Keywords: blood pressure, exercise, mechanoreflex, muscle afferents, sympathetic nerve activity
Abstract
Skeletal muscle reflexes play a crucial role in determining the magnitude of the cardiovascular response to exercise. However, evidence supporting an association between the magnitude of the pressor response and the velocity of muscle deformation has remained to be elucidated. Thus, we investigated the impact of different muscle deformation rates on the neural discharge of muscle afferents and pressor and sympathetic responses in Sprague–Dawley rats. In an ex vivo muscle-nerve preparation, action potentials elicited by sinusoidal mechanical stimuli (137 mN) at different frequencies (0.01, 0.05, 0.1, 0.2, and 0.25 Hz) were recorded in mechanosensitive group III and IV fibers. The afferent response magnitude to sine-wave stimulation significantly varied at different frequencies (ANOVA, P = 0.01). Specifically, as compared with 0.01 Hz (0.83 ± 0.96 spikes/s), the response magnitudes were significantly greater at 0.20 Hz (4.07 ± 5.04 spikes/s, P = 0.031) and 0.25 Hz (4.91 ± 5.30 spikes/s, P = 0.014). In an in vivo decerebrated rat preparation, renal sympathetic nerve activity (RSNA) and mean arterial pressure (MAP) responses to passive stretch (1 kg) of hindlimb skeletal muscle at different velocities of loading (slow, medium, and fast) were measured. Pressor responses to passive stretch were significantly associated with the velocity of muscle deformation (ANOVA, P < 0.001). The MAP response to fast stretch (Δ 56 ± 12 mmHg) was greater than slow (Δ 33 ± 11 mmHg, P = 0.006) or medium (Δ 30 ± 11 mmHg, P < 0.001) stretch. Likewise, the RSNA response was related to deformation velocity (ANOVA, P = 0.024). These findings suggest that the muscle neural afferent discharge and the cardiovascular response to mechanical stimulation are associated with muscle deformation velocity.
INTRODUCTION
Skeletal muscle contraction increases blood pressure (BP). The magnitude of the pressor response is determined by exercise intensity (1), muscle mass (2, 3), which limb is exercising (4–6), muscle fiber type (7), and muscle length (8, 9). An earlier study demonstrated that skeletal muscle contraction velocity affects the cardiorespiratory response to exercise in humans (10). In that study, it was shown that the BP response was greater during slow and fast contraction velocities than during middle contraction velocity at the same workload. However, the mechanisms underlying the variable BP responses to different muscle contraction velocities remain unclear. This is primarily due to the fact that it is difficult to experimentally isolate factors such as mechanical or chemical changes in working skeletal muscle in human studies. Moreover, in humans, it is difficult to differentiate the role of central factors (e.g., central command) from peripheral factors in mediating autonomic control of the circulation during exercise, especially in response to high-intensity exercise (11).
The exercise pressor reflex (EPR) is an important neural mechanism that contributes to the control of the cardiovascular system during exercise (12–14). The EPR is, in part, mediated by the muscle mechanoreflex, which is predominantly expressed via activation of mechanically sensitive group III and IV muscle sensory afferents during muscle contraction (12–14). Evidence suggests that skeletal muscle spindles are sensitive to changes in velocity and are innervated by proprioceptive afferents such as group Ia and group II fibers (15, 16). Specifically, the magnitude of responses in group Ia and group II fibers is highly dependent on the frequency of stimulation (15, 16). Thus, it is logical to suggest that sensory afferents (group III and group IV) innervating skeletal muscle involved in cardiovascular control during exercise exhibit similar characteristics. Moreover, the frequency-dependent properties of muscle afferents could possibly be responsible for evoking the distinct cardiovascular responses to different velocities of skeletal muscle mechanical deformation at the same workload as previously noted in humans (10).
In this study, therefore, it was hypothesized that muscle neural afferent discharge and characteristic cardiovascular responses are dependent on the velocity of muscle deformation. More specifically, the velocity at which the muscle is mechanically activated independent of central factors and metabolic peripheral factors. To test the hypothesis, we investigated 1) the impact of different frequencies of sinusoidal mechanical stimulation on mechanosensitive group III and IV muscle afferent neuronal responses ex vivo and 2) the impact of passive hindlimb muscle stretch at different velocities on renal sympathetic nerve activity (RSNA), mean arterial pressure (MAP), and heart rate (HR) responses in vivo.
MATERIALS AND METHODS
Animals
All experiments were performed in Sprague–Dawley male rats. For ex vivo experiments, 11 rats were used (368 ± 37 g body wt, Envigo RMS, LLC). For in vivo experiments, seven rats were used (407 ± 56 g body wt). The animals were housed in standard rodent cages on 12:12-h light/dark cycles and were fed a standard diet and water ad libitum. All studies were performed in accordance with the United States Department of Health and Human Services National Institutes of Health Guide for the Care and Use of Laboratory Animals. The procedures outlined were approved by the Institutional Animal Care and Use Committee of the University of Texas Southwestern Medical Center.
Experiment 1: Ex Vivo Single-Fiber Nerve Recordings
Muscle-nerve preparations.
