leproult R (2008). Metabolic consequences of sleep and sleep loss. Sleep medicine, 9, S23–S28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Linden D, Frese M, & Meijman TF (2003). Mental fatigue and the control of cognitive processes: effects on perseveration and planning. Acta psychologica, 113(1), 45–65. [DOI] [PubMed] [Google Scholar]
- van der Zweerde T, Lancee J, Slottje P, Bosmans J, Van Someren E, Reynolds C, … van Straten A (2016). Cost-effectiveness of i-Sleep, a guided online CBT intervention, for patients with insomnia in general practice: protocol of a pragmatic randomized controlled trial. BMC psychiatry, 16(1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dongen H, Maislin G, Mullington JM, & Dinges DF (2003). The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep, 26(2), 117–126. [DOI] [PubMed] [Google Scholar]
- Verona E, & Bozzay ML (2017). Biobehavioral Approaches to Aggression Implicate Perceived Threat and Insufficient Sleep: Clinical Relevance and Policy Implications. Policy Insights from the Behavioral and Brain Sciences, 4(2), 178–185. [Google Scholar]
- Verona E, & Bresin K (2015). Aggression proneness: Transdiagnostic processes involving negative valence and cognitive systems. International Journal of Psychophysiology, 98(2), 321–329. [DOI] [PubMed] [Google Scholar]
- Verona E, & Kilmer A (2007). Stress exposure and affective modulation of aggressive behavior in men and women. Journal of Abnormal Psychology, 116(2), 410. [DOI] [PubMed] [Google Scholar]
- Verona E, & Patrick CJ (2002). Suicide risk in externalizing syndromes: Temperamental and neurobiological underpinnings. In Suicide Science (pp. 137–173): Springer. [Google Scholar]
- Verona E, Sadeh N, & Curtin JJ (2009). Stress-induced asymmetric frontal brain activity and aggression risk. Journal of abnormal psychology, 118(1), 131. [DOI] [PubMed] [Google Scholar]
- Verona E, Sprague J, & Sadeh N (2012). Inhibitory control and negative emotional processing in psychopathy and antisocial personality disorder. Journal of Abnormal Psychology, 121(2), 498. [DOI] [PubMed] [Google Scholar]
- Vigil-Colet A, & Codorniu-Raga MJ (2004). Aggression and inhibition deficits, the role of functional and dysfunctional impulsivity. Personality and individual differences, 37(7), 1431–1440. [Google Scholar]
- Vogler N, Perkinson-Gloor N, Brand S, Grob A, & Lemola S (2014). Sleep, aggression, and psychosocial adjustment in male prisoners. Swiss journal of psychology. [Google Scholar]
- Vohs KD, Glass BD, Maddox WT, & Markman AB (2011). Ego depletion is not just fatigue: evidence from a total sleep deprivation experiment. Social Psychological and Personality Science, 2(2), 166–173. [Google Scholar]
- Volkow ND, Tomasi D, Wang G-J, Telang F, Fowler JS, Wang RL, … Swanson JM (2009). Hyperstimulation of striatal D2 receptors with sleep deprivation: Implications for cognitive impairment. Neuroimage, 45(4), 1232–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyazovskiy VV, & Delogu A (2014). NREM and REM sleep: complementary roles in recovery after wakefulness. The Neuroscientist, 20(3), 203–219. [DOI] [PubMed] [Google Scholar]
- Vyazovskiy VV, & Harris KD (2013). Sleep and the single neuron: the role of global slow oscillations in individual cell rest. Nature Reviews Neuroscience, 14(6), 443–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, & Clark L (1994). The PANAS-X: Manual for the positive and negative affect schedule – expanded form. Unpublished manuscript. [Google Scholar]
- Watson D, Clark LA, & Tellegen A (1988). Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of Personality and Social Psychology, 54(6), 1063. [DOI] [PubMed] [Google Scholar]
- Wilkowski BM, Robinson MD, Gordon RD, & Troop-Gordon W (2007). Tracking the Evil Eye: Trait Anger and Selective Attention within Ambiguously Hostile Scenes. J Res Pers, 41(3), 650–666. doi: 10.1016/j.jrp.2006.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkowski BM, Robinson MD, & Troop-Gordon W (2010). How does cognitive control reduce anger and aggression? The role of conflict monitoring and forgiveness processes. J Pers Soc Psychol, 98(5), 830–840. doi: 10.1037/a0018962 [DOI] [PubMed] [Google Scholar]
- Wyble B, Sharma D, & Bowman H (2008). Strategic regulation of cognitive control by emotional salience: A neural network model. Cognition and Emotion, 22(6), 1019–1051. [Google Scholar]
- Yoo S-S, Gujar N, Hu P, Jolesz FA, & Walker MP (2007). The human emotional brain without sleep—a prefrontal amygdala disconnect. Current Biology, 17(20), R877–R878. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.