The experimental procedures used have been described previously (17). In brief, the extensor digitorum longus (EDL) muscle was excised with the common peroneal nerve attached with the animal under isoflurane anesthesia (4% in 100% oxygen, 1.5%–2% during surgery) and intubated for mechanical ventilation. The isolated muscle-nerve preparation was then placed in a test chamber maintained at 34 ± 0.5°C (pH 7.4) with superfusion of modified Krebs–Henseleit solution (Krebs solution, 110.9 mM NaCl, 4.7 mM KCl, 2.5 mM CaCl2, 1.2 mM MgSO4, 1.2 mM KH2PO4, 25.0 mM NaHCO3, and 20.0 mM glucose) bubbled with a 5% CO2–95% O2 gas mixture. The common peroneal nerve was drawn through a hole to a recording chamber filled with paraffin oil, and small filaments of the nerve were repeatedly split with sharpened watchmaker forceps and a thin needle until single-fiber activity could be recorded. Animals were euthanized by inhalation of CO2 gas after the excision of tissues.
Thin fiber muscle afferents recordings.
As described previously, skeletal muscle single-afferent fibers that fulfilled the following criteria were studied (17): 1) the afferent fiber was sensitive to mechanical stimulation from gentle probing with a glass rod and 2) there was no intensity-dependent increase in the discharge rate of the afferent fiber in response to muscle stretch. We defined fibers with a conduction velocity of ≤2.0 m/s as group IV fibers and from 2.0 to < 15.0 m/s as group III fibers (18). Conduction velocity was calculated from the conduction delay of the action potential induced by electrically stimulating the receptive field in EDL muscle and the distance between the recording and stimulating electrodes.
Sinusoidal mechanical stimulus.
A mechanical stimulus was applied to the receptive field of the afferent fiber using a servocontrolled mechanical stimulator equipped with a rounded probe (2.3 mm2; 17). Action potentials elicited by a 137-mN sinusoidal mechanical stimulus at different frequencies (0.01, 0.05, 0.10, 0.20, and 0.25 Hz) were recorded near the receptive field on the EDL muscle.
Experiment 2: In Vivo Skeletal Muscle Mechanoreflex Testing
General surgical procedures for acute experiments.
Rats were anesthetized with 1%–4% isoflurane in oxygen and instrumented using methods previously described (19, 20). Subsequently, animals were ventilated after intubation and 1 M NaHCO3, 5% dextrose Ringer solution was continuously infused via the jugular vein at a rate of 3–5 mL·h−1·kg−1 to stabilize fluid balance and maintain baseline blood pressure. MAP was continuously measured through a left carotid arterial catheter connected to a pressure transducer. HR was obtained via an electrocardiogram signal and calculated from the time between R-R intervals. RSNA was recorded from a branch of the left renal nerve attached to bipolar stainless steel wire electrodes (Bioflex wire AS633; Cooner Wire) and insulated with silicone glue (Kwik-Sil; World Precision Instruments, Sarasota, FL). The preamplified nerve signal was band-pass filtered at 150–1,000 Hz (Neuro Amp EX; ADInstruments), and then full-wave rectified and low-pass filtered with a cutoff frequency of 30 Hz for quantification of RSNA. Animals were placed in a stereotaxic head unit (Kopf Instruments) and a precollicular decerebration procedure (brain tissue rostral to the colliculi was removed by aspiration) was performed to render the animal insentient. Immediately after the decerebration was complete, isoflurane anesthesia was discontinued. Animals were subsequently ventilated with oxygen for the remainder of the experiment to ensure adequate oxygenation of tissues during recovery from anesthesia and decerebration procedures. Experimental procedures were started 1 h after cessation of inhalant anesthesia to eliminate the depressive effects of isoflurane on the pressor and sympathetic responses to mechanoreflex activation (19). Throughout surgical procedures and experimental testing, animal body temperature was kept within a constant range (36.5°C–37.5°C) with a far-infrared heating pad (Kent Scientific).
Procedures for activation of mechanically sensitive muscle afferents with three different velocities of loading at the same mechanical force.
The calcaneal tendon was attached to a force transducer (FT-10) with a braided fishing line to measure tension during mechanoreflex activation. Mechanosensitive muscle afferents were stimulated at different velocities using a hanging water bottle apparatus connected to the braided line, the weight of the water bottle apparatus was ∼96 g. To stimulate mechanosensitive afferents slowly, water was poured into the bottle at a constant rate for 30 s until the tension peaked (900 mL of water + 100-g water bottle apparatus, or ∼1,000 g), at which point the bottle was manually lifted to end the maneuver. For medium-velocity stimulation of mechanosensitive fibers, the bottle was filled for 15 s then drained for 15 s at constant rates, the volume of water was 900 mL (peak tension: 1,000 g). For fast stimulation of mechanosensitive fibers, the bottle was prefilled with 900 mL of water, the bottle was then lowered by hand until the preload was steady at ∼100 g, and finally the bottle was carefully released before being drained at a constant rate for 30 s, the peak tension was 1,000 g. The slow, medium, and fast stimulus maneuvers were applied in random order, twice each for every experiment. The mechanoreflex response to the same stimulus can appreciably vary, thus an average of two trials was used to more accurately represent the responses evoked by the different velocities of passive stretch. The weight of both the empty and filled (900 mL) water bottle apparatus was used to calibrate the measured tension by the force transducer.
End-Experiment Procedures
At the end of the experiments, hexamethonium bromide (60 mg/kg) was administered intravenously to confirm that RSNA signals were recorded from postganglionic renal sympathetic fibers. RSNA background noise was determined 30-min after the animal was humanely euthanized by intravenous injection of saturated potassium chloride (4 M, 2 mL/kg). Measuring background noise after KCl administration allows for isolation of background electrical interference from the local environment, if any. Hearts were removed and weighed following KCl administration. In addition, the tibia was harvested and measured.
Data Acquisition and Analysis
For ex vivo experiments, action potentials were amplified, filtered, and displayed on an oscilloscope and continuously recorded on a computer via an A/D converter (PowerLab8/30; ADInstruments) and data acquisition software (LabChart; ADInstruments). For in vivo experiments, HR, MAP, RSNA, and tension were acquired, recorded, and analyzed using data acquisition software (LabChart; ADInstruments) and the PowerLab digital-to-analog converter (PowerLab8/30; ADInstruments). For each variable, the average of two values for each maneuver (slow, medium, and fast), in each experiment, was calculated and used for statistical analysis. Baseline values were determined from and averaged over a 30-s prestimulation period. Response values obtained during the 30-s mechanoreflex stimulation period were calculated as the difference from baseline. To quantify RSNA response to mechanoreflex activation, the baseline value was considered as 100% of basal RSNA. Changes in RSNA evoked during the mechanoreflex stimulation period were expressed as a percentage of the baseline, and the relative change in RSNA to the baseline was taken as the estimated change (ΔRSNA%). Datasets of 1-s averages were used for the analysis and the maximum response to mechanoreflex activation was defined as the peak change from baseline.
Statistical Analysis
Data were analyzed using SPSS and GraphPad Prism 9 software. All data are expressed as means ± SD. Data normality was assessed by using the Shapiro–Wilk test. Data were analyzed using one‐way repeated measures ANOVA, and post hoc analysis by Fisher’s least significant difference or Tukey’s multiple comparison test. The level of statistical significance was defined as P < 0.05.
RESULTS
Single-Fiber Nerve Recordings
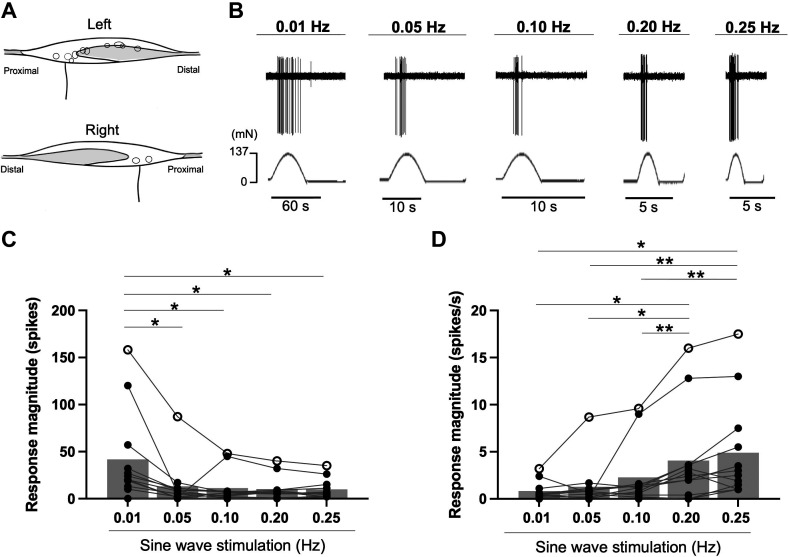
Average fiber conduction velocities in mechanosensitive group IV fibers were 1.08 ± 0.45 m/s (a range of 0.47–1.88 m/s, n = 11). Fiber conduction velocities in group III fibers were 5.15 m/s (n = 1). The position of the group III and IV muscle afferent receptive fields assessed in this study is shown in Fig. 1A. An original recording demonstrating the action potential responses to sinusoidal mechanical stimuli is shown in Fig. 1B. The absolute response magnitude to sine-wave stimulation significantly differed between varying frequencies in group III and IV fibers (ANOVA, P = 0.025, Fig. 1C). Specifically, as compared with 0.01 Hz, the response magnitudes were significantly lower at 0.05 (P = 0.01), 0.10 (P = 0.03), 0.20 (P = 0.02), and 0.25 Hz (P = 0.03). The response magnitude per second to sine-wave stimulation significantly differed among the variable frequencies in mechanosensitive group III and IV fibers (ANOVA, P = 0.01; Fig. 1D). Specifically, the response magnitudes were significantly greater at 0.25 Hz compared with 0.01 (P = 0.01), 0.05 (P = 0.008), and 0.10 Hz (P = 0.003). Moreover, the response magnitudes were significantly higher at 0.20 Hz than 0.01 (P = 0.03), 0.05 (P = 0.03), and 0.10 Hz (P = 0.008).
Figure 1.
Response of group III and IV muscle afferents to sinusoidal mechanical stimuli at different frequencies in rats. Each spot in the illustration represents the receptive field of a group III and IV muscle afferent. A: shading illustrates the tendinous area. B: representative raw recording of individual group IV fiber activity in response to a 137-mN sinusoidal mechanical stimulus at different frequencies (0.01, 0.05, 0.10, 0.20, and 0.25 Hz). C: average number of spikes evoked by a 137-mN sinusoidal mechanical stimulus at different frequencies in group III (○, n = 1) and IV (●, n = 11) fibers. D: average number of spikes per second to a 137-mN sinusoidal mechanical stimulus at different frequencies in group III (○, n = 1) and IV (●, n = 11) fibers. *P < 0.05; **P < 0.01. All data analyzed by one-way repeated-measures ANOVA followed by post hoc Fisher’s least significant difference (LSD).
Skeletal Muscle Mechanoreflex Testing
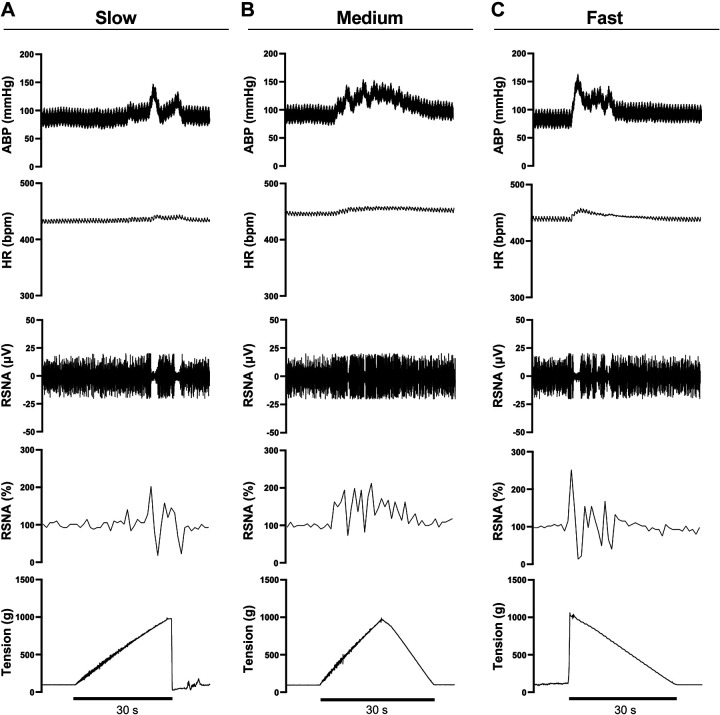
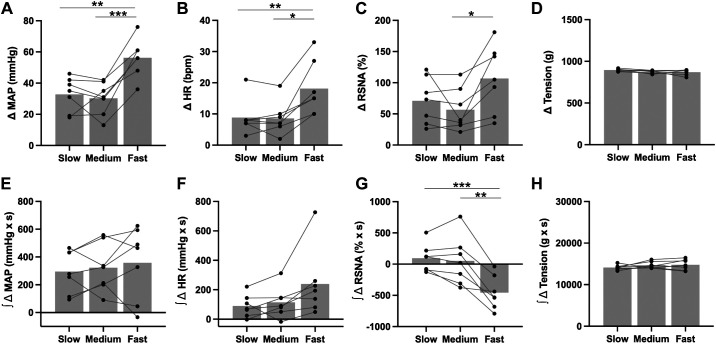
Table 1 lists the characteristics of the animals, including morphometry, and baseline hemodynamics. Slow, medium, and fast velocity tension development at the same mechanical force evoked mechanoreflex activation and subsequent changes in MAP, HR, and RSNA (Fig. 2, A–C). Fast mechanostimulation significantly changed the average peak MAP response (ANOVA, P < 0.001, Fig. 3A). Fast muscle deformation increased MAP (Δ56 ± 12 mmHg) relative to both medium (Δ30 ± 11 mmHg, P < 0.001) and slow deformation (Δ33 ± 11 mmHg, P = 0.006). The average integrated MAP response (slow = 296 ± 154, medium = 323 ± 175, fast = 358 ± 261 mmHg × s) was not different (ANOVA, P = 0.571, Fig. 3E).
Table 1.
Morphometric characteristics, baseline hemodynamics, and mechanoreflex threshold for in vivo experiments
| n | 7 |
| Body weight | 407 ± 56 |
| Heart weight/body weight, mg/g | 2.78 ± 0.37 |
| Heart weight/tibial length, mg/mm | 26.6 ± 2.0 |
| 1% Isoflurane | |
| HR, beats/min | 337 ± 31 |
| MAP, mmHg | 111 ± 10 |
| RSNA, signal to noise ratio | 4.5 ± 2.7 |
| After decerebration | |
| HR, beats/min | 447 ± 34 |
| MAP, mmHg | 113 ± 23 |
| RSNA, signal to noise ratio | 4.0 ± 2.9 |
Values are means ± SD. HR, heart rate; MAP, mean arterial pressure; RSNA, renal sympathetic nerve activity.
Figure 2.
Skeletal muscle mechanoreflex activation by passive stretch during slow, medium, and fast stimulation. Thick horizontal bar represents the 30-s period of mechanoreflex stimulation. Slow (A), medium (B), and fast (C) velocity muscle deformation by passive stretch-induced reflexive increases in blood pressure and heart rate as well as raw and normalized sympathetic nerve activity as can be clearly seen in the representative tracings from experimental animals. Fast mechanostimulation induced the largest increase in all physiological measures at the same mechanical force. ABP, arterial blood pressure; bpm, beats/min; HR, heart rate; RSNA, renal sympathetic nerve activity.
Figure 3.
Rapid stimulation of mechanosensitive fibers by passive stretch produces the most robust increases in heart rate, blood pressure, and sympathetic nerve activity. Fast mechanostimulation produced significantly greater increases in blood pressure (A), heart rate (B), and RSNA (C) relative to both slow and medium stimulations. The increase in mechanical force (D) was the same for all three velocities of muscle deformation. The mean between the integrated blood pressure (E) and integrated heart rate (F) response to muscle deformation at different velocities was not statistically significant. Fast deformation decreased integrated RSNA (G) relative to both slow and medium deformations. The integrated mechanical force (H) was the same for all three velocities of muscle deformation. n = 7. *P < 0.05; **P < 0.01; ***P < 0.001. All data analyzed by one-way repeated-measures ANOVA followed by Tukey’s multiple comparison test. HR, heart rate; bpm, beats/min; MAP, mean arterial pressure; RSNA, renal sympathetic nerve activity.
Likewise, fast muscle deformation significantly changed the average peak HR response (ANOVA, P = 0.002, Fig. 3B). Specifically, fast muscle deformation increased HR (Δ18 ± 9 beats/min) relative to both medium (Δ9 ± 5 beats/min, P = 0.011) and slow deformation (Δ9 ± 6 beats/min, P = 0.007). However, the average integrated HR response (slow = 90 ± 76, medium = 115 ± 103, fast = 239 ± 228 beats/min × s) was not different (ANOVA, P = 0.072, Fig. 3F).
Fast muscle deformation velocity increased the average peak change in RSNA (ANOVA, P = 0.024, Fig. 3C). Specifically, fast muscle deformation increased RSNA (Δ107 ± 54%) relative to medium deformation (Δ57 ± 34%, P = 0.030). However, fast muscle deformation relative to slow muscle deformation was not statistically significant (Δ71 ± 37%, P = 0.195). In addition, the average integrated SNA response was influenced by the velocity of muscle deformation (ANOVA, P < 0.001, Fig. 3G). Specifically, fast muscle deformation decreased the integrated RSNA (−456 ± 268% × s) relative to both medium (52 ± 391% × s, P = 0.004) and slow muscle deformations (95 ± 223% × s, P < 0.001). Finally, the average peak change in tension (slow = 872 ± 18, medium = 871 ± 34, fast = 897 ± 15 g) was not different between the three velocities of muscle deformation of mechanostimulation (ANOVA, P = 0.096, Fig. 3D), and likewise the integrated tension development (slow = 14,128 ± 604, medium = 14,791 ± 813, fast = 14,791 ± 1,280 g × s) was not different (ANOVA, P = 0.300, Fig. 3H).
DISCUSSION
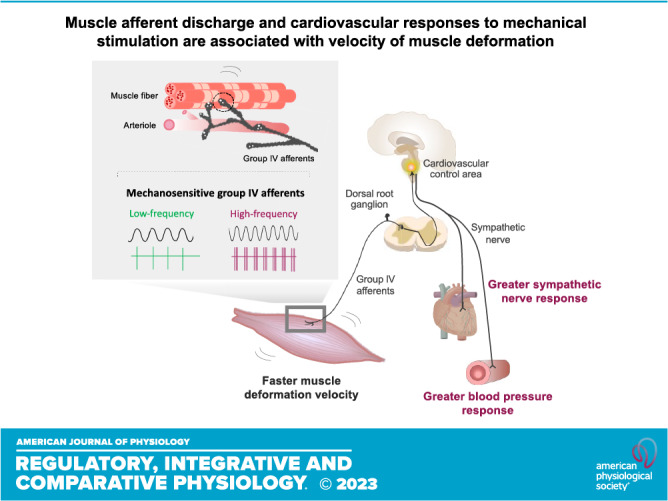
The major novel findings from this investigation were 1) the per second response magnitude of skeletal muscle group III and IV fibers was highly dependent on the rate of change of mechanical stimulation ex vivo and 2) the peak BP, HR, and RSNA responses to passive hindlimb stretch differed significantly with muscle deformation velocity in vivo. These findings are, to our best knowledge, the first evidence that both neural discharge per second response magnitude in muscle afferents and cardiovascular responses to mechanical stimulation are associated with muscle deformation rate in rodents independent of metabolic peripheral and central factors.
Neural Discharge of Group III and IV Muscle Afferents to Different Frequencies of Sinusoidal Mechanical Stimulation
Skeletal muscle group IV afferent fibers are known to display polymodal nociceptor characteristics having the ability to respond to mechanical, thermal, and chemical stimuli (21). Furthermore, previous evidence has demonstrated that 68% of group IV fibers in the skeletal muscle are mechanosensitive (22). These reported findings suggest that group IV muscle afferents play a crucial role in mechanical as well as chemical sensations. To our best knowledge, we observed for the first time (using an ex vivo preparation) that the mechanical neuronal group IV afferent response is rate of change dependent in rats (Fig. 1D). Evidence suggests that group Ia and group II proprioceptive afferents that innervate muscle spindles are highly dependent on the frequency of stimulation for their action (15, 16). Likewise, this study suggests that mechanosensitive group IV afferents display similar properties. Thus, our results provide insight into the potential contributions of group IV muscle afferents to detect rate of change of mechanical stimulation. As the cardiovascular response evoked by the EPR is engaged on stimulation of afferents in skeletal muscle (12, 23, 24), these data open the possibility that rate of change-dependent neural discharge of mechanosensitive group IV muscle afferents significantly contributes to determining the magnitude of the BP response to muscle contraction. The EPR is also mediated via activation of not only group IV fibers but also group III muscle sensory afferents (12–14). Because of the small number of group III afferents that could be successfully isolated in this study, only one mechanosensitive group III afferent response to sinusoidal mechanical stimulation was able to be examined in this investigation. Additional studies are warranted in the future to determine the responsiveness of group III muscle afferents to sinusoidal mechanical stimulation as well.
Cardiovascular and Sympathetic Responses to Different Velocities of Muscle Deformation
It is well known that skeletal muscle reflexes play a crucial role in determining the magnitude of the cardiovascular response to exercise (14). However, to date, the impact of velocity of muscle deformation on muscle afferent discharge has not been directly elucidated. As discussed previously, an earlier study demonstrated that cardiorespiratory responses to skeletal muscle contraction are greater with slow and fast velocities than with middle velocities at the same workload in healthy human subjects (10). Importantly, in that study, as oxygen uptake to the same workload varied among different muscle contraction velocities (10), it was difficult to distinguish the effects of different energy turnover and/or the recruitment of different muscle fiber types from the effects of mechanical deformation in the working muscle. To this end, using an in vivo preparation, this study clearly demonstrated that pressor responses to passive hindlimb stretch were associated with the velocity of muscle deformation. For example, peak changes in MAP, HR, and RSNA to fast-velocity stretch were greater than to slow- or medium-velocity stretch in decerebrated rats (Figs. 2 and 3).
There are several experimental limitations in this study. First, a potential limitation of the study was the method used to load the hindlimb muscles where tangential forces could have been experienced by the mechanosensitive fibers. This may explain why, in one experiment, slow stretch produced a greater peak RSNA response than the fast-stretch maneuver (Fig. 3C). Alternatively, that specific observation might also be explained by a heightened sensitivity to mechanoreflex activation in that particular animal. Nevertheless, the relationship between the muscle deformation velocity and the magnitude of the RSNA response to stretch for the group of animals tested was significant. Second, we may have observed graded changes in cardiovascular and sympathetic responses across all three velocities of passive stretch if we had used a 60-s stimulation period. Finally, although the outcomes of this study may not be impacted by elevated arterial oxygen levels, the titration of oxygen delivery during ventilation should be considered as elevated oxygen levels may influence relevant physiological factors such as heart rate, blood flow, or peripheral gas exchange during muscle contraction (25). Future studies designed to further examine mechanoreflex function should optimize these factors.
Velocity-Dependent Potential Mechanisms for Cardiovascular and Neuronal Discharge Responses
The mechanosensitive channels responsible for activation of the skeletal muscle mechanoreflex have yet to be fully identified. That being said, evidence suggests that mechanosensitive Piezo1/2 channels may underlie mechanosensation in skeletal muscle (26, 27). These channels have been clearly implicated in sensing static touch and vibration. Interestingly, a recent study showed that the amplitude of the initial peak current increased monotonically with changes in sinusoidal pressure frequency in HEK293t cells transfected with Piezo1 (28). Piezo1/2 is expressed in mouse and human dorsal root ganglion (DRG) neurons (26). Equally important, earlier studies suggest that Piezo channels contribute significantly to the expression of the skeletal muscle mechanoreflex in normal healthy rats (29–31), although evidence is somewhat controversial (32). Thus, these channels are good candidates for contribution to the observed rate of change-dependent neural discharge of group III and IV muscle afferents as well as the deformation velocity-dependent cardiovascular responses to mechanical stimulation in this study. As involvement of the Piezo channel pathway was not examined in this investigation, additional studies are warranted in the future to determine its role. In particular, its role in muscle nociceptive sensory afferents and cardiovascular responses to mechanical stimulation in high-intensity exercise.
Perspectives and Significance
In a recently published report, the contribution of the mechanoreflex to cardiovascular regulation in humans has been explicitly demonstrated and is dependent on the intensity of stimulation (33). As such, the findings of this study suggest that the velocity of muscle contraction, in addition to exercise intensity, is likely a key factor in determining the magnitude of cardiovascular response to physical activity in humans.
In conclusion, the findings of this study suggest the muscle afferent discharge and cardiovascular responses to mechanical stimulation are associated with velocity of muscle deformation.
DATA AVAILABILITY
Data will be made available upon reasonable request.
GRANTS
This research was supported by National Institutes of Health Heart, Lung, and Blood Institute Grants R01HL-133179 (to S.A.S. and W.V.) and R01HL-151632 (to M. M.), the University of Southwestern O’Brien Kidney Research Core Center Grant P30DK079328 (to W.V.), and a Jere H. Mitchell Distinguished Professorship in Clinical Research grant (to S.A.S.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.M. conceived and designed research; R.I. and J.E. performed experiments; R.I. and J.E. analyzed data; R.I., J.E., H.-K.K., N.H., A.F., G.A.I., S.A.S., W.V., and M.M. interpreted results of experiments; R.I., J.E., and M.M. prepared figures; R.I., J.E., and M.M. drafted manuscript; H.-K.K., N.H., A.F., G.A.I., S.A.S., and W.V. edited and revised manuscript; R.I., J.E., H.-K.K., N.H., A.F., G.A.I., S.A.S., W.V., and M.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Martha Romero for expert technical assistance. We acknowledge our mentor the late Dr. Jere H. Mitchell, a giant in the field of neural cardiovascular control. Note that the basic concept of this research was originated by Dr. Mitchell and modified for completion after his passing.
REFERENCES
- 1. Mitchell JH. J.B. Wolffe memorial lecture. Neural control of the circulation during exercise. Med Sci Sports Exerc 22: 141–154, 1990. [PubMed] [Google Scholar]
- 2. Mitchell JH, Payne FC, Saltin B, Schibye B. The role of muscle mass in the cardiovascular response to static contractions. J Physiol 309: 45–54, 1980. doi: 10.1113/jphysiol.1980.sp013492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Estrada JA, Ducrocq GP, Kaufman MP. The magnitude of the exercise pressor reflex is influenced by the active skeletal muscle mass in the decerebrate rat. Am J Physiol Regul Integr Comp Physiol 318: R30–R37, 2020. doi: 10.1152/ajpregu.00263.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bevegård S, Freyschuss U, Strandell T. Circulatory adaptation to arm and leg exercise in supine and sitting position. J Appl Physiol 21: 37–46, 1966. doi: 10.1152/jappl.1966.21.1.37. [DOI] [PubMed] [Google Scholar]
- 5. Tokizawa K, Mizuno M, Hayashi N, Muraoka I. Cardiovascular responses to static extension and flexion of arms and legs. Eur J Appl Physiol 97: 249–252, 2006. doi: 10.1007/s00421-006-0186-9. [DOI] [PubMed] [Google Scholar]
- 6. Hayashi N, Hayes SG, Kaufman MP. Comparison of the exercise pressor reflex between forelimb and hindlimb muscles in cats. Am J Physiol Regul Integr Comp Physiol 281: R1127–R1133, 2001. doi: 10.1152/ajpregu.2001.281.4.R1127. [DOI] [PubMed] [Google Scholar]
- 7. Fisher JP, White MJ. Muscle afferent contributions to the cardiovascular response to isometric exercise. Exp Physiol 89: 639–646, 2004. doi: 10.1113/expphysiol.2004.028639. [DOI] [PubMed] [Google Scholar]
- 8. Ng AV, Agre JC, Hanson P, Harrington MS, Nagle FJ. Influence of muscle length and force on endurance and pressor responses to isometric exercise. J Appl Physiol (1985) 76: 2561–2569, 1994. doi: 10.1152/jappl.1994.76.6.2561. [DOI] [PubMed] [Google Scholar]
- 9. Mizuno M, Tokizawa K, Muraoka I. Changes in perfusion related to muscle length affect the pressor response to isometric muscle contraction. Adv Exp Med Biol 662: 371–377, 2010. doi: 10.1007/978-1-4419-1241-1_54. [DOI] [PubMed] [Google Scholar]
- 10. Hayashi N, Koba S, Yoshida T. The effect of muscle contraction velocity on cardiorespiratory responses to repetitive isokinetic exercise in humans. Jpn J Physiol 53: 327–333, 2003. doi: 10.2170/jjphysiol.53.327. [DOI] [PubMed] [Google Scholar]
- 11. Victor RG, Secher NH, Lyson T, Mitchell JH. Central command increases muscle sympathetic nerve activity during intense intermittent isometric exercise in humans. Circ Res 76: 127–131, 1995. doi: 10.1161/01.res.76.1.127. [DOI] [PubMed] [Google Scholar]
- 12. McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol 224: 173–186, 1972. doi: 10.1113/jphysiol.1972.sp009887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J Appl Physiol Respir Environ Exerc Physiol 55: 105–112, 1983. doi: 10.1152/jappl.1983.55.1.105. [DOI] [PubMed] [Google Scholar]
- 14. Mitchell JH, Kaufman MP, Iwamoto GA. The exercise pressor reflex: its cardiovascular effects, afferent mechanisms, and central pathways. Annu Rev Physiol 45: 229–242, 1983. doi: 10.1146/annurev.ph.45.030183.001305. [DOI] [PubMed] [Google Scholar]
- 15. Roll JP, Vedel JP. Kinaesthetic role of muscle afferents in man, studied by tendon vibration and microneurography. Exp Brain Res 47: 177–190, 1982. doi: 10.1007/BF00239377. [DOI] [PubMed] [Google Scholar]
- 16. Roll JP, Vedel JP, Ribot E. Alteration of proprioceptive messages induced by tendon vibration in man: a microneurographic study. Exp Brain Res 76: 213–222, 1989. doi: 10.1007/BF00253639. [DOI] [PubMed] [Google Scholar]
- 17. Taguchi T, Sato J, Mizumura K. Augmented mechanical response of muscle thin-fiber sensory receptors recorded from rat muscle-nerve preparations in vitro after eccentric contraction. J Neurophysiol 94: 2822–2831, 2005. doi: 10.1152/jn.00470.2005. [DOI] [PubMed] [Google Scholar]
- 18. Collins WR Jr, Nulsen FE, Randt CT. Relation of peripheral nerve fiber size and sensation in man. Arch Neurol 3: 381–385, 1960. doi: 10.1001/archneur.1960.00450040031003. [DOI] [PubMed] [Google Scholar]
- 19. Mizuno M, Mitchell JH, Crawford S, Huang CL, Maalouf N, Hu MC, Moe OW, Smith SA, Vongpatanasin W. High dietary phosphate intake induces hypertension and augments exercise pressor reflex function in rats. Am J Physiol Regul Integr Comp Physiol 311: R39–R48, 2016. doi: 10.1152/ajpregu.00124.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Smith SA, Mitchell JH, Garry MG. Electrically induced static exercise elicits a pressor response in the decerebrate rat. J Physiol 537: 961–970, 2001. doi: 10.1113/jphysiol.2001.012918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kumazawa T, Mizumura K. Thin-fibre receptors responding to mechanical, chemical, and thermal stimulation in the skeletal muscle of the dog. J Physiol 273: 179–194, 1977. doi: 10.1113/jphysiol.1977.sp012088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Franz M, Mense S. Muscle receptors with group IV afferent fibres responding to application of bradykinin. Brain Res 92: 369–383, 1975. doi: 10.1016/0006-8993(75)90323-6. [DOI] [PubMed] [Google Scholar]
- 23. Smith SA, Leal AK, Williams MA, Murphy MN, Mitchell JH, Garry MG. The TRPv1 receptor is a mediator of the exercise pressor reflex in rats. J Physiol 588: 1179–1189, 2010. doi: 10.1113/jphysiol.2009.184952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li Q, Garry MG. A murine model of the exercise pressor reflex. J Physiol 598: 3155–3171, 2020. doi: 10.1113/JP277602. [DOI] [PubMed] [Google Scholar]
- 25. Pedersen PK, Kiens B, Saltin B. Hyperoxia does not increase peak muscle oxygen uptake in small muscle group exercise. Acta Physiol Scand 166: 309–318, 1999. doi: 10.1046/j.1365-201x.1999.00575.x. [DOI] [PubMed] [Google Scholar]
- 26. Roh J, Hwang SM, Lee SH, Lee K, Kim YH, Park CK. Functional expression of Piezo1 in dorsal root ganglion (DRG) neurons. Int J Mol Sci 21: 3834, 2020. doi: 10.3390/ijms21113834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Richardson J, Kotevski A, Poole K. From stretch to deflection: the importance of context in the activation of mammalian, mechanically activated ion channels. FEBS J 289: 4447–4469, 2022. doi: 10.1111/febs.16041. [DOI] [PubMed] [Google Scholar]
- 28. Lewis AH, Cui AF, McDonald MF, Grandl J. Transduction of repetitive mechanical stimuli by Piezo1 and Piezo2 ion channels. Cell Rep 19: 2572–2585, 2017. doi: 10.1016/j.celrep.2017.05.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Copp SW, Kim JS, Ruiz-Velasco V, Kaufman MP. The mechano-gated channel inhibitor GsMTx4 reduces the exercise pressor reflex in decerebrate rats. J Physiol 594: 641–655, 2016. doi: 10.1113/JP271714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sanderson BC, Rollins KS, Hopkins TD, Butenas AL, Felice KP, Ade CJ, Copp SW. GsMTx4 reduces the reflex pressor response during dynamic hindlimb skeletal muscle stretch in decerebrate rats. Physiol Rep 7: e13974, 2019. doi: 10.14814/phy2.13974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Copp SW, Kim JS, Ruiz-Velasco V, Kaufman MP. The mechano-gated channel inhibitor GsMTx4 reduces the exercise pressor reflex in rats with ligated femoral arteries. Am J Physiol Heart Circ Physiol 310: H1233–H1241, 2016. doi: 10.1152/ajpheart.00974.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Grotle AK, Garcia EA, Harrison ML, Huo Y, Crawford CK, Ybarbo KM, Stone AJ. Exaggerated mechanoreflex in early-stage type 1 diabetic rats: role of Piezo channels. Am J Physiol Regul Integr Comp Physiol 316: R417–R426, 2019. [Erratum in Am J Physiol Regul Integr Comp Physiol 316: R725, 2019]. doi: 10.1152/ajpregu.00294.2018. [DOI] [PubMed] [Google Scholar]
- 33. Nakamura N, Heng P, Hayashi N. Muscle stretching induces the mechanoreflex response in human arterial blood pressure. J Appl Physiol (1985) 134: 1–9, 2023. doi: 10.1152/japplphysiol.00418.2022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available upon reasonable request.